Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 257-268 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Osarieme Imade R, Ayinde B A, Onyebuchi C E, Asoya E V. Antifibroid Evaluation of Tetrapleura tetraptera Taubert (Fabaceae) Stem Bark Ethanol Extract and HPLC Analysis of Its Polyphenolic Compounds. Pharm Biomed Res 2024; 10 (3) :257-268
URL: http://pbr.mazums.ac.ir/article-1-624-en.html
URL: http://pbr.mazums.ac.ir/article-1-624-en.html
Rose Osarieme Imade *1 

 , Buniyamin Adesina Ayinde1
, Buniyamin Adesina Ayinde1 

 , Chinaza Esther Onyebuchi1
, Chinaza Esther Onyebuchi1 
 , Ekene Victor Asoya2
, Ekene Victor Asoya2 




 , Buniyamin Adesina Ayinde1
, Buniyamin Adesina Ayinde1 

 , Chinaza Esther Onyebuchi1
, Chinaza Esther Onyebuchi1 
 , Ekene Victor Asoya2
, Ekene Victor Asoya2 


1- Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin, Nigeria.
2- Department of Medical Biochemistry, School of Basic Medical Science, University of Benin, Benin, Nigeria.
2- Department of Medical Biochemistry, School of Basic Medical Science, University of Benin, Benin, Nigeria.
Keywords: Tetrapleura tetraptera, Fibroid, High-performance liquid chromatography (HPLC), Biochemical, Histopathology
Full-Text [PDF 2181 kb]
(672 Downloads)
| Abstract (HTML) (2165 Views)
Full-Text: (626 Views)
Introduction
Uterine fibroid is a benign smooth muscle tumor, also known as uterine myoma. However, it presents a primary global concern to the reproductive health of women [1]. It can be described as submucosal, subserosal, or intramural fibroids as it develops in different places along and inside the uterine walls or within its cavity. Its size and shape vary from a pea to a watermelon [2]. Some risk factors increase the likelihood of uterine leiomyoma: Age (women aged 20 to 44 are more vulnerable), food (it has been demonstrated that refined sugar, carbohydrates, and meals containing growth hormones raise the risk), overweight, ethnicity (compared to their Caucasian counterparts, African American women are more likely to have uterine fibroids), and consuming certain chemicals, such as monosodium glutamate (MSG) which has flavor-enhancing properties and induce fibroid formation in rat models [3, 4]. Furthermore, a person is more likely to develop uterine fibroids if she takes certain medications that may raise total protein, cholesterol, and estrogen levels [5, 6].
In many women, uterine fibroids may not exhibit symptoms, so they will not receive much clinical attention. The few who experience symptoms typically report infertility, complicated pregnancy, labor problems, lower back discomfort, pelvic fullness, and irregular uterine bleeding [2]. It is important to remember that the severity of these symptoms depends on the tumor's size and location.
High progesterone and estrogen levels increase the mitosis rate, contributing to the formation of a myoma, even if the etiology of uterine fibroid is not entirely understood [5-7]. It has been documented that progesterone and estrogen contribute to the tumor's continued growth during the menstrual cycle. Additional evidence originates from clinical findings, demonstrating that uterine leiomyoma rarely develops before the onset of menstruation and regresses after menopause. Due to the adverse effects of prolonged use, hormone replacement therapy is only beneficial for 6 to 12 months. Therefore, the usual accessible treatments for this condition are myomectomy and hysterectomy [7, 8].
More effective treatment options are required due to the side effects, expense, and duration of the symptomatic relief provided by conventional drugs. Pharmacological evaluation of some plants reveals their antifibroid potential. In a study, the aqueous extract of Spondias mombin and Aspilia africana leaves improved several parameters affected by the administration of MSG. Histopathological evaluation indicates that the treated groups are protected against leiomyoma development [4]. It has also been documented that the fruit of Emblica officinalis has phytochemicals that preserve the uterus against uterine fibroids [9]. Furthermore, morphological analysis shows that the entire Labisia pumila plant suppresses the growth of uterine fibroid tumors and triggers apoptosis on SK-UT-1 (uterine fibroid cells) [6].
Tetrapleura tetraptera Taubert is a deciduous plant in the Fabaceae family. It grows on the fringes of the rainforest region that covers West and Central Africa [10]. It raises well in the Gambia, Nigeria, Mauritania, Uganda, Mali, and Burkina Faso. The plant reaches 20–25 m in height and 1.2–3 m in girth. It is used to treat feverish diseases, infections, fibroids, postpartum contraction prevention, cholesterol control, and the promotion of breast milk supply [11, 12]. T. tetraptera stem bark has been the subject of numerous investigations, but its antifibroid activity has yet to be validated. This study was conducted to determine its preventive or curative capacities for fibroid tumors. Specifically, its effects on total cholesterol, protein, and estradiol content were evaluated. In addition, its ability to protect the uterus of Sprague-Dawley (SD) rats against MSG-induced fibroids was assessed through histopathological analysis. Finally, its phytochemical constituents were determined through high-performance liquid chromatography (HPLC) analysis.
Materials and Methods
Chemicals and reagents
The study’s reagents were all analytical graded and sourced from reliable local suppliers: MSG from Lobachem, India, ethanol from BDH Chemicals, England, AGAPPE test kit, and E2 AccuBind ELISA Kit.
Plant collection and preparation
The stem bark of T. tetraptera, collected in Ikire, Osun state, Nigeria, was validated at the Forest Research Institute of Nigeria (FRIN) with herbarium number FHI 113604. Absolute ethanol was used to extract the dried and crushed bark using a Soxhlet apparatus. A rotary evaporator was employed to dry the extract completely.
Animal handling conditions
The Department of Pharmacology at the Faculty of Pharmacy, University of Benin, Nigeria, supplied non-pregnant SD rats weighing between 160 and 200 g. The animals were grouped in fives in plastic cages with beddings made of shavings from wood and a wire screen top for aeration. Commercial rat pellets and clean water were given to the animals ad libitum. Guidelines issued by the National Institutes of Health for the Use and Treatment of Laboratory Animals were followed when handling the animals.
Acute toxicity test
Lorke’s method was used to assess the oral median lethal dosage (LD50) of T. tetraptera stem bark extract (TTS) [13]. The extract was given orally to three groups of rats in the first phase at 10, 100, and 1000 mg/kg, respectively. In the second phase, 1600, 2900, and 5000 mg/kg of the extract were given orally to three groups of one rat each. Throughout 24 hours, the animals in both phases were watched for indications of tremor, diarrhea, writhing, and mortality. This phase concluded with the determination of the stem bark extract’s LD50.
Dosing of experimental animals
This study used three doses of TTS (100, 200, and 400 mg/kg) chosen based on the stem barks LD50, which was more than 5000 mg/kg. The SD rats’ weekly body weight was used to determine the dose they received once a day. Since humans typically use the oral route, this route was chosen for administration.
Antifibroid effect of TTS
Preventive study
In this study, female SD rats were divided into five groups of five rats each. The 1st group received only food and water, the second group received 800 mg/kg MSG, and the third, fourth, and fifth groups received TTS with different doses: 100, 200, and 400 mg/kg, respectively. The method described in the literature [5] was followed. Throughout 30 days, all treatments were administered simultaneously. On the 31st day, the animals were sacrificed by anesthetizing them in a chamber saturated with chloroform, and their blood was drawn via cardiac puncture and placed in plain bottles for the measurement of total plasma cholesterol, protein, and estradiol content. The uteri were removed surgically and placed into sterile tissue containers having 10% neutral buffer formalin for assessment of histopathology.
Curative study
Female SD rats were given 800 mg/kg MSG for 30 days to cause uterine fibroid development, based on the procedure outlined in published research [5]. Each set of five rats in the housing was designated as a group. On the 31st day, the extract treatment began. Group A was the control group throughout the experiment, given food and water only without MSG. From day 30 to day 60, Group B received nothing but food and water following their 30-day administration of 800 mg/kg MSG. After the 30-day administration of MSG, groups C through E were treated with 100, 200, and 400 mg/kg of TTS, respectively, from day 31 to day 60. To measure the total plasma cholesterol, protein, and estradiol content, the animals were sacrificed by anesthetizing them in a chamber saturated with chloroform, and their blood was drawn by cardiac puncture into plain bottles. The uteri were removed surgically and placed in sterile containers with 10% neutral buffer formalin for assessment by histopathology.
Biochemical assays
Determination of total cholesterol content
The animals’ blood was taken one day after the conclusion of the administration (30 days for preventive treatment and 60 days for curative treatment), and the serum was separated for the experiment by centrifugation at 3000 rpm for 10 minutes.
The Agappe test kit and the Mindray BA-88A Reagent system, a semi-automated chemical analyzer, were used to determine the total cholesterol content. Using the data from the instruction manual, the semi-automated biochemistry analyzer was set to run the total cholesterol kit. A micro-tube labeled “blank” was filled with 1000 µL of cholesterol biuret reagent. In a tube labeled «standard,» 10 µL of standard cholesterol and 1000 µL of the reagent were added and thoroughly mixed. A tube labeled «A1» was filled with 10 µL of sample A1 and 1000 µL of cholesterol reagent, and the combination was well mixed. The remaining samples (A1-E5) were processed similarly in the corresponding tubes. Samples from both the curative and preventive studies were treated in this manner. Every tube was incubated at 37 °C for 10 minutes. Following the incubation period, the absorbance of the blank tube and standard tubes’ absorbance were measured by aspirating their respective contents into the analyzer’s flow cell. The absorbance for each sample was then measured by aspirating the reaction mixture into the flow cell. Each tube’s absorbance readings were noted appropriately [9].
Determination of total protein content
The kit was programmed on the semi-automated biochemistry analyzer using the data from the instruction booklet. To a micro-tube designated “blank,” 1000 µL of total protein biuret reagent was introduced. A tube labeled «standard» was filled with 20 µL of standard total protein and 1000 µL of the reagent, then thoroughly mixed. Sample A1 (20 µL) and total protein reagent (1000 µL) were added to and thoroughly mixed in a tube labeled «A». The exact process was applied to the remaining samples (A1-E5) from the curative and preventive tests in their corresponding tubes. Every tube was incubated at 37 ˚C for 10 minutes. Following the incubation period, the absorbance of the blank and standard tubes was measured by aspirating their respective contents into the analyzer’s flow cell. To quantify the absorbance, the reaction mixture for each sample was aspirated into the flow well after that. Each tube’s absorbance value was noted appropriately [9].
Determination of estradiol content
The E2 AccuBind ELISA Kit, the Mindray MR-96A microplate reader, and the Mindray MW-12A microplate washer were used to measure estradiol. Pipetting 25 µL of the serum reference and 25 µL of each sample (A1-E5) into the designated wells was followed by adding 50 µL of estradiol biotin reagent to each well. After gently swirling the plates for approximately 30 seconds, they were incubated for 30 minutes. Subsequently, 50 µL of estradiol enzyme reagent was added to each well, and the plates were left to incubate for 90 minutes at room temperature. Decanting the contents of the microplate was followed by drying the plates with absorbent paper. Then, 350 µL of the wash buffer was added thrice, and the content was decanted. Following the addition of 100 µL of substrate and 20 minutes of incubation, 50 µL of the stop solution was added to each well and gently mixed for an additional 20 minutes. Within 15 minutes of administering the stop solution, the absorbance at 450 nm was measured. From a dosage response curve, the amount of estradiol in the samples was extrapolated [9].
Histopathology studies of the uterus
For histological analysis, the animal’s uterus was stored in a 10% neutral buffered formalin solution. After being dehydrated in increasing alcohol grades (70%, 90%, 96%, and 100%), the tissue was cleared with xylene, saturated with melted paraffin wax, and sectioned into slides. These sections (4-5 μm thick) were stained with hematoxylin after dewaxing with xylene and hydrating in descending degrees of alcohol (100%, 96%, 70%) and water. Following 1% acid alcohol differentiation, eosin was used as a counterstain on the sections. Before being examined under a microscope, the slices were once more dehydrated in increasing grades of alcohol, cleaned in xylene, and mounted with polystyrene made of dibutyl phthalate using coverslips [5].
Polyphenolic compounds identification by HPLC-DAD
The HPLC technique was performed at the Bato Chemical Laboratory in Lagos, Nigeria. This HPLC was made by Shimadzu (Nexera mx). The ubondapak C18 reverse-phase chromatographic column was utilized. Its dimensions were as follows: Length of 100 mm, internal diameter of 4.6 mm, and thickness of 7 µm. Acetonitrile/water (70:30) was the mobile phase used. The HPLC system was attached to a UV–Vis Diode array detector (DAD) set at an analytical wavelength of 254 nm within the UV–Visible region. The used pump pressure was 15 MPa. The HPLC system was initially injected with standard solutions to produce a chromatogram with a specified peak and peak profile. These were employed to make a window to analyze the test sample. Also, 5 µL of extract was injected into the HPLC system at a constant flow rate of 2 mL/min to create a chromatogram with corresponding peak areas and profiles.
Data analysis
GraphPad Prism software for Windows, version 6.01, was used to create bar graphs, and data were subjected to one-way analysis of variance (ANOVA) using the Tukey-Kramer multiple comparison test. In all analyses, P≤0.05 was deemed statistically significant.
Results
Acute toxicity test
TTES showed no mortality or evidence of toxicity during both phase 1 and phase 2 of the acute toxicity test; normal eating habits, fecal matter, movements, and sleep were observed. The lethal median dose of the extract was estimated to be greater than 5000 mg/kg.
Total serum cholesterol in preventive and curative studies
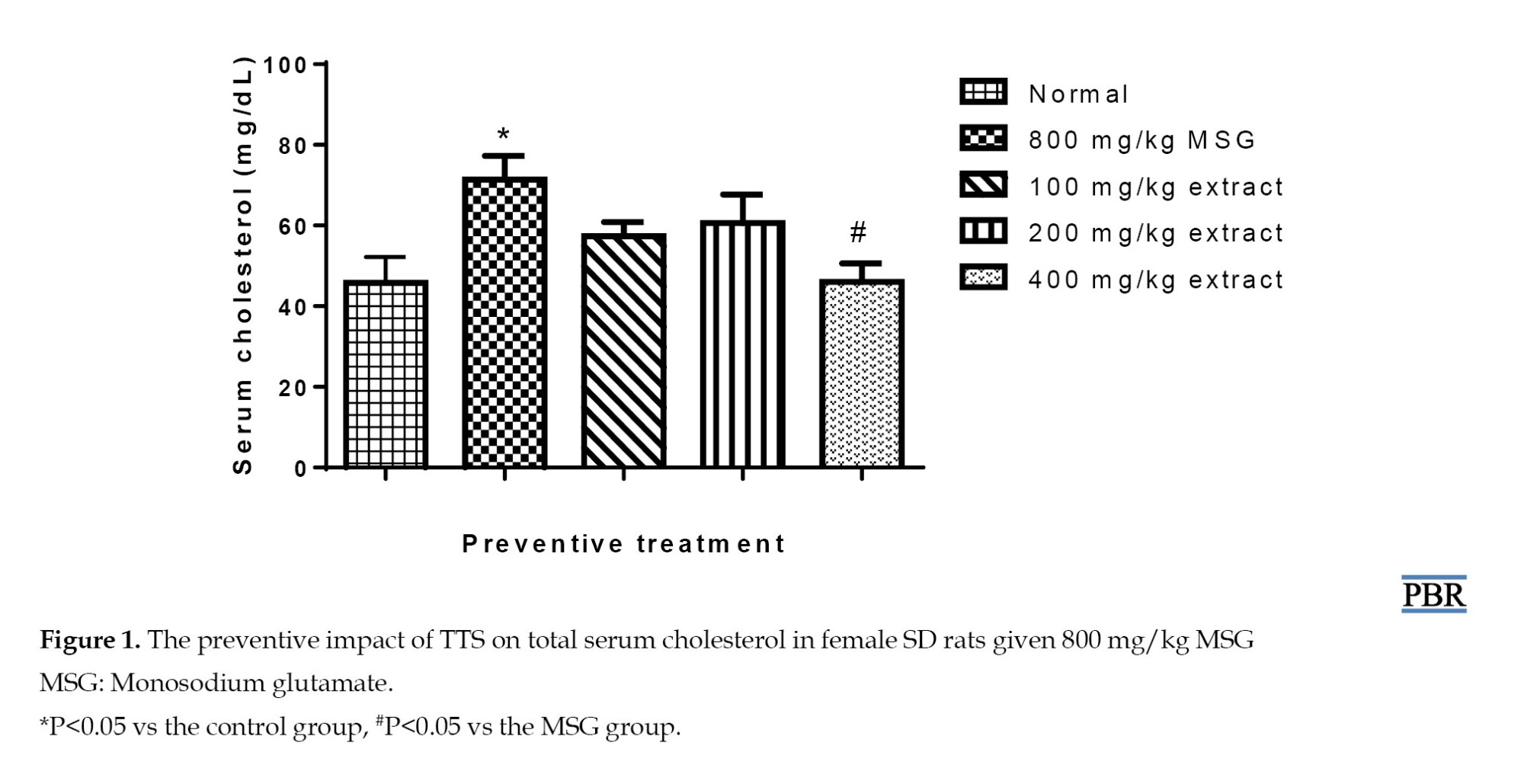
An increase in blood cholesterol was seen (56.28%, P≤0.05) in the group that received MSG alone in the preventive experiment. This effect was seen less when the extract and MSG were given together at 100 and 200 mg/kg. There was only a 25.68%–32.46% increase in blood cholesterol over the control (P≥0.05) (Figure 1). Following MSG-induced uterine leiomyoma formation, serum cholesterol levels were raised (45.10%).
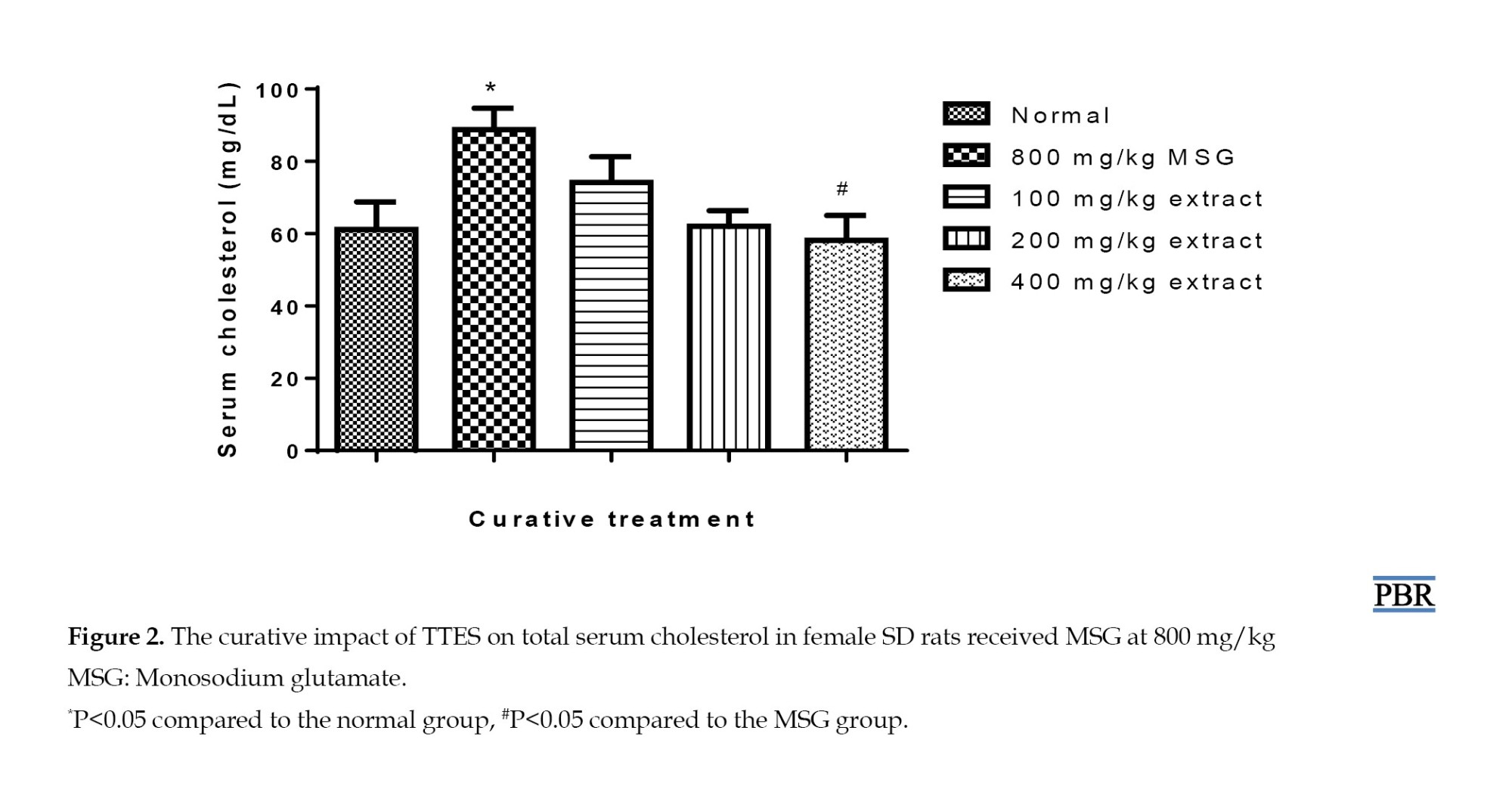
However, this parameter was reduced by the curative treatment using varying doses of the extract, with a 21.24% rise observed at 100 mg/kg. Higher concentrations of 200 and 400 mg/kg further reduced this parameter to within the normal range (P≥0.05) (Figure 2).
Total protein content in preventive and curative studies
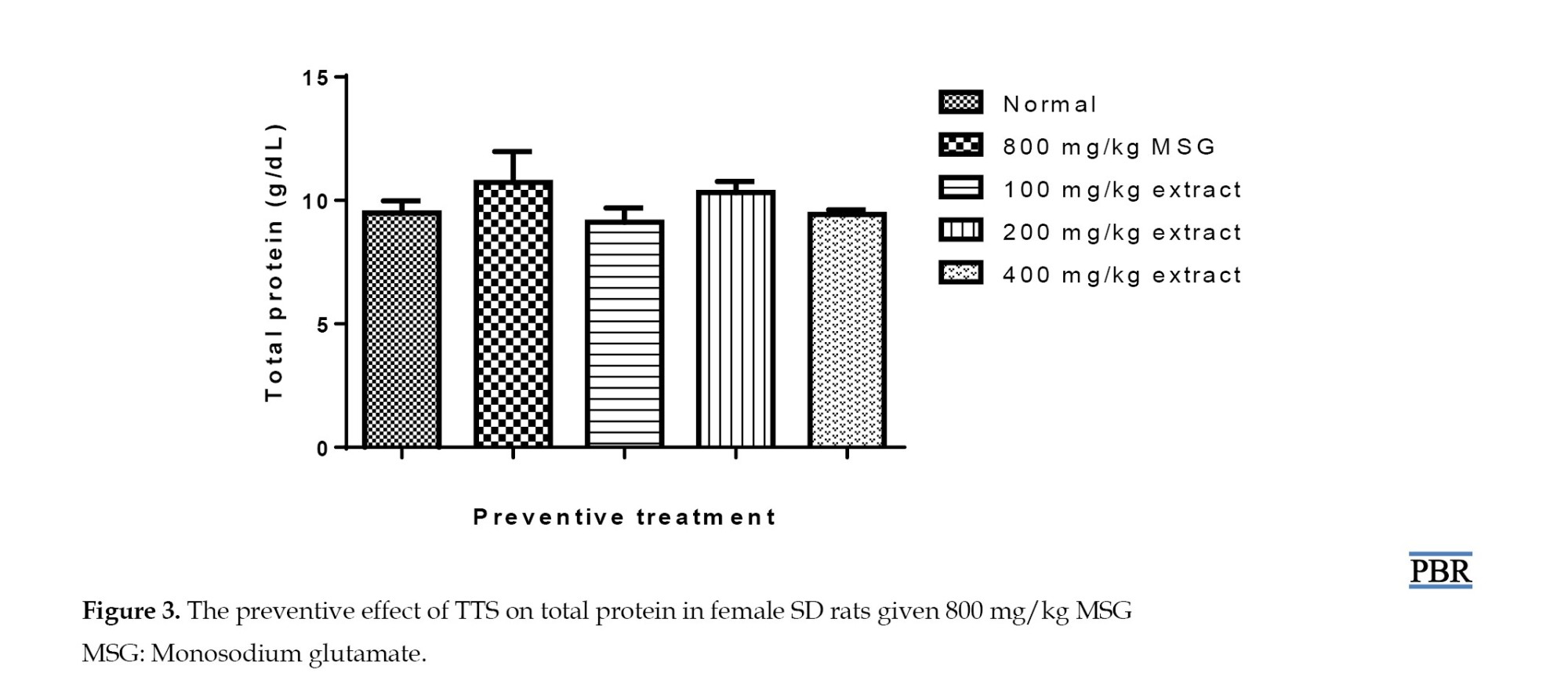
There was no obvious (P≥0.05) alteration in blood protein between the normal rats, the MSG-only, and MSG and extract-treated groups (Figure 3).
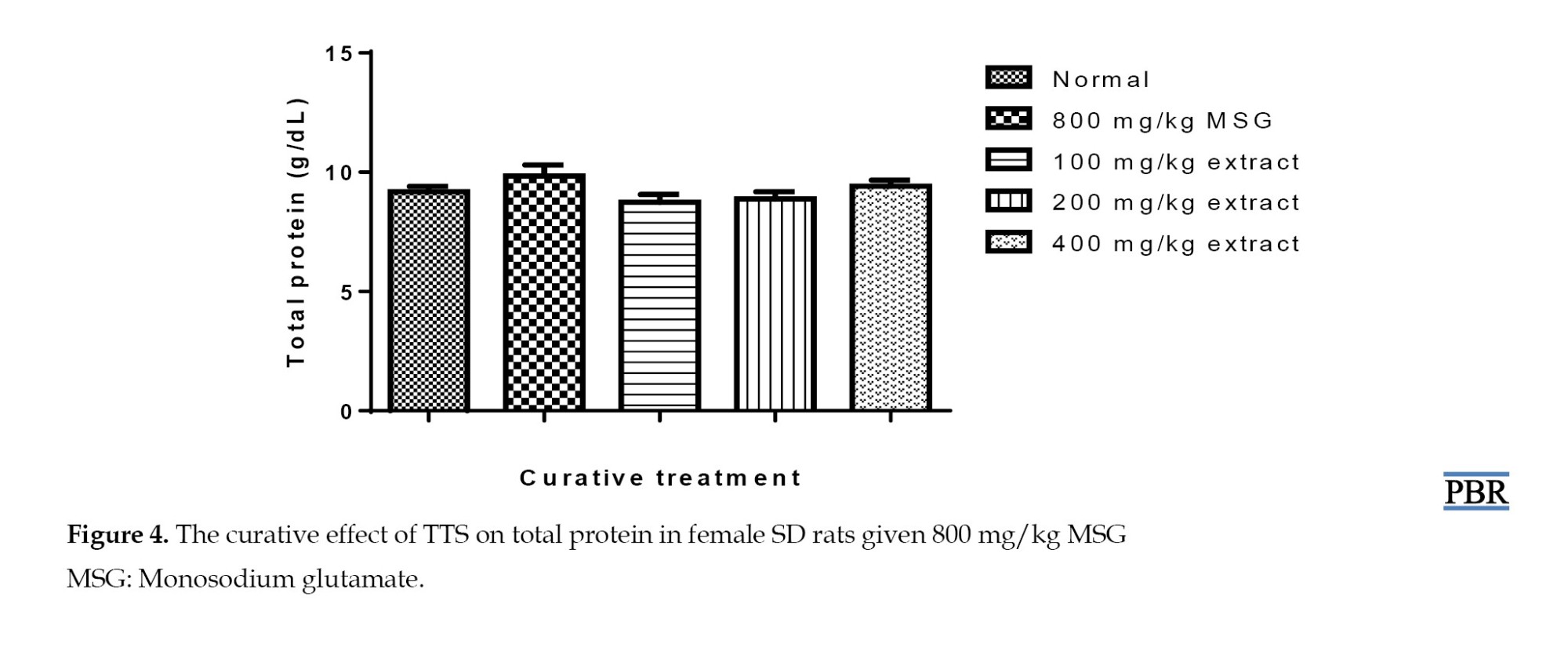
Additionally, pretreatment of normal rats with MSG did not result in a statistically significant increase in blood protein levels (Figure 4).
Total serum estradiol in preventive and curative studies
Treatment with MSG resulted in a highly significant increase in estradiol (90.98%, P≤0.05). Concurrent administration of MSG and extract at different doses decreased hormone levels, where a 28.77% increase was seen at 100 mg/kg. At 200 and 400 mg/kg, the estradiol content was reduced to within the normal range (P≥0.05) (Figure 5), with a significant difference between the MSG and the 400 mg/kg group (P≤0.05).
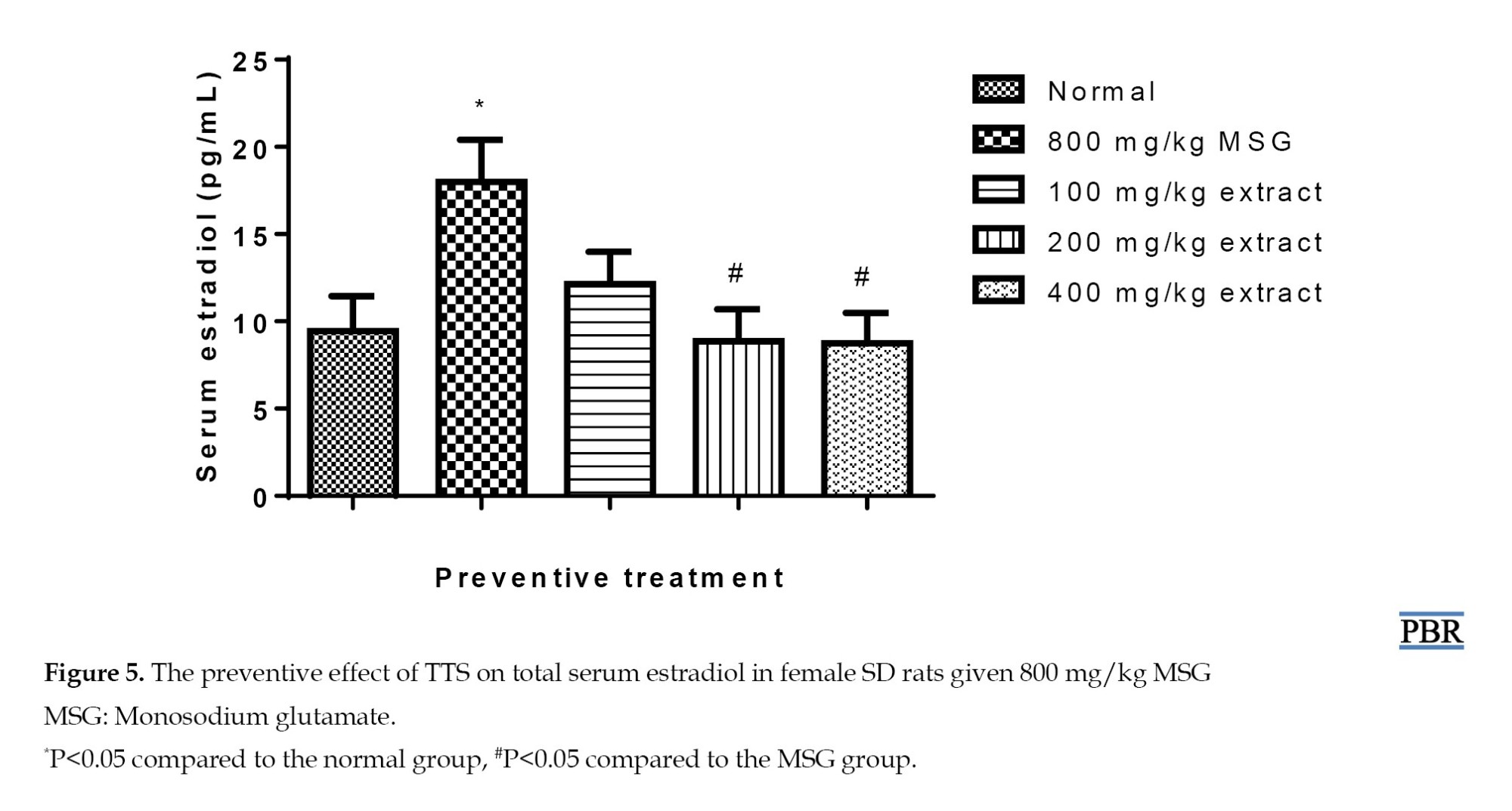
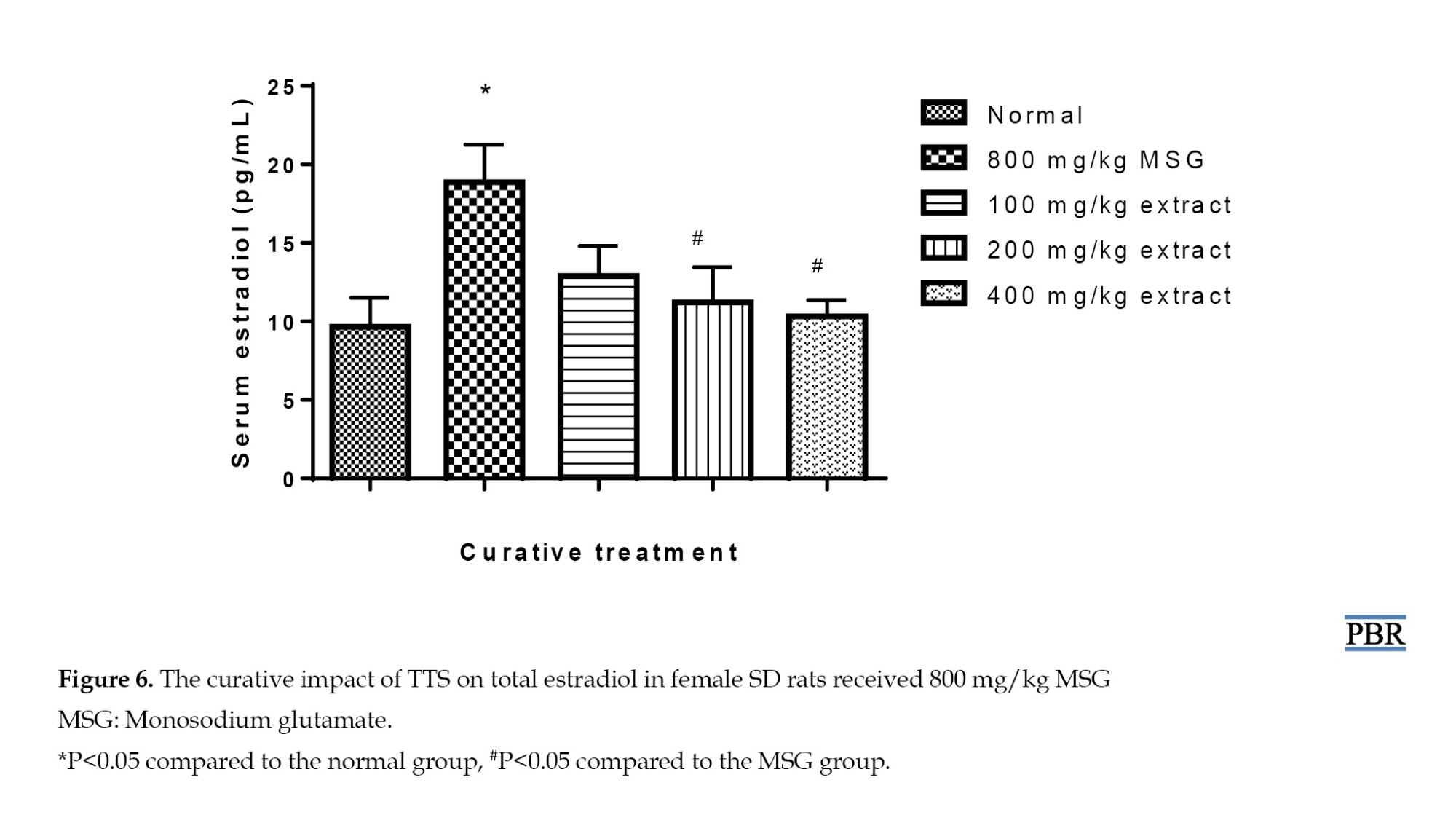
In the curative treatment, serum estradiol was significantly raised (95.23%, P≤0.05) in the MSG group relative to the control. The normal group’s parameter showed elevations of 33.44% and 16.15% with 100 and 200 mg/kg doses (Figure 6), indicating a considerable reduction after pre-treating the rats with MSG and treating them with the extract in graded doses. The MSG-induced rise in estradiol was significantly inhibited at 400 mg/kg (P≤0.05). The extract brought high serum estradiol down to normal in a dose-dependent manner.
Histopathology results of the uterus
A typical layout of tissues was seen in the section of the normal uterus, which included the uterine cavity (UC), endometrial glands, endometrial stroma, and endometrial lining (EL). The sections of the female rats treated with 800 mg/kg MSG alone revealed thick bundles of spindle-shaped Ss randomly organized and crisscrossing the stroma and endometrial glands, a feature typical of leiomyoma.
Gradually increasing doses of TTES administered concurrently with MSG appeared to slow the proliferation of fibroid cells. At 200 and 400 mg/kg, there was little evidence of the thick band of smooth muscle fibers (SFs) showing the development of leiomyoma, and normal tissue layout was primarily detected, indicating that the extract was active in the preventive experiment (Figure 7). Curative treatment still revealed some fibroid cells at 400 mg/kg.
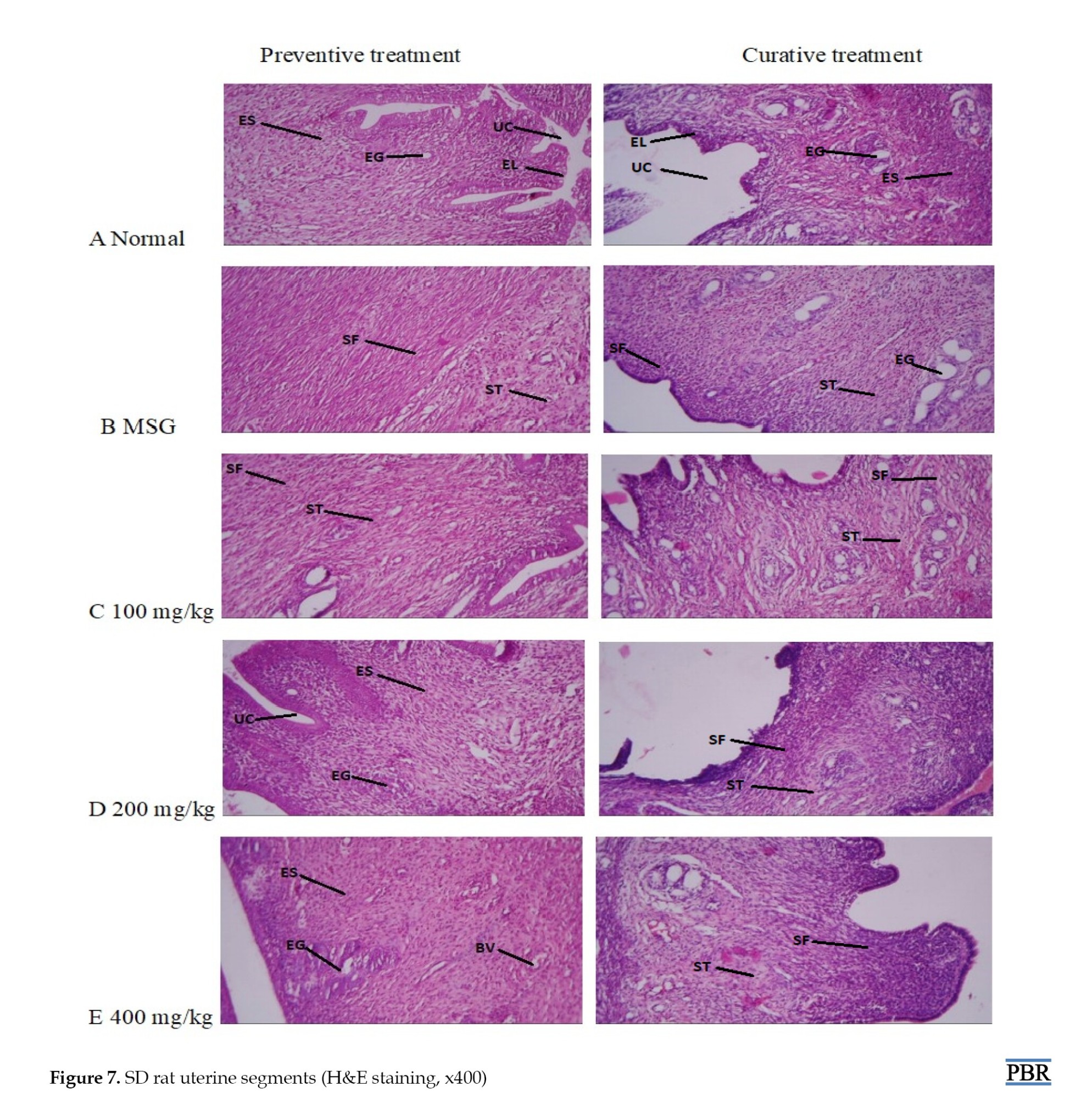
The UC, EL, endometrial stroma (ES), and endometrial glands (EG) are visible in panel A, which depicts the typical architecture of the uterus. Panel B shows MSG-only treatment of the rat uterine wall made up of stroma (ST) and endometrial glands (EG); this structure is typical of leiomyoma and is surrounded by spindle-shaped, proliferating bundles of SF. Panel C displays the 100 mg/kg treated group, which comprises less spindle-shaped proliferating SF bundles in both treatments and endometrial stroma (ST). Panel D comprises stroma (ES) with a minor form of proliferating SF bundles at 200 mg/kg in the curative treatment, and preventive treatment shows relatively normal cells. Panel E shows a 400 mg/kg treated group, comprising endometrial stroma (ES) blood vessel (BV) in the preventive group, which indicates primarily normal uterine architecture; curative treatment reveals mild form of proliferating bundles (SF).
HPLC analysis
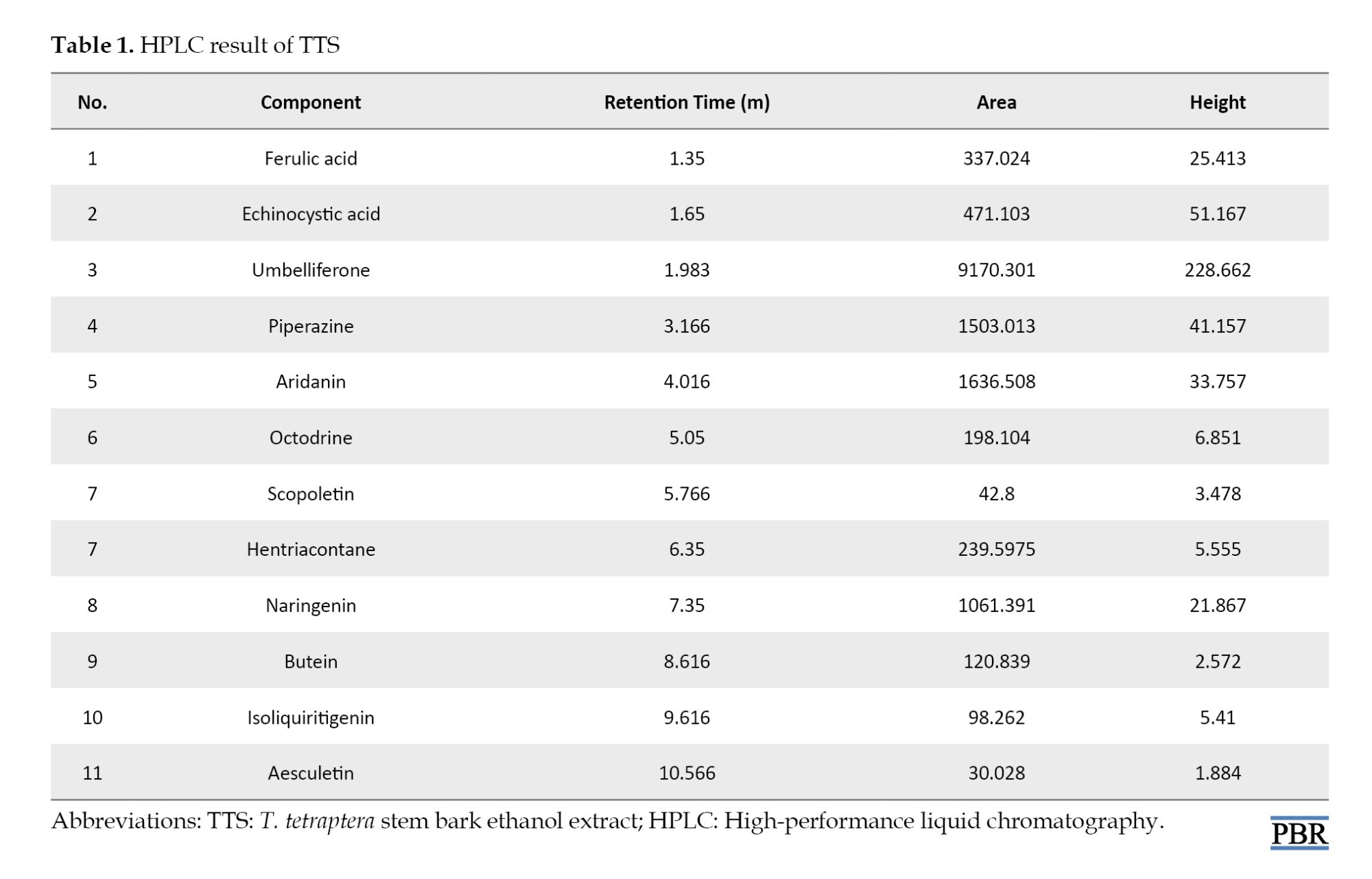
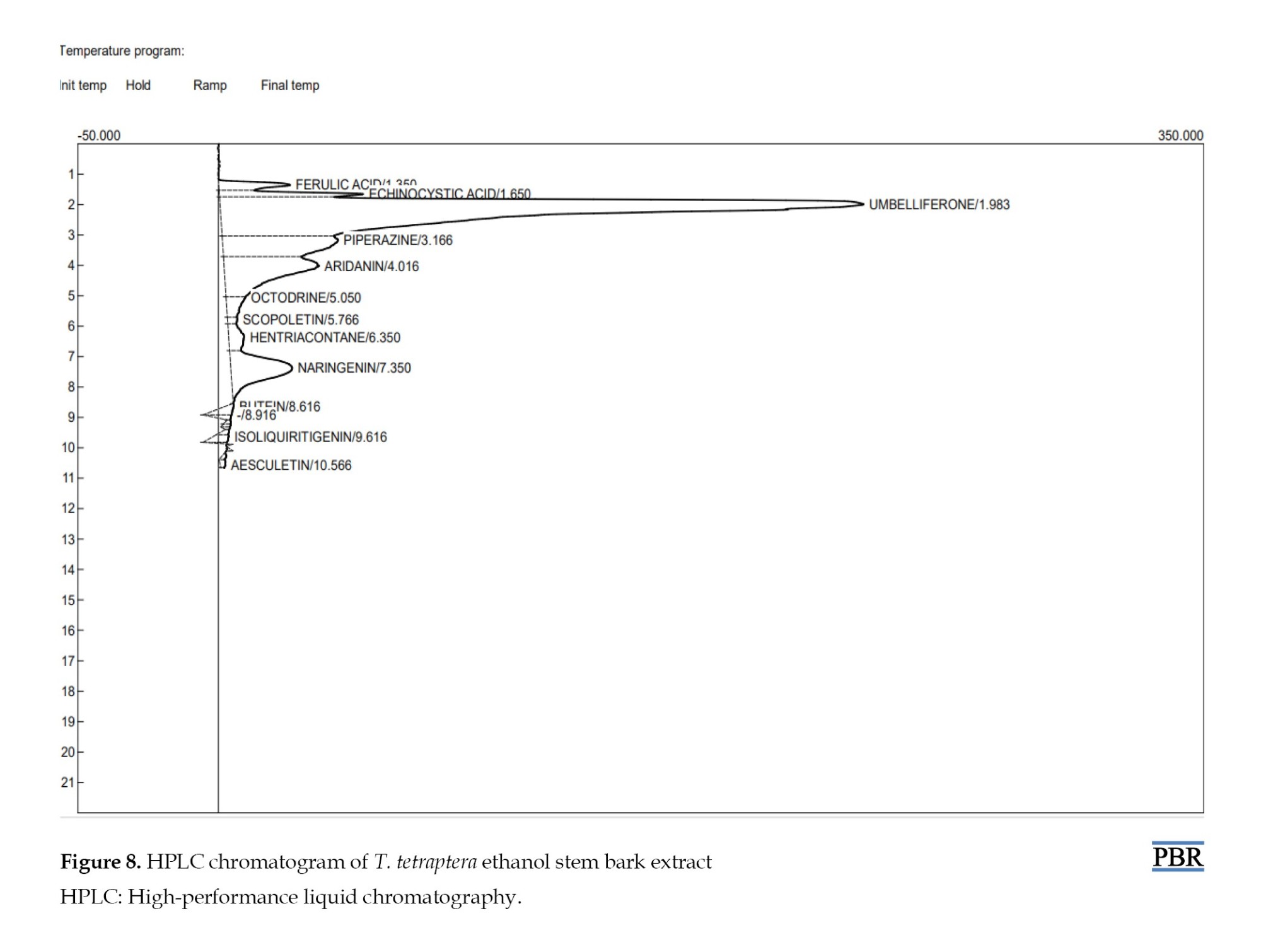
HPLC evaluation of TTS revealed the presence of major umbelliferone, piperazine, aridanin, and naringenin. Other phenolic compounds, such as echinocystic acid, ferulic acid, octodrine, hentriacontane, butein, are present but in smaller proportions (Table 1).
An HPLC chromatogram displaying the different peaks of the chemicals that were identified is presented in Figure 8.
Discussion
This study looked at the possible benefits of TTS in preventing/curing uterine fibroids in rat models. Several steroid hormones, potent signaling molecules that control a range of bodily processes, are produced with the help of cholesterol [14]. The enzyme 3-hydroxyl-3-methoxylglutamyl-CoA reductase (HMGR), which catalyzes the rate-limiting step in the manufacture of cholesterol by converting HMG-CoA to mevalonate, is often responsible for an increase in total blood cholesterol [9].
Ovarian steroid hormones are significant molecular markers connected to the emergence and proliferation of uterine fibroids. Estrogen has been largely linked to the development and creation of uterine fibroids, although progesterone and its receptors may also play a significant role [15]. Estradiol is unusual in that it can stimulate the proliferation of uterine cells because it binds to ERα receptors in the uterus and forms a complex that interacts with DNA in the nucleus to activate transcriptional promoter and enhancer regions that drive gene expression. This condition facilitates the binding of RNA polymerase II and the subsequent initiation of transcription, leading to the synthesis of proteins and increased proliferation of ovarian and uterine cells [15].
TTS is non-toxic at an oral dose of 5000 mg/kg. Therefore, this work investigated it at graded doses of 100, 200, and 400 mg/kg for its potential capacity to block MSG impact on specific biological markers measured as well as the production of fibroid cells in the uterus. Serum cholesterol levels in the MSG group were considerably (P<0.05) higher than those of the normal group (Figure 2). Both in the curative and preventive experiments, treatment with TTS brought the increased cholesterol levels nearly to normal (P>0.05). The extract's capacity to reduce cholesterol may result from a decline in dephosphorylated HMGR levels and a negative impact on cholesterol synthesis by glucagon and adrenaline activation [6].
There was no notable change in the total protein level between the MSG, treatment, and normal groups. This finding is comparable to the outcomes of earlier studies [9, 15].
In both the curative and preventive studies, treatment with the extract lowered the increased estradiol levels in a dose-dependent way (Figure 6). Its effect on estradiol may be related to inhibition of the enzyme aromatase, which is involved in the manufacture of estradiol from cholesterol and is responsible for the aromatization of androstenedione and testosterone to estrogens [16]. Furthermore, it might include phytochemicals that act as agonists of gonadotropin-releasing hormone (GnRH), which, when persistently activated, reduce or downregulate the production of GnRH receptors on the anterior pituitary. Another possible cause is a liver microsomal enzyme inducer, which speeds up the metabolism of estradiol [16]. As a result, less estradiol would be generated. There is also the possibility that the decrease in estradiol production is related to reducing cholesterol production.
Subsequent histology studies revealed the influence of T. tetraptera on uterine fibroid cell proliferation. A section of the uterus of rats fed only food and water showed normal tissue architecture; however, after the female rats were given 800 mg/kg of MSG, the section displayed thick bands of randomly arranged, spindle-shaped SFs that crossed the endometrial glands and stroma, indicating the formation of fibroid [5]. The extract and MSG were administered concurrently at progressively higher doses. The results showed a dose-dependent, progressive improvement of the leiomyoma lesions with the highest dose resulting in the most apparent change. Compared to the curative treatment, the preventive treatment demonstrated a higher suppression of leiomyoma cell development (Figure 8). According to reports, the stem bark of T. tetraptera contains sugars, tannins, saponins, phenols, and amino acids. It also possesses a high proportion of antioxidant activity, nearly equal to ascorbic acid [17]. Activation of the immune system and a decrease in cholesterol levels are the health advantages of saponins [18]. Furthermore, research has demonstrated that the enzyme aromatase [12], which is involved in the synthesis of estrogen, is inhibited by saponin. Phenols are critical in preventing chronic illnesses because they shield key components, including proteins, lipids, and DNA, from oxidative damage induced by reactive oxidant species.
Studies indicate several antioxidants may help control and manage uterine fibroids [19]. Major compounds, including umbelliferone, piperazine, aridanin, and naringenin, were found in TTS by HPLC analysis; smaller levels of hentriacontane, ferulic acid, echinocystic acid, butein, and octodrine were also detected (Table 1). Umbelliferone, a phenylpropanoid, has been shown to have antioxidant properties and to efficiently block type 3 17β-hydroxysteroid dehydrogenase, the primary enzyme that converts 4-androstene-3,17-dione to testosterone [20]. Among its many biological functions, ferulic acid is particularly active against oxidative stress, inflammation, and fibrosis [21]. There have been reports of antiviral, anti-inflammatory, and antioxidation effects for echinocystic acid and anti-inflammatory, antioxidant, lipid-lowering properties and antiproliferative activities for naringenin, a flavanone [10]. Several pharmacological characteristics, such as anti-inflammatory and anti-oxidative actions, are possessed by butein, hentriacontane, and isoliquiritigenin [10].
Conclusion
This study aimed to investigate the use of TTS extract in treating fibroids. The results indicate that the extract, primarily when used as a preventive measure, can lower the biochemical parameters elevated by MSG in SD rats and inhibit the formation of leiomyoma cells.
Ethical Considerations
Compliance with ethical guidelines
The Research Ethics Committee of the University of Benin, Faculty of Pharmacy, authorized all protocols for utilizing animals in the experiment (Code: EC/FP/023/19)
Funding
This research was funded by the TETfund Institutional Based Research, Abuja, Nigeria (2020-2022).
Authors' contributions
Study design: Rose Osarieme Imade and Buniyamin Adesina Ayinde); Experiments: Chinaza Esther Onyebuchi, Rose Osarieme Imade and Ekene Victor Asoya; Statistical analysis and writing: Rose Osarieme Imade; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge John Obasuyi and Queen Okoro from the University of Benin Teaching Hospital, Benin City, for analyzing the serum biochemical parameters and preparing the histology slides. Their profound appreciation also goes to Gerald Eze from the Anatomy Department for his aid in assessing the histology slides.
References
Uterine fibroid is a benign smooth muscle tumor, also known as uterine myoma. However, it presents a primary global concern to the reproductive health of women [1]. It can be described as submucosal, subserosal, or intramural fibroids as it develops in different places along and inside the uterine walls or within its cavity. Its size and shape vary from a pea to a watermelon [2]. Some risk factors increase the likelihood of uterine leiomyoma: Age (women aged 20 to 44 are more vulnerable), food (it has been demonstrated that refined sugar, carbohydrates, and meals containing growth hormones raise the risk), overweight, ethnicity (compared to their Caucasian counterparts, African American women are more likely to have uterine fibroids), and consuming certain chemicals, such as monosodium glutamate (MSG) which has flavor-enhancing properties and induce fibroid formation in rat models [3, 4]. Furthermore, a person is more likely to develop uterine fibroids if she takes certain medications that may raise total protein, cholesterol, and estrogen levels [5, 6].
In many women, uterine fibroids may not exhibit symptoms, so they will not receive much clinical attention. The few who experience symptoms typically report infertility, complicated pregnancy, labor problems, lower back discomfort, pelvic fullness, and irregular uterine bleeding [2]. It is important to remember that the severity of these symptoms depends on the tumor's size and location.
High progesterone and estrogen levels increase the mitosis rate, contributing to the formation of a myoma, even if the etiology of uterine fibroid is not entirely understood [5-7]. It has been documented that progesterone and estrogen contribute to the tumor's continued growth during the menstrual cycle. Additional evidence originates from clinical findings, demonstrating that uterine leiomyoma rarely develops before the onset of menstruation and regresses after menopause. Due to the adverse effects of prolonged use, hormone replacement therapy is only beneficial for 6 to 12 months. Therefore, the usual accessible treatments for this condition are myomectomy and hysterectomy [7, 8].
More effective treatment options are required due to the side effects, expense, and duration of the symptomatic relief provided by conventional drugs. Pharmacological evaluation of some plants reveals their antifibroid potential. In a study, the aqueous extract of Spondias mombin and Aspilia africana leaves improved several parameters affected by the administration of MSG. Histopathological evaluation indicates that the treated groups are protected against leiomyoma development [4]. It has also been documented that the fruit of Emblica officinalis has phytochemicals that preserve the uterus against uterine fibroids [9]. Furthermore, morphological analysis shows that the entire Labisia pumila plant suppresses the growth of uterine fibroid tumors and triggers apoptosis on SK-UT-1 (uterine fibroid cells) [6].
Tetrapleura tetraptera Taubert is a deciduous plant in the Fabaceae family. It grows on the fringes of the rainforest region that covers West and Central Africa [10]. It raises well in the Gambia, Nigeria, Mauritania, Uganda, Mali, and Burkina Faso. The plant reaches 20–25 m in height and 1.2–3 m in girth. It is used to treat feverish diseases, infections, fibroids, postpartum contraction prevention, cholesterol control, and the promotion of breast milk supply [11, 12]. T. tetraptera stem bark has been the subject of numerous investigations, but its antifibroid activity has yet to be validated. This study was conducted to determine its preventive or curative capacities for fibroid tumors. Specifically, its effects on total cholesterol, protein, and estradiol content were evaluated. In addition, its ability to protect the uterus of Sprague-Dawley (SD) rats against MSG-induced fibroids was assessed through histopathological analysis. Finally, its phytochemical constituents were determined through high-performance liquid chromatography (HPLC) analysis.
Materials and Methods
Chemicals and reagents
The study’s reagents were all analytical graded and sourced from reliable local suppliers: MSG from Lobachem, India, ethanol from BDH Chemicals, England, AGAPPE test kit, and E2 AccuBind ELISA Kit.
Plant collection and preparation
The stem bark of T. tetraptera, collected in Ikire, Osun state, Nigeria, was validated at the Forest Research Institute of Nigeria (FRIN) with herbarium number FHI 113604. Absolute ethanol was used to extract the dried and crushed bark using a Soxhlet apparatus. A rotary evaporator was employed to dry the extract completely.
Animal handling conditions
The Department of Pharmacology at the Faculty of Pharmacy, University of Benin, Nigeria, supplied non-pregnant SD rats weighing between 160 and 200 g. The animals were grouped in fives in plastic cages with beddings made of shavings from wood and a wire screen top for aeration. Commercial rat pellets and clean water were given to the animals ad libitum. Guidelines issued by the National Institutes of Health for the Use and Treatment of Laboratory Animals were followed when handling the animals.
Acute toxicity test
Lorke’s method was used to assess the oral median lethal dosage (LD50) of T. tetraptera stem bark extract (TTS) [13]. The extract was given orally to three groups of rats in the first phase at 10, 100, and 1000 mg/kg, respectively. In the second phase, 1600, 2900, and 5000 mg/kg of the extract were given orally to three groups of one rat each. Throughout 24 hours, the animals in both phases were watched for indications of tremor, diarrhea, writhing, and mortality. This phase concluded with the determination of the stem bark extract’s LD50.
Dosing of experimental animals
This study used three doses of TTS (100, 200, and 400 mg/kg) chosen based on the stem barks LD50, which was more than 5000 mg/kg. The SD rats’ weekly body weight was used to determine the dose they received once a day. Since humans typically use the oral route, this route was chosen for administration.
Antifibroid effect of TTS
Preventive study
In this study, female SD rats were divided into five groups of five rats each. The 1st group received only food and water, the second group received 800 mg/kg MSG, and the third, fourth, and fifth groups received TTS with different doses: 100, 200, and 400 mg/kg, respectively. The method described in the literature [5] was followed. Throughout 30 days, all treatments were administered simultaneously. On the 31st day, the animals were sacrificed by anesthetizing them in a chamber saturated with chloroform, and their blood was drawn via cardiac puncture and placed in plain bottles for the measurement of total plasma cholesterol, protein, and estradiol content. The uteri were removed surgically and placed into sterile tissue containers having 10% neutral buffer formalin for assessment of histopathology.
Curative study
Female SD rats were given 800 mg/kg MSG for 30 days to cause uterine fibroid development, based on the procedure outlined in published research [5]. Each set of five rats in the housing was designated as a group. On the 31st day, the extract treatment began. Group A was the control group throughout the experiment, given food and water only without MSG. From day 30 to day 60, Group B received nothing but food and water following their 30-day administration of 800 mg/kg MSG. After the 30-day administration of MSG, groups C through E were treated with 100, 200, and 400 mg/kg of TTS, respectively, from day 31 to day 60. To measure the total plasma cholesterol, protein, and estradiol content, the animals were sacrificed by anesthetizing them in a chamber saturated with chloroform, and their blood was drawn by cardiac puncture into plain bottles. The uteri were removed surgically and placed in sterile containers with 10% neutral buffer formalin for assessment by histopathology.
Biochemical assays
Determination of total cholesterol content
The animals’ blood was taken one day after the conclusion of the administration (30 days for preventive treatment and 60 days for curative treatment), and the serum was separated for the experiment by centrifugation at 3000 rpm for 10 minutes.
The Agappe test kit and the Mindray BA-88A Reagent system, a semi-automated chemical analyzer, were used to determine the total cholesterol content. Using the data from the instruction manual, the semi-automated biochemistry analyzer was set to run the total cholesterol kit. A micro-tube labeled “blank” was filled with 1000 µL of cholesterol biuret reagent. In a tube labeled «standard,» 10 µL of standard cholesterol and 1000 µL of the reagent were added and thoroughly mixed. A tube labeled «A1» was filled with 10 µL of sample A1 and 1000 µL of cholesterol reagent, and the combination was well mixed. The remaining samples (A1-E5) were processed similarly in the corresponding tubes. Samples from both the curative and preventive studies were treated in this manner. Every tube was incubated at 37 °C for 10 minutes. Following the incubation period, the absorbance of the blank tube and standard tubes’ absorbance were measured by aspirating their respective contents into the analyzer’s flow cell. The absorbance for each sample was then measured by aspirating the reaction mixture into the flow cell. Each tube’s absorbance readings were noted appropriately [9].
Determination of total protein content
The kit was programmed on the semi-automated biochemistry analyzer using the data from the instruction booklet. To a micro-tube designated “blank,” 1000 µL of total protein biuret reagent was introduced. A tube labeled «standard» was filled with 20 µL of standard total protein and 1000 µL of the reagent, then thoroughly mixed. Sample A1 (20 µL) and total protein reagent (1000 µL) were added to and thoroughly mixed in a tube labeled «A». The exact process was applied to the remaining samples (A1-E5) from the curative and preventive tests in their corresponding tubes. Every tube was incubated at 37 ˚C for 10 minutes. Following the incubation period, the absorbance of the blank and standard tubes was measured by aspirating their respective contents into the analyzer’s flow cell. To quantify the absorbance, the reaction mixture for each sample was aspirated into the flow well after that. Each tube’s absorbance value was noted appropriately [9].
Determination of estradiol content
The E2 AccuBind ELISA Kit, the Mindray MR-96A microplate reader, and the Mindray MW-12A microplate washer were used to measure estradiol. Pipetting 25 µL of the serum reference and 25 µL of each sample (A1-E5) into the designated wells was followed by adding 50 µL of estradiol biotin reagent to each well. After gently swirling the plates for approximately 30 seconds, they were incubated for 30 minutes. Subsequently, 50 µL of estradiol enzyme reagent was added to each well, and the plates were left to incubate for 90 minutes at room temperature. Decanting the contents of the microplate was followed by drying the plates with absorbent paper. Then, 350 µL of the wash buffer was added thrice, and the content was decanted. Following the addition of 100 µL of substrate and 20 minutes of incubation, 50 µL of the stop solution was added to each well and gently mixed for an additional 20 minutes. Within 15 minutes of administering the stop solution, the absorbance at 450 nm was measured. From a dosage response curve, the amount of estradiol in the samples was extrapolated [9].
Histopathology studies of the uterus
For histological analysis, the animal’s uterus was stored in a 10% neutral buffered formalin solution. After being dehydrated in increasing alcohol grades (70%, 90%, 96%, and 100%), the tissue was cleared with xylene, saturated with melted paraffin wax, and sectioned into slides. These sections (4-5 μm thick) were stained with hematoxylin after dewaxing with xylene and hydrating in descending degrees of alcohol (100%, 96%, 70%) and water. Following 1% acid alcohol differentiation, eosin was used as a counterstain on the sections. Before being examined under a microscope, the slices were once more dehydrated in increasing grades of alcohol, cleaned in xylene, and mounted with polystyrene made of dibutyl phthalate using coverslips [5].
Polyphenolic compounds identification by HPLC-DAD
The HPLC technique was performed at the Bato Chemical Laboratory in Lagos, Nigeria. This HPLC was made by Shimadzu (Nexera mx). The ubondapak C18 reverse-phase chromatographic column was utilized. Its dimensions were as follows: Length of 100 mm, internal diameter of 4.6 mm, and thickness of 7 µm. Acetonitrile/water (70:30) was the mobile phase used. The HPLC system was attached to a UV–Vis Diode array detector (DAD) set at an analytical wavelength of 254 nm within the UV–Visible region. The used pump pressure was 15 MPa. The HPLC system was initially injected with standard solutions to produce a chromatogram with a specified peak and peak profile. These were employed to make a window to analyze the test sample. Also, 5 µL of extract was injected into the HPLC system at a constant flow rate of 2 mL/min to create a chromatogram with corresponding peak areas and profiles.
Data analysis
GraphPad Prism software for Windows, version 6.01, was used to create bar graphs, and data were subjected to one-way analysis of variance (ANOVA) using the Tukey-Kramer multiple comparison test. In all analyses, P≤0.05 was deemed statistically significant.
Results
Acute toxicity test
TTES showed no mortality or evidence of toxicity during both phase 1 and phase 2 of the acute toxicity test; normal eating habits, fecal matter, movements, and sleep were observed. The lethal median dose of the extract was estimated to be greater than 5000 mg/kg.
Total serum cholesterol in preventive and curative studies
An increase in blood cholesterol was seen (56.28%, P≤0.05) in the group that received MSG alone in the preventive experiment. This effect was seen less when the extract and MSG were given together at 100 and 200 mg/kg. There was only a 25.68%–32.46% increase in blood cholesterol over the control (P≥0.05) (Figure 1). Following MSG-induced uterine leiomyoma formation, serum cholesterol levels were raised (45.10%).

However, this parameter was reduced by the curative treatment using varying doses of the extract, with a 21.24% rise observed at 100 mg/kg. Higher concentrations of 200 and 400 mg/kg further reduced this parameter to within the normal range (P≥0.05) (Figure 2).

Total protein content in preventive and curative studies
There was no obvious (P≥0.05) alteration in blood protein between the normal rats, the MSG-only, and MSG and extract-treated groups (Figure 3).

Additionally, pretreatment of normal rats with MSG did not result in a statistically significant increase in blood protein levels (Figure 4).

Total serum estradiol in preventive and curative studies
Treatment with MSG resulted in a highly significant increase in estradiol (90.98%, P≤0.05). Concurrent administration of MSG and extract at different doses decreased hormone levels, where a 28.77% increase was seen at 100 mg/kg. At 200 and 400 mg/kg, the estradiol content was reduced to within the normal range (P≥0.05) (Figure 5), with a significant difference between the MSG and the 400 mg/kg group (P≤0.05).

In the curative treatment, serum estradiol was significantly raised (95.23%, P≤0.05) in the MSG group relative to the control. The normal group’s parameter showed elevations of 33.44% and 16.15% with 100 and 200 mg/kg doses (Figure 6), indicating a considerable reduction after pre-treating the rats with MSG and treating them with the extract in graded doses. The MSG-induced rise in estradiol was significantly inhibited at 400 mg/kg (P≤0.05). The extract brought high serum estradiol down to normal in a dose-dependent manner.

Histopathology results of the uterus
A typical layout of tissues was seen in the section of the normal uterus, which included the uterine cavity (UC), endometrial glands, endometrial stroma, and endometrial lining (EL). The sections of the female rats treated with 800 mg/kg MSG alone revealed thick bundles of spindle-shaped Ss randomly organized and crisscrossing the stroma and endometrial glands, a feature typical of leiomyoma.
Gradually increasing doses of TTES administered concurrently with MSG appeared to slow the proliferation of fibroid cells. At 200 and 400 mg/kg, there was little evidence of the thick band of smooth muscle fibers (SFs) showing the development of leiomyoma, and normal tissue layout was primarily detected, indicating that the extract was active in the preventive experiment (Figure 7). Curative treatment still revealed some fibroid cells at 400 mg/kg.

The UC, EL, endometrial stroma (ES), and endometrial glands (EG) are visible in panel A, which depicts the typical architecture of the uterus. Panel B shows MSG-only treatment of the rat uterine wall made up of stroma (ST) and endometrial glands (EG); this structure is typical of leiomyoma and is surrounded by spindle-shaped, proliferating bundles of SF. Panel C displays the 100 mg/kg treated group, which comprises less spindle-shaped proliferating SF bundles in both treatments and endometrial stroma (ST). Panel D comprises stroma (ES) with a minor form of proliferating SF bundles at 200 mg/kg in the curative treatment, and preventive treatment shows relatively normal cells. Panel E shows a 400 mg/kg treated group, comprising endometrial stroma (ES) blood vessel (BV) in the preventive group, which indicates primarily normal uterine architecture; curative treatment reveals mild form of proliferating bundles (SF).
HPLC analysis
HPLC evaluation of TTS revealed the presence of major umbelliferone, piperazine, aridanin, and naringenin. Other phenolic compounds, such as echinocystic acid, ferulic acid, octodrine, hentriacontane, butein, are present but in smaller proportions (Table 1).

An HPLC chromatogram displaying the different peaks of the chemicals that were identified is presented in Figure 8.

Discussion
This study looked at the possible benefits of TTS in preventing/curing uterine fibroids in rat models. Several steroid hormones, potent signaling molecules that control a range of bodily processes, are produced with the help of cholesterol [14]. The enzyme 3-hydroxyl-3-methoxylglutamyl-CoA reductase (HMGR), which catalyzes the rate-limiting step in the manufacture of cholesterol by converting HMG-CoA to mevalonate, is often responsible for an increase in total blood cholesterol [9].
Ovarian steroid hormones are significant molecular markers connected to the emergence and proliferation of uterine fibroids. Estrogen has been largely linked to the development and creation of uterine fibroids, although progesterone and its receptors may also play a significant role [15]. Estradiol is unusual in that it can stimulate the proliferation of uterine cells because it binds to ERα receptors in the uterus and forms a complex that interacts with DNA in the nucleus to activate transcriptional promoter and enhancer regions that drive gene expression. This condition facilitates the binding of RNA polymerase II and the subsequent initiation of transcription, leading to the synthesis of proteins and increased proliferation of ovarian and uterine cells [15].
TTS is non-toxic at an oral dose of 5000 mg/kg. Therefore, this work investigated it at graded doses of 100, 200, and 400 mg/kg for its potential capacity to block MSG impact on specific biological markers measured as well as the production of fibroid cells in the uterus. Serum cholesterol levels in the MSG group were considerably (P<0.05) higher than those of the normal group (Figure 2). Both in the curative and preventive experiments, treatment with TTS brought the increased cholesterol levels nearly to normal (P>0.05). The extract's capacity to reduce cholesterol may result from a decline in dephosphorylated HMGR levels and a negative impact on cholesterol synthesis by glucagon and adrenaline activation [6].
There was no notable change in the total protein level between the MSG, treatment, and normal groups. This finding is comparable to the outcomes of earlier studies [9, 15].
In both the curative and preventive studies, treatment with the extract lowered the increased estradiol levels in a dose-dependent way (Figure 6). Its effect on estradiol may be related to inhibition of the enzyme aromatase, which is involved in the manufacture of estradiol from cholesterol and is responsible for the aromatization of androstenedione and testosterone to estrogens [16]. Furthermore, it might include phytochemicals that act as agonists of gonadotropin-releasing hormone (GnRH), which, when persistently activated, reduce or downregulate the production of GnRH receptors on the anterior pituitary. Another possible cause is a liver microsomal enzyme inducer, which speeds up the metabolism of estradiol [16]. As a result, less estradiol would be generated. There is also the possibility that the decrease in estradiol production is related to reducing cholesterol production.
Subsequent histology studies revealed the influence of T. tetraptera on uterine fibroid cell proliferation. A section of the uterus of rats fed only food and water showed normal tissue architecture; however, after the female rats were given 800 mg/kg of MSG, the section displayed thick bands of randomly arranged, spindle-shaped SFs that crossed the endometrial glands and stroma, indicating the formation of fibroid [5]. The extract and MSG were administered concurrently at progressively higher doses. The results showed a dose-dependent, progressive improvement of the leiomyoma lesions with the highest dose resulting in the most apparent change. Compared to the curative treatment, the preventive treatment demonstrated a higher suppression of leiomyoma cell development (Figure 8). According to reports, the stem bark of T. tetraptera contains sugars, tannins, saponins, phenols, and amino acids. It also possesses a high proportion of antioxidant activity, nearly equal to ascorbic acid [17]. Activation of the immune system and a decrease in cholesterol levels are the health advantages of saponins [18]. Furthermore, research has demonstrated that the enzyme aromatase [12], which is involved in the synthesis of estrogen, is inhibited by saponin. Phenols are critical in preventing chronic illnesses because they shield key components, including proteins, lipids, and DNA, from oxidative damage induced by reactive oxidant species.
Studies indicate several antioxidants may help control and manage uterine fibroids [19]. Major compounds, including umbelliferone, piperazine, aridanin, and naringenin, were found in TTS by HPLC analysis; smaller levels of hentriacontane, ferulic acid, echinocystic acid, butein, and octodrine were also detected (Table 1). Umbelliferone, a phenylpropanoid, has been shown to have antioxidant properties and to efficiently block type 3 17β-hydroxysteroid dehydrogenase, the primary enzyme that converts 4-androstene-3,17-dione to testosterone [20]. Among its many biological functions, ferulic acid is particularly active against oxidative stress, inflammation, and fibrosis [21]. There have been reports of antiviral, anti-inflammatory, and antioxidation effects for echinocystic acid and anti-inflammatory, antioxidant, lipid-lowering properties and antiproliferative activities for naringenin, a flavanone [10]. Several pharmacological characteristics, such as anti-inflammatory and anti-oxidative actions, are possessed by butein, hentriacontane, and isoliquiritigenin [10].
Conclusion
This study aimed to investigate the use of TTS extract in treating fibroids. The results indicate that the extract, primarily when used as a preventive measure, can lower the biochemical parameters elevated by MSG in SD rats and inhibit the formation of leiomyoma cells.
Ethical Considerations
Compliance with ethical guidelines
The Research Ethics Committee of the University of Benin, Faculty of Pharmacy, authorized all protocols for utilizing animals in the experiment (Code: EC/FP/023/19)
Funding
This research was funded by the TETfund Institutional Based Research, Abuja, Nigeria (2020-2022).
Authors' contributions
Study design: Rose Osarieme Imade and Buniyamin Adesina Ayinde); Experiments: Chinaza Esther Onyebuchi, Rose Osarieme Imade and Ekene Victor Asoya; Statistical analysis and writing: Rose Osarieme Imade; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge John Obasuyi and Queen Okoro from the University of Benin Teaching Hospital, Benin City, for analyzing the serum biochemical parameters and preparing the histology slides. Their profound appreciation also goes to Gerald Eze from the Anatomy Department for his aid in assessing the histology slides.
References
- Zakaria N, Mohd KS, Ahmed Saeed MA, Ahmed Hassan LE, Shafaei A, Al-Suede FSR, et al. Anti-uterine fibroid effect of standardized Labisia Pumila Var. Alata extracts in vitro and in human uterine fibroid cancer xenograft model. Asian Pacific J Cancer Prev. 2020; 21(4):943-51. [DOI:10.31557/APJCP.2020.21.4.943] [PMID]
- Navarro A, Bariani MV, Yang Q, Al-Hendy A. Understanding the impact of uterine fibroids on human endometrium function. Front Cell Dev Biol. 2021; 9:633180. [DOI:10.3389/fcell.2021.633180] [PMID]
- Uimari O, Nazri H, Tapmeier T. Endometriosis and uterine fibroids (leiomyomata): Comorbidity, risks and implications. Front Reprod Health. 2021; 3:750018. [DOI:10.3389/frph.2021.750018] [PMID]
- Eze-Steven PE, Udedi SC, Ude CM. Effects of Spondias mombin and Aspilia Africana aqueous extracts on rats with monosodium glutamate-induced leiomyoma. Cross Curr Int J Med Biosci. 2019; 1:26-32. [Link]
- Ezejiofor TI, Okoroafor CH. Effects of ethanol extracts of Diodia sarmentosa leaves on biochemical and histopathological indices of monosodium glutamate-induced uterine leiomyoma in rats. Biomed Res Ther. 2022; 9(7):5140-8. [Link]
- Zakaria N, Mohd KS, Saeed MA, Hassan LE, Shafaei A, Al-Suede F, et al. Anti-uterine fibroid effect of standardized Labisia Pumila Var. Alata extracts in vitro and in human uterine fibroid cancer xenograft model. Asian Pac J Cancer Prev. 2020; 21(4):943-51. [DOI:10.31557/APJCP.2020.21.4.943]
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G. Uterine fibroids: An update on current and emerging medical treatment options. Ther Clin Risk Manag. 2019; 15:157-78. [DOI:10.2147/TCRM.S147318] [PMID]
- Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q. 1,25 Dihydroxyvitamin D3 enhances the antifibroid effects of ulipristal acetate in human uterine fibroids. Reprod Sci. 2019; 26(6):812-28. [DOI:10.1177/1933719118812720] [PMID]
- Ahmed I, Ahmed N, Ahmed S, Ahmad F, Al-Subaie AM. Effect of emblica officinalis (Amla) on monosodium glutamate (MSG) induced uterine fibroids in wistar rats. Res J Pharm Technol. 2020; 13(6):2535-9. [DOI:10.5958/0974-360X.2020.00451.5]
- Adesina S, Iwalewa E, Johnny I. Tetrapleura tetraptera taub- ethnopharmacology, chemistry, medicinal and nutritional values- A Review. J Pharm Res Int. 2016; 12(3):1-22. [DOI:10.9734/BJPR/2016/26554]
- Adebisi MA. Ethnobotany survey of medicinal plants used in the treatment of fibroid in Ogun and Osun States, southwestern, Nigeria. J Res For Wildl Environ. 2019; 11:33-44. [Link]
- Anyamele T, Onwuegbuchu PN, Ugbogu EA, Ibe C. Phytochemical composition, bioactive properties, and toxicological profile of Tetrapleura tetraptera. Bioorg Chem. 2023; 131:106288. [DOI:10.1016/j.bioorg.2022.106288] [PMID]
- Abbas MY, Ejiofor JI, Yakubu MI. Acute and chronic toxicity profiles of the methanol leaf extract of Acacia ataxacantha D.C (Leguminosae) in Wistar rats. Bull Fac Pharm Cairo Univ. 2018; 56(2):185-9. [DOI:10.1016/j.bfopcu.2018.09.001]
- Odesanmi OS, Lawal RA, Ojokuku SA. Effects of ethanolic extract of Tetrapleura tetraptera fruit on serum lipid profile and kidney function in male Dutch-white rabbits. Nig Q J Hosp Med. 2011; 21(4):299-302. [PMID]
- Yashunina M. A study of the effect of an ethanolic extract of femitol on uterine fibroid in laboratory model. J Pharmacogn Nat Prod. 2021; 7:2462. [Link]
- Koyejo OD, Rotimi OA, Abikpa EN, Bello OA, Rotimi SO. A review of the anti-fibroid potential of medicinal plants: Mechanisms and targeted signaling pathways. Trop J Nat Prod Res. 2021; 5(5):792-804. [Link]
- Famobuwa O, Lajide L, Owolabi B, Osho I, Amuho U. Antioxidant activity of the fruit and stem bark of tetrapleura tetraptera taub (Mimosaceae). J Pharm Res. 2016; 9(3):1-4. [DOI:10.9734/BJPR/2016/21462]
- Erukainure OL, Onifade OF, Odjobo BO, Olasehinde TA, Adesioye TA, Tugbobo-Amisu AO, et al. Ethanol extract of Tetrapleura tetraptera fruit peels: Chemical characterization, and antioxidant potentials against free radicals and lipid peroxidation in hepatic tissues. J Taibah Univ Sci. 2017; 1196):861-7. [DOI:10.1016/j.jtusci.2017.03.007]
- AlAshqar A, Lulseged B, Mason-Otey A, Liang J, Begum UAM, Afrin S, et al. Oxidative stress and antioxidants in uterine fibroids: Pathophysiology and clinical implications. Antioxidants. 2023; 12(4). [Link]
- Mazimba O. Umbelliferone: Sources, chemistry and bioactivities review. Bull Fac Pharm Cairo Univ. 2017; 55(2):223-32. [DOI:10.1016/j.bfopcu.2017.05.001]
- Liu Y, Shi L, Qiu W, Shi Y. Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation. Mol Cell Toxicol. 2022; 18:509-19. [DOI:10.1007/s13273-021-00219-5]
Type of Study: Original Research |
Subject:
Pharmacognosy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






