Volume 10, Issue 4 (2024)
Pharm Biomed Res 2024, 10(4): 279-290 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sami H, Jain A, Singhai A K. A Review Study on Reverse Iontophoresis and Its Applications. Pharm Biomed Res 2024; 10 (4) :279-290
URL: http://pbr.mazums.ac.ir/article-1-636-en.html
URL: http://pbr.mazums.ac.ir/article-1-636-en.html
1- Lakshmi Narain College of Pharmacy, Bhopal, India.
2- School of Pharmacy, LNCT University, Bhopal, India.
2- School of Pharmacy, LNCT University, Bhopal, India.
Keywords: Reverse iontophoresis (RI), GlucoWatch biographer, Chronic kidney disease, Lithium monitoring, Neonates monitoring
Full-Text [PDF 1390 kb]
(890 Downloads)
| Abstract (HTML) (1698 Views)
Full-Text: (2438 Views)
Introduction
Early in the 1970s, therapeutic drug monitoring was implemented with the goals of reducing systemic toxicity, enhancing patient safety, and customizing dosing regimens. Medications with a narrow therapeutic index, undesirable effects related to concentration, and pharmacokinetic changes are regarded as potential therapeutic drug monitor candidates. The therapeutic monitoring approach currently in use is intrusive, time-consuming, and frequently leads to low patient compliance. A significant amount of work is being done to establish novel therapeutic medication monitoring strategies using screening non-invasive technologies. One such technique is transdermal reverse iontophoresis (RI). Using a needle-free method called transdermal RI, medications and biomolecules can be extracted via healthy skin. In the field of medical science that deals with diagnosis, it has enormous promise. The method drives both charged and uncharged polar moieties over undamaged skin by the use of a little electric current [1]. Transdermal RI is a needle-free technique that can be used to extract biomolecules and drugs via healthy skin. It holds great promise for the area of medical science that deals with diagnostics. The technique uses a small amount of electric current to move both charged and uncharged polar moieties over skin that is not harmed [2]. Iontophoretic delivery of drugs would be beneficial in the treatment of certain skin disorders, such as skin cancer, psoriasis, dermatitis, venous ulcers, keloid and hypertrophic scars [3]. By driving charged molecules through the skin with an electric current, the spectrum of medications that can be safely administered trans-dermally may be greatly increased. This mode of administration is being closely studied concerning higher-molecular-weight treatments (peptides and tiny proteins, in particular), which are now only available by invasive injection and cannot be absorbed after oral administration. Furthermore, the process known as RI is a noninvasive method for blood chemical diagnostic monitoring [4].
What is RI?
The goal of the research is to develop non-invasive techniques for tracking medication plasma levels. Skin serves as a special entry point for noninvasive transdermal drug monitoring in this competition. To extract the endogenous chemicals within or beneath the skin, an electric current could be applied across the skin. The technique of RI has been used for non-invasive medication monitoring. Usually, it facilitates the movement of both charged and neutral molecules by passing a small amount of electric current between two skin electrodes [3]. RI can track the subcutaneous concentration of medications and other biomolecules, as shown by several in vitro and in vivo investigations [5]. The previous technology of drug analysis from the extraction chamber has been supplanted by the development of biosensors, creating new, more practical, portable instruments. Utilizing the GlucoWatch for noninvasive glucose monitoring is one of the most effective uses of RI to date [6]. With a surface area of around 2 m2, the skin is the biggest organ in the body and offers a thorough and accessible surface for drug extraction from the subdermal area. The outermost layer of skin, known as the stratum corneum, is primarily responsible for the skin’s barrier function and is made up of dead, flat skin cells. Although many physical and chemical strategies have been devised, very few have been successful in breaking through this strong barrier [7]. RI is one of the techniques that has been created and is the most promising method according to research. In the 20th century, RI was developed as a transdermal enhancement method, mainly for the transport of big, charged molecules [8]. Drugs can be extracted from the interstitial fluid by passing a small amount of current through the skin, usually using a few skin electrodes and a conducting base to increase the extraction of both charged and neutral molecules by various methods. A suitable equipment is used to collect and analyze the interstitial fluid. Since this technology allows for the non-invasive sampling of endogenous chemicals, it may find significant use in medical diagnostics. In RI, the skin’s negative charge at a pH buffer makes it perm-selective to cations, which drives solvent flow in the cathode’s direction [9].
How does RI work?
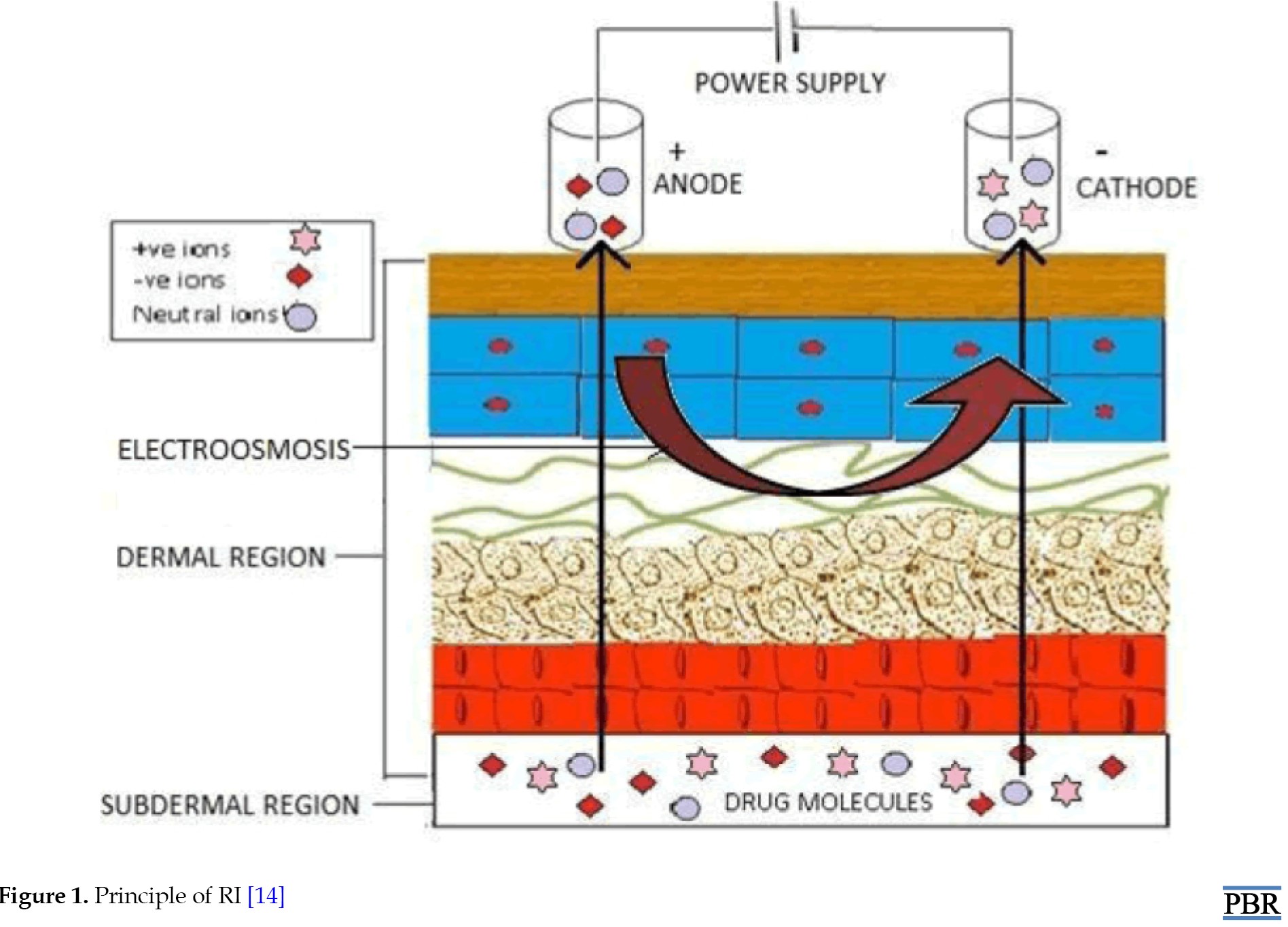
Analytes are also extracted by electroosmosis in RI. Sodium ions in the interstitial fluid are the main charge carriers through the skin because the skin has a net negative charge at neutral pH. This sodium ion migration causes a net convective solvent flow in the direction of the cathode [10]. In RI, analytes are also extracted using electroosmosis. Since the skin contains a net negative charge at neutral pH, sodium ions in the interstitial fluid are the primary charge carriers through the skin. There is a net convective solvent flow in the cathode’s direction as a result of this migration of sodium ions [11]; however, the volume of solvent flow is directly proportional to the potential gradient between the electrodes [12]. The electroosmosis transport is the basic mechanism underlying the RI extraction of glucose, a neutral and polar substance. Furthermore, this convective flow reinforces the transport of cations while acting against that of anions. Thus, extraction of a cationic analyte will always be easier than that of an anion of similar physio-chemical and pharmacokinetics properties [13] (Figure 1).
The success of RI technique
Many research scientist groups have been motivated by the RI extraction technique’s success in clinical tests, and this technology is now the focus of the current study. Numerous endogenous chemicals have been studied to broaden the application of this approach, including urea, prostaglandin, lactate, phenylalanine, amino acids, and lithium.
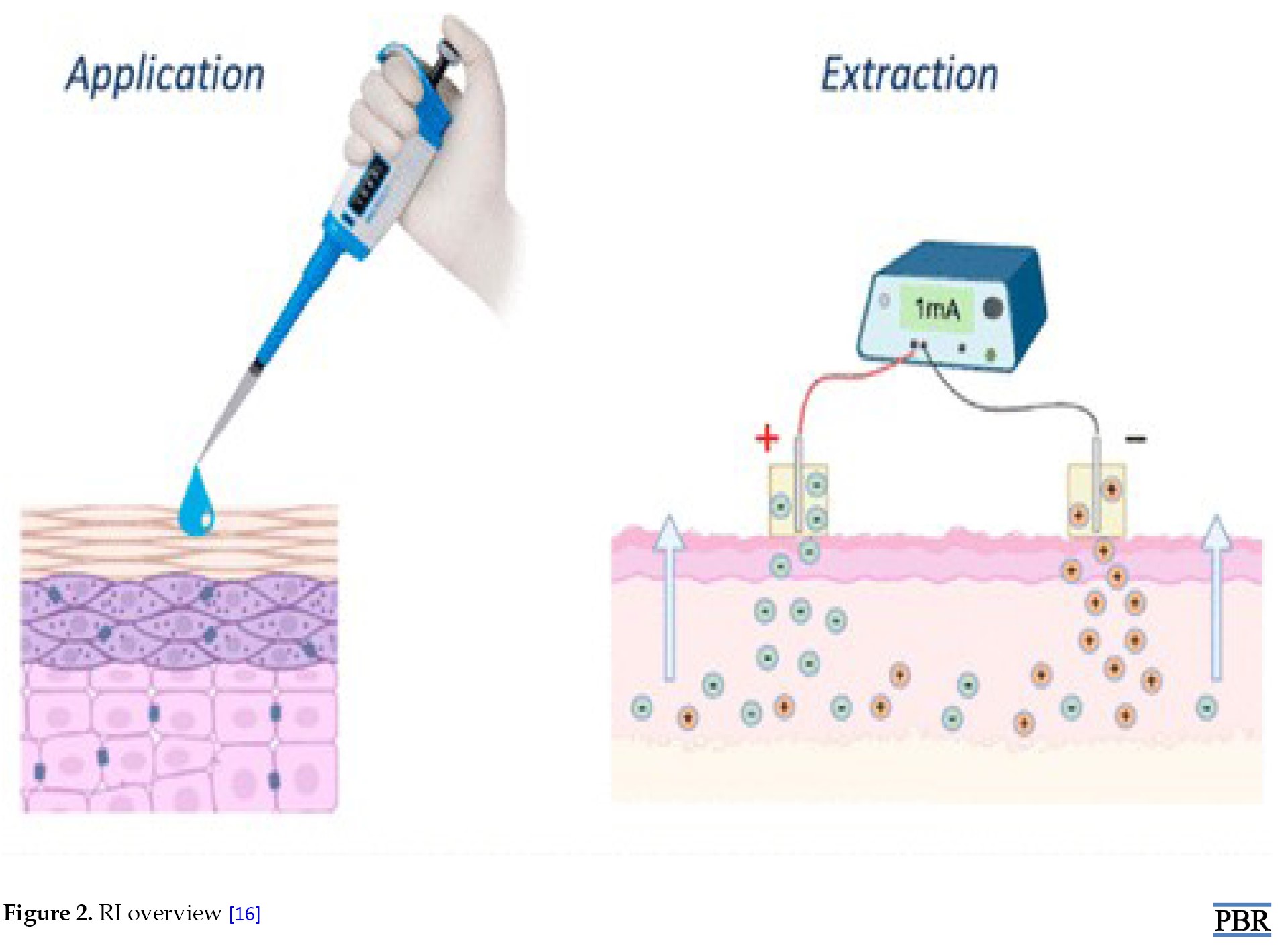
Lithium is a low molecular weight cationic chemical with a greater effective plasma concentration that is used to treat bipolar illnesses. To determine if it would be possible to extract this chemical, in vitro investigations were conducted. Excellent correlation and quick extraction from a physiological buffer were shown by the obtained results. Urine in plasma monitoring may be used to look into renal failure in people with diabetes and high blood pressure [14]. Noninvasive lactatemia monitoring is interesting as a performance indicator in sports training as well as for the comfort of very sick patients. Lactate is a suitable candidate for reverse iontophoretic extraction since it is a tiny, relatively concentrated anion. Both in vitro and in vivo extractions are quick and simple. Meanwhile, chloride could be extracted simultaneously in vivo, and it could be used as an internal standard to calibrate lactate reverse iontophoretic fluxes [15]. Another study used a unique model that resembles a newborn’s epidermal barrier to evaluate the possibility of RI in extracting theophylline and caffeine. In vitro experiments were conducted on full-thickness and tape-stripped porcine skin. High-performance liquid chromatography (HPLC) was used to measure the amount of medication extracted every hour while the electric potential was applied for five hours. Theophylline and caffeine may be extracted using this approach, as the results showed considerable drug extraction after iontophoresis [14] (Figure 2).
Advantages of RI
RI has the potential to effectively and noninvasively sample analytes that possess the necessary properties, according to the findings of numerous studies. RI has been developed, supporting its potential for therapeutic drug monitoring. The creation of GlucoWatch has demonstrated its enormous potential for glucose monitoring. The creation of this technique offers diabetes patients excellent care. This technology is critical for research purposes and other ways that patient care can be improved and pain and discomfort can be reduced. There are not many real-world issues that need to be worked out before this technology is used extensively. RI is a viable method for therapeutic drug monitoring that is expected to demonstrate a high degree of miniaturization, sensitivity, and specificity in the future. This will probably become the most effective noninvasive technique used shortly to monitor chemicals in the body, taking the role of a blood sample [14].
Applications of RI
The applications of RI are as follows: Blood glucose monitoring; diagnosis and monitoring of chronic kidney disease (CKD); monitoring in premature neonates; lithium monitoring; extraction of transdermal biomarkers.
Blood glucose monitoring
Glucose can be extracted through intact skin by electro‐osmotic flow (RI process) upon the application of a low‐level electrical current [17]. An electric current can be used to drive charged molecules through the skin, potentially expanding the range of drugs that can be safely applied trans-dermally. Higher-molecular-weight treatments, peptides, and small proteins, which are now only accessible by invasive injection and cannot be absorbed orally are being intensively researched in connection to this mode of administration. Moreover, RI is a noninvasive technique for tracking blood chemical diagnostics [18]. Strict blood glucose management lowers the incidence of long-term problems in both type 1 and type 2 diabetes. Multiple daily blood glucose tests are necessary for these strict control regimes to monitor insulin dosing. For many diabetics, strict glucose control has the disadvantage of increasing the risk of hypoglycemia [19].
Devices use to monitor blood glucose by RI method
The devices that are used to monitor blood glucose by the RI method are GlucoWatch biographer and smartphones.
GlucoWatch biographer
Intensive glucose management regimens would be substantially aided by a system that offered an easy and automated way to perform frequent glucose readings together with hypo- and hyper-glycemic alerts. Non-invasive measures are highly desirable since many patients would prefer to have fewer finger sticks made to gather blood samples. Presently, Cygnus, Inc., located in Redwood City, CA, USA, is developing the GlucoWatch1, a biographer gadget that possesses these features. RI is a unique technology used by this device to extract glucose via the skin, where an amperometric biosensor detects it. The biography is a compact wristwatch that has a digital display, electrical circuitry, and sampling and detection modules. Clinical experiments have examined the GlucoWatch biographer’s capacity to measure glucose in individuals with diabetes. The device’s working principles are explained in this report together with the findings of the clinical trials. By applying a small electrical current, glucose can be removed through undamaged skin using a technique known as RI or electro-osmotic flow. We have recently developed a gadget called the GlucoWatch1 biographer that combines an in-situ glucose sensor with iontophoretic extraction. Using a single blood glucose measurement as calibration, clinical results with this device demonstrate close tracking of blood glucose throughout a range of 2.2 to 22.2 mmol/L for up to 12 h. The biographer’s measurements are typically 18 min behind blood glucose values. GlucoWatch biography readings and blood glucose have a linear connection (r=0.88), according to an examination of data from 92 diabetic people in a controlled clinical setting [18]. For a maximum of 12 h, the GlucoWatch® blood glucose meter (Cygnus, Inc., Redwood City, CA, USA) offers automated, frequent, and noninvasive blood glucose readings. Through unbroken skin, the gadget collects glucose, which is then measured using an amperometric biosensor. When compared to serial fingerstick blood glucose measurements, clinical trials conducted in a range of settings have demonstrated that the biographer gives reliable and exact glucose measurements. In the home context, the mean difference between these values was 0.26 mmol/L (r=0.8). The therapeutically appropriate A+B zone of the Clarke Error Grid contained more than 94% of biographer readings. At low glucose levels, a small positive bias is seen in the biographer’s readings. The coefficient of variation percentage indicates that the precision of a biographer is roughly 10%. Up to 75% of hypoglycemic episodes were identified by the biographer’s low glucose warning feature with a low false alert level. Using the GlucoWatch® biographer to monitor trends and patterns in blood glucose levels is a safe and efficient way, which should help many patients achieve better glycemic management [20]. The choice of a low-glucose warning level that will sound an auditory alarm determines how well the hypoglycemia alert functions. The best way to assess the alert function’s performance is to examine the receiver operator characteristic curves [21].
Principle of GlucoWatch biographer
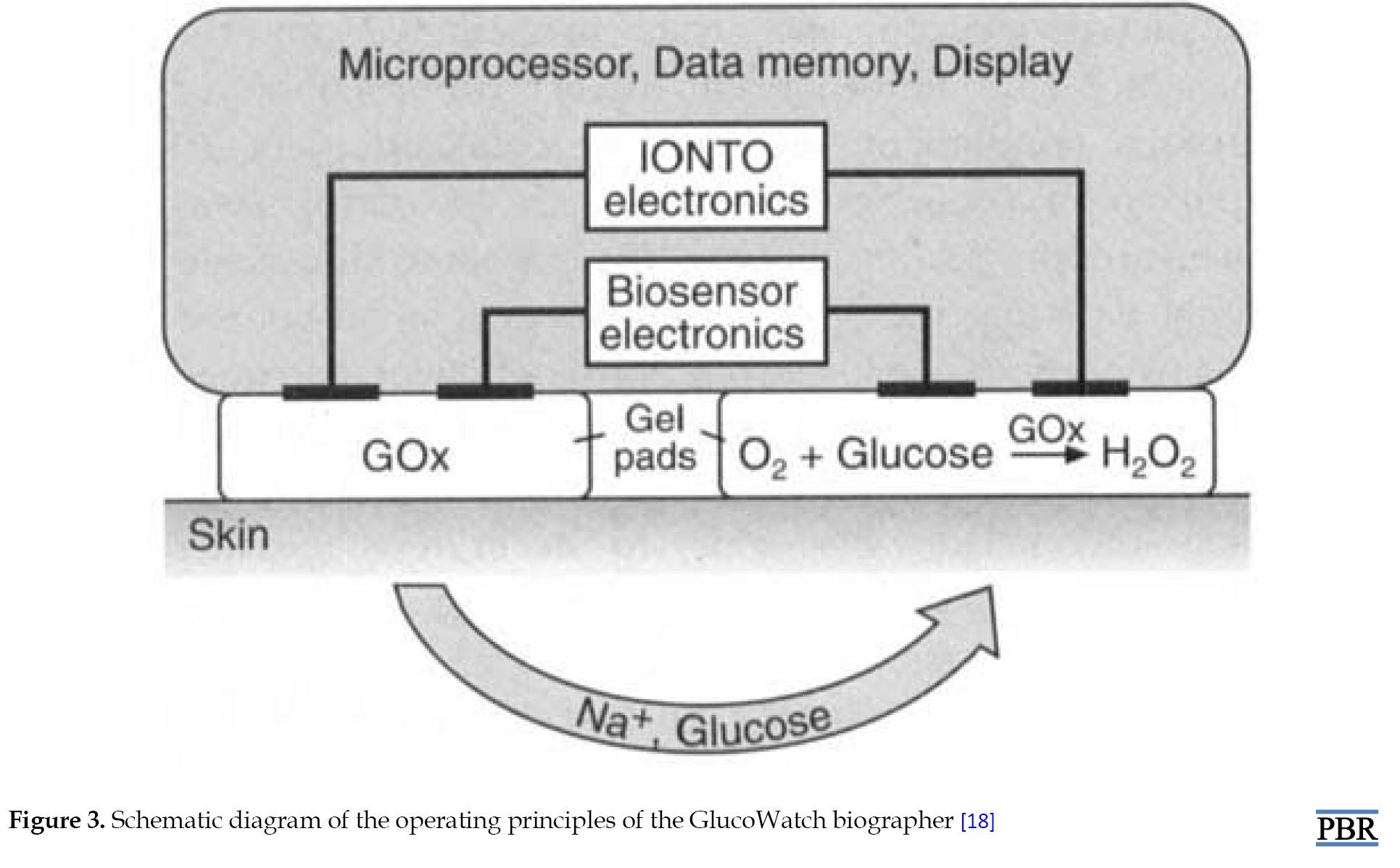
Drug delivery through transdermal application has been accomplished for more than a century employing electrical current passing through the skin. This method is known as RI because the glucose in the GlucoWatch biographer is transported in the other direction, from the skin outward. Because glucose is uncharged, electroosmosis is the method of transport. The pH of the skin is net negative at physiological levels. Na+ ions thus make up the bulk of current carriers through the skin. Convective solvent flow caused by the migration of these Na+ ions moves uncharged substances like glucose in the direction of the iontophoretic cathode. The biosensor chemistry makes use of direct measurement of H2O2 produced when glucose oxidase oxidizes glucose [22]. This device extracts glucose through intact skin via RI where it is detected by an amperometric biosensor [23]. GlucoWatch biographer incorporates three distinct technologies: glucose sample measurement using an amperometric biosensor, glucose sample extraction using RI, and data conversion and verification resulting in the glucose reading being displayed [18] (Figure 3).
GlucoWatch mechanism
By applying RI, the biographer collects glucose via the skin. An electrochemical biosensor is used to analyze the extracted sample, and a low-level electric current is applied across the skin between an anode and a cathode [24]. The migration of sodium ions in the direction of the cathode is the primary source of the current. Convective transport, or electroosmosis, is the mechanism by which uncharged molecules, like glucose, are transported [25]. The amount of glucose extracted at the cathode has been demonstrated to correlate with blood glucose in diabetic subjects [26]. In the biographer, the extracted glucose is measured by an amperometric biosensor using the detection of H2O2 generated by the glucose/glucose oxidase reaction [27]. After a 3-h equilibration period, the biographer uses a fingerstick blood glucose measurement to produce results every 20 min following calibration. This single-point calibration is utilized to translate future biosensor measurements into glucose readings, taking into account variations in skin permeability and biosensor sensitivity. The biography software has an algorithm for processing signals built-in [28]. The biography software’s data integrity screens identify erroneous data points that come from open or short circuits, excessive background currents, and electrical noise, among other sources. When there are data points that do not meet objective a priori criteria, a glucose reading is ignored (or skipped). Additionally, skin conductance and skin temperature sensors are included in the biography. The latter is closely associated with the quantity of perspiration on the skin’s surface. The glucose measurement can be complicated by significant temperature variations or the presence of glucose in perspiration. For this reason, the measurement for that cycle will be omitted if the output from either of these sensors is over a predefined threshold. Thus, these data-screening techniques shield against potentially erroneous glucose levels [29].
Diagnosis and monitoring of CKD
The global burden of CKD is increasing, due to several factors, such as the pandemics of diabetes and obesity, but partly due to improved screening [30]. For those already on dialysis, frequent monitoring of blood urea is also performed to determine the efficacy of the treatment [31]. Measurements of elevated urea and creatinine levels are the main basis for the clinical diagnosis of CKD. It is impossible to postpone the beginning of end-stage renal disease and the corresponding mortality and morbidity without this vital monitoring data. Renal replacement therapy (or hemodialysis [HD]) is necessary as the condition progresses. Enhancing the management of high-risk patients, such as those with diabetes and hypertension, as well as monitoring them during HD presents challenges, as does avoiding the clear drawbacks of traditional blood collection, which include the requirement for trained personnel, discomfort, and infection risk. The primary means of making a clinical diagnosis of CKD is through measurements of high urea and creatinine levels. Without these crucial monitoring data, it is impossible to delay the onset of end-stage renal disease and the associated mortality and morbidity. As the illness worsens, renal replacement therapy (also known as HD) becomes required. Improving the care of high-risk patients, with diabetes, hypertension, and so on, along with keeping an eye on them during HD are difficult tasks. Traditional blood collection has disadvantages, such as the need for skilled personnel, discomfort, and infection risk [32]. After applying a 0.8 mA current to the skin for 2 h, the urea collected iontophoretically can be used to separate patients with CKD from healthy volunteers. Subcutaneous urea concentrations in both healthy people and CKD patients are in balance with plasma urea under steady-state settings [33]. Hence, extracted subdermal interstitial urea could be considered to reflect blood urea [36].
Mechanism
The mechanism is based on three main steps: RI, sample analysis, and statistical analysis.
RI
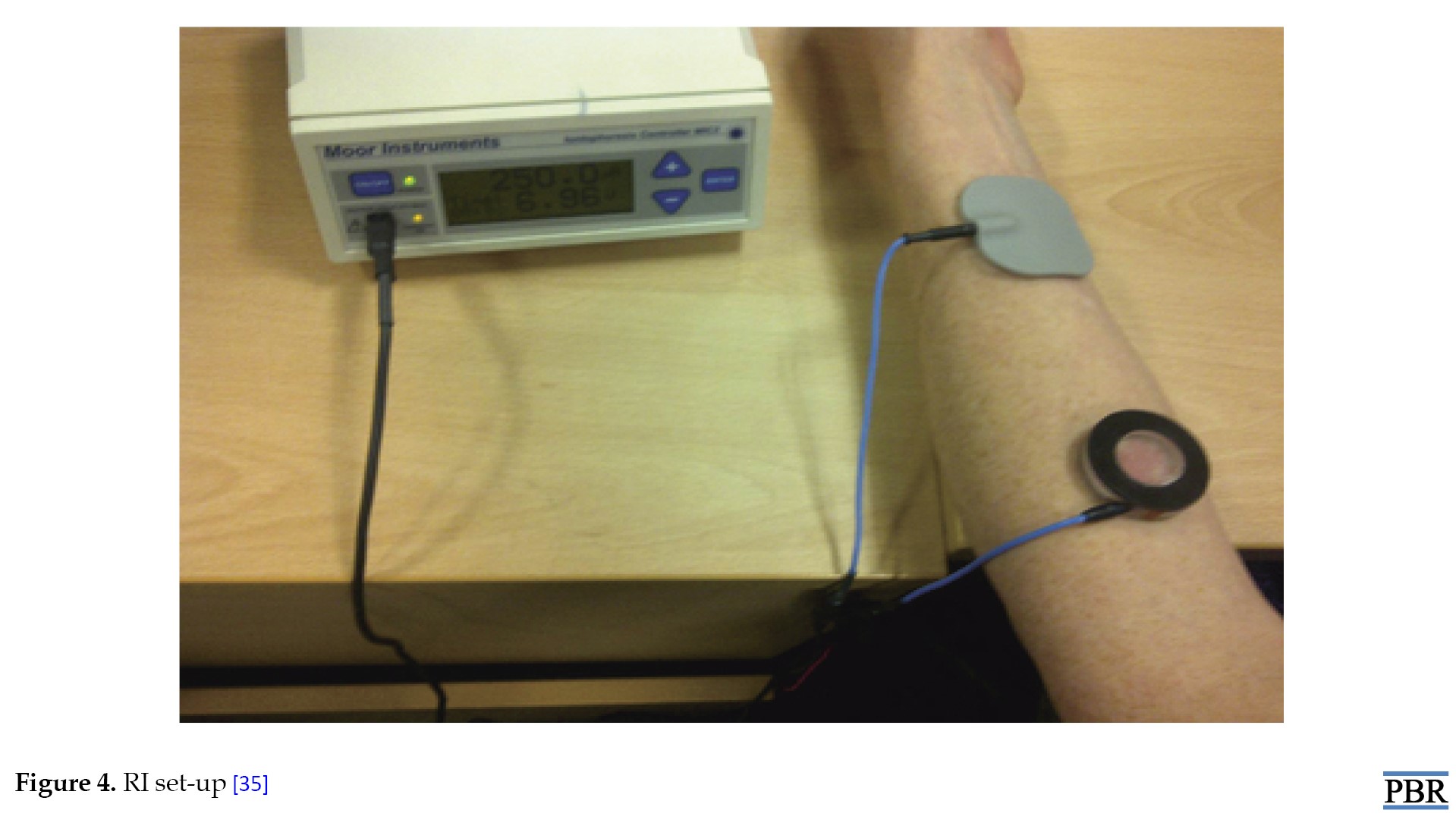
In patients with arteriovenous fistula, RI was performed with the non-dominant arm or the non-fistula arm. Patients undergoing hemodialysis were tested during dialysis. Peritoneal dialysis patients were dried before examinations. A MIC2® iontophoresis driver (Moor Instruments Inc., Wilmington, DE, USA) was used to deliver a constant current of 250 μA between two electrodes placed approximately 10 cm apart on an abdominal forearm that had been previously cleaned and defatted at 70 °C ethanol. The anode was an inactive gel electrode and the cathode was prepared from 1 mM TRIS (hydroxymethylaminomethane) buffer at pH 8.75 at 37 °C in a 1.5 mL chamber. The buffer contacted the skin over an area of 3.8 cm2. After 30 min, the controller was automatically turned off and the cathode chamber buffer (sample) was emptied into an Eppendorf flask. The skin was dried and the cycle was repeated at least five times for each subject and dialysis duration in HD patients. Where possible, one run was performed before starting dialysis (pre-dialysis sample) and after completing dialysis (post-dialysis sample). During HD, blood was drawn every 30 min from the arterial port. In others, a single blood sample was taken by venipuncture at the end of the session. Blood samples were collected in ethylenediamine tetraacetic acid tubes, centrifuged at 770 g for 10 min, plasma was aliquoted, and all samples were stored at -80 °C until analysis. Any pain, discomfort, skin irritation or damage resulting from application of the iontophoretic current and its duration and severity were recorded (Figure 4).
Sample analysis
Urea is measured using a colorimetric urease and glutamate dehydrogenase method with a lower detection limit of 0.5 mM. As the urea concentrations in the iontophoretic buffer were much smaller (of the micromolar order), this required a sensitive assay and the diacetylmonoxime method of urea assay described by Friedman. [36].
Statistical analysis
The GraphPad Prism® Software version 5.0 (GraphPad Software Inc., La Jolla, CA USA) was used for statistical analysis. The Mean±SD are used to express the results. The t-test (for regularly distributed data) and the Mann-Whitney test (for nonnormally distributed data) are the two test types that can be employed in statistics [35].
Monitoring of premature neonates
Premature neonates represent a fragile patient population, often subjected to intensive clinical care and multiple drug therapy, which must be monitored carefully and continuously. The difficult and painful nature of repetitive blood sampling, particularly in this population, has provided considerable impetus for the development of noninvasive methods for monitoring blood analytes. RI, a relatively new technology already used for the transdermal monitoring of blood glucose levels in adults, may be particularly well-suited to exploit the unique properties of preterm neonatal skin. The underdevelopment of the premature infant’s epidermis, and more specifically the stratum corneum, results in an increased permeability to molecular transport [37]. Therapeutic drug monitoring, or measuring plasma drug concentrations while a patient is under treatment, is a useful tool for clinicians to optimize patient therapy. However, this procedure is only employed in certain circumstances because of its expense and time-consuming nature [38]: 1) For drugs with a narrow therapeutic range (and therefore a high risk of toxicity) 2) In the absence of clinical response (e.g. due to pharmacodynamic variability), 3) To individualize treatment (due to interindividual variability in drug pharmacokinetics), and 4) For vulnerable patient groups requiring more careful drug concentration (e.g. in the elderly, neonates and premature infants). Continuous monitoring of premature infants is an important therapeutic issue. Due to prematurity and related complications, they are often treated with multiple drug therapies. However, minimal processing is also recommended [39] because every medical procedure performed on a premature newborn raises the possibility of infection and intensifies energy and heat losses. As a result, noninvasive monitoring techniques, like transcutaneous gas collection, are recommended [40]. The technique has the advantage of being noninvasive and therefore warrants consideration as an alternative to blood sampling for drug monitoring. To date, the major application of RI has centered on the development of a device (the GlucoWatch Biographer, Cygnus, Inc., USA) for the continuous monitoring of blood glucose levels in diabetics [41]. Other applications have been envisaged for both drug monitoring (e.g. clonidine and theophylline) [42]. Because of its limited therapeutic range, theophylline is a great choice for therapeutic monitoring in premature infants. It is given for the treatment and prevention of apnea, coupled with caffeine, another methylxanthine. Caffeine is N-methylated theophylline in preterm infants, at steady state, the concentration of the latter is approximately one-third that of the former; neonates have been shown to maintain this proportion consistently over a range of post-conceptional ages, from 28 to 42 weeks. [43].
Mechanism
A little, controlled electrical current is applied to the skin during RI. Either electromigration of charged species to the electrode with the opposite polarity, electroosmosis of neutral molecules to the cathode, or possibly a mix of the two for cations, is the mechanism of extraction [4].
Lithium monitoring
Lithium monitoring is essential to ensure efficacy as well as to prevent adverse effects [44]. A therapeutic response, such as in the treatment of manic episodes, is not apparent until two to three weeks following the start of treatment, even though steady-state plasma concentrations of the drug are reached in a matter of days [45]. A blood test known as the “standardized 12 h li+ serum concentration” is required for lithium monitoring. This means that patients must have their blood drawn early in the morning, approximately 12 h after their final dose of the previous day and before their first administration on the monitoring day. Therefore, one may argue that a fully noninvasive method of lithium monitoring would help bipolar patients adhere to their treatment plans and live better. Although the wide inter- and intraindividual variability of the saliva/plasma concentration ratio has been suggested as an alternate matrix for lithium monitoring, this approach’s utility is severely limited [46].
Mechanism
In iontophoresis, two transport processes are involved. Ions travel over the skin carrying charge and are drawn (attracted) to the electrode with the opposite polarity during electromigration. A net solvent flow from the anode to the cathode is known as electroosmosis, and it greatly improves cationic transport and allows for considerably better penetration of neutral molecules (like glucose) [47]. RI has been used to monitor lithium in vitro. This cationic medication is tiny, mobile, and a great fit for iontophoretic extraction. Utilizing electromigration, lithium is moved at a flux to the cathode or negative electrode. In the presence of competing co-ions, the transport number of a given ion is proportional to its concentration [13]. This is the case in RI, in which the physiologic milieu provides a panoply of competing co-ions under these circumstances [45].
Extraction of transdermal biomarkers
Lithium has been observed in vitro using RI. This cationic drug is small, easily transportable, and ideal for iontophoretic extraction. Lithium migrates to the cathode, or negative electrode, at a flux rate through electromigration. The transport number of a particular ion in the presence of competing co-ions is proportional to its concentration [48]. The most common types of skin cancer are non-melanoma skin cancers, which include squamous cell carcinoma and basal cell carcinoma [1]. Melanoma-related skin cancers, on the other hand, are less common but more dangerous because they can spread to other organs, and they are also one of the fastest-growing types of the disease [49]. The current gold standard for diagnosing skin cancer mostly uses visual examination of a lesion, tissue sampling, and staining. The features of the lesion and the experience of the doctor are two elements that affect this approach’s accuracy [50]. It is critically necessary to create a supplemental or alternative non-invasive technology to identify skin cancer in its early stages. Such an approach would help to decrease the number of unnecessary biopsies and provide justification for when surgical intervention is necessary because it would have improved specificity and sensitivity [51]. In the case of actinic keratoses and Bowen’s disease, it can eventually develop into squamous cell carcinoma [52]. Since persistent inflammation is a risk factor for cancer, identifying inflammatory biomarkers can help diagnose the disease before it manifests. Our understanding of the biomarkers linked to inflammation and cancer, such as interleukin 6, interferon-gamma, tumor necrosis factor-α, the enzyme indoleamine-2, 3-dioxygenase, and mutations in the BRAF gene, continually expands [53-55]. However, the modulation of the immune response is greatly aided by the degradation of the important amino acid tryptophan (Trp) via the kynurenine (Kyn) pathway [56], and changes in the Trp/Kyn ratio are associated with several diseases, including cancer [57]. Their polar nature raises concerns about whether enough can be extracted promptly to enable quantification later on, but their tiny molecular weights make them potentially good candidates for topical sampling [58]. RI greatly improved the extraction of Trp and Kyn, especially in the anode-to-cathode direction. Once the skin’s natural Trp is present. An alternate and more realistic (i.e. structurally stable) receptor into which Trp and Kyn could be extracted worked well: a fully hydrated, semi-solid, bi-continuous lipid cubic phase. More research is needed to fully understand RI as a comparatively non-invasive technique for sampling tiny biomarker chemicals associated with inflammation and cancer [59].
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the mnuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
Early in the 1970s, therapeutic drug monitoring was implemented with the goals of reducing systemic toxicity, enhancing patient safety, and customizing dosing regimens. Medications with a narrow therapeutic index, undesirable effects related to concentration, and pharmacokinetic changes are regarded as potential therapeutic drug monitor candidates. The therapeutic monitoring approach currently in use is intrusive, time-consuming, and frequently leads to low patient compliance. A significant amount of work is being done to establish novel therapeutic medication monitoring strategies using screening non-invasive technologies. One such technique is transdermal reverse iontophoresis (RI). Using a needle-free method called transdermal RI, medications and biomolecules can be extracted via healthy skin. In the field of medical science that deals with diagnosis, it has enormous promise. The method drives both charged and uncharged polar moieties over undamaged skin by the use of a little electric current [1]. Transdermal RI is a needle-free technique that can be used to extract biomolecules and drugs via healthy skin. It holds great promise for the area of medical science that deals with diagnostics. The technique uses a small amount of electric current to move both charged and uncharged polar moieties over skin that is not harmed [2]. Iontophoretic delivery of drugs would be beneficial in the treatment of certain skin disorders, such as skin cancer, psoriasis, dermatitis, venous ulcers, keloid and hypertrophic scars [3]. By driving charged molecules through the skin with an electric current, the spectrum of medications that can be safely administered trans-dermally may be greatly increased. This mode of administration is being closely studied concerning higher-molecular-weight treatments (peptides and tiny proteins, in particular), which are now only available by invasive injection and cannot be absorbed after oral administration. Furthermore, the process known as RI is a noninvasive method for blood chemical diagnostic monitoring [4].
What is RI?
The goal of the research is to develop non-invasive techniques for tracking medication plasma levels. Skin serves as a special entry point for noninvasive transdermal drug monitoring in this competition. To extract the endogenous chemicals within or beneath the skin, an electric current could be applied across the skin. The technique of RI has been used for non-invasive medication monitoring. Usually, it facilitates the movement of both charged and neutral molecules by passing a small amount of electric current between two skin electrodes [3]. RI can track the subcutaneous concentration of medications and other biomolecules, as shown by several in vitro and in vivo investigations [5]. The previous technology of drug analysis from the extraction chamber has been supplanted by the development of biosensors, creating new, more practical, portable instruments. Utilizing the GlucoWatch for noninvasive glucose monitoring is one of the most effective uses of RI to date [6]. With a surface area of around 2 m2, the skin is the biggest organ in the body and offers a thorough and accessible surface for drug extraction from the subdermal area. The outermost layer of skin, known as the stratum corneum, is primarily responsible for the skin’s barrier function and is made up of dead, flat skin cells. Although many physical and chemical strategies have been devised, very few have been successful in breaking through this strong barrier [7]. RI is one of the techniques that has been created and is the most promising method according to research. In the 20th century, RI was developed as a transdermal enhancement method, mainly for the transport of big, charged molecules [8]. Drugs can be extracted from the interstitial fluid by passing a small amount of current through the skin, usually using a few skin electrodes and a conducting base to increase the extraction of both charged and neutral molecules by various methods. A suitable equipment is used to collect and analyze the interstitial fluid. Since this technology allows for the non-invasive sampling of endogenous chemicals, it may find significant use in medical diagnostics. In RI, the skin’s negative charge at a pH buffer makes it perm-selective to cations, which drives solvent flow in the cathode’s direction [9].
How does RI work?
Analytes are also extracted by electroosmosis in RI. Sodium ions in the interstitial fluid are the main charge carriers through the skin because the skin has a net negative charge at neutral pH. This sodium ion migration causes a net convective solvent flow in the direction of the cathode [10]. In RI, analytes are also extracted using electroosmosis. Since the skin contains a net negative charge at neutral pH, sodium ions in the interstitial fluid are the primary charge carriers through the skin. There is a net convective solvent flow in the cathode’s direction as a result of this migration of sodium ions [11]; however, the volume of solvent flow is directly proportional to the potential gradient between the electrodes [12]. The electroosmosis transport is the basic mechanism underlying the RI extraction of glucose, a neutral and polar substance. Furthermore, this convective flow reinforces the transport of cations while acting against that of anions. Thus, extraction of a cationic analyte will always be easier than that of an anion of similar physio-chemical and pharmacokinetics properties [13] (Figure 1).
The success of RI technique
Many research scientist groups have been motivated by the RI extraction technique’s success in clinical tests, and this technology is now the focus of the current study. Numerous endogenous chemicals have been studied to broaden the application of this approach, including urea, prostaglandin, lactate, phenylalanine, amino acids, and lithium.
Lithium is a low molecular weight cationic chemical with a greater effective plasma concentration that is used to treat bipolar illnesses. To determine if it would be possible to extract this chemical, in vitro investigations were conducted. Excellent correlation and quick extraction from a physiological buffer were shown by the obtained results. Urine in plasma monitoring may be used to look into renal failure in people with diabetes and high blood pressure [14]. Noninvasive lactatemia monitoring is interesting as a performance indicator in sports training as well as for the comfort of very sick patients. Lactate is a suitable candidate for reverse iontophoretic extraction since it is a tiny, relatively concentrated anion. Both in vitro and in vivo extractions are quick and simple. Meanwhile, chloride could be extracted simultaneously in vivo, and it could be used as an internal standard to calibrate lactate reverse iontophoretic fluxes [15]. Another study used a unique model that resembles a newborn’s epidermal barrier to evaluate the possibility of RI in extracting theophylline and caffeine. In vitro experiments were conducted on full-thickness and tape-stripped porcine skin. High-performance liquid chromatography (HPLC) was used to measure the amount of medication extracted every hour while the electric potential was applied for five hours. Theophylline and caffeine may be extracted using this approach, as the results showed considerable drug extraction after iontophoresis [14] (Figure 2).
Advantages of RI
RI has the potential to effectively and noninvasively sample analytes that possess the necessary properties, according to the findings of numerous studies. RI has been developed, supporting its potential for therapeutic drug monitoring. The creation of GlucoWatch has demonstrated its enormous potential for glucose monitoring. The creation of this technique offers diabetes patients excellent care. This technology is critical for research purposes and other ways that patient care can be improved and pain and discomfort can be reduced. There are not many real-world issues that need to be worked out before this technology is used extensively. RI is a viable method for therapeutic drug monitoring that is expected to demonstrate a high degree of miniaturization, sensitivity, and specificity in the future. This will probably become the most effective noninvasive technique used shortly to monitor chemicals in the body, taking the role of a blood sample [14].
Applications of RI
The applications of RI are as follows: Blood glucose monitoring; diagnosis and monitoring of chronic kidney disease (CKD); monitoring in premature neonates; lithium monitoring; extraction of transdermal biomarkers.
Blood glucose monitoring
Glucose can be extracted through intact skin by electro‐osmotic flow (RI process) upon the application of a low‐level electrical current [17]. An electric current can be used to drive charged molecules through the skin, potentially expanding the range of drugs that can be safely applied trans-dermally. Higher-molecular-weight treatments, peptides, and small proteins, which are now only accessible by invasive injection and cannot be absorbed orally are being intensively researched in connection to this mode of administration. Moreover, RI is a noninvasive technique for tracking blood chemical diagnostics [18]. Strict blood glucose management lowers the incidence of long-term problems in both type 1 and type 2 diabetes. Multiple daily blood glucose tests are necessary for these strict control regimes to monitor insulin dosing. For many diabetics, strict glucose control has the disadvantage of increasing the risk of hypoglycemia [19].
Devices use to monitor blood glucose by RI method
The devices that are used to monitor blood glucose by the RI method are GlucoWatch biographer and smartphones.
GlucoWatch biographer
Intensive glucose management regimens would be substantially aided by a system that offered an easy and automated way to perform frequent glucose readings together with hypo- and hyper-glycemic alerts. Non-invasive measures are highly desirable since many patients would prefer to have fewer finger sticks made to gather blood samples. Presently, Cygnus, Inc., located in Redwood City, CA, USA, is developing the GlucoWatch1, a biographer gadget that possesses these features. RI is a unique technology used by this device to extract glucose via the skin, where an amperometric biosensor detects it. The biography is a compact wristwatch that has a digital display, electrical circuitry, and sampling and detection modules. Clinical experiments have examined the GlucoWatch biographer’s capacity to measure glucose in individuals with diabetes. The device’s working principles are explained in this report together with the findings of the clinical trials. By applying a small electrical current, glucose can be removed through undamaged skin using a technique known as RI or electro-osmotic flow. We have recently developed a gadget called the GlucoWatch1 biographer that combines an in-situ glucose sensor with iontophoretic extraction. Using a single blood glucose measurement as calibration, clinical results with this device demonstrate close tracking of blood glucose throughout a range of 2.2 to 22.2 mmol/L for up to 12 h. The biographer’s measurements are typically 18 min behind blood glucose values. GlucoWatch biography readings and blood glucose have a linear connection (r=0.88), according to an examination of data from 92 diabetic people in a controlled clinical setting [18]. For a maximum of 12 h, the GlucoWatch® blood glucose meter (Cygnus, Inc., Redwood City, CA, USA) offers automated, frequent, and noninvasive blood glucose readings. Through unbroken skin, the gadget collects glucose, which is then measured using an amperometric biosensor. When compared to serial fingerstick blood glucose measurements, clinical trials conducted in a range of settings have demonstrated that the biographer gives reliable and exact glucose measurements. In the home context, the mean difference between these values was 0.26 mmol/L (r=0.8). The therapeutically appropriate A+B zone of the Clarke Error Grid contained more than 94% of biographer readings. At low glucose levels, a small positive bias is seen in the biographer’s readings. The coefficient of variation percentage indicates that the precision of a biographer is roughly 10%. Up to 75% of hypoglycemic episodes were identified by the biographer’s low glucose warning feature with a low false alert level. Using the GlucoWatch® biographer to monitor trends and patterns in blood glucose levels is a safe and efficient way, which should help many patients achieve better glycemic management [20]. The choice of a low-glucose warning level that will sound an auditory alarm determines how well the hypoglycemia alert functions. The best way to assess the alert function’s performance is to examine the receiver operator characteristic curves [21].
Principle of GlucoWatch biographer
Drug delivery through transdermal application has been accomplished for more than a century employing electrical current passing through the skin. This method is known as RI because the glucose in the GlucoWatch biographer is transported in the other direction, from the skin outward. Because glucose is uncharged, electroosmosis is the method of transport. The pH of the skin is net negative at physiological levels. Na+ ions thus make up the bulk of current carriers through the skin. Convective solvent flow caused by the migration of these Na+ ions moves uncharged substances like glucose in the direction of the iontophoretic cathode. The biosensor chemistry makes use of direct measurement of H2O2 produced when glucose oxidase oxidizes glucose [22]. This device extracts glucose through intact skin via RI where it is detected by an amperometric biosensor [23]. GlucoWatch biographer incorporates three distinct technologies: glucose sample measurement using an amperometric biosensor, glucose sample extraction using RI, and data conversion and verification resulting in the glucose reading being displayed [18] (Figure 3).
GlucoWatch mechanism
By applying RI, the biographer collects glucose via the skin. An electrochemical biosensor is used to analyze the extracted sample, and a low-level electric current is applied across the skin between an anode and a cathode [24]. The migration of sodium ions in the direction of the cathode is the primary source of the current. Convective transport, or electroosmosis, is the mechanism by which uncharged molecules, like glucose, are transported [25]. The amount of glucose extracted at the cathode has been demonstrated to correlate with blood glucose in diabetic subjects [26]. In the biographer, the extracted glucose is measured by an amperometric biosensor using the detection of H2O2 generated by the glucose/glucose oxidase reaction [27]. After a 3-h equilibration period, the biographer uses a fingerstick blood glucose measurement to produce results every 20 min following calibration. This single-point calibration is utilized to translate future biosensor measurements into glucose readings, taking into account variations in skin permeability and biosensor sensitivity. The biography software has an algorithm for processing signals built-in [28]. The biography software’s data integrity screens identify erroneous data points that come from open or short circuits, excessive background currents, and electrical noise, among other sources. When there are data points that do not meet objective a priori criteria, a glucose reading is ignored (or skipped). Additionally, skin conductance and skin temperature sensors are included in the biography. The latter is closely associated with the quantity of perspiration on the skin’s surface. The glucose measurement can be complicated by significant temperature variations or the presence of glucose in perspiration. For this reason, the measurement for that cycle will be omitted if the output from either of these sensors is over a predefined threshold. Thus, these data-screening techniques shield against potentially erroneous glucose levels [29].
Diagnosis and monitoring of CKD
The global burden of CKD is increasing, due to several factors, such as the pandemics of diabetes and obesity, but partly due to improved screening [30]. For those already on dialysis, frequent monitoring of blood urea is also performed to determine the efficacy of the treatment [31]. Measurements of elevated urea and creatinine levels are the main basis for the clinical diagnosis of CKD. It is impossible to postpone the beginning of end-stage renal disease and the corresponding mortality and morbidity without this vital monitoring data. Renal replacement therapy (or hemodialysis [HD]) is necessary as the condition progresses. Enhancing the management of high-risk patients, such as those with diabetes and hypertension, as well as monitoring them during HD presents challenges, as does avoiding the clear drawbacks of traditional blood collection, which include the requirement for trained personnel, discomfort, and infection risk. The primary means of making a clinical diagnosis of CKD is through measurements of high urea and creatinine levels. Without these crucial monitoring data, it is impossible to delay the onset of end-stage renal disease and the associated mortality and morbidity. As the illness worsens, renal replacement therapy (also known as HD) becomes required. Improving the care of high-risk patients, with diabetes, hypertension, and so on, along with keeping an eye on them during HD are difficult tasks. Traditional blood collection has disadvantages, such as the need for skilled personnel, discomfort, and infection risk [32]. After applying a 0.8 mA current to the skin for 2 h, the urea collected iontophoretically can be used to separate patients with CKD from healthy volunteers. Subcutaneous urea concentrations in both healthy people and CKD patients are in balance with plasma urea under steady-state settings [33]. Hence, extracted subdermal interstitial urea could be considered to reflect blood urea [36].
Mechanism
The mechanism is based on three main steps: RI, sample analysis, and statistical analysis.
RI
In patients with arteriovenous fistula, RI was performed with the non-dominant arm or the non-fistula arm. Patients undergoing hemodialysis were tested during dialysis. Peritoneal dialysis patients were dried before examinations. A MIC2® iontophoresis driver (Moor Instruments Inc., Wilmington, DE, USA) was used to deliver a constant current of 250 μA between two electrodes placed approximately 10 cm apart on an abdominal forearm that had been previously cleaned and defatted at 70 °C ethanol. The anode was an inactive gel electrode and the cathode was prepared from 1 mM TRIS (hydroxymethylaminomethane) buffer at pH 8.75 at 37 °C in a 1.5 mL chamber. The buffer contacted the skin over an area of 3.8 cm2. After 30 min, the controller was automatically turned off and the cathode chamber buffer (sample) was emptied into an Eppendorf flask. The skin was dried and the cycle was repeated at least five times for each subject and dialysis duration in HD patients. Where possible, one run was performed before starting dialysis (pre-dialysis sample) and after completing dialysis (post-dialysis sample). During HD, blood was drawn every 30 min from the arterial port. In others, a single blood sample was taken by venipuncture at the end of the session. Blood samples were collected in ethylenediamine tetraacetic acid tubes, centrifuged at 770 g for 10 min, plasma was aliquoted, and all samples were stored at -80 °C until analysis. Any pain, discomfort, skin irritation or damage resulting from application of the iontophoretic current and its duration and severity were recorded (Figure 4).
Sample analysis
Urea is measured using a colorimetric urease and glutamate dehydrogenase method with a lower detection limit of 0.5 mM. As the urea concentrations in the iontophoretic buffer were much smaller (of the micromolar order), this required a sensitive assay and the diacetylmonoxime method of urea assay described by Friedman. [36].
Statistical analysis
The GraphPad Prism® Software version 5.0 (GraphPad Software Inc., La Jolla, CA USA) was used for statistical analysis. The Mean±SD are used to express the results. The t-test (for regularly distributed data) and the Mann-Whitney test (for nonnormally distributed data) are the two test types that can be employed in statistics [35].
Monitoring of premature neonates
Premature neonates represent a fragile patient population, often subjected to intensive clinical care and multiple drug therapy, which must be monitored carefully and continuously. The difficult and painful nature of repetitive blood sampling, particularly in this population, has provided considerable impetus for the development of noninvasive methods for monitoring blood analytes. RI, a relatively new technology already used for the transdermal monitoring of blood glucose levels in adults, may be particularly well-suited to exploit the unique properties of preterm neonatal skin. The underdevelopment of the premature infant’s epidermis, and more specifically the stratum corneum, results in an increased permeability to molecular transport [37]. Therapeutic drug monitoring, or measuring plasma drug concentrations while a patient is under treatment, is a useful tool for clinicians to optimize patient therapy. However, this procedure is only employed in certain circumstances because of its expense and time-consuming nature [38]: 1) For drugs with a narrow therapeutic range (and therefore a high risk of toxicity) 2) In the absence of clinical response (e.g. due to pharmacodynamic variability), 3) To individualize treatment (due to interindividual variability in drug pharmacokinetics), and 4) For vulnerable patient groups requiring more careful drug concentration (e.g. in the elderly, neonates and premature infants). Continuous monitoring of premature infants is an important therapeutic issue. Due to prematurity and related complications, they are often treated with multiple drug therapies. However, minimal processing is also recommended [39] because every medical procedure performed on a premature newborn raises the possibility of infection and intensifies energy and heat losses. As a result, noninvasive monitoring techniques, like transcutaneous gas collection, are recommended [40]. The technique has the advantage of being noninvasive and therefore warrants consideration as an alternative to blood sampling for drug monitoring. To date, the major application of RI has centered on the development of a device (the GlucoWatch Biographer, Cygnus, Inc., USA) for the continuous monitoring of blood glucose levels in diabetics [41]. Other applications have been envisaged for both drug monitoring (e.g. clonidine and theophylline) [42]. Because of its limited therapeutic range, theophylline is a great choice for therapeutic monitoring in premature infants. It is given for the treatment and prevention of apnea, coupled with caffeine, another methylxanthine. Caffeine is N-methylated theophylline in preterm infants, at steady state, the concentration of the latter is approximately one-third that of the former; neonates have been shown to maintain this proportion consistently over a range of post-conceptional ages, from 28 to 42 weeks. [43].
Mechanism
A little, controlled electrical current is applied to the skin during RI. Either electromigration of charged species to the electrode with the opposite polarity, electroosmosis of neutral molecules to the cathode, or possibly a mix of the two for cations, is the mechanism of extraction [4].
Lithium monitoring
Lithium monitoring is essential to ensure efficacy as well as to prevent adverse effects [44]. A therapeutic response, such as in the treatment of manic episodes, is not apparent until two to three weeks following the start of treatment, even though steady-state plasma concentrations of the drug are reached in a matter of days [45]. A blood test known as the “standardized 12 h li+ serum concentration” is required for lithium monitoring. This means that patients must have their blood drawn early in the morning, approximately 12 h after their final dose of the previous day and before their first administration on the monitoring day. Therefore, one may argue that a fully noninvasive method of lithium monitoring would help bipolar patients adhere to their treatment plans and live better. Although the wide inter- and intraindividual variability of the saliva/plasma concentration ratio has been suggested as an alternate matrix for lithium monitoring, this approach’s utility is severely limited [46].
Mechanism
In iontophoresis, two transport processes are involved. Ions travel over the skin carrying charge and are drawn (attracted) to the electrode with the opposite polarity during electromigration. A net solvent flow from the anode to the cathode is known as electroosmosis, and it greatly improves cationic transport and allows for considerably better penetration of neutral molecules (like glucose) [47]. RI has been used to monitor lithium in vitro. This cationic medication is tiny, mobile, and a great fit for iontophoretic extraction. Utilizing electromigration, lithium is moved at a flux to the cathode or negative electrode. In the presence of competing co-ions, the transport number of a given ion is proportional to its concentration [13]. This is the case in RI, in which the physiologic milieu provides a panoply of competing co-ions under these circumstances [45].
Extraction of transdermal biomarkers
Lithium has been observed in vitro using RI. This cationic drug is small, easily transportable, and ideal for iontophoretic extraction. Lithium migrates to the cathode, or negative electrode, at a flux rate through electromigration. The transport number of a particular ion in the presence of competing co-ions is proportional to its concentration [48]. The most common types of skin cancer are non-melanoma skin cancers, which include squamous cell carcinoma and basal cell carcinoma [1]. Melanoma-related skin cancers, on the other hand, are less common but more dangerous because they can spread to other organs, and they are also one of the fastest-growing types of the disease [49]. The current gold standard for diagnosing skin cancer mostly uses visual examination of a lesion, tissue sampling, and staining. The features of the lesion and the experience of the doctor are two elements that affect this approach’s accuracy [50]. It is critically necessary to create a supplemental or alternative non-invasive technology to identify skin cancer in its early stages. Such an approach would help to decrease the number of unnecessary biopsies and provide justification for when surgical intervention is necessary because it would have improved specificity and sensitivity [51]. In the case of actinic keratoses and Bowen’s disease, it can eventually develop into squamous cell carcinoma [52]. Since persistent inflammation is a risk factor for cancer, identifying inflammatory biomarkers can help diagnose the disease before it manifests. Our understanding of the biomarkers linked to inflammation and cancer, such as interleukin 6, interferon-gamma, tumor necrosis factor-α, the enzyme indoleamine-2, 3-dioxygenase, and mutations in the BRAF gene, continually expands [53-55]. However, the modulation of the immune response is greatly aided by the degradation of the important amino acid tryptophan (Trp) via the kynurenine (Kyn) pathway [56], and changes in the Trp/Kyn ratio are associated with several diseases, including cancer [57]. Their polar nature raises concerns about whether enough can be extracted promptly to enable quantification later on, but their tiny molecular weights make them potentially good candidates for topical sampling [58]. RI greatly improved the extraction of Trp and Kyn, especially in the anode-to-cathode direction. Once the skin’s natural Trp is present. An alternate and more realistic (i.e. structurally stable) receptor into which Trp and Kyn could be extracted worked well: a fully hydrated, semi-solid, bi-continuous lipid cubic phase. More research is needed to fully understand RI as a comparatively non-invasive technique for sampling tiny biomarker chemicals associated with inflammation and cancer [59].
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the mnuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
- Giri TK, Chakrabarty S, Ghosh B. Transdermal reverse iontophoresis: A novel technique for therapeutic drug monitoring. J Control Release. 2017; 246:30-38. [DOI:10.1016/j.jconrel.2016.12.007] [PMID]
- Zheng H, Pu Z, Wu H, Li C, Zhang X, Li D. Reverse iontophoresis with the development of flexible electronics: A review. Biosens Bioelectron. 2023; 223:115036. [DOI:10.1016/j.bios.2022.115036] [PMID]
- Kanikkannan N. Iontophoresis-based transdermal delivery systems. BioDrugs. 2002; 16(5):339-47. [DOI:10.2165/00063030-200216050-00003] [PMID]
- Merino V, Kalia YN, Guy RH. Transdermal therapy and diagnosis by iontophoresis. Trends Biotechnol. 1997; 15(8):288-90. [DOI:10.1016/S0167-7799(97)01069-X] [PMID]
- Paulley Y, Delgado-Charro MB, White KJ. Modelling formation of a drug reservoir in the stratum corneum and its impact on drug monitoring using RI. Comput Math Methods Med. 2010; 11(4):353-68. [DOI:10.1155/2010/284230]
- Suthakaran C, Adithan C. Chapter-7 therapeutic drug monitoring–concepts, methodology, clinical applications and limitations. Health Adm. 2006; 19(1):22-6. [Link]
- Nair A, Reddy C, Jacob S. Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Skin Res Technol. 2009; 15(2):187-94. [DOI:10.1111/j.1600-0846.2009.00350.x] [PMID]
- Batheja P, Thakur R, Michniak B. Transdermal iontophoresis. Expert Opin Drug Deliv. 2006; 3(1):127-38. [DOI:10.1517/17425247.3.1.127] [PMID]
- Merino V, López A, Hochstrasser D, Guy RH. Noninvasive sampling of phenylalanine by reverse iontophoresis. J Control Release. 1999; 61(1-2):65-9. [DOI:10.1016/S0168-3659(99)00102-9] [PMID]
- Santi P, Guy RH. RI-parameters determining electro-osmotic flow. II. Electrode chamber formulation. J Controlled Release. 1996; 42(1):29-36. [DOI:10.1016/0168-3659(96)01345-4]
- Phipps JB, Gyory JR. Transdermal ion migration. Adv Drug Deliv Rev. 1992; 9(2-3):137-76. [DOI:10.1016/0169-409X(92)90022-I]
- Marro D, Kalia YN, Delgado-Charro MB, Guy RH. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm Res. 2001; 18(12):1701-8. [DOI:10.1023/A:1013370529366] [PMID]
- Benjamin FB, Kempen R, Mulder AG, Ivy AC. Sodium-potassium ratio of human skin as obtained by reverse iontophoresis. J Appl Physiol. 1954; 6(7):401-7. [DOI:10.1152/jappl.1954.6.7.401] [PMID]
- Nair AB, Goel A, Prakash S, Kumar A. Therapeutic drug monitoring by reverse iontophoresis. J Basic Clin Pharm. 2011; 3(1):207-13. [DOI:10.4103/0976-0105.103825] [PMID] [PMCID]
- Nixon S, Sieg A, Delgado-Charro MB, Guy RH. Reverse iontophoresis of L-lactate: In vitro and in vivo studies. J Pharm Sci. 2007; 96(12):3457-65. [DOI:10.1002/jps.20989] [PMID]
- Moore K, Grégoire S, Eilstein J, Delgado-Charro MB, Guy RH. Reverse iontophoresis: Noninvasive assessment of topical drug bioavailability. Mol Pharm. 2024; 21(1):234-44. [DOI:10.1021/acs.molpharmaceut.3c00791] [PMID] [PMCID]
- Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002; 18(Suppl 1):S49-53. [DOI:10.1002/dmrr.210] [PMID]
- Chang T, Li H, Zhang N, Jiang X, Yu X, Yang Q, et al. Highly integrated watch for noninvasive continual glucose monitoring. Microsyst Nanoeng. 2022; 8:25. [DOI:10.1038/s41378-022-00355-5] [PMID] [PMCID]
- Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329(14):977-86. [DOI:10.1056/NEJM199309303291401] [PMID]
- Tierney MJ, Tamada JA, Potts RO, Eastman RC, Pitzer K, Ackerman NR, et al. The glucowatch biographer: A frequent automatic and noninvasive glucose monitor. Ann Med. 2000; 32(9):632-41. [DOI:10.3109/07853890009002034] [PMID]
- Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978; 8(4):283-98. [DOI:10.1016/S0001-2998(78)80014-2] [PMID]
- Tierney MJ, Jayalakshmi Y, Parris NA, Reidy MP, Uhegbu C, Vijayakumar P. Design of a biosensor for continual, transdermal glucose monitoring. Clin Chem. 1999; 45:1681-2. [DOI:10.1093/clinchem/45.9.1681]
- Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S; Cygnus Research Team. Clinical evaluation of the GlucoWatch biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001; 16(9-12):621-9. [DOI:10.1016/S0956-5663(01)00189-0] [PMID]
- Singh J, Dinh SM, Berner B. Electrical properties of skin. In: Berner B, Dinh SM, editors. Electronically controlled drug delivery. Berner B, Dinh SM, Eds. Boca Raton: CRC Press 1998. [DOI:10.1201/9780429262838-5]
- Rao G, Glikfeld P, Guy RH. Reverse iontophoresis: Development of a noninvasive approach for glucose monitoring. Pharm Res. 1993; 10(12):1751-5. [DOI:10.1023/A:1018926215306] [PMID]
- Tamada JA, Bohannon NJ, Potts RO. Measurement of glucose in diabetic subjects using noninvasive transdermal extraction. Nat Med. 1995; 1(11):1198-201. [DOI:10.1038/nm1195-1198] [PMID]
- Kurnik RT, Berner B, Tamada J, Potts RO. Design and simulation of a reverse iontophoretic glucose monitoring device. J Electrochem Soc. 1998; 145(12):4119-25. [DOI:10.1149/1.1838924]
- Kurnik RT, Oliver JJ, Waterhouse SR, Dunn T, Jayalakshmi Y, Lesho M, et al. Application of the mixtures of experts algorithm for signal processing in a non-invasive glucose monitoring system. Sensors Actuators B. 199; 60(1):19-26. [DOI:10.1016/S0925-4005(99)00239-7]
- Pitzer KR, Desai S, Dunn T, Edelman S, Jayalakshmi Y, Kennedy J, et al. Detection of hypoglycemia with the GlucoWatch biographer. Diab Care. 2001; 24(5):881-5. [DOI:10.2337/diacare.24.5.881] [PMID]
- Eknoyan G, Levey AS, Levin NW, Keane WF. The national epidemic of chronic kidney disease. What we know and what we can do. Postgrad Med. 2001; 110(3):23-9. [DOI:10.3810/pgm.2001.09.1024] [PMID]
- Manahan FJ, Ramanujam L, Ajam M, Ing TS, Gandhi VC, Daugirdas JT. Post to predialysis plasma urea nitrogen ratio, ultrafiltration and weight to estimate K.t/V. Use in auditing the amount of dialysis being administered. ASAIO Trans. 1989; 35(3):511-2. [DOI:10.1097/00002480-198907000-00109] [PMID]
- Wascotte V, Rozet E, Salvaterra A, Hubert P, Jadoul M, Guy RH, et al. Non-invasive diagnosis and monitoring of chronic kidney disease by reverse iontophoresis of urea in vivo. Eur J Pharm Biopharm. 2008; 69(3):1077-82. [DOI:10.1016/j.ejpb.2008.02.012] [PMID]
- Ebah L, Brenchley P, Coupes B, Mitra S. A modified in vivo flow variation technique of microdialysis for sampling uremic toxins in the subcutaneous interstitial compartment. Blood Purif. 2011; 32(2):96-103. [DOI:10.1159/000324207] [PMID]
- Wascotte V, Delgado-Charro MB, Rozet E, Wallemacq P, Hubert P, Guy RH, et al. Monitoring of urea and potassium by reverse iontophoresis in vitro. Pharm Res. 2007; 24(6):1131-7. [DOI:10.1007/s11095-007-9237-0] [PMID]
- Ebah LM, Read I, Sayce A, Morgan J, Chaloner C, Brenchley P, et al. Reverse iontophoresis of urea in health and chronic kidney disease: A potential diagnostic and monitoring tool? Eur J Clin Invest. 2012; 42(8):840-7. [DOI:10.1111/j.1365-2362.2012.02657.x] [PMID] [PMCID]
- Friedman HS. Modification of determination of urea by diacetyl monoxime method. Analytical Chem. 1953; 25(4):662-4. [DOI:10.1021/ac60076a040]
- Sekkat N, Naik A, Kalia YN, Glikfeld P, Guy RH. Reverse iontophoretic monitoring in premature neonates: Feasibility and potential. J Control Release. 2002; 81(1-2):83-9. [DOI:10.1016/S0168-3659(02)00046-9] [PMID]
- Brown GR, Miyata M, McCormack JP. Drug concentration monitoring. An approach to rational use. Clin Pharmacokinet. 1993; 24(3):187-94. [DOI:10.2165/00003088-199324030-00001] [PMID]
- Langer VS. Minimal handling protocol for the intensive care nursery. Neonatal Netw. 1990; 9(3):23-7. [PMID]
- Barrett DA, Rutter N. Transdermal delivery and the premature neonate. Crit Rev Ther Drug Carrier Syst. 1994; 11(1):1-30. [PMID]
- Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: Comprehensive clinical results. Cygnus Research Team. JAMA. 1999; 282(19):1839-44. [DOI:10.1001/jama.282.19.1839] [PMID]
- Glikfeld P, Hinz RS, Guy RH. Noninvasive sampling of biological fluids by iontophoresis. Pharm Res. 1989; 6(11):988-90. [DOI:10.1023/A:1015957816254] [PMID]
- Besunder JB, Reed MD, Blumer JL. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (Part I). Clin Pharmacokinet. 1988; 14(4):189-216. [DOI:10.2165/00003088-198814050-00001] [PMID]
- Amdisen A. Serum concentration and clinical supervision in monitoring of lithium treatment. Ther Drug Monit. 1980; 2(1):73-83. [DOI:10.1097/00007691-198001000-00009] [PMID]
- Leboulanger B, Fathi M, Guy RH, Delgado-Charro MB. Reverse iontophoresis as a noninvasive tool for lithium monitoring and pharmacokinetic profiling. Pharm Res. 2004; 21(7):1214-22. [DOI:10.1023/B:PHAM.0000033008.64915.c8] [PMID]
- Moody JP. Biologic variation of serum and salivary lithium. Ther Drug Monit. 1999; 21(1):97-101. [DOI:10.1097/00007691-199902000-00015] [PMID]
- Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 2001; 46(1-3):281-305. [DOI:10.1016/S0169-409X(00)00138-1] [PMID]
- Ching CT, Fu LS, Sun TP, Hsu TH, Chang KM. Use of electroporation and reverse iontophoresis for extraction of transdermal multibiomarkers. Int J Nanomedicine. 2012; 7:885-94. [DOI:10.2147/IJN.S27421] [PMID] [PMCID]
- Jalles C, Lepelley M, Mouret S, Charles J, Leccia MT, Trabelsi S. Skin cancers under Janus kinase inhibitors: A World Health Organization drug safety database analysis. Therapie. 2022; 77(6):649-56. [DOI:10.1016/j.therap.2022.04.005] [PMID]
- Fink C, Haenssle HA. Non-invasive tools for the diagnosis of cutaneous melanoma. Skin Res Technol. 2017; 23(3):261-71. [DOI:10.1111/srt.12350] [PMID]
- Malvehy J, Hauschild A, Curiel-Lewandrowski C, Mohr P, Hofmann-Wellenhof R, Motley R, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014; 171(5):1099-107. [DOI:10.1111/bjd.13121] [PMID] [PMCID]
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002; 146(Suppl 61):1-6. [DOI:10.1046/j.1365-2133.146.s61.2.x] [PMID]
- Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005; 26(6):318-25. [DOI:10.1016/j.it.2005.04.003] [PMID]
- Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 pathway in cancer: From bench to bedside. J Hematol Oncol. 2018; 11(1):100. [DOI:10.1186/s13045-018-0644-y] [PMID] [PMCID]
- Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006; 203(7):1651-6. [DOI:10.1084/jem.20051848] [PMID] [PMCID]
- Mbongue JC, Nicholas DA, Torrez TW, Kim NS, Firek AF, Langridge WH. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines. 2015; 3(3):703-29. [DOI:10.3390/vaccines3030703] [PMID] [PMCID]
- Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015; 22(12):1964-8. [DOI:10.1016/j.jocn.2015.06.018] [PMID] [PMCID]
- Leboulanger B, Guy RH, Delgado-Charro MB. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol Meas. 2004; 25(3):R35-50. [DOI:10.1088/0967-3334/25/3/R01] [PMID]
- Morin M, Björklund S, Jankovskaja S, Moore K, Delgado-Charro MB, Ruzgas T, et al. Reverse iontophoretic extraction of skin cancer-related biomarkers. Pharmaceutics. 2021; 14(1):79. [DOI:10.3390/pharmaceutics14010079] [PMID] [PMCID]
Type of Study: Review article |
Subject:
Pharmaceutics
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |