Volume 11, Issue 1 (2025)
Pharm Biomed Res 2025, 11(1): 49-60 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dawoud A D H, Abdalbagi M, Shayoub M E H. Formulation and Assessment of Drug Release and Stability of New Antipsoriatic Emulgel. Pharm Biomed Res 2025; 11 (1) :49-60
URL: http://pbr.mazums.ac.ir/article-1-664-en.html
URL: http://pbr.mazums.ac.ir/article-1-664-en.html
1- Department of Drug Formulation, Medicinal and Aromatic Plants & Traditional Medicine Research Institute, National Center of Research, Khartum, Sudan. & Department of Pharmaceutics, Faculty of Pharmacy, National Ribat University, Khartum, Sudan.
2- Department of Microbiology & Parasitology, Medicinal and Aromatic Plants & Traditional Medicine Research Institute, National Center of Research, Khartum, Sudan.
3- Department of Pharmaceutics, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan.
2- Department of Microbiology & Parasitology, Medicinal and Aromatic Plants & Traditional Medicine Research Institute, National Center of Research, Khartum, Sudan.
3- Department of Pharmaceutics, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan.
Full-Text [PDF 1272 kb]
(377 Downloads)
| Abstract (HTML) (1476 Views)
Full-Text: (655 Views)
Introduction
There is currently no cure for psoriasis, an auto-immune disease that affects the skin and is an abnormal proliferative skin disorder involving genetic and environmental factors [1]. The synthetic drugs currently available to treat psoriasis have not fully met the needs of those who suffer from it, primarily because of several issues, including resistance to treatment after prolonged drug exposure and the possibility that some treatments (like phototherapy) may increase the risk of cancer [2]. Additionally, the effectiveness of the treatment may decrease over time and require replacement by another therapy.
Given everything mentioned above, we gathered various Sudanese medicinal herbs that have long been used to treat psoriasis at the Medicinal and Aromatic Plant and Traditional Medicine Research Institute. We then conducted tests to identify, assess, and choose the best antipsoriatic plants. The results show Aloe sinkatana was the most effective antipsoriatic herb [3].
The antipsoriatic activity of the plant was assessed by examining the effects of 0.1% and 0.2% extracts of A. sinkatana on five mouse groups (imiquimod-induced psoriasis mice model) and using dexamethasone as a control. According to the data, the groups that received 0.2% A. sinkatana extract, 0.1% A. sinkatana extract, and the usual medication dexamethasone experienced a reduction in psoriasis-like symptoms of 86%, 84%, and 68%, respectively [3].
Additionally, the safety of A. sinkatana was investigated by performing acute dermal toxicity using two groups of ten rats and applying 2000 mg/kg of A. sinkatana extract topically on the skin of experimental animals. The results show that the A. sinkatana plant is safe and has no harmful effects when applied topically, which supports its use in traditional Sudanese medicine to treat psoriasis [3].
The medicinal plant A. sinkatana grows natively in the Red Sea Mountains in Eastern Sudan, primarily in the Sinkat region. Aloe-emodin (AE) and microdontin chemicals are present. These two compounds were reported to exert strong suppressive effects on T-cell proliferation with an IC50 of 9.2 and 7.4 mg/L, respectively, and a suppressive interleukin (IL)-2 generation activity with an IC50 of 1.1 and 1.9 mg/L for AE and microdontin, respectively [4]. A hydroxyanthraquinone generated from plants, AE, has been shown to have several biological properties, including antiproliferative activity [5]. At 5 μM, AE dramatically reduced the growth of cultured human keratinocytes [6]. According to Pandey et al., psoriatic skin treated with topical hydrogel filled with AE showed decreased epidermal thickness [7].
In light of all of this, the primary goal of this research is to create and assess a novel emulgel using plant extract from A. sinkatana as an antipsoriatic treatment.
Applying a drug-containing formulation directly to the skin to treat cutaneous conditions is known as topical drug delivery [8]. Topical drugs have the primary benefit of avoiding first-pass metabolism.
One easy way to get localized medication into the bloodstream is topical administration. For their systemic or local action, drugs are applied topically [9].
Other medications can now enter the bloodstream and be absorbed via the skin thanks to new technologies. These can treat the entire body, not only the skin’s afflicted parts [10]. When other topical medication delivery methods cannot directly treat cutaneous problems, emulgel (gellified emulsion) is typically utilized [11]. Emulsion gels have gained significant traction in pharmaceutical semisolid dosage forms since the mid-1980s. Nowadays, emulgel is a widely used drug delivery system [12].
Emulgel is a blend of gels and emulsions; the emulsions might be water-in-oil or oil-in-water. The emulsion is transformed into an emulgel by the gelling agent found in the water phase. While water-in-oil systems encapsulate hydrophilic pharmaceuticals, oil-in-water systems directly entrap lipophilic medications [13, 14]. Gel and the emulsion control the systems’ regulated medication release [15]. The two types of gels are water-based, hydrophilic, or hydrogels and organic solvent-based, hydrophobic, or organogels [16].
Emulgel exhibits superior drug release and stability and can be utilized to extend the effects of medications with shorter half-lives. Additionally, emulgel preparation components are readily available and less expensive, which lowers the cost of producing emulgels [17, 18, 19].
Materials and Methods
The standard compound
The standard compound of AE was a gift from Professor Masaki of Kobe University in Japan.
Plant collection and authentication
The A. sinkatana plants were collected from the Sinkat area, Red Sea state, Sudan, and authenticated by the Department of Chemistry, Medicinal, and Aromatic Plants Research Institute and Traditional Medicine, National Research Center, Khartoum, Sudan.
Extraction of A. sinkatana
Fresh water was used to wash mature, healthy, fresh A. sinkatana leaves between 75 and 90 cm long.
The inside gel was cut into pieces by scraping it off. Aloe leaves were processed using the age-old hand filleting method. Sharp blades cut off the lower leaf base, the tapering tip at the leaf apex, and the short spines along the leaf edges. After that, the top rind was cut off, and the blade was inserted into the mucilage layer beneath the green rind to avoid the vascular bundles. The leaves firm, colorless mucilaginous gel was chopped into pieces after the epidermis was peeled off. Afterward, 500 mL of solvent (ethanol) was added to a 1000 mL flask containing 250 g of gel. For 60 minutes, ultrasound-assisted extraction was carried out at 60 °C. The solution was then filtered, and the solvent was extracted in a rotary evaporator at a lower pressure until it was scorched [20].
Formulation design using 2³ full factorial design
To obtain the “best” or an “optimized product”, eight different formulations were generated using two levels, three-factor, full factorial design.
A 2³ factorial design for three factors at two levels each was selected to optimize the varied response variables. The amount of gelling agent X1, emulsifying agent X2, and liquid paraffin X3 were chosen as independent formulation variables, and the factor levels were suitably coded (Table 1).
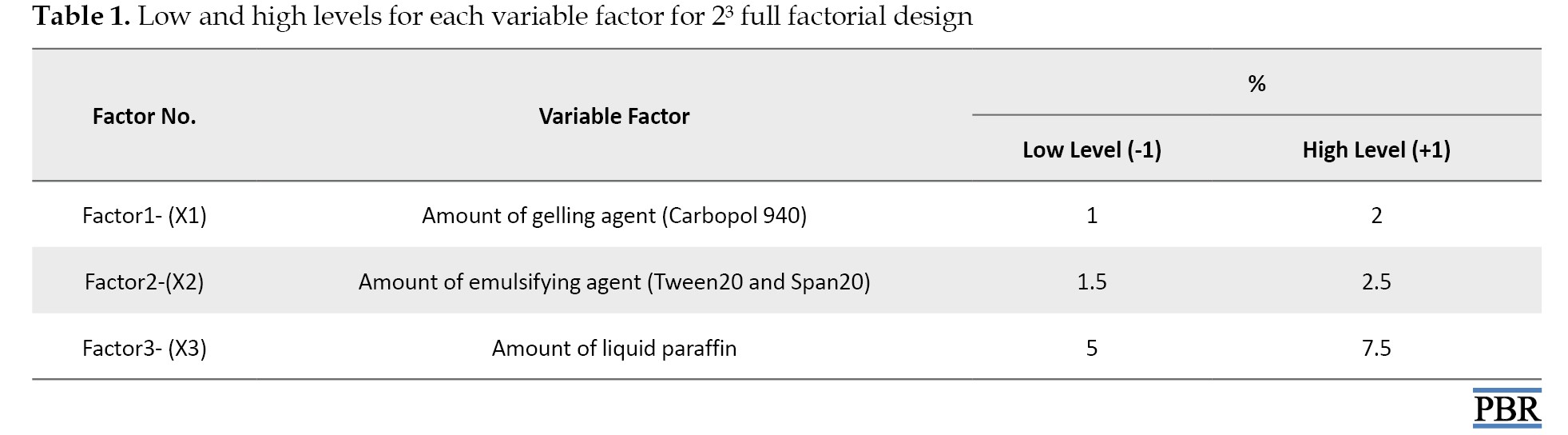
Experimental trials were performed at all 8 possible combinations (Table 2).
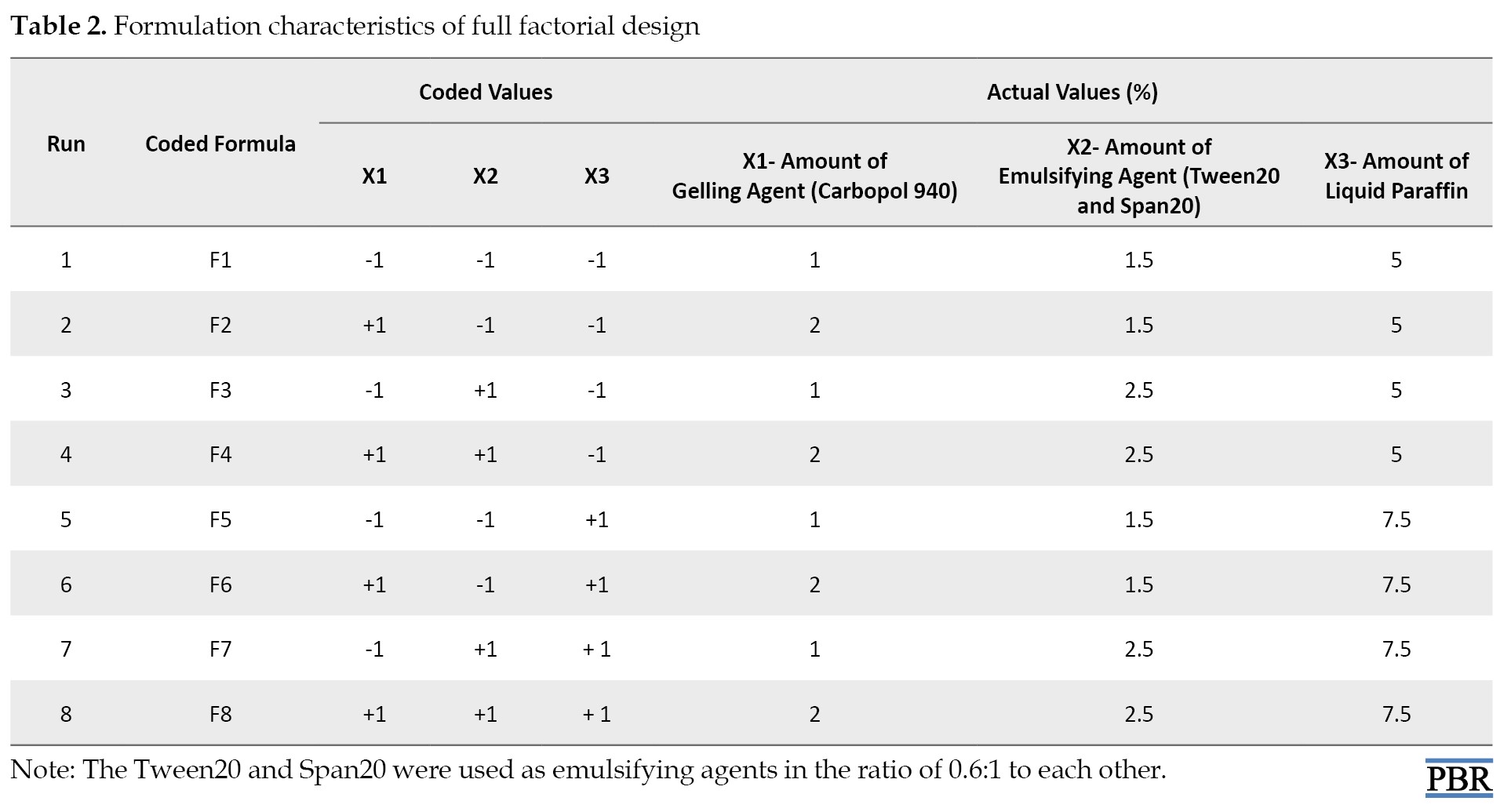
All other formulation and processing variables were kept invariant throughout the study [21].
Design-Expert software, version 8 was used to generate and evaluate the statistical experimental design.
Preparation of emulgel
Table 3 displays the composition of emulgel formulations.
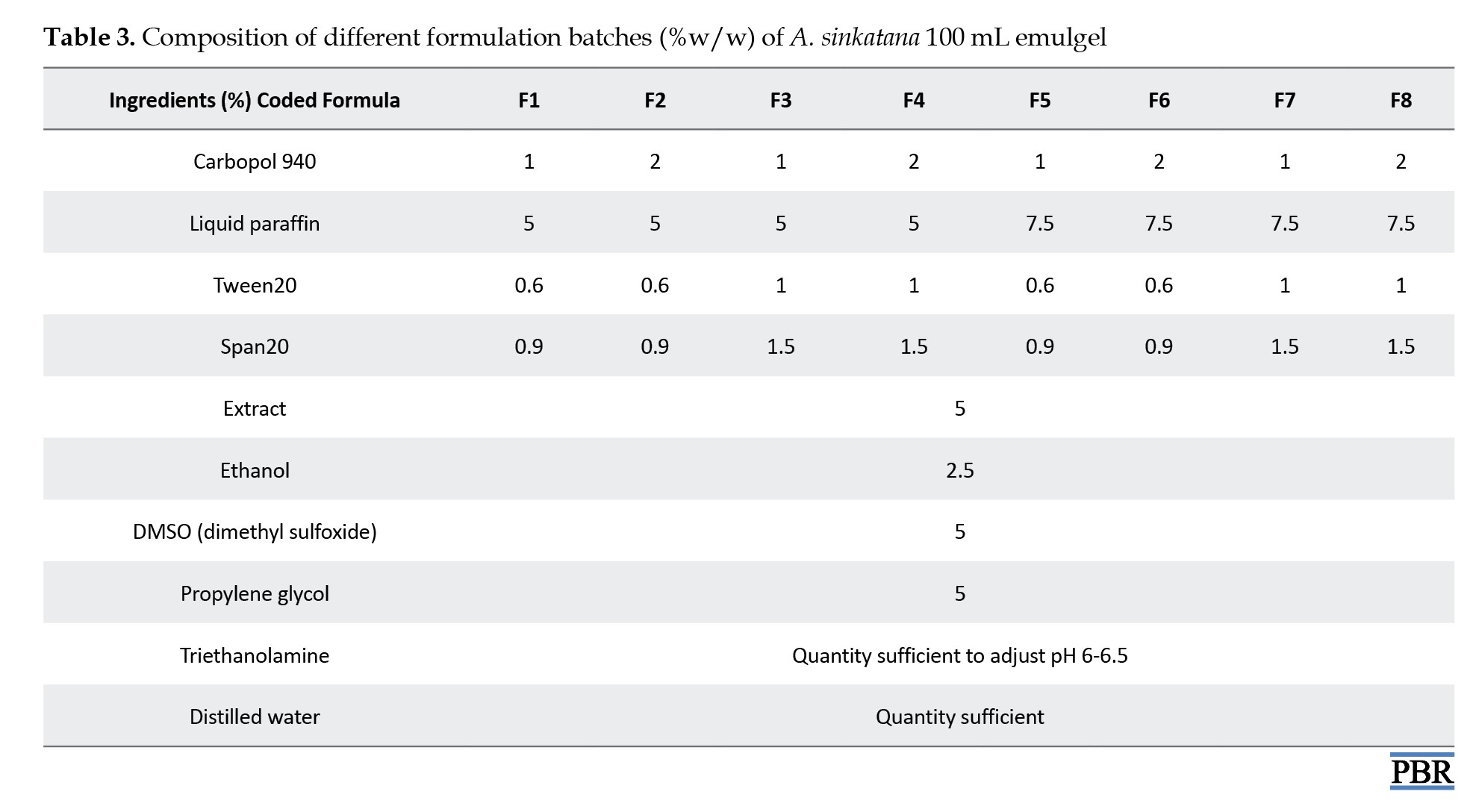
Carbopol 934 was first dissolved in 80 °C hot filtered water to create the gel, which was then allowed to cool and sit overnight. Span20 was dissolved in liquid paraffin to create the emulsion’s oil phase, while Tween20 was dissolved in filtered water to make the aqueous phase. Both solutions were combined with the aqueous phase after propylene glycol was dissolved in ethanol and plant extract in DMSO. After heating the aqueous and oily phases independently to 70 °C to 80 °C, the oily phase was gradually introduced to the aqueous phase while constantly stirring until it cooled to room temperature.
To formulate the emulgel, the resulting emulsion and gel were combined in a 1:1 ratio while gently stirring. Lastly, triethanolamine changed the emulgel’s pH [22, 23].
Macroscopic evaluations
The color, texture, homogeneity, consistency, and phase separation of the 8 batches of emulgel formulas were examined under a microscope [24, 25].
Measurement of pH
A pH meter (Max Instruments Chandigarh, India) calibrated before each reading was used to measure the pH of 1% aqueous solutions of the prepared A. sinkatana emulgel using buffered solutions at pH 4.0 and 7.0. Each formulation’s pH was measured thrice, and the average results were computed [26-28].
Viscosity test
The viscometer VR 3000 (Viscotech, Spain) was used to measure viscosity at room temperature by selecting the proper spindle number (L4) and speed (100 rpm). The spindle groove was dipped, the rpm was set, and a reasonable amount of each emulgel formulation was maintained in a suitable beaker. Viscosity measurements were initiated, and each formulation’s viscosity was computed by measuring the data after one minute [29, 30].
Spreadability test
The spreadability was determined by the parallel plate method, which is widely used to measure the spreading diameter of 1 gm of emulgel between two horizontal plates (30×30 cm) after one minute [31].
Calibration curve of standard AE compound
A standard 100 µg/mL (stock solution) was obtained by dissolving the precisely weighed AE standard material (0.01 g) in methanol using an ultrasonic shaker in a 100 mL volumetric flask. Different stock solution concentrations (2 µg/mL, 4 µg/mL, 6 µg/mL, 8 µg/mL, 10 µg/mL, and 12 µg/mL) were prepared, and a UV spectrophotometer was used to measure the absorbance of the various dilutions at 430 nm. Plotting the absorbance against concentration allowed for the determination of the calibration curve.
Determination of AE content in A. sinkatana extract
About 0.5 g of dried extracts were dissolved by ultrasonic shaker in 100 mL of methanol (0.5%). The absorbance of the resultant solution was read at wavelength 430 nm using a UV spectrophotometer.
Determination of drug content
To determine the AE compound content in the different formulas of A. sinkatana extract was weighted 5 g of emulgel formula and dissolved by an ultrasonic shaker in 50 mL of methanol (10%). The absorbance of the resultant solution was read at a wavelength of 430 nm using a UV spectrophotometer. The percentage of drug content was calculated.
In vitro drug released study
The drug-released research was conducted using a manually constructed apparatus replicating Franz diffusion cells via a dialysis membrane. Diffusion investigations were conducted at 37 °C using methanol as the dissolution medium after a 1 g sample of emulgel was placed in a dialysis membrane that had been soaked in methanol. Five milliliters of the sample were taken out at intervals of 0, 1, 2, 3, 5, 6, 7, and 8 hours. A similar volume of dissolving media was used to replace each sample. Methanol was used as a plank to evaluate the samples for drug content [32].
Finally, % drug released=concentration of drug×volume of receptor compartment/amount of drug in the donor compartment
Drug release kinetics studies
Drug release kinetics was examined by fitting the results from ex vivo drug release investigations into several drug release kinetics models. Zero-order, first-order, and Higuchi plots were made for this investigation. Plotting the cumulative percent medication penetrated on the vertical axis against time (in hours) on the horizontal axis created zero-order plots. Plotting the log cumulative percent of medication remaining against time (in hours) allowed researchers to examine the formulations for first-order kinetics.
The Higuchi plots were also obtained by comparing the X-axis’s square root of time (in hours) with the cumulative percent drug permeated taken on the Y-axis. The goodness-of-fit test was used to determine which model was best. The plot that produced the highest correlation coefficient value, or R2, was considered the most suitable for the formulation [33].
The mechanism of drug release from the topical gel analyzed by fitting the release data
Zero-order equation
Q=k0t
, where Q is the amount of drug released at time t, and k0 is the zero-order release rate.
First–order equation
In (100 –Q)=In 100–k1t
, where Q is the percentage of drug release at time t, and k1 is the first–order release rate constant.
Higuchi’s equation
Q=k2√t
, where Q is the percentage of drug release at time t, and k2 is the diffusion rate constant.
Accelerated stability studies
The emulgel formula was packed in a clean, dry, ampere container and subjected to stability studies at 25°C/60% RH, 30°C/65% RH, and 40°C/75% RH for 3 months. Samples were drawn at predetermined intervals 1, 2, and 3 and evaluated for physical appearance (visually inspected for any change in color and odor), phase separation, drug content, pH, and microbial growth [34].
Statistical analysis
The data obtained was statistically analyzed by the Design-Expert software, version 8 statistical program.
Results and Discussion
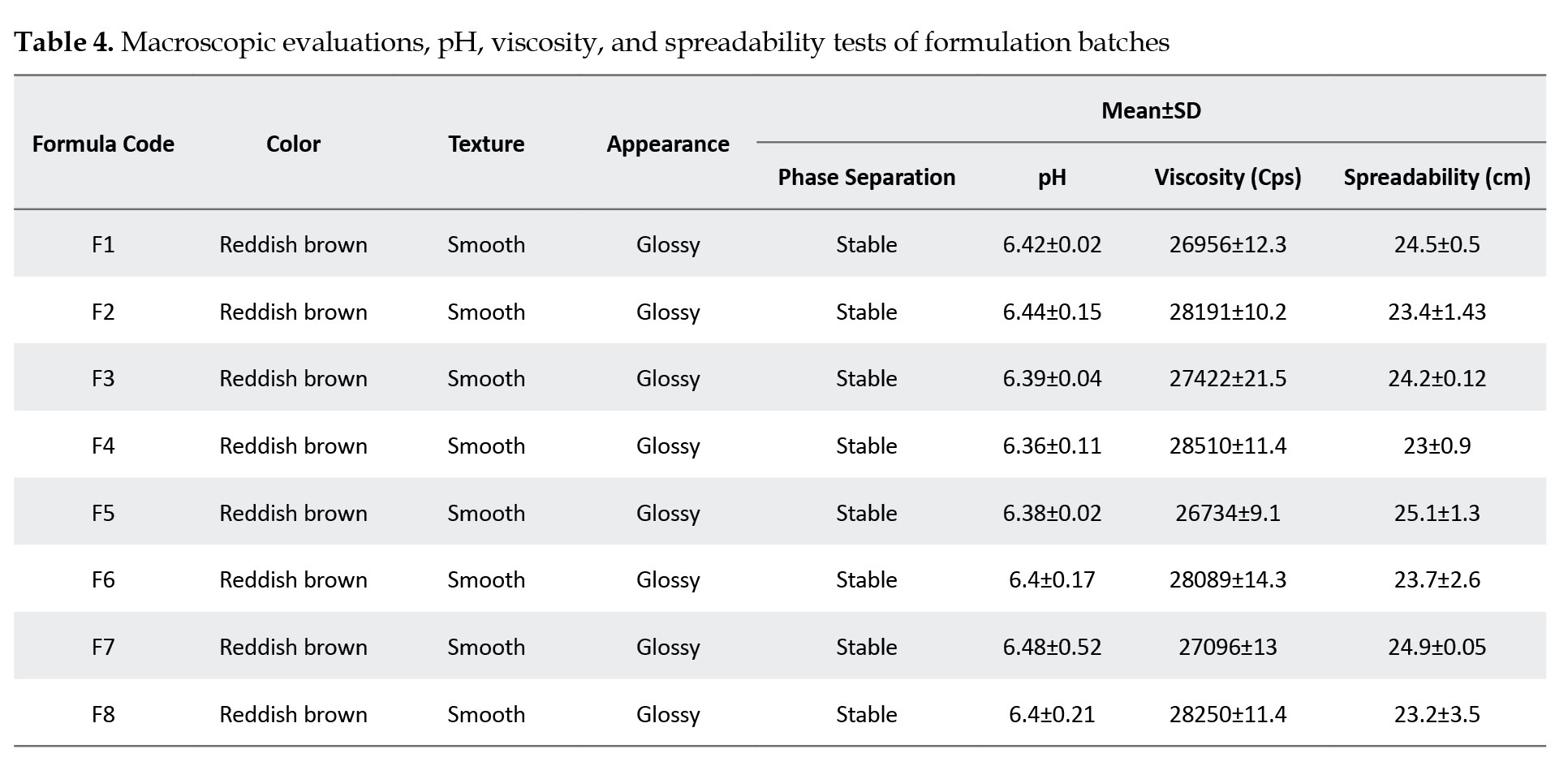
The macroscopic evaluations, measured pH, and viscosity values of the prepared emulgel formulations are given in Table 1. The emulgel formulas are glossy and reddish brown; all formulations are homogeneous, stable, and smooth in texture.
The pH values of the formulations range from 6.36 to 6.48, which is considered acceptable to avoid the risk of irritation upon application to the skin (within the pH range of the human skin). The incorporation of triethanolamine achieved this index. Historically, an acidic skin surface’s physiologic role was considered a defense mechanism against invading organisms. More recently, it has been demonstrated that several key enzymes involved in the synthesis and maintenance of a competent skin barrier are largely impacted by pH. Hence, a broader view of the importance of pH in the function and integrity of the skin is emerging [35].
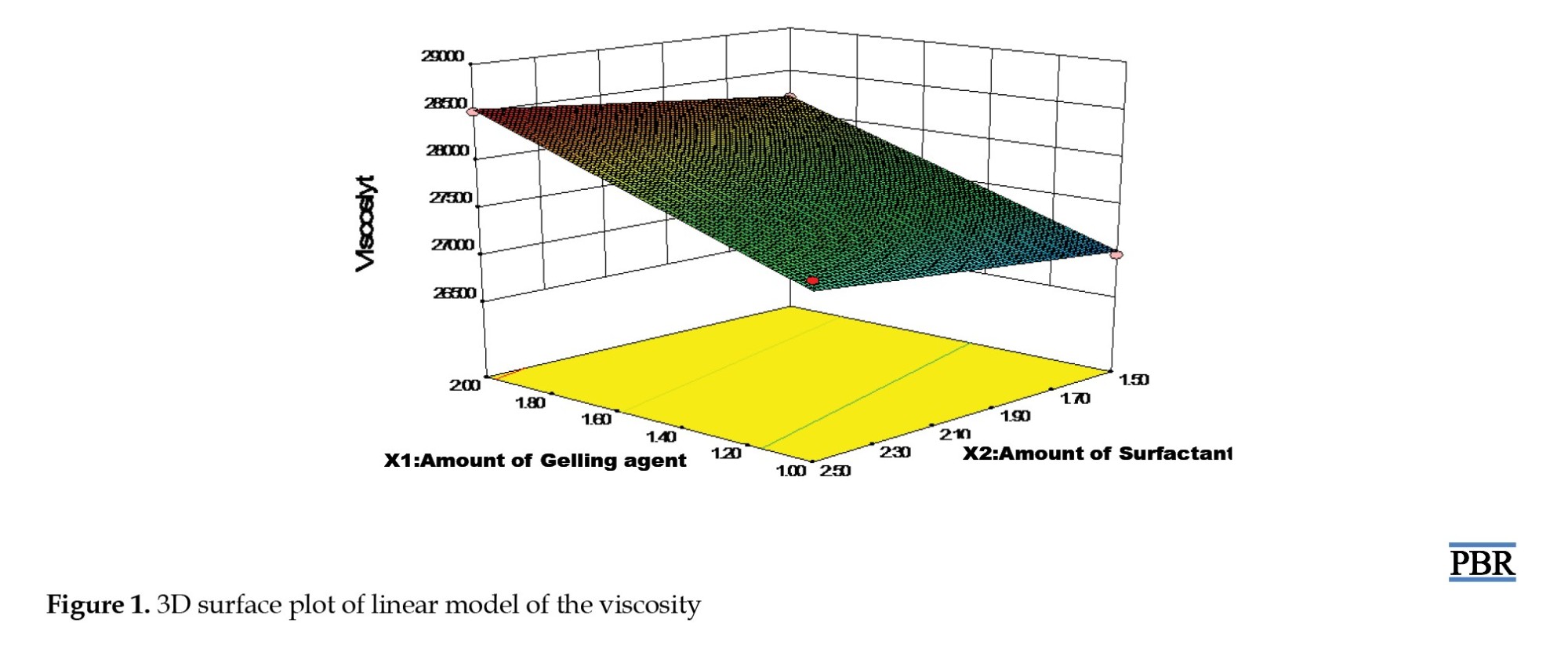
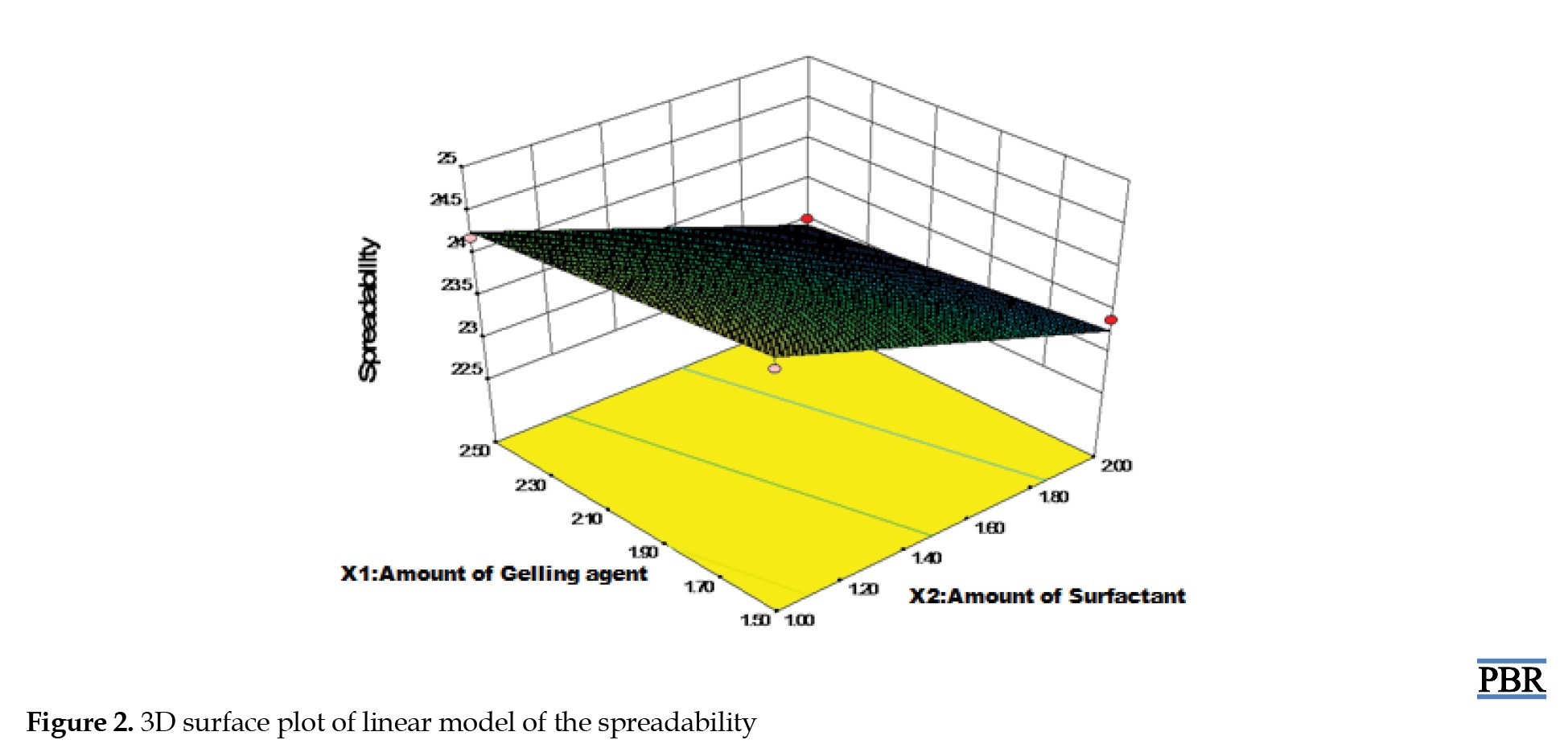
Results of the viscosity test showed that the viscosity increased as the amount of gelling agent and a surfactant increased and vice versa; the viscosity ranged between 26734 to 28510 Cps. The results for spreadability ranged between 23.2 cm and 25.1 cm.
Figure 1 displays the viscosity model graph. According to the figure, viscosity rose as a gelling agent, surfactant concentrations rose, and vice versa. The spreadability is directly related to the amount of liquid paraffin (linear model) and indirectly proportional to the amount of gelling agent and surfactant, according to the graphical result in Figure 2. The absorbance for the concentration of the AE standard compound is shown in Table 5.
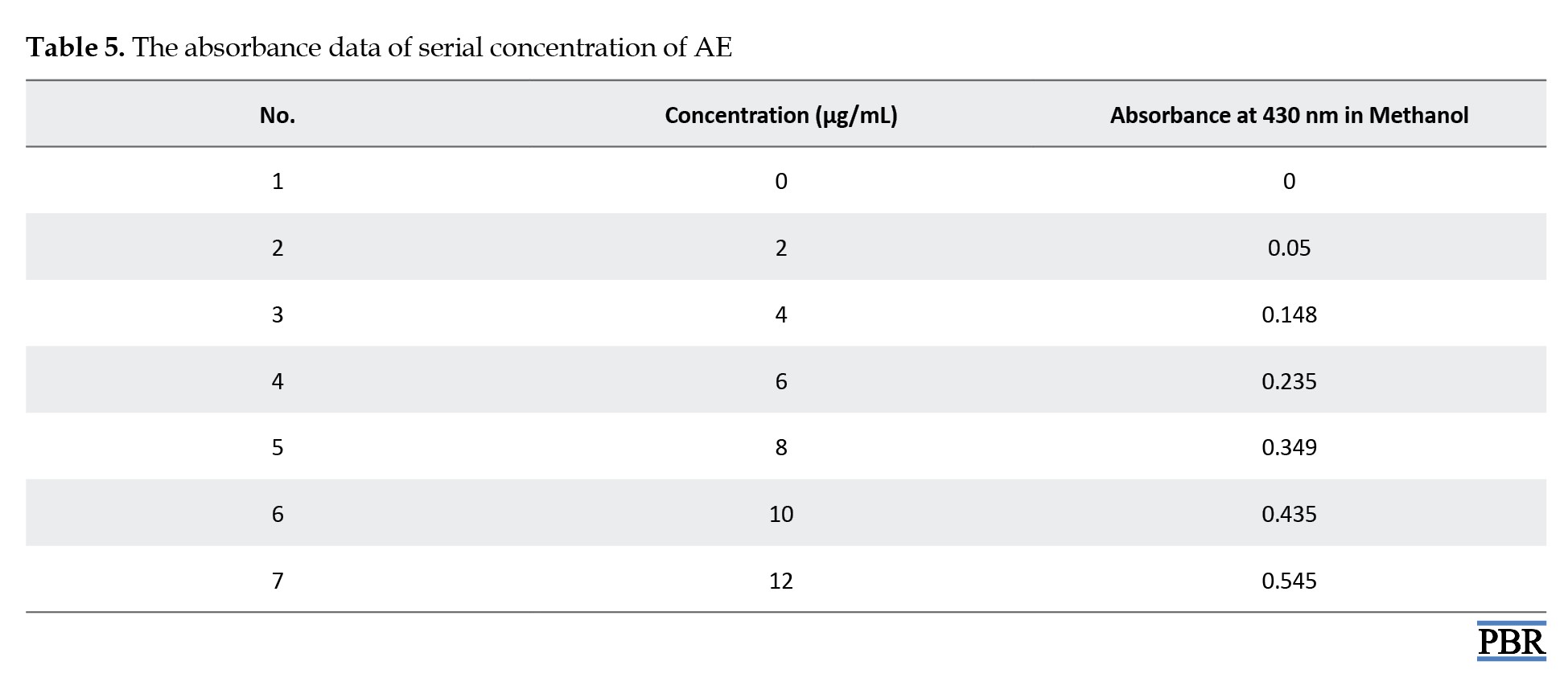
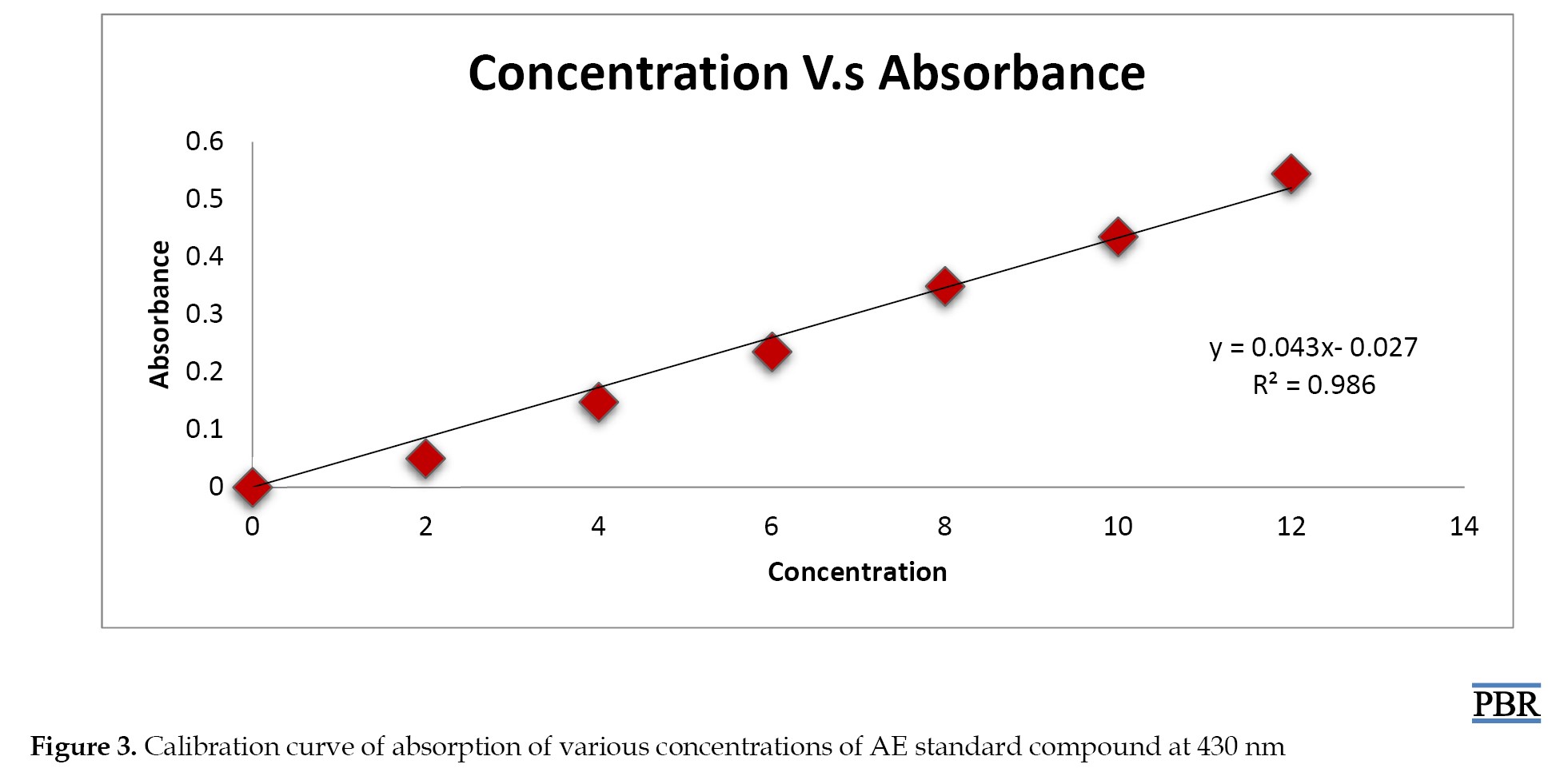
The calibration curve is illustrated in Figure 3. The standard graph of the AE compound shows good linearity with an R² value of 0.986, which indicates that it obeys Beer-Lambert Law in the concentration range of 0-100 µg/mL.
The AE concentration in A. sinkatana extract and the drug content were estimated from Equation 1 of the calibration curve. The final concentration of AE in the plant extract was 4 µg/5 mg=0.08%.
Y= 0.046×X - 0.027
The drug content of the 8 formulae ranged between 92.34% and 99.48%, while the F3 formula shows the highest percent of drug content (Table 6).
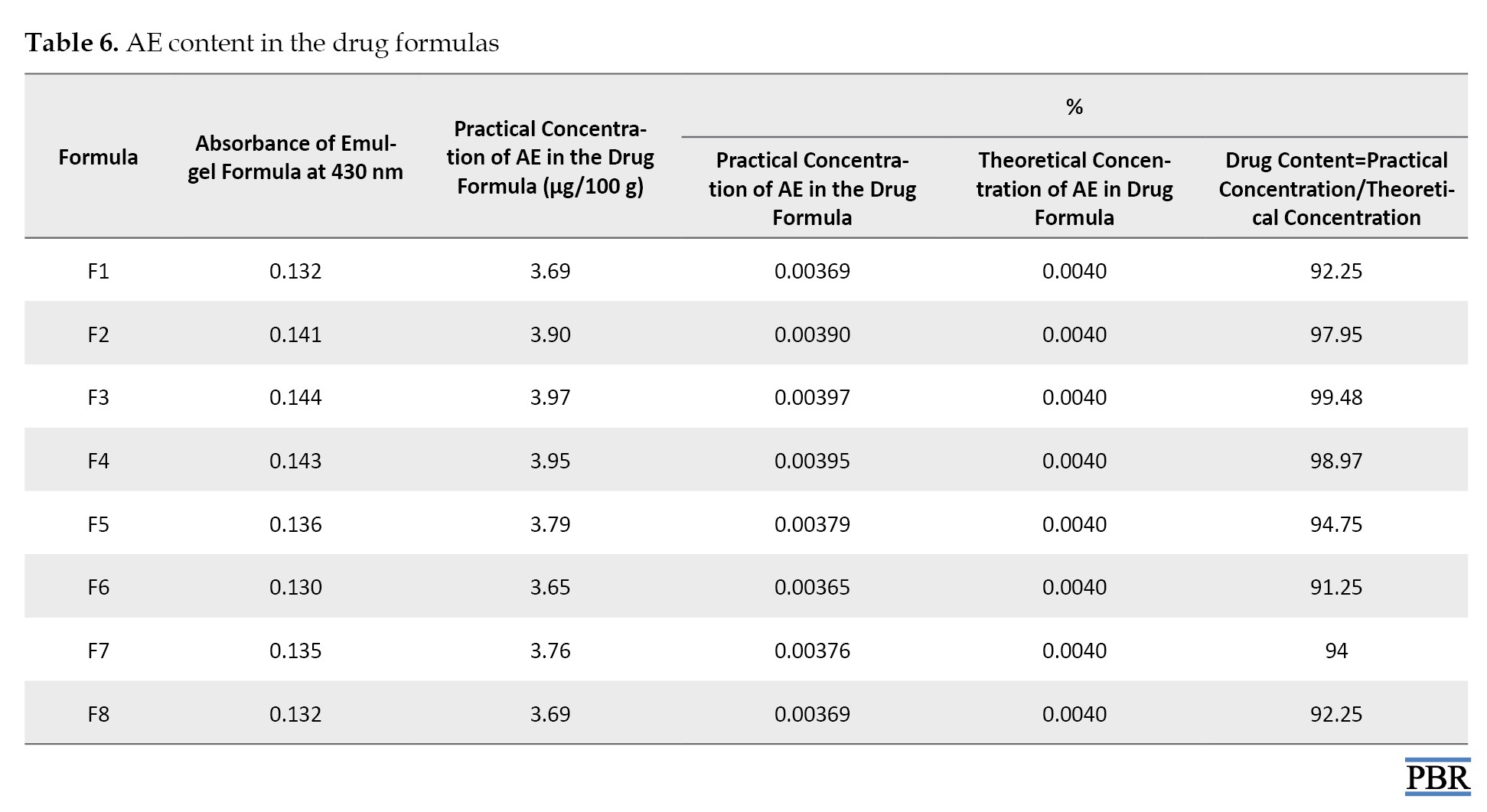
Table 7 presents the diffusion data for the formulations F1 to F8.
