Volume 6, Issue 2 (2020)
Pharm Biomed Res 2020, 6(2): 105-114 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gholizadeh Shirdarreh G, Eslami G, Soleimani A, Salehifar E. Evaluation of Aminoglycosides Consumption in Open Heart Surgery Based on the Defined Daily Dose Index. Pharm Biomed Res 2020; 6 (2) :105-114
URL: http://pbr.mazums.ac.ir/article-1-270-en.html
URL: http://pbr.mazums.ac.ir/article-1-270-en.html
1- Department of Clinical Pharmacy, Cardiovascular Research Center, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Anaesthesiology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Anaesthesiology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Keywords: Intensive Care Units (ICU), Rational use, Cardiac surgery, Microbial resistance, Prophylactic antibiotic, Aminoglycosides, Defined Daily Dose (DDD)
Full-Text [PDF 883 kb]
(1427 Downloads)
| Abstract (HTML) (2770 Views)
Full-Text: (1957 Views)
Introduction
Although prescription of antibiotics is essential in infectious diseases and unwise use of them might threaten life, most studies have indicated that 30% to 60% of prescriptions are improper [1]. Irrational consumption of antibiotics can cause various problems, including increased microbial resistance, more side effects, and increased healthcare costs for both the patient and healthcare centers. Since antibiotics are among the major drugs prescribed for hospitalized patients, performing studies for rational use of these drugs can be very valuable [2].
Drug Use Evaluation (DUE) is defined as a progressive and suitable program dealing with collecting information across all steps of prescription, delivery, and consumption in a healthcare center and comparing the extent of their correspondence with the standards. Successful implementation of these studies contribute to gaining confidence about proper, safe, and effective consumption of drugs [2, 3]. Thus DUE main purpose is to facilitate the rational use of the drug, which means administering the drug at the correct dose and for its right indication [4].
In this regard, a valid index called the anatomical therapeutic chemical classification system with Defined Daily Dose (DDD) was defined by the World Health Organization (WHO) to study drugs statistically [5]. According to WHO’s definition, DDD refers to the average daily dose of a drug in an adult person used for its main indication [6].
Aminoglycosides are potent, broad-spectrum antibiotics that play a significant role in treating life-threatening infections and have desirable characteristics for this purpose [7]. The bactericidal effect of this group of drugs is dose-dependent, and as the concentration of drug rises, the rate of bacterial death increases. The post-antibiotic effect is another characteristic of aminoglycosides, where the duration of this effect depends also on their serum concentration [8]. Prescription of their single daily dose results in optimal activity and prevention of drug resistance [9]. The most important side effects of this group of drugs are nephrotoxicity, ototoxicity, and rarely neuromuscular blockade [10, 11].
One of the important side effects of cardiovascular surgery is the surgical site infection, which is seen in 2%-20% of patients following the surgery [12]. Among different methods in preventing these infections, antibiotic prophylaxis is of great importance [13]. Irrational use of prophylactic antibiotics increases the side effects caused by drug consumption, imposes inessential costs to both the patient and healthcare system, and finally increases microbial resistance species and diminished efficacy of antibiotics [14]. Therefore, rational drug therapy is very important. In this study, we aimed to evaluate the clinical points of aminoglycosides consumption and drug utilization pattern based on the Defined Daily Dose (DDD) index in open heart surgery.
Materials and Methods
This study was conducted retrospectively by referring to the medical records of 268 patients who underwent heart surgery during 2015 and 2016 (18 months) and received aminoglycosides in Fatima Zahra Cardiology Hospital, a referral teaching center affiliated to Mazandaran University of Medical Sciences, Sari City, Iran.
After going through the administrative process, the authors referred to the medical records of patients and collected the required information including age, gender, duration of hospitalization, duration of antibiotic administration, the time of initiating aminoglycosides, the duration of receiving aminoglycosides, the received dose of drug, comorbidities, and so on. The data were collected and recorded in a data-gathering form that was designed by authors.
Drug consumption data were expressed as Defined Daily Doses (DDD) per 100 inhabitants per day (DID). To calculate DID, ATC (Anatomical Therapeutic Chemical) codes and DDD for each antibiotic were obtained from WHO website. The following formula was used to calculate DID:
DDD per 100 inhabitant per day (DID) = (total consumption in DDDs x 100)/(covered inhabitants x days in the period of data collection) [15].
Creatinine clearance was calculated by using the Cockcroft-Gault Formula 1:
Although prescription of antibiotics is essential in infectious diseases and unwise use of them might threaten life, most studies have indicated that 30% to 60% of prescriptions are improper [1]. Irrational consumption of antibiotics can cause various problems, including increased microbial resistance, more side effects, and increased healthcare costs for both the patient and healthcare centers. Since antibiotics are among the major drugs prescribed for hospitalized patients, performing studies for rational use of these drugs can be very valuable [2].
Drug Use Evaluation (DUE) is defined as a progressive and suitable program dealing with collecting information across all steps of prescription, delivery, and consumption in a healthcare center and comparing the extent of their correspondence with the standards. Successful implementation of these studies contribute to gaining confidence about proper, safe, and effective consumption of drugs [2, 3]. Thus DUE main purpose is to facilitate the rational use of the drug, which means administering the drug at the correct dose and for its right indication [4].
In this regard, a valid index called the anatomical therapeutic chemical classification system with Defined Daily Dose (DDD) was defined by the World Health Organization (WHO) to study drugs statistically [5]. According to WHO’s definition, DDD refers to the average daily dose of a drug in an adult person used for its main indication [6].
Aminoglycosides are potent, broad-spectrum antibiotics that play a significant role in treating life-threatening infections and have desirable characteristics for this purpose [7]. The bactericidal effect of this group of drugs is dose-dependent, and as the concentration of drug rises, the rate of bacterial death increases. The post-antibiotic effect is another characteristic of aminoglycosides, where the duration of this effect depends also on their serum concentration [8]. Prescription of their single daily dose results in optimal activity and prevention of drug resistance [9]. The most important side effects of this group of drugs are nephrotoxicity, ototoxicity, and rarely neuromuscular blockade [10, 11].
One of the important side effects of cardiovascular surgery is the surgical site infection, which is seen in 2%-20% of patients following the surgery [12]. Among different methods in preventing these infections, antibiotic prophylaxis is of great importance [13]. Irrational use of prophylactic antibiotics increases the side effects caused by drug consumption, imposes inessential costs to both the patient and healthcare system, and finally increases microbial resistance species and diminished efficacy of antibiotics [14]. Therefore, rational drug therapy is very important. In this study, we aimed to evaluate the clinical points of aminoglycosides consumption and drug utilization pattern based on the Defined Daily Dose (DDD) index in open heart surgery.
Materials and Methods
This study was conducted retrospectively by referring to the medical records of 268 patients who underwent heart surgery during 2015 and 2016 (18 months) and received aminoglycosides in Fatima Zahra Cardiology Hospital, a referral teaching center affiliated to Mazandaran University of Medical Sciences, Sari City, Iran.
After going through the administrative process, the authors referred to the medical records of patients and collected the required information including age, gender, duration of hospitalization, duration of antibiotic administration, the time of initiating aminoglycosides, the duration of receiving aminoglycosides, the received dose of drug, comorbidities, and so on. The data were collected and recorded in a data-gathering form that was designed by authors.
Drug consumption data were expressed as Defined Daily Doses (DDD) per 100 inhabitants per day (DID). To calculate DID, ATC (Anatomical Therapeutic Chemical) codes and DDD for each antibiotic were obtained from WHO website. The following formula was used to calculate DID:
DDD per 100 inhabitant per day (DID) = (total consumption in DDDs x 100)/(covered inhabitants x days in the period of data collection) [15].
Creatinine clearance was calculated by using the Cockcroft-Gault Formula 1:
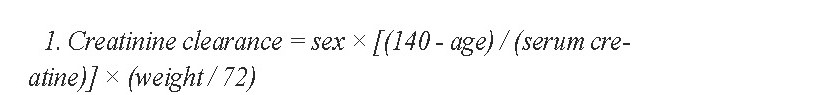
1. Creatinine clearance = sex × [(140 - age) / (serum creatine)] × (weight / 72)
The collected data were fed into SPSS V. 20. Furthermore, the quantitative variables were assessed by the independent sample t test, while the qualitative variables were evaluated by the Chi-square test with P<0.05 reported as a significant difference.
Results
Based on the demographic information, out of the 268 patients receiving aminoglycosides, 36.6% (n=98) and 63.4% (n=170) were female and male, respectively. The Mean±SD age of the patients was 60.65±10.07 years (Table 1).
The collected data were fed into SPSS V. 20. Furthermore, the quantitative variables were assessed by the independent sample t test, while the qualitative variables were evaluated by the Chi-square test with P<0.05 reported as a significant difference.
Results
Based on the demographic information, out of the 268 patients receiving aminoglycosides, 36.6% (n=98) and 63.4% (n=170) were female and male, respectively. The Mean±SD age of the patients was 60.65±10.07 years (Table 1).
Aminoglycosides consumption during the study period (18 months) was calculated based on DID (DDD/100 inhabitant/day). The bed-day inhabitant in ICU was 6400 during this period and according to our calculation, DID of gentamicin and amikacin were 15.92 and 4.78, respectively.
By investigation, the drugs used by patients hospitalized in the ICU, the significant history of drug consumption (in addition to aminoglycosides), was related to cephalosporins, which had been used by 69% of patients during the hospitalization (Figure 1).
By investigation, the drugs used by patients hospitalized in the ICU, the significant history of drug consumption (in addition to aminoglycosides), was related to cephalosporins, which had been used by 69% of patients during the hospitalization (Figure 1).
The most antibiotics used by patients during hospitalization were cefazolin (95%) and then gentamicin (89.9%) (Figure 2).
The reasons for prescribing aminoglycosides in ICU were prophylaxis (94%), followed by urinary tract infection (3%), and then endocarditis (1%). The most prescribed antibiotics concurrent with gentamicin were cefazolin (92%) and then ceftriaxone (4%). In the case of amikacin, the concurrent antibiotics were cefazolin (45%), ceftriaxone (14%), vancomycin (7%), and imipenem (7%). The precise dose of aminoglycosides in surgical site infection prophylaxis was calculated based on the creatinine clearance level, according to the guideline for each patient. The results indicated that 48.1% of the received dose by the patients was not correct and, in most cases, was insufficient.
Serum creatinine level was measured in patients before (90.67% of patients), after (66.04% of patients), and during (99.6% of patients) the administration of aminoglycosides. However, the clearance of creatinine had not been calculated in any of the patients (Table 2).
Serum creatinine level was measured in patients before (90.67% of patients), after (66.04% of patients), and during (99.6% of patients) the administration of aminoglycosides. However, the clearance of creatinine had not been calculated in any of the patients (Table 2).
In 9 patients (3.3%), serum creatinine increased by 50%. The results indicated that 134 patients (50.2%) had a creatinine clearance of less than 60 during the intake of aminoglycosides. However, dose modification occurred only in 11 patients (4.10%) after 24 hours (n=7) and 48 hours (n=4). This dose modification was in the form of a decrease in drug dose or an increase in the usage interval. The mean creatinine clearance of patients before, during, and after injection of aminoglycosides are presented in Table 3.
The culture was requested for 25 (9.3%) patients, in which 6 of them were sputum culture, and 19 were urine culture. Seventeen samples were taken for culture (out of 25) at the initiation of antibiotic therapy, 4 samples after 24 hours, 2 samples after 48 hours, and the rest were taken later. Overall, 15 cultures (60%) had positive results. Among the microorganisms which grew in the samples, the maximum growth percentage (40%) was related to E. Coli (Table 4). This microorganism was sensitive to amikacin by 66% and to gentamicin by 16%, while 50% revealed resistance to gentamicin.
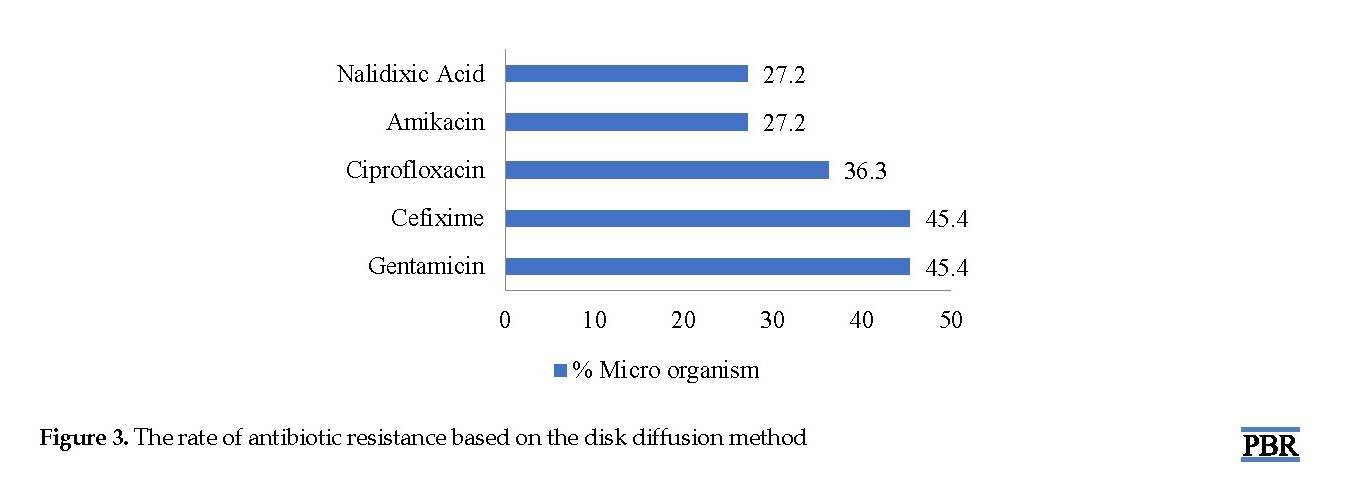
According to the investigations, it was found that 54% of the microorganisms isolated from the culture were sensitive to amikacin (E. coli), and 45% were sensitive to gentamicin (Pseudomonas). Also, 45% of the microorganisms isolated from the culture were resistant to gentamicin, and the most resistant organism was E. coli (27% to amikacin). The rate of antibiotic resistance based on the disk diffusion method is displayed in Figure 3.
Discussion
This study was conducted to evaluate the pattern of aminoglycosides consumption in patients undergoing open heart surgery. According to the American Society of Health-System Pharmacists (ASHP) guideline, the primary indication for using aminoglycosides is to prevent surgical site infection when patients are allergic to β-lactam, and gram-negative pathogens are a concern [16]. Although the main cause of prescribing aminoglycosides in our study was prophylaxis, no documented β-lactam allergy was found.
The result of our study demonstrates that 48.1% of patients did not receive the prescribed dose of aminoglycosides according to guidelines [7, 16]. In other words, the drug dose was inappropriate to the patient’s weight and creatinine clearance. In Safargholi et al. study in the Surgery Ward of Buali Hospital in Tehran, only in 30.6% of cases, the prescription dose of drugs for prophylaxis followed the guidelines [20].
Serum creatinine had not been measured or at least recorded in 9.7% of patients. However, after initiating the drug, the serum creatinine level had been measured routinely for all patients. Also, it should be noted that the creatinine clearance had not been calculated in any of the patients. Since one of the important side effects of aminoglycosides is nephrotoxicity, which occurs in a significant number of patients receiving aminoglycosides [11] and is associated with 50% reduction in creating clearance of patients or 0.5-1 mg/dL increase in serum creatinine [21], monitoring the kidneys during consumption of aminoglycosides is necessary.
According to our calculations, serum creatinine increased by 50% in 3.35% of the patients. In Baciewicz et al. study on the toxicity caused by aminoglycosides in elderly individuals, a 50% increase in serum creatinine was observed in 12.4% of patients. The difference between our study and theirs is justifiable given the old age of their patients [22].
Nephrotoxicity usually manifests 7-10 days after treatment initiation [11, 23], and if the duration of treatment goes beyond 14 days, the probability of this adverse effect increases to 50% [24]. In our study, none of the patients received the drug for more than 14 days.
Furthermore, 26% of patients had received at least one nephrotoxic drug concurrent with aminoglycosides, out of which the maximum concurrency was related to ACE Is, followed by vancomycin. In Gerlach et al. study, 51% of patients received at least one other nephrotoxic drug alongside aminoglycosides, where the maximum concurrency was related to iodine-containing contrast agents [24].
In our study, a significant number of hospitalized patients (94%) received aminoglycosides for prophylaxis before surgery, and by the initiation of drug therapy, they had no symptoms. Besides, only 7.8% of these patients received amikacin for prophylaxis, while for others (92.2%) gentamicin had been prescribed. However, many guidelines have recommended that aminoglycosides should be used only in patients with β-lactam allergy in addition to vancomycin or clindamycin to cover gram-negative microorganisms [25].
DDD methodology provides standardized data to allow comparisons between various drugs and among different wards, hospitals, or countries. This assessment helps to improve the quality of antimicrobial use [17, 26]. Calculation of DID of aminoglycosides in this study indicated that out of every 100 hospitalized patients in ICU ward per day, 20.7 patients underwent treatment with this group of drugs. In Shelat et al. study, the DID of aminoglycosides had been reported as 16.1, which was lower than that in our study [27]. In Ebrahimzadeh et al. study, the DID of aminoglycosides increased from 20.18 in 2000 to 30.9 in 2005 in the ICU of a hospital in Sari City, Iran [5].
Despite the recommendation of all references for prophylaxis before heart surgery, the duration of this prophylaxis is still controversial [28]. Gupta et al. found that 48-h prophylaxis is as effective as 72-h prophylaxis, and the only difference is the incidence of resistant microorganisms in prophylaxis for 72 hours [29]. Similarly, the Harbarth et al. study indicated that the continuation of heart surgery prophylaxis beyond 48 hours does not decrease wound site infection and is ineffective. Actually, it will only cause an increase in microbial resistance and thus should be avoided [13]. Hamouda et al. found that prolongation of the duration of prophylaxis in heart surgery would not cause a significant reduction in surgical site infection. They reported that generally short duration of prophylaxis before heart surgery is as effective as prophylaxis with a longer duration. Nevertheless, the recommendations for preoperative prophylaxis are variable, ranging from a single dose of up to 72 hours [28].
In our study, the Mean±SD duration of receiving aminoglycosides for prophylaxis was 6.3±2.8 and 3.22±1.09 days, respectively using amikacin and gentamicin and only in 10.4% of the patients, they were according to the guidelines. Also, most patients (63%) received prophylaxis for 72 hours. The results of our study were similar to the findings of Safargholi et al., who reported only 19.4% correspondence between the duration of receiving prophylaxis and guidelines. In most cases, it had been longer than the recommended duration of the antibiotic prescribed, without observing any evidence of infection [20].
In our study, the serum level of aminoglycosides had not been measured for any of the patients. Nevertheless, calculation of the serum level of the drug has been recommended in old aged patients, those with a treatment course of longer than 10 days, patients who received both glycosides and other nephrotoxic drugs, and patients with serious underlying diseases [30].
In our study, culture was requested for 25 patients (9.3%), where 15 cases were positive. The main microorganism isolated from the culture medium was E. Coli. In Salehifar et al. study, the same findings were observed [30]. Similarly, in Fraisse et al. study on the consumption of aminoglycosides in individuals older than 75 years, the major microorganism isolated from the culture medium was S. aureus followed by E. Coli. [31].
The extent of E. Coli. resistance to gentamicin in our study was 50%, which was the same as the number obtained by Salehifar et al. [15]. Also, the extent of sensitivity of this organism to amikacin was 66%, which was reported as 72% in Eslami et al. [32].
Overall, the microorganisms isolated from the culture medium were sensitive to amikacin and gentamicin by 54% and 45%, respectively, while 45% and 27% were resistant to gentamicin and amikacin. This lower percentage of resistance to amikacin could be due to the more limited usage of this drug. In Yaghubi et al. study on patients undergoing heart surgery, gentamicin was one of the antibiotics which revealed the maximum resistance of bacteria isolated from patients [33].
Conclusion
This study shows that the prescribed dose was not sufficient in most cases because of imprecise dose adjustment. Additionally, aminoglycosides were consumed longer than what is recommended in the guidelines so it can increase the risk of the microbial resistance. If it is required to use aminoglycosides, the standard duration of use should be observed. Creatinine clearance was not calculated in any of the patients and although almost half the patients had a creatinine clearance of less than 60 during intake of aminoglycosides, dose modification was done only in a few of them. Because of nephrotoxicity risk, dose adjustment is highly recommended.
The small sample size was one of the study limitations that makes it impossible to generalize the results of the study. Besides, as the study design was retrospective, some information was lost and had not been recorded in the patients’ files. For example, the results of some cultures and patients’ serum creatinine during and after aminoglycosides consumption were not available.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
Zarezade M, Shaterzade F, Abedini S, Raadabadi M. Evaluating pattern of prescribing antibiotics in surgical wards of shahid rahnemon hospital compared to standard methods in 2015. JSSU. 2015; 23(7):679-90
Shimels T, Bilal AI, Mulugeta A. Evaluation of Ceftriaxone utilization in internal medicine wards of general hospitals in Addis Ababa, Ethiopia: A comparative retrospective study. J Pharm Policy Pract. 2015; 8:26.
Khalili H, Gholami Kh, Hajiabdolbaghi M, Sairafipoor Z. Investigating the pattern of vancomycin consumption in the infection ward of Imam Khjomeini hospital in Tehran. Tehran Univ Med J. 2007; 64(12):64-8.
Ala SH, Eslami G, Sayftabar A. “Enoxaparin utilization evaluation in inpatients with or at risk of thromboembolic disorders: A one-year, single-centered, retrospective Study.” Pharm Biomed Res. 2016, 2(1):55-64 . [DOI:10.18869/acadpub.pbr.2.1.55]
Ebrahimzadeh M, Ansari F, Ramezani A, Shokrzadeh M, Shabankhani B, Saeedi S, et al. Utilization pattern of antibiotics in different wards of Sari Imam Khomeini Teaching Hospital. J Mazandaran Univ Med Sci. 2007; 17(61):166-9.
Rønning M, Blix HS, Harbø BT, Strøm H. Different versions of the anatomical therapeutic chemical classification system and the defined daily dose-are drug utilisation data comparable? Eur J Clin Pharmacol. 2000; 56(9-10):723-7 [DOI:10.1007/s002280000200] [PMID]
Eliopoulos GM, Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, et al. Back to the future: Using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007; 45(6):753-60. [DOI:10.1086/520991] [PMID]
Eslami G, Salehifar E, Behbudi M, Rezai MS. Rational use of amikacin in Buali-Sina Hospital in Sari 2011. J Mazandaran University Med Sci. 2013; 23(100):2-9.
Sweileh WM. Gender differences in aminoglycoside induced nephrotoxicity: A prospective, hospital-based study. Curr Clin Pharmacol. 2009; 4(3):229-32. [DOI:10.2174/157488409789375339] [PMID]
Salehifar E, Rafati MR. Extended-Interval dosing of aminoglycosides in pediatrics: A narrative review. J Pediatr Rev. 2015; 3(2):31-34 [DOI:10.17795/jpr-2652]
Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors foraminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009; 53(7):2887-91 [DOI:10.1128/AAC.01430-08] [PMID] [PMCID]
Ghafari R, Baradari AG, Nouraei M, Khademloo M, Esmaieli M. Comparing the effects of cefazolin and cefazolin plus gentamicin on surgical site infection in cabg patients. J Mazandaran Univ Med Sci. 2012; 22(89):1-9.
Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circ. 2000; 101(25):2916-21. [DOI:10.1161/01.CIR.101.25.2916] [PMID]
Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg. 2002; 123(2):326-32. [DOI:10.1067/mtc.2002.119698] [PMID]
Salehifar E, Nasehi M, Eslami G, Sahraei S, Alizadeh Navaei R. Determination of antibiotics consumption in Buali-Sina Pediatric Hospital, Sari 2010-2011. Iran J Pharm Res. 2014; 13(3):995.
American Society of Health-System Pharmacists. ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 1999; 56:1839-88. [DOI:10.1093/ajhp/56.18.1839] [PMID]
Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR. The society of thoracic surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part I: Duration. Ann Thorac Surg. 2006; 1:397-404 [DOI:10.1016/j.athoracsur.2005.06.034] [PMID]
Baddour LM, Wilson WR, Bayer AS, Fowler Jr VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circ. 2015; 132(15):1435-86. [DOI:10.1161/CIR.0000000000000296] [PMID]
Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings S, & Wagenlehner F, et al. Urological infections. Arnhem: European Association of Urology; 2018.
Safargholi S, Mousavi F, Faghani Y, Najari S. Adherence to international and national prophylaxis guidelines in surgicalwards of boali hospital in tehran in 2013. Iran J Infect Trop Med. 2013; 17(59):23-7.
Leibovici L, Vidal L, Paul M. Aminoglycoside drugs in clinical practice: An evidence-based approach. J Antimicrob Chemother. 2009; 63(2):246-51. [DOI:10.1093/jac/dkn469] [PMID]
Baciewicz AM, Sokos DR, Cowan RI. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003; 37(2):182-6. [DOI:10.1345/aph.1A395] [PMID]
Seyed Mohammad Javad H, Reza R, Ali M. [Comparison of the effect of amikacin and gentamicin on renal tubular function (Persion)]. Infec Trop Dis Iran J. 1387; 49(13):1-4.
Gerlach AT, Stawicki SP, Cook CH, Murphy C. Risk factors for aminoglycoside-associated nephrotoxicity in surgical intensive care unit patients. Int J Crit Illn Inj Sci. 2011; 1(1):17. [DOI:10.4103/2229-5151.79277] [PMID] [PMCID]
Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013; 14(1):73-156. [DOI:10.1089/sur.2013.9999] [PMID]
Kiivet RA, Dahl ML, Llerena A, Maimets M, Wettermark B, Berecz R. Antibiotic use in 3 European university hospitals. Scand J Infect Dis. 1998; 30(3):277-80. [DOI:10.1080/00365549850160936] [PMID]
Shelat PR, Gandhi AM, Parth P. A study of drug utilization pattern according to daily define dose in Intensive Care Unit (ICU) s at Tertiary Care Teaching Hospital, India. J Young Pharm. 2015; 7(4):349. [DOI:10.5530/jyp.2015.4.9]
Hamouda K, Oezkur M, Sinha B, Hain J, Menkel H, Leistner M, et al. Different duration strategies of perioperative antibiotic prophylaxis in adult patients undergoing cardiac surgery: An observational study. J Cardiothorac Surg. 2015; 10(1):1. [DOI:10.1186/s13019-015-0225-x] [PMID] [PMCID]
Gupta A, Hote MP, Choudhury M, Kapil A, Bisoi AK. Comparison of 48 h and 72 h of prophylactic antibiotic therapy in adult cardiac surgery: A randomized double blind controlled trial. J Antimicrob Chemother. 2010; 65(5):1036-41 [DOI:10.1093/jac/dkq080] [PMID]
Salehifar E, Eslami G, Ahangar N, Rafati MR, Eslami S. How aminoglycosides are used in critically ill patients in a teaching hospital in North of Iran. aspian J Intern Med. 2015; 6(4):238.
Fraisse T, Paccalin M, Vitrat V, De Wazieres B, Baudoux V, Lechiche C, et al. Aminoglycosides use in patients over 75 years old. Age Ageing. 2014; 43(5):676–81. [DOI:10.1093/ageing/afu023] [PMID]
Eslami G, Salehifar E, Behbudi M, Rezai M S. Rational use of amikacin in Buali-Sina Hospital in Sari 2011. J Mazandaran Univ Med Sci. 2013; 23(100):2-9.
Yaghoubi A, Ghotaslou R, Safaei N, Jahanroshan J, Mahmoudian R. [Study on bacteria and factors involved in wound infections after cardiac surgery in Shahid Madani Hospital (Persian)]. Med J Tabriz Univ Med Sci. 2011; 32(6):83-9.
According to the investigations, it was found that 54% of the microorganisms isolated from the culture were sensitive to amikacin (E. coli), and 45% were sensitive to gentamicin (Pseudomonas). Also, 45% of the microorganisms isolated from the culture were resistant to gentamicin, and the most resistant organism was E. coli (27% to amikacin). The rate of antibiotic resistance based on the disk diffusion method is displayed in Figure 3.
Discussion
This study was conducted to evaluate the pattern of aminoglycosides consumption in patients undergoing open heart surgery. According to the American Society of Health-System Pharmacists (ASHP) guideline, the primary indication for using aminoglycosides is to prevent surgical site infection when patients are allergic to β-lactam, and gram-negative pathogens are a concern [16]. Although the main cause of prescribing aminoglycosides in our study was prophylaxis, no documented β-lactam allergy was found.
The result of our study demonstrates that 48.1% of patients did not receive the prescribed dose of aminoglycosides according to guidelines [7, 16]. In other words, the drug dose was inappropriate to the patient’s weight and creatinine clearance. In Safargholi et al. study in the Surgery Ward of Buali Hospital in Tehran, only in 30.6% of cases, the prescription dose of drugs for prophylaxis followed the guidelines [20].
Serum creatinine had not been measured or at least recorded in 9.7% of patients. However, after initiating the drug, the serum creatinine level had been measured routinely for all patients. Also, it should be noted that the creatinine clearance had not been calculated in any of the patients. Since one of the important side effects of aminoglycosides is nephrotoxicity, which occurs in a significant number of patients receiving aminoglycosides [11] and is associated with 50% reduction in creating clearance of patients or 0.5-1 mg/dL increase in serum creatinine [21], monitoring the kidneys during consumption of aminoglycosides is necessary.
According to our calculations, serum creatinine increased by 50% in 3.35% of the patients. In Baciewicz et al. study on the toxicity caused by aminoglycosides in elderly individuals, a 50% increase in serum creatinine was observed in 12.4% of patients. The difference between our study and theirs is justifiable given the old age of their patients [22].
Nephrotoxicity usually manifests 7-10 days after treatment initiation [11, 23], and if the duration of treatment goes beyond 14 days, the probability of this adverse effect increases to 50% [24]. In our study, none of the patients received the drug for more than 14 days.
Furthermore, 26% of patients had received at least one nephrotoxic drug concurrent with aminoglycosides, out of which the maximum concurrency was related to ACE Is, followed by vancomycin. In Gerlach et al. study, 51% of patients received at least one other nephrotoxic drug alongside aminoglycosides, where the maximum concurrency was related to iodine-containing contrast agents [24].
In our study, a significant number of hospitalized patients (94%) received aminoglycosides for prophylaxis before surgery, and by the initiation of drug therapy, they had no symptoms. Besides, only 7.8% of these patients received amikacin for prophylaxis, while for others (92.2%) gentamicin had been prescribed. However, many guidelines have recommended that aminoglycosides should be used only in patients with β-lactam allergy in addition to vancomycin or clindamycin to cover gram-negative microorganisms [25].
DDD methodology provides standardized data to allow comparisons between various drugs and among different wards, hospitals, or countries. This assessment helps to improve the quality of antimicrobial use [17, 26]. Calculation of DID of aminoglycosides in this study indicated that out of every 100 hospitalized patients in ICU ward per day, 20.7 patients underwent treatment with this group of drugs. In Shelat et al. study, the DID of aminoglycosides had been reported as 16.1, which was lower than that in our study [27]. In Ebrahimzadeh et al. study, the DID of aminoglycosides increased from 20.18 in 2000 to 30.9 in 2005 in the ICU of a hospital in Sari City, Iran [5].
Despite the recommendation of all references for prophylaxis before heart surgery, the duration of this prophylaxis is still controversial [28]. Gupta et al. found that 48-h prophylaxis is as effective as 72-h prophylaxis, and the only difference is the incidence of resistant microorganisms in prophylaxis for 72 hours [29]. Similarly, the Harbarth et al. study indicated that the continuation of heart surgery prophylaxis beyond 48 hours does not decrease wound site infection and is ineffective. Actually, it will only cause an increase in microbial resistance and thus should be avoided [13]. Hamouda et al. found that prolongation of the duration of prophylaxis in heart surgery would not cause a significant reduction in surgical site infection. They reported that generally short duration of prophylaxis before heart surgery is as effective as prophylaxis with a longer duration. Nevertheless, the recommendations for preoperative prophylaxis are variable, ranging from a single dose of up to 72 hours [28].
In our study, the Mean±SD duration of receiving aminoglycosides for prophylaxis was 6.3±2.8 and 3.22±1.09 days, respectively using amikacin and gentamicin and only in 10.4% of the patients, they were according to the guidelines. Also, most patients (63%) received prophylaxis for 72 hours. The results of our study were similar to the findings of Safargholi et al., who reported only 19.4% correspondence between the duration of receiving prophylaxis and guidelines. In most cases, it had been longer than the recommended duration of the antibiotic prescribed, without observing any evidence of infection [20].
In our study, the serum level of aminoglycosides had not been measured for any of the patients. Nevertheless, calculation of the serum level of the drug has been recommended in old aged patients, those with a treatment course of longer than 10 days, patients who received both glycosides and other nephrotoxic drugs, and patients with serious underlying diseases [30].
In our study, culture was requested for 25 patients (9.3%), where 15 cases were positive. The main microorganism isolated from the culture medium was E. Coli. In Salehifar et al. study, the same findings were observed [30]. Similarly, in Fraisse et al. study on the consumption of aminoglycosides in individuals older than 75 years, the major microorganism isolated from the culture medium was S. aureus followed by E. Coli. [31].
The extent of E. Coli. resistance to gentamicin in our study was 50%, which was the same as the number obtained by Salehifar et al. [15]. Also, the extent of sensitivity of this organism to amikacin was 66%, which was reported as 72% in Eslami et al. [32].
Overall, the microorganisms isolated from the culture medium were sensitive to amikacin and gentamicin by 54% and 45%, respectively, while 45% and 27% were resistant to gentamicin and amikacin. This lower percentage of resistance to amikacin could be due to the more limited usage of this drug. In Yaghubi et al. study on patients undergoing heart surgery, gentamicin was one of the antibiotics which revealed the maximum resistance of bacteria isolated from patients [33].
Conclusion
This study shows that the prescribed dose was not sufficient in most cases because of imprecise dose adjustment. Additionally, aminoglycosides were consumed longer than what is recommended in the guidelines so it can increase the risk of the microbial resistance. If it is required to use aminoglycosides, the standard duration of use should be observed. Creatinine clearance was not calculated in any of the patients and although almost half the patients had a creatinine clearance of less than 60 during intake of aminoglycosides, dose modification was done only in a few of them. Because of nephrotoxicity risk, dose adjustment is highly recommended.
The small sample size was one of the study limitations that makes it impossible to generalize the results of the study. Besides, as the study design was retrospective, some information was lost and had not been recorded in the patients’ files. For example, the results of some cultures and patients’ serum creatinine during and after aminoglycosides consumption were not available.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
References
Zarezade M, Shaterzade F, Abedini S, Raadabadi M. Evaluating pattern of prescribing antibiotics in surgical wards of shahid rahnemon hospital compared to standard methods in 2015. JSSU. 2015; 23(7):679-90
Shimels T, Bilal AI, Mulugeta A. Evaluation of Ceftriaxone utilization in internal medicine wards of general hospitals in Addis Ababa, Ethiopia: A comparative retrospective study. J Pharm Policy Pract. 2015; 8:26.
Khalili H, Gholami Kh, Hajiabdolbaghi M, Sairafipoor Z. Investigating the pattern of vancomycin consumption in the infection ward of Imam Khjomeini hospital in Tehran. Tehran Univ Med J. 2007; 64(12):64-8.
Ala SH, Eslami G, Sayftabar A. “Enoxaparin utilization evaluation in inpatients with or at risk of thromboembolic disorders: A one-year, single-centered, retrospective Study.” Pharm Biomed Res. 2016, 2(1):55-64 . [DOI:10.18869/acadpub.pbr.2.1.55]
Ebrahimzadeh M, Ansari F, Ramezani A, Shokrzadeh M, Shabankhani B, Saeedi S, et al. Utilization pattern of antibiotics in different wards of Sari Imam Khomeini Teaching Hospital. J Mazandaran Univ Med Sci. 2007; 17(61):166-9.
Rønning M, Blix HS, Harbø BT, Strøm H. Different versions of the anatomical therapeutic chemical classification system and the defined daily dose-are drug utilisation data comparable? Eur J Clin Pharmacol. 2000; 56(9-10):723-7 [DOI:10.1007/s002280000200] [PMID]
Eliopoulos GM, Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, et al. Back to the future: Using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007; 45(6):753-60. [DOI:10.1086/520991] [PMID]
Eslami G, Salehifar E, Behbudi M, Rezai MS. Rational use of amikacin in Buali-Sina Hospital in Sari 2011. J Mazandaran University Med Sci. 2013; 23(100):2-9.
Sweileh WM. Gender differences in aminoglycoside induced nephrotoxicity: A prospective, hospital-based study. Curr Clin Pharmacol. 2009; 4(3):229-32. [DOI:10.2174/157488409789375339] [PMID]
Salehifar E, Rafati MR. Extended-Interval dosing of aminoglycosides in pediatrics: A narrative review. J Pediatr Rev. 2015; 3(2):31-34 [DOI:10.17795/jpr-2652]
Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors foraminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother. 2009; 53(7):2887-91 [DOI:10.1128/AAC.01430-08] [PMID] [PMCID]
Ghafari R, Baradari AG, Nouraei M, Khademloo M, Esmaieli M. Comparing the effects of cefazolin and cefazolin plus gentamicin on surgical site infection in cabg patients. J Mazandaran Univ Med Sci. 2012; 22(89):1-9.
Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circ. 2000; 101(25):2916-21. [DOI:10.1161/01.CIR.101.25.2916] [PMID]
Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg. 2002; 123(2):326-32. [DOI:10.1067/mtc.2002.119698] [PMID]
Salehifar E, Nasehi M, Eslami G, Sahraei S, Alizadeh Navaei R. Determination of antibiotics consumption in Buali-Sina Pediatric Hospital, Sari 2010-2011. Iran J Pharm Res. 2014; 13(3):995.
American Society of Health-System Pharmacists. ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 1999; 56:1839-88. [DOI:10.1093/ajhp/56.18.1839] [PMID]
Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR. The society of thoracic surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part I: Duration. Ann Thorac Surg. 2006; 1:397-404 [DOI:10.1016/j.athoracsur.2005.06.034] [PMID]
Baddour LM, Wilson WR, Bayer AS, Fowler Jr VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circ. 2015; 132(15):1435-86. [DOI:10.1161/CIR.0000000000000296] [PMID]
Bonkat G, Pickard R, Bartoletti R, Bruyère F, Geerlings S, & Wagenlehner F, et al. Urological infections. Arnhem: European Association of Urology; 2018.
Safargholi S, Mousavi F, Faghani Y, Najari S. Adherence to international and national prophylaxis guidelines in surgicalwards of boali hospital in tehran in 2013. Iran J Infect Trop Med. 2013; 17(59):23-7.
Leibovici L, Vidal L, Paul M. Aminoglycoside drugs in clinical practice: An evidence-based approach. J Antimicrob Chemother. 2009; 63(2):246-51. [DOI:10.1093/jac/dkn469] [PMID]
Baciewicz AM, Sokos DR, Cowan RI. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003; 37(2):182-6. [DOI:10.1345/aph.1A395] [PMID]
Seyed Mohammad Javad H, Reza R, Ali M. [Comparison of the effect of amikacin and gentamicin on renal tubular function (Persion)]. Infec Trop Dis Iran J. 1387; 49(13):1-4.
Gerlach AT, Stawicki SP, Cook CH, Murphy C. Risk factors for aminoglycoside-associated nephrotoxicity in surgical intensive care unit patients. Int J Crit Illn Inj Sci. 2011; 1(1):17. [DOI:10.4103/2229-5151.79277] [PMID] [PMCID]
Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013; 14(1):73-156. [DOI:10.1089/sur.2013.9999] [PMID]
Kiivet RA, Dahl ML, Llerena A, Maimets M, Wettermark B, Berecz R. Antibiotic use in 3 European university hospitals. Scand J Infect Dis. 1998; 30(3):277-80. [DOI:10.1080/00365549850160936] [PMID]
Shelat PR, Gandhi AM, Parth P. A study of drug utilization pattern according to daily define dose in Intensive Care Unit (ICU) s at Tertiary Care Teaching Hospital, India. J Young Pharm. 2015; 7(4):349. [DOI:10.5530/jyp.2015.4.9]
Hamouda K, Oezkur M, Sinha B, Hain J, Menkel H, Leistner M, et al. Different duration strategies of perioperative antibiotic prophylaxis in adult patients undergoing cardiac surgery: An observational study. J Cardiothorac Surg. 2015; 10(1):1. [DOI:10.1186/s13019-015-0225-x] [PMID] [PMCID]
Gupta A, Hote MP, Choudhury M, Kapil A, Bisoi AK. Comparison of 48 h and 72 h of prophylactic antibiotic therapy in adult cardiac surgery: A randomized double blind controlled trial. J Antimicrob Chemother. 2010; 65(5):1036-41 [DOI:10.1093/jac/dkq080] [PMID]
Salehifar E, Eslami G, Ahangar N, Rafati MR, Eslami S. How aminoglycosides are used in critically ill patients in a teaching hospital in North of Iran. aspian J Intern Med. 2015; 6(4):238.
Fraisse T, Paccalin M, Vitrat V, De Wazieres B, Baudoux V, Lechiche C, et al. Aminoglycosides use in patients over 75 years old. Age Ageing. 2014; 43(5):676–81. [DOI:10.1093/ageing/afu023] [PMID]
Eslami G, Salehifar E, Behbudi M, Rezai M S. Rational use of amikacin in Buali-Sina Hospital in Sari 2011. J Mazandaran Univ Med Sci. 2013; 23(100):2-9.
Yaghoubi A, Ghotaslou R, Safaei N, Jahanroshan J, Mahmoudian R. [Study on bacteria and factors involved in wound infections after cardiac surgery in Shahid Madani Hospital (Persian)]. Med J Tabriz Univ Med Sci. 2011; 32(6):83-9.
Type of Study: Original Research |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |