Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 95-104 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Olapeju Bolanl I, Erharuyi O, Akhigbemen A M, Ugiagbe A I, Ekugum E, Nwusulor S O, et al . Valsartan and Amlodipine Improved Haematological and Biochemical Indices of Vildagliptin-treated Streptozotocin-induced Diabetic Rats. Pharm Biomed Res 2025; 11 (2) :95-104
URL: http://pbr.mazums.ac.ir/article-1-651-en.html
URL: http://pbr.mazums.ac.ir/article-1-651-en.html
Israel Olapeju Bolanl *1 

 , Osayemwenre Erharuyi2
, Osayemwenre Erharuyi2 

 , Abigail M. Akhigbemen3
, Abigail M. Akhigbemen3 

 , Amenaghawon Imuetinyan Ugiagbe3
, Amenaghawon Imuetinyan Ugiagbe3 
 , Edobor Ekugum4
, Edobor Ekugum4 
 , Samuel O. Nwusulor1
, Samuel O. Nwusulor1 
 , Keziah E. Adudu1
, Keziah E. Adudu1 
 , Abiodun Falodun2
, Abiodun Falodun2 




 , Osayemwenre Erharuyi2
, Osayemwenre Erharuyi2 

 , Abigail M. Akhigbemen3
, Abigail M. Akhigbemen3 

 , Amenaghawon Imuetinyan Ugiagbe3
, Amenaghawon Imuetinyan Ugiagbe3 
 , Edobor Ekugum4
, Edobor Ekugum4 
 , Samuel O. Nwusulor1
, Samuel O. Nwusulor1 
 , Keziah E. Adudu1
, Keziah E. Adudu1 
 , Abiodun Falodun2
, Abiodun Falodun2 


1- Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria.
2- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Benin, Benin City, Nigeria.
3- Department of Pharmacy, University of Benin Teaching Hospital, University of Benin, Benin City, Nigeria.
4- Department of Pharmaceutical Technology, Edo State Polytechnic Usen, Usen, Nigeria.
2- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Benin, Benin City, Nigeria.
3- Department of Pharmacy, University of Benin Teaching Hospital, University of Benin, Benin City, Nigeria.
4- Department of Pharmaceutical Technology, Edo State Polytechnic Usen, Usen, Nigeria.
Full-Text [PDF 1198 kb]
(288 Downloads)
| Abstract (HTML) (687 Views)
Full-Text: (206 Views)
Introduction
Diabetes mellitus (DM) is still a leading non-communicable cause of death globally, partly due to the increase in the aging population and unhealthy lifestyle changes brought on by rapid urbanization and westernization [1-3]. Since the last century, there have been advances in the management of DM; however, much more research is still required to fully comprehend the etiology of the disease, pharmacological treatment, and other associated pathologies and risk factors. Furthermore, DM and hypertension frequently co-exist in sufferers and are two leading risk factors for cardiovascular diseases, including coronary artery disease and atherosclerosis [4]. Up to 75% of adults living with DM also have hypertension, and patients with hypertension alone often exhibit insulin resistance [5]. Consequently, identifying overlapping disease mechanisms and pharmacological drug interactions will be a more proactive approach to controlling and preventing the complications of DM and hypertension.
Antihypertensive drugs can have a substantial impact on the likelihood that otherwise healthy individuals may develop type 2 DM (T2DM) or metabolic syndrome [6, 7]. For example, the prodiabetic potentials of diuretics and beta blockers have been suggested [8, 9]. Meanwhile, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers may prevent diabetes more effectively than metabolically neutral calcium channel blockers [10, 11]. Therefore, the impact of co-administration of known antihypertensive drugs on the treatment outcomes of antidiabetic agents is not fully understood. As a proactive approach, this study assessed the effectiveness and treatment outcomes of amlodipine, valsartan, and vildagliptin combinations in the treatment of streptozotocin-induced diabetes in rats. Our focus was on the hematological and biochemical differentials, which are measures of organ function. The drugs for this study were rationally selected, with vildagliptin being the most prescribed and studied drug among the dipeptidyl peptidase-4 inhibitors [12]. Amlodipine and valsartan, on the other hand, are well-prescribed antihypertensive drugs that block calcium channels and angiotensin II receptors, respectively, and are not notorious for hypotension [13-15].
Aim
This study aimed to evaluate the effect of valsartan and amlodipine on the hematological and biochemical differentials of vildagliptin-treated streptozotocin-induced diabetic rats.
Materials and Methods
Materials
Drugs and chemicals
Generic samples of drugs (vildagliptin, amlodipine, and valsartan) in the form of tablet dosage forms were obtained from community pharmacies in Benin City, Nigeria. The drugs were dissolved in distilled water for oral administration using a gastric tube. Sodium citrate, citric acid, and streptozotocin were acquired from Santa Cruz Biotechnology. Wet reagent diagnostic kits were obtained from Randox Laboratories Ltd (UK).
Animals
Male Wistar rats (200 – 350 g) were obtained from the Animal House of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria. The rats were kept in plastic cages and housed at room temperature, with controlled humidity and a light/dark cycle of 12 hours each. They were fed dry rodent pellet feed and allowed free access to water. The bedding materials (wood shavings) in the cages were changed daily. The bedding materials (wood shavings) of the cages were changed daily.
Methods
Induction of DM
The animals were fasted overnight, and DM was induced by a single intraperitoneal injection of streptozotocin at a dose of 40 mg/kg body weight, dissolved in freshly prepared 0.1 M citrate buffer, pH 4.5. After administration, the animals were allowed free access to food and water. After 48 hours, the animals were tested for DM using the Accu-Chek® Active glucometer (Roche, USA), and any animal with a blood sugar level of ≥200 mg/dL was considered diabetic [16].
Experimental design
The animals were divided into five groups of four rats each and treated orally for three weeks as follows;
Group 1: Healthy animals with no treatment.
Group 2: Diabetic animals treated with 5 mg/kg of vildagliptin only.
Group 3: Diabetic animals treated with 5 mg/kg and 2.5 mg/kg of vildagliptin and amlodipine, respectively.
Group 4: Diabetic animals treated with 5 mg/kg and 30 mg/kg of vildagliptin and valsartan, respectively.
Group 5: Untreated diabetic animals.
Determination of lipid profile
Total plasma cholesterol, high- and low-density lipoproteins, and triglycerides were estimated by collecting blood from the abdominal aorta in the rats under chloroform anesthesia after three weeks of treatment.
Total cholesterol
Total cholesterol levels in plasma were determined by the enzymatic method using a wet reagent diagnostic kit (Randox Laboratories Ltd, UK), which is a modification of the method of Abell et al. [17].
Total triglycerides
Total triglyceride levels were determined by the enzymatic method using wet reagent diagnostic kits (Randox Laboratories Ltd, UK), a modification of the method by Jacobs and Vandermark [18].
High-density lipoprotein
High-density lipoprotein levels were determined by the enzymatic method using wet reagent diagnostic kits (Randox Laboratories Ltd UK) [19].
Hematological analysis
Following three weeks of treatment, the animals were anesthetized with chloroform, and blood samples were obtained from the aorta after dissecting the animals. The blood samples were transferred into ethylene diamine tetra-acetic acid (EDTA) sample bottles and analyzed immediately after blood collection using the Human Automated Haematology System analyzer (ERMA PCE 210, ERMA, Japan). The parameters analyzed included haemoglobin (Hb), packed cell volume (PCV), white blood cell count (WBC) and differential count (granulocyte, lymphocyte, and monocyte), platelets (PLT), mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC), and random blood glucose [20].
Biochemical analyses
The assays for alanine aminotransferase, aspartate amino transaminase, and alkaline phosphatase were carried out according to previous studies [21, 22]. The assay for electrolytes (sodium, chloride, bicarbonate, and potassium) was done as described by Van Slyke and Neil [23]. Chloride ions were assayed as described by Schales and Schales [24]. The assay for sodium and potassium was done according to the method by Margoshes and Vallee [25]. The assay for bilirubin was carried out according to the method described by Schmidt and Schmidt [22], and proteins were assayed using the method described by Tietz [26].
Data collection and statistical analysis
Statistical analysis was carried out using GraphPad Prism software, version 6 (San Diego, USA). Results were expressed as Mean±SE. Statistical analyses were performed using a one-way analysis of variance followed by the Dunnett post-hoc test for multiple comparisons. A P of ≤0.05 was considered significant.
Results
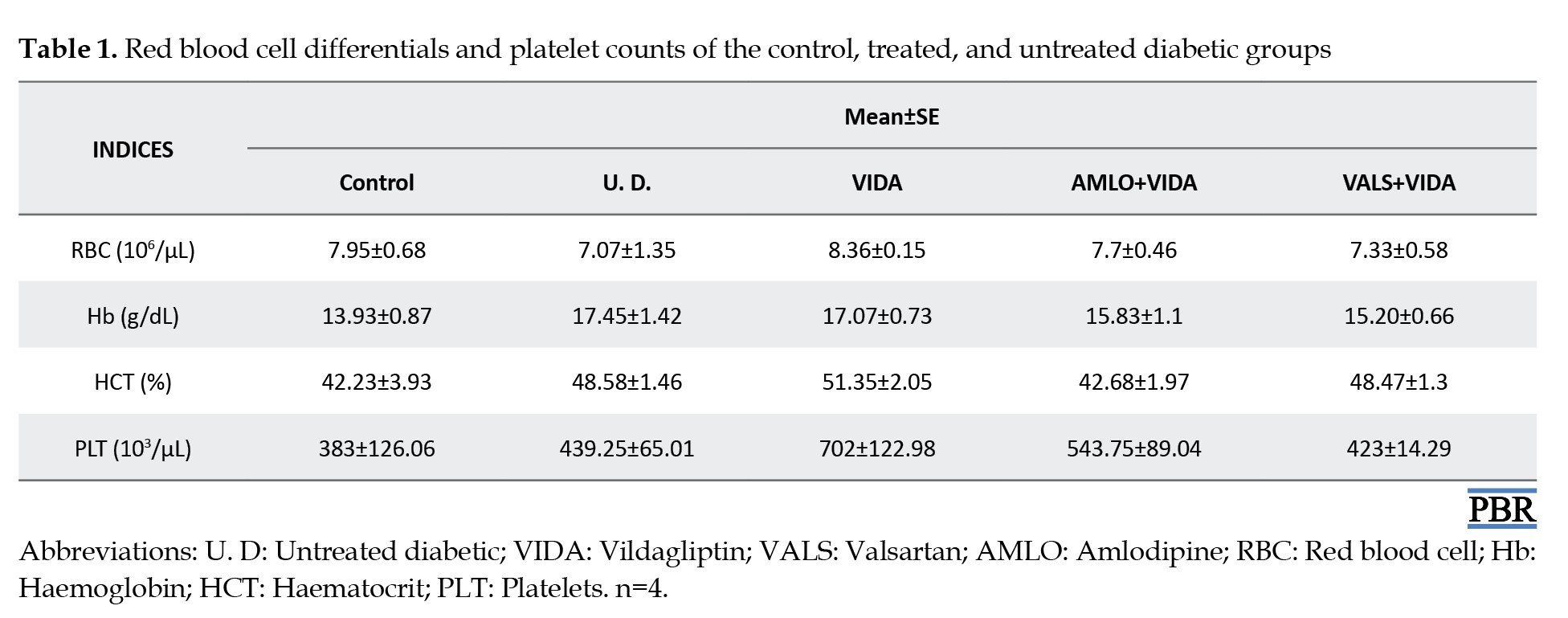
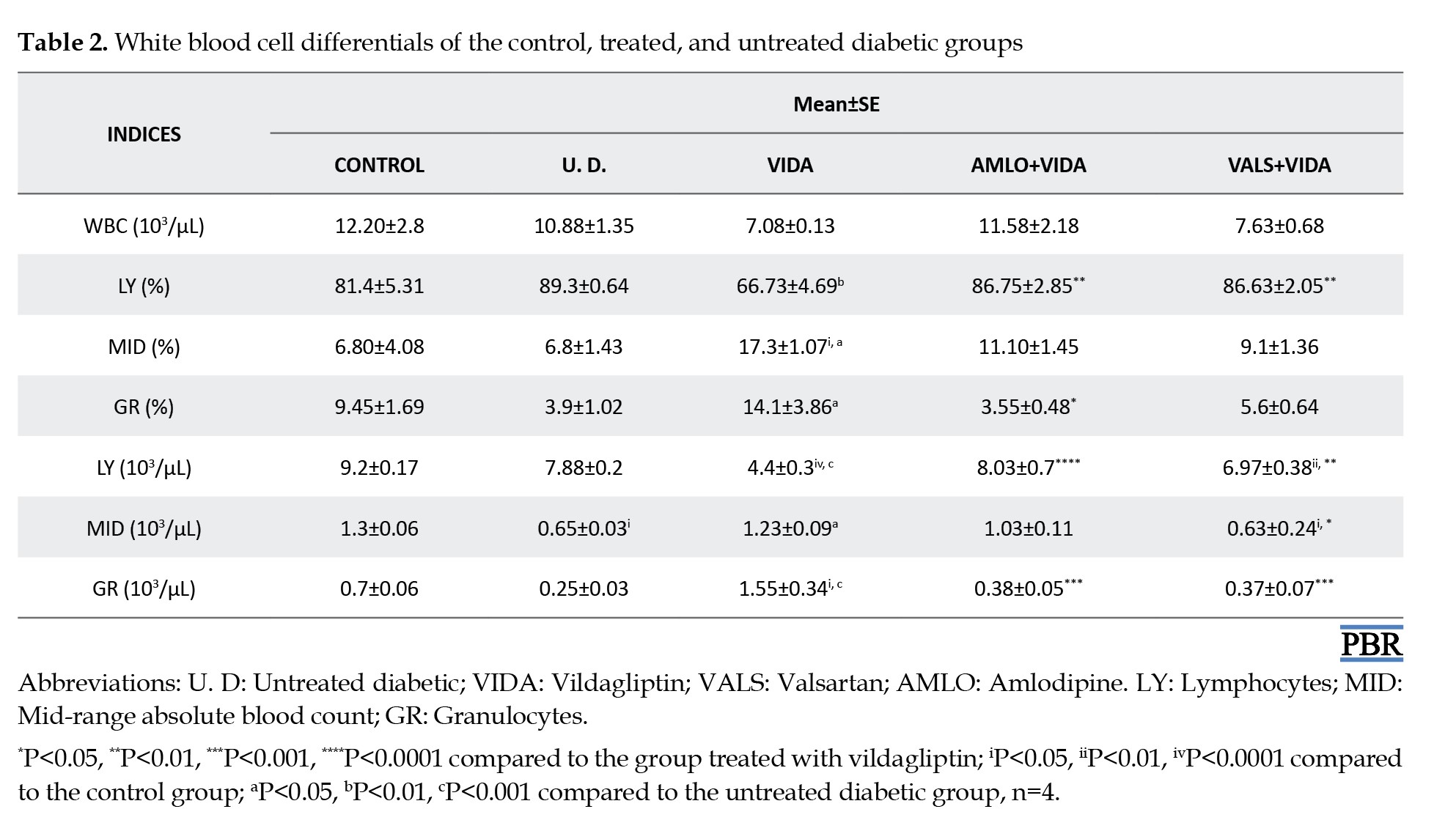
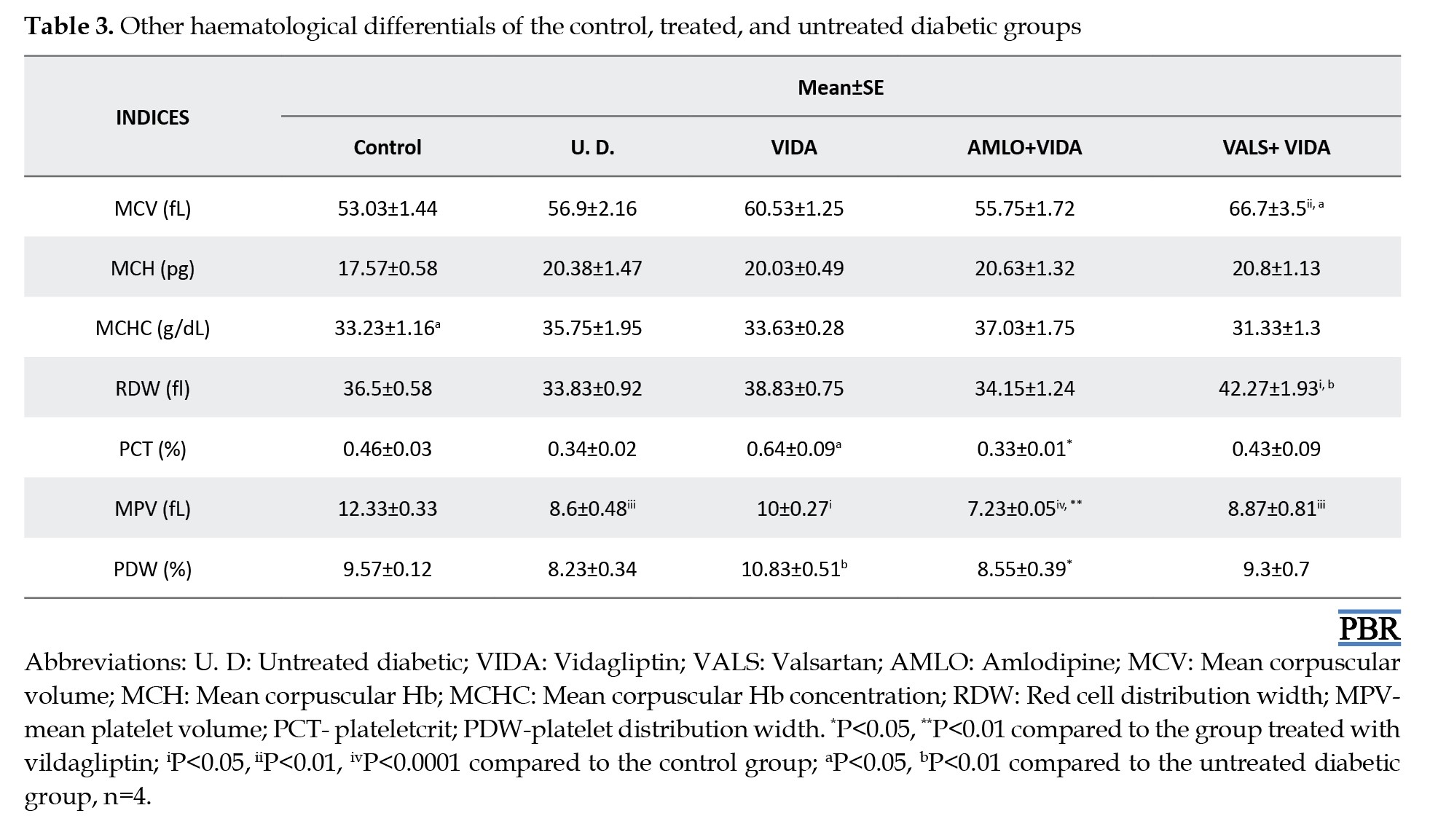
Hematological differentials of the control, treated, and untreated diabetic groups
Tables 1, 2 and 3 show the mean hematological differentials of the rats in the treatment groups, the untreated diabetic rats, and the control group, compared to one another after 3 weeks of treatment.
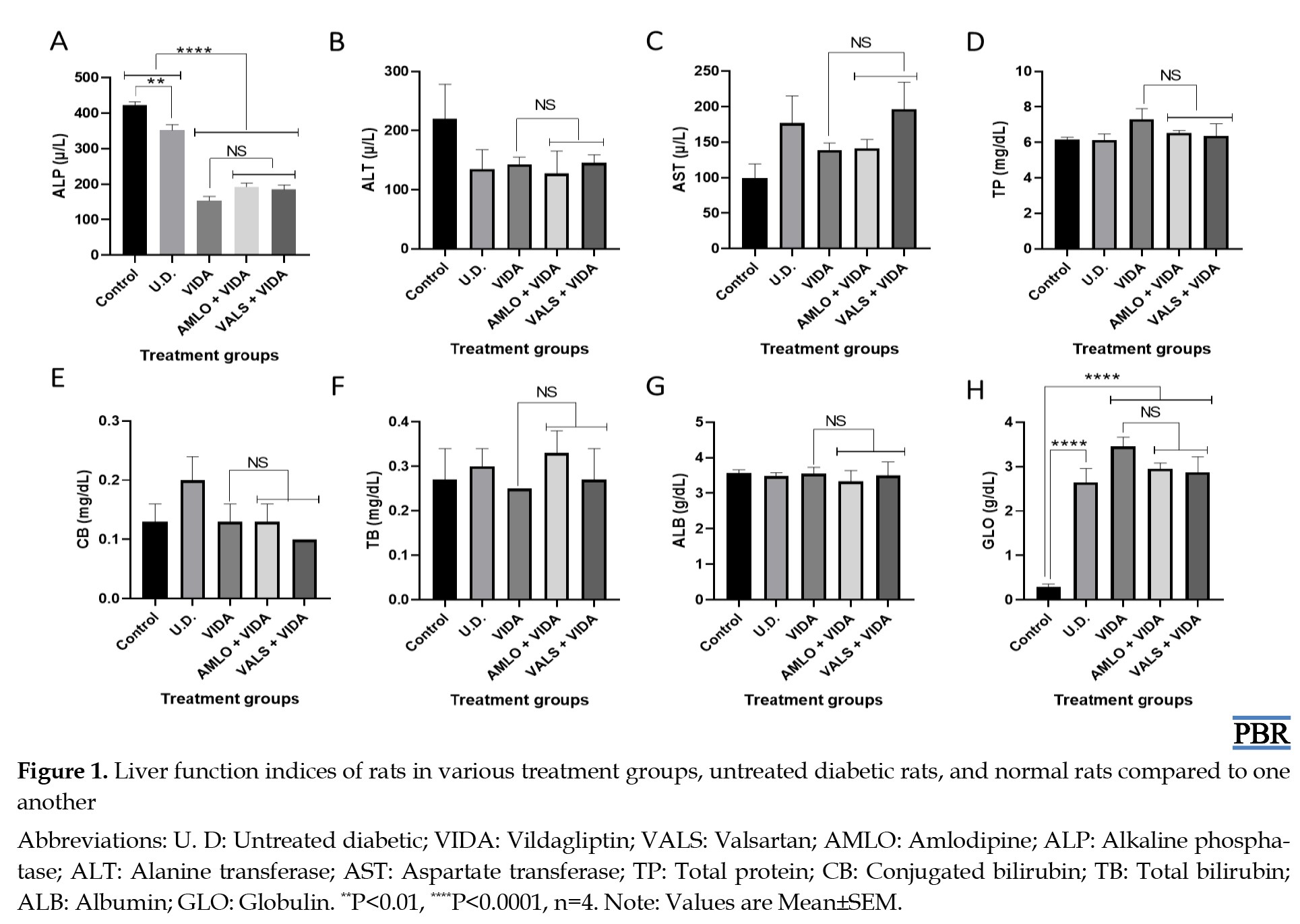
Effects of treatments on liver function
Figure 1 shows the mean values of some liver enzymes and indices of the rats in the treatment groups, untreated diabetic group, and control group compared to one another after three weeks of treatment. The results showed significant changes in liver function as follows: (i) a significant decrease (P<0.05) in alkaline phosphatase in untreated diabetic rats and a decrease (P<0.0001) in groups treated with vildagliptin, amlodipine plus vildagliptin, and valsartan plus vildagliptin, all compared to normal rats; (ii) a significant increase (P<0.0001) in globulin in groups treated with vildagliptin alone, amlodipine plus vildagliptin, and valsartan plus vildagliptin, as well as in untreated diabetic rats, all compared to normal rats.
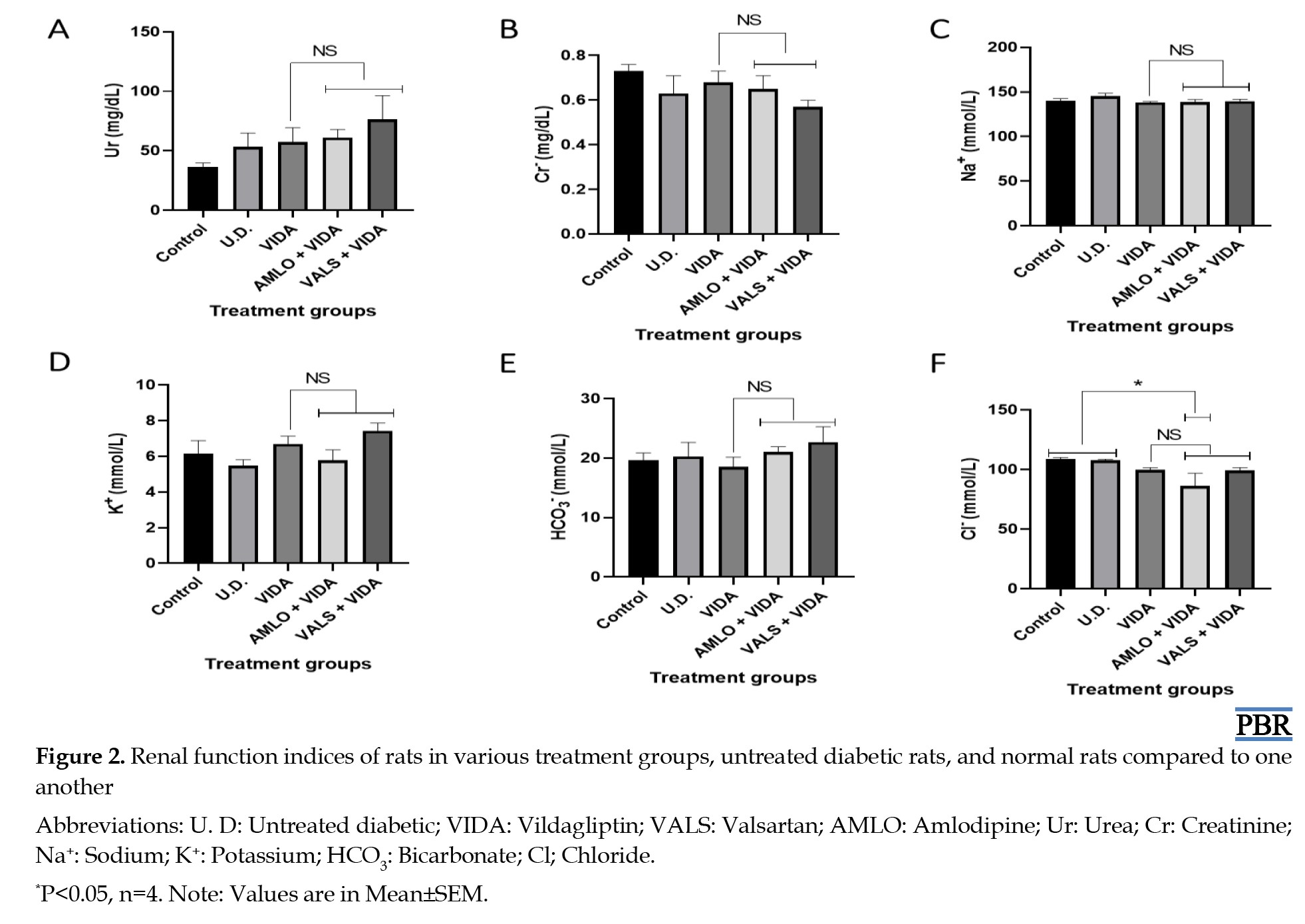
Effects of treatments on renal function
Figure 2 shows the mean values of some renal function indices of the rats in the treatment groups, untreated diabetic group, and control group after three weeks of treatment compared to one another. The results showed a significant (P<0.05) decrease in Cl- in the group treated with amlodipine and vildagliptin compared to both untreated diabetic and normal rats.
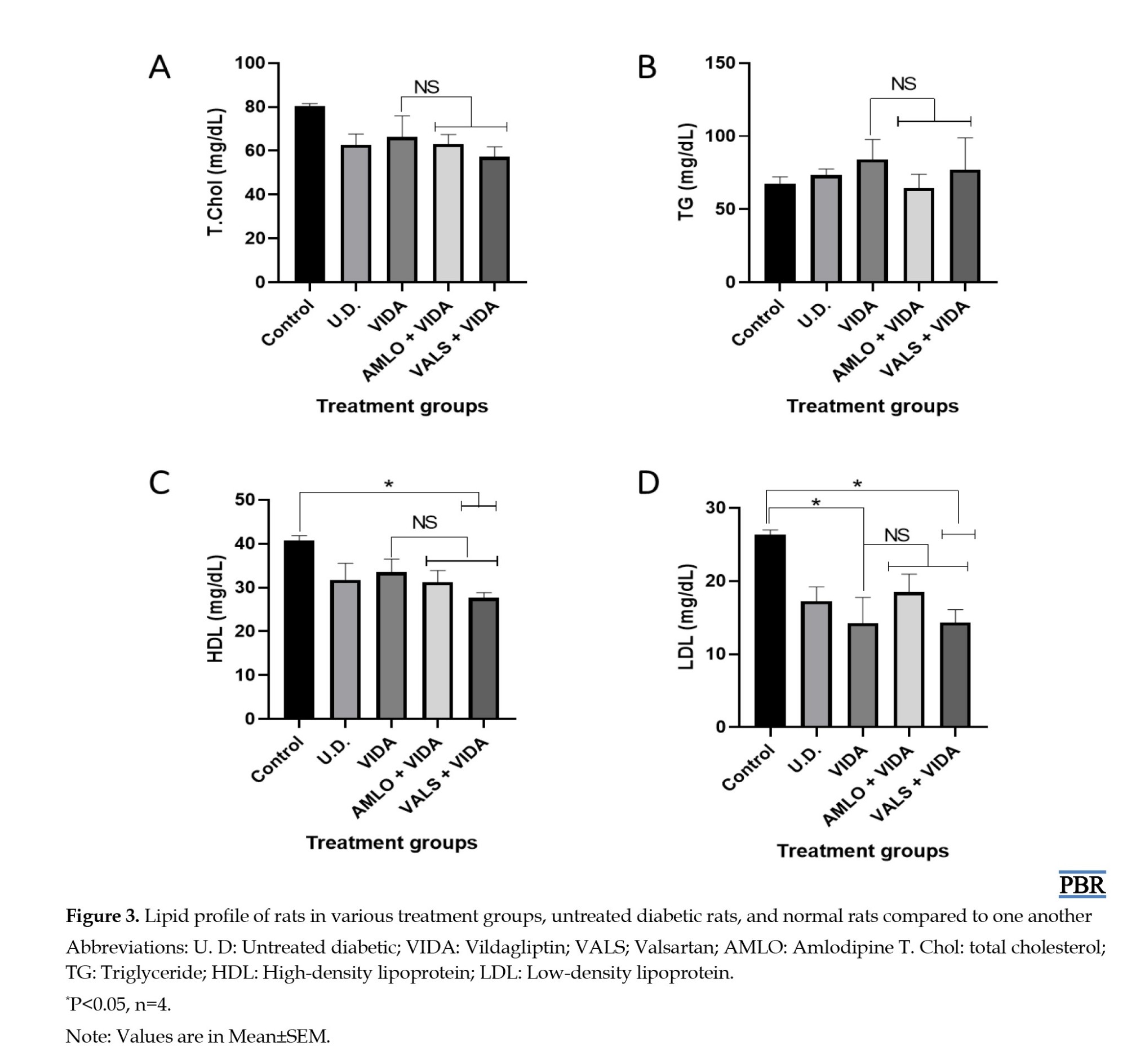
Effects of treatments on lipid profile
Figure 3 shows the mean values of the lipid profile of the rats in the treatment groups, the untreated diabetic group, and the control group after three weeks of treatment, all compared to one another. The results indicated a significant (P<0.05) decrease as follows: (i) in high density lipoprotein (HDL), valsartan plus vildagliptin compared to the normal control, and (ii) in LDL, vildagliptin and valsartan plus vildagliptin, both compared to the normal control.
Discussion
Exacerbated cardiovascular complications associated with the co-existence of DM and hypertension are major causes of the end-organ damage responsible for the mortality associated with DM [27-29]. The macrovascular and microvascular complications of DM and hypertension overlap in such a way that they may share common mechanisms [30]. Therefore, it is important to implement a multifactorial approach with concurrent emphasis on optimizing glycemic control and reducing the rate of pathological progression of diabetic complications, which will ultimately lower the mortality rate of diabetic patients. Hence, the focus of this study was to investigate the effect of valsartan and amlodipine on the hematological and biochemical differentials of vildagliptin-treated, streptozotocin-induced diabetic rats.
In this study, a single dose of streptozotocin (40 mg/kg body weight) was administered intraperitoneally for the induction of DM [7, 16, 20]. Streptozotocin is a structural analog of N-acetyl glucosamine that acts as a potent alkylating agent, resulting in disrupted glucose transport and glucokinase activity, as well as the breakdown of multiple DNA strands [31]. A single high dose STZ injection (>60 mg/kg body weight) leads to massive pancreatic beta cell destruction, which is a feature of type 1 DM. In contrast, intermediate dosages of STZ injections (between 40 and 55 mg/kg body weight) cause only partial impairment of insulin secretory mechanisms, leading to the hyperglycemic conditions present in type 2 DM (T2DM) [32].
Although not significant, there were marked increases in the RBC counts of the diabetic rats in the various treatment groups compared to untreated diabetic rats. Patients with diabetes over an extended period often experience anemia [33]. Hyperglycemia correlates with increased non-enzymatic glycosylation of RBC membrane proteins [34, 35]. Additionally, lipid peroxides, which cause hemolysis of RBC, are elevated due to the oxidation of RBC proteins and hyperglycemia in DM [36]. Furthermore, the hematological differentials showed that the groups treated with the antihypertensives, amlodipine, and valsartan, had slightly higher lymphocyte counts compared to those of the diabetic rats treated with vildagliptin only. This indicates that vildagliptin alone possesses good antidiabetic activity, as evidenced by a reduced lymphocyte count, suggesting regulated blood glucose control. Lymphocytes are generally increased in disease states as systemic markers of inflammation [37, 38]. In cases of poor glycemic control, as exhibited by the untreated diabetic rats, the lymphocyte count was elevated. This can exacerbate the progression of diabetes to complications, increasing the risk of a first cardiovascular event in untreated but diagnosed diabetic subjects [39]. Also, MCV values are generally decreased in the diabetic state. This indicates abnormal Hb synthesis, failure of blood osmoregulation, and altered plasma osmolarity [40]. The increase in MCV and RDW values for the diabetic rats was more pronounced in those treated with valsartan plus vildagliptin compared to the control and untreated diabetic rats. The reason for this observation is currently unclear.
The biochemical analysis results for liver function tests showed a significant reduction in alkaline phosphatase enzymes for the groups treated with vildagliptin only, vildagliptin plus amlodipine, and valsartan plus vildagliptin compared to the control and untreated diabetic groups. Alkaline phosphatase is used as a diagnostic indicator for bone or liver disease [41]. Among the various complications associated with diabetes, diabetic liver disease, along with diabetic bone disease, indicates the severity of the condition, as revealed by another finding [42], which showed elevated serum levels of alkaline phosphatase that further validate this study. The decrease in the enzyme, as shown in this study, could be attributed to the ameliorated effects of diabetes by all the treatment groups. This suggests that the progression and complications of diabetes were slowed down. Additionally, our results indicated that globulin levels in all treatment groups and untreated diabetic rats were significantly higher than those in the control group. Although the exact cause of this is unknown, live damage caused by DM may play a role, and this finding is consistent with previous studies [7, 20].
Analysis of renal function showed that chloride ion levels were reduced in the group treated with amlodipine plus vildagliptin compared to the control group. This observation may be due to the increased utilization of chloride ions in this group, with the treatment helping to ameliorate this effect. We have demonstrated similar findings in another study [7]. Additionally, lipid profile analysis showed that the addition of valsartan to vildagliptin caused a significant reduction in LDL levels when compared to the control group. The potential benefit of angiotensin receptor blockers, like valsartan as an effective adjunct therapy in the management of hypertension with a hyperlipidemia component, as previously demonstrated [43-45], is further highlighted by this intriguing finding, although more research may be needed to validate it.
Conclusion
Our results showed that the progression of diabetes was limited in the treated groups compared to the untreated diabetic group. Furthermore, the addition of valsartan and amlodipine, two known antihypertensives, to vildagliptin significantly improved certain hematological differentials and the lipid profile of diabetic rats. Valsartan, in particular, appeared to have a more pronounced overall effect than amlodipine when combined with vildagliptin. Therefore, valsartan may provide greater organ protection and reduce cardiovascular events in patients with T2DM, leading to improved clinical outcomes. However, further research is strongly needed, especially concerning long-term treatment, to validate these findings.
Ethical Considerations
Compliance with ethical guidelines
The experiment was carried out following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 2002. The Ethics Committee of the Faculty of Pharmacy, University of Benin, granted ethical permission (reference number EC/FP/017/01) for this study.
Funding
This work was supported by the Tertiary Education Trust Fund (TETFund) Institution-Based Research (IBR) Grant (Code: TETFund/DRSS/ESITMUSEN/2016/RP/VOL.1).
Authors' contributions
Supervision, and project administration: Israel Olapeju Bolanle, Abigail M. Aghigbemen, Osayemwenre Erharuyi, and Abiodun Falodun; Formal analysis: Israel Olapeju Bolanle, Amenaghawon Imuetinyan Ugiagbe, Edobor Ekugum, Samuel O. Nwusulor, and Keziah E. Adudu; Conceptualization, writing the original draft: Israel Olapeju Bolanle; Review, editing: Israel Olapeju Bolanle, Abigail M. Aghigbemen, Osayemwenre Erharuyi, and Abiodun Falodun; Investigation, methodology and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the technical support of Philip Obarisiagbon and Collins Osaigbovo, both from the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin, Nigeria.
References
Diabetes mellitus (DM) is still a leading non-communicable cause of death globally, partly due to the increase in the aging population and unhealthy lifestyle changes brought on by rapid urbanization and westernization [1-3]. Since the last century, there have been advances in the management of DM; however, much more research is still required to fully comprehend the etiology of the disease, pharmacological treatment, and other associated pathologies and risk factors. Furthermore, DM and hypertension frequently co-exist in sufferers and are two leading risk factors for cardiovascular diseases, including coronary artery disease and atherosclerosis [4]. Up to 75% of adults living with DM also have hypertension, and patients with hypertension alone often exhibit insulin resistance [5]. Consequently, identifying overlapping disease mechanisms and pharmacological drug interactions will be a more proactive approach to controlling and preventing the complications of DM and hypertension.
Antihypertensive drugs can have a substantial impact on the likelihood that otherwise healthy individuals may develop type 2 DM (T2DM) or metabolic syndrome [6, 7]. For example, the prodiabetic potentials of diuretics and beta blockers have been suggested [8, 9]. Meanwhile, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers may prevent diabetes more effectively than metabolically neutral calcium channel blockers [10, 11]. Therefore, the impact of co-administration of known antihypertensive drugs on the treatment outcomes of antidiabetic agents is not fully understood. As a proactive approach, this study assessed the effectiveness and treatment outcomes of amlodipine, valsartan, and vildagliptin combinations in the treatment of streptozotocin-induced diabetes in rats. Our focus was on the hematological and biochemical differentials, which are measures of organ function. The drugs for this study were rationally selected, with vildagliptin being the most prescribed and studied drug among the dipeptidyl peptidase-4 inhibitors [12]. Amlodipine and valsartan, on the other hand, are well-prescribed antihypertensive drugs that block calcium channels and angiotensin II receptors, respectively, and are not notorious for hypotension [13-15].
Aim
This study aimed to evaluate the effect of valsartan and amlodipine on the hematological and biochemical differentials of vildagliptin-treated streptozotocin-induced diabetic rats.
Materials and Methods
Materials
Drugs and chemicals
Generic samples of drugs (vildagliptin, amlodipine, and valsartan) in the form of tablet dosage forms were obtained from community pharmacies in Benin City, Nigeria. The drugs were dissolved in distilled water for oral administration using a gastric tube. Sodium citrate, citric acid, and streptozotocin were acquired from Santa Cruz Biotechnology. Wet reagent diagnostic kits were obtained from Randox Laboratories Ltd (UK).
Animals
Male Wistar rats (200 – 350 g) were obtained from the Animal House of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Nigeria. The rats were kept in plastic cages and housed at room temperature, with controlled humidity and a light/dark cycle of 12 hours each. They were fed dry rodent pellet feed and allowed free access to water. The bedding materials (wood shavings) in the cages were changed daily. The bedding materials (wood shavings) of the cages were changed daily.
Methods
Induction of DM
The animals were fasted overnight, and DM was induced by a single intraperitoneal injection of streptozotocin at a dose of 40 mg/kg body weight, dissolved in freshly prepared 0.1 M citrate buffer, pH 4.5. After administration, the animals were allowed free access to food and water. After 48 hours, the animals were tested for DM using the Accu-Chek® Active glucometer (Roche, USA), and any animal with a blood sugar level of ≥200 mg/dL was considered diabetic [16].
Experimental design
The animals were divided into five groups of four rats each and treated orally for three weeks as follows;
Group 1: Healthy animals with no treatment.
Group 2: Diabetic animals treated with 5 mg/kg of vildagliptin only.
Group 3: Diabetic animals treated with 5 mg/kg and 2.5 mg/kg of vildagliptin and amlodipine, respectively.
Group 4: Diabetic animals treated with 5 mg/kg and 30 mg/kg of vildagliptin and valsartan, respectively.
Group 5: Untreated diabetic animals.
Determination of lipid profile
Total plasma cholesterol, high- and low-density lipoproteins, and triglycerides were estimated by collecting blood from the abdominal aorta in the rats under chloroform anesthesia after three weeks of treatment.
Total cholesterol
Total cholesterol levels in plasma were determined by the enzymatic method using a wet reagent diagnostic kit (Randox Laboratories Ltd, UK), which is a modification of the method of Abell et al. [17].
Total triglycerides
Total triglyceride levels were determined by the enzymatic method using wet reagent diagnostic kits (Randox Laboratories Ltd, UK), a modification of the method by Jacobs and Vandermark [18].
High-density lipoprotein
High-density lipoprotein levels were determined by the enzymatic method using wet reagent diagnostic kits (Randox Laboratories Ltd UK) [19].
Hematological analysis
Following three weeks of treatment, the animals were anesthetized with chloroform, and blood samples were obtained from the aorta after dissecting the animals. The blood samples were transferred into ethylene diamine tetra-acetic acid (EDTA) sample bottles and analyzed immediately after blood collection using the Human Automated Haematology System analyzer (ERMA PCE 210, ERMA, Japan). The parameters analyzed included haemoglobin (Hb), packed cell volume (PCV), white blood cell count (WBC) and differential count (granulocyte, lymphocyte, and monocyte), platelets (PLT), mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC), and random blood glucose [20].
Biochemical analyses
The assays for alanine aminotransferase, aspartate amino transaminase, and alkaline phosphatase were carried out according to previous studies [21, 22]. The assay for electrolytes (sodium, chloride, bicarbonate, and potassium) was done as described by Van Slyke and Neil [23]. Chloride ions were assayed as described by Schales and Schales [24]. The assay for sodium and potassium was done according to the method by Margoshes and Vallee [25]. The assay for bilirubin was carried out according to the method described by Schmidt and Schmidt [22], and proteins were assayed using the method described by Tietz [26].
Data collection and statistical analysis
Statistical analysis was carried out using GraphPad Prism software, version 6 (San Diego, USA). Results were expressed as Mean±SE. Statistical analyses were performed using a one-way analysis of variance followed by the Dunnett post-hoc test for multiple comparisons. A P of ≤0.05 was considered significant.
Results
Hematological differentials of the control, treated, and untreated diabetic groups
Tables 1, 2 and 3 show the mean hematological differentials of the rats in the treatment groups, the untreated diabetic rats, and the control group, compared to one another after 3 weeks of treatment.



Effects of treatments on liver function
Figure 1 shows the mean values of some liver enzymes and indices of the rats in the treatment groups, untreated diabetic group, and control group compared to one another after three weeks of treatment. The results showed significant changes in liver function as follows: (i) a significant decrease (P<0.05) in alkaline phosphatase in untreated diabetic rats and a decrease (P<0.0001) in groups treated with vildagliptin, amlodipine plus vildagliptin, and valsartan plus vildagliptin, all compared to normal rats; (ii) a significant increase (P<0.0001) in globulin in groups treated with vildagliptin alone, amlodipine plus vildagliptin, and valsartan plus vildagliptin, as well as in untreated diabetic rats, all compared to normal rats.
Effects of treatments on renal function
Figure 2 shows the mean values of some renal function indices of the rats in the treatment groups, untreated diabetic group, and control group after three weeks of treatment compared to one another. The results showed a significant (P<0.05) decrease in Cl- in the group treated with amlodipine and vildagliptin compared to both untreated diabetic and normal rats.
Effects of treatments on lipid profile
Figure 3 shows the mean values of the lipid profile of the rats in the treatment groups, the untreated diabetic group, and the control group after three weeks of treatment, all compared to one another. The results indicated a significant (P<0.05) decrease as follows: (i) in high density lipoprotein (HDL), valsartan plus vildagliptin compared to the normal control, and (ii) in LDL, vildagliptin and valsartan plus vildagliptin, both compared to the normal control.
Discussion
Exacerbated cardiovascular complications associated with the co-existence of DM and hypertension are major causes of the end-organ damage responsible for the mortality associated with DM [27-29]. The macrovascular and microvascular complications of DM and hypertension overlap in such a way that they may share common mechanisms [30]. Therefore, it is important to implement a multifactorial approach with concurrent emphasis on optimizing glycemic control and reducing the rate of pathological progression of diabetic complications, which will ultimately lower the mortality rate of diabetic patients. Hence, the focus of this study was to investigate the effect of valsartan and amlodipine on the hematological and biochemical differentials of vildagliptin-treated, streptozotocin-induced diabetic rats.
In this study, a single dose of streptozotocin (40 mg/kg body weight) was administered intraperitoneally for the induction of DM [7, 16, 20]. Streptozotocin is a structural analog of N-acetyl glucosamine that acts as a potent alkylating agent, resulting in disrupted glucose transport and glucokinase activity, as well as the breakdown of multiple DNA strands [31]. A single high dose STZ injection (>60 mg/kg body weight) leads to massive pancreatic beta cell destruction, which is a feature of type 1 DM. In contrast, intermediate dosages of STZ injections (between 40 and 55 mg/kg body weight) cause only partial impairment of insulin secretory mechanisms, leading to the hyperglycemic conditions present in type 2 DM (T2DM) [32].
Although not significant, there were marked increases in the RBC counts of the diabetic rats in the various treatment groups compared to untreated diabetic rats. Patients with diabetes over an extended period often experience anemia [33]. Hyperglycemia correlates with increased non-enzymatic glycosylation of RBC membrane proteins [34, 35]. Additionally, lipid peroxides, which cause hemolysis of RBC, are elevated due to the oxidation of RBC proteins and hyperglycemia in DM [36]. Furthermore, the hematological differentials showed that the groups treated with the antihypertensives, amlodipine, and valsartan, had slightly higher lymphocyte counts compared to those of the diabetic rats treated with vildagliptin only. This indicates that vildagliptin alone possesses good antidiabetic activity, as evidenced by a reduced lymphocyte count, suggesting regulated blood glucose control. Lymphocytes are generally increased in disease states as systemic markers of inflammation [37, 38]. In cases of poor glycemic control, as exhibited by the untreated diabetic rats, the lymphocyte count was elevated. This can exacerbate the progression of diabetes to complications, increasing the risk of a first cardiovascular event in untreated but diagnosed diabetic subjects [39]. Also, MCV values are generally decreased in the diabetic state. This indicates abnormal Hb synthesis, failure of blood osmoregulation, and altered plasma osmolarity [40]. The increase in MCV and RDW values for the diabetic rats was more pronounced in those treated with valsartan plus vildagliptin compared to the control and untreated diabetic rats. The reason for this observation is currently unclear.
The biochemical analysis results for liver function tests showed a significant reduction in alkaline phosphatase enzymes for the groups treated with vildagliptin only, vildagliptin plus amlodipine, and valsartan plus vildagliptin compared to the control and untreated diabetic groups. Alkaline phosphatase is used as a diagnostic indicator for bone or liver disease [41]. Among the various complications associated with diabetes, diabetic liver disease, along with diabetic bone disease, indicates the severity of the condition, as revealed by another finding [42], which showed elevated serum levels of alkaline phosphatase that further validate this study. The decrease in the enzyme, as shown in this study, could be attributed to the ameliorated effects of diabetes by all the treatment groups. This suggests that the progression and complications of diabetes were slowed down. Additionally, our results indicated that globulin levels in all treatment groups and untreated diabetic rats were significantly higher than those in the control group. Although the exact cause of this is unknown, live damage caused by DM may play a role, and this finding is consistent with previous studies [7, 20].
Analysis of renal function showed that chloride ion levels were reduced in the group treated with amlodipine plus vildagliptin compared to the control group. This observation may be due to the increased utilization of chloride ions in this group, with the treatment helping to ameliorate this effect. We have demonstrated similar findings in another study [7]. Additionally, lipid profile analysis showed that the addition of valsartan to vildagliptin caused a significant reduction in LDL levels when compared to the control group. The potential benefit of angiotensin receptor blockers, like valsartan as an effective adjunct therapy in the management of hypertension with a hyperlipidemia component, as previously demonstrated [43-45], is further highlighted by this intriguing finding, although more research may be needed to validate it.
Conclusion
Our results showed that the progression of diabetes was limited in the treated groups compared to the untreated diabetic group. Furthermore, the addition of valsartan and amlodipine, two known antihypertensives, to vildagliptin significantly improved certain hematological differentials and the lipid profile of diabetic rats. Valsartan, in particular, appeared to have a more pronounced overall effect than amlodipine when combined with vildagliptin. Therefore, valsartan may provide greater organ protection and reduce cardiovascular events in patients with T2DM, leading to improved clinical outcomes. However, further research is strongly needed, especially concerning long-term treatment, to validate these findings.
Ethical Considerations
Compliance with ethical guidelines
The experiment was carried out following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 2002. The Ethics Committee of the Faculty of Pharmacy, University of Benin, granted ethical permission (reference number EC/FP/017/01) for this study.
Funding
This work was supported by the Tertiary Education Trust Fund (TETFund) Institution-Based Research (IBR) Grant (Code: TETFund/DRSS/ESITMUSEN/2016/RP/VOL.1).
Authors' contributions
Supervision, and project administration: Israel Olapeju Bolanle, Abigail M. Aghigbemen, Osayemwenre Erharuyi, and Abiodun Falodun; Formal analysis: Israel Olapeju Bolanle, Amenaghawon Imuetinyan Ugiagbe, Edobor Ekugum, Samuel O. Nwusulor, and Keziah E. Adudu; Conceptualization, writing the original draft: Israel Olapeju Bolanle; Review, editing: Israel Olapeju Bolanle, Abigail M. Aghigbemen, Osayemwenre Erharuyi, and Abiodun Falodun; Investigation, methodology and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the technical support of Philip Obarisiagbon and Collins Osaigbovo, both from the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin, Nigeria.
References
- Arokiasamy P, Salvi S, Selvamani Y. Global burden of diabetes mellitus. In: Haring R, Kickbusch I, Ganten D, Moeti M, editors. Handbook of global health. Cham: Springer International Publishing; 2021. [DOI:10.1007/978-3-030-05325-3_28-1]
- Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 2020; 18(2):104-9. [DOI:10.2174/1570161117666190405165911] [PMID]
- Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci Rep. 2024; 7(3):e2004. [DOI:10.1002/hsr2.2004] [PMID]
- Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol. 2005; 42(Suppl 1):S17-25. [DOI:10.1007/s00592-005-0177-z] [PMID]
- Centers for Disease Control and Prevention. National diabetes statistics report: Estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014. [Link]
- Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016; 134(23):e535-78. [DOI:10.1161/CIR.0000000000000450]
- Bolanle IO, Omogbai EKI, Bafor EE. Effects of amlodipine and valsartan on glibenclamide-treated streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018; 106:566-574. [DOI:10.1016/j.biopha.2018.06.152] [PMID]
- Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008; 117(20):2706-15. [DOI:10.1161/CIRCULATIONAHA.107.695007] [PMID]
- Zappe DH, Sowers JR, Hsueh WA, Haffner SM, Deedwania PC, Fonseca VA, et al. Metabolic and antihypertensive effects of combined angiotensin receptor blocker and diuretic therapy in prediabetic hypertensive patients with the cardiometabolic syndrome. J Clin Hypertens. 2008; 10(12):894-903. [DOI:10.1111/j.1751-7176.2008.00054.x] [PMID]
- Wen H, Lin S. [Renal protective effect of valsartan in diabetic rats (Chinese)]. Zhonghua nei ke za zhi. 1999; 38(3):157-60. [PMID]
- Cure E, Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr. 2020; 14(4):349-50. [DOI:10.1016/j.dsx.2020.04.019] [PMID]
- Ahrén B, Schweizer A, Dejager S, Villhauer EB, Dunning BE, Foley JE. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011; 13(9):775-83. [DOI:10.1111/j.1463-1326.2011.01414.x] [PMID]
- Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015; 38(1):33-54. [DOI:10.1007/s40264-014-0239-7] [PMID]
- Oparil S, Giles T, Ofili EO, Pitt B, Seifu Y, Hilkert R, et al. Moderate versus intensive treatment of hypertension with amlodipine/valsartan for patients uncontrolled on angiotensin receptor blocker monotherapy. J Hypertens. 2011; 29(1):161-70. [DOI:10.1097/HJH.0b013e32834000a7] [PMID]
- Bolanle IO, Akhigbemen AM, Omogbai EK. Amlodipine-and valsartan-enhanced end organ protection in streptozotocin-induced type 2 diabetic rats treated with metformin. JMPAS. 2023; 20(1):3859-67. [Link]
- Owolabi OJ, Omogbai EK. Effect of metformin on potassium-adapted and nonadapted diabetic rats. Trop J Pharm Res. 2012; 11(5):747-52. [DOI:10.4314/tjpr.v11i5.7]
- Abell LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. Trop J Pharm Res. 1952; 1952:357-66. [DOI:10.1016/S0021-9258(19)50907-3]
- Jacobs N, Vandermark PJ. Determination of serum triacylglycerol. Arch. Biochem. Biophys. 1960; 88(2):250-61. [DOI:10.1016/0003-9861(60)90230-7]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499-502. [DOI:10.1093/clinchem/18.6.499] [PMID]
- Bolanle IO, Omogbai EK, Bafor EE. Amlodipine and valsartan improving effect on the survival rate and deleterious pathological changes in streptozotocin-induced diabetic rats treated with metformin. PBR. Pharm Biomed. 2019; 5(2):25. [DOI:10.18502/pbr.v5i2.1582]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28(1):56-63. [DOI:10.1093/ajcp/28.1.56] [PMID]
- Schmidt E, Schmidt FW. Determination of serum GOT and GPT. Enzyme Biol Clin. 1963; 3(1):1. [DOI:10.1159/000458038]
- Van Slyke DD, Neill JM. The determination of gases in blood and other solutions by vacuum extraction and manometric measurement I. J Biol Chem. 1924; 61(2):523-73. [DOI:10.1016/S0021-9258(18)85145-6]
- Schales O, Schales SS. A simple and accurate method for the determination of chloride in biological fluids. J Biol Chem. 1941; 140(5):879-82. [DOI:10.1016/S0021-9258(18)72872-X]
- Margoshes M, Vallee BL. Flame photometry and spectrometry; principles and applications. Methods Biochem Anal. 1956; 3:353-407. [DOI:10.1002/9780470110195.ch12] [PMID]
- Tietz NW. Clinical guide to laboratory tests. Philadelphia: Saunders; 1995. [Link]
- Waeber B, Feihl F, Ruilope L. Diabetes and hypertension. Blood Press. 2001; 10(5-6):311-21. [DOI:10.1080/080370501753400610] [PMID]
- Mytas DZ, Stougiannos PN, Zairis MN, Foussas SG, Pyrgakis VN, Kyriazis IA. Diabetic myocardial disease: Pathophysiology, early diagnosis and therapeutic options. J Diabetes Complications. 2009; 23(4):273-82. [DOI:10.1016/j.jdiacomp.2007.12.005] [PMID]
- Kaplan NM. Primary hypertension: Natural history, special population, and evaluation. Clin Hypertens; 1994. [Link]
- Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J Clin Hypertens. 2011; 13(4):244-51. [DOI:10.1111/j.1751-7176.2011.00434.x] [PMID]
- Bolzán AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002; 512(2-3):121-34. [DOI:10.1016/S1383-5742(02)00044-3] [PMID]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005; 52(4):313-20. [DOI:10.1016/j.phrs.2005.05.004] [PMID]
- Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009; 32(7):1320-6. [DOI:10.2337/dc08-0779] [PMID]
- Adeshara KA, Diwan AG, Jagtap TR, Advani K, Siddiqui A, Tupe RS. Relationship between plasma glycation with membrane modification, oxidative stress and expression of glucose trasporter-1 in type 2 diabetes patients with vascular complications. J Diabetes Complications. 2017; 31(2):439-48. [DOI:10.1016/j.jdiacomp.2016.10.012] [PMID]
- Mamoun Rajab A, Haider KH. Hyperglycemia and RBCs: Too sweet to survive. Int J Diabetes Dev Ctries. 2018; 38(4):357-65. [DOI:10.1007/s13410-018-0613-6]
- Shenoy AG, Goyal RK. Improvement of insulin sensitivity by perindopril in spontaneously hypertensive and streptozotocin-diabetic rats. Indian J Pharmacol. 2002; 34(3):156-64. [Link]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001; 286(3):327-34 [DOI:10.1001/jama.286.3.327] [PMID]
- Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the west of Scotland coronary prevention study. Circulation. 2003; 108(4):414-9. [DOI:10.1161/01.CIR.0000080897.52664.94] [PMID]
- Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: Exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011; 32(10):1214-26. [DOI:10.1093/eurheartj/ehq499] [PMID]
- Stookey JD, Burg M, Sellmeyer DE, Greenleaf JE, Arieff A, Van Hove L, et al. A proposed method for assessing plasma hypertonicity in vivo. Eur J Clin Nutr. 2007; 61(1):143-6. [DOI:10.1038/sj.ejcn.1602481] [PMID]
- Fernandez NJ, Kidney BA. Alkaline phosphatase: Beyond the liver. Vet Clin Pathol. 2007; 36(3):223-33. [DOI:10.1111/j.1939-165X.2007.tb00216.x] [PMID]
- Maxwell DB, Fisher EA, Ross-Clunis HA 3rd, Estep HL. Serum alkaline phosphatase in diabetes mellitus. J Am Coll Nutr. 1986; 5(1):55-9. [DOI:10.1080/07315724.1986.1072011] [PMID]
- Hussein O, Shneider J, Rosenblat M, Aviram M. Valsartan therapy has additive anti-oxidative effect to that of fluvastatin therapy against low-density lipoprotein oxidation: studies in hypercholesterolemic and hypertensive patients. J Cardiovasc Pharmacol. 2002; 40(1):28-34. [DOI:10.1097/00005344-200207000-00004] [PMID]
- Atacan I, Parlak A, Aydoğan Ü, Sari O, Gök DE, Cayci T, et al. The effects of valsartan treatment on visfatin levels and lipid profiles in newly diagnosed hypertensives. Turk J Med Sci. 2013; 43(1):57-62. [DOI:10.3906/sag-1112-50]
- Koh KK, Lim S, Choi H, Lee Y, Han SH, Lee K, et al. Combination pravastatin and valsartan treatment has additive beneficial effects to simultaneously improve both metabolic and cardiovascular phenotypes beyond that of monotherapy with either drug in patients with primary hypercholesterolemia. Diabetes. 2013; 62(10):3547-52. [DOI:10.2337/db13-0566] [PMID]
Type of Study: Original Research |
Subject:
Pharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |