Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 203-228 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tanaka H, Maezawa M, Nakao S, Miyasaka K, Hirofuji S, Yamashita M, et al . Analysis of Adverse Events With Janus Kinase Inhibitors Reported to Spontaneous Reporting System. Pharm Biomed Res 2024; 10 (3) :203-228
URL: http://pbr.mazums.ac.ir/article-1-560-en.html
URL: http://pbr.mazums.ac.ir/article-1-560-en.html
Hideyuki Tanaka1 

 , Mika Maezawa1
, Mika Maezawa1 

 , Satoshi Nakao1
, Satoshi Nakao1 

 , Koumi Miyasaka1
, Koumi Miyasaka1 

 , Sakiko Hirofuji1
, Sakiko Hirofuji1 

 , Moe Yamashita1
, Moe Yamashita1 

 , Kensuke Matsui1
, Kensuke Matsui1 

 , Nanaka Ichihara1
, Nanaka Ichihara1 

 , Yuka Nokura1
, Yuka Nokura1 

 , Mari Iwata1
, Mari Iwata1 

 , Mayumi Kitamura2
, Mayumi Kitamura2 

 , Megumi Horibe2
, Megumi Horibe2 

 , Hirofumi Tamaki3
, Hirofumi Tamaki3 

 , Kazuhiro Iguchi3
, Kazuhiro Iguchi3 

 , Mitsuhiro Nakamura *1
, Mitsuhiro Nakamura *1 




 , Mika Maezawa1
, Mika Maezawa1 

 , Satoshi Nakao1
, Satoshi Nakao1 

 , Koumi Miyasaka1
, Koumi Miyasaka1 

 , Sakiko Hirofuji1
, Sakiko Hirofuji1 

 , Moe Yamashita1
, Moe Yamashita1 

 , Kensuke Matsui1
, Kensuke Matsui1 

 , Nanaka Ichihara1
, Nanaka Ichihara1 

 , Yuka Nokura1
, Yuka Nokura1 

 , Mari Iwata1
, Mari Iwata1 

 , Mayumi Kitamura2
, Mayumi Kitamura2 

 , Megumi Horibe2
, Megumi Horibe2 

 , Hirofumi Tamaki3
, Hirofumi Tamaki3 

 , Kazuhiro Iguchi3
, Kazuhiro Iguchi3 

 , Mitsuhiro Nakamura *1
, Mitsuhiro Nakamura *1 


1- Laboratory of Drug Informatics, Gifu Pharmaceutical University, Gifu, Japan.
2- Department of Nursing, School of Health Science, Asahi University, Gifu, Japan.
3- Laboratory of Community Pharmacy, Gifu Pharmaceutical University, Gifu, Japan.
2- Department of Nursing, School of Health Science, Asahi University, Gifu, Japan.
3- Laboratory of Community Pharmacy, Gifu Pharmaceutical University, Gifu, Japan.
Full-Text [PDF 1228 kb]
(766 Downloads)
| Abstract (HTML) (2457 Views)
Full-Text: (1080 Views)
Introduction
Janus kinase (JAK) inhibitors act on cytokine signaling pathways involved in inflammatory diseases and immune system abnormalities [1-4]. JAKs are essential for many cytokine families, and biological therapies targeting inflammatory cytokines have critically changed the treatment of rheumatoid arthritis (RA) and other autoimmune diseases. RA, for instance, is associated with the overproduction of interleukin (IL)-6, IL-12, IL-15, IL-23, granulocyte macrophage-colony stimulating factor, and interferons [4].
Four members of the JAK family, JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), are involved in different cytokine signaling pathways. JAK1, JAK2, and TYK2 are ubiquitously expressed, while expression of JAK3 is mainly restricted to cells of hematopoietic origin [2]. JAK1 is primarily involved in inflammatory and innate immune responses. Inhibition of JAK1 suppresses IL-6 signaling, which is central to inflammatory disease [1]. JAK2 is essential for erythropoiesis, myelopoiesis, and platelet production. JAK3 is vital for lymphocyte proliferation and homeostasis [2-4]. The IL-2 family of cytokines (IL-2, -4, -7, -9, -15, and -21) signal through JAK3-bound receptors [3]. IL-2 is a cytokine secreted by antigen stimulation of T cell receptors that drives T cell growth, augments natural killer (NK) cytolytic activity, induces the differentiation of regulatory T cells, and mediates activation-induced cell death [5-7]. The function of IL-15 is the maintenance of NK cells and CD8+CD44h memory T cells to provide a long-term immune response to pathogens [7]. TYK2 is associated with antiviral responses [1-4]. Therefore, differences in JAK inhibitor selectivity for cytokine signaling via distinct JAK pairs may provide a mechanistic rationale for reported differences in safety profiles (Figure 1) [1].
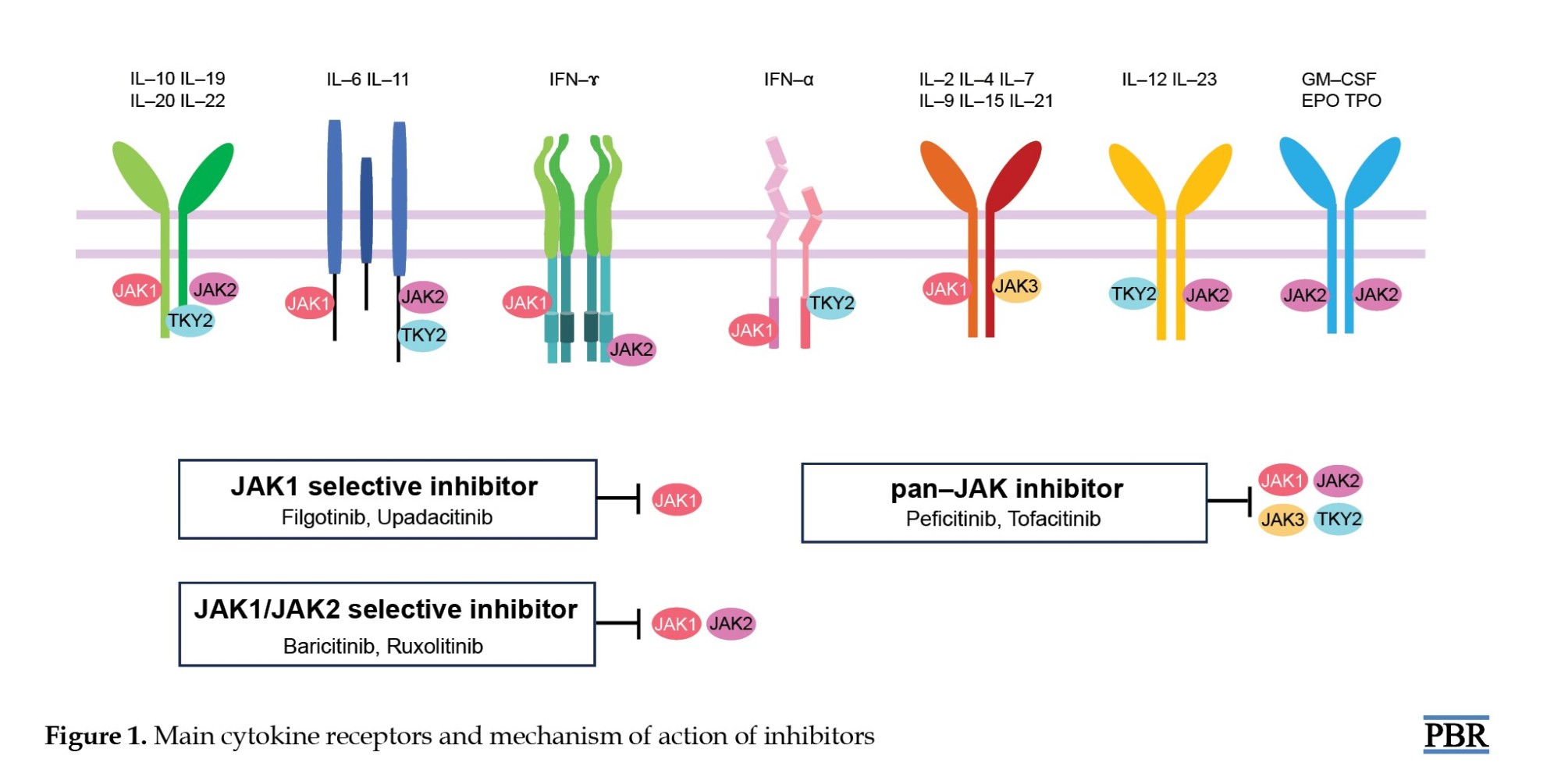
The main action of JAK inhibitors is suppression of inflammation and immune responses [8-12]. Drugs that selectively inhibit JAK1 and JAK3 treat autoimmune diseases such as RA, psoriatic arthritis, ulcerative colitis (UC), and Crohn disease [8-12]. JAK2 inhibitors, however, are used in the treatment of myeloid tumors such as myelofibrosis (MF) and primary myeloproliferative disorders [13, 14].
Tofacitinib is a first-generation selective oral JAK1 and JAK3 inhibitor with low activity against JAK2 and TYK2 [15]. Baricitinib is a JAK1 or JAK2 inhibitor with moderate activity against JYK2 and minimal activity against JAK3 [15]. Upadacitinib and filgotinib increase efficacy by selectively inhibiting JAK1, which is involved in the transmission of inflammatory cytokines, and reduce the risk of hematological adverse events (AEs) by preventing JAK2 inhibition [16-18]. Peficitinib inhibits the enzymatic activity of JAK1, JAK2, JAK3, and TYK2 and is expected to have moderate selectivity for JAK3 inhibition and relatively mild inhibition of JAK2, which may have less impact on red blood cells and platelets [19]. Ruxolitinib, a JAK1 and JAK2 inhibitor, is used for the treatment of polycythemia vera (PV), which is known to be associated with inappropriate JAK2 activation and intermediate- and high-risk primary MF [13, 14].
The common AEs of JAK inhibitor usage include infection, anemia, lymphopenia, liver dysfunction, renal dysfunction, and tumor exacerbation. Upper respiratory tract infections, pneumonia, bronchitis, and gastroenteritis are higher in patients treated with JAK inhibitors than in the general population [20]. Opportunistic infections such as herpes zoster (HZ), tuberculosis, and candidiasis have also been reported. Mainly, HZ is widely known as a high-frequency AE associated with JAK inhibitors, with a high incidence in Japan and other Asian countries [21]. The use of JAK inhibitors may increase the risk of thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), cardiovascular events, and malignancy [22, 23]. Safety data have been critical to the research and development of JAK inhibitors in recent years [23].
Understanding the incidence profile of AEs in patients with complex backgrounds and drug treatments is essential in clinical practice. The spontaneous reporting system (SRS) is a valuable tool for pharmacovigilance, reflecting the realities of clinical practice. In Japan, post-marketing AEs are managed by the Pharmaceuticals and Medical Devices Agency (PMDA), a regulatory authority. The Japanese adverse drug event report (JADER) database is an SRS that compiles data voluntarily submitted by healthcare professionals, pharmaceutical companies, and patients. The incidence profile for AEs associated with JAK inhibitors is unclear, and there are relatively fewer reports affecting the onset time of AEs caused by JAK inhibitors in actual clinical practice. This study evaluated the incidence profiles of AEs associated with JAK inhibitors by analyzing data from the JADER database.
Materials and Methods
Data source
Relevant information from the JADER database, from April 2004 to March 2023, was downloaded from the PMDA website [24]. The data from the JADER database was fully anonymized by the regulatory authority (PMDA) before we accessed it. The JADER database comprises four tables: DEMO table, including patient’s demographic information like gender, age, weight, etc.; DRUG table, including drug information like drug name, causality of drug, etc.; REAC table, including adverse drug reaction, name, outcome, etc.; and HIST table, including medical history like primary diseases, etc. The DEMO table was linked to the DRUG, REAC, and HIST tables using ID numbers. We integrated the relational databases of the four tables from the JADER dataset using MariaDB version. 10.5 [25]. In the DRUG table, each drug was categorized into three codes according to its association with an AE: Suspected drug, concomitant drug, and interacting drug. We only analyzed cases that were categorized as suspected drugs.
Definition of AEs
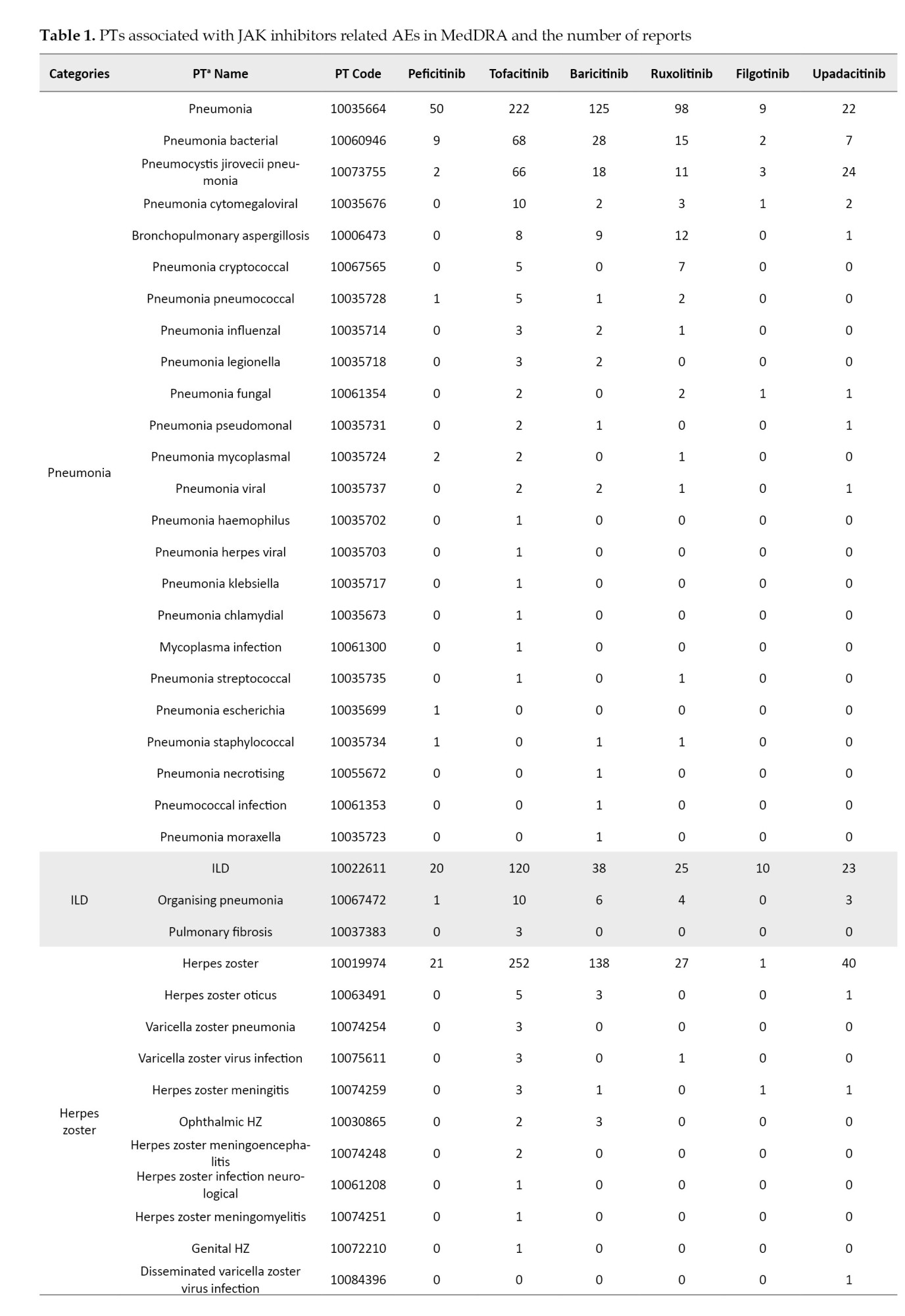
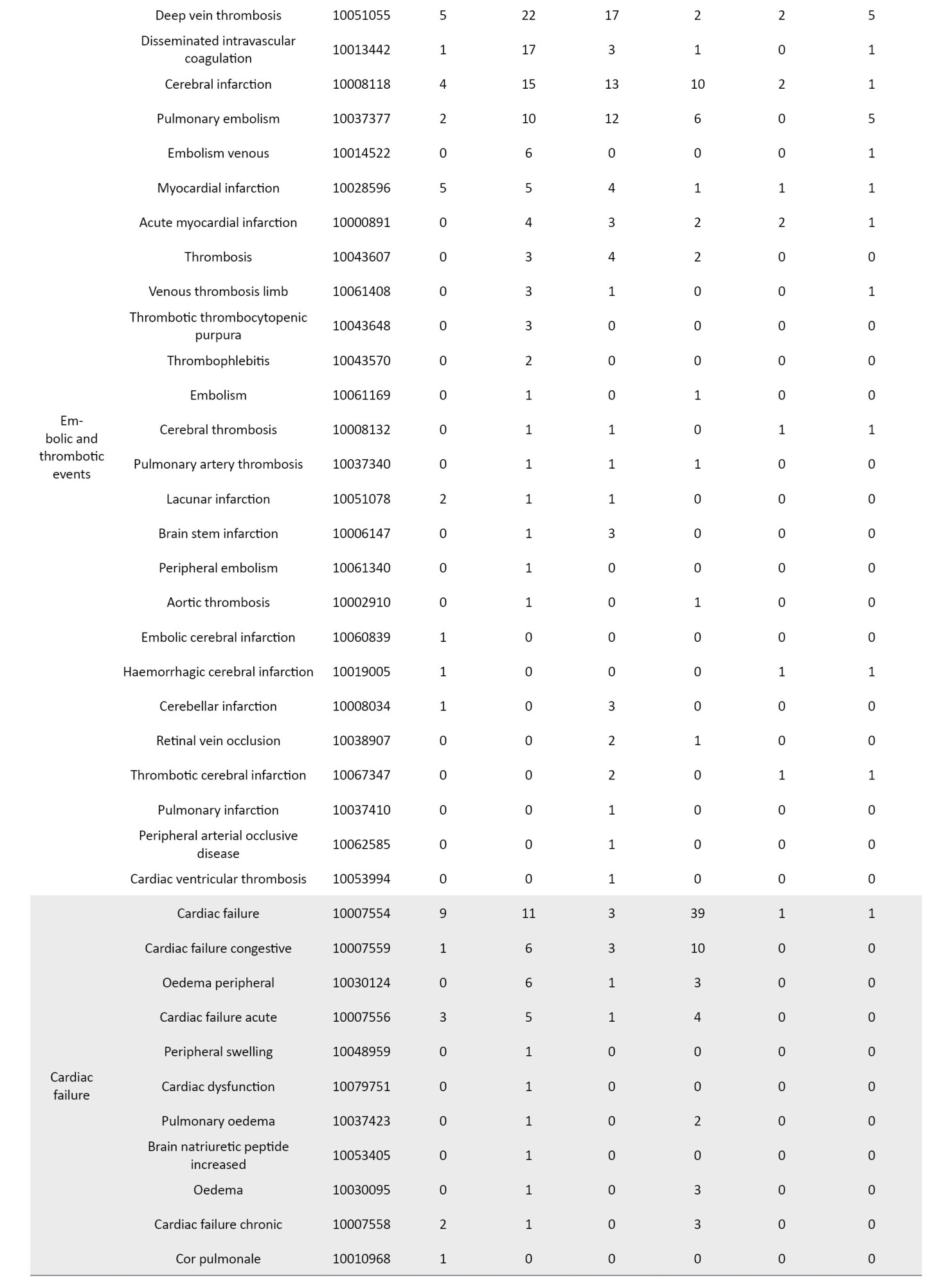
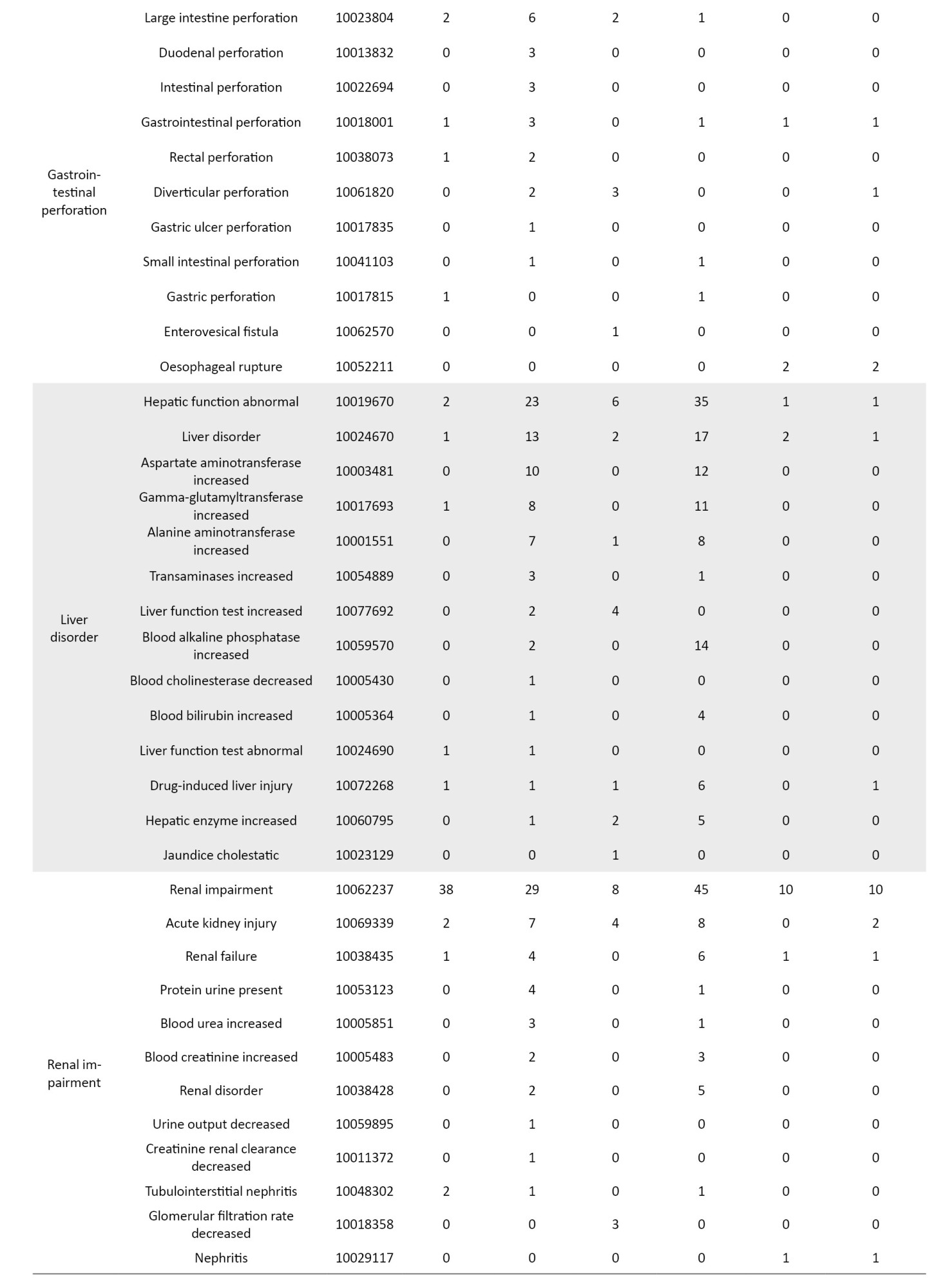
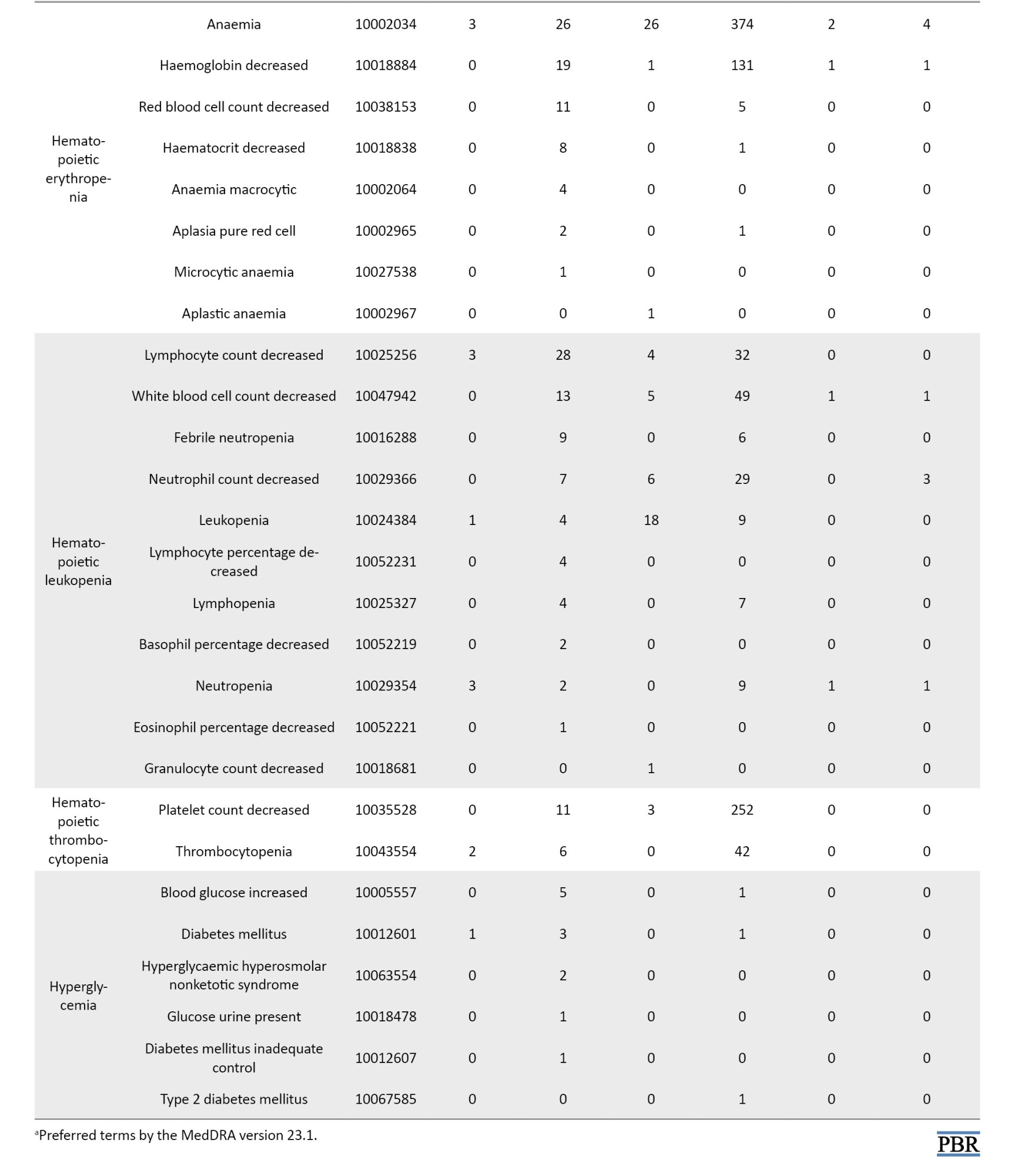
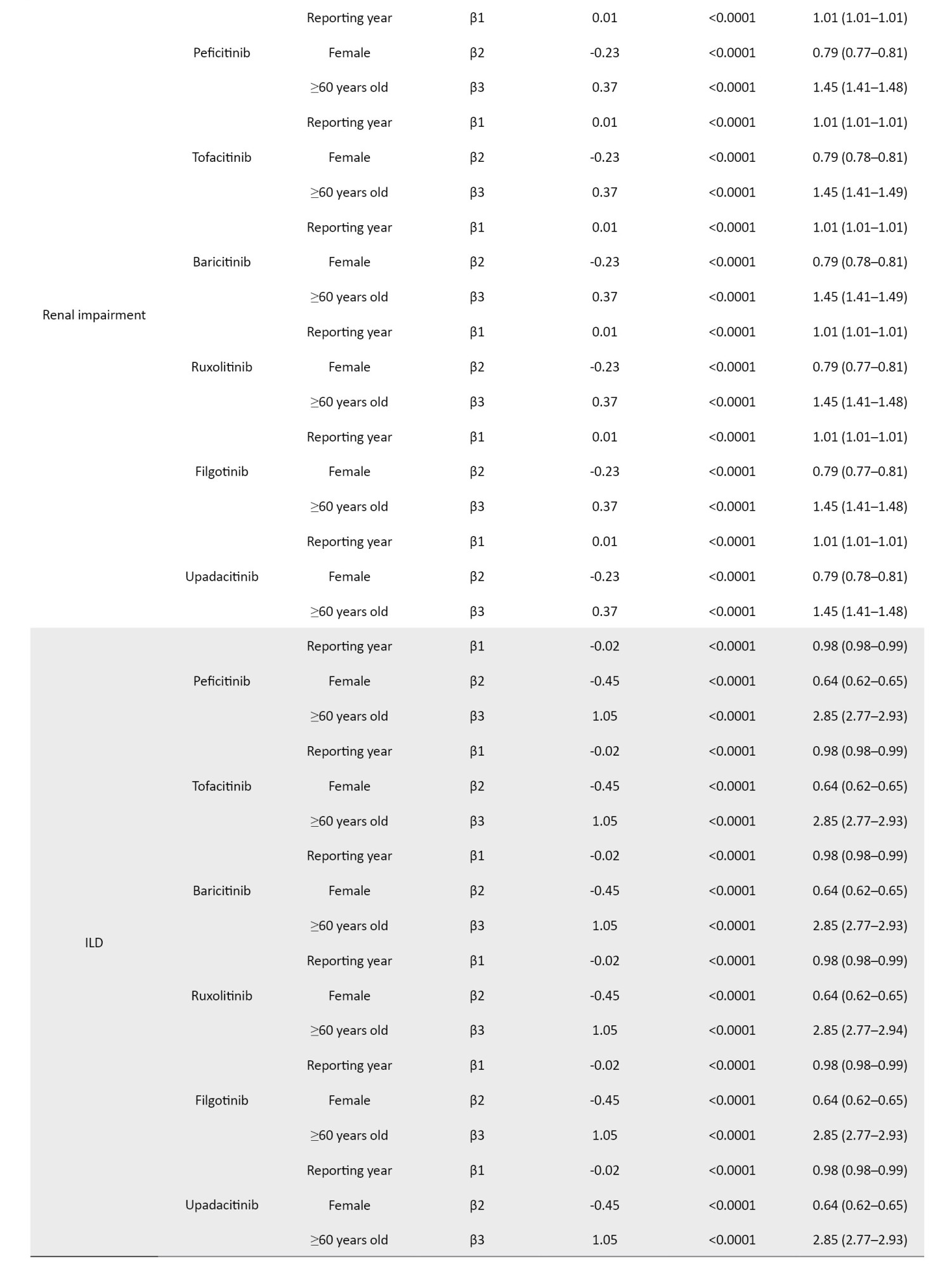
The definition of AEs used in the JADER database is based on MedDRA (medical dictionary for regulatory activities) version 23.1 [26] (Table 1). Standardized MedDRA queries (SMQs) are widely used to analyze SRS reports [26]. SMQs, built by the Maintenance and Support Services Organization, are groups of preferred terms (PTs) categorized according to the level related to a defined medical condition. The grouping of SMQs allows for valuable data retrieval and the presentation of relevant individual case safety reports. The PTs retrieved with the respective SMQs were as follows: “Pneumonia” with 24 PTs for infective pneumonia (SMQ code: 20000231); “interstitial lung disease (ILD)” with 3 PTs for ILD (SMQ code: 20000042); “herpes zoster” with 11 PTs for ocular infections (SMQ code: 20000183); infective pneumonia (SMQ code: 20000231); opportunistic infections (SMQ code: 20000235); “embolic and thrombotic events” with 26 PTs for embolic and thrombotic events, arterial (SMQ code: 20000082); embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ code: 20000083); embolic and thrombotic events, venous (SMQ code: 20000084); “gastrointestinal perforation (GP)” with 11 PTs for GP (SMQ code: 20000107); “liver disorder” with 14 PTs for liver related investigations, signs and symptoms (SMQ code: 20000008); cholestasis and jaundice of hepatic origin (SMQ code: 20000009); hepatic failure, fibrosis and cirrhosis, and other liver damage-related conditions (SMQ code: 20000013); “renal impairment” with 12 PTs for acute renal failure (SMQ code: 20000003); drug reaction with eosinophilia and systemic symptoms syndrome (SMQ code: 20000225); “cardiac failure” with 11 PTs for cardiac failure (SMQ code: 20000004); “hematopoietic erythropenia” with 8 PTs for hematopoietic erythropenia (SMQ code: 20000029); “hematopoietic leukopenia” with 11 PTs for hematopoietic leukopenia (SMQ code: 20000030); “hematopoietic thrombocytopenia” with 2 PTs for hematopoietic thrombocytopenia (SMQ code: 20000031); and “hyperglycemia” with 6 PTs for hyperglycemia/new onset diabetes mellitus (SMQ code: 20000041) (Table 1).
Drug selection
Six oral JAK inhibitors approved in Japan as of 2022 were examined. The JAK inhibitors analyzed in this study, along with the indications in parenthesis, are ruxolitinib (MF, PV), tofacitinib (RA, UC), baricitinib (RA, atopic dermatitis, pneumonia due to SARS-CoV-2, alopecia areata), upadacitinib (RA, psoriatic arthritis, ankylosing spondylitis, atopic dermatitis), filgotinib (RA, UC), and peficitinib (RA, UC).
Signal detection
To detect AEs associated with JAK inhibitors, we calculated the crude reporting odds ratio (ROR) using a 2-by-2 contingency table. Using multiple-logistic regression analysis, we also calculated the adjusted RORs to control for the covariates. We considered a signal detected if the estimated ROR and the lower limit of the corresponding 95% confidence interval (CI) was greater than one and if at least two cases were reported [27]. The reports were stratified by reporting age ≤59 and ≥60 years and sex (male and female). The following multiple logistic regression model (Equation 1) was used for the analysis:
1. log (odds)=β0 + β1Y + β2S + β3A + β4D
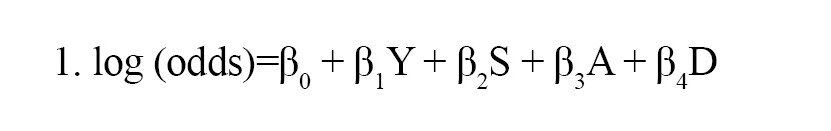
where, Y is the reporting year, S denotes sex, A is the stratified age group, and D is the JAK inhibitor. Data analyses were performed using the statsmodels version 0.13.2 in Python (version 3.8.13) [28].
Time-to-onset analysis
Recently, time-to-onset analysis has been proposed to detect AE signals in SRSs. It has been reported that the incidence of AEs after prescription depends on causative factors and often changes over time. In contrast, AEs not drug-related occur constantly as a background. Therefore, changes in the incidence of AEs over time may indicate a relationship between a drug and the AE [29, 30]. The specific parameter α of the Weibull probability density function determines the scale of the function, and the shape parameter β determines the shape of the function. The shape parameter β of the Weibull distribution was used to display the hazard without a reference population as follows. If the 95% CI of β included 1, the hazard was estimated to be constant (random failure type). If the lower limit of the 95% CI of β was greater than 1, the hazard was considered to increase over time (wear-out failure type). Finally, if the upper limit of the 95% CI of β was less than 1, the hazard was considered to decrease over time (initial failure type). Reports in which the time of occurrence of the AE and the time of prescription initiation were incomplete were excluded from the analysis. We calculated the period from the date of the dose to the date of the first onset of the AE and fitted it to a Weibull function using the parametric SurPyval model in SurPyval (version 0.10.1.0) in Python (version 3.8.13) [31].
Results
A total of 823662 reports were submitted to the JADER database during the study period. Also, 5524 cases were reported with JAK inhibitors as suspected drugs, with the following reporting rates: Peficitinib 8.6% (n=476), tofacitinib 39.2% (n=2164), baricitinib 19.1% (n=1054), ruxolitinib 25.8% (n=1427), filgotinib 2.3% (n=127), and upadacitinib 5.7% (n=314). Women reported 67.6% use of the JAK inhibitors, excluding ruxolitinib, whereas men reported 57.6% use of ruxolitinib. The reporting ratios of AEs in patients in their 80s, 70s, 60s, and 50s who were administered the JAK inhibitors, except for ruxolitinib, were 13.0%, 38.1%, 21.9%, and 7.6%, respectively. Among the reports on ruxolitinib, the percentages of patients with MF and PV were 76.9% and 20.6%, respectively. Of the reports on peficitinib, tofacitinib, baricitinib, filgotinib, and upadacitinib, the percentages of patients with RA history were 98.5%, 83.4%, 85.8%, 81.8%, and 89.7%, respectively.
ROR analysis
The crude and adjusted RORs for the categorized AEs of JAK inhibitors are summarized in Table 2.
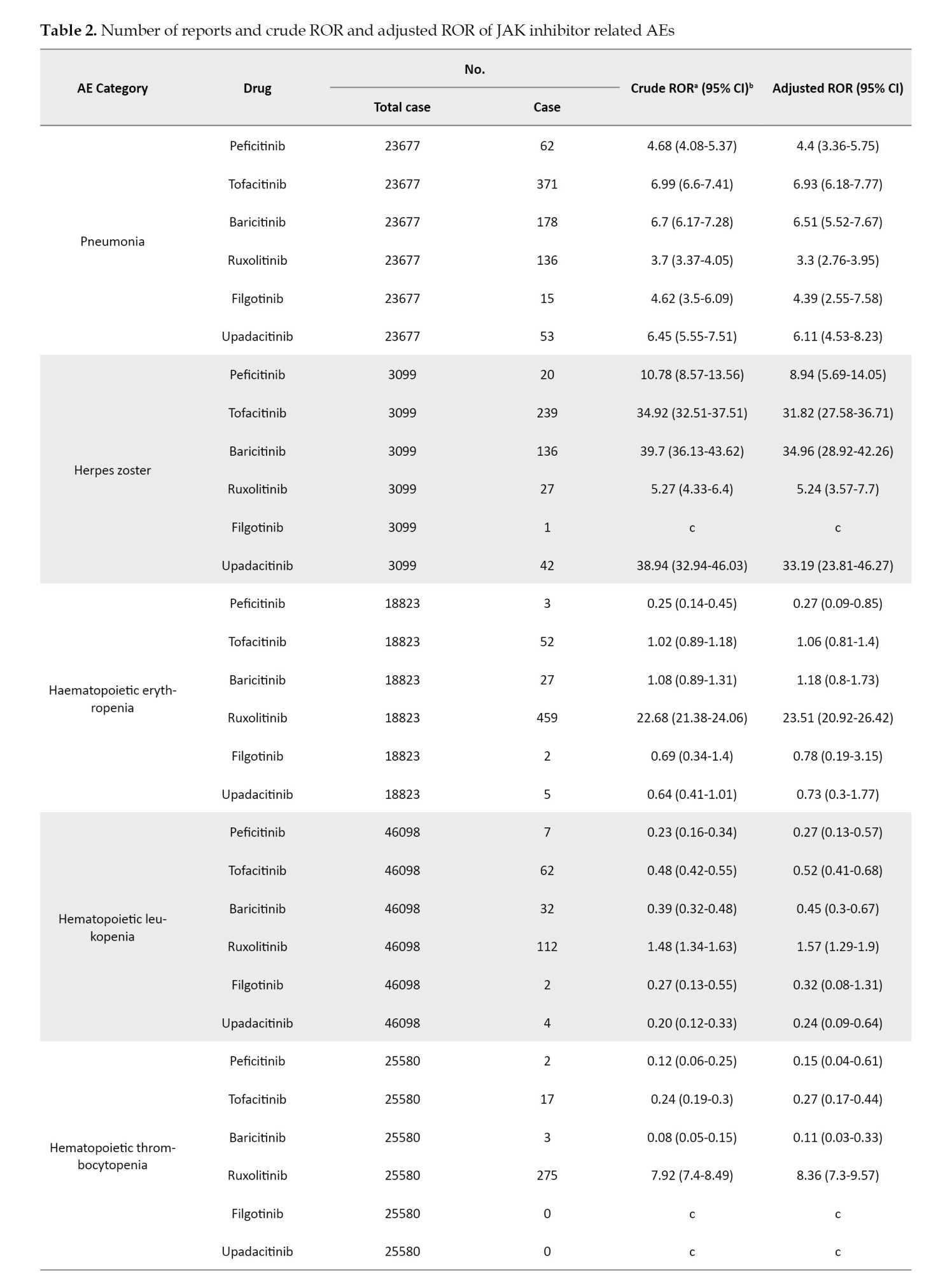
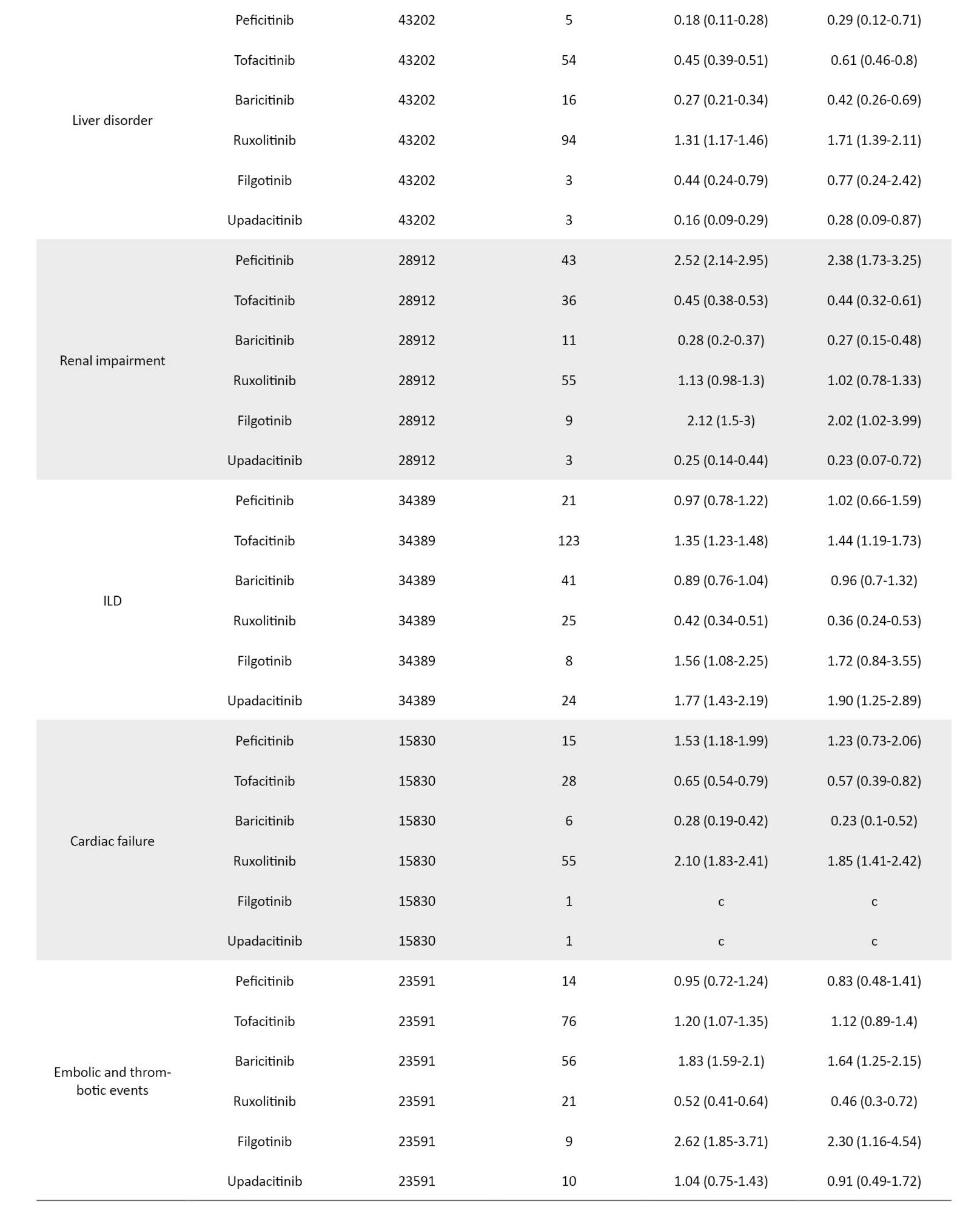
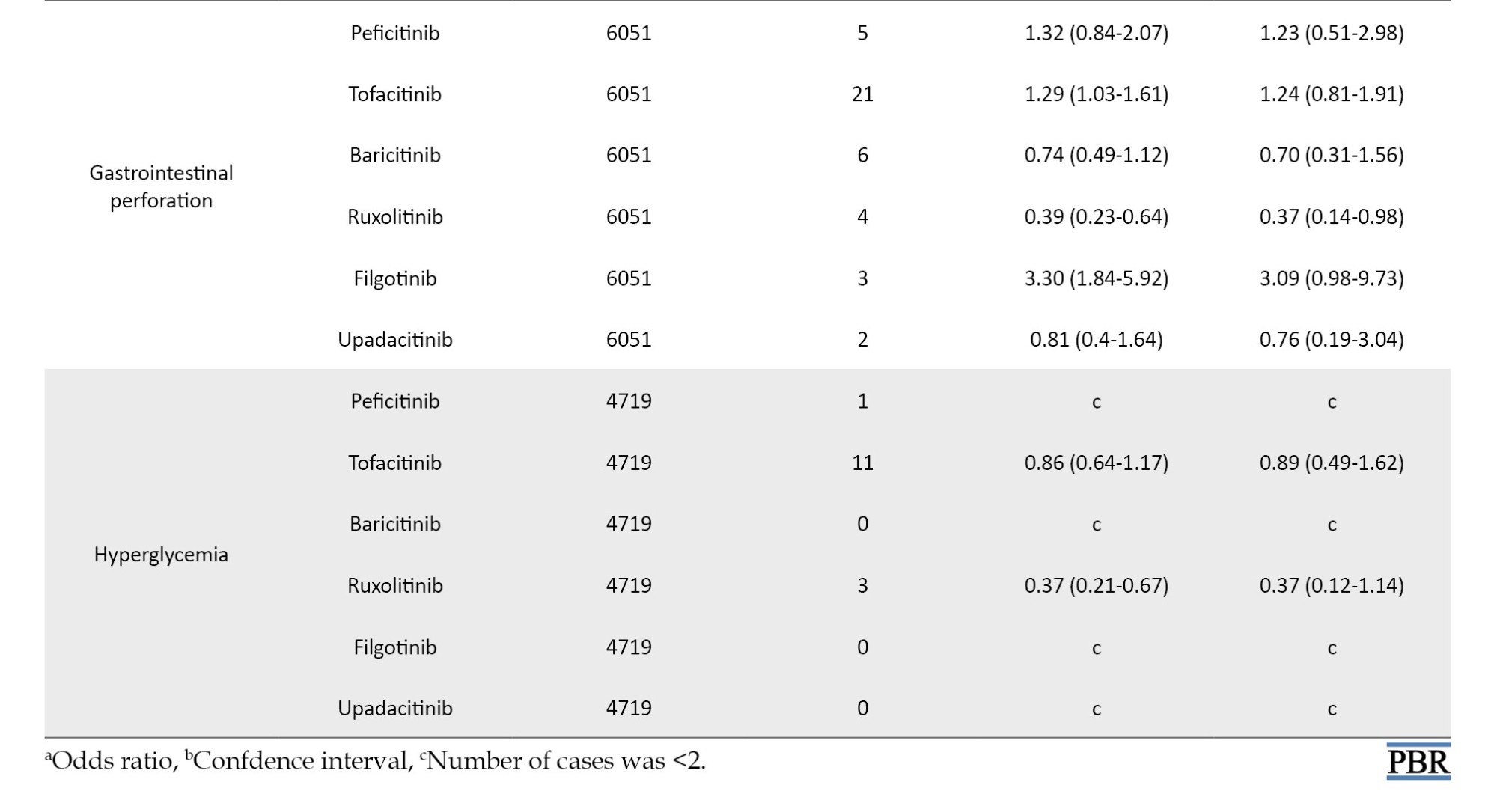
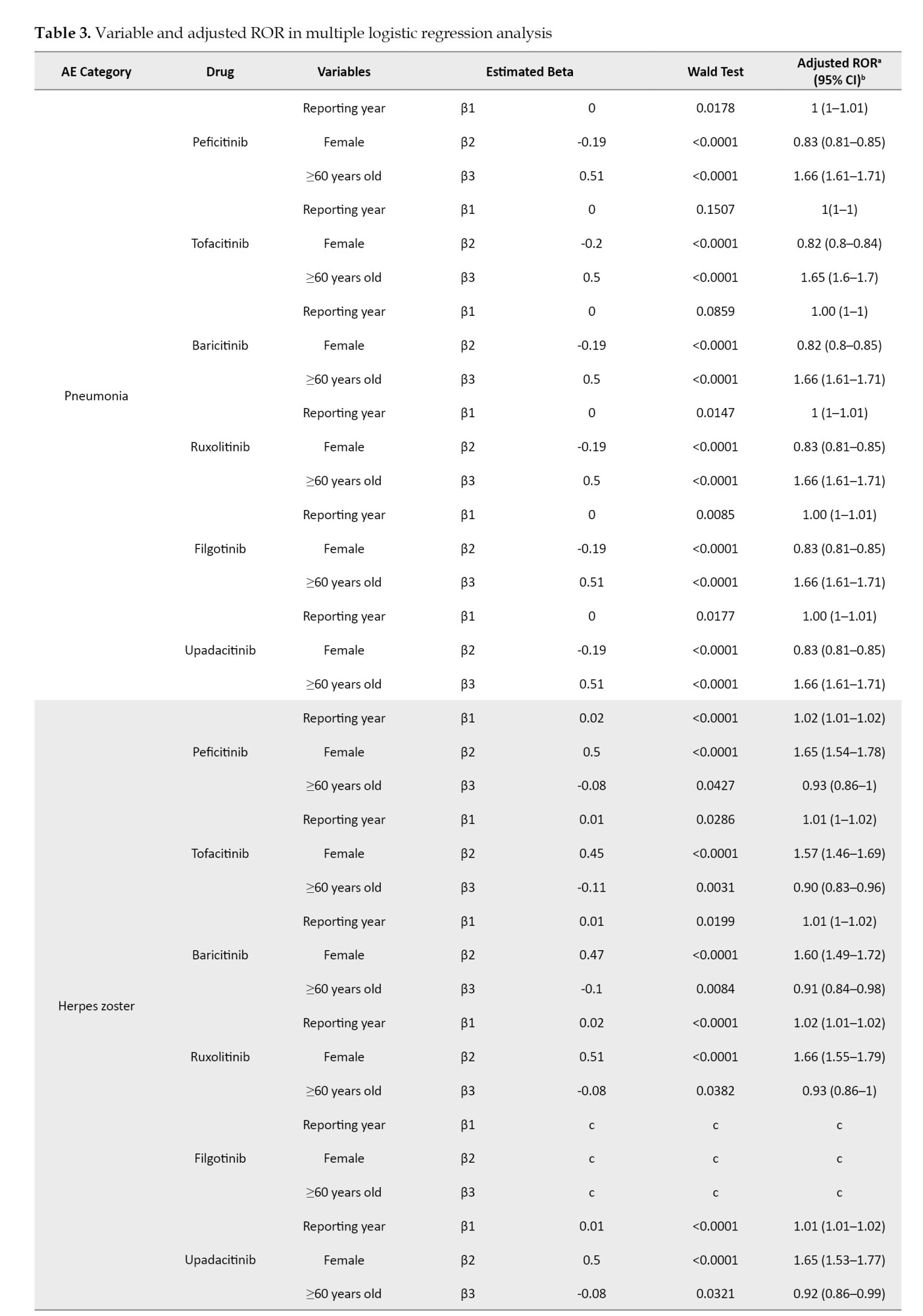
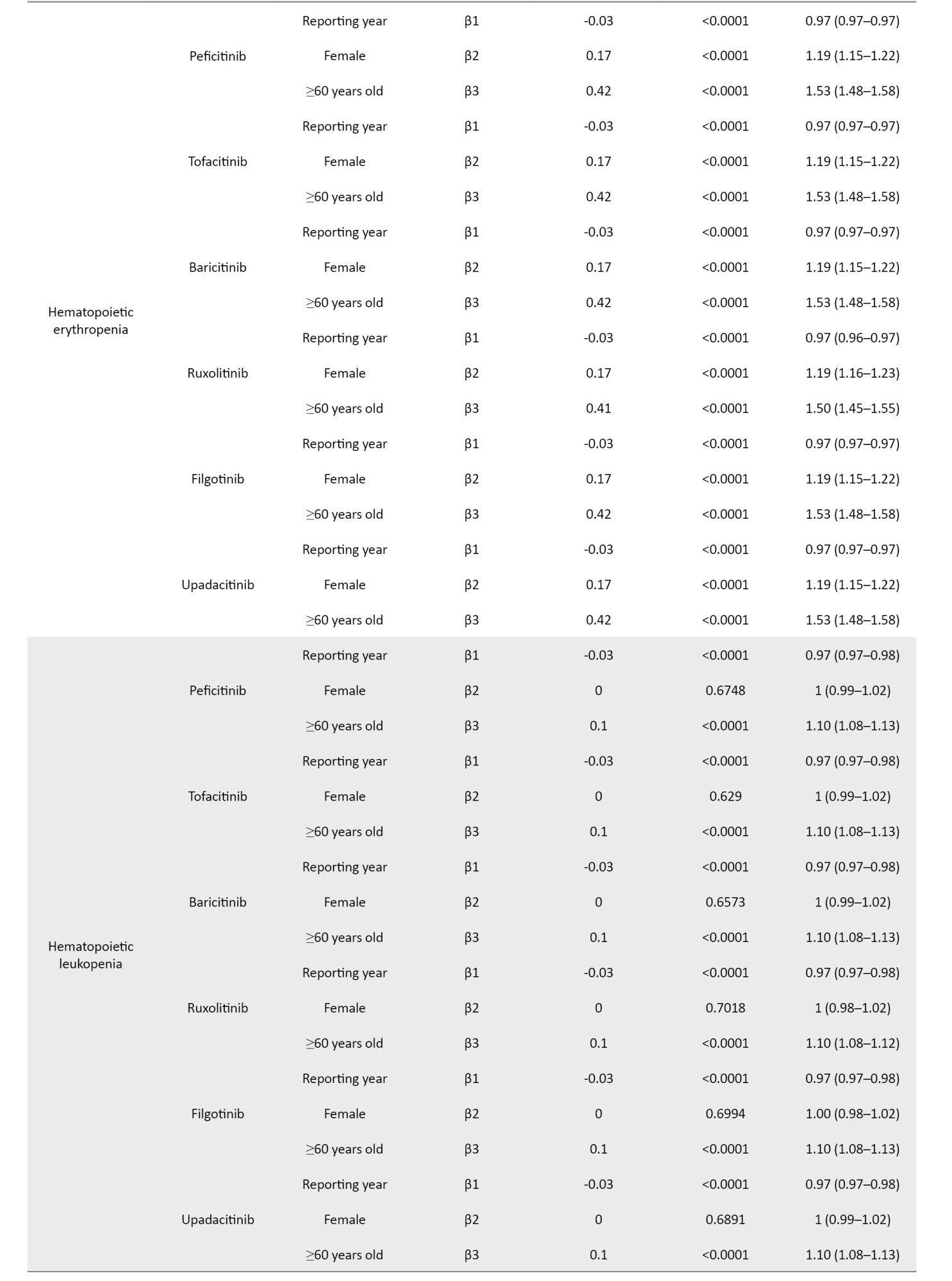
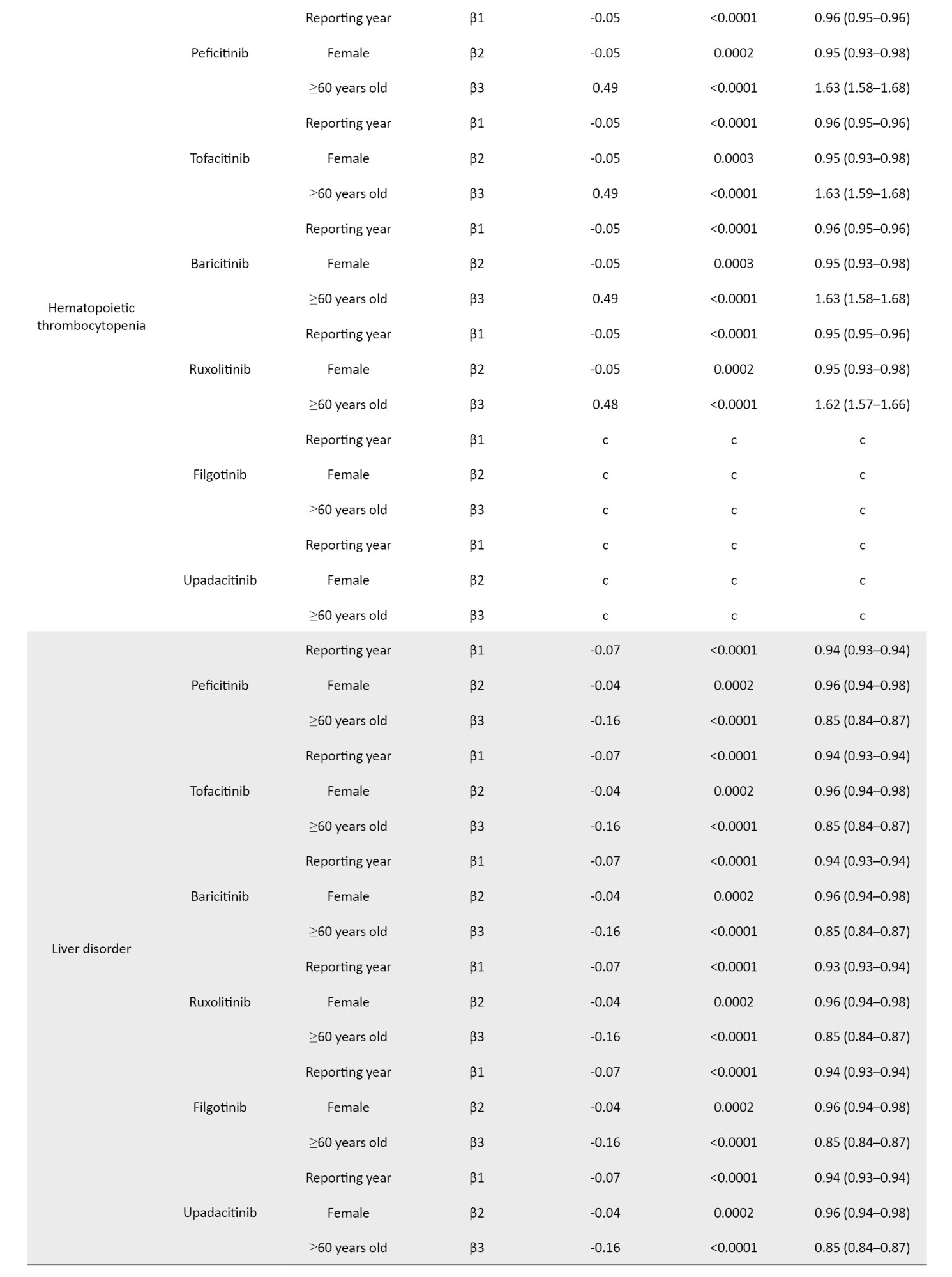
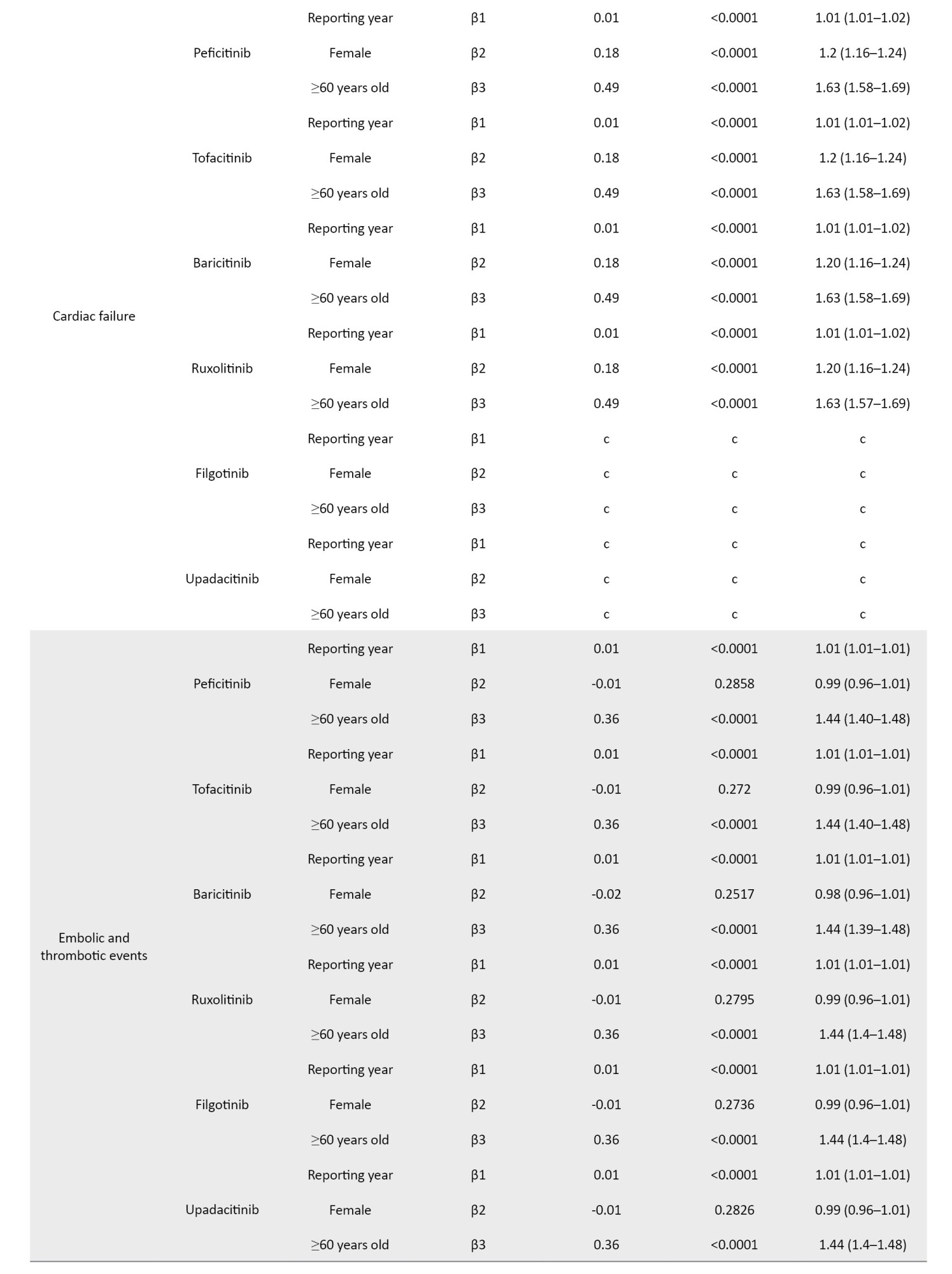
The coefficients of the confounding factors adjusted by multiple logistic regression are shown in Table 3. Adjusted RORs for pneumonia with peficitinib, tofacitinib, baricitinib, ruxolitinib, filgotinib, and upadacitinib were 4.40 (95% CI, 3.36%, 5.75%), 6.93 (95% CI, 6.18%, 7.77%), 6.51 (95% CI, 5.52%, 7.67%), 3.30 (95% CI, 2.76%, 3.95%), 4.39 (95% CI, 2.55%, 7.58%), and 6.11 (95% CI, 4.53%, 8.23%). The adjusted RORs for HZ with peficitinib, tofacitinib, baricitinib, ruxolitinib, and upadacitinib were 8.94 (95% CI, 5.69%, 14.05%), 31.82 (95% CI, 27.58%, 36.71%), 34.96 (95% CI, 28.92%, 42.26%), 5.24 (95% CI, 3.57%, 7.7%), and 33.19 (95% CI, 23.81%, 46.27%), respectively. Adjusted RORs with ruxolitinib for hematopoietic erythropenia, hematopoietic leukopenia, hematopoietic thrombocytopenia, liver disorder, and cardiac failure were 23.51 (95% CI: 20.92%, 26.42%), 1.57 (95% CI: 1.29%, 1.9%), 8.36 (95% CI, 7.3%, 9.57%), 1.71 (95% CI, 1.39%, 2.11%), and 1.85 (95% CI, 1.41%, 2.42%), respectively.
Time-to-onset analysis
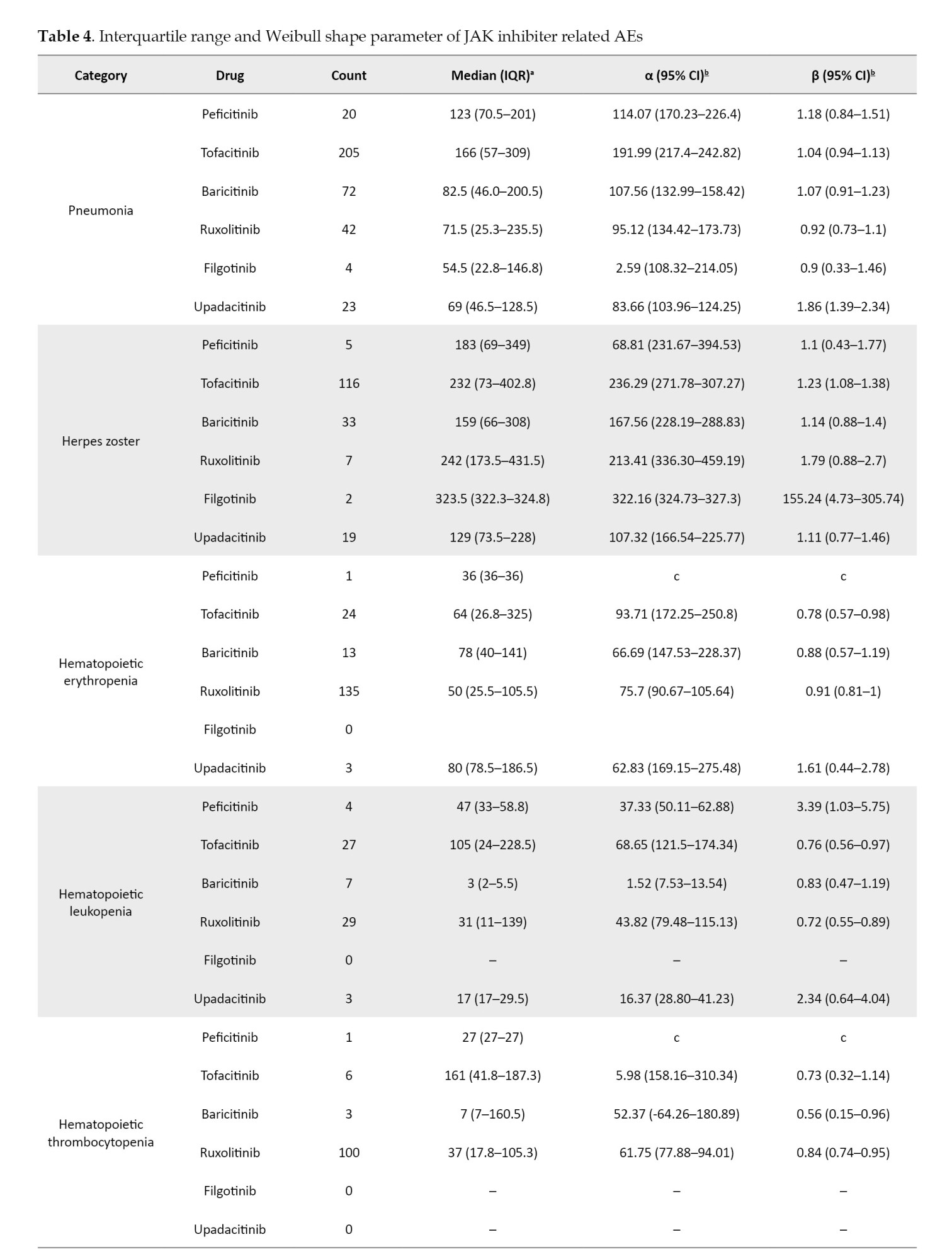
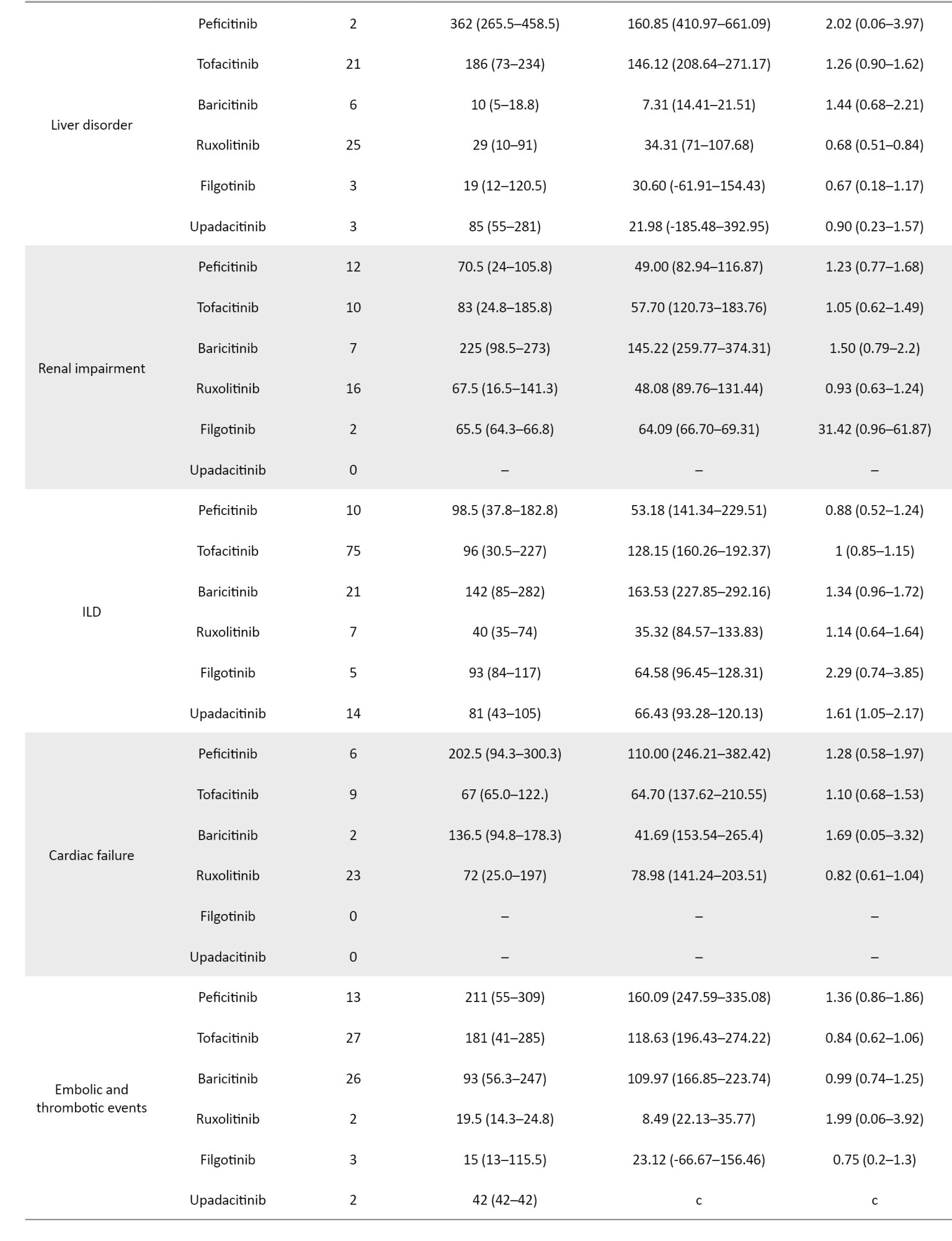
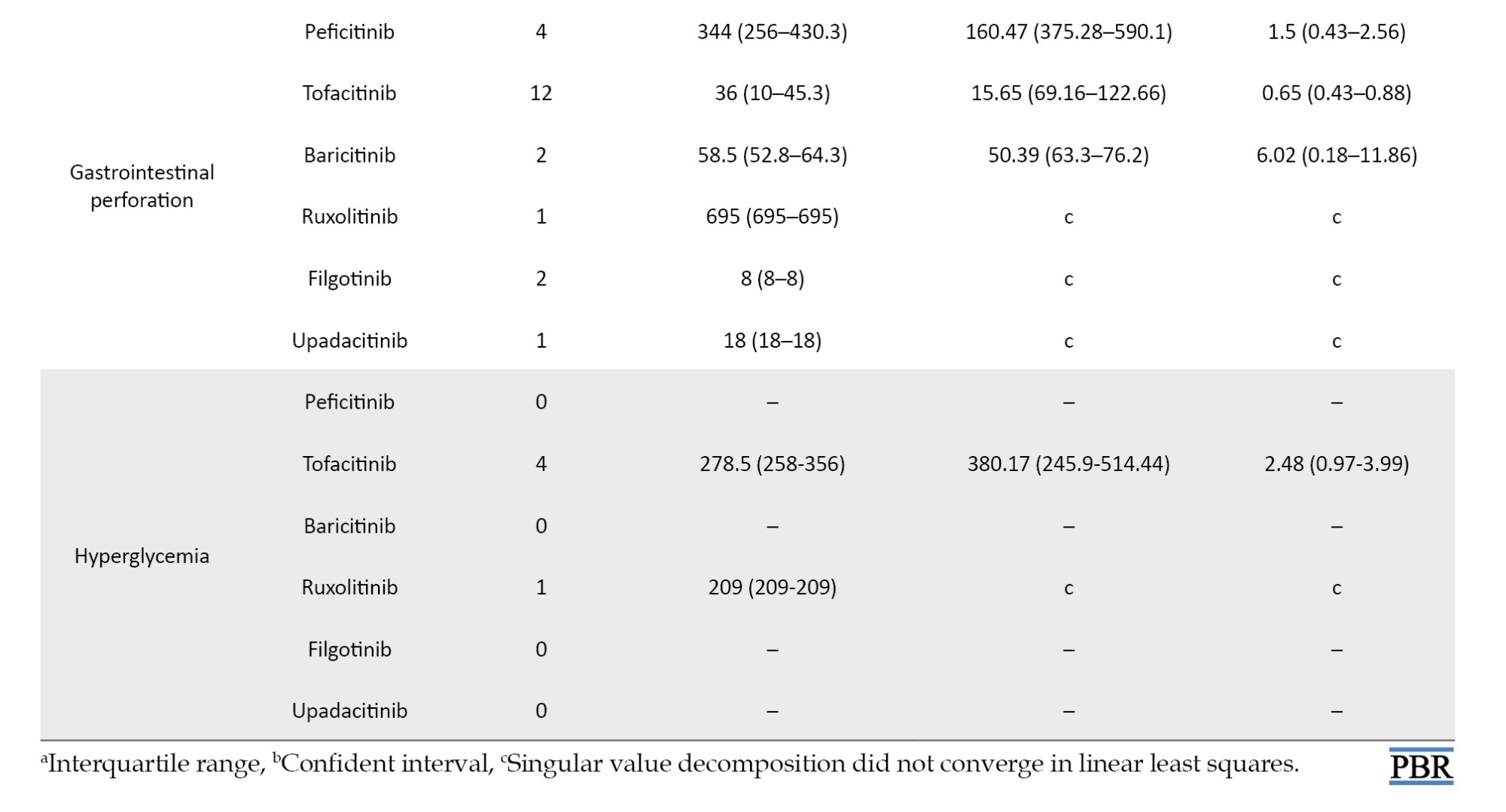
For the time-to-onset analysis, we used only combinations for which we had complete information on the drug start date and AE onset date. Boxplots were created for each JAK inhibitor with respect to the time of onset of each AE (Figure 2).
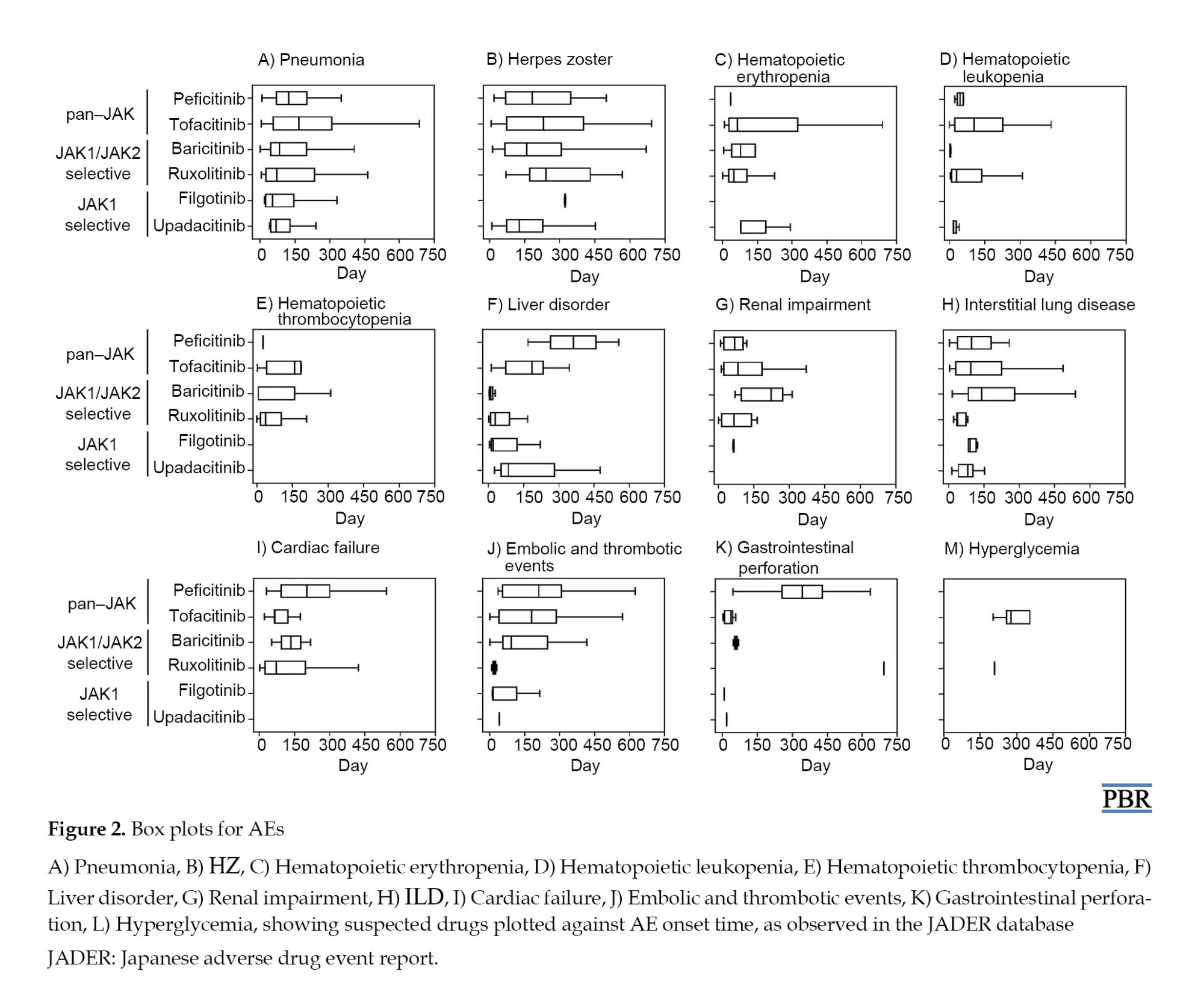
We summarize the median number of days, from the start of treatment to disease onset, and the calculated Weibull parameters in Table 4. The time-to-onset of pneumonia and HZ were highly variable for all JAK inhibitors. For tofacitinib, which was reported most frequently, the median time-to-onset (interquartile range: IQR) and the Weibull parameter β (95% CI) for pneumonia were 166.0 (IQR: 57–309) days and 1.04 (95% CI, 0.94%, 1.13%), and for HZ they were 232 (IQR: 73–402.8) days and 1.23 (95% CI, 1.08%, 1.38%), respectively. Hematopoietic erythropenia, leukopenia, and thrombocytopenia were most frequently reported with ruxolitinib and occurred early in the initiation of treatment. The median time-to-onset (IQR) and the Weibull β values were 50.0 (IQR: 25.5–105.5) days and 0.91 (95% CI, 0.81%, 1.00%) for erythropenia, 31.0 (IQR: 11–139) days and 0.72 (95% CI, 0.55%, 0.89%) for leukopenia, and 37.0 (IQR: 17.8–105.3) days and 0.84 (95% CI, 0.74%, 0.95%) for thrombocytopenia. The incidence of hepatic and renal disorders varied according to the formulation; however, the number of reports was small. ILD was reported at a median of 3 to 4 months after drug initiation. Cardiac failure, embolic and thrombotic events, and GP were reported less frequently; trends in the duration of these AEs could not be ascertained.
Discussion
Pneumonia is a serious infectious event observed in clinical trials involving JAK inhibitors [13, 32-36]. In our study, pneumonia was the most reported infection-related AE, with ROR signals detected. The median time-to-onset of pneumonia ranged from 2 to 6 months with all JAK inhibitors. Multiple logistic regression analysis showed that pneumonia was reported more frequently in males over 60. In safety reports of tofacitinib, males and older people are reported to be at risk of serious infections, including pneumonia [32].
Herpes zoster is also a frequent AE of JAK inhibitor use, as per our study. Severe cases have been reported to date, but infrequently so [32-36]. Irreversible ganglion cell necrosis may result in residual postherpetic neuralgia and sequelae, such as physical disability and mental anguish, making it an AE that requires sufficient vigilance, with a poor prognosis in severe cases of HZ [37]. The degree of inhibition of the JAK3-dependent pathway is low and thus appears to have little effect on homeostatic immune functions that control infection and HZ [1]. A family history of HZ, physical trauma, older age, female gender, psychological stress, and the presence of comorbidities such as diabetes, RA, cardiovascular diseases, kidney disease, systemic lupus erythematosus, and inflammatory bowel disease have been reported as risk factors for HZ and should be addressed according to patient risk [38]. In our study, multiple logistic regression results indicate that HZ is more commonly reported in females under 60. HZ may be underreported in older age groups. The median time-to-onset of HZ with JAK inhibitors ranges from 4 to 7 months. The incidence of severe infections, including pneumonia and HZ, has not been shown to increase with long-term JAK inhibitor use and remains stable over time [32, 33], suggesting the need for constant monitoring of signs of infection during JAK inhibitor use.
The ROR signals for hematopoietic erythropenia, leukopenia, and thrombocytopenia were detected only with ruxolitinib. The AEs associated with ruxolitinib are often anemia and thrombocytopenia due to JAK2 inhibition; however, the treatment discontinuation rate is low [13, 14, 39]. Rates of new or worsening grade 3 or 4 anemia and thrombocytopenia have been reported in clinical trials of MF, with most new or worsening events occurring within the first 6 months of treatment [14, 40]. In a double-blind, placebo-controlled trial of ruxolitinib for MF, anemia, and thrombocytopenia were more common in ruxolitinib-treated patients than in placebo-treated patients. Still, the discontinuation rate was reported to be low (1 patient in each group for each event), and these AEs were manageable [39]. In our study, the time-to-onset of AEs for ruxolitinib was concentrated around 4 months after the initiation of treatment, which is consistent with clinical trial reports. Erythropenia, leukopenia, and thrombocytopenia have also been reported in response to JAK inhibitors other than ruxolitinib. In a safety analysis of tofacitinib, lymphocytopenia was shown to be a risk factor for severe infections and HZ [41, 42]. Laboratory values in patients prescribed with JAK inhibitors should be closely monitored because lymphopenia may be missed if white blood cell percentages are not calculated.
Drug-induced liver injury is the leading cause of acute liver injury. In this study, AEs related to hepatitis B virus (HBV) reactivation were excluded to detect only drug-induced liver dysfunction signals. In previous studies, JAK inhibitors did not appear to be drugs with an exceptionally high risk of HBV-reactivation [43, 44]. The exact mechanism underlying JAK inhibitor-induced liver injury is not understood yet. The ROR signals of liver injury were detected only with ruxolitinib but not with the other JAK inhibitors. Liver injury is not the major AE for JAK inhibitors; however, monitoring liver enzymes is necessary because severe outcomes, including the development of liver failure, have been reported [45]. In our study, with ruxolitinib, most reports of liver injury occur about 3 months after initiation of treatment. Tofacitinib has a wider distribution at the time of hepatic injury onset than ruxolitinib. Therefore, it is considered necessary to monitor liver function not only during the initial period of drug initiation but also throughout its use.
The ROR signals for renal impairment were detected using peficitinib and filgotinib. A safety analysis of clinical trials of tofacitinib in RA reported a serum creatinine elevation of 1.5% as an AE, leading to discontinuation. In contrast, no serum creatinine elevation with long-term use was reported [41]. Serum creatinine should always be monitored during JAK inhibitor use, with early action warranted for elevated creatinine.
JAK inhibitors have not yielded consistent results in detecting ROR signals for interstitial pneumonia. ILD is the most common pulmonary manifestation of lung disease due to RA; hence, JAK inhibitors may not be a risk factor for interstitial pneumonia [46]. Although difficult in patients with pre-existing RA-related ILD, the development of parenchymal abnormalities should be appropriately evaluated before treatment, to differentiate between drug-induced pulmonary toxicity and pulmonary involvement due to the underlying disease. In our study, the shortest median time-to-onset of ILD with JAK inhibitors was 40.0 days for ruxolitinib, and the longest was 142.0 days for baricitinib. Patients should be monitored for symptoms of interstitial pneumonia, such as fever, cough, and dyspnea, especially during the first four months of using JAK inhibitors. They should be instructed to discontinue the drug immediately upon symptom onset.
ROR signals for thromboembolic-related events were detected with baricitinib and filgotinib, whereas those for heart failure were detected only with ruxolitinib. Dose-dependent increases in total, high-density, and low-density lipoprotein cholesterol levels have been reported following JAK inhibitor treatment [47]. However, no association has been reported with major adverse cardiovascular events [48, 49]. Inflammation causes hypercoagulability and is a risk factor for venous thromboembolism (VTE) and arterial thromboembolism, including DVT and PE. It has been reported that RA disease correlates with an increased risk of VTE [50]. Currently, there are reports of DVT and PE during JAK inhibitor use. However, the relationship between JAK inhibitors and an increased risk of these events is unclear [49]. In addition to the underlying disease, patient risk factors and case history should be carefully considered.
Gastrointestinal perforation can occur at any site in the gut, with the contents of the stomach or intestinal tract released into the abdominal cavity. In our study, JAK inhibitors were not associated with ROR signals for GP-related AEs. In a report on tofacitinib, most GPs occurred in the lower gastrointestinal tract, and most cases were treated with concomitant NSAIDs or glucocorticoids [32].
The incidence of GP has been reported to be higher with interleukin (IL)-6 inhibitors than with other anti-rheumatic drugs [51]. Although the detailed mechanism is unclear, IL-6 regulates vascular endothelial growth factor, which is involved in angiogenesis and tissue repair [52]. Dendritic cells derived from Peyer’s patches in the intestinal submucosa express high levels of IL-6 and strongly induce IgA [53], which may derange the intestinal microbiota and delay tissue repair processes. Suppression of IL-6 signaling by JAK1 inhibition may result in GP. Timely diagnosis of GP is difficult as it is infrequent, and the timing of its occurrence is unpredictable. Nevertheless, it is a potentially serious AE that should not be overlooked.
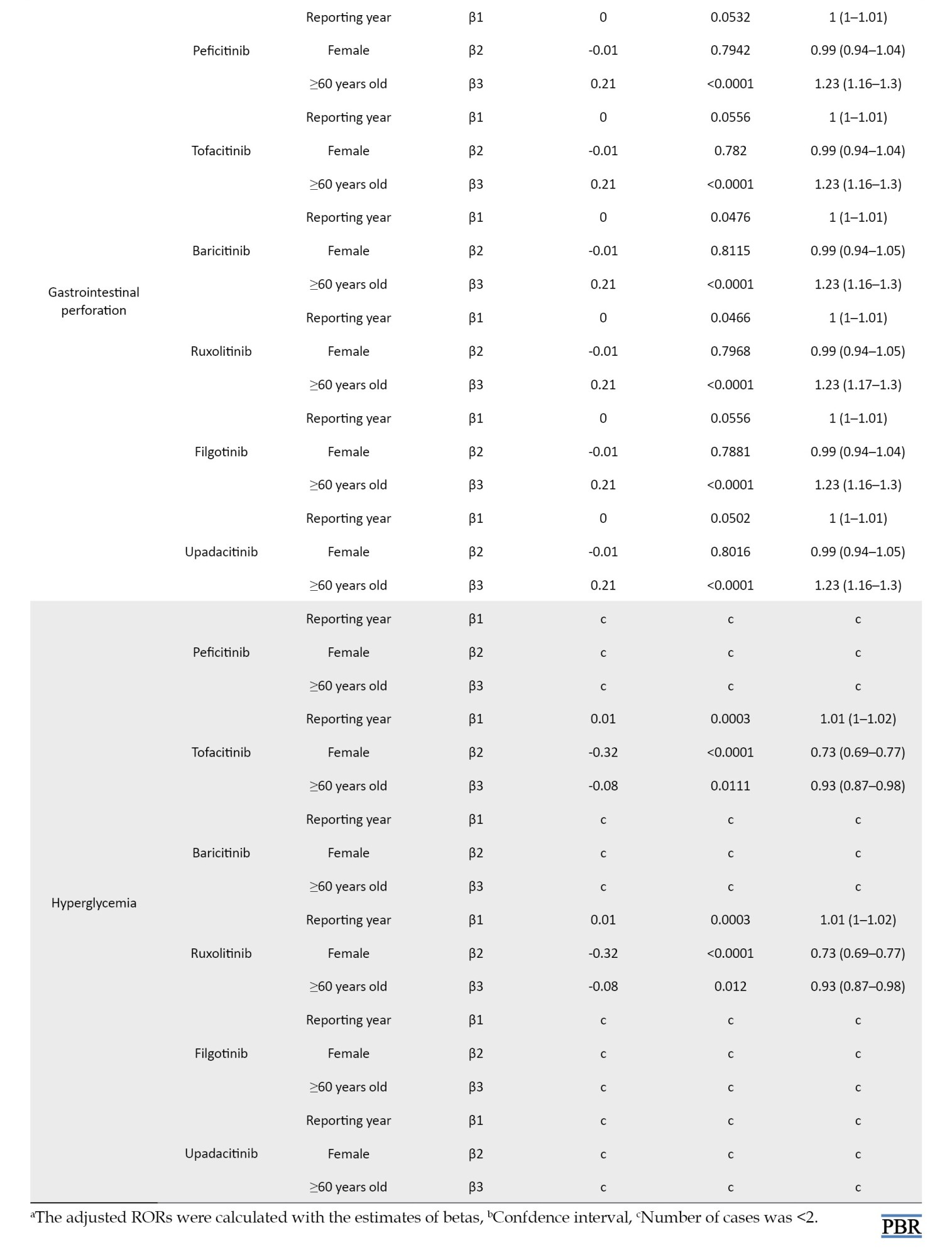
Although reported infrequently, our study found no association between JAK inhibitor use and hyperglycemia (Table 1). To our knowledge, few reports exist on the relationship between JAKs and impaired glucose tolerance.
Analyses using SRS, such as the JADER database, have several notable limitations. Intrinsic problems with the SRS data include over-reporting, under-reporting, missing data, exclusion of healthy individuals, lack of denominators, and confounding factors [54]. It is improbable that the “true” risk of AEs will be evaluated without information on the total number of patients administered with JAK inhibitors. We adjusted for possible confounders in the database using multiple logistic regression methods. The adjusted ROR offers a rough indication of the signal strength, which can be used to generate hypotheses to search for unknown potential AEs. In clinical practice, the AE profile of JAK inhibitors in post-marketing real-world data remains to be established. The JADER is the primary tool available for pharmacovigilance because it is the world’s largest and one of the most widely used databases. Although our results only support the basis of a phenomenon already known in the literature, our results, based on the evaluation of JADER, provide essential knowledge to improve our understanding of this issue. The timing of AE occurrence is also affected by different patient backgrounds. However, our results provide useful findings that reflect real-life scenarios, including the median timing of AE occurrence. This information may be particularly beneficial to prescribers.
Conclusion
Despite the inherent limitations of the SRS, we demonstrated the potential risks of JAK inhibitor use with real-world data. The present analysis shows that patients receiving peficitinib, tofacitinib, baricitinib, ruxolitinib, filgotinib, or upadacitinib should be closely monitored for AEs. The median onset of pneumonia and HZ with JAK inhibitor usage ranged from 2 to 6 months and 4 to 7 months, respectively. We believe that the data presented in this study will aid in detecting various AEs associated with JAK inhibitors early.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was not sought for this study because the study was a database-related observational study without directly involving any research subjects. All results were obtained from data openly available online from the PMDA website. All data from the JADER database were fully anonymized by the relevant regulatory authority before we accessed them.
Funding
This research was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No.: 21K06646 and 21K11100).
Authors' contributions
Conceptualization: Mitsuhiro Nakamura; Methodology: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Data collection: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Data analysis: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Investigation: Hideyuki Tanaka and Mika Maezawa; Writing the original draft: Hideyuki Tanaka, Mika Maezawa, and Mitsuhiro Nakamura; Review and editing: Mari Iwata, Mayumi Kitamura, Megumi Horibe, Hirofumi Tamaki, and Kazuhiro Iguchi; Supervision: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, Yuka Nokura, and Mitsuhiro Nakamura; Funding administration: Mitsuhiro Nakamura.
Conflict of interest
The authors declared no conflict of interest.
References
Janus kinase (JAK) inhibitors act on cytokine signaling pathways involved in inflammatory diseases and immune system abnormalities [1-4]. JAKs are essential for many cytokine families, and biological therapies targeting inflammatory cytokines have critically changed the treatment of rheumatoid arthritis (RA) and other autoimmune diseases. RA, for instance, is associated with the overproduction of interleukin (IL)-6, IL-12, IL-15, IL-23, granulocyte macrophage-colony stimulating factor, and interferons [4].
Four members of the JAK family, JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), are involved in different cytokine signaling pathways. JAK1, JAK2, and TYK2 are ubiquitously expressed, while expression of JAK3 is mainly restricted to cells of hematopoietic origin [2]. JAK1 is primarily involved in inflammatory and innate immune responses. Inhibition of JAK1 suppresses IL-6 signaling, which is central to inflammatory disease [1]. JAK2 is essential for erythropoiesis, myelopoiesis, and platelet production. JAK3 is vital for lymphocyte proliferation and homeostasis [2-4]. The IL-2 family of cytokines (IL-2, -4, -7, -9, -15, and -21) signal through JAK3-bound receptors [3]. IL-2 is a cytokine secreted by antigen stimulation of T cell receptors that drives T cell growth, augments natural killer (NK) cytolytic activity, induces the differentiation of regulatory T cells, and mediates activation-induced cell death [5-7]. The function of IL-15 is the maintenance of NK cells and CD8+CD44h memory T cells to provide a long-term immune response to pathogens [7]. TYK2 is associated with antiviral responses [1-4]. Therefore, differences in JAK inhibitor selectivity for cytokine signaling via distinct JAK pairs may provide a mechanistic rationale for reported differences in safety profiles (Figure 1) [1].

The main action of JAK inhibitors is suppression of inflammation and immune responses [8-12]. Drugs that selectively inhibit JAK1 and JAK3 treat autoimmune diseases such as RA, psoriatic arthritis, ulcerative colitis (UC), and Crohn disease [8-12]. JAK2 inhibitors, however, are used in the treatment of myeloid tumors such as myelofibrosis (MF) and primary myeloproliferative disorders [13, 14].
Tofacitinib is a first-generation selective oral JAK1 and JAK3 inhibitor with low activity against JAK2 and TYK2 [15]. Baricitinib is a JAK1 or JAK2 inhibitor with moderate activity against JYK2 and minimal activity against JAK3 [15]. Upadacitinib and filgotinib increase efficacy by selectively inhibiting JAK1, which is involved in the transmission of inflammatory cytokines, and reduce the risk of hematological adverse events (AEs) by preventing JAK2 inhibition [16-18]. Peficitinib inhibits the enzymatic activity of JAK1, JAK2, JAK3, and TYK2 and is expected to have moderate selectivity for JAK3 inhibition and relatively mild inhibition of JAK2, which may have less impact on red blood cells and platelets [19]. Ruxolitinib, a JAK1 and JAK2 inhibitor, is used for the treatment of polycythemia vera (PV), which is known to be associated with inappropriate JAK2 activation and intermediate- and high-risk primary MF [13, 14].
The common AEs of JAK inhibitor usage include infection, anemia, lymphopenia, liver dysfunction, renal dysfunction, and tumor exacerbation. Upper respiratory tract infections, pneumonia, bronchitis, and gastroenteritis are higher in patients treated with JAK inhibitors than in the general population [20]. Opportunistic infections such as herpes zoster (HZ), tuberculosis, and candidiasis have also been reported. Mainly, HZ is widely known as a high-frequency AE associated with JAK inhibitors, with a high incidence in Japan and other Asian countries [21]. The use of JAK inhibitors may increase the risk of thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), cardiovascular events, and malignancy [22, 23]. Safety data have been critical to the research and development of JAK inhibitors in recent years [23].
Understanding the incidence profile of AEs in patients with complex backgrounds and drug treatments is essential in clinical practice. The spontaneous reporting system (SRS) is a valuable tool for pharmacovigilance, reflecting the realities of clinical practice. In Japan, post-marketing AEs are managed by the Pharmaceuticals and Medical Devices Agency (PMDA), a regulatory authority. The Japanese adverse drug event report (JADER) database is an SRS that compiles data voluntarily submitted by healthcare professionals, pharmaceutical companies, and patients. The incidence profile for AEs associated with JAK inhibitors is unclear, and there are relatively fewer reports affecting the onset time of AEs caused by JAK inhibitors in actual clinical practice. This study evaluated the incidence profiles of AEs associated with JAK inhibitors by analyzing data from the JADER database.
Materials and Methods
Data source
Relevant information from the JADER database, from April 2004 to March 2023, was downloaded from the PMDA website [24]. The data from the JADER database was fully anonymized by the regulatory authority (PMDA) before we accessed it. The JADER database comprises four tables: DEMO table, including patient’s demographic information like gender, age, weight, etc.; DRUG table, including drug information like drug name, causality of drug, etc.; REAC table, including adverse drug reaction, name, outcome, etc.; and HIST table, including medical history like primary diseases, etc. The DEMO table was linked to the DRUG, REAC, and HIST tables using ID numbers. We integrated the relational databases of the four tables from the JADER dataset using MariaDB version. 10.5 [25]. In the DRUG table, each drug was categorized into three codes according to its association with an AE: Suspected drug, concomitant drug, and interacting drug. We only analyzed cases that were categorized as suspected drugs.
Definition of AEs
The definition of AEs used in the JADER database is based on MedDRA (medical dictionary for regulatory activities) version 23.1 [26] (Table 1). Standardized MedDRA queries (SMQs) are widely used to analyze SRS reports [26]. SMQs, built by the Maintenance and Support Services Organization, are groups of preferred terms (PTs) categorized according to the level related to a defined medical condition. The grouping of SMQs allows for valuable data retrieval and the presentation of relevant individual case safety reports. The PTs retrieved with the respective SMQs were as follows: “Pneumonia” with 24 PTs for infective pneumonia (SMQ code: 20000231); “interstitial lung disease (ILD)” with 3 PTs for ILD (SMQ code: 20000042); “herpes zoster” with 11 PTs for ocular infections (SMQ code: 20000183); infective pneumonia (SMQ code: 20000231); opportunistic infections (SMQ code: 20000235); “embolic and thrombotic events” with 26 PTs for embolic and thrombotic events, arterial (SMQ code: 20000082); embolic and thrombotic events, vessel type unspecified and mixed arterial and venous (SMQ code: 20000083); embolic and thrombotic events, venous (SMQ code: 20000084); “gastrointestinal perforation (GP)” with 11 PTs for GP (SMQ code: 20000107); “liver disorder” with 14 PTs for liver related investigations, signs and symptoms (SMQ code: 20000008); cholestasis and jaundice of hepatic origin (SMQ code: 20000009); hepatic failure, fibrosis and cirrhosis, and other liver damage-related conditions (SMQ code: 20000013); “renal impairment” with 12 PTs for acute renal failure (SMQ code: 20000003); drug reaction with eosinophilia and systemic symptoms syndrome (SMQ code: 20000225); “cardiac failure” with 11 PTs for cardiac failure (SMQ code: 20000004); “hematopoietic erythropenia” with 8 PTs for hematopoietic erythropenia (SMQ code: 20000029); “hematopoietic leukopenia” with 11 PTs for hematopoietic leukopenia (SMQ code: 20000030); “hematopoietic thrombocytopenia” with 2 PTs for hematopoietic thrombocytopenia (SMQ code: 20000031); and “hyperglycemia” with 6 PTs for hyperglycemia/new onset diabetes mellitus (SMQ code: 20000041) (Table 1).




Drug selection
Six oral JAK inhibitors approved in Japan as of 2022 were examined. The JAK inhibitors analyzed in this study, along with the indications in parenthesis, are ruxolitinib (MF, PV), tofacitinib (RA, UC), baricitinib (RA, atopic dermatitis, pneumonia due to SARS-CoV-2, alopecia areata), upadacitinib (RA, psoriatic arthritis, ankylosing spondylitis, atopic dermatitis), filgotinib (RA, UC), and peficitinib (RA, UC).
Signal detection
To detect AEs associated with JAK inhibitors, we calculated the crude reporting odds ratio (ROR) using a 2-by-2 contingency table. Using multiple-logistic regression analysis, we also calculated the adjusted RORs to control for the covariates. We considered a signal detected if the estimated ROR and the lower limit of the corresponding 95% confidence interval (CI) was greater than one and if at least two cases were reported [27]. The reports were stratified by reporting age ≤59 and ≥60 years and sex (male and female). The following multiple logistic regression model (Equation 1) was used for the analysis:
1. log (odds)=β0 + β1Y + β2S + β3A + β4D
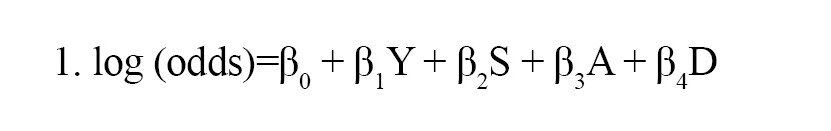
where, Y is the reporting year, S denotes sex, A is the stratified age group, and D is the JAK inhibitor. Data analyses were performed using the statsmodels version 0.13.2 in Python (version 3.8.13) [28].
Time-to-onset analysis
Recently, time-to-onset analysis has been proposed to detect AE signals in SRSs. It has been reported that the incidence of AEs after prescription depends on causative factors and often changes over time. In contrast, AEs not drug-related occur constantly as a background. Therefore, changes in the incidence of AEs over time may indicate a relationship between a drug and the AE [29, 30]. The specific parameter α of the Weibull probability density function determines the scale of the function, and the shape parameter β determines the shape of the function. The shape parameter β of the Weibull distribution was used to display the hazard without a reference population as follows. If the 95% CI of β included 1, the hazard was estimated to be constant (random failure type). If the lower limit of the 95% CI of β was greater than 1, the hazard was considered to increase over time (wear-out failure type). Finally, if the upper limit of the 95% CI of β was less than 1, the hazard was considered to decrease over time (initial failure type). Reports in which the time of occurrence of the AE and the time of prescription initiation were incomplete were excluded from the analysis. We calculated the period from the date of the dose to the date of the first onset of the AE and fitted it to a Weibull function using the parametric SurPyval model in SurPyval (version 0.10.1.0) in Python (version 3.8.13) [31].
Results
A total of 823662 reports were submitted to the JADER database during the study period. Also, 5524 cases were reported with JAK inhibitors as suspected drugs, with the following reporting rates: Peficitinib 8.6% (n=476), tofacitinib 39.2% (n=2164), baricitinib 19.1% (n=1054), ruxolitinib 25.8% (n=1427), filgotinib 2.3% (n=127), and upadacitinib 5.7% (n=314). Women reported 67.6% use of the JAK inhibitors, excluding ruxolitinib, whereas men reported 57.6% use of ruxolitinib. The reporting ratios of AEs in patients in their 80s, 70s, 60s, and 50s who were administered the JAK inhibitors, except for ruxolitinib, were 13.0%, 38.1%, 21.9%, and 7.6%, respectively. Among the reports on ruxolitinib, the percentages of patients with MF and PV were 76.9% and 20.6%, respectively. Of the reports on peficitinib, tofacitinib, baricitinib, filgotinib, and upadacitinib, the percentages of patients with RA history were 98.5%, 83.4%, 85.8%, 81.8%, and 89.7%, respectively.
ROR analysis
The crude and adjusted RORs for the categorized AEs of JAK inhibitors are summarized in Table 2.



The coefficients of the confounding factors adjusted by multiple logistic regression are shown in Table 3. Adjusted RORs for pneumonia with peficitinib, tofacitinib, baricitinib, ruxolitinib, filgotinib, and upadacitinib were 4.40 (95% CI, 3.36%, 5.75%), 6.93 (95% CI, 6.18%, 7.77%), 6.51 (95% CI, 5.52%, 7.67%), 3.30 (95% CI, 2.76%, 3.95%), 4.39 (95% CI, 2.55%, 7.58%), and 6.11 (95% CI, 4.53%, 8.23%). The adjusted RORs for HZ with peficitinib, tofacitinib, baricitinib, ruxolitinib, and upadacitinib were 8.94 (95% CI, 5.69%, 14.05%), 31.82 (95% CI, 27.58%, 36.71%), 34.96 (95% CI, 28.92%, 42.26%), 5.24 (95% CI, 3.57%, 7.7%), and 33.19 (95% CI, 23.81%, 46.27%), respectively. Adjusted RORs with ruxolitinib for hematopoietic erythropenia, hematopoietic leukopenia, hematopoietic thrombocytopenia, liver disorder, and cardiac failure were 23.51 (95% CI: 20.92%, 26.42%), 1.57 (95% CI: 1.29%, 1.9%), 8.36 (95% CI, 7.3%, 9.57%), 1.71 (95% CI, 1.39%, 2.11%), and 1.85 (95% CI, 1.41%, 2.42%), respectively.






Time-to-onset analysis
For the time-to-onset analysis, we used only combinations for which we had complete information on the drug start date and AE onset date. Boxplots were created for each JAK inhibitor with respect to the time of onset of each AE (Figure 2).

We summarize the median number of days, from the start of treatment to disease onset, and the calculated Weibull parameters in Table 4. The time-to-onset of pneumonia and HZ were highly variable for all JAK inhibitors. For tofacitinib, which was reported most frequently, the median time-to-onset (interquartile range: IQR) and the Weibull parameter β (95% CI) for pneumonia were 166.0 (IQR: 57–309) days and 1.04 (95% CI, 0.94%, 1.13%), and for HZ they were 232 (IQR: 73–402.8) days and 1.23 (95% CI, 1.08%, 1.38%), respectively. Hematopoietic erythropenia, leukopenia, and thrombocytopenia were most frequently reported with ruxolitinib and occurred early in the initiation of treatment. The median time-to-onset (IQR) and the Weibull β values were 50.0 (IQR: 25.5–105.5) days and 0.91 (95% CI, 0.81%, 1.00%) for erythropenia, 31.0 (IQR: 11–139) days and 0.72 (95% CI, 0.55%, 0.89%) for leukopenia, and 37.0 (IQR: 17.8–105.3) days and 0.84 (95% CI, 0.74%, 0.95%) for thrombocytopenia. The incidence of hepatic and renal disorders varied according to the formulation; however, the number of reports was small. ILD was reported at a median of 3 to 4 months after drug initiation. Cardiac failure, embolic and thrombotic events, and GP were reported less frequently; trends in the duration of these AEs could not be ascertained.



Discussion
Pneumonia is a serious infectious event observed in clinical trials involving JAK inhibitors [13, 32-36]. In our study, pneumonia was the most reported infection-related AE, with ROR signals detected. The median time-to-onset of pneumonia ranged from 2 to 6 months with all JAK inhibitors. Multiple logistic regression analysis showed that pneumonia was reported more frequently in males over 60. In safety reports of tofacitinib, males and older people are reported to be at risk of serious infections, including pneumonia [32].
Herpes zoster is also a frequent AE of JAK inhibitor use, as per our study. Severe cases have been reported to date, but infrequently so [32-36]. Irreversible ganglion cell necrosis may result in residual postherpetic neuralgia and sequelae, such as physical disability and mental anguish, making it an AE that requires sufficient vigilance, with a poor prognosis in severe cases of HZ [37]. The degree of inhibition of the JAK3-dependent pathway is low and thus appears to have little effect on homeostatic immune functions that control infection and HZ [1]. A family history of HZ, physical trauma, older age, female gender, psychological stress, and the presence of comorbidities such as diabetes, RA, cardiovascular diseases, kidney disease, systemic lupus erythematosus, and inflammatory bowel disease have been reported as risk factors for HZ and should be addressed according to patient risk [38]. In our study, multiple logistic regression results indicate that HZ is more commonly reported in females under 60. HZ may be underreported in older age groups. The median time-to-onset of HZ with JAK inhibitors ranges from 4 to 7 months. The incidence of severe infections, including pneumonia and HZ, has not been shown to increase with long-term JAK inhibitor use and remains stable over time [32, 33], suggesting the need for constant monitoring of signs of infection during JAK inhibitor use.
The ROR signals for hematopoietic erythropenia, leukopenia, and thrombocytopenia were detected only with ruxolitinib. The AEs associated with ruxolitinib are often anemia and thrombocytopenia due to JAK2 inhibition; however, the treatment discontinuation rate is low [13, 14, 39]. Rates of new or worsening grade 3 or 4 anemia and thrombocytopenia have been reported in clinical trials of MF, with most new or worsening events occurring within the first 6 months of treatment [14, 40]. In a double-blind, placebo-controlled trial of ruxolitinib for MF, anemia, and thrombocytopenia were more common in ruxolitinib-treated patients than in placebo-treated patients. Still, the discontinuation rate was reported to be low (1 patient in each group for each event), and these AEs were manageable [39]. In our study, the time-to-onset of AEs for ruxolitinib was concentrated around 4 months after the initiation of treatment, which is consistent with clinical trial reports. Erythropenia, leukopenia, and thrombocytopenia have also been reported in response to JAK inhibitors other than ruxolitinib. In a safety analysis of tofacitinib, lymphocytopenia was shown to be a risk factor for severe infections and HZ [41, 42]. Laboratory values in patients prescribed with JAK inhibitors should be closely monitored because lymphopenia may be missed if white blood cell percentages are not calculated.
Drug-induced liver injury is the leading cause of acute liver injury. In this study, AEs related to hepatitis B virus (HBV) reactivation were excluded to detect only drug-induced liver dysfunction signals. In previous studies, JAK inhibitors did not appear to be drugs with an exceptionally high risk of HBV-reactivation [43, 44]. The exact mechanism underlying JAK inhibitor-induced liver injury is not understood yet. The ROR signals of liver injury were detected only with ruxolitinib but not with the other JAK inhibitors. Liver injury is not the major AE for JAK inhibitors; however, monitoring liver enzymes is necessary because severe outcomes, including the development of liver failure, have been reported [45]. In our study, with ruxolitinib, most reports of liver injury occur about 3 months after initiation of treatment. Tofacitinib has a wider distribution at the time of hepatic injury onset than ruxolitinib. Therefore, it is considered necessary to monitor liver function not only during the initial period of drug initiation but also throughout its use.
The ROR signals for renal impairment were detected using peficitinib and filgotinib. A safety analysis of clinical trials of tofacitinib in RA reported a serum creatinine elevation of 1.5% as an AE, leading to discontinuation. In contrast, no serum creatinine elevation with long-term use was reported [41]. Serum creatinine should always be monitored during JAK inhibitor use, with early action warranted for elevated creatinine.
JAK inhibitors have not yielded consistent results in detecting ROR signals for interstitial pneumonia. ILD is the most common pulmonary manifestation of lung disease due to RA; hence, JAK inhibitors may not be a risk factor for interstitial pneumonia [46]. Although difficult in patients with pre-existing RA-related ILD, the development of parenchymal abnormalities should be appropriately evaluated before treatment, to differentiate between drug-induced pulmonary toxicity and pulmonary involvement due to the underlying disease. In our study, the shortest median time-to-onset of ILD with JAK inhibitors was 40.0 days for ruxolitinib, and the longest was 142.0 days for baricitinib. Patients should be monitored for symptoms of interstitial pneumonia, such as fever, cough, and dyspnea, especially during the first four months of using JAK inhibitors. They should be instructed to discontinue the drug immediately upon symptom onset.
ROR signals for thromboembolic-related events were detected with baricitinib and filgotinib, whereas those for heart failure were detected only with ruxolitinib. Dose-dependent increases in total, high-density, and low-density lipoprotein cholesterol levels have been reported following JAK inhibitor treatment [47]. However, no association has been reported with major adverse cardiovascular events [48, 49]. Inflammation causes hypercoagulability and is a risk factor for venous thromboembolism (VTE) and arterial thromboembolism, including DVT and PE. It has been reported that RA disease correlates with an increased risk of VTE [50]. Currently, there are reports of DVT and PE during JAK inhibitor use. However, the relationship between JAK inhibitors and an increased risk of these events is unclear [49]. In addition to the underlying disease, patient risk factors and case history should be carefully considered.
Gastrointestinal perforation can occur at any site in the gut, with the contents of the stomach or intestinal tract released into the abdominal cavity. In our study, JAK inhibitors were not associated with ROR signals for GP-related AEs. In a report on tofacitinib, most GPs occurred in the lower gastrointestinal tract, and most cases were treated with concomitant NSAIDs or glucocorticoids [32].
The incidence of GP has been reported to be higher with interleukin (IL)-6 inhibitors than with other anti-rheumatic drugs [51]. Although the detailed mechanism is unclear, IL-6 regulates vascular endothelial growth factor, which is involved in angiogenesis and tissue repair [52]. Dendritic cells derived from Peyer’s patches in the intestinal submucosa express high levels of IL-6 and strongly induce IgA [53], which may derange the intestinal microbiota and delay tissue repair processes. Suppression of IL-6 signaling by JAK1 inhibition may result in GP. Timely diagnosis of GP is difficult as it is infrequent, and the timing of its occurrence is unpredictable. Nevertheless, it is a potentially serious AE that should not be overlooked.
Although reported infrequently, our study found no association between JAK inhibitor use and hyperglycemia (Table 1). To our knowledge, few reports exist on the relationship between JAKs and impaired glucose tolerance.
Analyses using SRS, such as the JADER database, have several notable limitations. Intrinsic problems with the SRS data include over-reporting, under-reporting, missing data, exclusion of healthy individuals, lack of denominators, and confounding factors [54]. It is improbable that the “true” risk of AEs will be evaluated without information on the total number of patients administered with JAK inhibitors. We adjusted for possible confounders in the database using multiple logistic regression methods. The adjusted ROR offers a rough indication of the signal strength, which can be used to generate hypotheses to search for unknown potential AEs. In clinical practice, the AE profile of JAK inhibitors in post-marketing real-world data remains to be established. The JADER is the primary tool available for pharmacovigilance because it is the world’s largest and one of the most widely used databases. Although our results only support the basis of a phenomenon already known in the literature, our results, based on the evaluation of JADER, provide essential knowledge to improve our understanding of this issue. The timing of AE occurrence is also affected by different patient backgrounds. However, our results provide useful findings that reflect real-life scenarios, including the median timing of AE occurrence. This information may be particularly beneficial to prescribers.
Conclusion
Despite the inherent limitations of the SRS, we demonstrated the potential risks of JAK inhibitor use with real-world data. The present analysis shows that patients receiving peficitinib, tofacitinib, baricitinib, ruxolitinib, filgotinib, or upadacitinib should be closely monitored for AEs. The median onset of pneumonia and HZ with JAK inhibitor usage ranged from 2 to 6 months and 4 to 7 months, respectively. We believe that the data presented in this study will aid in detecting various AEs associated with JAK inhibitors early.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was not sought for this study because the study was a database-related observational study without directly involving any research subjects. All results were obtained from data openly available online from the PMDA website. All data from the JADER database were fully anonymized by the relevant regulatory authority before we accessed them.
Funding
This research was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No.: 21K06646 and 21K11100).
Authors' contributions
Conceptualization: Mitsuhiro Nakamura; Methodology: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Data collection: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Data analysis: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, and Yuka Nokura; Investigation: Hideyuki Tanaka and Mika Maezawa; Writing the original draft: Hideyuki Tanaka, Mika Maezawa, and Mitsuhiro Nakamura; Review and editing: Mari Iwata, Mayumi Kitamura, Megumi Horibe, Hirofumi Tamaki, and Kazuhiro Iguchi; Supervision: Hideyuki Tanaka, Mika Maezawa, Satoshi Nakao, Sakiko Hirofuji, Moe Yamashita, Kensuke Matsui, Nanaka Ichihara, Yuka Nokura, and Mitsuhiro Nakamura; Funding administration: Mitsuhiro Nakamura.
Conflict of interest
The authors declared no conflict of interest.
References
- Traves PG, Murray B, Campigotto F, Galien R, Meng A, Di Paolo JA. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signaling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021; 80(7):865-75. [DOI:10.1136/annrheumdis-2020-219012][PMID]
- Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019; 118:48-63. [DOI:10.1016/j.cyto.2018.03.041] [PMID]
- Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018; 27(12):1984-2009. [DOI:10.1002/pro.3519] [PMID]
- O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013; 72 Suppl 2(0 2):ii111-5. [DOI:10.1136/annrheumdis-2012-202576] [PMID]
- Cantrell DA, Collins MK, Crumpton MJ. Autocrine regulation of T-lymphocyte proliferation: Differential induction of IL-2 and IL-2 receptor. Immunology. 1988; 65(3):343-9. [PMID]
- Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011; 23(5):598-604. [DOI:10.1016/j.coi.2011.08.003] [PMID]
- Waldmann TA. The shared and contrasting roles of interleukin-2 (IL-2) and IL-15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol Res. 2015; 3(3):219-27. [DOI:10.1158/2326-6066.CIR-15-0009] [PMID]
- Harigai M, Honda S. Selectivity of janus kinase inhibitors in rheumatoid arthritis and other immune-mediated inflammatory diseases: Is expectation the root of all headache? Drugs. 2020; 80(12):1183-201. [DOI:10.1007/s40265-020-01349-1] [PMID]
- Sarabia S, Ranjith B, Koppikar S, Wijeratne DT. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: A systematic review and meta-analysis. BMC Rheumatol. 2022; 6(1):71. [DOI:10.1186/s41927-022-00287-7] [PMID]
- Tran V, Shammas RM, Sauk JS, Padua D. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: Design, development and positioning of therapy. Clin Exp Gastroenterol. 2019; 12:179-91. [DOI:10.2147/CEG.S150908] [PMID]
- Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: An updated network meta-analysis. Clin Gastroenterol Hepatol. 2020; 18(10):2179-91.e6. [DOI:10.1016/j.cgh.2020.01.008] [PMID]
- Rogler G. Efficacy of JAK inhibitors in Crohn’s Disease. J Crohns Colitis. 2020; 14(Supplement_2):S746-54. [DOI:10.1093/ecco-jcc/jjz186] [PMID]
- Kiladjian JJ, Zachee P, Hino M, Pane F, Masszi T, Harrison CN, et al. Long-term efficacy Lancet Haematol and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020; 7(3):e226-37. [DOI:10.1016/S2352-3026(19)30207-8] [PMID]
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: Results of a median 3-year follow-up of COMFORT-I. Haematologica 2015; 100(4):479-88. [DOI:10.3324/haematol.2014.115840] [PMID]
- Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019; 58(Suppl 1):i17-i26. [DOI:10.1093/rheumatology/key225] [PMID]
- Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018; 2:23. [DOI:10.1186/s41927-018-0031-x] [PMID]
- Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013; 191(7):3568-77. [DOI:10.4049/jimmunol.1201348] [PMID]
- Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG. Upadacitinib and filgotinib: The role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context. 2019; 8:212595. [DOI:10.7573/dic.212595] [PMID]
- Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: A12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016; 75(6):1057-64. [DOI:10.1136/annrheumdis-2015-208279] [PMID]
- Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: An evidence-based review on the emerging clinical data. J Inflamm. Res. 2020; 13:519-31. [DOI:10.2147/JIR.S219586] [PMID]
- Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014; 66(10):2675-84. [DOI:10.1002/art.38745] [PMID]
- Curtis JR, Yamaoka K, Chen YH, Bhatt DL, Gunay LM, Sugiyama N, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis. 2023; 82(3):331-43. [DOI:10.1136/ard-2022-222543] [PMID]
- Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Maryland: Food and Drug Administration; 2022. [Link]
- Pharmaceuticals and Medical Devices Agency. Japanese Adverse Drug Event Report (JADER) database. Tokyo: Pharmaceuticals and Medical Devices Agency; 2024.
- MariaDB. MariaDB documentation [Internet]. 2024 [Updated 11 November 2024]. Available from: [Link]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).Medical dictionary for regulatory activities [Internet]. 2023 [Updated 24 June 2023]. Available from: [Link]
- Poluzzi E, Raschi E, Piccinni C, De F. Data mining techniques in pharmacovigilance: Analysis of the publicly accessible FDA adverse event reporting system (AERS). In: Karahoca A, editor. Data mining applications in engineering and medicine. London: InTech; 2012. [DOI:10.5772/50095]
- Statsmodels. Statsmodels: statistical models in python. Version 0.13.2 [Internet]. 2022 [Updated 2023 June 24]. Available from: [Link]
- Nakamura M, Umetsu R, Abe J, Matsui T, Ueda N, Kato Y, et al. Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J Pharm Health Care Sci. 2015; 1:34. [DOI:10.1186/s40780-015-0035-2] [PMID]
- Hatahira H, Abe J, Hane Y, Matsui T, Sasaoka S, Motooka Y, et al. Drug-induced gingival hyperplasia: A retrospective study using spontaneous reporting system databases. J Pharm Health Care Sci. 2017; 3:19. [DOI:10.1186/s40780-017-0088-5] [PMID]
- Taylor J, Ross T. SurPyval: Survival and reliability analysis in python. Version 0.10.1.0 [Internet]. 2022 [Updated 11 November 2024]. Available from: [Link]
- Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: A comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020; 6(3):e001395. [DOI:10.1136/rmdopen-2020-001395] [PMID]
- Taylor PC, Takeuchi T, Burmester GR, Durez P, Smolen JS, Deberdt W, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: Final results from long-term extension study and integrated database. Ann Rheum Dis. 2022; 81(3):335-43. [DOI:10.1136/annrheumdis-2021-221276] [PMID]
- Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, et al. Safety profile of upadacitinib in rheumatoid arthritis: Integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021; 80(3):304-311. [DOI:10.1136/annrheumdis-2020-218510] [PMID]
- Takeuchi T, Tanaka Y, Tanaka S, Kawakami A, Song YW, Chen YH, et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: final results (32 months of mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Rheumatol Ther. 2021 ;8(1):425-42. [DOI:10.1007/s40744-021-00280-5] [PMID]
- Winthrop KL, Tanaka Y, Takeuchi T, Kivitz A, Matzkies F, Genovese MC, et al. Integrated safety analysis of filgotinib in patients with moderately to severely active rheumatoid arthritis receiving treatment over a median of 1.6 years. Ann Rheum Dis. 2022; 81(2):184-92. [DOI:10.1136/annrheumdis-2021-221051] [PMID]
- Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: Prevention and management. Am Fam Physician. 2017; 96(10):656-63. [PMID]
- Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: A meta-analysis. Open Forum Infect Dis. 2020; 7(1):ofaa005. [DOI:10.1093/ofid/ofaa005] [PMID]
- Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366(9):799-807. [DOI:10.1056/NEJMoa1110557] [PMID]
- Arana Yi C, Tam CS, Verstovsek S. Efficacy and safety of ruxolitinib in the treatment of patients with myelofibrosis. Future Oncol. 2015; 11(5):719-33. [DOI:10.2217/fon.14.272] [PMID]
- Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019; 21(1):89. [DOI:10.1186/s13075-019-1866-2] [PMID]
- van Vollenhoven R, Lee EB, Strengholt S, Mojcik C, Valdez H, Krishnaswami S, et al. Evaluation of the short-, mid-, and long-term effects of tofacitinib on lymphocytes in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019; 71(5):685-95. [DOI:10.1002/art.40780] [PMID]
- Harigai M, Winthrop K, Takeuchi T, Hsieh TY, Chen YM, Smolen JS, et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open. 2020; 6(1):e001095. [DOI:10.1136/rmdopen-2019-001095] [PMID]
- Pan C, Cao M, Yan C, Ou X, Zhang X, Xu W, et al. Hepatitis B virus reactivation associated with Janus kinase (JAK) inhibitors: A retrospective study of pharmacovigilance databases and review of the literature. Expert Opin Drug Saf. 2023; 22(6):469-76. [DOI:10.1080/14740338.2023.2181339] [PMID]
- Mardani M, Mohammadshahi J, Abolghasemi S, Teimourpour R. Drug-induced liver injury due to tofacitinib: A case report. J Med Case Rep. 2023; 17(1):97. [DOI:10.1186/s13256-023-03821-4] [PMID]
- Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. 2015; 24(135):1-16. [DOI:10.1183/09059180.00008014] [PMID]
- Taylor PC, Kremer JM, Emery P, Zuckerman SH, Ruotolo G, Zhong J, et al. Lipid profile and effect of statin treatment in pooled phase II and phase III baricitinib studies. Ann Rheum Dis. 2018; 77(7):988-95. [DOI:10.1136/annrheumdis-2017-212461] [PMID]
- Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, Boy M, Zuckerman A, Soma K, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016; 46(3):261-71. [DOI:10.1016/j.semarthrit.2016.05.014] [PMID]
- Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019; 71(7):1042-55. [DOI:10.1002/art.40841] [PMID]
- Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: A nationwide cohort study from Sweden. Ann Rheum Dis. 2021; 80(2):169-75. [DOI:10.1136/annrheumdis-2020-218419] [PMID]
- Strangfeld A, Richter A, Siegmund B, Herzer P, Rockwitz K, Demary W, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017; 76(3):504-10. [DOI:10.1136/annrheumdis-2016-209773] [PMID]
- Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003; 48(6):1521-9. [DOI:10.1002/art.11143] [PMID]
- Sato A, Hashiguchi M, Toda E, Iwasaki A, Hachimura S, Kaminogawa S. CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003; 171(7):3684-90. [DOI:10.4049/jimmunol.171.7.3684] [PMID]
- van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002; 11(1):3-10. [DOI:10.1002/pds.668] [PMID]
Type of Study: Original Research |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






