Volume 10, Issue 2 (2024)
Pharm Biomed Res 2024, 10(2): 147-156 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ohanme E O, Nwakelu B N, Nwoke E E, Ofonakara U, Etu K E, Oborolo A J, et al . Anticonvulsant Effect of Ethyl Acetate and n-hexane Fractions of Celosia leptostachya Leaf Extracts in Mice. Pharm Biomed Res 2024; 10 (2) :147-156
URL: http://pbr.mazums.ac.ir/article-1-588-en.html
URL: http://pbr.mazums.ac.ir/article-1-588-en.html
Eugene O Ohanme *1 

 , Benjamin N Nwakelu1
, Benjamin N Nwakelu1 

 , Ekene E Nwoke2
, Ekene E Nwoke2 

 , Uzochukwu Ofonakara1
, Uzochukwu Ofonakara1 

 , Kenneth E Etu1
, Kenneth E Etu1 

 , Angelinah J Oborolo3
, Angelinah J Oborolo3 

 , Mansur A Ramalan4
, Mansur A Ramalan4 

 , Godwin C Akuodor5
, Godwin C Akuodor5 

 , Kingsley C Chilaka5
, Kingsley C Chilaka5 




 , Benjamin N Nwakelu1
, Benjamin N Nwakelu1 

 , Ekene E Nwoke2
, Ekene E Nwoke2 

 , Uzochukwu Ofonakara1
, Uzochukwu Ofonakara1 

 , Kenneth E Etu1
, Kenneth E Etu1 

 , Angelinah J Oborolo3
, Angelinah J Oborolo3 

 , Mansur A Ramalan4
, Mansur A Ramalan4 

 , Godwin C Akuodor5
, Godwin C Akuodor5 

 , Kingsley C Chilaka5
, Kingsley C Chilaka5 


1- Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Alex Ekwueme Federal University, Abakaliki, Nigeria.
2- Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Rivers State University, Port-Harcourt, Nigeria.
3- Department of Nursing Services, Rivers State Ministry of Health, Port Harcourt, Nigeria.
4- Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Bayero University, Kano, Nigeria.
5- Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria.
2- Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Rivers State University, Port-Harcourt, Nigeria.
3- Department of Nursing Services, Rivers State Ministry of Health, Port Harcourt, Nigeria.
4- Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Bayero University, Kano, Nigeria.
5- Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria.
Full-Text [PDF 860 kb]
(409 Downloads)
| Abstract (HTML) (1196 Views)
Full-Text: (403 Views)
Introduction
Epilepsy is known to be a critical and frequent distress of man [1]. At least fifty million people suffer from epilepsy, which is responsible for about 1% of the total sickness concern worldwide [2]. Epilepsy is framed from the Greek word “epilembanein,” which is referred to as “seize.” Epilepsy is a persistent neurological chaos that disturbs males and females equally [3]. It is the next most frequent inveterate neurological state observed by neurologists globally [4]. Although there are many other brain diseases, epilepsy is more noticeable not out of its commonness and the gravity of occurrence but the superstitious beliefs of people of different traditions and world views, especially in Africa. This condition affects not only the person with epilepsy but also the family and the entire community [5]. Hence, epilepsy is still well known as one of the most stigmatized brain diseases. This stigmatization is common among developing nations, possibly due to low levels of education, lack of exposure, and poor information concerning the real nature of the disease. The embarrassment faced by victims makes it difficult to seek treatment. This situation usually worsens their conditions and reduces their chances of being educated, employed, and socially significant [6].
Thus, research into the best medical care and treatment options for epilepsy will continue to be a priority and a complex problem for various nations. For a very long time, food therapy, acupuncture, moxibustion, surgery, and medicine have been the mainstays of epilepsy treatment [7]. Currently, drugs are the most recommended option for managing and treating epilepsy. The global pharmaceutical industry currently offers over 50 distinct antiepileptic drugs (AEDs), such as stiripentol, carbamazepine, oxcarbazepine, sodium valproate, gabapentin, lamotrigine, topiramate, levetiracetam, lacosamide and pregabalin, etc. They are all available in the pharmaceutical industry globally. However, utilizing conventional AEDs may cause adverse effects in 30%–40% of people with epilepsy. In addition, about 30% of patients have drug resistance.
Consequently, the need for epilepsy treatment remains unfulfilled [8]. Moreover, prolonged usage of certain AEDs has been linked to adverse effects and an increased chance of drug-drug interactions [9]. Therefore, research and development of novel AEDs with multiple targets and minimal side effects is both serious and challenging.
One useful resource for discovering novel forms of AEDs is the kingdom of plants. Traditional medicine has a lengthy history and a wealth of real-world expertise in treating epilepsy. There is historical evidence supporting the use of herbs as medicine to treat epilepsy. Specifically, medicinal plants for epilepsy are frequently regarded as a gentle and secure substitute for chemical AEDs. Herbal medicine has been utilized globally as a complementary or alternative medication for the treatment of epilepsy [10]. More than 20 Chinese patent medicines composed of herbs have been documented for clinical use. In addition, herbal treatments are extensively used in traditional medicine, such as infusion or decoction, for epileptic patients in Japan, Korea, Australia, India, Mexico, African and South American countries, etc. [11].
The leaves, roots and or bark of medicinal plants prescribed by traditional medical healers are taken orally or used as a bath to treat convulsions [12]. For example, the decoction of Celosia leptostachya is used in Nigeria to treat convulsions [12].
Provided that efforts to cure epilepsy are not clinically successful, there is an increasing concern that the efficacy of drug management of epilepsy has not yielded better outcomes despite the development of new drugs for it.
C. leptostachya is classified under the family of Amaranthaceae. The stem lacks hairs with slim angle projections and decumbent support in nature. This plant can grow up to 300 mm in height and reach 600 mm high when lifted from the stalk. It does have ovate leaves, but they are smooth and noticeable. C. leptostachya is well distributed in Abia State, South East of Nigeria. The plant’s leaves are edible and serve as cooking vegetables for soup. C. leptostachya possesses numerous medicinal properties, which traditional medicine practitioners exploit mainly in curing illnesses such as boils, fever, snake bites, scorpion stings, eye infections, wounds and pain, and most notably, epilepsy. Based on personal interaction with traditional healers, when an epileptic patient is brought to the healer, the plant’s fresh leaves would be fetched and squeezed manually to get at least 10 mL of the liquid content and administered orally and through the eyes. The patients usually recover between 5-10 minutes after treatment. Hence, this work was designed to investigate its anticonvulsant activity using animal experimental design for epilepsy.
Materials and Methods
Collection and identification of the plant
C. leptostachya leaf (fresh) was collected at Umuhu Nvosi in Abia State, Nigeria, at 6:00 AM. The identification was done by Ibe M. of the Forestry Department of the Michael Okpara University of Agriculture Umudike, Abia State, Nigeria and was given a voucher number: “FHI3081.”
Preparation of extraction-fractionation
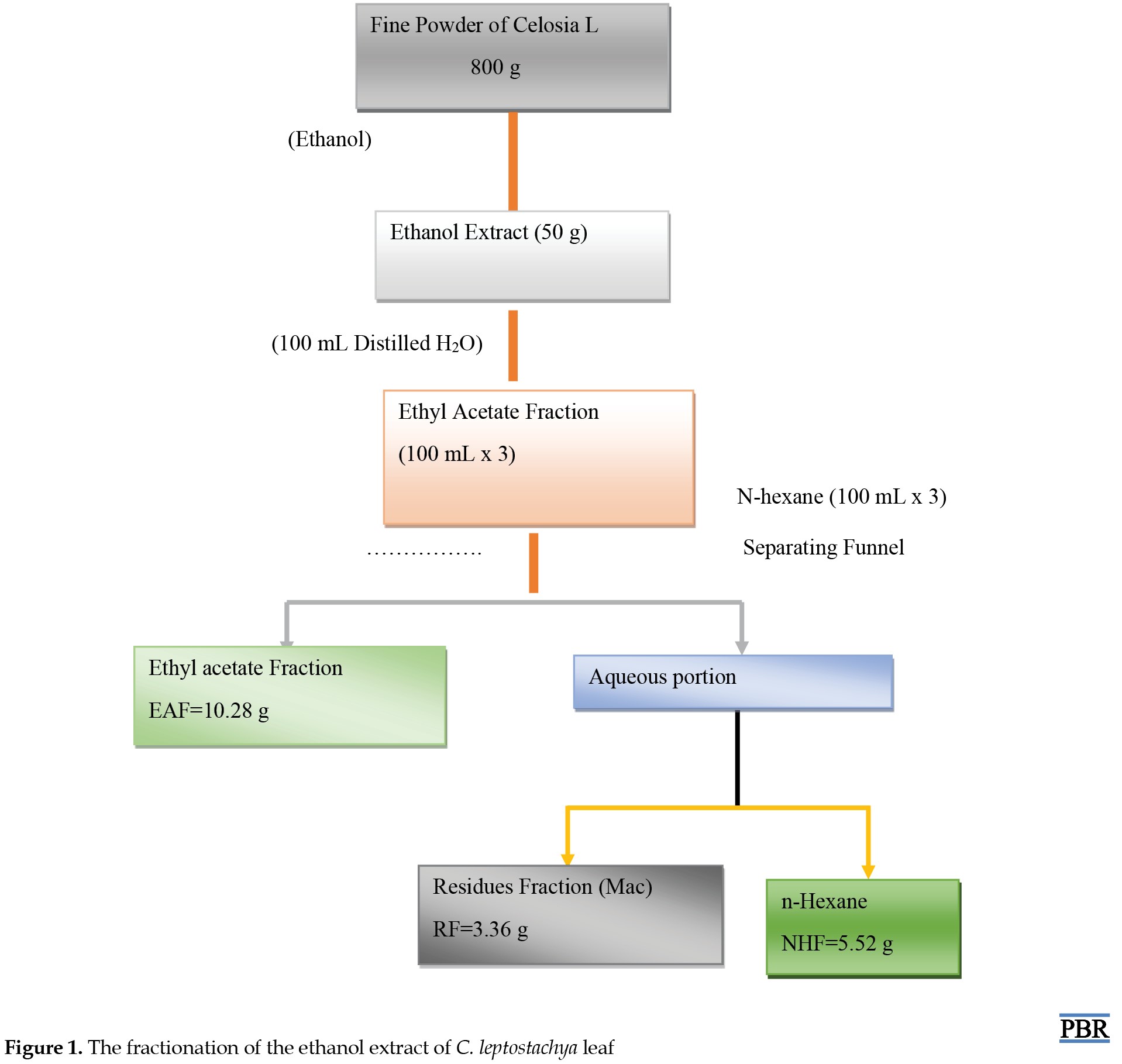
The collected leaves of C. leptostachya were washed with clean tap water to remove any dirt. It was then dried at room temperature of about 25oC. After that, it was pounded into fine particles with a local grinder. A maceration extraction technique was used whereby 800 g of fine particles of C. leptostachya were immersed in absolute ethanol for three days. It was filtered and allowed to dry under a controlled temperature (40oC) in a water bath. The method described by Shi et al. (2018) [2] was used for fractionation. The outcome of the extraction (30 g of ethanol extract) was liquefied using 100 mL of purified water alongside ethyl acetate (300 mL) as a partitioner, which produced ethyl acetate fraction (EAF) with the application of separating funnel and light heat (40oC) in a water bath. The remainder of the solution was further fractionated with 300 mL of n-hexane, which was collected using a separating funnel called n-hexane fraction (NHF) (Figure 1).
Phytochemical screening
The preliminary phytochemistry of C. leptostachya ethanol leaf extract was carried out to determine various phytochemical components of the plant. Here, we used the ferric chloride test for tannins, Mayer and Dragendorff test for alkaloids, Froth test for saponins, Liebermann-Burchard test for steroids, Salkowski test for terpenoids, ammonia and the sulphuric acid test for flavonoids and Borntrager’s test for anthraquinones.
Chemicals, drugs and equipment
The following chemicals and machine were used to induce convulsion and epileptic seizure in animals: Pentylenetetrazole (PTZ) (supplied by Sigma Aldrich Germany) (GABAA antagonist), brucine (provided by Sigma Aldrich Germany) (Glycine antagonist), and electroshock machine (supplied the University of Jos, Plateau State Nigeria). Also, standard drugs used for positive control were diazepam (NAFDAC Reg. 04-4402, Batch No: 151209. Mf, Wuhu Kangai, Pharm. Co. Ltd), phenytoin, and phenobarbital, all purchased from Delimi Pharmacy Masalachi-njuma Jos Plateau State Nigeria. Solvents used for the extraction were absolute ethanol (supplied by Sigma Aldrich Germany), aqueous EAF (Sigma Aldrich Germany), n-hexane (provided by Sigma Aldrich Germany), and Tween 80 (supplied by Cole-Parmer Illinois, USA).
Animals
At the animal house, which belongs to the Pharmacology Department, fully grown mice of both genders weighing 20-25 g were gotten and supplied with clean water and feeds from Guinea Feeds Plc Nigeria. Their movements were at will. All policies guiding the use of animals for experimental purposes, as stipulated by Ebonyi State University, were followed strictly.
Acute toxicity tests
The lethal dose (LD50) of the extracts was assayed using Lork’s (1983) method. The study was carried out in two ways. Firstly, the mice were arranged into three groups, each containing three mice. EAF was given to the mice orally at 10, 100 and 1000 mg/kg body weight. Evidence of toxicity and deaths were carefully scrutinized within 24 hours. Secondly, this procedure was repeated but with doses of 1600, 2900 and 5000 mg/kg body weight for 72 hours.
Anticonvulsant studies
The experimental model described by Swinyard (1989), modified by Shimada and Yamagata (2018) [10], was used in the PTZ-induced test. Thirty mice were divided into five groups of six mice each. The first and second groups were the negative and positive control and were given 10 mL/kg of normal saline and 10 mg/kg of diazepam in that order. Groups 3, 4 and 5 were given EAF from C. leptostachya at doses of 100, 150 and 200 mg/kg body weight intraperitoneally. Thirty minutes later, 90 mg/kg of PTZ solution was introduced to each mouse via subcutaneous injection. The mice were examined closely for 30 minutes to check for a period of clonic spasm of at least five seconds duration (presence or absence of seizure) and mortality. This procedure was repeated with NHF [10].
For brucine-induced seizure, the same procedure was repeated but using 110 mg/kg body weight brucine. Also, 20 mg of phenobarbital was used as the standard drug in this case.
For maximal electroshock (MES), the procedure described above was adopted. Phenytoin was used as a standard drug. However, after 30 minutes of administration, an alternating current (50 Hz and 35 mA) was delivered to the animals in each group through ear electrodes for 0.2 s. Electrodes were wet with normal saline before attaching the mouse’s ear for better electrical contact. During MES-induced seizures, initial onset staggering, mortality, recovering period and percentage of mice protected against seizures were meticulously observed. Also, physical evidence of seizure was measured regarding the hind limb tonic extensor (HLTE). This means that the extracts inhibited HLTE as the extract is effective against MES-induced seizures [12].
Statistical examination
The results were presented in terms of Mean±SE of the mean and analyzed with one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test in SPSS software, version 20. P<0.05 were considered significant.
Results
Phytochemical analysis
Preliminary phytochemistry of extract from C. leptostachya leaf using different solvents (EAF and NHF) indicated the occurrence of alkaloids, flavonoids, carbohydrates, terpenoids, steroids, saponins, balsam, resins, while anthraquinone, cardiac glycoside and tannins were absent in both EAF and NHF. However, saponins and flavonoids were abundant in EAF and less in NHF (Table 1).

The fingerprint of thin layer chromatography (TLC) with retention factor
The fingerprint of TLC revealed that saponins (retention factor [Rf]=0.04) and flavonoids (Rf=0.05) have the least retention factor, showing that the plant is rich in these secondary metabolites. Also, carbohydrates (Rf=0.80), alkaloids (Rf=0.8), balsam (Rf=0.70) and resins (Rf=0.65) have high retention factors. This is because they have less affinity towards the stationary face of the TLC plate, hence traveling more distance than saponins and flavonoids. This is why they were more abundant during preliminary quantitative phytochemical screening (Table 2).

Median LD50 value in mice
There was no visible evidence of toxicity during this test from EAF and NHF of C. leptostachya extracts from 10 mg/kg to 5000 mg/kg post-injection for up to three days.
PTZ-induced seizure in mice
During the test for seizures induced by PTZ, EAF at 100 mg/kg and NHF at 100, 150 and 200 mg/kg did not prevent mortality and also failed to make a significant (P>0.05) effect on both onset-period and meantime of convulsion compared with the mice that received normal saline. This study further indicated that 200 mg/kg of AEF prevented death in 100% of the animals and significantly (P<0.05) delayed the onset-period of convulsion similar to the effects elicited by the standard drug, diazepam (Table 3).
Brucine-induced seizure in mice
The study findings indicated that 100 mg/kg of EAF alongside 100, 150, and 200 mg/kg of NHF could not prevent seizure or death and did not significantly (P>0.05) affect both onset time and recovery time in convulsed mice. However, 150 mg/kg of EAF prevented mortality in mice up to 50% and significantly reduced the convulsion’s mean recovery time (P<0.05). On the other hand, 200 mg/kg of EAF prevented death up to 100% with a significant (P<0.05) reduction in mean recovering time, just like the standard drug: phenobarbital (Table 4).

MES seizure-induced
This work indicated that EAF at 100 mg/kg could not prevent convulsion among the mice but reduced the mean recovering period considerably (P<0.05). Meanwhile, 150 mg/kg and 200 mg/kg stopped HLTE during the MES seizure-induced test at 16.67% and 50% in that order. A significant (P<0.05) reduction in mean time recovering in convulsed mice was noted. Notably, 100 mg/kg of NHF could not protect mice. Similarly, 150 and 200 mg/kg could not prevent hind limb tonic extension but reduced significantly (P<0.05) the mean time of recovery in convulsed mice (Table 5).

Discussion
Preliminary phytochemistry of the C. leptostachya leaf with different extracts (crude, EAF, and n-hexane extracts) showed the existence of alkaloids, saponin, flavonoids, carbohydrates, steroids, terpenoids, balsam, and resins. However, the quantitative phytochemistry of the leaf of C. leptostachya revealed that saponins, flavonoids, and steroids are found more abundantly in the EAF fraction.
Based on the results obtained from the phytochemical screening, the observed anticonvulsant activity of C. leptostachya cannot be entirely credited to one or numerous secondary metabolites seen in the phytochemical analysis. Nevertheless, triterpene steroids and saponins have been demonstrated to elicit anticonvulsant effects in some animal models of epilepsy, like MES and subcutaneous PTZ [13]. Available records showed that flavonoids and alkaloids have demonstrated protective effects against PTZ convulsions [14]. Studies have revealed that flavonoids have a neuroprotective effect against electrical igniting in rats [15]. These phytoconstituents could account for the noticeable pharmacological activities of EAF and NHF in the tests conducted. The notable anticonvulsant activity of the EAF contrasted NHF, which means that the anticonvulsant principles present in the C. leptostachya plant are mostly non-polar.
The calculation of the median LD50 value of herbal plants applied by herbal healers based on acute toxicity tests is of supreme importance since it gives a reliable clue concerning the therapeutic index of the plant. Because the value of LD50 (orally) of the EAF and NHF is above 5000 mg/kg body weight in mice, it is reasonable to declare that acute toxicity tests of C. leptostachya gave an LD50 which could be considered as ‘non-toxic’ [16].
The causes of epilepsy are enormously varied, ranging from genetic, developmental defects, infections and traumatic occurrences to neoplastic and degenerative processes [17]. Consequently, it is very doubtful that a single drug can be used as an effective treatment for the disorder.
The inability of the NHF to offer protection against convulsion during MES-induced seizure may be a result of fractionation with solvents of different polarity, which may have separated the plants or decreased the number of bioactive principles capable of exerting anticonvulsant effects against electrically induced seizures. However, the ability of EAF to offer 50% protection against convulsion in proportion to dose indicates drug dependency. This property is also demonstrated in the mean HLTE recovering time or duration where all the various graded doses of 100 mg/kg, 150 mg/kg, 200 mg/kg of EAF (0.52±0.03, 0.26±0.06 and 0.14±0.06, respectively) and NHF at 150, and 200 mg/kg (0.73±0.10 and 0.52±0.02, respectively) show a decrease in mean recovering time and statistical significance (P<0.05) relatively proportional to the graded dosages compare to the control, i.e. normal saline (1.24±0.05). Based on these facts, it can be said that EAF and NHF are very effective against electrically induced seizures.
However, the ability of these extracts to significantly decrease the mean recovering time of convulsed mice compared to normal saline-group post-exposure to MES stimulus showed that these extracts probably antagonized the electrically induced seizures by blocking voltage-gated sodium ion (Na+) channels or by antagonizing glutamatergic excitation mediated by N-methyl-D-aspartate receptor complex. Inhibition of Na+ ion channels would always stabilize neuronal membranes, protecting mice against convulsions induced by MES stimuli.
The MES test model for anticonvulsant screening has a clearly defined (consistent) endpoint (inhibition of the tonic hind limb extension phase) and is highly reproducible [18]. MES is a reliable experimental design that can predict a drug’s effectiveness against tonic-clonic seizures (generalized seizures) [19]. The two standard drugs used here, i.e. carbamazepine and phenytoin, had been reported to be highly potent against HLTE-stimulated MES. It has been revealed that this kind of seizure, as shown in electrographs, is consistent with that of human disorder [20]. This model (MES) detects plant metabolites that stop seizures from spreading through neural tissue. Hence, the ability of EAF and NHF from C. leptostachya to hinder seizure circulation and reduce the recovery period from convulsion generated by MES stimulus suggests that these extracts have activity against generalized seizure.
PTZ, made from tetrazole, is a convulsant agent that acts in the system [21]. The parenteral injection of PTZ has always produced a reliable convulsant effect in rodents, cats, and other primates. Initially, PTZ is characterized by myoclonic jerks, which in turn lead to a generalized seizure (tonic-clonic). Previous works have proven PTZ to be a reducer of gamma-aminobutyric acid (GABA) ergic tone [22]. The two main neurotransmitters in the brain are GABA and glutamic acid. GABA plays an inhibitory role, while glutamic acid plays an excitatory role. Also, the promotion of GABA antagonizes seizures, whereas inhibition of GABA generates seizures [23]. Drugs that decrease T-type calcium current and agents that enhance the inhibition of neurotransmitters by GABA receptors are known to block seizures generated by PTZ. Hence, agents that could suppress PTZ-generated seizures are presumed to treat absence seizures [24].
The ability of prior injection of 200 mg/kg of EAF to considerably (P<0.05) attenuate PTZ-induced seizure activity in the mice measured in terms of mean duration of time and also significantly calm PTZ seizure-induced measured in terms of the mean onset time of seizure. ‘Straub’s tail’ phenomenon (a state by which an animal carries its tail erected or nearly vertical position), jerky body movements, and convulsion strongly suggest EAF from C. leptostachya affects GABAergic neurotransmission.
The seizure threshold has been proven to be reduced by the activity of dopamine in the brain. Specific agents that antagonize dopamine have been shown to have protective effects in animals against seizures stimulated by the injection of PTZ [25]. PTZ triggers a high influx of calcium and sodium ions into the neuron [26]. It has been established that the activity of PTZ is mainly on calcium channels, which lose selectivity by calcium and activate sodium ion influx uncontrollably [27]. Since the EAF was very potent in protecting against seizure produced by PTZ injection, this presumes that it may probably act by modulation of GABA receptor arbitrated by inhibition of neurotransmitters, which can be used in the absence of seizure therapy. Similarly, brucine, an alkaloid derivative, is a glycine synthesis inhibitor. Brucine antagonizes certain excitatory monotransmitters such as N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid. The blocking of glycine triggers a kind of seizure known as clonic and tonic in animal demonstrations. This brucine is used to induce seizures in animals during research exploration.
In addition, during the experiment, 150 and 200 mg/kg of EAF reduced the time frame of convulsion significantly (P<0.05). Apart from this effect, 150 and 200 mg/kg gave the mice 50% and 100% protection against death just in that order. These effects against brucine-induced seizures suggest strongly that the extract affects glycine receptors. Brucine usually exerts an antagonistic selective impact against glycine receptor inhibitory activity in the spinal cord, producing excitatory action in the central nervous system. This mechanism of action could suggest the mode of action of C. leptostachya.
Conclusion
The present study supports that C. leptostachya leaves contain anticonvulsant metabolites, which could account for its use as a convulsion remedy. Also, the therapeutic effect of this plant was found to be more practically evident in its EAF fraction than in the NHF.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by Ethics Committee of Ebonyi State University (Code: EBSU/DRIC/UREC/VOL.04/098).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
Authors express their profound gratitude to Diyen Bulus Auta and Sunday Azi of the University of Jos Nigeria for their unquantifiable technical assistance during this work.
References
Epilepsy is known to be a critical and frequent distress of man [1]. At least fifty million people suffer from epilepsy, which is responsible for about 1% of the total sickness concern worldwide [2]. Epilepsy is framed from the Greek word “epilembanein,” which is referred to as “seize.” Epilepsy is a persistent neurological chaos that disturbs males and females equally [3]. It is the next most frequent inveterate neurological state observed by neurologists globally [4]. Although there are many other brain diseases, epilepsy is more noticeable not out of its commonness and the gravity of occurrence but the superstitious beliefs of people of different traditions and world views, especially in Africa. This condition affects not only the person with epilepsy but also the family and the entire community [5]. Hence, epilepsy is still well known as one of the most stigmatized brain diseases. This stigmatization is common among developing nations, possibly due to low levels of education, lack of exposure, and poor information concerning the real nature of the disease. The embarrassment faced by victims makes it difficult to seek treatment. This situation usually worsens their conditions and reduces their chances of being educated, employed, and socially significant [6].
Thus, research into the best medical care and treatment options for epilepsy will continue to be a priority and a complex problem for various nations. For a very long time, food therapy, acupuncture, moxibustion, surgery, and medicine have been the mainstays of epilepsy treatment [7]. Currently, drugs are the most recommended option for managing and treating epilepsy. The global pharmaceutical industry currently offers over 50 distinct antiepileptic drugs (AEDs), such as stiripentol, carbamazepine, oxcarbazepine, sodium valproate, gabapentin, lamotrigine, topiramate, levetiracetam, lacosamide and pregabalin, etc. They are all available in the pharmaceutical industry globally. However, utilizing conventional AEDs may cause adverse effects in 30%–40% of people with epilepsy. In addition, about 30% of patients have drug resistance.
Consequently, the need for epilepsy treatment remains unfulfilled [8]. Moreover, prolonged usage of certain AEDs has been linked to adverse effects and an increased chance of drug-drug interactions [9]. Therefore, research and development of novel AEDs with multiple targets and minimal side effects is both serious and challenging.
One useful resource for discovering novel forms of AEDs is the kingdom of plants. Traditional medicine has a lengthy history and a wealth of real-world expertise in treating epilepsy. There is historical evidence supporting the use of herbs as medicine to treat epilepsy. Specifically, medicinal plants for epilepsy are frequently regarded as a gentle and secure substitute for chemical AEDs. Herbal medicine has been utilized globally as a complementary or alternative medication for the treatment of epilepsy [10]. More than 20 Chinese patent medicines composed of herbs have been documented for clinical use. In addition, herbal treatments are extensively used in traditional medicine, such as infusion or decoction, for epileptic patients in Japan, Korea, Australia, India, Mexico, African and South American countries, etc. [11].
The leaves, roots and or bark of medicinal plants prescribed by traditional medical healers are taken orally or used as a bath to treat convulsions [12]. For example, the decoction of Celosia leptostachya is used in Nigeria to treat convulsions [12].
Provided that efforts to cure epilepsy are not clinically successful, there is an increasing concern that the efficacy of drug management of epilepsy has not yielded better outcomes despite the development of new drugs for it.
C. leptostachya is classified under the family of Amaranthaceae. The stem lacks hairs with slim angle projections and decumbent support in nature. This plant can grow up to 300 mm in height and reach 600 mm high when lifted from the stalk. It does have ovate leaves, but they are smooth and noticeable. C. leptostachya is well distributed in Abia State, South East of Nigeria. The plant’s leaves are edible and serve as cooking vegetables for soup. C. leptostachya possesses numerous medicinal properties, which traditional medicine practitioners exploit mainly in curing illnesses such as boils, fever, snake bites, scorpion stings, eye infections, wounds and pain, and most notably, epilepsy. Based on personal interaction with traditional healers, when an epileptic patient is brought to the healer, the plant’s fresh leaves would be fetched and squeezed manually to get at least 10 mL of the liquid content and administered orally and through the eyes. The patients usually recover between 5-10 minutes after treatment. Hence, this work was designed to investigate its anticonvulsant activity using animal experimental design for epilepsy.
Materials and Methods
Collection and identification of the plant
C. leptostachya leaf (fresh) was collected at Umuhu Nvosi in Abia State, Nigeria, at 6:00 AM. The identification was done by Ibe M. of the Forestry Department of the Michael Okpara University of Agriculture Umudike, Abia State, Nigeria and was given a voucher number: “FHI3081.”
Preparation of extraction-fractionation
The collected leaves of C. leptostachya were washed with clean tap water to remove any dirt. It was then dried at room temperature of about 25oC. After that, it was pounded into fine particles with a local grinder. A maceration extraction technique was used whereby 800 g of fine particles of C. leptostachya were immersed in absolute ethanol for three days. It was filtered and allowed to dry under a controlled temperature (40oC) in a water bath. The method described by Shi et al. (2018) [2] was used for fractionation. The outcome of the extraction (30 g of ethanol extract) was liquefied using 100 mL of purified water alongside ethyl acetate (300 mL) as a partitioner, which produced ethyl acetate fraction (EAF) with the application of separating funnel and light heat (40oC) in a water bath. The remainder of the solution was further fractionated with 300 mL of n-hexane, which was collected using a separating funnel called n-hexane fraction (NHF) (Figure 1).
Phytochemical screening
The preliminary phytochemistry of C. leptostachya ethanol leaf extract was carried out to determine various phytochemical components of the plant. Here, we used the ferric chloride test for tannins, Mayer and Dragendorff test for alkaloids, Froth test for saponins, Liebermann-Burchard test for steroids, Salkowski test for terpenoids, ammonia and the sulphuric acid test for flavonoids and Borntrager’s test for anthraquinones.
Chemicals, drugs and equipment
The following chemicals and machine were used to induce convulsion and epileptic seizure in animals: Pentylenetetrazole (PTZ) (supplied by Sigma Aldrich Germany) (GABAA antagonist), brucine (provided by Sigma Aldrich Germany) (Glycine antagonist), and electroshock machine (supplied the University of Jos, Plateau State Nigeria). Also, standard drugs used for positive control were diazepam (NAFDAC Reg. 04-4402, Batch No: 151209. Mf, Wuhu Kangai, Pharm. Co. Ltd), phenytoin, and phenobarbital, all purchased from Delimi Pharmacy Masalachi-njuma Jos Plateau State Nigeria. Solvents used for the extraction were absolute ethanol (supplied by Sigma Aldrich Germany), aqueous EAF (Sigma Aldrich Germany), n-hexane (provided by Sigma Aldrich Germany), and Tween 80 (supplied by Cole-Parmer Illinois, USA).
Animals
At the animal house, which belongs to the Pharmacology Department, fully grown mice of both genders weighing 20-25 g were gotten and supplied with clean water and feeds from Guinea Feeds Plc Nigeria. Their movements were at will. All policies guiding the use of animals for experimental purposes, as stipulated by Ebonyi State University, were followed strictly.
Acute toxicity tests
The lethal dose (LD50) of the extracts was assayed using Lork’s (1983) method. The study was carried out in two ways. Firstly, the mice were arranged into three groups, each containing three mice. EAF was given to the mice orally at 10, 100 and 1000 mg/kg body weight. Evidence of toxicity and deaths were carefully scrutinized within 24 hours. Secondly, this procedure was repeated but with doses of 1600, 2900 and 5000 mg/kg body weight for 72 hours.
Anticonvulsant studies
The experimental model described by Swinyard (1989), modified by Shimada and Yamagata (2018) [10], was used in the PTZ-induced test. Thirty mice were divided into five groups of six mice each. The first and second groups were the negative and positive control and were given 10 mL/kg of normal saline and 10 mg/kg of diazepam in that order. Groups 3, 4 and 5 were given EAF from C. leptostachya at doses of 100, 150 and 200 mg/kg body weight intraperitoneally. Thirty minutes later, 90 mg/kg of PTZ solution was introduced to each mouse via subcutaneous injection. The mice were examined closely for 30 minutes to check for a period of clonic spasm of at least five seconds duration (presence or absence of seizure) and mortality. This procedure was repeated with NHF [10].
For brucine-induced seizure, the same procedure was repeated but using 110 mg/kg body weight brucine. Also, 20 mg of phenobarbital was used as the standard drug in this case.
For maximal electroshock (MES), the procedure described above was adopted. Phenytoin was used as a standard drug. However, after 30 minutes of administration, an alternating current (50 Hz and 35 mA) was delivered to the animals in each group through ear electrodes for 0.2 s. Electrodes were wet with normal saline before attaching the mouse’s ear for better electrical contact. During MES-induced seizures, initial onset staggering, mortality, recovering period and percentage of mice protected against seizures were meticulously observed. Also, physical evidence of seizure was measured regarding the hind limb tonic extensor (HLTE). This means that the extracts inhibited HLTE as the extract is effective against MES-induced seizures [12].
Statistical examination
The results were presented in terms of Mean±SE of the mean and analyzed with one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test in SPSS software, version 20. P<0.05 were considered significant.
Results
Phytochemical analysis
Preliminary phytochemistry of extract from C. leptostachya leaf using different solvents (EAF and NHF) indicated the occurrence of alkaloids, flavonoids, carbohydrates, terpenoids, steroids, saponins, balsam, resins, while anthraquinone, cardiac glycoside and tannins were absent in both EAF and NHF. However, saponins and flavonoids were abundant in EAF and less in NHF (Table 1).

The fingerprint of thin layer chromatography (TLC) with retention factor
The fingerprint of TLC revealed that saponins (retention factor [Rf]=0.04) and flavonoids (Rf=0.05) have the least retention factor, showing that the plant is rich in these secondary metabolites. Also, carbohydrates (Rf=0.80), alkaloids (Rf=0.8), balsam (Rf=0.70) and resins (Rf=0.65) have high retention factors. This is because they have less affinity towards the stationary face of the TLC plate, hence traveling more distance than saponins and flavonoids. This is why they were more abundant during preliminary quantitative phytochemical screening (Table 2).

Median LD50 value in mice
There was no visible evidence of toxicity during this test from EAF and NHF of C. leptostachya extracts from 10 mg/kg to 5000 mg/kg post-injection for up to three days.
PTZ-induced seizure in mice
During the test for seizures induced by PTZ, EAF at 100 mg/kg and NHF at 100, 150 and 200 mg/kg did not prevent mortality and also failed to make a significant (P>0.05) effect on both onset-period and meantime of convulsion compared with the mice that received normal saline. This study further indicated that 200 mg/kg of AEF prevented death in 100% of the animals and significantly (P<0.05) delayed the onset-period of convulsion similar to the effects elicited by the standard drug, diazepam (Table 3).

Brucine-induced seizure in mice
The study findings indicated that 100 mg/kg of EAF alongside 100, 150, and 200 mg/kg of NHF could not prevent seizure or death and did not significantly (P>0.05) affect both onset time and recovery time in convulsed mice. However, 150 mg/kg of EAF prevented mortality in mice up to 50% and significantly reduced the convulsion’s mean recovery time (P<0.05). On the other hand, 200 mg/kg of EAF prevented death up to 100% with a significant (P<0.05) reduction in mean recovering time, just like the standard drug: phenobarbital (Table 4).

MES seizure-induced
This work indicated that EAF at 100 mg/kg could not prevent convulsion among the mice but reduced the mean recovering period considerably (P<0.05). Meanwhile, 150 mg/kg and 200 mg/kg stopped HLTE during the MES seizure-induced test at 16.67% and 50% in that order. A significant (P<0.05) reduction in mean time recovering in convulsed mice was noted. Notably, 100 mg/kg of NHF could not protect mice. Similarly, 150 and 200 mg/kg could not prevent hind limb tonic extension but reduced significantly (P<0.05) the mean time of recovery in convulsed mice (Table 5).

Discussion
Preliminary phytochemistry of the C. leptostachya leaf with different extracts (crude, EAF, and n-hexane extracts) showed the existence of alkaloids, saponin, flavonoids, carbohydrates, steroids, terpenoids, balsam, and resins. However, the quantitative phytochemistry of the leaf of C. leptostachya revealed that saponins, flavonoids, and steroids are found more abundantly in the EAF fraction.
Based on the results obtained from the phytochemical screening, the observed anticonvulsant activity of C. leptostachya cannot be entirely credited to one or numerous secondary metabolites seen in the phytochemical analysis. Nevertheless, triterpene steroids and saponins have been demonstrated to elicit anticonvulsant effects in some animal models of epilepsy, like MES and subcutaneous PTZ [13]. Available records showed that flavonoids and alkaloids have demonstrated protective effects against PTZ convulsions [14]. Studies have revealed that flavonoids have a neuroprotective effect against electrical igniting in rats [15]. These phytoconstituents could account for the noticeable pharmacological activities of EAF and NHF in the tests conducted. The notable anticonvulsant activity of the EAF contrasted NHF, which means that the anticonvulsant principles present in the C. leptostachya plant are mostly non-polar.
The calculation of the median LD50 value of herbal plants applied by herbal healers based on acute toxicity tests is of supreme importance since it gives a reliable clue concerning the therapeutic index of the plant. Because the value of LD50 (orally) of the EAF and NHF is above 5000 mg/kg body weight in mice, it is reasonable to declare that acute toxicity tests of C. leptostachya gave an LD50 which could be considered as ‘non-toxic’ [16].
The causes of epilepsy are enormously varied, ranging from genetic, developmental defects, infections and traumatic occurrences to neoplastic and degenerative processes [17]. Consequently, it is very doubtful that a single drug can be used as an effective treatment for the disorder.
The inability of the NHF to offer protection against convulsion during MES-induced seizure may be a result of fractionation with solvents of different polarity, which may have separated the plants or decreased the number of bioactive principles capable of exerting anticonvulsant effects against electrically induced seizures. However, the ability of EAF to offer 50% protection against convulsion in proportion to dose indicates drug dependency. This property is also demonstrated in the mean HLTE recovering time or duration where all the various graded doses of 100 mg/kg, 150 mg/kg, 200 mg/kg of EAF (0.52±0.03, 0.26±0.06 and 0.14±0.06, respectively) and NHF at 150, and 200 mg/kg (0.73±0.10 and 0.52±0.02, respectively) show a decrease in mean recovering time and statistical significance (P<0.05) relatively proportional to the graded dosages compare to the control, i.e. normal saline (1.24±0.05). Based on these facts, it can be said that EAF and NHF are very effective against electrically induced seizures.
However, the ability of these extracts to significantly decrease the mean recovering time of convulsed mice compared to normal saline-group post-exposure to MES stimulus showed that these extracts probably antagonized the electrically induced seizures by blocking voltage-gated sodium ion (Na+) channels or by antagonizing glutamatergic excitation mediated by N-methyl-D-aspartate receptor complex. Inhibition of Na+ ion channels would always stabilize neuronal membranes, protecting mice against convulsions induced by MES stimuli.
The MES test model for anticonvulsant screening has a clearly defined (consistent) endpoint (inhibition of the tonic hind limb extension phase) and is highly reproducible [18]. MES is a reliable experimental design that can predict a drug’s effectiveness against tonic-clonic seizures (generalized seizures) [19]. The two standard drugs used here, i.e. carbamazepine and phenytoin, had been reported to be highly potent against HLTE-stimulated MES. It has been revealed that this kind of seizure, as shown in electrographs, is consistent with that of human disorder [20]. This model (MES) detects plant metabolites that stop seizures from spreading through neural tissue. Hence, the ability of EAF and NHF from C. leptostachya to hinder seizure circulation and reduce the recovery period from convulsion generated by MES stimulus suggests that these extracts have activity against generalized seizure.
PTZ, made from tetrazole, is a convulsant agent that acts in the system [21]. The parenteral injection of PTZ has always produced a reliable convulsant effect in rodents, cats, and other primates. Initially, PTZ is characterized by myoclonic jerks, which in turn lead to a generalized seizure (tonic-clonic). Previous works have proven PTZ to be a reducer of gamma-aminobutyric acid (GABA) ergic tone [22]. The two main neurotransmitters in the brain are GABA and glutamic acid. GABA plays an inhibitory role, while glutamic acid plays an excitatory role. Also, the promotion of GABA antagonizes seizures, whereas inhibition of GABA generates seizures [23]. Drugs that decrease T-type calcium current and agents that enhance the inhibition of neurotransmitters by GABA receptors are known to block seizures generated by PTZ. Hence, agents that could suppress PTZ-generated seizures are presumed to treat absence seizures [24].
The ability of prior injection of 200 mg/kg of EAF to considerably (P<0.05) attenuate PTZ-induced seizure activity in the mice measured in terms of mean duration of time and also significantly calm PTZ seizure-induced measured in terms of the mean onset time of seizure. ‘Straub’s tail’ phenomenon (a state by which an animal carries its tail erected or nearly vertical position), jerky body movements, and convulsion strongly suggest EAF from C. leptostachya affects GABAergic neurotransmission.
The seizure threshold has been proven to be reduced by the activity of dopamine in the brain. Specific agents that antagonize dopamine have been shown to have protective effects in animals against seizures stimulated by the injection of PTZ [25]. PTZ triggers a high influx of calcium and sodium ions into the neuron [26]. It has been established that the activity of PTZ is mainly on calcium channels, which lose selectivity by calcium and activate sodium ion influx uncontrollably [27]. Since the EAF was very potent in protecting against seizure produced by PTZ injection, this presumes that it may probably act by modulation of GABA receptor arbitrated by inhibition of neurotransmitters, which can be used in the absence of seizure therapy. Similarly, brucine, an alkaloid derivative, is a glycine synthesis inhibitor. Brucine antagonizes certain excitatory monotransmitters such as N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid. The blocking of glycine triggers a kind of seizure known as clonic and tonic in animal demonstrations. This brucine is used to induce seizures in animals during research exploration.
In addition, during the experiment, 150 and 200 mg/kg of EAF reduced the time frame of convulsion significantly (P<0.05). Apart from this effect, 150 and 200 mg/kg gave the mice 50% and 100% protection against death just in that order. These effects against brucine-induced seizures suggest strongly that the extract affects glycine receptors. Brucine usually exerts an antagonistic selective impact against glycine receptor inhibitory activity in the spinal cord, producing excitatory action in the central nervous system. This mechanism of action could suggest the mode of action of C. leptostachya.
Conclusion
The present study supports that C. leptostachya leaves contain anticonvulsant metabolites, which could account for its use as a convulsion remedy. Also, the therapeutic effect of this plant was found to be more practically evident in its EAF fraction than in the NHF.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by Ethics Committee of Ebonyi State University (Code: EBSU/DRIC/UREC/VOL.04/098).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
Authors express their profound gratitude to Diyen Bulus Auta and Sunday Azi of the University of Jos Nigeria for their unquantifiable technical assistance during this work.
References
- de Boer HM. Epilepsy stigma: Moving from a global problem to global solutions. Seizure. 2010; 19(10):630-6. [DOI:10.1016/j.seizure.2010.10.017] [PMID]
- Shi Y, Wang S, Ying J, Zhang M, Liu P, Zhang H, et al. Correlates of perceived stigma for people living with epilepsy: A meta-analysis. Epilepsy Behav. 2017; 70(Pt A):198-203. [DOI:10.1016/j.yebeh.2017.02.022] [PMID]
- Panebianco M, Al-Bachari S, Weston J, Hutton JL, Marson AG. Gabapentin add-on treatment for drug-resistant focal epilepsy. Cochrane Database Syst Rev. 2018; 10(10):CD001415. [DOI:10.1002/14651858.CD001415.pub3] [PMID] [PMCID]
- Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME. Neurological disorders in mental, neurological, and substance use disorders: Disease control priorities. Washington: The international bank for reconstruction and development/The world bank; 2016. [Link]
- von Gaudecker JR, Taylor AG, Keeling AW, Buelow JM, Benjamin S. Living in the epilepsy treatment gap in rural South India: A focused ethnography of women and problems associated with stigma. Health Care Women Int. 2017; 38(7):753-64. [DOI:10.1080/07399332.2017.1321000] [PMID]
- Keikelame MJ, Swartz L. "I wonder if I did not mess up….": Shame and resistance among women with epilepsy in Cape Town, South Africa. Seizure. 2018; 61:50-6. [DOI:10.1016/j.seizure.2018.07.021] [PMID]
- Weaver DF, Pohlmann-Eden B. Pharmacoresistant epilepsy: Unmet needs in solving the puzzle(s). Epilepsia. 2013; 54(Suppl 2):80-5. [DOI:10.1111/epi.12191] [PMID]
- Schulze-Bonhage A. A 2017 review of pharmacotherapy for treating focal epilepsy: Where are we now and how will treatment develop? Expert Opin Pharmacother. 2017; 18(17):1845-53. [DOI:10.1080/14656566.2017.1391788] [PMID]
- Mula M, Zaccara G, Galimberti CA, Ferrò B, Canevini MP, Mascia A, et al. Validated outcome of treatment changes according to International League Against Epilepsy criteria in adults with drug-resistant focal epilepsy. Epilepsia. 2019; 60(6):1114-23. [DOI:10.1111/epi.14685] [PMID] [PMCID]
- Shimada T, Yamagata K. Pentylenetetrazole-induced kindling mouse model. J Vis Exp. 2018; (136):56573. [DOI:10.3791/56573] [PMID] [PMCID]
- Balestrini S, Sisodiya SM. Pharmacogenomics in epilepsy. Neurosci Lett. 2018; 667:27-39. [DOI:10.1016/j.neulet.2017.01.014] [PMID] [PMCID]
- Tutka P, Wlaź A, Florek-Łuszczki M, Kołodziejczyk P, Bartusik-Aebisher D, Łuszczki JJ. Arvanil, olvanil, AM 1172 and LY 2183240 (various cannabinoid CB1 receptor agonists) increase the threshold for maximal electroshock-induced seizures in mice. Pharmacol Rep. 2018; 70(1):106-9. [DOI:10.1016/j.pharep.2017.08.006] [PMID]
- Malami S, Kyari H, Danjuma NM, Ya'u J, Hussaini IM. Anticonvulsant properties of methanol leaf extract of Laggera Aurita Linn. F. (Asteraceae) in laboratory animals. J Ethnopharmacol. 2016; 191:301-6. [DOI:10.1016/j.jep.2016.06.035] [PMID]
- Ye M, Bi YF, Ding L, Zhu WW, Gao W. Saikosaponin a functions as anti-epileptic effect in pentylenetetrazol induced rats through inhibiting mTOR signaling pathway. Biomed Pharmacother. 2016; 81:281-7. [DOI:10.1016/j.biopha.2016.04.012] [PMID]
- Muke S, Kaikini A, Peshattiwar V, Bagle S, Dighe V, Sathaye S. Neuroprotective effect of coumarin nasal formulation: kindling model assessment of epilepsy. Front Pharmacol. 2018; 9:992. [DOI:10.3389/fphar.2018.00992] [PMID] [PMCID]
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54(4):275-87. [DOI:10.1007/BF01234480] [PMID]
- Al-Eitan LN, Al-Dalalah IM, Mustafa MM, Alghamdi MA, Elshammari AK, Khreisat WH, et al. Effects of MTHFR and ABCC2 gene polymorphisms on antiepileptic drug responsiveness in Jordanian epileptic patients. Pharmgenomics Pers Med. 2019; 12:87-95. [DOI:10.2147/PGPM.S211490] [PMID] [PMCID]
- Borowicz-Reutt KK, Popławska M, Banach M, Wróblewska D. Influence of propafenone on the anticonvulsant activity of various novel antiepileptic drugs in the mouse maximal electroshock model. Pharmacol Rep. 2018; 70(3):481-7. [DOI:10.1016/j.pharep.2017.11.014] [PMID]
- Joshi R, Reeta KH, Sharma SK, Tripathi M, Gupta YK. Pharmacodynamic and pharmacokinetic interaction of Panchagavya Ghrita with phenytoin and carbamazepine in maximal electroshock induced seizures in rats. Ayu. 2015; 36(2):196-202. [DOI:10.4103/0974-8520.175538] [PMID] [PMCID]
- Baker EM, Thompson CH, Hawkins NA, Wagnon JL, Wengert ER, Patel MK, et al. The novel sodium channel modulator GS-458967 (GS967) is an effective treatment in a mouse model of SCN8A encephalopathy. Epilepsia 2018; 59(6):1166-1176. [DOI:10.1111/epi.14196] [PMID]
- Zhang B, Wong M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia. 2012; 53(3):506-11. [DOI:10.1111/j.1528-1167.2011.03384.x] [PMID] [PMCID]
- Bae MH, Bissonette GB, Mars WM, Michalopoulos GK, Achim CL, Depireux DA, et al. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Exp Neurol. 2010; 221(1):129-35. [DOI:10.1016/j.expneurol.2009.10.011] [PMID] [PMCID]
- Atherton JF, Menard A, Urbain N, Bevan MD. Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. J Neurosci. 2013; 33(17):7130-44. [DOI:10.1523/JNEUROSCI.3576-12.2013] [PMID] [PMCID]
- Karami R, Hosseini M, Mohammadpour T, Ghorbani A, Sadeghnia HR, Rakhshandeh H, et al. Effects of hydroalcoholic extract of Coriandrum sativum on oxidative damage in pentylenetetrazole-induced seizures in rats. Iran J Neurol. 2015; 14(2):59-66. [PMID] [PMCID]
- Bozzi Y, Borrelli E. The role of dopamine signaling in epileptogenesis. Front Cell Neurosci. 2013; 7:157. [DOI:10.3389/fncel.2013.00157] [PMID] [PMCID]
- N'Gouemo P. Probing the role of the sodium/calcium exchanger in pentylenetetrazole-induced generalized seizures in rats. Brain Res Bull. 2013; 90:52-7. [DOI:10.1016/j.brainresbull.2012.09.007] [PMID] [PMCID]
- Nieoczym D, Socała K, Łuszczki JJ, Czuczwar SJ, Wlaz P. Influence of sildenafil on the anticonvulsant action of selected antiepileptic drugs against pentylenetetrazole-induced clonic seizures in mice. J Neural Transm. 2012; 119(8):923-31. [DOI:10.1007/s00702-012-0767-1] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Pharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |