Volume 10, Issue 1 (2024)
Pharm Biomed Res 2024, 10(1): 33-46 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
salehi A. Effects of Melissa officinalis Alcoholic Extract on Kidney Tissue and Apoptosis Gene Expression in a Wistar Rat Model With Spinal Cord Injury. Pharm Biomed Res 2024; 10 (1) :33-46
URL: http://pbr.mazums.ac.ir/article-1-578-en.html
URL: http://pbr.mazums.ac.ir/article-1-578-en.html
Department of Cellular and Molecular Biology, Faculty of New Science and Technology, Tehran Medical Branch, Islamic Azad University, Tehran, Iran.
Full-Text [PDF 2062 kb]
(647 Downloads)
| Abstract (HTML) (1423 Views)
Full-Text: (407 Views)
Introduction
Spinal cord injury (SCI) is a severe medical condition that leads to physical, emotional, social, and financial challenges for the affected individuals and their communities. These patients face significant disruptions in multiple areas of their lives [1-3]. This is because the spinal cord, as part of the central nervous system, is responsible for receiving sensory signals and transmitting motor instructions. It also triggers spinal reflexes that protect the body from potential harm. Consequently, damaging the spinal cord can result in long-term disability. SCI occurs when the vertebral column experiences a sudden impact or blow, leading to fractures or dislocations of the vertebrae [2, 4-6].
SCI is categorized as primary or secondary based on their underlying cause. Primary injuries involve the initial impact on the spinal cord during an injury, which can lead to the movement of bone fragments, disc ruptures, and ligament damage. Following the initial injury, a complex secondary process occurs within a few hours, resulting in the death of nerve and support cells, reduced blood flow, and the beginning of inflammation [7, 8, 9]. The ongoing secondary damage causes the creation of a harmful environment at the cellular level, leading to the loss of functional cells and harm to the tissue’s surroundings [10]; the inflammatory environment plays a significant role in advancing further damage, causing inflammation to spread to other parts of the body. This leads to disruptions in the functioning of different organs, as the spinal cord plays a crucial role in coordinating bodily functions. This can result in dysfunction or impairment in several organs, such as neurogenic pain, breathing problems, heart disease, and kidney dysfunction, among others [11, 12].
Research has indicated that damage to spinal cord tissue can trigger the release of inflammatory cytokines, such as TNF-α, and pro-apoptotic factors, such as caspase-3 and Bax. These elements are essential in the mechanisms and phases that lead to SCI, particularly in cellular death and the body’s inflammatory reaction [13, 14]. Bax and Bcl-2 are key genes within the Bcl-2 family that regulate apoptosis. Bax is recognized as a critical factor in promoting apoptosis, while Bcl-2 functions to inhibit apoptosis. Furthermore, caspase-3 is an essential protein in the final steps of apoptosis. On the other hand, TNF-α is an inflammatory cytokine that is essential for the body’s inflammatory responses. At a molecular level, both proteins are crucial for advancing our comprehension of the impact of SCI [13, 15]. SCI leads to an increase in TNF-α, contributing to the amplification of apoptotic factors and damaging spinal cord tissue. This greatly aids in the management of spinal cord injuries [16].
Additionally, SCI can influence the kidneys, which are indirectly impacted by this condition. This can disrupt the function and overall well-being of this essential organ. The kidney is responsible for crucial tasks, such as maintaining fluid balance, removing waste materials, regulating electrolyte levels, balancing acidity, and supporting the endocrine glands in the body [17, 18]. The kidneys function similarly to endocrine glands by producing renin and erythropoietin and assisting in the hydroxylation of vitamin D. Additionally, they receive 25% of the cardiac output. They are crucial in regulating blood pressure and cardiac function. Consequently, any harm to the kidneys can have a significant impact on a person’s life [19, 20]. This research investigates the intricate connections between SCI, inflammation, apoptotic processes, and their effects on kidney function, recognizing the vital importance of the kidneys in people’s lives [21]. The kidneys are at risk of being severely affected by SCI, posing a threat to the individual’s life and potentially leading to death in severe cases. The secondary consequences of SCI, including widespread inflammation that affects the kidneys, can result in chronic kidney disease, renal failure, and other kidney-related conditions. Over time, this can cause a decline in kidney function and give rise to various serious health issues, which may ultimately lead to death in certain instances [11, 22]. Research has indicated that kidney problems are a significant predictor for individuals with SCI; doctors recommend that people with SCI monitor their kidney function to manage their health effectively [23, 24].
Studies have demonstrated that Melissa officinalis, a member of the mint family, is a critical medicinal plant. It has a long history of use as a soothing and pain-relieving herb for various ailments, such as anxiety, stress, and sleep disorders. It is extensively utilized in the food, pharmaceutical, and cosmetic sectors due to the beneficial compounds present in the plant and its extract [25]. One of the critical elements of this mixture contains vital substances, like citronellal, nerol, geraniol, and citronellal, which are accountable for the delightful scent of this plant. It also includes phenolic acids, such as rosmarinic acid, which has antioxidant qualities. Additionally, it contains flavonoids like luteolin, which have antioxidant and anti-inflammatory properties. Terpenoids, other fatty acids, and various other compounds are also present [26, 27, 28]. The plant M. officinalis also called the lemon balm, is recognized for its soothing properties. The components found in its alcohol extract attach to gamma-aminobutyric acid (GABA) receptors in the brain, which helps control neural function. This can lead to heightened relaxation and decreased stress. The phenolic and flavonoid compounds in the alcohol extract have anti-inflammatory and antioxidant characteristics, which can aid in diminishing inflammation and shielding nerve cells from harm [25, 29].
A study conducted by Hosseini et al. [30] showed that the alcoholic extract of M. officinalis can potentially be used to treat SCI. Other research has indicated that the alcoholic extract of this plant could have a beneficial effect on improving mood and reducing anxiety. Additionally, the extract may contain flavonoid compounds that have antioxidant properties [30, 31]. The plant extract has various functional mechanisms, including the inhibition of microbial and fungal growth and antimicrobial and antifungal properties. It can also help with digestive issues, such as bloating, heartburn, and other significant problems. According to this information, the alcoholic extract of M. officinalis shows promise as a potential medicinal plant for preventing and treating different neurological diseases. This is attributed to its chemical compounds, which have strong antioxidant and anti-inflammatory effects, particularly at higher concentrations. It can also have preventive effects on conditions such as cerebral ischemia, SCI, and Alzheimer’s disease, making it useful in treating diseases resulting from SCI [31-33]. Accordingly, this research investigates how M. officinalis extract affects the activity of genes related to apoptosis and the inflammatory factor TNFα in kidney tissue. The study also explored the extract’s influence on alterations in the kidney tissue of rats with SCI.
Materials and Methods
Gathering and obtaining a hydroalcoholic extract from the M. officinalis plant
The plants were sourced from the Firoozeh Medicinal Plant Garden in Tehran City, Iran, and approved by the Faculty of Pharmaceutical Sciences of Tehran Azad University to produce alcoholic extracts. The fresh leaves were dried, crushed, and mixed with 250 mL of 70% ethanol to create the hydroalcoholic extract. The mixture was left for 48 h, shaken every 12 h, and filtered. The remaining residues were washed with 70% ethanol, filtered again, and combined with the previous extract. The solvent was evaporated using a vacuum distillation device [32], producing 2.4 g of plant extract. This extract was then combined with 50 mL of physiological serum and left at room temperature for a day to dissolve completely. The resulting solution was injected intraperitoneally into each animal daily for four weeks.
Study animals
For the study, 36 adult male Wistar rats weighing 200-250 g were acquired from the Faculty of Veterinary Medicine at Tehran University and housed in standard laboratory conditions. The rats were maintained in an environment with a temperature of 22±2°C, a relative humidity between 10% and 50%, and a 12-h light/dark cycle, with access to food and water. The study specifically involved healthy rats within the 200-250 g weight range, excluding unhealthy, overweight, or without SCI.
Creating a model for spinal cord injuries
The animals were given ketamine (80 mg/kg) and xylazine (15 mg/kg) through an intraperitoneal injection to anesthetize them. The surgical area on the animal’s back was sterilized with alcohol and betadine, and the hair was shaved. A longitudinal incision was made along the midline from the eighth to the twelfth thoracic vertebrae. The superficial fascia and muscles were moved aside to expose the spinous and transverse processes. During the laminectomy procedure, a specialized retractor was used under a surgical microscope to identify the tenth vertebra to avoid damaging the dura mater. Spinal cord decompression surgery was then carried out [34, 35]. The animal was given 2-3 mL of normal saline solution under the skin to prevent dehydration. Stitches were used to close the surgical area after the injury. A subcutaneous injection of 15 mg/kg of gentamicin was given one day before the surgery and continued for two days after to lower the chances of infection at the surgical site and in the central nervous and urinary systems [36].
Animal groups and drug administration
The animals were divided into four groups, each consisting of nine animals, through a random process: In the control group (Co), the rats underwent laminectomy without SCI; in the vehicle group (V), the rats with a SCI model were given daily subcutaneous injections of normal saline at a dose of 100 mg/kg for four weeks, starting 24 h after the injury; in the treatment group 1 (MO1), the rats with an SCI model, 24 h post-injury, received a daily subcutaneous injection of a hydroalcoholic extract of M. officinalis at a dosage of 100 mg/kg for four weeks, and in the treatment group 2 (MO2), the rats with a SCI model, 24 h post-injury, received a daily subcutaneous injection of a 150 mg/kg dose of hydroalcoholic extract from M. officinalis for four weeks.
Validation of the spinal cord injury model
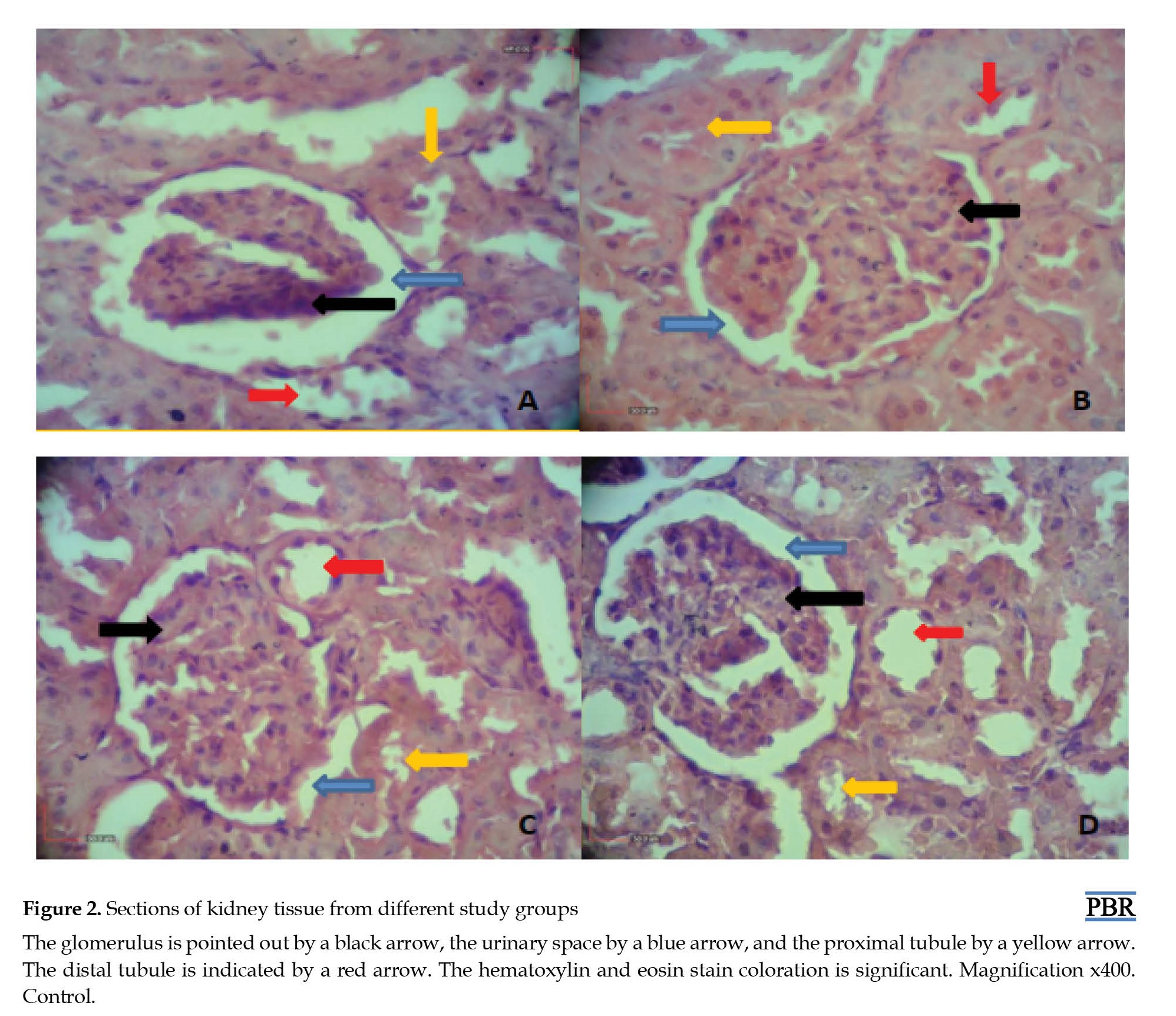
A tissue sample was taken from the injury site to confirm the SCI model. Following staining with hematoxylin-eosin, analysis under a light microscope showed a compressed region, indicating damage to the right side of the spinal cord in the sample. The sections also displayed empty spaces, suggesting the loss or deterioration of axons, thus confirming the SCI model (Figure 1).
Preparation of kidney tissue samples
After establishing the SCI model and providing treatment for four weeks, the animals were anesthetized using the earlier method. Subsequently, a midline incision was made in the abdominal skin. The right kidney of 5 rats from each group was randomly extracted and immediately frozen in liquid nitrogen for rapid preservation. The samples were then stored at -80 degrees Celsius for biochemical analysis, while the remaining four rats in each group were placed in 10% formalin for histological and tissue examinations.
The process of preparing tissue sections involved a meticulous sequence of seven steps as follows:
Fixation in 10% formalin: The tissue samples were initially fixed in a 10% formalin solution;
Hydration process: The fixed samples underwent a hydration process by sequentially immersing them in solutions, including 70% ethanol, 90% ethanol, and three rounds of absolute ethanol (100%);
Clearing process: Subsequently, the samples were cleared by being immersed in xylene three times, with each immersion lasting 1 h;
Wax infiltration: The samples were infiltrated with wax by embedding them in paraffin;
Embedding in molds: After the wax was infiltrated, the samples were embedded in molds that were partially filled with molten paraffin. Any remaining space in the mold was filled with molten paraffin;
Sectioning with a microtome: Thin slices, approximately 5 µm thick, were prepared using a microtome;
Staining with hematoxylin and eosin: Finally, the tissue sections were stained using the hematoxylin and eosin method.
This comprehensive process ensures the precise preparation of kidney tissue samples, facilitating subsequent biochemical analysis and histological examination to gain valuable insights into the experimental results.
Assessment of alterations in kidney tissue structure
The kidney tissue samples were observed using a light microscope. In histology and histological research, three different views were assessed in each sample using the Dino Capture software, version 2 (Dino-Lite Companyl; Netherlands) with a µm unit of measurement, and the results were then documented. The measurements encompassed the size of the Bowman’s capsule, glomerulus, urinary space, proximal tubule, lumen of the proximal tubule, height of the proximal tubule epithelium, diameter of the distal tubule, lumen of the distal tubule, and height of the distal tubule epithelium. These measurements were conducted using software.
Assessment of gene expression
Ribonucleic acid extraction
To extract the ribonucleic acid (RNA), TRIzol was added to the cell or tissue samples and homogenized for complete lysis. Then, chloroform was added, and the microtube contents were thoroughly mixed and incubated at room temperature for 5 min. The sample was centrifuged at 4°C with a relative centrifugal force of 12 000 for 1-5 min, forming three phases. The RNA was found in the supernatant phase. After isolating a sterile microorganism, the upper phase of isopropanol was separated, and more isopropanol was added before placing the samples in a 20°C freezer for 1 h. The samples were centrifuged at 4°C with a relative centrifugal force of 12 000, using a refrigerated centrifuge for 15 min. The RNA was found in the sediment at the bottom of the tube. The supernatant was removed, and 75% ethanol was added to the sediment to improve RNA purity. After adding ethanol, the sample was gently shaken and centrifuged at 4°C for 10 min at a relative centrifugal force of 8000. The supernatant was removed, and the ethanol precipitates were assessed for 15 min at room temperature for complete removal. RNA quantification and purity assessment were performed using nanodroplets.
Synthesis of cDNA from total ribonucleic acid
The first step in initiating a real-time polymerase chain reaction involves synthesizing cDNA from RNA using universal primers, such as dT-Oligo or Hexamer Random. Accurately determining mRNA levels is crucial for studying gene expression. Polymerase chain reaction is based on DNA replication as a template for nucleic acid and the action of the reverse transcriptase enzyme. cDNA was generated using the Premix PCR-2xRT 2-Step Kit from Biofact.
Real-time polymerase chain reaction
The reaction schedule was initially designed, and then the thermocycler device (ABI 7500, USA) was set up according to the manufacturer’s instructions.
Primer design
The CASP3, Bax, and Bcl-2 gene sequences were acquired from the National Center for Biotechnology Information website, and specific primers were created based on these sequences using the ABI software. These primers were carefully crafted for use in molecular experiments to ensure accuracy. The ABI software is a valuable tool in molecular research, assisting researchers in creating precise primers. These primers are highly effective for conducting various molecular tests related to CASP3, Bax, and Bcl-2. The precise design of primers is essential as it directly impacts the precision and dependability of molecular test outcomes. The primers designed for this study can be found in Table 1.

Statistical analysis
The data were analyzed using the SPSS software, version 23. Descriptive analysis included determining the average and variability. Furthermore, the one-way analysis of variance and the Tukey post hoc test were employed to assess variations among the data in the experimental groups. The significance level, was determined to be P<0.05.
Results
Outcomes of alterations in texture
As shown in Figure 2 tissue composition is altered in rats with an SCI model that was given an alcoholic extract of M. officinalis. As shown in Figure 3, the renal corpuscle’s diameter in the SCI group is notably smaller compared to the other groups. There is also a significant difference in the treated cases compared to the SCI group (Figure 3a). Additionally, the glomerulus diameter in the SCI group decreased compared to the control group; however, this decrease was significantly reduced in the treated groups (Figure 3b). Moreover, the urinary space diameter in the SCI group increased compared to the control group but was significantly restored in the treatment groups (Figure 3c).
Based on Figure 3, the analysis of variance, and the Tukey test results, the average diameter of the proximal renal tube in the SCI group significantly decreased compared to the other groups. Additionally, the mean diameter of the proximal renal tube in the treatment groups also showed a significant difference compared to the SCI group (Figure 4a). Furthermore, the diameter of the distal tube in the SCI group exhibited a significant difference. At the same time, there was no notable variation in the treated groups compared to the SCI group in group 1 (Figure 4b).
As shown in Figure 5, the SCI group had a notably smaller renal proximal tubule diameter than other groups. Conversely, the treatment groups showed a significant difference in the lumen diameter of the proximal renal tubule compared to the SCI group (Figure 5a). Additionally, the diameter of the lumen of the distal renal tubule in the SCI group was significantly larger compared to the other groups. The average diameter of the renal distal lumen in the treatment groups did not differ considerably from the SCI group (Figure 5b).
Figure 6 shows a notable decrease in the height of epithelial cells in the proximal tube in the SCI group compared to the other groups. Additionally, there was a significant difference in the average height of proximal epithelial cells between the treatment groups and the SCI group (Figure 6a). Furthermore, the height of epithelial cells in the distal renal tube in the SCI group also exhibited a significant decrease compared to other groups. Moreover, the average height of distal epithelial cells in the treatment groups differed significantly from those in the SCI group (Figure 6b).
Results of gene expression assessment
According to the findings, there is a notable rise in TNFα gene expression in the SCI group compared to the control group, as evidenced by the one-way analysis of variance and the Tukey test results. Additionally, the expression in the treatment groups was significantly lower than in the SCI group (Figure 7a). Furthermore, the Bax gene expression was elevated in the SCI group compared to the control group. In contrast, its expression in the treatment groups was notably decreased compared to the SCI group (Figure 7b). The study’s results demonstrated a significant decrease in Bcl-2 gene expression in the SCI group compared to the control group; however, in the treatment groups, there was partial compensation for the reduction of Bcl-2 gene expression compared to the SCI group (Figure 7c). The expression of the CASP3 gene increased in the SCI group compared to the control group. The increase in gene expression was significant, but this heightened expression was partially reduced in the treatment groups compared to the control group (Figure 7d).
Discussion
Recent research indicates that SCI can result in widespread inflammation throughout the body, marked by changes in the activity of genes linked to cell death and inflammatory processes. This inflammation can lead to the condition’s progression to other organs and tissues, such as the kidneys, essential for maintaining the body’s internal environment in response to external changes. This can cause irreversible harm to the body [1, 37]. However, studies have demonstrated that the M. officinalis plant can effectively help manage and alleviate the secondary effects of SCI due to its potent anti-inflammatory and antioxidant properties [31-33]. In this investigation, we explored the impact of the plant’s alcoholic extract on kidney tissue changes and the activity of genes associated with cell death (Bax, Bcl-2, and CASP3), as well as the inflammatory factor TNF-α, which is triggered by the secondary effects of SCI in rat models. We induced SCI in the rats using the compression method.
After a SCI, there is an increase in the production of inflammatory factors, including TNF-α, which contributes to the development of inflammation and secondary injuries. This also leads to an increase in the expression of apoptosis-related genes, such as Bax and CASP3, which promote the progression of apoptosis and inflammation. Additionally, there is a reduction in the expression of Bcl-2, an anti-apoptotic protein that inhibits apoptosis. The study found a significant decrease in the expression of the Bcl-2 gene in the SCI group compared to the control group. In contrast, the expression levels of Bax, CASP3, and TNF-α genes showed a significant increase in the SCI group compared to the control group (P<0.05). A study carried out in 2011 by a team of researchers discovered that SCI can activate mitochondrial apoptosis, which is likely to be a significant process. This mechanism involves a sequence of internal biochemical changes that lead to apoptosis. The study’s findings suggest that cell death is controlled by p53 and occurs when there is a reduction in the presence of Bcl-2 and an increase in the presence of Bax and CASP3 [38].
the alcoholic extract of M. officinalis impacts pro-inflammatory cytokines, such as TNF-α, and the expression of apoptosis-related genes, including CASP3, Bax, and Bcl-2. The study observed a significant increase in the expression of the TNF-α gene in the SCI group compared to the control group. TNF-α is a crucial inflammatory factor contributing to the onset and spread of SCI to other organs, such as the kidneys. After an SCI, TNF-α expression increases, peaks a few hours after the injury, remains at that level for several hours, and then decreases. This inflammatory factor is expressed in specific cell types, including glial cells, neurons, and injured endothelial cells [39]. Researchers suggest the post-injury inflammatory process may be linked to increased TNF-α expression. A 2020 study by Liu et al. also confirmed that SCI increases TNF-α expression in patients [40].
Research conducted by Zhao et al. [41] has indicated that the programmed death of neural cells is a critical factor in the secondary cellular events that occur after SCI. Apoptosis can happen through two pathways, dependent on CASP3 and independent of it. CASP3 is considered a critical factor in the final stages of apoptosis. Studies suggest inhibiting its expression could effectively control or reduce apoptosis after SCI. Additionally, Bax and Bcl-2, both members of the Bcl-2 protein family, play essential roles in regulating apoptosis. Bax initiates and advances apoptosis, while Bcl-2 can decrease or inhibit apoptosis [13-15, 41]. Research, including a 2011 study, has demonstrated the crucial role of Bcl-2 in reducing apoptosis caused by SCI [42]. Another study has shown the significant role of Bax in apoptosis after SCI [38]. Furthermore, a study by Zhao et al. has highlighted the crucial roles of Bax, Bcl-2, and CASP3 in neural cell apoptosis following SCI. These findings align with the present study, indicating the critical roles of these factors in initiating inflammation and promoting apoptosis after SCI [43].
Recent research has indicated that SCI causes an increase in the production of inflammatory factors, including TNF-α, in individuals with such injuries. This leads to widespread inflammation that affects the kidneys’ structure and function. Donnelly et al. conducted a study showing the potential of specific substances to relieve the secondary effects of SCI related explicitly to kidney and bladder dysfunction. The study found that simvastatin influenced changes in the bladder and kidneys of rats and reduced the expression of the CASP3 gene in the kidneys of SCI patients. Additionally, the study showed that the alcoholic extract of M. officinalis affected the expression levels of genes related to the apoptosis process and inflammatory factor TNF-α, which are altered due to SCI [39]. The extract was found to significantly impact the expression levels of these genes beneficially, reducing the secondary effects of SCI. Furthermore, the study revealed that the treatment groups using M. officinalis exhibited a significant decrease in the expression levels of CASP3, Bax, and TNF-α genes compared to the group with SCI. In contrast, the expression of the Bcl-2 gene significantly increased in the treatment groups. Another study in 2020 demonstrated that the alcoholic extract of M. officinalis reduced anxiety-like behaviors, depression, oxidative stress, and apoptotic markers in mice exposed to stress. It also decreased the expression of pro-apoptotic markers and increased the expression of anti-apoptotic proteins in the cortex and hippocampus of rats exposed to stress [31]. Additionally, a separate research study demonstrated that giving specific substances, like mangiferin, to rats with spinal cord injuries resulted in a notable decrease in Bcl-2 and suppressed the expression of CASP3. As a result, there was a reduction in oxidative stress in the rats with spinal cord injuries by controlling the Bax and Bcl-2 pathways, aligning with current research discoveries [44].
The current research also found that the hydroalcoholic extract of M. officinalis can potentially address SCI and its subsequent effects, such as inflammation that could result in complications in other organs like the kidneys. This is due to the presence of medicinal compounds in the extract. Previous research has demonstrated the diverse therapeutic effects of the extract, which are linked to its various medicinal compounds. For instance, studies have indicated that the extract contains a significant amount of rosmarinic acid, which has analgesic properties by activating mechanisms related to the cholinergic system [45]. Another research study has found that the hydroalcoholic extract has neuroprotective effects and can be used to treat neurological diseases because of its anti-inflammatory and antioxidant properties at high concentrations. The presence of phenolic compounds in the extract, including hydroxycinnamic acid, flavonoids, rosmarinic acid, and caffeic acid, is responsible for its neuroprotective effect. These compounds work by inhibiting acetylcholinesterase, preserving cellular integrity, and neutralizing free radicals [46]. The alcoholic extract may also have antimicrobial effects due to the presence of luteolin, rosmarinic acid, and salvianolic acid. The hydroalcoholic extract of M. officinalis has various properties, such as antispasmodic, antiviral, and anti-infectious effects, as well as anti-inflammatory and therapeutic benefits for the digestive system, among others [47]. In a study from 2016, researchers found that the alcoholic extract of M. officinalis contains substances with strong antioxidant properties, particularly folate, which contribute to its neuroprotective effects [30]. A 2023 study by another group of researchers confirmed that the alcoholic extract of M. officinalis can be utilized to prevent and treat apoptosis, showcasing its potential for addressing SCI and other central nervous system conditions [48].
Analysis of kidney tissue samples stained using the hematoxylin and eosin technique in various groups indicated that SCI could result in structural and tissue alterations in the kidney. The study observed significant changes in the diameter of the renal corpuscle, glomerulus, and urinary space in the SCI group compared to the control group. Specifically, the study revealed a notable reduction in the diameter of the renal corpuscle and glomerulus in the SCI group compared to the control group. Additionally, the urinary space’s diameter was larger in the SCI group compared to the control group (P<0.05).
Research conducted in 2022 by Nasiri et al. aimed to demonstrate the protective effects of M. officinalis Plant extract on kidney function. The study focused on the impact of the extract on gentamicin-induced renal failure in diabetic rats. The findings indicated that the M. officinalis extract could reduce inflammation and kidney tissue damage caused by gentamicin and diabetes. Accordingly, the extract significantly mitigated complications from kidney inflammation and necrosis due to diabetes and gentamicin use [49]. The study also revealed that specific groups treated with M. officinalis extract showed significant improvements in the diameter of the renal corpuscle, glomerulus, and urinary space, which had been affected by SCI. Additionally, the height of the epithelial cells in the proximal and distal convoluted tubules showed a significant decrease in the injury group compared to the control group. However, this significance showed a smaller decline in the distal group than in the control group. The slight reduction in the height of the distal tubule could be attributed to the proximity of the proximal tubule cells to the glomerulus and renal corpuscle, enabling them to exert a stronger influence on the height of the epithelial cells that receive the filtered material. The results also showed a significant reduction in the diameter of the proximal tube compared to the control group. In contrast, the diameter of the distal tube did not exhibit a significant change compared to the control group. This change can also be related to the previously mentioned alteration in the height of epithelial cells. In this context, previous research has indicated that SCI is a complex process that can affect distant organs, such as the heart, kidneys, lungs, and lower limbs. The researchers found that degeneration and necrosis occurred in the urinary tubular cells due to SCI. They also noted that dexmedetomidine can effectively improve the kidney’s histopathology.
In the present research, we assessed the size of the distal and proximal convoluted tubules, as well as the height of the epithelial cells in the proximal and distal tubules in the groups that were administered the alcoholic extract of M. officinalis compared to the injury group, the subjects showed improved condition, and their histomorphometric analysis indicated that they were more similar to the control group. Therefore, further investigation of the alcoholic extract of M. officinalis is warranted, as it may potentially facilitate abnormal changes in the urinary tract. A study conducted in 2022 by Şengel et al. demonstrated that dexmedetomidine can alleviate the harmful effects of kidney damage in rats with SCI through various mechanisms [50].
In a separate investigation carried out by Bahrke and Yesalis in 2004, it was determined that the intraperitoneal injection of nandrolone decanoate in adult female Balb/c rats led to a notable increase in the size of both distal and proximal tubules in the experimental groups compared to the control group. Additionally, Research has shown that nandrolone attaches to androgen receptors located on the surface of epithelial cells in the proximal and distal tubules. This binding influences the structure of chromatin, the expression of genes, and the synthesis of mRNA and stimulates the synthesis of proteins [51]. As mentioned, the kidney is highly sensitive in purifying the blood plasma and eliminating waste materials from the body. The kidney’s size can vary. In a 2008 study [52], scientists examined the impact of garlic extract on animals with induced diabetes. They observed enlargement of the glomeruli and alterations in glomerular volume, indicating that the kidneys were responsive to and underwent changes in reaction to exposure to different substances. This study is linked to research conducted by Welt in 2007 [52, 53].
In a study conducted in 2017 by Orr and Briggs, it was found that heavy metal consumers had an increased volume in their distal tubules compared to the control group, indicating the kidneys’ responsiveness to different substances [54]. Similarly, a 2018 study by the European :union: Safety Organization showed that certain substances, such as aspartame, could significantly reduce parameters like the diameter of the renal corpuscle, glomerular capsule, and the height of the proximal convoluted tubules in the experimental groups compared to the control group. This aligns with previous research by Petros and Humphries (2009), who demonstrated that dietary substances like aspartame decreased the diameter of the Bowman’s capsule and subsequent expansion of the urinary space. Additionally, a 2022 study by Tah, Wang, and colleagues revealed that the combination of metformin, barberry, and parsley in diabetic mice could affect kidney histopathology and modify the vacuolar cells of the urinary tubules [55].
Conclusion
The occurrence of SCI represents a significant medical concern with profound implications for the affected individuals and society at large. Our recent study has shown that SCI in rats causes widespread inflammation throughout the body shortly after the injury, resulting in secondary effects that spread the condition to other organs and tissues. This cascade of events triggers systemic inflammation, exacerbating the spread of the disease to various bodily organs and tissues. Notably, the kidneys, vital for blood purification, blood pressure regulation, and overall health maintenance, are particularly impacted. Consequently, this injury poses a potential threat to life.
The investigation findings suggest that the development and progression of systemic inflammation are initiated by increased expression of inflammatory markers like TNF-α, upregulation of genes linked to the apoptosis process (such as Bax and CASP3), and a decrease in Bcl-2 expression due to secondary injury resulting from SCI. This inflammatory spread in kidney tissues causes damage and disease progression. The advancement of the disease impacts renal tissues, resulting in histological changes and affecting their function. This condition causes modifications in renal morphology, affecting parameters, such as the diameter of renal corpuscles, glomerular diameter, height of proximal tubule epithelial cells, diameter of proximal tubules, height of distal tubule epithelial cells, and diameter of distal tubules. Furthermore, it leads to a decrease in the renal urinary space’s diameter, proximal tubules’ luminal diameter, and distal tubules’ luminal diameter. These histomorphometric and structural changes influence renal function, leading to impaired performance.
This study shows that the alcoholic extract of the borage plant may be able to treat SCI because of its anti-inflammatory, antioxidant, neuroprotective, gastrointestinal, and urinary properties. The results indicate that the extract can decrease the activity of genes involved in cell death, such as Bax and CASP3, while increasing the activity of Bcl-2. It also reduces the activity of the inflammatory factor TNF-α, which is disrupted in SCI. Additionally, the extract affects the kidney’s tissue and histomorphometric changes caused by SCI, demonstrating its therapeutic potential. The alcoholic borage extract can potentially address spinal cord lesions and their secondary effects on spinal tissue.
Ethical Considerations
Compliance with ethical guidelines
In this experimental research, following approval from the Ethics Committee of the Islamic Azad University, Tehran Medical Sciences Branch. All procedures were carried out in compliance with the guidelines of the Animal Protection Ethics Committee, sanctioned by Tehran Medical Sciences Branch, Islamic Azad University (Code: IR.IAU.PS.REC.1400.525).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
References
Spinal cord injury (SCI) is a severe medical condition that leads to physical, emotional, social, and financial challenges for the affected individuals and their communities. These patients face significant disruptions in multiple areas of their lives [1-3]. This is because the spinal cord, as part of the central nervous system, is responsible for receiving sensory signals and transmitting motor instructions. It also triggers spinal reflexes that protect the body from potential harm. Consequently, damaging the spinal cord can result in long-term disability. SCI occurs when the vertebral column experiences a sudden impact or blow, leading to fractures or dislocations of the vertebrae [2, 4-6].
SCI is categorized as primary or secondary based on their underlying cause. Primary injuries involve the initial impact on the spinal cord during an injury, which can lead to the movement of bone fragments, disc ruptures, and ligament damage. Following the initial injury, a complex secondary process occurs within a few hours, resulting in the death of nerve and support cells, reduced blood flow, and the beginning of inflammation [7, 8, 9]. The ongoing secondary damage causes the creation of a harmful environment at the cellular level, leading to the loss of functional cells and harm to the tissue’s surroundings [10]; the inflammatory environment plays a significant role in advancing further damage, causing inflammation to spread to other parts of the body. This leads to disruptions in the functioning of different organs, as the spinal cord plays a crucial role in coordinating bodily functions. This can result in dysfunction or impairment in several organs, such as neurogenic pain, breathing problems, heart disease, and kidney dysfunction, among others [11, 12].
Research has indicated that damage to spinal cord tissue can trigger the release of inflammatory cytokines, such as TNF-α, and pro-apoptotic factors, such as caspase-3 and Bax. These elements are essential in the mechanisms and phases that lead to SCI, particularly in cellular death and the body’s inflammatory reaction [13, 14]. Bax and Bcl-2 are key genes within the Bcl-2 family that regulate apoptosis. Bax is recognized as a critical factor in promoting apoptosis, while Bcl-2 functions to inhibit apoptosis. Furthermore, caspase-3 is an essential protein in the final steps of apoptosis. On the other hand, TNF-α is an inflammatory cytokine that is essential for the body’s inflammatory responses. At a molecular level, both proteins are crucial for advancing our comprehension of the impact of SCI [13, 15]. SCI leads to an increase in TNF-α, contributing to the amplification of apoptotic factors and damaging spinal cord tissue. This greatly aids in the management of spinal cord injuries [16].
Additionally, SCI can influence the kidneys, which are indirectly impacted by this condition. This can disrupt the function and overall well-being of this essential organ. The kidney is responsible for crucial tasks, such as maintaining fluid balance, removing waste materials, regulating electrolyte levels, balancing acidity, and supporting the endocrine glands in the body [17, 18]. The kidneys function similarly to endocrine glands by producing renin and erythropoietin and assisting in the hydroxylation of vitamin D. Additionally, they receive 25% of the cardiac output. They are crucial in regulating blood pressure and cardiac function. Consequently, any harm to the kidneys can have a significant impact on a person’s life [19, 20]. This research investigates the intricate connections between SCI, inflammation, apoptotic processes, and their effects on kidney function, recognizing the vital importance of the kidneys in people’s lives [21]. The kidneys are at risk of being severely affected by SCI, posing a threat to the individual’s life and potentially leading to death in severe cases. The secondary consequences of SCI, including widespread inflammation that affects the kidneys, can result in chronic kidney disease, renal failure, and other kidney-related conditions. Over time, this can cause a decline in kidney function and give rise to various serious health issues, which may ultimately lead to death in certain instances [11, 22]. Research has indicated that kidney problems are a significant predictor for individuals with SCI; doctors recommend that people with SCI monitor their kidney function to manage their health effectively [23, 24].
Studies have demonstrated that Melissa officinalis, a member of the mint family, is a critical medicinal plant. It has a long history of use as a soothing and pain-relieving herb for various ailments, such as anxiety, stress, and sleep disorders. It is extensively utilized in the food, pharmaceutical, and cosmetic sectors due to the beneficial compounds present in the plant and its extract [25]. One of the critical elements of this mixture contains vital substances, like citronellal, nerol, geraniol, and citronellal, which are accountable for the delightful scent of this plant. It also includes phenolic acids, such as rosmarinic acid, which has antioxidant qualities. Additionally, it contains flavonoids like luteolin, which have antioxidant and anti-inflammatory properties. Terpenoids, other fatty acids, and various other compounds are also present [26, 27, 28]. The plant M. officinalis also called the lemon balm, is recognized for its soothing properties. The components found in its alcohol extract attach to gamma-aminobutyric acid (GABA) receptors in the brain, which helps control neural function. This can lead to heightened relaxation and decreased stress. The phenolic and flavonoid compounds in the alcohol extract have anti-inflammatory and antioxidant characteristics, which can aid in diminishing inflammation and shielding nerve cells from harm [25, 29].
A study conducted by Hosseini et al. [30] showed that the alcoholic extract of M. officinalis can potentially be used to treat SCI. Other research has indicated that the alcoholic extract of this plant could have a beneficial effect on improving mood and reducing anxiety. Additionally, the extract may contain flavonoid compounds that have antioxidant properties [30, 31]. The plant extract has various functional mechanisms, including the inhibition of microbial and fungal growth and antimicrobial and antifungal properties. It can also help with digestive issues, such as bloating, heartburn, and other significant problems. According to this information, the alcoholic extract of M. officinalis shows promise as a potential medicinal plant for preventing and treating different neurological diseases. This is attributed to its chemical compounds, which have strong antioxidant and anti-inflammatory effects, particularly at higher concentrations. It can also have preventive effects on conditions such as cerebral ischemia, SCI, and Alzheimer’s disease, making it useful in treating diseases resulting from SCI [31-33]. Accordingly, this research investigates how M. officinalis extract affects the activity of genes related to apoptosis and the inflammatory factor TNFα in kidney tissue. The study also explored the extract’s influence on alterations in the kidney tissue of rats with SCI.
Materials and Methods
Gathering and obtaining a hydroalcoholic extract from the M. officinalis plant
The plants were sourced from the Firoozeh Medicinal Plant Garden in Tehran City, Iran, and approved by the Faculty of Pharmaceutical Sciences of Tehran Azad University to produce alcoholic extracts. The fresh leaves were dried, crushed, and mixed with 250 mL of 70% ethanol to create the hydroalcoholic extract. The mixture was left for 48 h, shaken every 12 h, and filtered. The remaining residues were washed with 70% ethanol, filtered again, and combined with the previous extract. The solvent was evaporated using a vacuum distillation device [32], producing 2.4 g of plant extract. This extract was then combined with 50 mL of physiological serum and left at room temperature for a day to dissolve completely. The resulting solution was injected intraperitoneally into each animal daily for four weeks.
Study animals
For the study, 36 adult male Wistar rats weighing 200-250 g were acquired from the Faculty of Veterinary Medicine at Tehran University and housed in standard laboratory conditions. The rats were maintained in an environment with a temperature of 22±2°C, a relative humidity between 10% and 50%, and a 12-h light/dark cycle, with access to food and water. The study specifically involved healthy rats within the 200-250 g weight range, excluding unhealthy, overweight, or without SCI.
Creating a model for spinal cord injuries
The animals were given ketamine (80 mg/kg) and xylazine (15 mg/kg) through an intraperitoneal injection to anesthetize them. The surgical area on the animal’s back was sterilized with alcohol and betadine, and the hair was shaved. A longitudinal incision was made along the midline from the eighth to the twelfth thoracic vertebrae. The superficial fascia and muscles were moved aside to expose the spinous and transverse processes. During the laminectomy procedure, a specialized retractor was used under a surgical microscope to identify the tenth vertebra to avoid damaging the dura mater. Spinal cord decompression surgery was then carried out [34, 35]. The animal was given 2-3 mL of normal saline solution under the skin to prevent dehydration. Stitches were used to close the surgical area after the injury. A subcutaneous injection of 15 mg/kg of gentamicin was given one day before the surgery and continued for two days after to lower the chances of infection at the surgical site and in the central nervous and urinary systems [36].
Animal groups and drug administration
The animals were divided into four groups, each consisting of nine animals, through a random process: In the control group (Co), the rats underwent laminectomy without SCI; in the vehicle group (V), the rats with a SCI model were given daily subcutaneous injections of normal saline at a dose of 100 mg/kg for four weeks, starting 24 h after the injury; in the treatment group 1 (MO1), the rats with an SCI model, 24 h post-injury, received a daily subcutaneous injection of a hydroalcoholic extract of M. officinalis at a dosage of 100 mg/kg for four weeks, and in the treatment group 2 (MO2), the rats with a SCI model, 24 h post-injury, received a daily subcutaneous injection of a 150 mg/kg dose of hydroalcoholic extract from M. officinalis for four weeks.
Validation of the spinal cord injury model
A tissue sample was taken from the injury site to confirm the SCI model. Following staining with hematoxylin-eosin, analysis under a light microscope showed a compressed region, indicating damage to the right side of the spinal cord in the sample. The sections also displayed empty spaces, suggesting the loss or deterioration of axons, thus confirming the SCI model (Figure 1).
Preparation of kidney tissue samples
After establishing the SCI model and providing treatment for four weeks, the animals were anesthetized using the earlier method. Subsequently, a midline incision was made in the abdominal skin. The right kidney of 5 rats from each group was randomly extracted and immediately frozen in liquid nitrogen for rapid preservation. The samples were then stored at -80 degrees Celsius for biochemical analysis, while the remaining four rats in each group were placed in 10% formalin for histological and tissue examinations.
The process of preparing tissue sections involved a meticulous sequence of seven steps as follows:
Fixation in 10% formalin: The tissue samples were initially fixed in a 10% formalin solution;
Hydration process: The fixed samples underwent a hydration process by sequentially immersing them in solutions, including 70% ethanol, 90% ethanol, and three rounds of absolute ethanol (100%);
Clearing process: Subsequently, the samples were cleared by being immersed in xylene three times, with each immersion lasting 1 h;
Wax infiltration: The samples were infiltrated with wax by embedding them in paraffin;
Embedding in molds: After the wax was infiltrated, the samples were embedded in molds that were partially filled with molten paraffin. Any remaining space in the mold was filled with molten paraffin;
Sectioning with a microtome: Thin slices, approximately 5 µm thick, were prepared using a microtome;
Staining with hematoxylin and eosin: Finally, the tissue sections were stained using the hematoxylin and eosin method.
This comprehensive process ensures the precise preparation of kidney tissue samples, facilitating subsequent biochemical analysis and histological examination to gain valuable insights into the experimental results.
Assessment of alterations in kidney tissue structure
The kidney tissue samples were observed using a light microscope. In histology and histological research, three different views were assessed in each sample using the Dino Capture software, version 2 (Dino-Lite Companyl; Netherlands) with a µm unit of measurement, and the results were then documented. The measurements encompassed the size of the Bowman’s capsule, glomerulus, urinary space, proximal tubule, lumen of the proximal tubule, height of the proximal tubule epithelium, diameter of the distal tubule, lumen of the distal tubule, and height of the distal tubule epithelium. These measurements were conducted using software.
Assessment of gene expression
Ribonucleic acid extraction
To extract the ribonucleic acid (RNA), TRIzol was added to the cell or tissue samples and homogenized for complete lysis. Then, chloroform was added, and the microtube contents were thoroughly mixed and incubated at room temperature for 5 min. The sample was centrifuged at 4°C with a relative centrifugal force of 12 000 for 1-5 min, forming three phases. The RNA was found in the supernatant phase. After isolating a sterile microorganism, the upper phase of isopropanol was separated, and more isopropanol was added before placing the samples in a 20°C freezer for 1 h. The samples were centrifuged at 4°C with a relative centrifugal force of 12 000, using a refrigerated centrifuge for 15 min. The RNA was found in the sediment at the bottom of the tube. The supernatant was removed, and 75% ethanol was added to the sediment to improve RNA purity. After adding ethanol, the sample was gently shaken and centrifuged at 4°C for 10 min at a relative centrifugal force of 8000. The supernatant was removed, and the ethanol precipitates were assessed for 15 min at room temperature for complete removal. RNA quantification and purity assessment were performed using nanodroplets.
Synthesis of cDNA from total ribonucleic acid
The first step in initiating a real-time polymerase chain reaction involves synthesizing cDNA from RNA using universal primers, such as dT-Oligo or Hexamer Random. Accurately determining mRNA levels is crucial for studying gene expression. Polymerase chain reaction is based on DNA replication as a template for nucleic acid and the action of the reverse transcriptase enzyme. cDNA was generated using the Premix PCR-2xRT 2-Step Kit from Biofact.
Real-time polymerase chain reaction
The reaction schedule was initially designed, and then the thermocycler device (ABI 7500, USA) was set up according to the manufacturer’s instructions.
Primer design
The CASP3, Bax, and Bcl-2 gene sequences were acquired from the National Center for Biotechnology Information website, and specific primers were created based on these sequences using the ABI software. These primers were carefully crafted for use in molecular experiments to ensure accuracy. The ABI software is a valuable tool in molecular research, assisting researchers in creating precise primers. These primers are highly effective for conducting various molecular tests related to CASP3, Bax, and Bcl-2. The precise design of primers is essential as it directly impacts the precision and dependability of molecular test outcomes. The primers designed for this study can be found in Table 1.

Statistical analysis
The data were analyzed using the SPSS software, version 23. Descriptive analysis included determining the average and variability. Furthermore, the one-way analysis of variance and the Tukey post hoc test were employed to assess variations among the data in the experimental groups. The significance level, was determined to be P<0.05.
Results
Outcomes of alterations in texture
As shown in Figure 2 tissue composition is altered in rats with an SCI model that was given an alcoholic extract of M. officinalis. As shown in Figure 3, the renal corpuscle’s diameter in the SCI group is notably smaller compared to the other groups. There is also a significant difference in the treated cases compared to the SCI group (Figure 3a). Additionally, the glomerulus diameter in the SCI group decreased compared to the control group; however, this decrease was significantly reduced in the treated groups (Figure 3b). Moreover, the urinary space diameter in the SCI group increased compared to the control group but was significantly restored in the treatment groups (Figure 3c).
Based on Figure 3, the analysis of variance, and the Tukey test results, the average diameter of the proximal renal tube in the SCI group significantly decreased compared to the other groups. Additionally, the mean diameter of the proximal renal tube in the treatment groups also showed a significant difference compared to the SCI group (Figure 4a). Furthermore, the diameter of the distal tube in the SCI group exhibited a significant difference. At the same time, there was no notable variation in the treated groups compared to the SCI group in group 1 (Figure 4b).
As shown in Figure 5, the SCI group had a notably smaller renal proximal tubule diameter than other groups. Conversely, the treatment groups showed a significant difference in the lumen diameter of the proximal renal tubule compared to the SCI group (Figure 5a). Additionally, the diameter of the lumen of the distal renal tubule in the SCI group was significantly larger compared to the other groups. The average diameter of the renal distal lumen in the treatment groups did not differ considerably from the SCI group (Figure 5b).
Figure 6 shows a notable decrease in the height of epithelial cells in the proximal tube in the SCI group compared to the other groups. Additionally, there was a significant difference in the average height of proximal epithelial cells between the treatment groups and the SCI group (Figure 6a). Furthermore, the height of epithelial cells in the distal renal tube in the SCI group also exhibited a significant decrease compared to other groups. Moreover, the average height of distal epithelial cells in the treatment groups differed significantly from those in the SCI group (Figure 6b).
Results of gene expression assessment
According to the findings, there is a notable rise in TNFα gene expression in the SCI group compared to the control group, as evidenced by the one-way analysis of variance and the Tukey test results. Additionally, the expression in the treatment groups was significantly lower than in the SCI group (Figure 7a). Furthermore, the Bax gene expression was elevated in the SCI group compared to the control group. In contrast, its expression in the treatment groups was notably decreased compared to the SCI group (Figure 7b). The study’s results demonstrated a significant decrease in Bcl-2 gene expression in the SCI group compared to the control group; however, in the treatment groups, there was partial compensation for the reduction of Bcl-2 gene expression compared to the SCI group (Figure 7c). The expression of the CASP3 gene increased in the SCI group compared to the control group. The increase in gene expression was significant, but this heightened expression was partially reduced in the treatment groups compared to the control group (Figure 7d).
Discussion
Recent research indicates that SCI can result in widespread inflammation throughout the body, marked by changes in the activity of genes linked to cell death and inflammatory processes. This inflammation can lead to the condition’s progression to other organs and tissues, such as the kidneys, essential for maintaining the body’s internal environment in response to external changes. This can cause irreversible harm to the body [1, 37]. However, studies have demonstrated that the M. officinalis plant can effectively help manage and alleviate the secondary effects of SCI due to its potent anti-inflammatory and antioxidant properties [31-33]. In this investigation, we explored the impact of the plant’s alcoholic extract on kidney tissue changes and the activity of genes associated with cell death (Bax, Bcl-2, and CASP3), as well as the inflammatory factor TNF-α, which is triggered by the secondary effects of SCI in rat models. We induced SCI in the rats using the compression method.
After a SCI, there is an increase in the production of inflammatory factors, including TNF-α, which contributes to the development of inflammation and secondary injuries. This also leads to an increase in the expression of apoptosis-related genes, such as Bax and CASP3, which promote the progression of apoptosis and inflammation. Additionally, there is a reduction in the expression of Bcl-2, an anti-apoptotic protein that inhibits apoptosis. The study found a significant decrease in the expression of the Bcl-2 gene in the SCI group compared to the control group. In contrast, the expression levels of Bax, CASP3, and TNF-α genes showed a significant increase in the SCI group compared to the control group (P<0.05). A study carried out in 2011 by a team of researchers discovered that SCI can activate mitochondrial apoptosis, which is likely to be a significant process. This mechanism involves a sequence of internal biochemical changes that lead to apoptosis. The study’s findings suggest that cell death is controlled by p53 and occurs when there is a reduction in the presence of Bcl-2 and an increase in the presence of Bax and CASP3 [38].
the alcoholic extract of M. officinalis impacts pro-inflammatory cytokines, such as TNF-α, and the expression of apoptosis-related genes, including CASP3, Bax, and Bcl-2. The study observed a significant increase in the expression of the TNF-α gene in the SCI group compared to the control group. TNF-α is a crucial inflammatory factor contributing to the onset and spread of SCI to other organs, such as the kidneys. After an SCI, TNF-α expression increases, peaks a few hours after the injury, remains at that level for several hours, and then decreases. This inflammatory factor is expressed in specific cell types, including glial cells, neurons, and injured endothelial cells [39]. Researchers suggest the post-injury inflammatory process may be linked to increased TNF-α expression. A 2020 study by Liu et al. also confirmed that SCI increases TNF-α expression in patients [40].
Research conducted by Zhao et al. [41] has indicated that the programmed death of neural cells is a critical factor in the secondary cellular events that occur after SCI. Apoptosis can happen through two pathways, dependent on CASP3 and independent of it. CASP3 is considered a critical factor in the final stages of apoptosis. Studies suggest inhibiting its expression could effectively control or reduce apoptosis after SCI. Additionally, Bax and Bcl-2, both members of the Bcl-2 protein family, play essential roles in regulating apoptosis. Bax initiates and advances apoptosis, while Bcl-2 can decrease or inhibit apoptosis [13-15, 41]. Research, including a 2011 study, has demonstrated the crucial role of Bcl-2 in reducing apoptosis caused by SCI [42]. Another study has shown the significant role of Bax in apoptosis after SCI [38]. Furthermore, a study by Zhao et al. has highlighted the crucial roles of Bax, Bcl-2, and CASP3 in neural cell apoptosis following SCI. These findings align with the present study, indicating the critical roles of these factors in initiating inflammation and promoting apoptosis after SCI [43].
Recent research has indicated that SCI causes an increase in the production of inflammatory factors, including TNF-α, in individuals with such injuries. This leads to widespread inflammation that affects the kidneys’ structure and function. Donnelly et al. conducted a study showing the potential of specific substances to relieve the secondary effects of SCI related explicitly to kidney and bladder dysfunction. The study found that simvastatin influenced changes in the bladder and kidneys of rats and reduced the expression of the CASP3 gene in the kidneys of SCI patients. Additionally, the study showed that the alcoholic extract of M. officinalis affected the expression levels of genes related to the apoptosis process and inflammatory factor TNF-α, which are altered due to SCI [39]. The extract was found to significantly impact the expression levels of these genes beneficially, reducing the secondary effects of SCI. Furthermore, the study revealed that the treatment groups using M. officinalis exhibited a significant decrease in the expression levels of CASP3, Bax, and TNF-α genes compared to the group with SCI. In contrast, the expression of the Bcl-2 gene significantly increased in the treatment groups. Another study in 2020 demonstrated that the alcoholic extract of M. officinalis reduced anxiety-like behaviors, depression, oxidative stress, and apoptotic markers in mice exposed to stress. It also decreased the expression of pro-apoptotic markers and increased the expression of anti-apoptotic proteins in the cortex and hippocampus of rats exposed to stress [31]. Additionally, a separate research study demonstrated that giving specific substances, like mangiferin, to rats with spinal cord injuries resulted in a notable decrease in Bcl-2 and suppressed the expression of CASP3. As a result, there was a reduction in oxidative stress in the rats with spinal cord injuries by controlling the Bax and Bcl-2 pathways, aligning with current research discoveries [44].
The current research also found that the hydroalcoholic extract of M. officinalis can potentially address SCI and its subsequent effects, such as inflammation that could result in complications in other organs like the kidneys. This is due to the presence of medicinal compounds in the extract. Previous research has demonstrated the diverse therapeutic effects of the extract, which are linked to its various medicinal compounds. For instance, studies have indicated that the extract contains a significant amount of rosmarinic acid, which has analgesic properties by activating mechanisms related to the cholinergic system [45]. Another research study has found that the hydroalcoholic extract has neuroprotective effects and can be used to treat neurological diseases because of its anti-inflammatory and antioxidant properties at high concentrations. The presence of phenolic compounds in the extract, including hydroxycinnamic acid, flavonoids, rosmarinic acid, and caffeic acid, is responsible for its neuroprotective effect. These compounds work by inhibiting acetylcholinesterase, preserving cellular integrity, and neutralizing free radicals [46]. The alcoholic extract may also have antimicrobial effects due to the presence of luteolin, rosmarinic acid, and salvianolic acid. The hydroalcoholic extract of M. officinalis has various properties, such as antispasmodic, antiviral, and anti-infectious effects, as well as anti-inflammatory and therapeutic benefits for the digestive system, among others [47]. In a study from 2016, researchers found that the alcoholic extract of M. officinalis contains substances with strong antioxidant properties, particularly folate, which contribute to its neuroprotective effects [30]. A 2023 study by another group of researchers confirmed that the alcoholic extract of M. officinalis can be utilized to prevent and treat apoptosis, showcasing its potential for addressing SCI and other central nervous system conditions [48].
Analysis of kidney tissue samples stained using the hematoxylin and eosin technique in various groups indicated that SCI could result in structural and tissue alterations in the kidney. The study observed significant changes in the diameter of the renal corpuscle, glomerulus, and urinary space in the SCI group compared to the control group. Specifically, the study revealed a notable reduction in the diameter of the renal corpuscle and glomerulus in the SCI group compared to the control group. Additionally, the urinary space’s diameter was larger in the SCI group compared to the control group (P<0.05).
Research conducted in 2022 by Nasiri et al. aimed to demonstrate the protective effects of M. officinalis Plant extract on kidney function. The study focused on the impact of the extract on gentamicin-induced renal failure in diabetic rats. The findings indicated that the M. officinalis extract could reduce inflammation and kidney tissue damage caused by gentamicin and diabetes. Accordingly, the extract significantly mitigated complications from kidney inflammation and necrosis due to diabetes and gentamicin use [49]. The study also revealed that specific groups treated with M. officinalis extract showed significant improvements in the diameter of the renal corpuscle, glomerulus, and urinary space, which had been affected by SCI. Additionally, the height of the epithelial cells in the proximal and distal convoluted tubules showed a significant decrease in the injury group compared to the control group. However, this significance showed a smaller decline in the distal group than in the control group. The slight reduction in the height of the distal tubule could be attributed to the proximity of the proximal tubule cells to the glomerulus and renal corpuscle, enabling them to exert a stronger influence on the height of the epithelial cells that receive the filtered material. The results also showed a significant reduction in the diameter of the proximal tube compared to the control group. In contrast, the diameter of the distal tube did not exhibit a significant change compared to the control group. This change can also be related to the previously mentioned alteration in the height of epithelial cells. In this context, previous research has indicated that SCI is a complex process that can affect distant organs, such as the heart, kidneys, lungs, and lower limbs. The researchers found that degeneration and necrosis occurred in the urinary tubular cells due to SCI. They also noted that dexmedetomidine can effectively improve the kidney’s histopathology.
In the present research, we assessed the size of the distal and proximal convoluted tubules, as well as the height of the epithelial cells in the proximal and distal tubules in the groups that were administered the alcoholic extract of M. officinalis compared to the injury group, the subjects showed improved condition, and their histomorphometric analysis indicated that they were more similar to the control group. Therefore, further investigation of the alcoholic extract of M. officinalis is warranted, as it may potentially facilitate abnormal changes in the urinary tract. A study conducted in 2022 by Şengel et al. demonstrated that dexmedetomidine can alleviate the harmful effects of kidney damage in rats with SCI through various mechanisms [50].
In a separate investigation carried out by Bahrke and Yesalis in 2004, it was determined that the intraperitoneal injection of nandrolone decanoate in adult female Balb/c rats led to a notable increase in the size of both distal and proximal tubules in the experimental groups compared to the control group. Additionally, Research has shown that nandrolone attaches to androgen receptors located on the surface of epithelial cells in the proximal and distal tubules. This binding influences the structure of chromatin, the expression of genes, and the synthesis of mRNA and stimulates the synthesis of proteins [51]. As mentioned, the kidney is highly sensitive in purifying the blood plasma and eliminating waste materials from the body. The kidney’s size can vary. In a 2008 study [52], scientists examined the impact of garlic extract on animals with induced diabetes. They observed enlargement of the glomeruli and alterations in glomerular volume, indicating that the kidneys were responsive to and underwent changes in reaction to exposure to different substances. This study is linked to research conducted by Welt in 2007 [52, 53].
In a study conducted in 2017 by Orr and Briggs, it was found that heavy metal consumers had an increased volume in their distal tubules compared to the control group, indicating the kidneys’ responsiveness to different substances [54]. Similarly, a 2018 study by the European :union: Safety Organization showed that certain substances, such as aspartame, could significantly reduce parameters like the diameter of the renal corpuscle, glomerular capsule, and the height of the proximal convoluted tubules in the experimental groups compared to the control group. This aligns with previous research by Petros and Humphries (2009), who demonstrated that dietary substances like aspartame decreased the diameter of the Bowman’s capsule and subsequent expansion of the urinary space. Additionally, a 2022 study by Tah, Wang, and colleagues revealed that the combination of metformin, barberry, and parsley in diabetic mice could affect kidney histopathology and modify the vacuolar cells of the urinary tubules [55].
Conclusion
The occurrence of SCI represents a significant medical concern with profound implications for the affected individuals and society at large. Our recent study has shown that SCI in rats causes widespread inflammation throughout the body shortly after the injury, resulting in secondary effects that spread the condition to other organs and tissues. This cascade of events triggers systemic inflammation, exacerbating the spread of the disease to various bodily organs and tissues. Notably, the kidneys, vital for blood purification, blood pressure regulation, and overall health maintenance, are particularly impacted. Consequently, this injury poses a potential threat to life.
The investigation findings suggest that the development and progression of systemic inflammation are initiated by increased expression of inflammatory markers like TNF-α, upregulation of genes linked to the apoptosis process (such as Bax and CASP3), and a decrease in Bcl-2 expression due to secondary injury resulting from SCI. This inflammatory spread in kidney tissues causes damage and disease progression. The advancement of the disease impacts renal tissues, resulting in histological changes and affecting their function. This condition causes modifications in renal morphology, affecting parameters, such as the diameter of renal corpuscles, glomerular diameter, height of proximal tubule epithelial cells, diameter of proximal tubules, height of distal tubule epithelial cells, and diameter of distal tubules. Furthermore, it leads to a decrease in the renal urinary space’s diameter, proximal tubules’ luminal diameter, and distal tubules’ luminal diameter. These histomorphometric and structural changes influence renal function, leading to impaired performance.
This study shows that the alcoholic extract of the borage plant may be able to treat SCI because of its anti-inflammatory, antioxidant, neuroprotective, gastrointestinal, and urinary properties. The results indicate that the extract can decrease the activity of genes involved in cell death, such as Bax and CASP3, while increasing the activity of Bcl-2. It also reduces the activity of the inflammatory factor TNF-α, which is disrupted in SCI. Additionally, the extract affects the kidney’s tissue and histomorphometric changes caused by SCI, demonstrating its therapeutic potential. The alcoholic borage extract can potentially address spinal cord lesions and their secondary effects on spinal tissue.
Ethical Considerations
Compliance with ethical guidelines
In this experimental research, following approval from the Ethics Committee of the Islamic Azad University, Tehran Medical Sciences Branch. All procedures were carried out in compliance with the guidelines of the Animal Protection Ethics Committee, sanctioned by Tehran Medical Sciences Branch, Islamic Azad University (Code: IR.IAU.PS.REC.1400.525).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
References
- Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J Spinal Cord Med. 2017; 40(6):665-75. [DOI:10.1080/10790268.2017.1329076] [PMID] [PMCID]
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019; 10:282. [DOI:10.3389/fneur.2019.00282] [PMID] [PMCID]
- Schmidt EKA, Torres-Espin A, Raposo PJF, Madsen KL, Kigerl KA, Popovich PG, et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. Plos One. 2020; 15(1):e0226128. [DOI:10.1371/journal.pone.0226128] [PMID] [PMCID]
- Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol Exp. 2011; 71(2):281-99. [DOI:10.55782/ane-2011-1848] [PMID]
- Lv B, Zhang X, Yuan J, Chen Y, Ding H, Cao X, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021; 12(1):36. [DOI:10.1186/s13287-020-02090-y] [PMID] [PMCID]
- Greaves CY, Gadala MS, Oxland TR. A three-dimensional finite element model of the cervical spine with spinal cord: An investigation of three injury mechanisms. Ann Biomed Eng. 2008; 36(3):396-405. [DOI:10.1007/s10439-008-9440-0] [PMID]
- Gumbel JH, Montgomery LR, Yang CB, Hubscher CH. Activity-based training reverses spinal cord injury-induced changes in kidney receptor densities and membrane proteins. J Neurotrauma. 2020; 37(3):555-63. [DOI:10.1089/neu.2019.6670] [PMID]
- Liau LL, Looi QH, Chia WC, Subramaniam T, Ng MH, Law JX. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020; 10:112. [DOI:10.1186/s13578-020-00475-3] [PMID] [PMCID]
- Meng YF, Zhang JW, Tong AN, Tang HH, Bai JZ, Wang FY, et al. Prognosis of traumatic spinal cord injury in children: Follow-up of 86 patients. Chin J Traumatol. 2023; 26(1):14-9. [DOI:10.1016/j.cjtee.2022.05.001] [PMID] [PMCID]
- Freire MAM, Lima RR, Bittencourt LO, Guimaraes JS, Falcao D, Gomes-Leal W. Astrocytosis, inflammation, axonal damage and myelin impairment in the internal capsule following striatal ischemic injury. Cells. 2023; 12(3):457. [DOI:10.3390/cells12030457] [PMID] [PMCID]
- Sun X, Jones ZB, Chen XM, Zhou L, So KF, Ren Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury: A complex relationship. J Neuroinflammation. 2016; 13(1):260. [DOI:10.1186/s12974-016-0736-y] [PMID] [PMCID]
- Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015; 53(1):14-8. [DOI:10.1038/sc.2014.184] [PMID]
- Liu L, Pei FX, Tang KL, Xu JZ, Li QH. Expression and effect of Caspase-3 in neurons after tractive spinal cord injury in rats. Chin J Traumatol. 2005; 8(4):220-4. [PMID]
- Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014; 258:62-77. [DOI:10.1016/j.expneurol.2014.04.006] [PMID]
- Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019; 20(3):175-93. [DOI:10.1038/s41580-018-0089-8] [PMID] [PMCID]
- Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, et al. Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma. 2003; 20(2):207-19. [DOI:10.1089/08977150360547116] [PMID]
- Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007; 26(2):228-33. [DOI:10.1002/nau.20319] [PMID]
- Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, et al. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020; 21(20):7533. [DOI:10.3390/ijms21207533] [PMID] [PMCID]
- Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005; 115(5):1275-80. [DOI:10.1172/JCI24066] [PMID] [PMCID]
- Rahyussalim AJ, Saleh I, Kurniawati T, Lutfi APWY. Improvement of renal function after human umbilical cord mesenchymal stem cell treatment on chronic renal failure and thoracic spinal cord entrapment: A case report. J Med Case Rep. 2017; 11(1):334. [DOI:10.1186/s13256-017-1489-7] [PMID] [PMCID]
- Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 2012; 135(3):287-96. [PMID] [PMCID]
- Wulf MJ, Tom VJ. Consequences of spinal cord injury on the sympathetic nervous system. Front Cell Neurosci. 2023; 17:999253. [DOI:10.3389/fncel.2023.999253] [PMID] [PMCID]
- Shunmugavel A, Khan M, Te Chou PC, Dhindsa RK, Martin MM, Copay AG, et al. Simvastatin protects bladder and renal functions following spinal cord injury in rats. J Inflamm. 2010; 7:17. [DOI:10.1186/1476-9255-7-17] [PMID] [PMCID]
- Ko HY. Neurogenic bladder dysfunction. In: Ko HY, editor. Management and Rehabilitation of Spinal Cord Injuries. New York: Springer; 2019. [DOI:10.1007/978-981-10-7033-4_24]
- Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L. - A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2016; 188:204-28. [DOI:10.1016/j.jep.2016.05.010] [PMID]
- Miraj S, Azizi N, Kiani S. A review of chemical components and pharmacological effects of Melissa officinalis L. Der Pharmacia Lettre. 2016; 8(6):229-37. [Link]
- Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C J Biosci. 2007; 62(7-8):507-13. [DOI:10.1515/znc-2007-7-808] [PMID]
- Ohashi Y, Huang S, Maeda I. Biosyntheses of geranic acid and citronellic acid from monoterpene alcohols by Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2021; 85(6):1530-5. [DOI:10.1093/bbb/zbab039] [PMID]
- Kennedy DO, Little W, Scholey AB. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (lemon balm). Psychosom Med. 2004; 66(4):607-13. [DOI:10.1097/01.psy.0000132877.72833.71] [PMID]
- Hosseini SR, Kaka G, Joghataei MT, Hooshmandi M, Sadraie SH, Yaghoobi K, et al. Assessment of neuroprotective properties of melissa officinalis in combination with human umbilical cord blood stem cells after spinal cord injury. ASN Neuro. 2016; 8(6):1759091416674833. [DOI:10.1177/1759091416674833] [PMID] [PMCID]
- Ghazizadeh J, Hamedeyazdan S, Torbati M, Farajdokht F, Fakhari A, Mahmoudi J, et al. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp Physiol. 2020; 105(4):707-20. [DOI:10.1113/EP088254] [PMID]
- Bayat M, Azami Tameh A, Hossein Ghahremani M, Akbari M, Mehr SE, Khanavi M, et al. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. Daru. 2012; 20(1):42. [DOI:10.1186/2008-2231-20-42] [PMID] [PMCID]
- Hosseini SR, Kaka G, Joghataei MT, Hooshmandi M, Sadraie SH, Yaghoobi K, et al. Coadministration of dexamethasone and melissa officinalis has neuroprotective effects in rat animal model with spinal cord injury. Cell J. 2017; 19(1):102-16. [PMID] [PMCID]
- Santos DRD, Teixeira RKC, Araújo NP, Calvo FC, Duarte TB, Ataíde LAP, et al. A new anesthetic protocol to medullary nerve roots access in rats. Acta Cir Bras. 2021; 36(9):e360908. [DOI:10.1590/acb360908] [PMID] [PMCID]
- Kharazinejad E, Hassanzadeh G, Sahebkar A, Yousefi B, Sameni HR, Majidpoor J, et al. The comparative effects of schwann cells and wharton's jelly mesenchymal stem cells on the aim2 inflammasome activity in an experimental model of spinal cord injury. Neuroscience. 2023; 535:1-2. [DOI:10.1016/j.neuroscience.2023.10.011]
- Tam VH, Cohen DN, Ledesma KR, Guillory B, Chan K, Garey KW. Local tissue response to subcutaneous administration of ceftriaxone in an animal model. Antimicrob Agents Chemother. 2020; 64(3):e02090-19. [DOI:10.1128/AAC.02090-19] [PMID] [PMCID]
- Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016; 2016:9476020. [DOI:10.1155/2016/9476020] [PMID] [PMCID]
- Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS. p53- and bax-mediated apoptosis in injured rat spinal cord. Neurochem Res. 2011; 36(11):2063-74. [DOI:10.1007/s11064-011-0530-2] [PMID]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008; 209(2):378-88. [DOI:10.1016/j.expneurol.2007.06.009] [PMID] [PMCID]
- Liu J, Peng L, Li J. The lipoxin A4 receptor agonist BML-111 alleviates inflammatory injury and oxidative stress in spinal cord injury. Med Sci Monit. 2020; 26:e919883-1. [DOI:10.12659/MSM.919883]
- Zhao W, Li H, Hou Y, Jin Y, Zhang L. Combined administration of poly-ADP-ribose polymerase-1 and caspase-3 inhibitors alleviates neuronal apoptosis after spinal cord injury in rats. World Neurosurg. 2019; 127:e346-52. [DOI:10.1016/j.wneu.2019.03.116] [PMID]
- Wang Y, Sun ZY, Zhang KM, Xu GQ, Li G. Bcl-2 in suppressing neuronal apoptosis after spinal cord injury. World J Emerg Med. 2011; 2(1):38-44. [PMID] [PMCID]
- Zhao D, Zhang M, Yuan H, Meng C, Zhang B, Wu H. Ginsenoside Rb1 protects against spinal cord ischemia-reperfusion injury in rats by downregulating the Bax/Bcl-2 ratio and caspase-3 and p-Ask-1 levels. Exp Mol Pathol. 2018; 105(3):229-35. [DOI:10.1016/j.yexmp.2018.09.001] [PMID]
- Luo Y, Fu C, Wang Z, Zhang Z, Wang H, Liu Y. Mangiferin attenuates contusive spinal cord injury in rats through the regulation of oxidative stress, inflammation and the Bcl‑2 and Bax pathway. Mol Med Rep. 2015; 12(5):7132-8. [DOI:10.3892/mmr.2015.4274] [PMID]
- Guginski G, Luiz AP, Silva MD, Massaro M, Martins DF, Chaves J, et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 2009; 93(1):10-6. [DOI:10.1016/j.pbb.2009.03.014] [PMID]
- Ferreira A, Proença C, Serralheiro ML, Araújo ME. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2006; 108(1):31-7. [DOI:10.1016/j.jep.2006.04.010] [PMID]
- Petrisor G, Motelica L, Craciun LN, Oprea OC, Ficai D, Ficai A. Melissa officinalis: Composition, pharmacological effects and derived release systems-A review. Int J Mol Sci. 2022; 23(7):3591. [DOI:10.3390/ijms23073591] [PMID] [PMCID]
- Abo-Zaid OA, Moawed FS, Taha EF, Ahmed ESA, Kawara RS. Melissa officinalis extract suppresses endoplasmic reticulum stress-induced apoptosis in the brain of hypothyroidism-induced rats exposed to γ-radiation. Cell Stress Chaperones. 2023; 28(6):709-20. [DOI:10.1007/s12192-023-01363-8] [PMID] [PMCID]
- Nasiri AR, Karamibonari AR. Protective effects of Melissa officinalis L. extract on gentamicin-induced renal failure in diabetic rats. Med Lab J. 2023; 17(1):35-41. [Link]
- Şengel N, Köksal Z, Dursun AD, Kurtipek Ö, Sezen ŞC, Arslan M, et al. Effects of dexmedetomidine administered through different routes on kidney tissue in rats with spinal cord ischaemia-reperfusion injury. Drug Des Devel Ther. 2022; 16:2229-39. [DOI:10.2147/DDDT.S361618] [PMID] [PMCID]
- Abbas Y. Abuse of anabolic androgenic steroids. J Stress Physiol Biochem. 2009; 5(3):22-32. [Link]
- Al-Qattan K, Thomson M, Ali M. Garlic (allium sativum) and ginger (zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. e-SPEN Eur J Clin Nutr Metab. 2008; 3(2):e62-71. [DOI:10.1016/j.eclnm.2007.12.001]
- Welt K, Weiss J, Martin R, Hermsdorf T, Drews S, Fitzl G. Ginkgo biloba extract protects rat kidney from diabetic and hypoxic damage. Phytomedicine. 2007; 14(2-3):196-203. [DOI:10.1016/j.phymed.2006.03.023] [PMID]
- Orr SE, Bridges CC. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci. 2017; 18(5):1039. [DOI:10.3390/ijms18051039] [PMID] [PMCID]
- Choi JY, Jang TW, Song PH, Choi SH, Ku SK, Song CH. Combination effects of metformin and a mixture of lemon balm and dandelion on high-fat diet-induced metabolic alterations in mice. Antioxidants. 2022; 11(3):580. [DOI:10.3390/antiox11030580] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Cellular and Molecular Biology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |