Volume 10, Issue 4 (2024)
Pharm Biomed Res 2024, 10(4): 319-330 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Awatef E, Chaabane M, Ghorbel I, Jebahi S, Marrekchi R, Boudawara T et al . Effects of Barium Chloride on Metallothioneins Induction and Biochemical and Histomorphological Changes in the Kidney of Adult Female Rats. Pharm Biomed Res 2024; 10 (4) :319-330
URL: http://pbr.mazums.ac.ir/article-1-604-en.html
URL: http://pbr.mazums.ac.ir/article-1-604-en.html
Elwej Awatef *1 

 , Mariem Chaabane1
, Mariem Chaabane1 
 , Imen Ghorbel1
, Imen Ghorbel1 
 , Samira Jebahi2
, Samira Jebahi2 
 , Rim Marrekchi3
, Rim Marrekchi3 
 , Tahia Boudawara1
, Tahia Boudawara1 
 , Najiba Zeghal4
, Najiba Zeghal4 



 , Mariem Chaabane1
, Mariem Chaabane1 
 , Imen Ghorbel1
, Imen Ghorbel1 
 , Samira Jebahi2
, Samira Jebahi2 
 , Rim Marrekchi3
, Rim Marrekchi3 
 , Tahia Boudawara1
, Tahia Boudawara1 
 , Najiba Zeghal4
, Najiba Zeghal4 

1- Animal Physiology Laborator, Sfax Faculty of Sciences, Sfax University, Sfax, Tunisia.
2- Biochemistry Laboratory, CHU Hedi Chaker, University of Sfax, Sfax, Tunisia.
3- Anatomopathology Laboratory, CHU Habib Bourguiba, Sfax University, Sfax, Tunisia.
4- Animal Physiology Laboratory. Sfax Faculty of Sciences.BP1171, 3000 Sfax. Sfax University, Tunisia
2- Biochemistry Laboratory, CHU Hedi Chaker, University of Sfax, Sfax, Tunisia.
3- Anatomopathology Laboratory, CHU Habib Bourguiba, Sfax University, Sfax, Tunisia.
4- Animal Physiology Laboratory. Sfax Faculty of Sciences.BP1171, 3000 Sfax. Sfax University, Tunisia
Full-Text [PDF 1344 kb]
(504 Downloads)
| Abstract (HTML) (1694 Views)
Full-Text: (604 Views)
Introduction
During the last years, our environment as well as our health have been exposed to various toxicants, and the rapid growth of industry has led to large amounts of industrial wastes that discharging into soil and water. Environmental pollutants can induce oxidative stress by overwhelming the body’s antioxidant defenses [1] with excessive free radical production leading to cellular and tissue damage. Cells have developed enzymatic and non-enzymatic antioxidant systems to convert reactive oxygen species into harmless metabolites, working together to prevent damage caused by pesticides [2] and metal exposure [3]. Barium, a dense alkaline earth metal found as divalent cations in various salts [4] is released into the environment through natural events and industrial processes. This contamination affects surface waters, soils, foods, and the food chain in both rural and urban areas [4, 5]. Barium is extensively used in various industries, such as plastics, electronics, textile pharmaceuticals and cosmetics [6]. Studies have shown that barium chloride (BaCl2) can lead to toxicity in various soft tissues [7, 8] and potentially increase the risk of congenital heart defects [9] and orofacial clefts [10] in offspring.
Since the kidneys play many vital functions to maintain normal homeostasis in the body, alterations in renal cells can promote the formation of several sufferings of people worldwide. The kidney is highly vulnerable to drugs and environmental chemicals due to high blood flow and oxygen requirement via the circulation leading to a high risk of nephrotoxicity [11-13]. Liu et al. [14] report that barium may impair the human kidney with renal injury being more prominent in women. This dysfunction could be attributed to the adverse effects of barium on female reproductive hormones [15] since it plays a pivotal role in protecting the kidney [16]. Furthermore, alterations in renal functional capacity have been also reported in a case of barium poisoning of patients treated with intravenous barium sulfate where renal tubules are obstructed leading to necrosis [17, 18]. Accordingly, this study evaluates the nephrotoxicity induced by BaCl2 administered orally to adult rats for 21 consecutive days at different doses with a view of the systemic oxidative stress, biochemical parameters, and histopathological changes.
Materials and Methods
Study chemicals
BaCl2 was obtained from Loba Chemie (Mumbai, India). All experiments were performed using other reagents of analytical grade purchased from standard commercial suppliers.
Animals and experimental protocols
A total of 24 female rats weighing from 168 to 179 g, were purchased from the Central Pharmacy (SIPHAT, Tunisia). They were reared under standard laboratory conditions (temperature 22±2 °C, relative humidity 40%, and 12-h light/dark cycle). A commercial pellet diet and tap water were provided ad libitum. After sufficient time, the rats were randomly allocated into four groups (n=6): the first group served as a control and received distilled water. Group 2 received 67 parts per million (ppm) of barium (D1), group 3 received 150 ppm of barium (D2), and Group 4 received barium at a dose of 300 ppm (D3). The barium doses used in the experiment were determined based on our previous study [19] and they induced toxicity without mortality.
Sampling procedure
On the 20th day, the rats were transferred to individual metabolic cages for a 24-h urine collection. Subsequently, urinary samples were collected in bottles and the volume of each sample was recorded then centrifuged for 5 min at 5000 g.
At the end of the 21-day study period, the animals were sacrificed by cervical decapitation. Whole blood samples were collected into serum vacutainer tubes, then centrifuged at 2200 ×g for 10 min at 4 °C and finally stored at -80 °C until biochemical analysis. The kidneys were removed from the abdominal cavities, washed in ice-cold, and frozen immediately until further analysis. To evaluate oxidative stress, some kidney was cut into small pieces, homogenized in appropriate phosphate buffer (10 mM, pH=7.4), and then subjected to centrifugation. Aliquots of supernatant were kept at -80 °C for further biochemical assays. Other kidneys were collected, and fixed in 10% neutral formaldehyde for histological examination.
Biochemical analysis
Determination of kidney protein content
Total protein content in kidney homogenates was determined according to the method by Lowry et al. [20], using bovine serum albumin as standard.
Biochemical assay
The levels of urea, uric acid, and creatinine in plasma and urine were estimated spectrophotometrically using commercial diagnostic kits (ref 20151, 20143, 20092; Biomaghreb, Tunisia). Creatinine clearance, an index of glomerular filtration rate was calculated by the UV/P equation [21], where U was the urinary creatinine level, V was the volume of a urine sample collected within 24 h, and P was the plasma creatinine concentration.
Measurement of kidney oxidative stress and antioxidant defence levels
Lipid peroxidation assay was estimated by following the method of Draper and Hadley [22]. Absorbance was measured at 532 nm and results were expressed as nM of TBARS per milligram protein. Hydrogen peroxide (H2O2) was carried out by the method of Ou and Wolff [23]. The amount of H2O2 was determined at 560 nm and values were expressed as µmoles per milligram of protein. Advanced oxidation protein product (AOPP) levels were evaluated by the method of Kayali et al. [24]. The absorbance was recorded at 340 nm and the results were expressed as nanomoles per milligram protein.
Kidney glutathione (GSH) content was performed according to the method of Ellman [25] and modified by Jollow et al. [26]. The absorbance was measured at 412 nm and total GSH content was expressed as micrograms per milligram of protein. Non-protein thiols (NPSH) kidney tissue levels were determined according to the method of Ellman [25]. Absorbance was measured at 412 nm and the results were expressed as nanomoles per milligram of protein. Metallothionein (MT) content in the kidney was measured spectrophotometrically following the protocol of Viarengo et al. [27] and modified by Petrovic et al. [28]. Ascorbic acid (vitamin C) content in kidney tissue was assayed by the dinitrophenylhydrazine method described by Jacques-Silva et al. [29] and results were expressed as µmoles per milligram of protein. Superoxide dismutase (SOD) activity was performed according to Beauchamp and Fridovich [30] and results were expressed as enzyme units per milligram of protein. Meanwhile, catalase (CAT) activity was assayed by the method of Aebi [31] and the activities were expressed as micromoles of H2O2 consumed per min per milligram of protein. Glutathione peroxidase (GPx) activity was performed according to the method of Flohe and Gunzler [32] and the enzyme activity was expressed as moles of GSH oxidized per min per mg protein.
Plasma and kidney lactate dehydrogenase (LDH) activities
Plasma and kidney LDH activities, were determined using commercial reagent kits (Biomagreb, Ariana, Tunisia, Ref 20012).
Histopathological analysis
After their fixation in neutral formaldehyde, the kidney was processed using a graded ethanol series and embedded in paraffin blocks. Hematoxylin–Eosin (H&E) staining was applied to 5 mm thick sections and examined under light microscopy (ZEISS, Axiolab) using a Canon PowerShot camera (model A640). All sections were evaluated for the degree of tubular and glomerular injury and necrosis.
Statistical analysis
The results were analyzed using GraphPad Prism software, version 9.2 for Windows (GraphPad Software, San Diego, CA). The statistical analysis was performed by one-way analysis of variance followed by the Tukey post hoc test. In all statistical analyses, differences were considered significant for P<0.05.
Results
General assessment
In the experimental investigation, the general condition of rats following the oral administration of BaCl2 was changed. Rats subjected to graded doses of BaCl2 (67, 150, and 300 ppm) displayed mild to moderate alterations and exhibited symptoms, such as abdominal pain and increased urination. However, there were no fatalities recorded in all groups during the experimental period.
Effect on renal function
Table 1 exhibits data concerning kidney oxidative stress analyses (creatinine, urea, and uric acid levels).
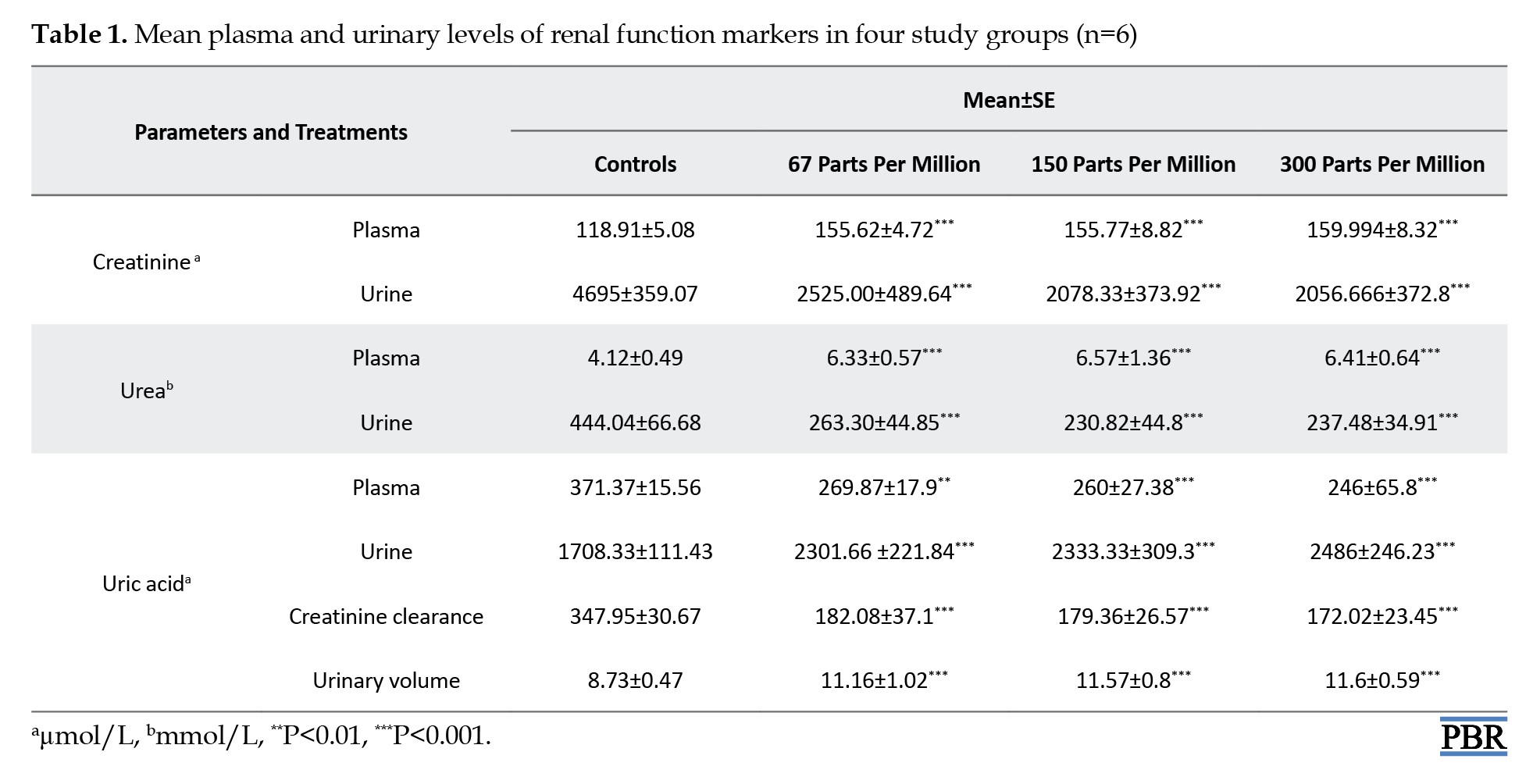
The results showed that barium administration at graded doses (67, 150, and 300 ppm) led to a significant increase in creatinine and urea levels in plasma as +30%, +31% and +34%, and 53%, +57% and +54% and a decrease in urine as ‒46%, ‒55%, and ‒56% and ‒40%, ‒48%, and ‒46%, respectively, as compared to controls. On the other hand, uric acid levels exhibited a dose-dependent pattern, with decreases of ‒27%, ‒30%, and ‒34% in plasma and increases of +34%, +37%, and +45% in urine, when compared with controls. Furthermore, additionally, compared to controls, rats treated with BaCl2 showed disorders in renal function which are manifested by an increase in urine volume (+27%, +32%, +32%) and a decrease in creatinine clearance (-47%, -48% and -50%) considered as an indicator of glomerular dysfunction.
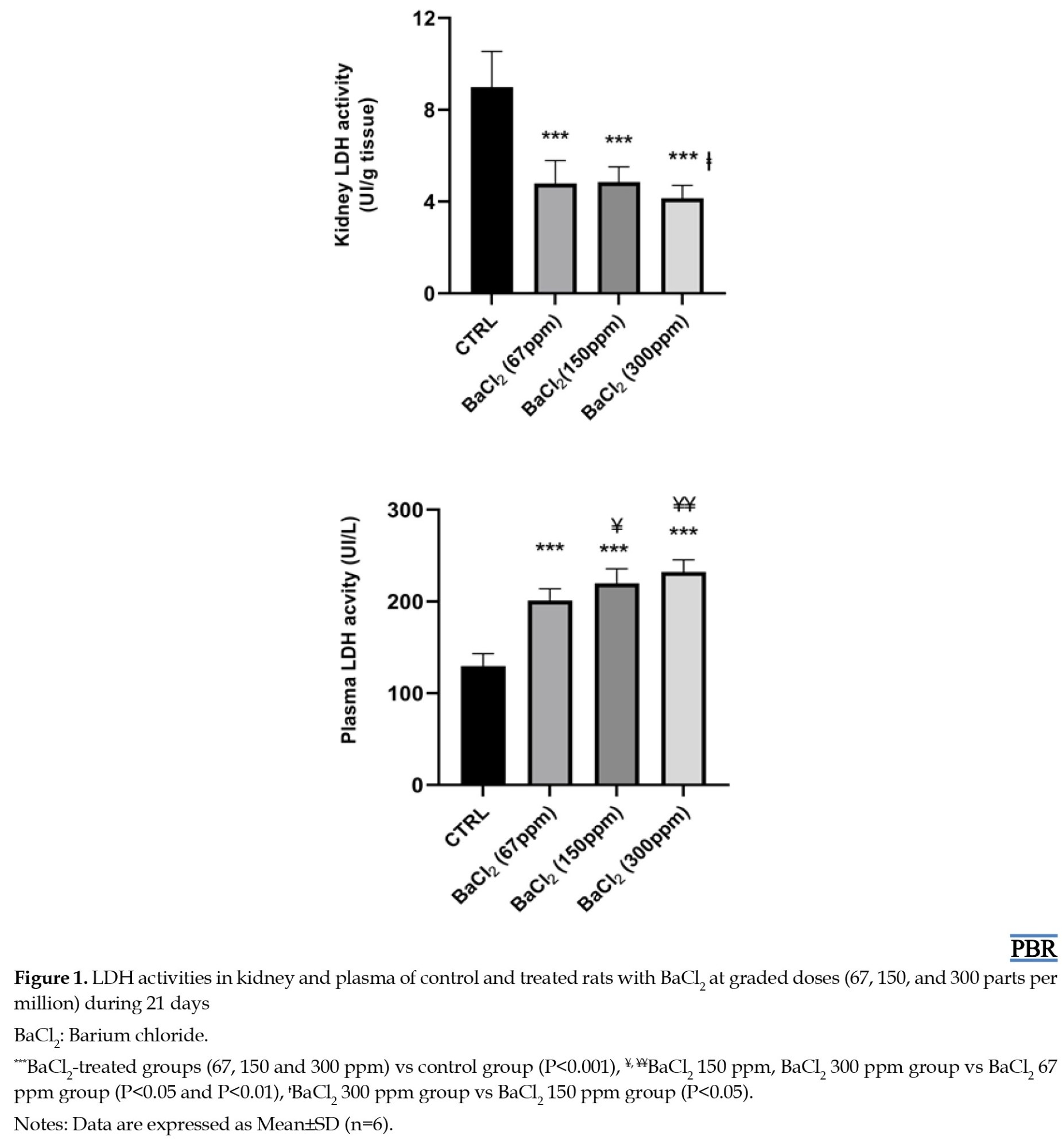
LDH activities in plasma and kidney homogenates
Activities of LDH in the different groups of rats are illustrated in Figure 2. The results showed statistically significant increases in plasma LDH activity by 55%, 70%, and 79% and decrease in kidney LDH activity by 46%, 46%, and 54%, were observed in the three groups treated with BaCl2 (doses=67, 150, and 300 ppm, respectively) as compared to control rats.
Effect on renal oxidative stress and antioxidant enzymes
The effect of BaCl2 on renal oxidative stress of the experimental groups is provided in Table 2.
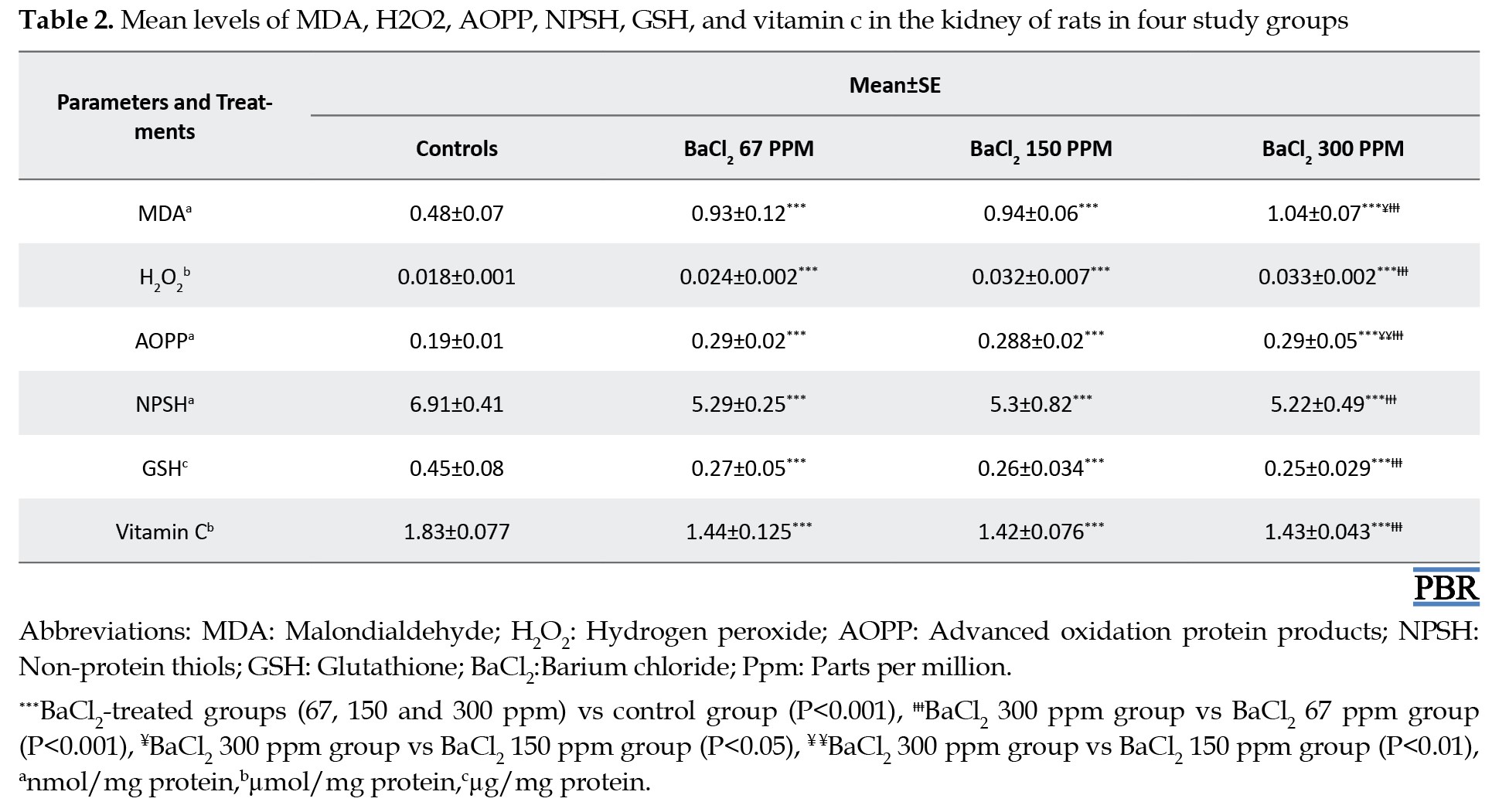
The results revealed a significant increase of tissue lipid peroxidation and H2O2 levels in the kidney of the BaCl2-treated group by +92%, +93%, and +113%, in addition to 48%, 78%, and 80% respectively, when compared to controls. The oxidative damage of kidney protein was demonstrated by a significant increase in AOPP levels of exposed rats to BaCl2-graded doses when compared to those of controls (Table 2).
Nonenzymatic and enzymatic antioxidant status in kidney
The deleterious effects of BaCl2 on the renal tissue of the experimental groups are shown in Tables 2 and 3.
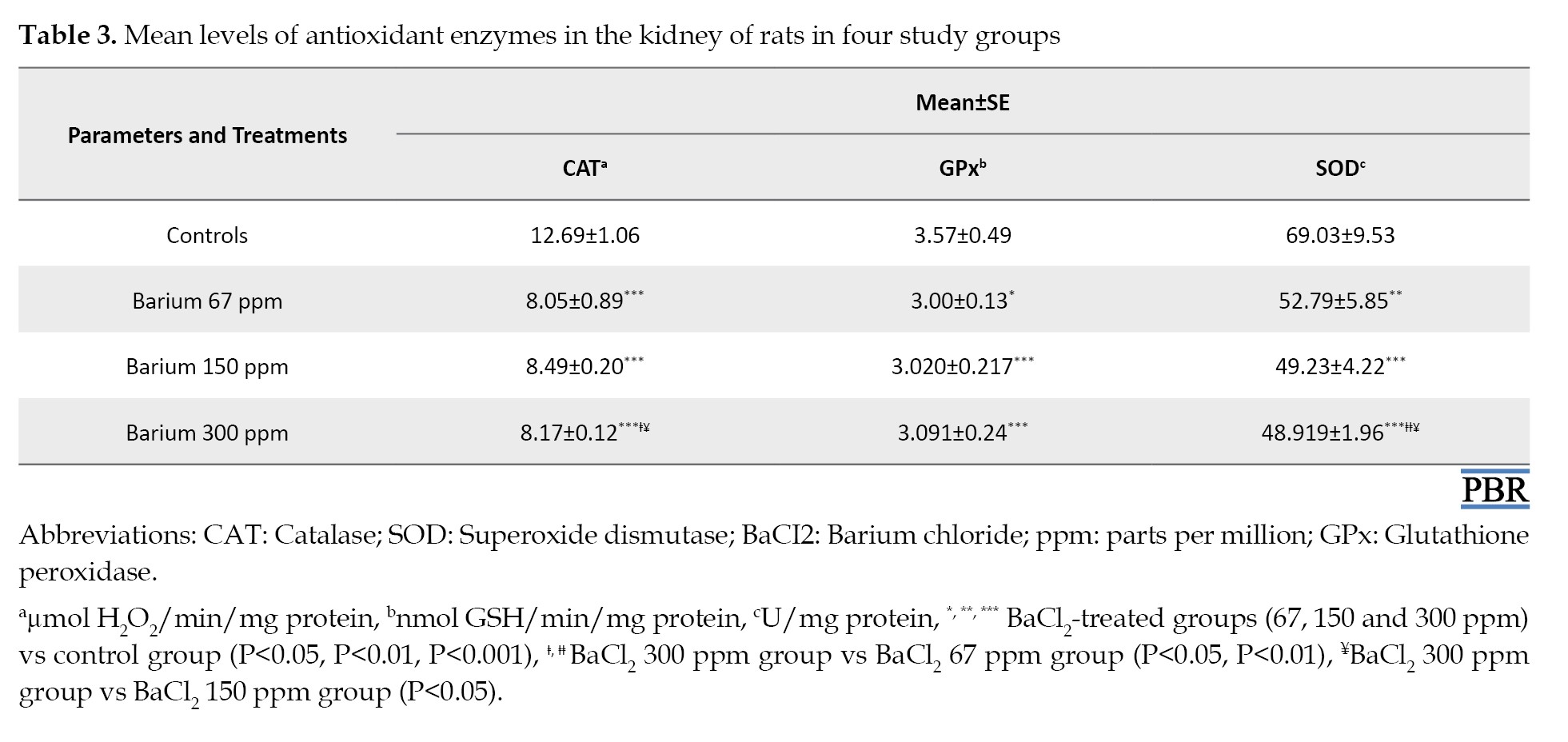
A significant (P<0.001) depletion of nonenzymatic antioxidants as evidenced by a decrease in NPSH (-23%, -23%, and -42%), GSH (-37%, -42%, and -45%) and vitamin C (-19%, -22%, and -22%) was shown in the kidney of exposed rats to doses of BaCl2 (67, 150 and 300 ppm, respectively) when compared to the controls (Table 2). On the other hand, BaCl2 induced the depletion of renal antioxidant enzyme activities as illustrated in Table 3. The activities of CAT, GPx, and SOD decreased significantly (P<0.001) in the barium-treated group’s control group at graded doses compared to controls (Table 3).
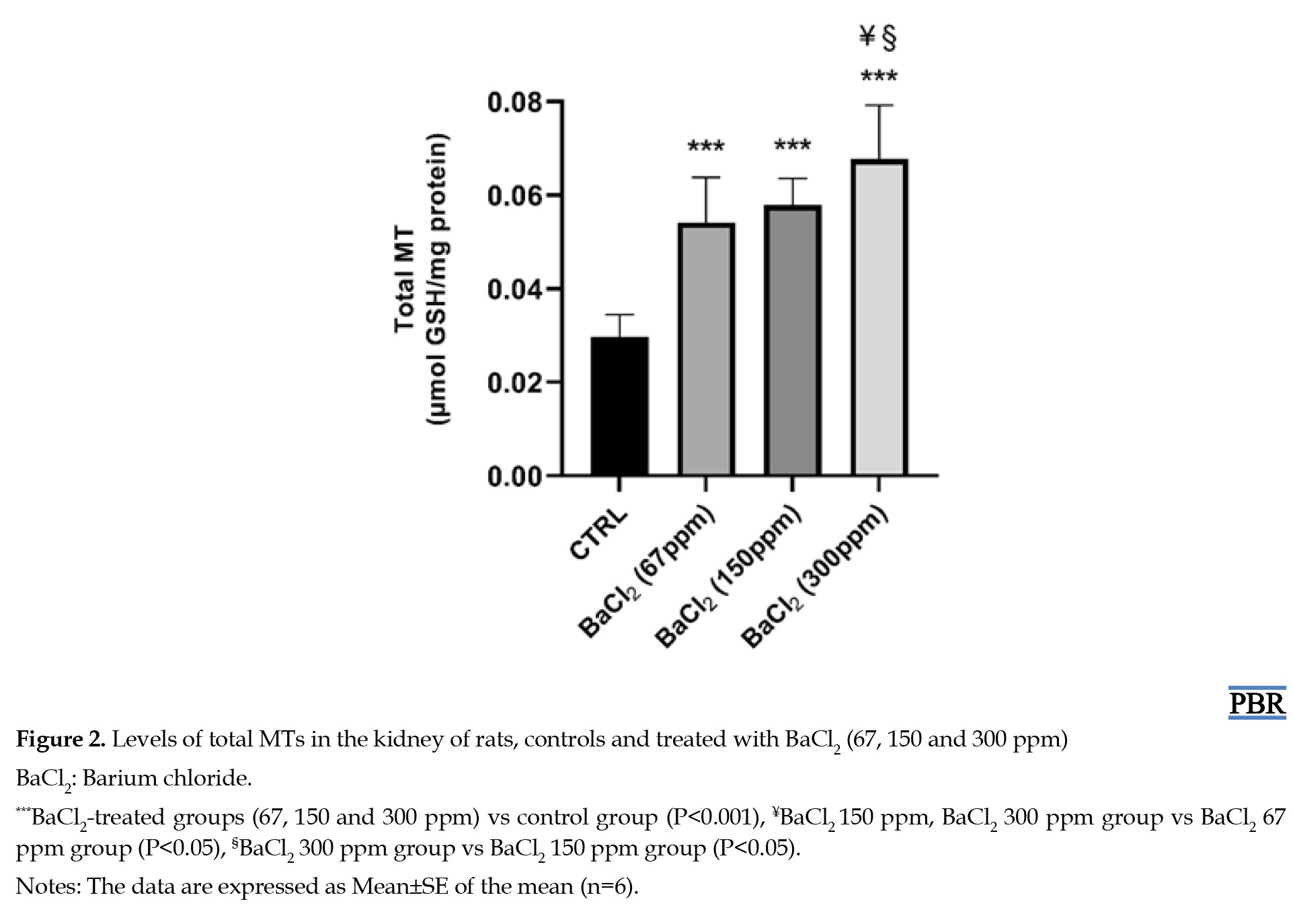
MTs content
A significant increase of MT levels was observed in the kidneys of rats exposed to doses of BaCl2 (67, 150, and 300 ppm) by 82%, 95%, and 128% respectively when compared to those of controls (Figure 2).
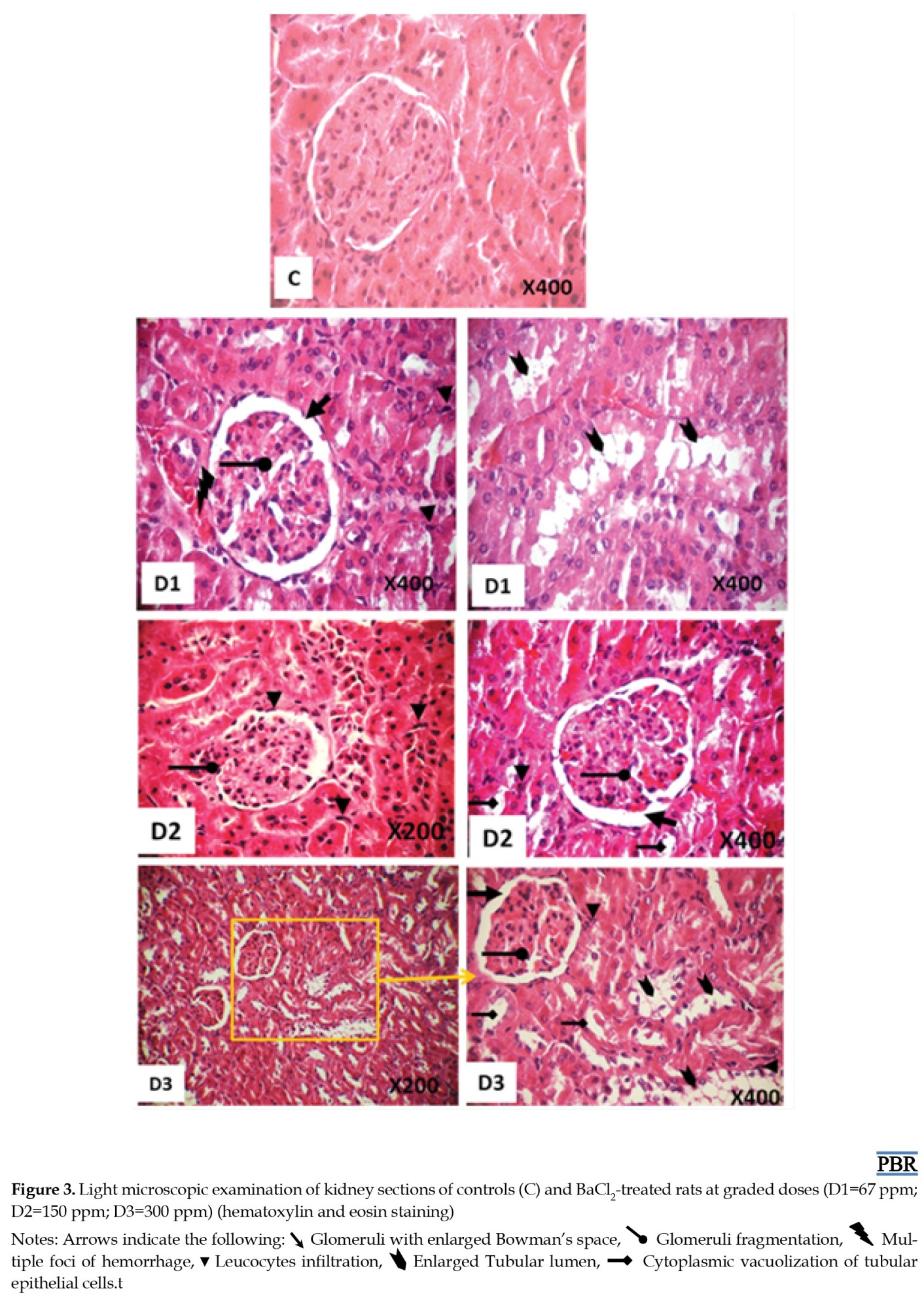
Light microscopy results
The kidney histological assessment and attributed scores of BaCl2-treated and control groups of rats were shown in Figure 3 and Table 4 after 3 weeks of treatment.
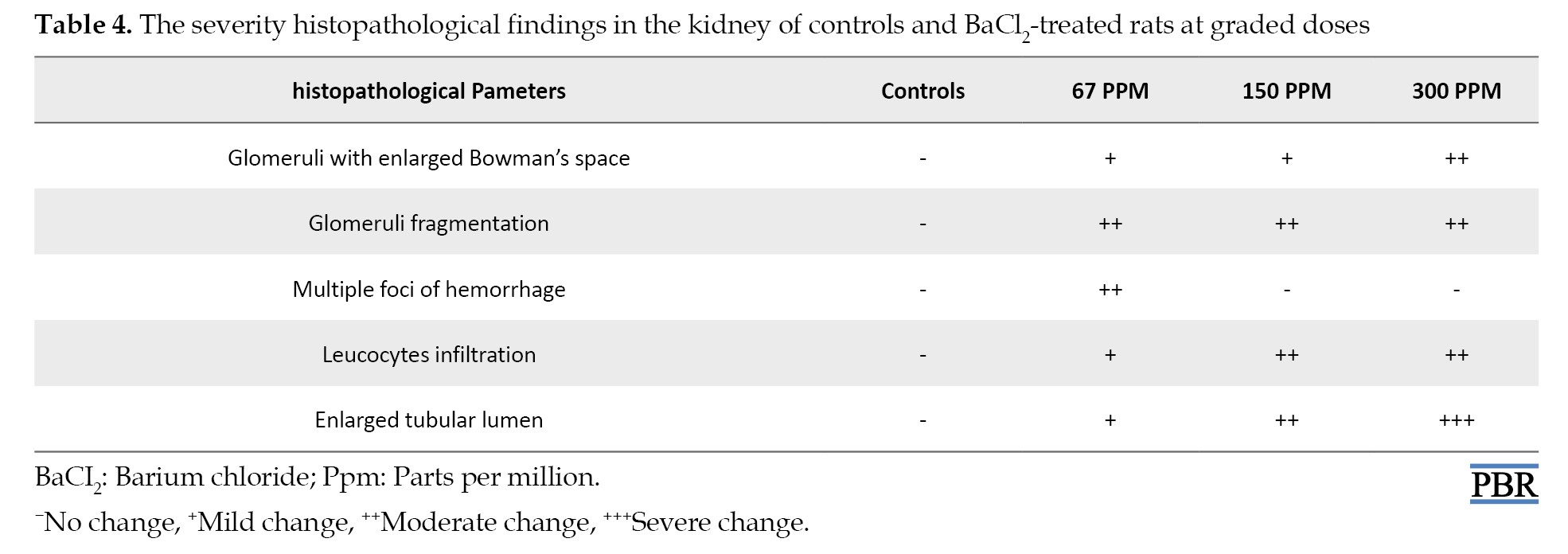
The kidney tissues of the control group showed a typical histological appearance with normal glomerulus and tubules displaying regular morphology (Figure 3C). While kidney sections after BaCl2 exposure induced renal damage and various alterations such as multiple foci of hemorrhage (Figure 3D1), fragmentation of glomeruli with enlarged Bowman’s space, leucocytes infiltration, and cytoplasmic vacuolization of tubular epithelial cells (Figures 3D1, D2, and D3). The severity scores of histopathological findings are summarized in Table 4.
Discussion
Barium, one of the main environmental and occupational pollutants, is widely employed in different industries [33-35]. The extensive use of barium compounds leads to its environmental release health risks and fatalities [4, 9, 36]. Exposure to barium compounds causes deleterious effects in soft tissues, particularly the kidney. This study focused on the potential nephrotoxicity of BaCl2 by disrupting the pro-oxidant/antioxidant balance and causing histological changes in kidney tissue.
The kidney excretes excess xenobiotics and endogenous substances. Urea, uric acid, creatinine, and creatinine clearance, are used as indicators of renal function. In the present study, exposure rats to barium-graded doses led to a decrease in their glomerular filtration rate, as evidenced by an increase in plasma creatinine and urea and a decrease of creatinine clearance and accompanied by an increase in a 24-h urinary volume output in all barium-treated rats.
Uric acid was another parameter used in the present study to assess kidney function. This compound is widely recognized as an antioxidant and it is potentially associated with kidney dysfunction. In this study, exposure of rats to barium led to a decrease in plasma uric acid levels and an increase in its urinary excretion, reflecting the body’s response to an excess of reactive oxygen species production. Our results are consistent with previous in vivo findings [37-39], already confirmed tubular damage in rats exposed to heavy metals.
Although reactive oxygen species are normally produced through cellular metabolism, their overproduction in abnormal conditions, such as toxicity leads to the accumulation of H2O2, which promotes lipid peroxidation, oxidative stress, and cell death [40]. In the present study, exposure of rats to BaCl2 resulted in a significant increase in malondialdehyde (MDA) and H2O2 levels in renal tissue. Barium ions (Ba2+) disrupt the balance between antioxidants and reactive oxygen species by damaging mitochondria [41]. This imbalance leads to excessive reactive oxygen species production, which not only damages cell membranes by altering their structure and function [42] but also causes deleterious effects on proteins. Our present study showed a significant increase in AOPP levels, suggesting the formation of protein oxidative damages in the kidney after exposure of the rats to BaCl2.
The antioxidant system is one of the several mechanisms that protect renal tissues from oxidative stress, lipid peroxidation, or inflammation, thereby preventing the occurrence of renal damage. Among them, CAT, SOD, and GPx are major antioxidant enzyme systems. In the present study, the barium-treated group showed a marked diminution in CAT, SOD, and GPx activities. Our results were following the recent study of Omole [43] and Mohammed [44], who have reported that rats’ exposure to graded barium doses significantly disrupts the antioxidant enzyme levels in their soft tissue.
Over-accumulation of free radicals is maintained by both enzymatic and nonenzymatic antioxidant defense systems like GSH, NPSH, and vitamin C as well as MT that can work synergistically. In particular, GSH and its metabolizing enzymes have a crucial role in modulating oxidative stress damage. MTs are well-known for their potent metal-binding properties and maintaining heavy metal homeostasis. This protein shares with GSH an important similarity which they contain both cysteine residues. In line with our findings, a significant decrease in GSH, NPSH, and vitamin C levels was shown after exposure of rats to graded barium doses. Our results are concordant with anterior investigations that demonstrated the failure of antioxidant defense systems in kidney rats after barium exposure [44].
MTs are recognized as key cellular defense mechanisms against oxidative stress due to their low molecular weight and cysteine-rich polypeptide and tripeptide structures. Our results demonstrated that to counteract the oxidative stress induced by barium, the body upregulated MT levels in a dose-dependent manner. While MT is typically involved in metal detoxification [45-48] its induction suggests a protective role against barium-induced toxicity. Although MT can bind and transport barium from the liver to the kidney, the subsequent release of barium within renal tubules may contribute to tubular injury, thus highlighting a potential role for MT in the protection of barium-induced nephrotoxicity.
The biochemical findings are associated with histopathological observations. The histoarchitecture investigation in the kidney of barium-treated rats revealed structural degeneration in glomerular vessels and renal tubules. Our findings on the kidneys of rats exposed to BaCl2 showed various alterations such as interstitial hemorrhage and glomerular fragmentation with enlarged glomeruli Bowman’s space, renal tubular epithelial cell damage with cytoplasmic vacuolization, and leucocytes infiltration. Our results corroborate the previous finding [49] that lead-induced similar histopathological observations. The most harmful outcome associated with large doses of barium exposure was tubular cell damage, as indicated by scoring changes in the kidney histoarchitecture. The histopathological alterations in the renal tissue of barium-treated rats are likely attributable to an accumulation of free radicals resulting from enhanced lipid peroxidation.
Conclusion
To the best of our knowledge, the findings of this study provide for the first time the mechanism underlying the induction of MT production and renal damage based on biochemical disorders as well as histological changes in the kidney of rats treated with doses of BaCl2 (67, 150, and 300 ppm). These findings provide substantial evidence regarding the toxicity of BaCl2 exposure, contribute to increasing public awareness to understand the risks of environmental pollutants and help to inform clinical practice and public health policies to minimize BaCl2 exposure and protect children, pregnant women, and occupationally exposed workers.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of Sciences Faculty, Sfax University, Sfax, Tunisia. All experimental procedures were performed according to the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research, Tunis, Tunisia.
Authors' contributions
Data analysis, interpretation and writing the original draft: Awatef Elwej; Experiments and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are indebted to Raoudha Ben Amar Abdennadher for her skilful technical assistance.
References
During the last years, our environment as well as our health have been exposed to various toxicants, and the rapid growth of industry has led to large amounts of industrial wastes that discharging into soil and water. Environmental pollutants can induce oxidative stress by overwhelming the body’s antioxidant defenses [1] with excessive free radical production leading to cellular and tissue damage. Cells have developed enzymatic and non-enzymatic antioxidant systems to convert reactive oxygen species into harmless metabolites, working together to prevent damage caused by pesticides [2] and metal exposure [3]. Barium, a dense alkaline earth metal found as divalent cations in various salts [4] is released into the environment through natural events and industrial processes. This contamination affects surface waters, soils, foods, and the food chain in both rural and urban areas [4, 5]. Barium is extensively used in various industries, such as plastics, electronics, textile pharmaceuticals and cosmetics [6]. Studies have shown that barium chloride (BaCl2) can lead to toxicity in various soft tissues [7, 8] and potentially increase the risk of congenital heart defects [9] and orofacial clefts [10] in offspring.
Since the kidneys play many vital functions to maintain normal homeostasis in the body, alterations in renal cells can promote the formation of several sufferings of people worldwide. The kidney is highly vulnerable to drugs and environmental chemicals due to high blood flow and oxygen requirement via the circulation leading to a high risk of nephrotoxicity [11-13]. Liu et al. [14] report that barium may impair the human kidney with renal injury being more prominent in women. This dysfunction could be attributed to the adverse effects of barium on female reproductive hormones [15] since it plays a pivotal role in protecting the kidney [16]. Furthermore, alterations in renal functional capacity have been also reported in a case of barium poisoning of patients treated with intravenous barium sulfate where renal tubules are obstructed leading to necrosis [17, 18]. Accordingly, this study evaluates the nephrotoxicity induced by BaCl2 administered orally to adult rats for 21 consecutive days at different doses with a view of the systemic oxidative stress, biochemical parameters, and histopathological changes.
Materials and Methods
Study chemicals
BaCl2 was obtained from Loba Chemie (Mumbai, India). All experiments were performed using other reagents of analytical grade purchased from standard commercial suppliers.
Animals and experimental protocols
A total of 24 female rats weighing from 168 to 179 g, were purchased from the Central Pharmacy (SIPHAT, Tunisia). They were reared under standard laboratory conditions (temperature 22±2 °C, relative humidity 40%, and 12-h light/dark cycle). A commercial pellet diet and tap water were provided ad libitum. After sufficient time, the rats were randomly allocated into four groups (n=6): the first group served as a control and received distilled water. Group 2 received 67 parts per million (ppm) of barium (D1), group 3 received 150 ppm of barium (D2), and Group 4 received barium at a dose of 300 ppm (D3). The barium doses used in the experiment were determined based on our previous study [19] and they induced toxicity without mortality.
Sampling procedure
On the 20th day, the rats were transferred to individual metabolic cages for a 24-h urine collection. Subsequently, urinary samples were collected in bottles and the volume of each sample was recorded then centrifuged for 5 min at 5000 g.
At the end of the 21-day study period, the animals were sacrificed by cervical decapitation. Whole blood samples were collected into serum vacutainer tubes, then centrifuged at 2200 ×g for 10 min at 4 °C and finally stored at -80 °C until biochemical analysis. The kidneys were removed from the abdominal cavities, washed in ice-cold, and frozen immediately until further analysis. To evaluate oxidative stress, some kidney was cut into small pieces, homogenized in appropriate phosphate buffer (10 mM, pH=7.4), and then subjected to centrifugation. Aliquots of supernatant were kept at -80 °C for further biochemical assays. Other kidneys were collected, and fixed in 10% neutral formaldehyde for histological examination.
Biochemical analysis
Determination of kidney protein content
Total protein content in kidney homogenates was determined according to the method by Lowry et al. [20], using bovine serum albumin as standard.
Biochemical assay
The levels of urea, uric acid, and creatinine in plasma and urine were estimated spectrophotometrically using commercial diagnostic kits (ref 20151, 20143, 20092; Biomaghreb, Tunisia). Creatinine clearance, an index of glomerular filtration rate was calculated by the UV/P equation [21], where U was the urinary creatinine level, V was the volume of a urine sample collected within 24 h, and P was the plasma creatinine concentration.
Measurement of kidney oxidative stress and antioxidant defence levels
Lipid peroxidation assay was estimated by following the method of Draper and Hadley [22]. Absorbance was measured at 532 nm and results were expressed as nM of TBARS per milligram protein. Hydrogen peroxide (H2O2) was carried out by the method of Ou and Wolff [23]. The amount of H2O2 was determined at 560 nm and values were expressed as µmoles per milligram of protein. Advanced oxidation protein product (AOPP) levels were evaluated by the method of Kayali et al. [24]. The absorbance was recorded at 340 nm and the results were expressed as nanomoles per milligram protein.
Kidney glutathione (GSH) content was performed according to the method of Ellman [25] and modified by Jollow et al. [26]. The absorbance was measured at 412 nm and total GSH content was expressed as micrograms per milligram of protein. Non-protein thiols (NPSH) kidney tissue levels were determined according to the method of Ellman [25]. Absorbance was measured at 412 nm and the results were expressed as nanomoles per milligram of protein. Metallothionein (MT) content in the kidney was measured spectrophotometrically following the protocol of Viarengo et al. [27] and modified by Petrovic et al. [28]. Ascorbic acid (vitamin C) content in kidney tissue was assayed by the dinitrophenylhydrazine method described by Jacques-Silva et al. [29] and results were expressed as µmoles per milligram of protein. Superoxide dismutase (SOD) activity was performed according to Beauchamp and Fridovich [30] and results were expressed as enzyme units per milligram of protein. Meanwhile, catalase (CAT) activity was assayed by the method of Aebi [31] and the activities were expressed as micromoles of H2O2 consumed per min per milligram of protein. Glutathione peroxidase (GPx) activity was performed according to the method of Flohe and Gunzler [32] and the enzyme activity was expressed as moles of GSH oxidized per min per mg protein.
Plasma and kidney lactate dehydrogenase (LDH) activities
Plasma and kidney LDH activities, were determined using commercial reagent kits (Biomagreb, Ariana, Tunisia, Ref 20012).
Histopathological analysis
After their fixation in neutral formaldehyde, the kidney was processed using a graded ethanol series and embedded in paraffin blocks. Hematoxylin–Eosin (H&E) staining was applied to 5 mm thick sections and examined under light microscopy (ZEISS, Axiolab) using a Canon PowerShot camera (model A640). All sections were evaluated for the degree of tubular and glomerular injury and necrosis.
Statistical analysis
The results were analyzed using GraphPad Prism software, version 9.2 for Windows (GraphPad Software, San Diego, CA). The statistical analysis was performed by one-way analysis of variance followed by the Tukey post hoc test. In all statistical analyses, differences were considered significant for P<0.05.
Results
General assessment
In the experimental investigation, the general condition of rats following the oral administration of BaCl2 was changed. Rats subjected to graded doses of BaCl2 (67, 150, and 300 ppm) displayed mild to moderate alterations and exhibited symptoms, such as abdominal pain and increased urination. However, there were no fatalities recorded in all groups during the experimental period.
Effect on renal function
Table 1 exhibits data concerning kidney oxidative stress analyses (creatinine, urea, and uric acid levels).

The results showed that barium administration at graded doses (67, 150, and 300 ppm) led to a significant increase in creatinine and urea levels in plasma as +30%, +31% and +34%, and 53%, +57% and +54% and a decrease in urine as ‒46%, ‒55%, and ‒56% and ‒40%, ‒48%, and ‒46%, respectively, as compared to controls. On the other hand, uric acid levels exhibited a dose-dependent pattern, with decreases of ‒27%, ‒30%, and ‒34% in plasma and increases of +34%, +37%, and +45% in urine, when compared with controls. Furthermore, additionally, compared to controls, rats treated with BaCl2 showed disorders in renal function which are manifested by an increase in urine volume (+27%, +32%, +32%) and a decrease in creatinine clearance (-47%, -48% and -50%) considered as an indicator of glomerular dysfunction.

LDH activities in plasma and kidney homogenates
Activities of LDH in the different groups of rats are illustrated in Figure 2. The results showed statistically significant increases in plasma LDH activity by 55%, 70%, and 79% and decrease in kidney LDH activity by 46%, 46%, and 54%, were observed in the three groups treated with BaCl2 (doses=67, 150, and 300 ppm, respectively) as compared to control rats.
Effect on renal oxidative stress and antioxidant enzymes
The effect of BaCl2 on renal oxidative stress of the experimental groups is provided in Table 2.

The results revealed a significant increase of tissue lipid peroxidation and H2O2 levels in the kidney of the BaCl2-treated group by +92%, +93%, and +113%, in addition to 48%, 78%, and 80% respectively, when compared to controls. The oxidative damage of kidney protein was demonstrated by a significant increase in AOPP levels of exposed rats to BaCl2-graded doses when compared to those of controls (Table 2).
Nonenzymatic and enzymatic antioxidant status in kidney
The deleterious effects of BaCl2 on the renal tissue of the experimental groups are shown in Tables 2 and 3.

A significant (P<0.001) depletion of nonenzymatic antioxidants as evidenced by a decrease in NPSH (-23%, -23%, and -42%), GSH (-37%, -42%, and -45%) and vitamin C (-19%, -22%, and -22%) was shown in the kidney of exposed rats to doses of BaCl2 (67, 150 and 300 ppm, respectively) when compared to the controls (Table 2). On the other hand, BaCl2 induced the depletion of renal antioxidant enzyme activities as illustrated in Table 3. The activities of CAT, GPx, and SOD decreased significantly (P<0.001) in the barium-treated group’s control group at graded doses compared to controls (Table 3).
MTs content
A significant increase of MT levels was observed in the kidneys of rats exposed to doses of BaCl2 (67, 150, and 300 ppm) by 82%, 95%, and 128% respectively when compared to those of controls (Figure 2).
Light microscopy results
The kidney histological assessment and attributed scores of BaCl2-treated and control groups of rats were shown in Figure 3 and Table 4 after 3 weeks of treatment.

The kidney tissues of the control group showed a typical histological appearance with normal glomerulus and tubules displaying regular morphology (Figure 3C). While kidney sections after BaCl2 exposure induced renal damage and various alterations such as multiple foci of hemorrhage (Figure 3D1), fragmentation of glomeruli with enlarged Bowman’s space, leucocytes infiltration, and cytoplasmic vacuolization of tubular epithelial cells (Figures 3D1, D2, and D3). The severity scores of histopathological findings are summarized in Table 4.
Discussion
Barium, one of the main environmental and occupational pollutants, is widely employed in different industries [33-35]. The extensive use of barium compounds leads to its environmental release health risks and fatalities [4, 9, 36]. Exposure to barium compounds causes deleterious effects in soft tissues, particularly the kidney. This study focused on the potential nephrotoxicity of BaCl2 by disrupting the pro-oxidant/antioxidant balance and causing histological changes in kidney tissue.
The kidney excretes excess xenobiotics and endogenous substances. Urea, uric acid, creatinine, and creatinine clearance, are used as indicators of renal function. In the present study, exposure rats to barium-graded doses led to a decrease in their glomerular filtration rate, as evidenced by an increase in plasma creatinine and urea and a decrease of creatinine clearance and accompanied by an increase in a 24-h urinary volume output in all barium-treated rats.
Uric acid was another parameter used in the present study to assess kidney function. This compound is widely recognized as an antioxidant and it is potentially associated with kidney dysfunction. In this study, exposure of rats to barium led to a decrease in plasma uric acid levels and an increase in its urinary excretion, reflecting the body’s response to an excess of reactive oxygen species production. Our results are consistent with previous in vivo findings [37-39], already confirmed tubular damage in rats exposed to heavy metals.
Although reactive oxygen species are normally produced through cellular metabolism, their overproduction in abnormal conditions, such as toxicity leads to the accumulation of H2O2, which promotes lipid peroxidation, oxidative stress, and cell death [40]. In the present study, exposure of rats to BaCl2 resulted in a significant increase in malondialdehyde (MDA) and H2O2 levels in renal tissue. Barium ions (Ba2+) disrupt the balance between antioxidants and reactive oxygen species by damaging mitochondria [41]. This imbalance leads to excessive reactive oxygen species production, which not only damages cell membranes by altering their structure and function [42] but also causes deleterious effects on proteins. Our present study showed a significant increase in AOPP levels, suggesting the formation of protein oxidative damages in the kidney after exposure of the rats to BaCl2.
The antioxidant system is one of the several mechanisms that protect renal tissues from oxidative stress, lipid peroxidation, or inflammation, thereby preventing the occurrence of renal damage. Among them, CAT, SOD, and GPx are major antioxidant enzyme systems. In the present study, the barium-treated group showed a marked diminution in CAT, SOD, and GPx activities. Our results were following the recent study of Omole [43] and Mohammed [44], who have reported that rats’ exposure to graded barium doses significantly disrupts the antioxidant enzyme levels in their soft tissue.
Over-accumulation of free radicals is maintained by both enzymatic and nonenzymatic antioxidant defense systems like GSH, NPSH, and vitamin C as well as MT that can work synergistically. In particular, GSH and its metabolizing enzymes have a crucial role in modulating oxidative stress damage. MTs are well-known for their potent metal-binding properties and maintaining heavy metal homeostasis. This protein shares with GSH an important similarity which they contain both cysteine residues. In line with our findings, a significant decrease in GSH, NPSH, and vitamin C levels was shown after exposure of rats to graded barium doses. Our results are concordant with anterior investigations that demonstrated the failure of antioxidant defense systems in kidney rats after barium exposure [44].
MTs are recognized as key cellular defense mechanisms against oxidative stress due to their low molecular weight and cysteine-rich polypeptide and tripeptide structures. Our results demonstrated that to counteract the oxidative stress induced by barium, the body upregulated MT levels in a dose-dependent manner. While MT is typically involved in metal detoxification [45-48] its induction suggests a protective role against barium-induced toxicity. Although MT can bind and transport barium from the liver to the kidney, the subsequent release of barium within renal tubules may contribute to tubular injury, thus highlighting a potential role for MT in the protection of barium-induced nephrotoxicity.
The biochemical findings are associated with histopathological observations. The histoarchitecture investigation in the kidney of barium-treated rats revealed structural degeneration in glomerular vessels and renal tubules. Our findings on the kidneys of rats exposed to BaCl2 showed various alterations such as interstitial hemorrhage and glomerular fragmentation with enlarged glomeruli Bowman’s space, renal tubular epithelial cell damage with cytoplasmic vacuolization, and leucocytes infiltration. Our results corroborate the previous finding [49] that lead-induced similar histopathological observations. The most harmful outcome associated with large doses of barium exposure was tubular cell damage, as indicated by scoring changes in the kidney histoarchitecture. The histopathological alterations in the renal tissue of barium-treated rats are likely attributable to an accumulation of free radicals resulting from enhanced lipid peroxidation.
Conclusion
To the best of our knowledge, the findings of this study provide for the first time the mechanism underlying the induction of MT production and renal damage based on biochemical disorders as well as histological changes in the kidney of rats treated with doses of BaCl2 (67, 150, and 300 ppm). These findings provide substantial evidence regarding the toxicity of BaCl2 exposure, contribute to increasing public awareness to understand the risks of environmental pollutants and help to inform clinical practice and public health policies to minimize BaCl2 exposure and protect children, pregnant women, and occupationally exposed workers.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of Sciences Faculty, Sfax University, Sfax, Tunisia. All experimental procedures were performed according to the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research, Tunis, Tunisia.
Authors' contributions
Data analysis, interpretation and writing the original draft: Awatef Elwej; Experiments and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are indebted to Raoudha Ben Amar Abdennadher for her skilful technical assistance.
References
- Sadiq IZ. Free radicals and oxidative stress: Signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Curr Mol Med. 2023; 23(1):13-35. [DOI:10.2174/1566524022666211222161637] [PMID]
- Sule RO, Condon L, Gomes AV. A common feature of pesticides: Oxidative stress-the role of oxidative stress in pesticide-induced toxicity. Oxid Med Cell Longev. 2022; 2022:5563759. [DOI:10.1155/2022/5563759] [PMID] [PMCID]
- Wang J, Zhu H, Wang K, Yang Z, Liu Z. Protective effect of quercetin on rat testes against cadmium toxicity by alleviating oxidative stress and autophagy. Environ Sci Pollut Res Int. 2020; 27(20):25278-86. [DOI:10.1007/s11356-020-08947-2] [PMID]
- Peana M, Medici S, Dadar M, Zoroddu MA, Pelucelli A, Chasapis CT, et al. Environmental barium: Potential exposure and health-hazards. Arch Toxicol. 2021; 95(8):2605-12. [DOI:10.1007/s00204-021-03049-5] [PMID]
- Daphne M, Cassandra SS, Yee-Wan S. Toxicological profile for barium and barium compounds. Atlanta: CDC Library collection; 2007. [Link]
- Agarwal J. Waste management of residual black ash in barium industries. Materialstoday. 2019; 18(7):4810‑5. [DOI:10.1016/j.matpr.2019.07.469]
- Ueha R, Nativ-Zeltzer N, Sato T, Goto T, Yamauchi A, Belafsky PC, et al. The effects of barium concentration levels on the pulmonary inflammatory response in a rat model of aspiration. Eur Arch Otorhinolaryngol. 2020; 277(1):189-96. [DOI:10.1007/s00405-019-05666-4] [PMID]
- Elwej A, Grojja Y, Ghorbel I, Boudawara O, Jarraya R, Boudawara T, et al. Barium chloride induces redox status unbalance, upregulates cytokine genes expression and confers hepatotoxicity in rats-alleviation by pomegranate peel. Environ Sci Pollut Res Int. 2016; 23(8):7559-71. [DOI:10.1007/s11356-015-6023-0] [PMID]
- Zhang N, Liu Z, Tian X, Chen M, Deng Y, Guo Y, et al. Barium exposure increases the risk of congenital heart defects occurrence in offspring. Clin Toxicol. 2018; 56(2):132-9. [DOI:10.1080/15563650.2017.1343479] [PMID]
- Pi X, Jin L, Li Z, Liu J, Zhang Y, Wang L, et al. Association between concentrations of barium and aluminum in placental tissues and risk for orofacial clefts. Sci Total Environ. 2019; 652:406-12. [DOI:10.1016/j.scitotenv.2018.10.262] [PMID]
- Yuksel Y, Yuksel R, Yagmurca M, Haltas H, Erdamar H, Toktas M, et al. Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum Exp Toxicol. 2017; 36(1):51-61. [DOI:10.1177/0960327116637414] [PMID]
- Nyarko RA, Larbie C, Anning AK, Baidoo PK, Emikpe BO, Oyagbemi AA, et al. Griffonia simplicifolia (DC.) Baill. attenuates gentamicin and cisplatin-induced nephrotoxicty in rats. Comp Clin Pathol. 2019; 28(5):1293‑304. [DOI:10.1007/s00580-019-02934-x]
- Ghorbel I, Elwej A, Fendri N, Mnif H, Jamoussi K, Boudawara T, et al. Olive oil abrogates acrylamide induced nephrotoxicity by modulating biochemical and histological changes in rats. Ren Fail. 2017; 39(1):236-45. [DOI:10.1080/0886022X.2016.1256320] [PMID] [PMCID]
- Liu Y, Yuan Y, Xiao Y, Li Y, Yu Y, Mo T, et al. Associations of plasma metal concentrations with the decline in kidney function: A longitudinal study of Chinese adults. Ecotoxicol Environ Saf. 2020; 189:110006. [DOI:10.1016/j.ecoenv.2019.110006] [PMID]
- Ashrap P, Sánchez BN, Téllez-Rojo MM, Basu N, Tamayo-Ortiz M, Peterson KE, et al. In utero and peripubertal metals exposure in relation to reproductive hormones and sexual maturation and progression among girls in Mexico City. Environ Res. 2019; 177:108630. [DOI:10.1016/j.envres.2019.108630] [PMID] [PMCID]
- Valdivielso JM, Jacobs-Cachá C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019; 28(1):1-9. [DOI:10.1097/MNH.0000000000000463] [PMID]
- Agarwal AK, Ahlawat SK, Gupta S, Singh B, Singh CP, Wadhwa S, et al. Hypokalaemic paralysis secondary to acute barium carbonate toxicity. Trop Doct. 1995; 25(3):101-3. [DOI:10.1177/004947559502500304] [PMID]
- Dallas CE, Williams PL. Barium: Rationale for a new oral reference dose. J Toxicol Environ Health B Crit Rev. 2001; 4(4):395-429. [DOI:10.1080/109374001753146216] [PMID]
- Elwej A, Chaabane M, Ghorbel I, Chelly S, Boudawara T, Zeghal N. Effects of barium graded doses on redox status, membrane bound ATPases and histomorphological aspect of the liver in adult rats. Toxicol Mech Methods. 2017; 27(9):677-86. [DOI:10.1080/15376516.2017.1351016] [PMID]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265-75. [DOI:10.1016/S0021-9258(19)52451-6] [PMID]
- Charrel M. Urea and creatinine [French]. In: Charrel M (editor). Biochemical Semiology. Paris: Ellipses Publisher; 1991. [Link]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990; 186:421-31. [DOI:10.1016/0076-6879(90)86135-I] [PMID]
- Ou P, Wolff SP. A discontinuous method for catalase determination at 'near physiological' concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods. 1996; 31(1-2):59-67. [DOI:10.1016/0165-022X(95)00039-T] [PMID]
- Kayali R, Cakatay U, Akçay T, Altuğ T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct. 2006; 24(1):79-85. [DOI:10.1002/cbf.1190] [PMID]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82(1):70-7. [DOI:10.1016/0003-9861(59)90090-6] [PMID]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974; 11(3):151-69. [DOI:10.1159/000136485] [PMID]
- Viarengo A, Ponzano E, Dondero F, Fabbri R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to mediterranean and antarctic molluscs. Mar Environ Res. 1997; 44(1):69‑84. [DOI:10.1016/S0141-1136(96)00103-1]
- Petrović S, Ozretić B, Krajnović-Ozretić M, Bobinac D. Lysosomal membrane stability and metallothioneins in digestive gland of Mussels (Mytilus galloprovincialis Lam.) as biomarkers in a field study. Mar Pollut Bull. 2001; 42(12):1373-8. [DOI:10.1016/S0025-326X(01)00167-9] [PMID]
- Jacques-Silva MC, Nogueira CW, Broch LC, Flores EM, Rocha JB. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 2001; 88(3):119-25. [DOI:10.1034/j.1600-0773.2001.d01-92.x] [PMID]
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44(1):276-87. [DOI:10.1016/0003-2697(71)90370-8] [PMID]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121-6. [DOI:10.1016/s0076-6879(84)05016-3] [PMID]
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984; 105:114-21. [DOI:10.1016/S0076-6879(84)05015-1] [PMID]
- Migliaccio V, Lionetti L, Putti R, Scudiero R. Exposure to dichlorodiphenyldichloroethylene (DDE) and metallothionein levels in rats fed with normocaloric or high-fat diet: A review. Int J Mol Sci. 2020; 21(5):1903 [DOI:10.3390/ijms21051903] [PMID] [PMCID]
- Pragst F, Stieglitz K, Runge H, Runow KD, Quig D, Osborne R, et al. High concentrations of lead and barium in hair of the rural population caused by water pollution in the Thar Jath oilfields in South Sudan. Forensic Sci Int. 2017; 274:99-106. [DOI:10.1016/j.forsciint.2016.12.022] [PMID]
- World Health Organization (WHO). Water sanitation and health. Barium [Internet]. 2024 [Updated 2024 December 11]. Available from: [Link]
- Kravchenko J, Darrah TH, Miller RK, Lyerly HK, Vengosh A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health. 2014; 36(4):797-814. [DOI:10.1007/s10653-014-9622-7] [PMID]
- Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, et al. Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environ Sci Pollut Res Int. 2019; 26(4):3909-20. [DOI:10.1007/s11356-018-3819-8] [PMID]
- Zhang Z, Gao X, Guo M, Jiang H, Cao Y, Zhang N. The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol Trace Elem Res. 2017; 175(1):129-35. [DOI:10.1007/s12011-016-0731-2] [PMID]
- Kahalerras L, Otmani I, Abdennour C. The allium triquetrum l. leaves mitigated hepatotoxicity and nephrotoxicity induced by lead acetate in wistar rats. Biol Trace Elem Res. 2022; 200(11):4733-43. [DOI:10.1007/s12011-021-03052-y] [PMID]
- Babizhayev MA. Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin. 2016; 6:49-68. [DOI:10.1016/j.bbacli.2016.04.004] [PMID] [PMCID]
- Mores L, França EL, Silva NA, Suchara EA, Honorio-França AC. Nanoparticles of barium induce apoptosis in human phagocytes. Int J Nanomedicine. 2015; 10:6021-6. [DOI:10.2147/IJN.S90382] [PMID] [PMCID]
- Drevet JR, Aitken RJ. Oxidation of sperm nucleus in mammals: A physiological necessity to some extent with adverse impacts on oocyte and offspring. Antioxidants. 2020; 9(2):95. [DOI:10.3390/antiox9020095] [PMID] [PMCID]
- Omole JG, Alabi QK, Aturamu A, Adefisayo MA, Oluwayomi O, Dada MB, et al. Barium chloride dose-dependently induced heart and lung injury in Wistar rats. Environ Toxicol. 2019; 34(12):1303-12. [DOI:10.1002/tox.22831] [PMID]
- Mohammed AT, Ismail HTH. Hematological, biochemical, and histopathological impacts of barium chloride and barium carbonate accumulation in soft tissues of male Sprague-Dawley rats. Environ Sci Pollut Res Int. 2017; 24(34):26634-45. [DOI:10.1007/s11356-017-0282-x] [PMID]
- Gungor H, Kara H. Effects of selenium, zinc, insulin and metallothionein on cadmium-induced oxidative stress and metallothionein gene expression levels in diabetic rats. J Basic Clin Physiol Pharmacol. 2020; 31(2):/j/jbcpp.2020.31.issue-2/jbcpp-2019-0198/jbcpp-2019-0198.xml. [DOI:10.1515/jbcpp-2019-0198] [PMID]
- Alam RTM, Abu Zeid EH, Khalifa BA, Arisha AH, Reda RM. Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ Sci Pollut Res Int. 2021; 28(24):31391-402. [DOI:10.1007/s11356-021-13014-5] [PMID]
- Ghorbel I, Elwej A, Chaabene M, Boudawara O, Marrakchi R, Jamoussi K, et al. Effects of acrylamide graded doses on metallothioneins I and II induction and DNA fragmentation: Bochemical and histomorphological changes in the liver of adult rats. Toxicol Ind Health. 2017; 33(8):611-622. [DOI:10.1177/0748233717696613] [PMID]
- Elwej A, Ghorbel I, Marrekchi R, Boudawara O, Jamoussi K, Boudawara T, et al. Improvement of kidney redox states contributes to the beneficial effects of dietary pomegranate peel against barium chloride-induced nephrotoxicity in adult rats. Arch Physiol Biochem. 2016; 122(3):130-40. [DOI:10.3109/13813455.2016.1150298] [PMID]
- Amin I, Hussain I, Rehman MU, Mir BA, Ganaie SA, Ahmad SB, et al. Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J Food Biochem. 2021; 45(3):e13241. [DOI:10.1111/jfbc.13241] [PMID]
Type of Study: Original Research |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |