Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 139-146 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fagbohun B O, Ogar O E, Messiah A I, Adewuyi H A, Adio W S, Oluwatoyin A H, et al . Antihypertensive Potential of Euphorbia hirta and Leptadenia hastata in Adrenaline-induced Wistar Rats. Pharm Biomed Res 2025; 11 (2) :139-146
URL: http://pbr.mazums.ac.ir/article-1-659-en.html
URL: http://pbr.mazums.ac.ir/article-1-659-en.html
Bushirat Oyenike Fagbohun1 

 , Ochonung Emmanuel Ogar2
, Ochonung Emmanuel Ogar2 

 , Atapia Innocent Messiah2
, Atapia Innocent Messiah2 

 , Hassan Abdulsalam Adewuyi *3
, Hassan Abdulsalam Adewuyi *3 

 , Waheed Sakariyau Adio4
, Waheed Sakariyau Adio4 

 , Adebimpe Hameedah Oluwatoyin5
, Adebimpe Hameedah Oluwatoyin5 
 , Chizoba Victory Obunadike6
, Chizoba Victory Obunadike6 
 , Adeleye Adegboyega Edema7
, Adeleye Adegboyega Edema7 

 , Samad Hussein3
, Samad Hussein3 
 , Usman Abiola Mohammed8
, Usman Abiola Mohammed8 
 , Tolulope Olukayode Jaiyeola9
, Tolulope Olukayode Jaiyeola9 




 , Ochonung Emmanuel Ogar2
, Ochonung Emmanuel Ogar2 

 , Atapia Innocent Messiah2
, Atapia Innocent Messiah2 

 , Hassan Abdulsalam Adewuyi *3
, Hassan Abdulsalam Adewuyi *3 

 , Waheed Sakariyau Adio4
, Waheed Sakariyau Adio4 

 , Adebimpe Hameedah Oluwatoyin5
, Adebimpe Hameedah Oluwatoyin5 
 , Chizoba Victory Obunadike6
, Chizoba Victory Obunadike6 
 , Adeleye Adegboyega Edema7
, Adeleye Adegboyega Edema7 

 , Samad Hussein3
, Samad Hussein3 
 , Usman Abiola Mohammed8
, Usman Abiola Mohammed8 
 , Tolulope Olukayode Jaiyeola9
, Tolulope Olukayode Jaiyeola9 


1- Department of Pharmacy Technician, Ogun State College of Health Science and Technology, Ogun, Nigeria.
2- Department of Chemical Engineering, University of Benin, Benin City, Nigeria.
3- Department of Biochemistry, Federal University of Technology, Minna, Nigeria.
4- Department of Chemistry and Biochemistry, College of Science, Old Dominion University, Norfolk, United States.
5- Department of Chemistry and Biochemistry, College of Health and Natural Sciences-Keplinger Hall, The University of Tulsa, Oklahoma, United States.
6- Department of Industrial Chemistry, College of Pure and Applied Chemistry, Osun State University Osogbo, Osun, Nigeria.
7- Department of Biochemistry, University of Ibadan, Oyo State Nigeria.
8- Department of Biological Sciences, AhmanPategi University, Pategi, Nigeria.
9- Department of Biological Sciences, Crawford University, Ketu Adieowe, Nigeria.
2- Department of Chemical Engineering, University of Benin, Benin City, Nigeria.
3- Department of Biochemistry, Federal University of Technology, Minna, Nigeria.
4- Department of Chemistry and Biochemistry, College of Science, Old Dominion University, Norfolk, United States.
5- Department of Chemistry and Biochemistry, College of Health and Natural Sciences-Keplinger Hall, The University of Tulsa, Oklahoma, United States.
6- Department of Industrial Chemistry, College of Pure and Applied Chemistry, Osun State University Osogbo, Osun, Nigeria.
7- Department of Biochemistry, University of Ibadan, Oyo State Nigeria.
8- Department of Biological Sciences, AhmanPategi University, Pategi, Nigeria.
9- Department of Biological Sciences, Crawford University, Ketu Adieowe, Nigeria.
Keywords: Antihypertensive, Euphorbia hirta, Leptadenia hastata, traditional medicine, Cardiovascular disorders, Phytotherapy, Cardiovascular
Full-Text [PDF 600 kb]
(274 Downloads)
| Abstract (HTML) (786 Views)
Full-Text: (264 Views)
Introduction
Cardiovascular diseases, encompassing hypertension, arteriosclerosis, and heart disease, pose significant global health concerns, accounting for approximately 17.9 million deaths annually [1]. Hypertension, a primary risk factor, affects over 1 billion individuals worldwide, with prevalence rates projected to increase [2]. In developing countries, hypertension often remains untreated or inadequately managed, exacerbating cardiovascular morbidity and mortality.
Traditional medicinal plants, such as Euphorbia hirta and Leptadenia hastata, offer potential therapeutic strategies. L. hastata, commonly used in West African folk medicine, exhibits diverse health applications [3]. Its leaf extract has been employed to treat onchocerciasis [4], scabies [5], hypertension, catarrh, and skin diseases [6]. Phytochemical studies reveal phenolic glycosides, tannins, flavonoids, proanthocyanidins, alkaloids, and saponins, contributing to its therapeutic efficacy [7].
E. hirta, another medicinal plant, demonstrates antihypertensive, anti-inflammatory, and antioxidant properties [8]. Its phytochemical constituents, including flavonoids, alkaloids, and terpenoids, contribute to cardiovascular protection [9].
The pathophysiology of hypertension involves complex interactions between fluid dynamics, vascular resistance, and pressor factors [10]. Oxidative stress and inflammation play critical roles in hypertension’s development and progression [11]. Therefore, investigating medicinal plants with antioxidant and anti-inflammatory properties, such as L. hastata and E. hirta, may provide novel therapeutic approaches.
This study aimed to investigate the antihypertensive effects of L. hastata leaf extract on biochemical and hematological parameters in adrenaline-induced hypertensive rats, complementing existing research on E. hirta.
Materials and Methods
Experimental Animals: fifty adult male Wistar rats (120±5 g) were obtained from the Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria Animal Holding Unit. Animals were handled in accordance with the Canadian Council on animal care guidelines and review protocol [12].
Sample extraction
E. hirta and L. hastata leaves were washed, chopped, dried (37 °C, 2 weeks) and ground. A 50 g sample of each was extracted with 200 mL of ethanol and aqueous solution using a Soxhlet apparatus. The resulting extract was concentrated on a rotary evaporator [6].
Experimental design: This experimental study was conducted on 50 Wistar rats, divided into eight groups (A-H), with 5 rats in each group, except for one group that included 10 rats serving as the normal control group, resulting in 8 rats after the removal of 2 rats. The groups were as follows:
Group A: Normal control (no treatment)
Group B: Hypertensive control (adrenaline-induced)
Group C: Amlodipine-treated standard antihypertensive drug (0.5 mg/kg) and the extract (50 mg/kg).
D: E. hirta extract-treated (low dose) (100 mg/kg).
Group E: E. hirta extract-treated (high dose) (200 mg/kg).
Group F: L. hastata extract-treated (low dose) (50 mg/kg)
Group G: L. hastata extract-treated (high dose) (100 mg/kg).
Group H: Rats receiving adrenaline (0.5 mg/kg) and L. hastata extract (200 mg/kg).
We measured blood pressure, antioxidant activity, lipid profiles, liver function tests (LFTs), and hematological parameters on days three and seven.
Blood and serum collection
Following the study period, animals were sacrificed under ether anesthesia, and blood samples were collected into EDTA bottles for hematological analyses and EDTA-free bottles for serum collection [6]. Serum samples were obtained after clotting and centrifugation (3000 rpm, 10 minutes) and stored at -20 °C for further analysis [9].
Analyses of biochemical parameters
Biochemical parameters, including transaminases [13], total proteins [14], albumin and bilirubin [15], urea, creatinine, and electrolytes, were assayed using standard protocols [16, 17]. Serum concentrations of total cholesterol, triglycerides, and high-density lipoprotein (HDL) were determined using enzymatic and colorimetric methods with commercial kits [18, 19]. Very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) were calculated using Friedewald’s formula [19]. The activities or concentrations of aspartate transaminase (AST), alanine transaminase (ALT) [13], and alkaline phosphatase (ALP) [16], were determined by standard methods.
Analyses of hematological parameters
Hematological parameters, including hemoglobin (Hb), packed cell value (PCV), red blood cells (RBC), white blood cells (WBC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC), were determined using an automated hematologic analyzer (SYSMEX KX21, Japan) [20].
Statistical analyses
Data were analyzed by software and described as Mean±SD. One-way analysis of variance and Duncan’s multiple range test were used for group comparisons at P<0.05 [21].
Results
The present study investigated the antihypertensive effects of E. hirta and L. hastata leaf extracts in adrenaline-induced hypertensive rats. Our findings demonstrate significant improvements in systolic blood pressure, biochemical parameters, hematological parameters, and liver function tests (LFTs), supporting the traditional use of these plants in hypertension management [22, 23].
The observed significant (P<0.05) reduction in systolic blood pressure in both E. hirta- and L. hastata-treated groups suggests potential vasodilatory effects, possibly mediated by the extracts’ flavonoid and phenolic content (Table 1).
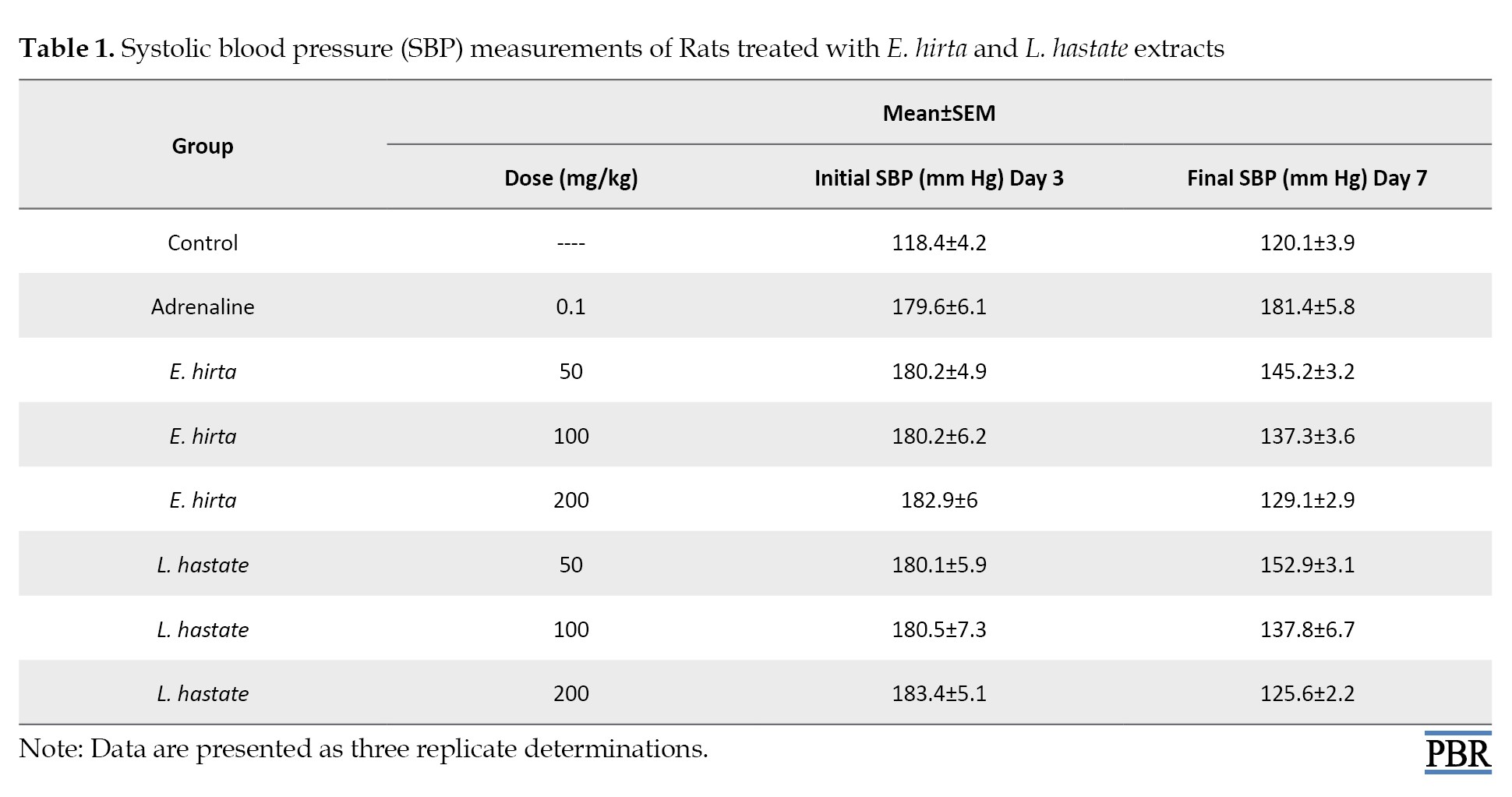
Flavonoids have been shown to relax vascular smooth muscle, leading to decreased blood pressure. Additionally, the extracts’ antioxidant properties may have contributed to the observed effects by reducing oxidative stress and improving endothelial function [24].
The results indicate significant (P<0.05) reductions in SBP in both E. hirta- and L. hastata-treated groups compared to the adrenaline-induced hypertensive group. L. hastata showed slightly higher efficacy in reducing SBP. These findings suggest potential antihypertensive effects of both plant extracts [23] (Table 1).
The observed significant (P<0.05) reduction in triglyceride levels suggests enhanced lipid metabolism, which may be attributed to the extracts’ flavonoid and polyphenol content (Table 2).
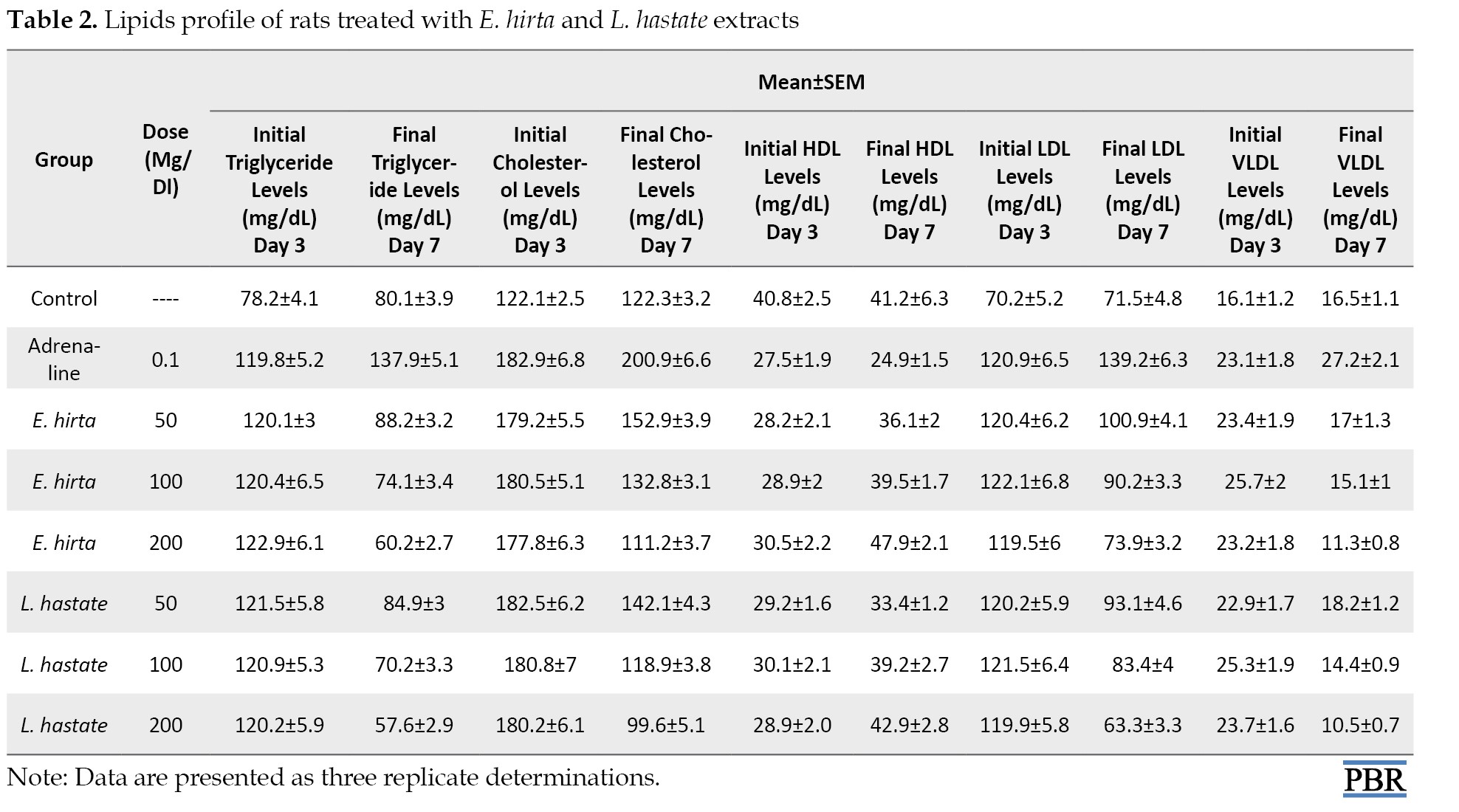
Polyphenols have been shown to inhibit triglyceride synthesis and enhance lipolysis [24].
Regarding triglyceride, there were significant alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated total triglyceride levels (Table 2). In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Total cholesterol: Decreased total cholesterol levels in both extract-treated groups imply improved lipid profiles, potentially mediated by the extracts’ ability to inhibit cholesterol synthesis [24] (Table 2).
Regarding total cholesterol, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated total cholesterol levels (Table 2), In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Increased HDL levels in both extract-treated groups suggest enhanced reverse cholesterol transport, potentially contributing to improved cardiovascular health [24].
Regarding HDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated HDL (Table 3).
Also, HDL levels increased significantly in E. hirta (200 mg/kg) and L. hastata (200 mg/kg) groups (Table 2), suggesting enhanced reverse cholesterol transport.
The significant (P<0.05) decreased LDL levels indicate reduced atherogenic risk, potentially mediated by the extracts’ antioxidant and anti-inflammatory properties [22].
Regarding LDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated LDL levels (Table 2), In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
The significant (P<0.05) decreased VLDL levels indicate reduced atherogenic risk, potentially mediated by the extracts’ antioxidant and anti-inflammatory properties [22].
Regarding VLDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated LDL levels (Table 2). In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Adrenaline induced significant electrolyte imbalances (P<0.05), including hypochloremia (Table 4).
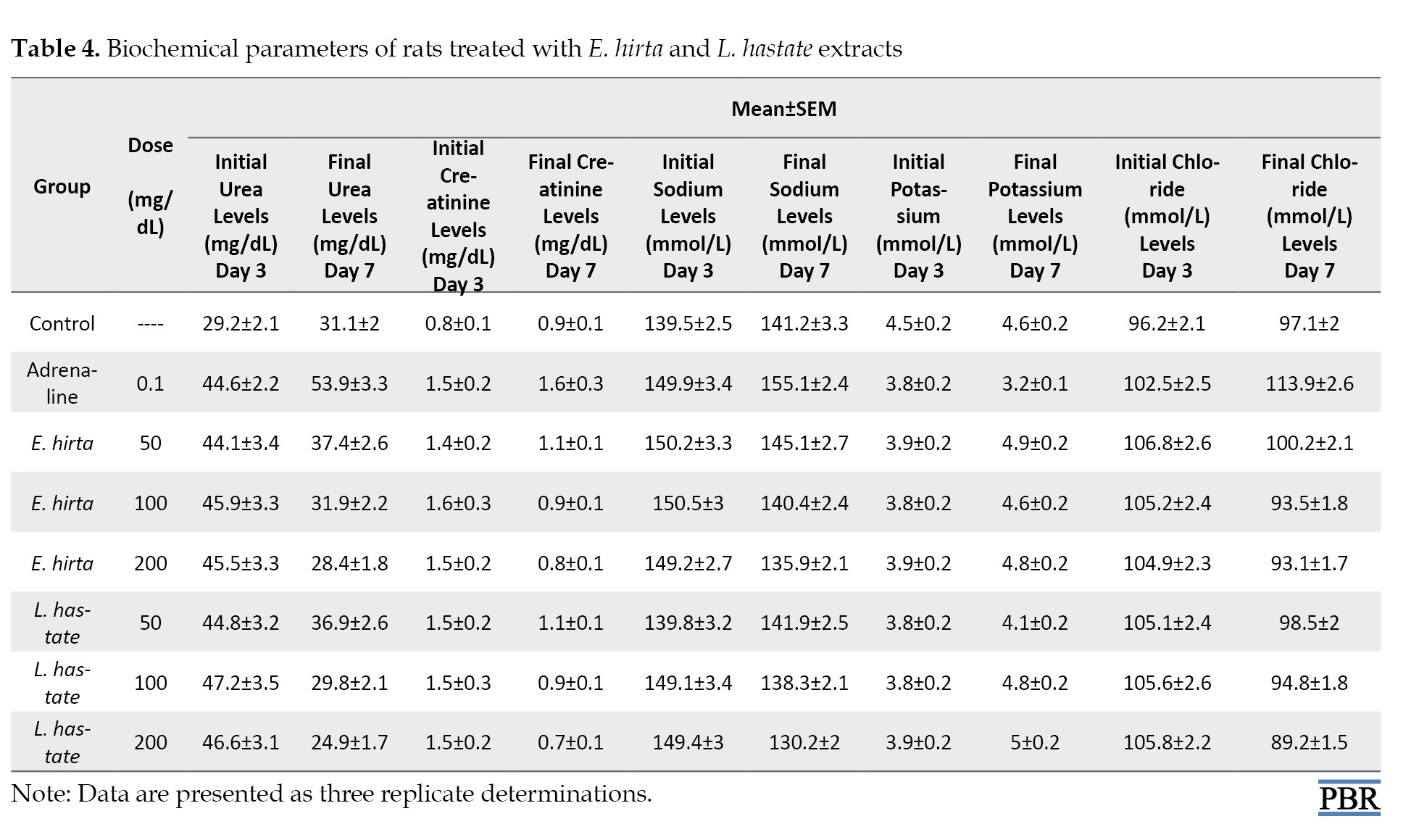
E. hirta and L. hastata extracts normalized electrolyte levels.
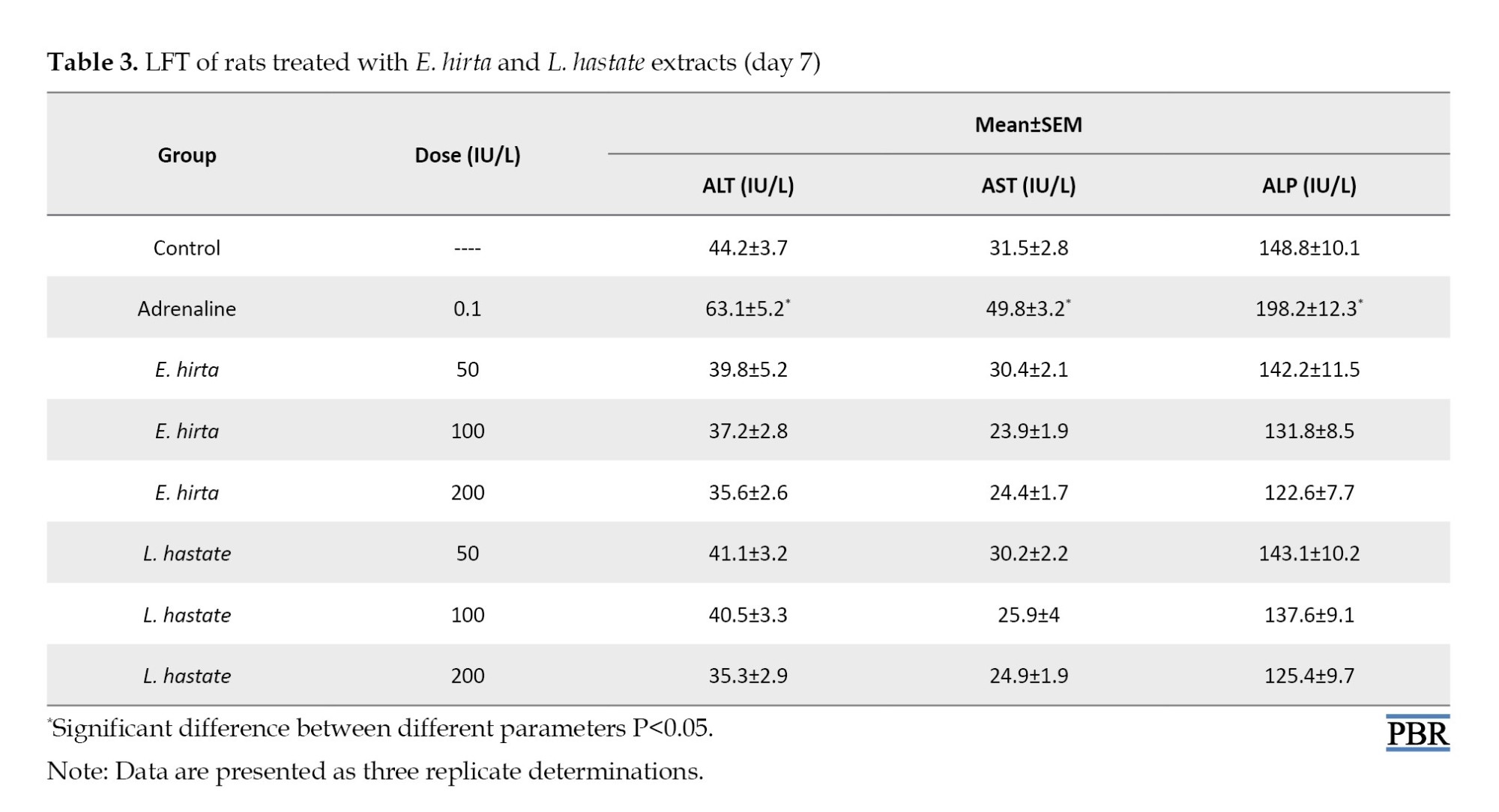
LFT parameters are essential indicators of liver health and function. In this study, the assessed LFT parameters included:
ALT: Decreased ALT levels in both extract-treated groups suggest improved hepatocellular integrity [22]. ALT is a liver enzyme involved in amino acid metabolism.
AST: Reduced AST levels indicate enhanced liver function [23]. AST is a liver enzyme involved in amino acid metabolism.
ALP: Decreased ALP levels suggest improved hepatic function [24]. ALP is a liver enzyme involved in bile acid synthesis.
Adrenaline administration induced hepatotoxicity, evidenced by elevated ALT, AST, and ALP levels. E. hirta and L. hastata extracts demonstrated hepatoprotective effects, as indicated by significant (P<0.05) reduced liver enzyme levels (Table 3).
RBC: Increased RBC count in both extract-treated groups suggests improved erythropoiesis [22]. RBCs are responsible for oxygen transport, and increased counts may indicate enhanced oxygen-carrying capacity.
Adrenaline caused anemia, characterized by decreased hemoglobin (Hb), packed cell volume (PCV), and MCH levels in adrenaline and amlodipine-induced untreated rats (Table 5).
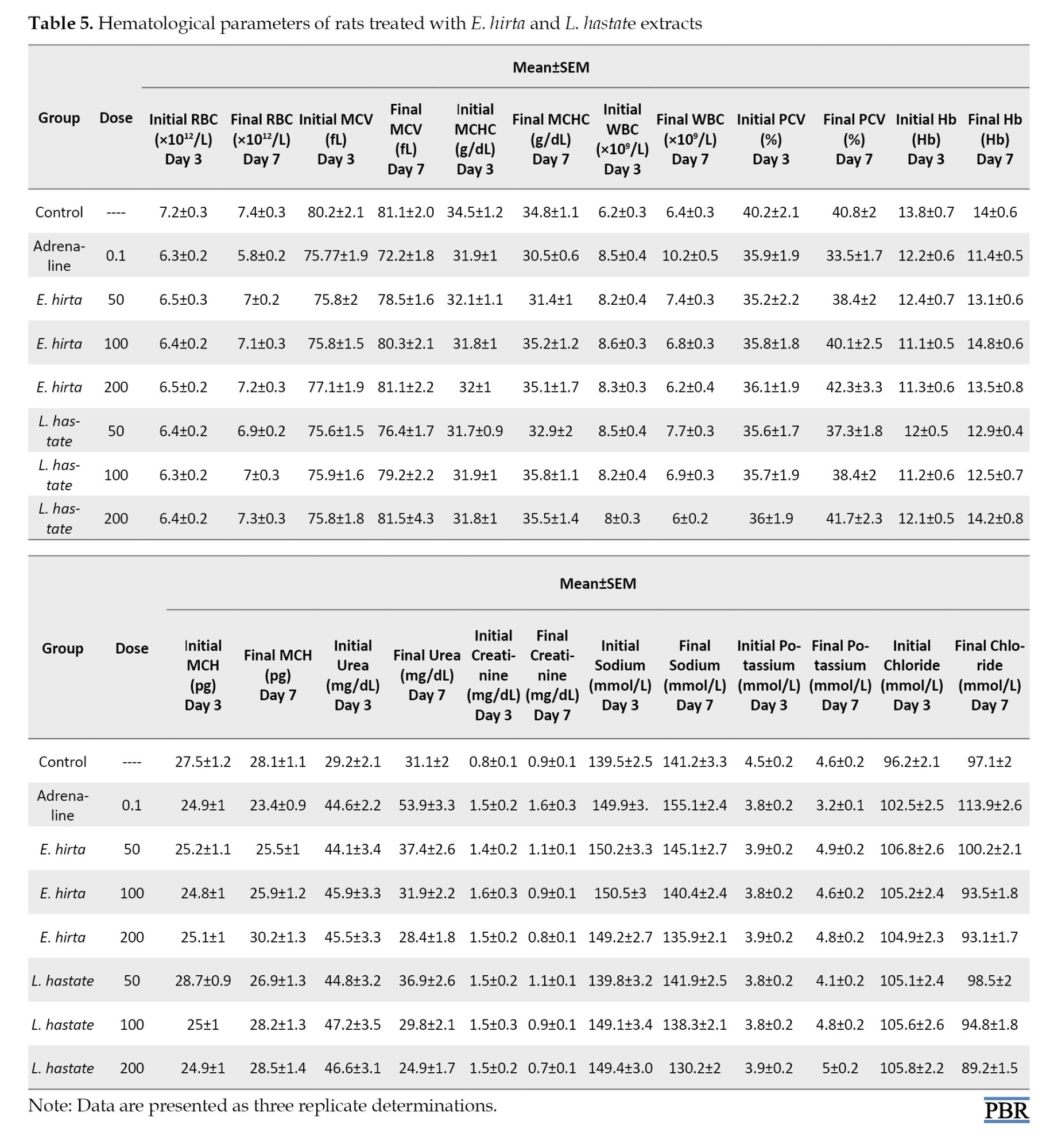
E. hirta and L. hastata extracts improved hematological parameters in extract-treated groups (Table 5).
MCV and MCHC (Table 5): Improved MCV and MCHC values indicate enhanced erythrocyte health [24]. MCV measures the average size of RBCs, while MCHC measures the average Hb concentration within RBCs.
WBC: Normalized WBC count in both extract-treated groups implies enhanced immune function [23]. WBCs play a crucial role in immune defense, and normalized counts may indicate improved immune response.
PCV): Improved PCV values indicate enhanced erythrocyte production and/or reduced erythrocyte destruction [24]. PCV is a measure of the proportion of blood volume occupied by RBCs.
Hb: Increased Hb levels in both E. hirta- and L. hastata-treated groups suggest improved erythropoiesis, potentially due to the extracts’ iron-chelating properties [23]. Hb is essential for oxygen transport, and increased levels may indicate enhanced oxygen delivery to tissues.
Discussion
The present study demonstrated the antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing effects of E. hirta and L. hastata extracts. The findings suggest that these plant extracts may be useful in managing hypertension and related complications. Our findings corroborate existing scientific literature on the antioxidant and protective effects of these plant extracts [23, 25].
The observed anti-hyperlipidemic effects are consistent with research demonstrating the lipid-lowering properties of E. hirta [26] and L. hastate [27]. The hepatoprotective and renoprotective effects can be attributed to their antioxidant activity, reducing oxidative stress and inflammation in liver and kidney tissues [28].
Notably, our study provides novel insights into the combined antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing properties of E. hirta and L. hastata extracts. These findings suggest potential therapeutic applications in managing oxidative stress-related disorders, such as atherosclerosis, hepatotoxicity, and nephrotoxicity.
Studies have demonstrated the antioxidant and protective effects of various plant extracts [7, 25]. However, our study provides a comprehensive evaluation of the antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing effects of E. hirta and L. hastata extracts.
Our findings are consistent with existing scientific literature on the antioxidant and protective effects of plant extracts [7, 25]. However, our comprehensive evaluation of E. hirta and L. hastata extracts provides novel insights into their therapeutic potential.
Conclusion
The study highlights the potential of E. hirta and L. hastata extracts as a phytotherapeutic approach to hypertension. Further studies are needed to elucidate the mechanisms of action and to translate these findings to human studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria (Code: OG/CHT/2023/011). The animal study was approved by the University’s Animal Ethics Committee (Code: AEC/2024/022).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their appreciation to the technical staff of the Pharmacy Technician Department of the Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria, for their technical assistance.
References
Cardiovascular diseases, encompassing hypertension, arteriosclerosis, and heart disease, pose significant global health concerns, accounting for approximately 17.9 million deaths annually [1]. Hypertension, a primary risk factor, affects over 1 billion individuals worldwide, with prevalence rates projected to increase [2]. In developing countries, hypertension often remains untreated or inadequately managed, exacerbating cardiovascular morbidity and mortality.
Traditional medicinal plants, such as Euphorbia hirta and Leptadenia hastata, offer potential therapeutic strategies. L. hastata, commonly used in West African folk medicine, exhibits diverse health applications [3]. Its leaf extract has been employed to treat onchocerciasis [4], scabies [5], hypertension, catarrh, and skin diseases [6]. Phytochemical studies reveal phenolic glycosides, tannins, flavonoids, proanthocyanidins, alkaloids, and saponins, contributing to its therapeutic efficacy [7].
E. hirta, another medicinal plant, demonstrates antihypertensive, anti-inflammatory, and antioxidant properties [8]. Its phytochemical constituents, including flavonoids, alkaloids, and terpenoids, contribute to cardiovascular protection [9].
The pathophysiology of hypertension involves complex interactions between fluid dynamics, vascular resistance, and pressor factors [10]. Oxidative stress and inflammation play critical roles in hypertension’s development and progression [11]. Therefore, investigating medicinal plants with antioxidant and anti-inflammatory properties, such as L. hastata and E. hirta, may provide novel therapeutic approaches.
This study aimed to investigate the antihypertensive effects of L. hastata leaf extract on biochemical and hematological parameters in adrenaline-induced hypertensive rats, complementing existing research on E. hirta.
Materials and Methods
Experimental Animals: fifty adult male Wistar rats (120±5 g) were obtained from the Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria Animal Holding Unit. Animals were handled in accordance with the Canadian Council on animal care guidelines and review protocol [12].
Sample extraction
E. hirta and L. hastata leaves were washed, chopped, dried (37 °C, 2 weeks) and ground. A 50 g sample of each was extracted with 200 mL of ethanol and aqueous solution using a Soxhlet apparatus. The resulting extract was concentrated on a rotary evaporator [6].
Experimental design: This experimental study was conducted on 50 Wistar rats, divided into eight groups (A-H), with 5 rats in each group, except for one group that included 10 rats serving as the normal control group, resulting in 8 rats after the removal of 2 rats. The groups were as follows:
Group A: Normal control (no treatment)
Group B: Hypertensive control (adrenaline-induced)
Group C: Amlodipine-treated standard antihypertensive drug (0.5 mg/kg) and the extract (50 mg/kg).
D: E. hirta extract-treated (low dose) (100 mg/kg).
Group E: E. hirta extract-treated (high dose) (200 mg/kg).
Group F: L. hastata extract-treated (low dose) (50 mg/kg)
Group G: L. hastata extract-treated (high dose) (100 mg/kg).
Group H: Rats receiving adrenaline (0.5 mg/kg) and L. hastata extract (200 mg/kg).
We measured blood pressure, antioxidant activity, lipid profiles, liver function tests (LFTs), and hematological parameters on days three and seven.
Blood and serum collection
Following the study period, animals were sacrificed under ether anesthesia, and blood samples were collected into EDTA bottles for hematological analyses and EDTA-free bottles for serum collection [6]. Serum samples were obtained after clotting and centrifugation (3000 rpm, 10 minutes) and stored at -20 °C for further analysis [9].
Analyses of biochemical parameters
Biochemical parameters, including transaminases [13], total proteins [14], albumin and bilirubin [15], urea, creatinine, and electrolytes, were assayed using standard protocols [16, 17]. Serum concentrations of total cholesterol, triglycerides, and high-density lipoprotein (HDL) were determined using enzymatic and colorimetric methods with commercial kits [18, 19]. Very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) were calculated using Friedewald’s formula [19]. The activities or concentrations of aspartate transaminase (AST), alanine transaminase (ALT) [13], and alkaline phosphatase (ALP) [16], were determined by standard methods.
Analyses of hematological parameters
Hematological parameters, including hemoglobin (Hb), packed cell value (PCV), red blood cells (RBC), white blood cells (WBC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC), were determined using an automated hematologic analyzer (SYSMEX KX21, Japan) [20].
Statistical analyses
Data were analyzed by software and described as Mean±SD. One-way analysis of variance and Duncan’s multiple range test were used for group comparisons at P<0.05 [21].
Results
The present study investigated the antihypertensive effects of E. hirta and L. hastata leaf extracts in adrenaline-induced hypertensive rats. Our findings demonstrate significant improvements in systolic blood pressure, biochemical parameters, hematological parameters, and liver function tests (LFTs), supporting the traditional use of these plants in hypertension management [22, 23].
The observed significant (P<0.05) reduction in systolic blood pressure in both E. hirta- and L. hastata-treated groups suggests potential vasodilatory effects, possibly mediated by the extracts’ flavonoid and phenolic content (Table 1).

Flavonoids have been shown to relax vascular smooth muscle, leading to decreased blood pressure. Additionally, the extracts’ antioxidant properties may have contributed to the observed effects by reducing oxidative stress and improving endothelial function [24].
The results indicate significant (P<0.05) reductions in SBP in both E. hirta- and L. hastata-treated groups compared to the adrenaline-induced hypertensive group. L. hastata showed slightly higher efficacy in reducing SBP. These findings suggest potential antihypertensive effects of both plant extracts [23] (Table 1).
The observed significant (P<0.05) reduction in triglyceride levels suggests enhanced lipid metabolism, which may be attributed to the extracts’ flavonoid and polyphenol content (Table 2).

Polyphenols have been shown to inhibit triglyceride synthesis and enhance lipolysis [24].
Regarding triglyceride, there were significant alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated total triglyceride levels (Table 2). In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Total cholesterol: Decreased total cholesterol levels in both extract-treated groups imply improved lipid profiles, potentially mediated by the extracts’ ability to inhibit cholesterol synthesis [24] (Table 2).
Regarding total cholesterol, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated total cholesterol levels (Table 2), In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Increased HDL levels in both extract-treated groups suggest enhanced reverse cholesterol transport, potentially contributing to improved cardiovascular health [24].
Regarding HDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated HDL (Table 3).

Also, HDL levels increased significantly in E. hirta (200 mg/kg) and L. hastata (200 mg/kg) groups (Table 2), suggesting enhanced reverse cholesterol transport.
The significant (P<0.05) decreased LDL levels indicate reduced atherogenic risk, potentially mediated by the extracts’ antioxidant and anti-inflammatory properties [22].
Regarding LDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated LDL levels (Table 2), In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
The significant (P<0.05) decreased VLDL levels indicate reduced atherogenic risk, potentially mediated by the extracts’ antioxidant and anti-inflammatory properties [22].
Regarding VLDL, there were significant (P<0.05) alterations in the experimental groups compared to the control group. The adrenaline group exhibited hyperlipidemia, characterized by elevated LDL levels (Table 2). In contrast, E. hirta and L. hastata extracts mitigated lipid profile alterations, indicating potential anti-hyperlipidemic effects.
Adrenaline induced significant electrolyte imbalances (P<0.05), including hypochloremia (Table 4).

E. hirta and L. hastata extracts normalized electrolyte levels.
LFT parameters are essential indicators of liver health and function. In this study, the assessed LFT parameters included:
ALT: Decreased ALT levels in both extract-treated groups suggest improved hepatocellular integrity [22]. ALT is a liver enzyme involved in amino acid metabolism.
AST: Reduced AST levels indicate enhanced liver function [23]. AST is a liver enzyme involved in amino acid metabolism.
ALP: Decreased ALP levels suggest improved hepatic function [24]. ALP is a liver enzyme involved in bile acid synthesis.
Adrenaline administration induced hepatotoxicity, evidenced by elevated ALT, AST, and ALP levels. E. hirta and L. hastata extracts demonstrated hepatoprotective effects, as indicated by significant (P<0.05) reduced liver enzyme levels (Table 3).
RBC: Increased RBC count in both extract-treated groups suggests improved erythropoiesis [22]. RBCs are responsible for oxygen transport, and increased counts may indicate enhanced oxygen-carrying capacity.
Adrenaline caused anemia, characterized by decreased hemoglobin (Hb), packed cell volume (PCV), and MCH levels in adrenaline and amlodipine-induced untreated rats (Table 5).

E. hirta and L. hastata extracts improved hematological parameters in extract-treated groups (Table 5).
MCV and MCHC (Table 5): Improved MCV and MCHC values indicate enhanced erythrocyte health [24]. MCV measures the average size of RBCs, while MCHC measures the average Hb concentration within RBCs.
WBC: Normalized WBC count in both extract-treated groups implies enhanced immune function [23]. WBCs play a crucial role in immune defense, and normalized counts may indicate improved immune response.
PCV): Improved PCV values indicate enhanced erythrocyte production and/or reduced erythrocyte destruction [24]. PCV is a measure of the proportion of blood volume occupied by RBCs.
Hb: Increased Hb levels in both E. hirta- and L. hastata-treated groups suggest improved erythropoiesis, potentially due to the extracts’ iron-chelating properties [23]. Hb is essential for oxygen transport, and increased levels may indicate enhanced oxygen delivery to tissues.
Discussion
The present study demonstrated the antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing effects of E. hirta and L. hastata extracts. The findings suggest that these plant extracts may be useful in managing hypertension and related complications. Our findings corroborate existing scientific literature on the antioxidant and protective effects of these plant extracts [23, 25].
The observed anti-hyperlipidemic effects are consistent with research demonstrating the lipid-lowering properties of E. hirta [26] and L. hastate [27]. The hepatoprotective and renoprotective effects can be attributed to their antioxidant activity, reducing oxidative stress and inflammation in liver and kidney tissues [28].
Notably, our study provides novel insights into the combined antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing properties of E. hirta and L. hastata extracts. These findings suggest potential therapeutic applications in managing oxidative stress-related disorders, such as atherosclerosis, hepatotoxicity, and nephrotoxicity.
Studies have demonstrated the antioxidant and protective effects of various plant extracts [7, 25]. However, our study provides a comprehensive evaluation of the antioxidant, anti-hyperlipidemic, hepatoprotective, renoprotective, and hematological-enhancing effects of E. hirta and L. hastata extracts.
Our findings are consistent with existing scientific literature on the antioxidant and protective effects of plant extracts [7, 25]. However, our comprehensive evaluation of E. hirta and L. hastata extracts provides novel insights into their therapeutic potential.
Conclusion
The study highlights the potential of E. hirta and L. hastata extracts as a phytotherapeutic approach to hypertension. Further studies are needed to elucidate the mechanisms of action and to translate these findings to human studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria (Code: OG/CHT/2023/011). The animal study was approved by the University’s Animal Ethics Committee (Code: AEC/2024/022).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their appreciation to the technical staff of the Pharmacy Technician Department of the Ogun State College of Health Science and Technology, Illese-Ijebu, Nigeria, for their technical assistance.
References
- World Health Organization (WHO). Cardiovascular diseases (CVDs). Geneva: World Health Organization; 2020. [Link]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2018; 365(9455):217-23. [Link]
- Ogah OS, Okpechi I, Chukwuonye II, Akinyemi JO, Onwubere BJ, Falase AO, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: A review. World J Cardiol. 2012; 4(12):327-40. [DOI:10.4330/wjc.v4.i12.327]
- Leonti M, Cabras S, Castellanos ME, Challenger A, Gertsch J, Casu L. Bioprospecting: evolutionary implications from a post-olmec pharmacopoeia and the relevance of widespread taxa. J Ethnopharmacol. 2013; 147(1):92-107. [DOI:10.1016/j.jep.2013.02.012] [PMID]
- Martins DF, Emer AA, Batisti AP, Donatello N, Carlesso MG, Mazzardo-Martins L, et al. Inhalation of Cedrus atlantica essential oil alleviates pain behavior through activation of descending pain modulation pathways in a mouse model of postoperative pain. J Ethnopharmacol. 2015; 175:30-8. [DOI:10.1016/j.jep.2015.08.048] [PMID]
- Hassan Abdulsalam A, Hadiza ML, Chiamaka OS, Jonathan I, Alfa S, Rahmatallah Adenike A, et al. Protective role of leptadenia hastata on the haematological and biochemical alterations in adrenaline-induced hypertensive rats. Iran J Toxicol. 2020; 14(1):25-32. [DOI:10.32598/ijt.14.1.25]
- Yang WS, Jeong D, Nam G, Yi YS, Yoon DH, Kim TW, et al. AP-1 pathway-targeted inhibition of inflammatory responses in LPS-treated macrophages and EtOH/HCl-treated stomach by Archidendron clypearia methanol extract. J Ethnopharmacol. 2013; 146(2):637-44. [DOI:10.1016/j.jep.2013.01.034]
- Chin CY, Jalil J, Ng PY, Ng SF. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J Ethnopharmacol. 2018; 212:188-199. [DOI:10.1016/j.jep.2017.10.016]
- Zhong Q, Shi Z, Zhang L, Zhong R, Xia Z, Wang J, et al. The potential of Epimedium koreanum Nakai for herb-drug interaction. J Pharm Pharmacol. 2017; 69(10):1398-408.[DOI:10.1111/jphp.12773]
- Guyton AC, Hall JE. Textbook of medical physiology. 14th ed. Philadelphia: Saunders; 2020. [Link]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003; 42(6):1206-52. [DOI:10.1161/01.HYP.0000107251.49515.c2] [PMID]
- Canadian Council on Animal Care. CCAC Guidelines on Animal Use. Ottawa: Canadian Council on Animal Care; 2017. [Link]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1):56-63. [DOI:10.1093/ajcp/28.1.56] [PMID]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265-75. [DOI:10.1016/S0021-9258(19)52451-6] [PMID]
- Malloy HT, Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biol Chem. 1937; 119(2):481-90. [DOI:10.1016/S0021-9258(18)74392-5]
- Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia: Saunders; 1995. [Link]
- Young DS. Effects of preanalytical variables on clinical laboratory tests. Clin Chem 2001; 47(8):1447-54. [Link]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974; 20(4):470-5. [DOI:10.1093/clinchem/20.4.470] [PMID]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499-502. [DOI:10.1093/clinchem/18.6.499] [PMID]
- Lewis SM, Bain BJ, Bates I. Dacie and Lewis Practical Haematology. London: Churchill Livingstone; 2006. [Link]
- Duncan DB. Multiple range and multiple F tests. Biometrics 1955; 11(1):1-42. [DOI:10.2307/3001478]
- Singh D, Murugaiyah V, Hamid SBS, Kasinather V, Chan MSA, Ho ETW, et al. Assessment of gonadotropins and testosterone hormone levels in regular Mitragyna speciosa (Korth.) users. J Ethnopharmacol. 2018; 221:30-36. [DOI:10.1016/j.jep.2018.04.005]
- Xiao H, Fang Z, He X, Ding P, Cao Y, Chan S, et al. Recombinant ling zhi-8 enhances Tregs function to restore glycemic control in streptozocin-induced diabetic rats. J Pharm Pharmacol. 2020; 72(12):1946-55. [DOI:10.1111/jphp.13360]
- Ayyanar M, Ignacimuthu S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J Ethnopharmacol. 2011; 134(3):851-64. [PMID]
- Wele A. The Hungarian initiative for Ayurveda: European Institute of Ayurvedic Sciences. J Ayurveda Integr Med. 2018; 9(2):155-8. [DOI:10.1016/j.jaim.2017.12.002]
- Rao M. Anti-hyperlipidemic activity of Euphorbia hirta in high-fat diet-induced hyperlipidemic rats. J Clin Diagn Res. 2015; 9(9): 27-30.
- Rajaram A, Vanaja GR, Vyakaranam P, Rachamallu A, Reddy GV, Anilkumar K, et al. Anti-inflammatory profile of Aegle marmelos (L) Correa (Bilva) with special reference to young roots grown in different parts of India. J Ayurveda Integr Med. 2018; 9(2):90-8. [DOI:10.1016/j.jaim.2017.03.006]
- Wang Z, Zhao P, Zhang Y, Shi S, Chen X. The hepatoprotective effect and mechanism of lotus leaf on liver injury induced by Genkwa Flos. J Pharm Pharmacol. 2020; 72(12):1909-20. [DOI:10.1111/jphp.13355] [PMID]
Type of Study: Original Research |
Subject:
Ehtnopharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