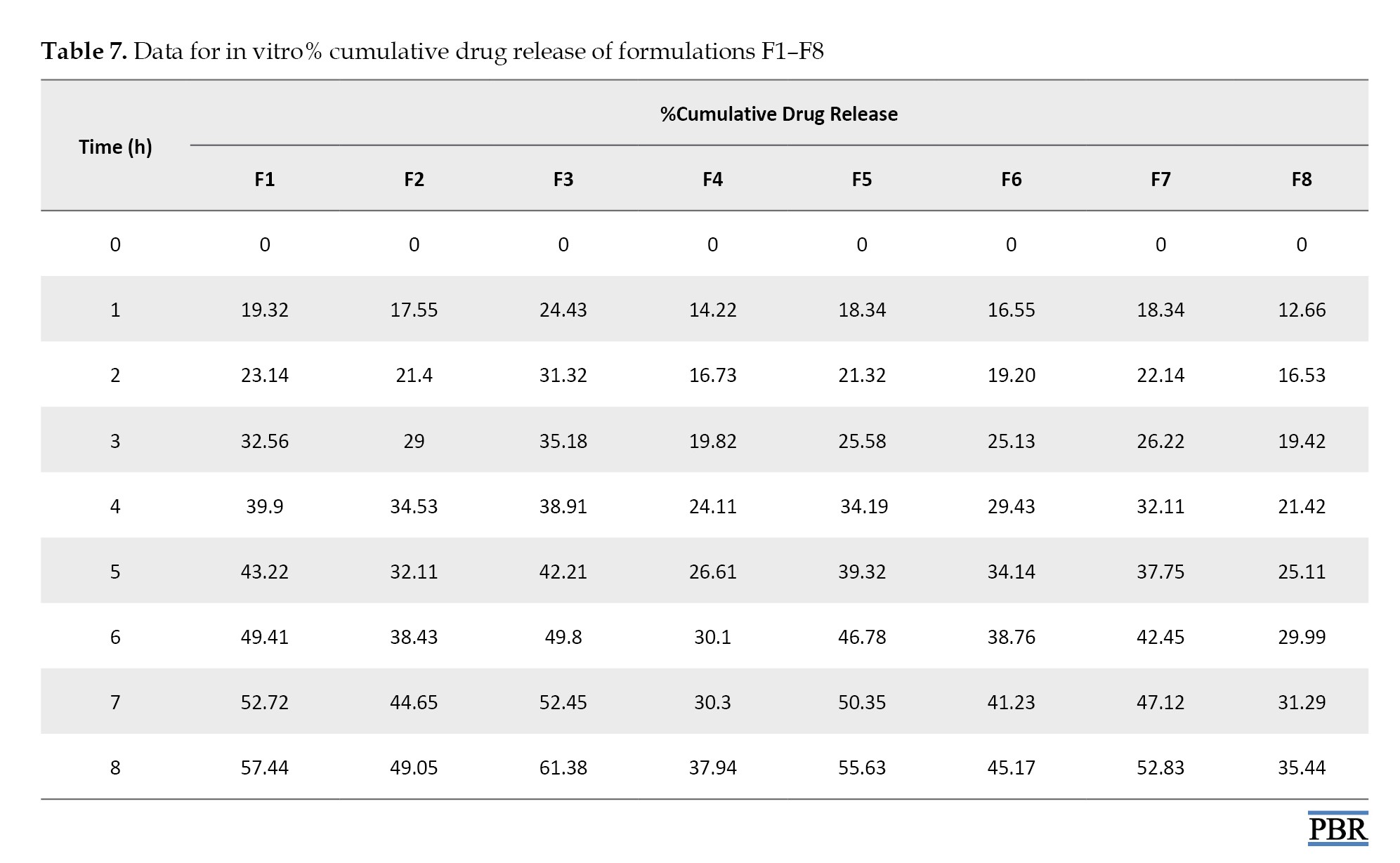
The best release of the drug after 8 h from all emulgel formulations can be observed by F3 (61.38%) and can be ranked in the following descending order: F3 > F1 > F5 >F7 > F2 > F6 >F4>F8.
Based on all evaluation tests performed for the emulgel formulation, the F3 formula shows the best results among all the formulations. Therefore, the F3 formulation was considered the optimized emulgel formula. The in vitro drug-released kinetics studies were performed on the most promising emulgel formula, i.e. F3 (Table 8).
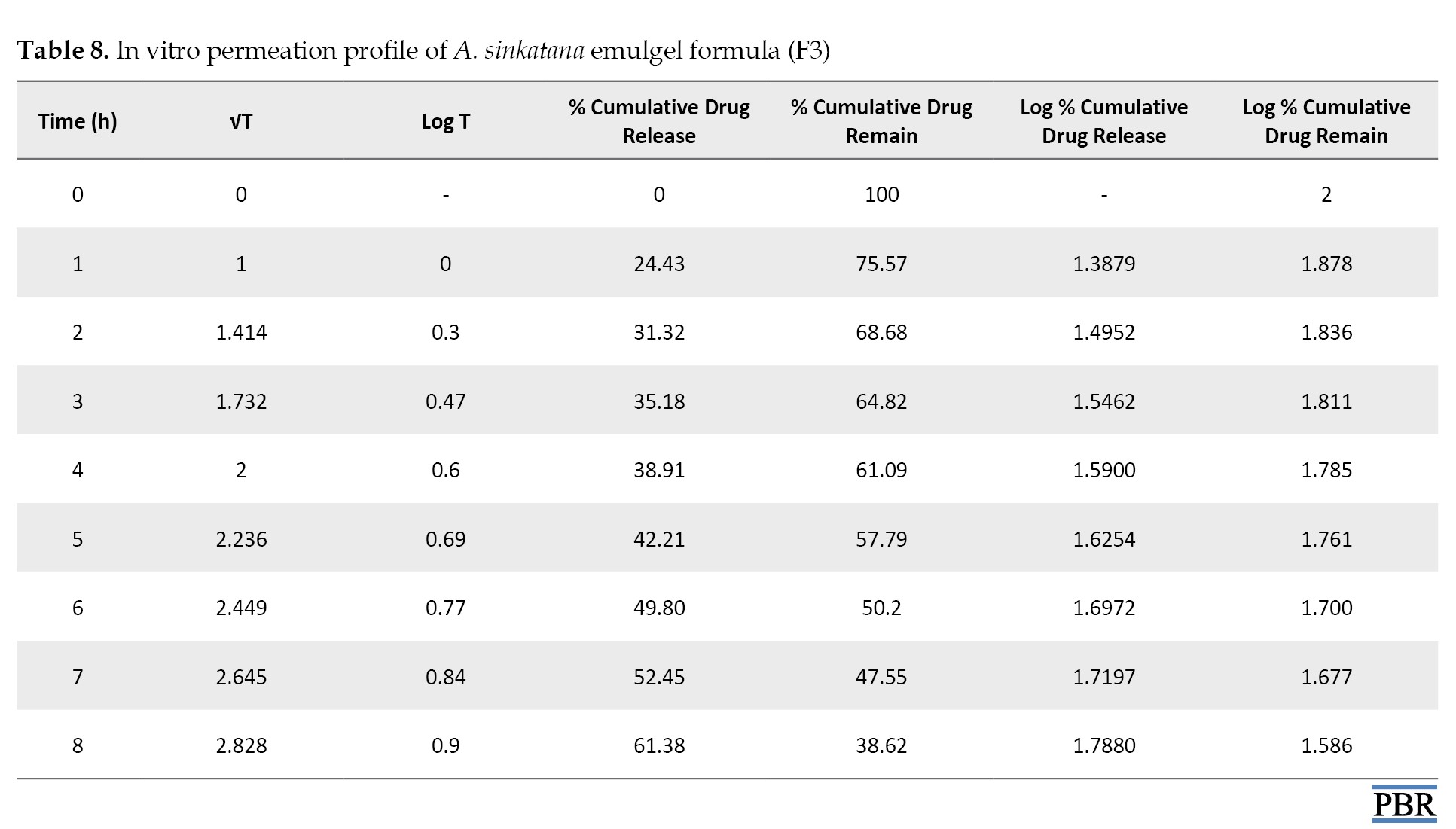
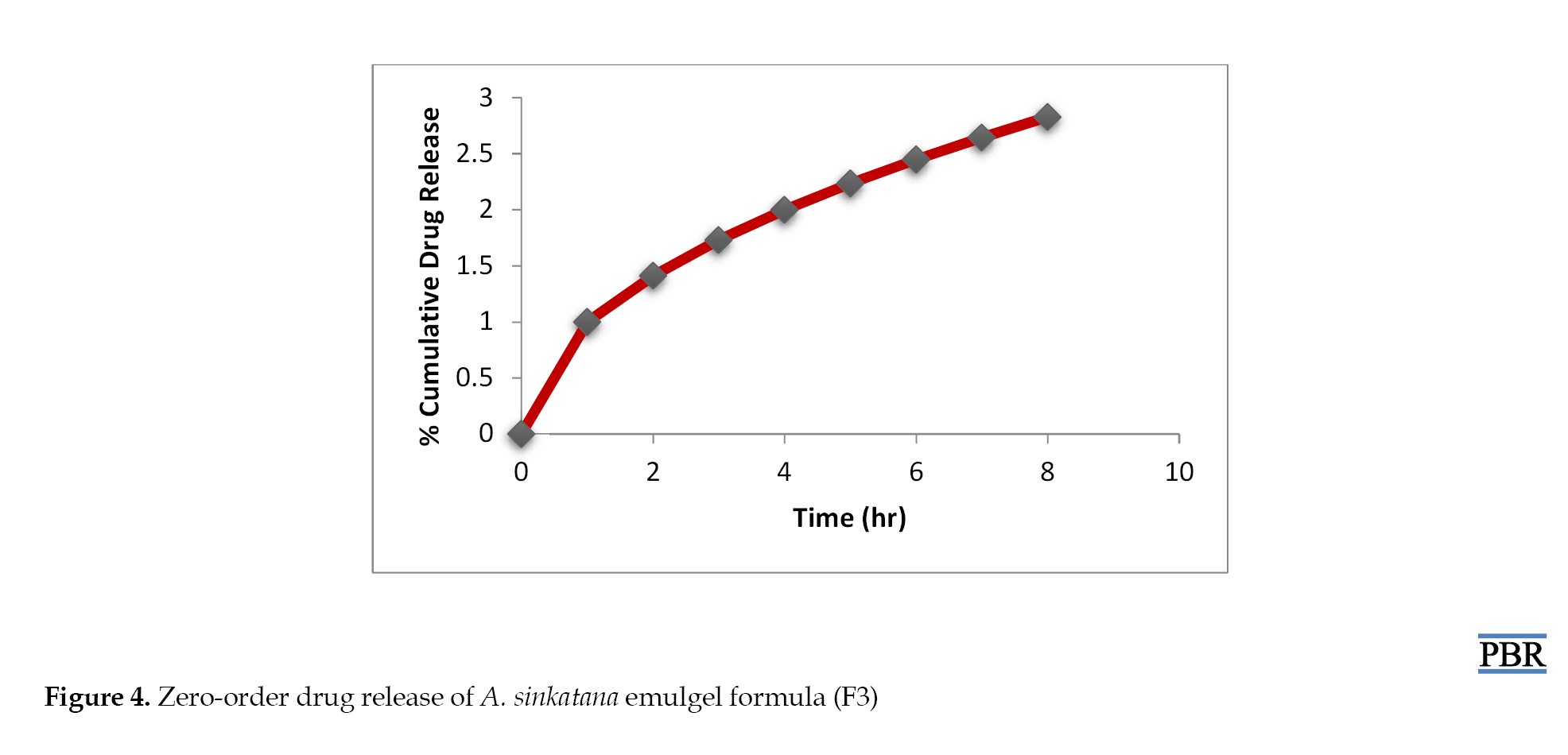
The data from in vitro drug release studies were treated using various conventional mathematical models (zero-order, first-order, and Higuchi) to determine the release mechanism from the designed emulgel formulations.
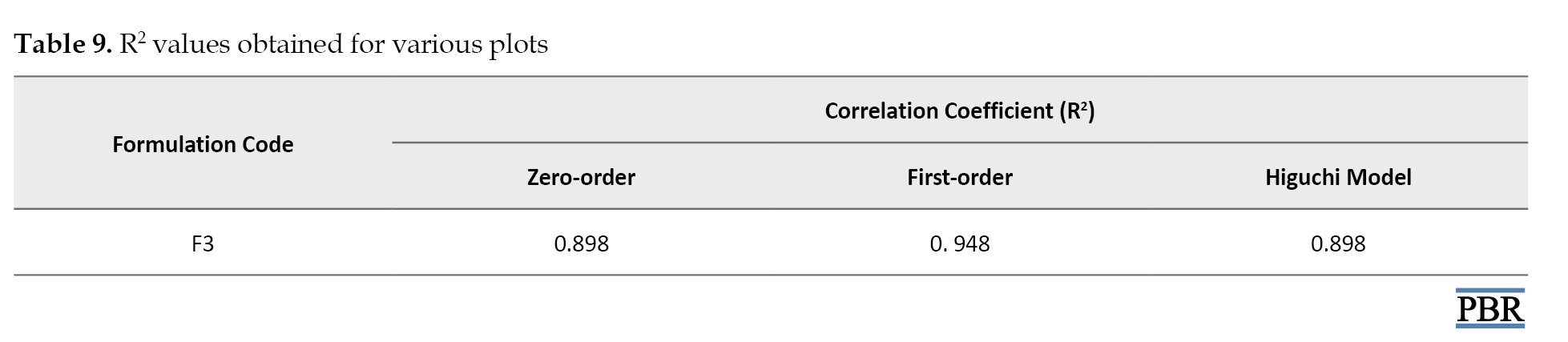
A suitable release model was selected based on the R² (regression coefficient) values.
The regression coefficient of the formula F3 is shown in Table 9.
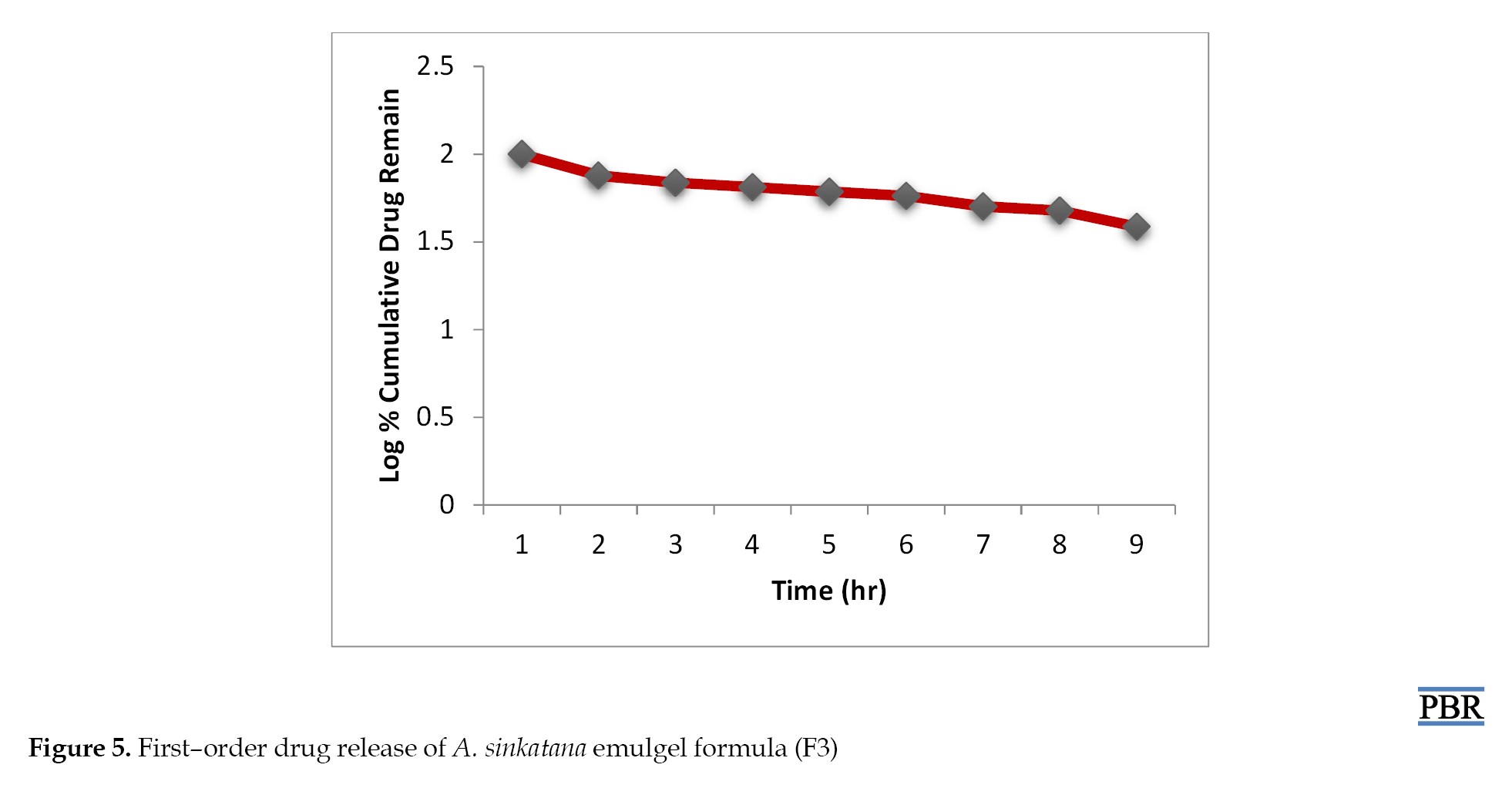
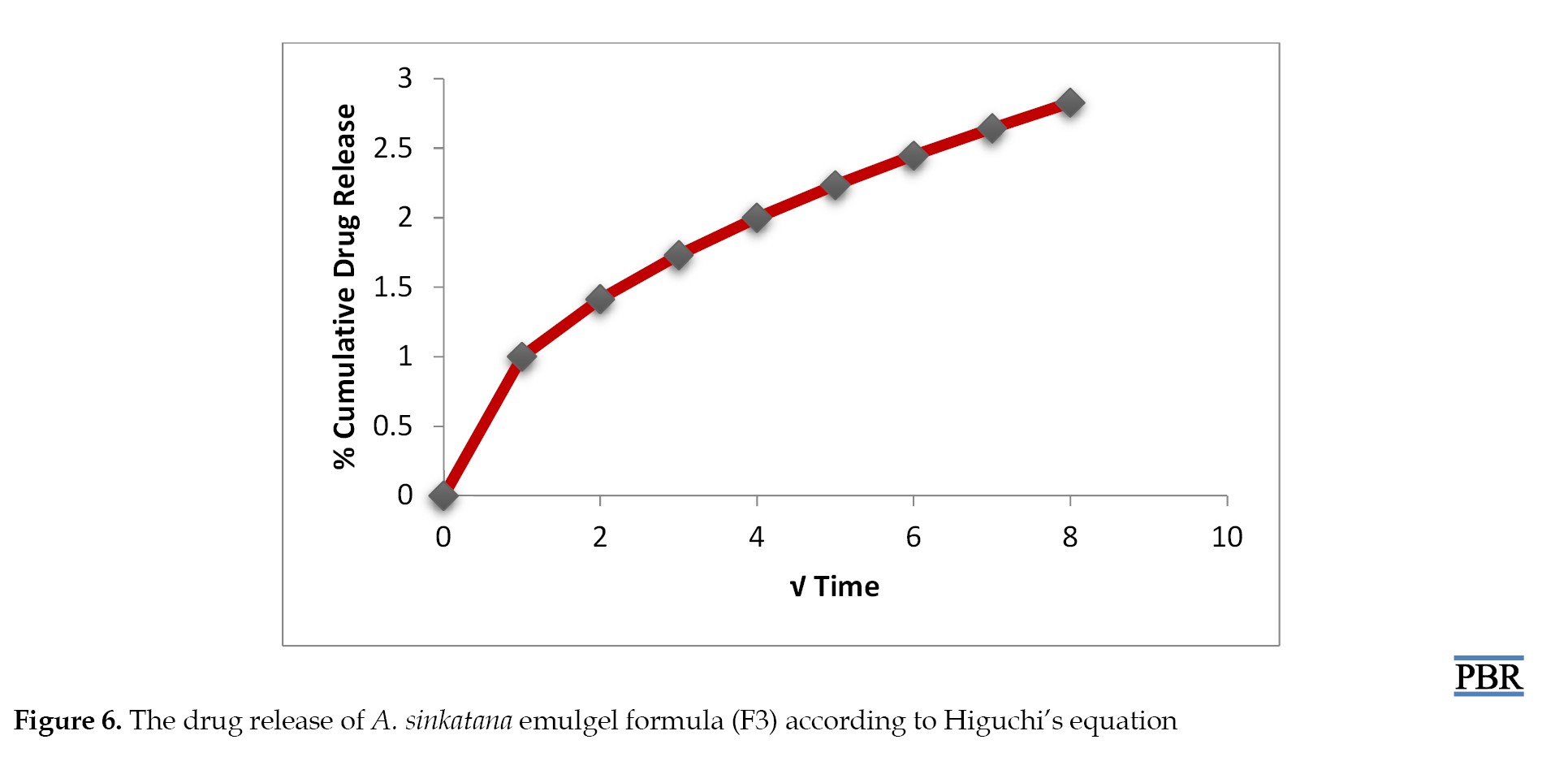
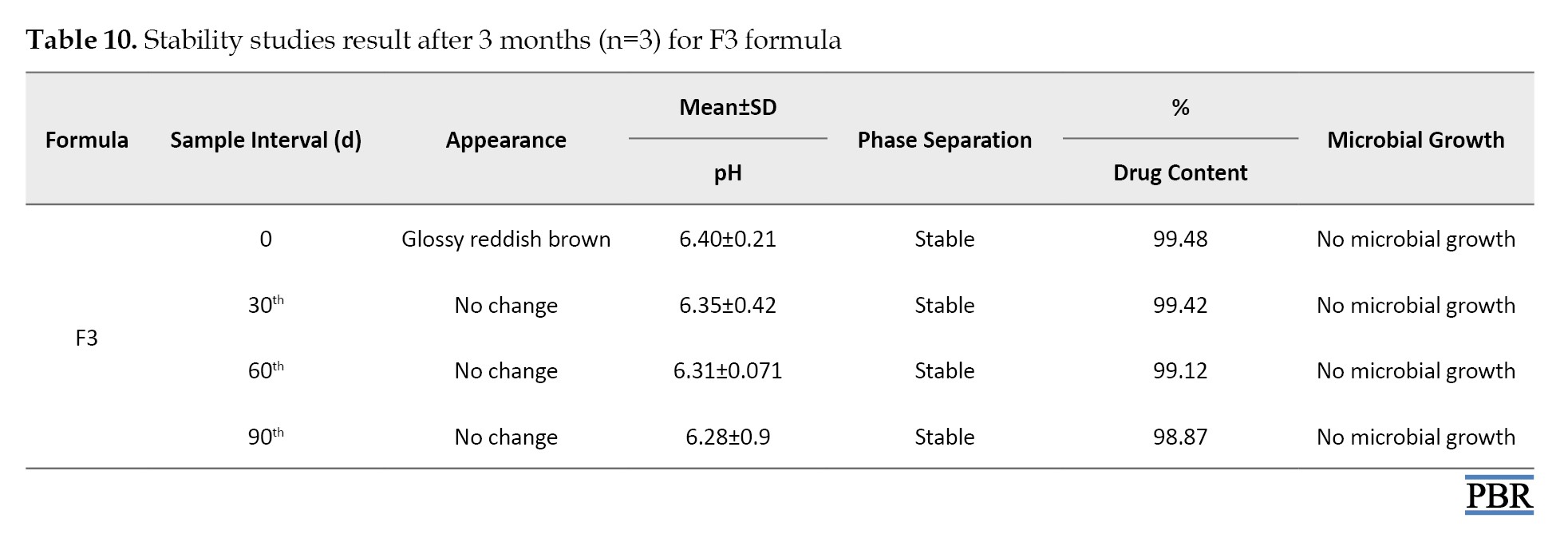
It was found that the formula F3 follows the first-order kinetics (Figures 4, 5 and 6). The stability studies were performed on formula F3. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of various environmental factors such as temperature, humidity, and light and enables recommended storage conditions and shelf lives to be established.
The F3 formula was stable upon storage for 3 months, and no significant change was observed in their physical appearance, pH, or drug content. No microbial growth was observed, and there was no liquefaction or phase separation till the end of the study period in any sample. These results depicted that formulation was stable even at high humidity and temperature of 75% and 40 °C, respectively, as shown in Table 10.
Conclusion
The present study deals with the formulation development and optimization of A. sinkatana emulgel. The optimization was done based on the drug content and in vitro drug release. The kinetic modeling revealed that A. sinkatana emulgel follows the first-order kinetics model. Formulation batch F3 showed the best drug release and was stable upon storage for 3 months, even at high humidity and temperature. Thus, a successful attempt was made to formulate emulgel.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations in this research.
Funding
This research was funded by the Ministry of Higher Education and Scientific Research, National Center for Research, Khartum, Sudan.
Authors' contributions
Investigation, data collection, and writing the original draft: Azza Dawoud Hussien Dawoud; Data analysis: Mohammed Abdalbagi; Supervision: Mohamed El Hassan Shayoub; Review, and editing: Azza Dawoud Hussien Dawoud, and Mohammed Abdalbagi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors take this opportunity to thank Pharmaceutical Department of Faculty of Pharmacy, National Ribat University, Khartoum, Sudan and Medicinal and Aromatic Plants & Traditional Medicine Research Institute, Sudan.
References
There is currently no cure for psoriasis, an auto-immune disease that affects the skin and is an abnormal proliferative skin disorder involving genetic and environmental factors [1]. The synthetic drugs currently available to treat psoriasis have not fully met the needs of those who suffer from it, primarily because of several issues, including resistance to treatment after prolonged drug exposure and the possibility that some treatments (like phototherapy) may increase the risk of cancer [2]. Additionally, the effectiveness of the treatment may decrease over time and require replacement by another therapy.
Given everything mentioned above, we gathered various Sudanese medicinal herbs that have long been used to treat psoriasis at the Medicinal and Aromatic Plant and Traditional Medicine Research Institute. We then conducted tests to identify, assess, and choose the best antipsoriatic plants. The results show Aloe sinkatana was the most effective antipsoriatic herb [3].
The antipsoriatic activity of the plant was assessed by examining the effects of 0.1% and 0.2% extracts of A. sinkatana on five mouse groups (imiquimod-induced psoriasis mice model) and using dexamethasone as a control. According to the data, the groups that received 0.2% A. sinkatana extract, 0.1% A. sinkatana extract, and the usual medication dexamethasone experienced a reduction in psoriasis-like symptoms of 86%, 84%, and 68%, respectively [3].
Additionally, the safety of A. sinkatana was investigated by performing acute dermal toxicity using two groups of ten rats and applying 2000 mg/kg of A. sinkatana extract topically on the skin of experimental animals. The results show that the A. sinkatana plant is safe and has no harmful effects when applied topically, which supports its use in traditional Sudanese medicine to treat psoriasis [3].
The medicinal plant A. sinkatana grows natively in the Red Sea Mountains in Eastern Sudan, primarily in the Sinkat region. Aloe-emodin (AE) and microdontin chemicals are present. These two compounds were reported to exert strong suppressive effects on T-cell proliferation with an IC50 of 9.2 and 7.4 mg/L, respectively, and a suppressive interleukin (IL)-2 generation activity with an IC50 of 1.1 and 1.9 mg/L for AE and microdontin, respectively [4]. A hydroxyanthraquinone generated from plants, AE, has been shown to have several biological properties, including antiproliferative activity [5]. At 5 μM, AE dramatically reduced the growth of cultured human keratinocytes [6]. According to Pandey et al., psoriatic skin treated with topical hydrogel filled with AE showed decreased epidermal thickness [7].
In light of all of this, the primary goal of this research is to create and assess a novel emulgel using plant extract from A. sinkatana as an antipsoriatic treatment.
Applying a drug-containing formulation directly to the skin to treat cutaneous conditions is known as topical drug delivery [8]. Topical drugs have the primary benefit of avoiding first-pass metabolism.
One easy way to get localized medication into the bloodstream is topical administration. For their systemic or local action, drugs are applied topically [9].
Other medications can now enter the bloodstream and be absorbed via the skin thanks to new technologies. These can treat the entire body, not only the skin’s afflicted parts [10]. When other topical medication delivery methods cannot directly treat cutaneous problems, emulgel (gellified emulsion) is typically utilized [11]. Emulsion gels have gained significant traction in pharmaceutical semisolid dosage forms since the mid-1980s. Nowadays, emulgel is a widely used drug delivery system [12].
Emulgel is a blend of gels and emulsions; the emulsions might be water-in-oil or oil-in-water. The emulsion is transformed into an emulgel by the gelling agent found in the water phase. While water-in-oil systems encapsulate hydrophilic pharmaceuticals, oil-in-water systems directly entrap lipophilic medications [13, 14]. Gel and the emulsion control the systems’ regulated medication release [15]. The two types of gels are water-based, hydrophilic, or hydrogels and organic solvent-based, hydrophobic, or organogels [16].
Emulgel exhibits superior drug release and stability and can be utilized to extend the effects of medications with shorter half-lives. Additionally, emulgel preparation components are readily available and less expensive, which lowers the cost of producing emulgels [17, 18, 19].
Materials and Methods
The standard compound
The standard compound of AE was a gift from Professor Masaki of Kobe University in Japan.
Plant collection and authentication
The A. sinkatana plants were collected from the Sinkat area, Red Sea state, Sudan, and authenticated by the Department of Chemistry, Medicinal, and Aromatic Plants Research Institute and Traditional Medicine, National Research Center, Khartoum, Sudan.
Extraction of A. sinkatana
Fresh water was used to wash mature, healthy, fresh A. sinkatana leaves between 75 and 90 cm long.
The inside gel was cut into pieces by scraping it off. Aloe leaves were processed using the age-old hand filleting method. Sharp blades cut off the lower leaf base, the tapering tip at the leaf apex, and the short spines along the leaf edges. After that, the top rind was cut off, and the blade was inserted into the mucilage layer beneath the green rind to avoid the vascular bundles. The leaves firm, colorless mucilaginous gel was chopped into pieces after the epidermis was peeled off. Afterward, 500 mL of solvent (ethanol) was added to a 1000 mL flask containing 250 g of gel. For 60 minutes, ultrasound-assisted extraction was carried out at 60 °C. The solution was then filtered, and the solvent was extracted in a rotary evaporator at a lower pressure until it was scorched [20].
Formulation design using 2³ full factorial design
To obtain the “best” or an “optimized product”, eight different formulations were generated using two levels, three-factor, full factorial design.
A 2³ factorial design for three factors at two levels each was selected to optimize the varied response variables. The amount of gelling agent X1, emulsifying agent X2, and liquid paraffin X3 were chosen as independent formulation variables, and the factor levels were suitably coded (Table 1).

Experimental trials were performed at all 8 possible combinations (Table 2).

All other formulation and processing variables were kept invariant throughout the study [21].
Design-Expert software, version 8 was used to generate and evaluate the statistical experimental design.
Preparation of emulgel
Table 3 displays the composition of emulgel formulations.

Carbopol 934 was first dissolved in 80 °C hot filtered water to create the gel, which was then allowed to cool and sit overnight. Span20 was dissolved in liquid paraffin to create the emulsion’s oil phase, while Tween20 was dissolved in filtered water to make the aqueous phase. Both solutions were combined with the aqueous phase after propylene glycol was dissolved in ethanol and plant extract in DMSO. After heating the aqueous and oily phases independently to 70 °C to 80 °C, the oily phase was gradually introduced to the aqueous phase while constantly stirring until it cooled to room temperature.
To formulate the emulgel, the resulting emulsion and gel were combined in a 1:1 ratio while gently stirring. Lastly, triethanolamine changed the emulgel’s pH [22, 23].
Macroscopic evaluations
The color, texture, homogeneity, consistency, and phase separation of the 8 batches of emulgel formulas were examined under a microscope [24, 25].
Measurement of pH
A pH meter (Max Instruments Chandigarh, India) calibrated before each reading was used to measure the pH of 1% aqueous solutions of the prepared A. sinkatana emulgel using buffered solutions at pH 4.0 and 7.0. Each formulation’s pH was measured thrice, and the average results were computed [26-28].
Viscosity test
The viscometer VR 3000 (Viscotech, Spain) was used to measure viscosity at room temperature by selecting the proper spindle number (L4) and speed (100 rpm). The spindle groove was dipped, the rpm was set, and a reasonable amount of each emulgel formulation was maintained in a suitable beaker. Viscosity measurements were initiated, and each formulation’s viscosity was computed by measuring the data after one minute [29, 30].
Spreadability test
The spreadability was determined by the parallel plate method, which is widely used to measure the spreading diameter of 1 gm of emulgel between two horizontal plates (30×30 cm) after one minute [31].
Calibration curve of standard AE compound
A standard 100 µg/mL (stock solution) was obtained by dissolving the precisely weighed AE standard material (0.01 g) in methanol using an ultrasonic shaker in a 100 mL volumetric flask. Different stock solution concentrations (2 µg/mL, 4 µg/mL, 6 µg/mL, 8 µg/mL, 10 µg/mL, and 12 µg/mL) were prepared, and a UV spectrophotometer was used to measure the absorbance of the various dilutions at 430 nm. Plotting the absorbance against concentration allowed for the determination of the calibration curve.
Determination of AE content in A. sinkatana extract
About 0.5 g of dried extracts were dissolved by ultrasonic shaker in 100 mL of methanol (0.5%). The absorbance of the resultant solution was read at wavelength 430 nm using a UV spectrophotometer.
Determination of drug content
To determine the AE compound content in the different formulas of A. sinkatana extract was weighted 5 g of emulgel formula and dissolved by an ultrasonic shaker in 50 mL of methanol (10%). The absorbance of the resultant solution was read at a wavelength of 430 nm using a UV spectrophotometer. The percentage of drug content was calculated.
In vitro drug released study
The drug-released research was conducted using a manually constructed apparatus replicating Franz diffusion cells via a dialysis membrane. Diffusion investigations were conducted at 37 °C using methanol as the dissolution medium after a 1 g sample of emulgel was placed in a dialysis membrane that had been soaked in methanol. Five milliliters of the sample were taken out at intervals of 0, 1, 2, 3, 5, 6, 7, and 8 hours. A similar volume of dissolving media was used to replace each sample. Methanol was used as a plank to evaluate the samples for drug content [32].
Finally, % drug released=concentration of drug×volume of receptor compartment/amount of drug in the donor compartment
Drug release kinetics studies
Drug release kinetics was examined by fitting the results from ex vivo drug release investigations into several drug release kinetics models. Zero-order, first-order, and Higuchi plots were made for this investigation. Plotting the cumulative percent medication penetrated on the vertical axis against time (in hours) on the horizontal axis created zero-order plots. Plotting the log cumulative percent of medication remaining against time (in hours) allowed researchers to examine the formulations for first-order kinetics.
The Higuchi plots were also obtained by comparing the X-axis’s square root of time (in hours) with the cumulative percent drug permeated taken on the Y-axis. The goodness-of-fit test was used to determine which model was best. The plot that produced the highest correlation coefficient value, or R2, was considered the most suitable for the formulation [33].
The mechanism of drug release from the topical gel analyzed by fitting the release data
Zero-order equation
Q=k0t
, where Q is the amount of drug released at time t, and k0 is the zero-order release rate.
First–order equation
In (100 –Q)=In 100–k1t
, where Q is the percentage of drug release at time t, and k1 is the first–order release rate constant.
Higuchi’s equation
Q=k2√t
, where Q is the percentage of drug release at time t, and k2 is the diffusion rate constant.
Accelerated stability studies
The emulgel formula was packed in a clean, dry, ampere container and subjected to stability studies at 25°C/60% RH, 30°C/65% RH, and 40°C/75% RH for 3 months. Samples were drawn at predetermined intervals 1, 2, and 3 and evaluated for physical appearance (visually inspected for any change in color and odor), phase separation, drug content, pH, and microbial growth [34].
Statistical analysis
The data obtained was statistically analyzed by the Design-Expert software, version 8 statistical program.
Results and Discussion
The macroscopic evaluations, measured pH, and viscosity values of the prepared emulgel formulations are given in Table 1. The emulgel formulas are glossy and reddish brown; all formulations are homogeneous, stable, and smooth in texture.
The pH values of the formulations range from 6.36 to 6.48, which is considered acceptable to avoid the risk of irritation upon application to the skin (within the pH range of the human skin). The incorporation of triethanolamine achieved this index. Historically, an acidic skin surface’s physiologic role was considered a defense mechanism against invading organisms. More recently, it has been demonstrated that several key enzymes involved in the synthesis and maintenance of a competent skin barrier are largely impacted by pH. Hence, a broader view of the importance of pH in the function and integrity of the skin is emerging [35].
Results of the viscosity test showed that the viscosity increased as the amount of gelling agent and a surfactant increased and vice versa; the viscosity ranged between 26734 to 28510 Cps. The results for spreadability ranged between 23.2 cm and 25.1 cm.
Figure 1 displays the viscosity model graph. According to the figure, viscosity rose as a gelling agent, surfactant concentrations rose, and vice versa. The spreadability is directly related to the amount of liquid paraffin (linear model) and indirectly proportional to the amount of gelling agent and surfactant, according to the graphical result in Figure 2. The absorbance for the concentration of the AE standard compound is shown in Table 5.

The calibration curve is illustrated in Figure 3. The standard graph of the AE compound shows good linearity with an R² value of 0.986, which indicates that it obeys Beer-Lambert Law in the concentration range of 0-100 µg/mL.
The AE concentration in A. sinkatana extract and the drug content were estimated from Equation 1 of the calibration curve. The final concentration of AE in the plant extract was 4 µg/5 mg=0.08%.
Y= 0.046×X - 0.027
The drug content of the 8 formulae ranged between 92.34% and 99.48%, while the F3 formula shows the highest percent of drug content (Table 6).

Table 7 presents the diffusion data for the formulations F1 to F8.

The best release of the drug after 8 h from all emulgel formulations can be observed by F3 (61.38%) and can be ranked in the following descending order: F3 > F1 > F5 >F7 > F2 > F6 >F4>F8.
Based on all evaluation tests performed for the emulgel formulation, the F3 formula shows the best results among all the formulations. Therefore, the F3 formulation was considered the optimized emulgel formula. The in vitro drug-released kinetics studies were performed on the most promising emulgel formula, i.e. F3 (Table 8).

The data from in vitro drug release studies were treated using various conventional mathematical models (zero-order, first-order, and Higuchi) to determine the release mechanism from the designed emulgel formulations.

A suitable release model was selected based on the R² (regression coefficient) values.
The regression coefficient of the formula F3 is shown in Table 9.

It was found that the formula F3 follows the first-order kinetics (Figures 4, 5 and 6). The stability studies were performed on formula F3. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of various environmental factors such as temperature, humidity, and light and enables recommended storage conditions and shelf lives to be established.
The F3 formula was stable upon storage for 3 months, and no significant change was observed in their physical appearance, pH, or drug content. No microbial growth was observed, and there was no liquefaction or phase separation till the end of the study period in any sample. These results depicted that formulation was stable even at high humidity and temperature of 75% and 40 °C, respectively, as shown in Table 10.

Conclusion
The present study deals with the formulation development and optimization of A. sinkatana emulgel. The optimization was done based on the drug content and in vitro drug release. The kinetic modeling revealed that A. sinkatana emulgel follows the first-order kinetics model. Formulation batch F3 showed the best drug release and was stable upon storage for 3 months, even at high humidity and temperature. Thus, a successful attempt was made to formulate emulgel.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations in this research.
Funding
This research was funded by the Ministry of Higher Education and Scientific Research, National Center for Research, Khartum, Sudan.
Authors' contributions
Investigation, data collection, and writing the original draft: Azza Dawoud Hussien Dawoud; Data analysis: Mohammed Abdalbagi; Supervision: Mohamed El Hassan Shayoub; Review, and editing: Azza Dawoud Hussien Dawoud, and Mohammed Abdalbagi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors take this opportunity to thank Pharmaceutical Department of Faculty of Pharmacy, National Ribat University, Khartoum, Sudan and Medicinal and Aromatic Plants & Traditional Medicine Research Institute, Sudan.
References
- Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005; 52(1):23-6. [DOI:10.1016/j.jaad.2004.07.045] [PMID]
- Kormeili T, Lowe NJ, Yamauchi PS. Psoriasis: immunopathogenesis and evolving immunomodulators and systemic therapies; U.S. experiences. Br J Dermatol. 2004; 151(1):3-15. [DOI:10.1111/j.1365-2133.2004.06009.x] [PMID]
- Dawoud AD. Formulation and evaluation of aloe Sinkatana gel as Antipsoriatic agent [PhD dissertation]. Khartoum: University of Khartoum; 2021. [Link]
- Elhassan GOM, Adhikari A, Yousuf S, Rahman MH, Khalid A, Omer H, et al. Phytochemistry and antiglycation activity of Aloe sinkatana Reynolds. Phytochem Lett. 2012; 5(4):725-8. [DOI:10.1016/j.phytol.2012.07.012]
- Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of aloeandongensis extract, aloe andongensis leaf juice, aloe arborescens leaf extract, aloe arborescens leaf juice, aloe arborescens leaf protoplasts, aloe barbadensis flower extract, aloe barbadensis leaf, aloe barbadensis leaf extract, aloe barbadensis leaf juice, aloe barbadensis leaf polysaccharides, aloe barbadensis leaf water, aloe ferox leaf extract, aloe ferox leaf juice, and aloe ferox leaf juice extract. Int J Toxicol. 2007; 26(S 2):1-50. [DOI:10.1080/10915810701351186] [PMID]
- Popadic D, Savic E, Ramic Z, Djordjevic V, Trajkovic V, Medenica L, et al. Aloe-emodin inhibits proliferation of adult human keratinocytes in vitro. J Cosmet Sci. 2012; 63(5):297-302. [PMID]
- Pandey A, Srivastava N, Dubey RC, Dhaneshwar S, Shukla AK. Anti-psoriatic evaluation of aloe emodin-loaded topical hydrogel in an imiquimod-induced human plaque-type psoriasis in BALB/c mice. J Appl Pharm Sci. 2024; 14(9):279-91. [DOI:10.7324/JAPS.2024.199942]
- Khullar R, Saini S, Seth N, Rana AC. Emulgels: A surrogate approach for topically used hydrophobic drugs. Int J Pharm Bio Sci. 2011; 1(3):117-28. [Link]
- Ayub AC, Gomes AD, Lima MV, Vianna-Soares CD, Ferreira LA. Topical delivery of fluconazole: in vitro skin penetration and permeation using emulsions as dosage forms. Drug Dev Ind Pharm. 2007; 33(3):273-80. [DOI:10.1080/03639040600829989] [PMID]
- Poucher WA. Poucher’s perfumes, cosmetics and soaps: Modern cosmetics. 8th ed. Berlin: Springer; 2013. [Link]
- Stam-Posthuma JJ, Vink J, le Cessie S, Bruijn JA, Bergman W, Pavel S. Effect of topical tretinoin under occlusion on atypical naevi. Melanoma Res. 1998; 8(6):539-48. [DOI:10.1097/00008390-199812000-00009] [PMID]
- Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004; 27(12):1993-9. [DOI:10.1248/bpb.27.1993] [PMID]
- Single V, Saini S, Joshi B, Rana AC. Emulgel: A new platform for topical drug delivery. Int J Pharm Biol Sci. 2012; 3(1):485-98. [Link]
- Mohamed MI. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004; 6(3):e26. [DOI:10.1208/aapsj060326] [PMID] [PMCID]
- Mulye SP, Wadkar KA, Kondawar MS. Formulation development and evaluation of Indomethacin emulgel. J Pharm Sci. 2013; 4(5):31-45. [Link]
- Mona Agrawal MA, Yogesh Agrawal YA, Prakash Itankar PI, Arun Patil AP, Jayshree Vyas JV, Amruta Kelkar AK. Phytochemical and HPTLC studies of various extracts of Annona squamosa (Annonaceae). Int J Pharm Tech Res. 2012; 4(1):364-8. [Link]
- Chandel A, Kumari N, Gupta R, Nazir A, Varghese AC. An overview on emulgel. Asian J Pharm Res. 2023; 13(3):196-9. [DOI:10.52711/2231-5691.2023.00037]
- Wang M, Fang L. Percutaneous absorption of diclofenac acid and its salts from emulgel. Asian J Pharmaceut Sci. 2008; 3(3):131-8. [Link]
- Ahmed SA, Verma S, Khan S, Sharma A. Emulgel: A revolution in topical drug delivery system. Int J Health Sci. 2022; 6(S4):10834-56. [DOI:10.53730/ijhs.v6nS6.10872]
- Bandar H, Hijazi A, Rammal H, Hachem A, Saad Z, Badran B. Techniques for the extraction of bioactive compounds from Lebanese Urtica Dioica. Am J Phytomed Clin Ther. 2013; 1(6):507-13. [Link]
- Kumar L, Sreenivasa Reddy M, Managuli RS, Pai KG. Full factorial design for optimization, development and validation of HPLC method to determine valsartan in nanoparticles. Saudi Pharm J. 2015; 23(5):549-55. [DOI:10.1016/j.jsps.2015.02.001] [PMID] [PMCID]
- Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J. 2012; 20(1):63-7. [DOI:10.1016/j.jsps.2011.08.001] [PMID] [PMCID]
- Yılmaz G, Tanrıverdi Tuncay S, Aksu B, Yeğen G, Özer O. Preparation and in vitro characterization of etofenamate emulgels using quality by design. J Res Pharm. 2019; 23(6):1033-9. [DOI:10.35333/jrp.2019.67]
- Ambala R, Vemula SK. Formulation and characterization of ketoprofen emulgels. J Appl Pharm Sci. 2015; 5(7):112-7. [DOI:10.7324/JAPS.2015.50717]
- Plumb AP, Rowe RC, York P, Brown M. Optimisation of the predictive ability of artificial neural network (ANN) models: A comparison of three ANN programs and four classes of training algorithm. Eur J Pharm Sci. 2005; 25(4-5):395-405. [DOI:10.1016/j.ejps.2005.04.010] [PMID]
- Kamble A, Adnaik R, Bhutkar MA. Formulation and evaluation of Itraconazole emulgel for topical drug delivery. Int J Uni Pharm Biosci. 2014; 3(5):37-45. [Link]
- Mohite S, Salunkhe A. Formulation and evaluation of Emulgel containing Coriandrum sativum seeds oil for Antiinflammatory activity. J Drug Deliv Ther. 2019; 9(3 S):124-30. [DOI:10.22270/jddt.v9i3-s.2808]
- Burki IK, Khan MK, Khan BA, Uzair B, Braga VA, Jamil QA. Formulation development, characterization, and evaluation of a novel Dexibuprofen-capsaicin skin Emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech. 2020; 21(6):211. [DOI:10.1208/s12249-020-01760-7] [PMID]
- Yapar EA, Ynal O, Erdal MS. Design and in vivo evaluation of emulgel formulations including green tea extract and rose oil. Acta Pharm. 2013; 63(4):531-44. [DOI:10.2478/acph-2013-0037] [PMID]
- Shelke O, Kulkarni A. Formulation, development and evaluation of nifedipine emulgel for treatment of anal fissures using polymeric emulsifiers. Indian J Pharm Educ Res. 2019; 53(2 S):S74-81. [DOI:10.5530/ijper.53.2s.51]
- Ashara KC, Paun JS, Soniwala MM, Chavada JR, Mori NM. Micro-emulsion based emulgel: A novel topical drug delivery system. Asian Pac J Trop Dis. 2014; 4(Supp 1):S27-32. [DOI:10.1016/S2222-1808(14)60411-4]
- Ansong JA, Asante E, Johnson R, Boakye-Gyasi ME, Kuntworbe N, Owusu FWA, et al. Formulation and evaluation of herbal-based antiacne gel preparations. Biomed Res Int. 2023; 2023:7838299. [DOI:10.1155/2023/7838299] [PMID] [PMCID]
- Barel AO, Paye M, Maibach HI. Handbook of cosmetic science and technology. 3th ed. New York: Informa Healthcare; 2009. [Link]
- ICH. Stability testing of new drug substances and products. 2003. [Link]
- Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013; 93(3):261-7. [DOI:10.2340/00015555-1531] [PMID]
Type of Study: Original Research |
Subject:
Pharmaceutics
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |