Volume 10, Issue 1 (2024)
Pharm Biomed Res 2024, 10(1): 47-56 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ansari A P, Arokiarajan M S, Syed Akbar W A, Ahmed N Z, Anwar N. Investigating the Quality Control, Antimicrobial, and Antioxidant Evaluation of the Ointment “Marham-i-Raal” Used in the Unani System of Medicine. Pharm Biomed Res 2024; 10 (1) :47-56
URL: http://pbr.mazums.ac.ir/article-1-572-en.html
URL: http://pbr.mazums.ac.ir/article-1-572-en.html
Athar Parvez Ansari *1 

 , Mary Shamya Arokiarajan1
, Mary Shamya Arokiarajan1 

 , Wasim Akram Syed Akbar1
, Wasim Akram Syed Akbar1 

 , N Zaheer Ahmed2
, N Zaheer Ahmed2 

 , Noman Anwar1
, Noman Anwar1 




 , Mary Shamya Arokiarajan1
, Mary Shamya Arokiarajan1 

 , Wasim Akram Syed Akbar1
, Wasim Akram Syed Akbar1 

 , N Zaheer Ahmed2
, N Zaheer Ahmed2 

 , Noman Anwar1
, Noman Anwar1 


1- Regional Research Institute of Unani Medicine, Chennai (NABH Accredited), New Delhi, India.
2- Central Council for Research in Unani Medicine, Ministry of Ayush, Government of India, New Delhi, India.
2- Central Council for Research in Unani Medicine, Ministry of Ayush, Government of India, New Delhi, India.
Full-Text [PDF 4863 kb]
(696 Downloads)
| Abstract (HTML) (1615 Views)
Full-Text: (824 Views)
Introduction
Currently, the global propensity for using synthetic drugs has been shifted toward herbal medicines, which is also referred to as the return to nature. Botanicals are considered a rich source of medicinal properties for the management of many ailments of the body. The renaissance of complementary and alternative medical systems, including herbs, has deliberated to raise the demand and trade of herbal medicines, which has turned down their quality, mainly because of deficient quality control parameters and regulations for herbal drugs. The World Health Organization (WHO) has stressed the necessity of checking the quality of medicinal products used in complementary and alternative medical systems by applying modern methodologies and standards. To conquer the deficiencies found in the pharmacopoeial monographs, other important parameters to explore the quality and purity of herbal products may also be used [1]. The Unani system of medicine is one of the oldest systems proven to have productive treatment strategies. In Unani medicine, several systemic and topical dosage forms are used to treat various diseases [2]. Marham-i-Raal, a pharmacopoeial formulation, acts as dāfi‘-i-ta‘affun (antiseptic) and mudammil (healing agent) and is topically used in the treatment of qurūh-i-ātshak (syphilitic ulcers), qurūh al-anf (nasal ulcers), nāsūr (fistula) [3], qurūh (ulcers), jurūh (wounds) [4], etc. This ointment helps remove the slough materials and necrosed tissues from the site of the wound and produces granulation tissues, which help heal wounds. It also augments the collagen concentration along with diminishing fibrous tissues at the site of wounds, which causes increased epithelialization and contraction of the wound [5]. This pharmacopoeial formulation contains four plant-derived drugs, including Kafoor (Cinnamomum camphora [Linn.] Nees & Eberm.), Raal (Vateria indica L.), Kath Safaid (Acacia catechu [Linn. f.] Willd.), and Roghan Kunjad (Sesamum indicum L. oil) and one animal-derived drug, i.e. Mom Safaid (Beeswax) [3]. The standardization of Unani formulations is the current need for their worldwide acceptance [6]. The quality control of Marham-i-Raal has not yet been carried out in Unani medicine. Hence, its standardization was carried out using physicochemical evaluation, heavy metal analysis, microbial load, and high-performance thin-layer chromatography (HPTLC). Moreover, the antimicrobial and antioxidant activities of Marham-i-Raal were also carried out to explore its antimicrobial and antioxidant properties scientifically.
Materials and Methods
Source of data collection
The data were collected from the GMP-certified Pharmacy and Drug Testing Laboratory approved by the State Drug Licensing Authority, Tamil Nadu, i.e. Drug Standardization Research Unit and Microbiology Lab of the Regional Research Institute of Unani Medicine, Chennai, Central Council for Research in Unani Medicine, New Delhi, Ministry of Ayush, Government of India. The study was carried out in January 2023.
Collection of materials and chemicals
The ingredients of Marham-i-Raal were procured from an authorized drug supplier in Chennai, Tamil Nadu, and authenticated by Mageswari, pharmacognosist, Department of Pharmacognosy, Regional Research Institute of Unani Medicine, Chennai. The chemicals and reagents used in the quality control evaluation of Marham-i-Raal were obtained from authorized and registered suppliers in Chennai.
Preparation of Marham-i-Raal
The ointment was prepared as per the composition of ingredients and method described in the National Formulary of Unani Medicine, Part I [3] at the GMP-certified pharmacy of Regional Research Institute of Unani Medicine, Chennai. The botanical ingredients, such as Kafoor (Cinnamomum camphora), Raal (Vateria indica), and Kath Safaid (Acacia catechu), were ground separately by using a pulverizer. The powdered drug was then sieved using the sieve number 100. After sieving the powder, individual ingredients were uniformly mixed using a double-cone blender. The given ratio of Mom Safaid (beeswax) was then added to Roghan Kunjad (Sesamum indicum L. oil) and heated over a low flame. The powdered drug was mixed in the base and well-mixed using a planetary mixer machine. The prepared ointment was stored in a tightly sealed container [3]. The prepared formulation of 10 g contains Kafoor (2 g), Raal (2 g), Kath Safaid (2 g), Mom (2 g), and Roghan Kunjad (2 mL).
Quality control
The quality control parameters, including organoleptic characters, foreign matter, weight loss on drying, pH, ash values, microbial load, aflatoxins, heavy metals, and high-performance thin-layer chromatography, were carried out using standard methods.
Foreign matter
The foreign matters present in the individual ingredients and the formulation were checked in daylight with the naked eye [6].
Loss of weight on drying at 105°C
The weight loss on drying at 105°C is designed to measure the water in a dried sample under specified conditions. An accurately weighed 10 g of Marham-i-Raal was placed without preliminary drying after being accurately weighed in a tarred evaporated dish. This was dried at 105°C for 5 h and weighed. Drying and weighing were repeated at every 1-h interval until the weight was found to be constant. A constant weight was reached when two consecutive weights, after drying for 30 min and cooling for 30 min in a desiccator, showed not more than a 0.1 g difference evaluated based on the guidelines mentioned in the Unani Pharmacopoeia of India, part II, volume land WHO guidelines for quality control of herbal materials [7, 8].
pH of 5% solution
The pH of a 5% aqueous solution of the test drug was determined by following the standard methods described in the Unani pharmacopoeia of India (UPI) [7]. A weight of 5 g of the drug was dissolved in a measured 100 mL of water and filtered, and the pH of the filtrate was checked with a standardized glass electrode.
Ash values
The test sample’s total ash and acid insoluble ash values were also determined following the standard methods described in the UPI and WHO guidelines for quality control of herbal materials [7, 8].
Heavy metal analysis by atomic absorption spectrometer
The quantitative analysis of heavy metals, including cadmium and lead, was accomplished using an atomic absorption spectrometer (Thermo Scientific, iCE 3000 model) using SOLAAR, AA software, version 11.10.
Sample preparation for atomic absorption spectrometer
An amount of 0.5 g of the ointment was transferred into a TFM-PTFE Microwave digestion tube (50 mL), and 6 mL of concentrated HNO3 was added. The sample was then allowed to undergo hot digestion using the Anton Paar Microwave digestion system (Multi-wave GO Plus) from 0°C to 200°C for 30 min. After that, the pressure was released slowly from the TFM digestion tube, and it was allowed to release nitric acid fumes, followed by two portions of 5 mL of deionized water. The digested sample was transferred into a 100 mL volumetric flask using Whatman (No. 1) filter paper. The sample was made into 100 mL by adding deionized water. We have modified and adopted the procedures mentioned in the following references [9] for microwave digestion.
Microbial load
The microbial limit tests for the bacterial count, total fungal count, and specific microorganisms were carried out using the standard methods mentioned in the Unani Pharmacopoeia of India, part II, volume I [7].
High-performance thin layer chromatography
A total of 5 µL of the alcohol extract of the formulation was applied on an aluminum plate pre-coated with silica gel 60 F254 HPTLC plate (5×10 cm, E. Merck) by employing a CAMAG ATS4 sample applicator. The plates were developed up to a distance of 8 cm in the CAMAG twin-through glass chamber (10×10), using 10 mL of the developing system (toluene: Ethyl acetate: Formic acid; 9.0:1.0:0.01) as the mobile phase, previously saturated with solvent for 15 min at 26°C, and dried at room temperature. The mobile phase was chosen after testing different solvent systems of varying polarity. Data was processed using Win CATS planar chromatography manager software and scanned under UV 254 and 366 nm [10].
Antimicrobial activity
Agar disc diffusion method
The antimicrobial activity of Marham-i-Raal was investigated by the disc diffusion method [11]. The test strains, including Staphylococcus aureus (ATCC 27853), Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 700603), Bacillus cereus (Clinical Cochin University), and C. albicans (Clinical Cochin University), were collected from the Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai. The plates were prepared with 20 to 25 mL of Muller-Hinton agar. One loopful of inoculum from each bacterial culture to be tested was spread on agar plates with a sterile swab moistened with the bacterial suspension. Subsequently, the sterile disc was dropped with 50 µL of test extract and allowed to diffuse at room temperature. The plates were incubated at 37°C for 24 h. After 24 h of incubation, the test determines the efficacy of the test drug in terms of the zone of inhibition of the organism [12].
Antioxidant activity
Diphenyl-1-picrylhydrazyl radical scavenging activity
The free radical scavenging capacity of Marham-i-Raal was evaluated based on the method described by Brand-Williams et al. (1995) [13] with slight modifications. An amount of 1.5 mL of 0.1 mM diphenyl-1-picrylhydrazyl solution in methanol was mixed with 1 mL of the Unani formulation solution of different concentrations, i.e. 25, 50, 75, and 100 μg/mL. Blank samples were prepared, and L-ascorbic acid was used as a standard. These tubes were thoroughly mixed and kept in the dark for 30 min, and their absorbance was measured at 517 nm using an ultraviolet-visible spectrophotometer [14]. The percentage of inhibition was calculated using the Equation 1:
1. % of inhibition=A(control)-A(test)×100/A(control)
2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid assay
The free radical scavenging activity of Marham-i-Raal was also determined by the 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) radical cation decolorization assay. The ABTS+ cation radical was produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate (1:1), stored in the dark at room temperature for 12-16 h before use. The ABTS+ solution was then diluted with methanol to obtain an absorbance of 0.700 at 734 nm. After adding 500 μL of the Unani formulation to 3.5 mL of diluted ABTS+solution, the absorbance was measured for 30 min after the initial mixing. An appropriate solvent blank was run in each assay. All the measurements were carried out at least three times. The percent inhibition of absorbance at 734 nm was calculated using the Equation 2:
2. ABTS+Scavenging effect (%)=((AB–AA)/AB)×100,
Where AB is the absorbance of ABTS radical+methanol, and AA is the absorbance of ABTS radical+sample extract/standard. Trolox was used as a standard substance [15].
Statistical analysis
The antimicrobial and antioxidant potentials of Marham-i-Raal were determined in duplicate in all trials. The Mean±SD are used to express the data. The data was statistically examined for standard deviation using MS Excel, and a graph was created using Origin Pro3.
Results
Quality control
Organoleptic characters
The organoleptic evaluation of Marham-i-Raal revealed that it was tasteless, reddish-brown in color, and had a characteristic odor.
Physicochemical standardization
No foreign matters were detected in any ingredients of the formulation or in the preparation itself. The moisture content in Marham-i-Raal was 11.15%. The pH of a 5% aqueous solution of the test drug was determined to be 6.7%. The total ash and acid-insoluble ash values were recorded at 3.96% and 1.55%, respectively.
Heavy metal analysis
The findings showed that lead was not found in Marham-i-Raal, whereas cadmium was 0.0077 mg/L.
Microbial load
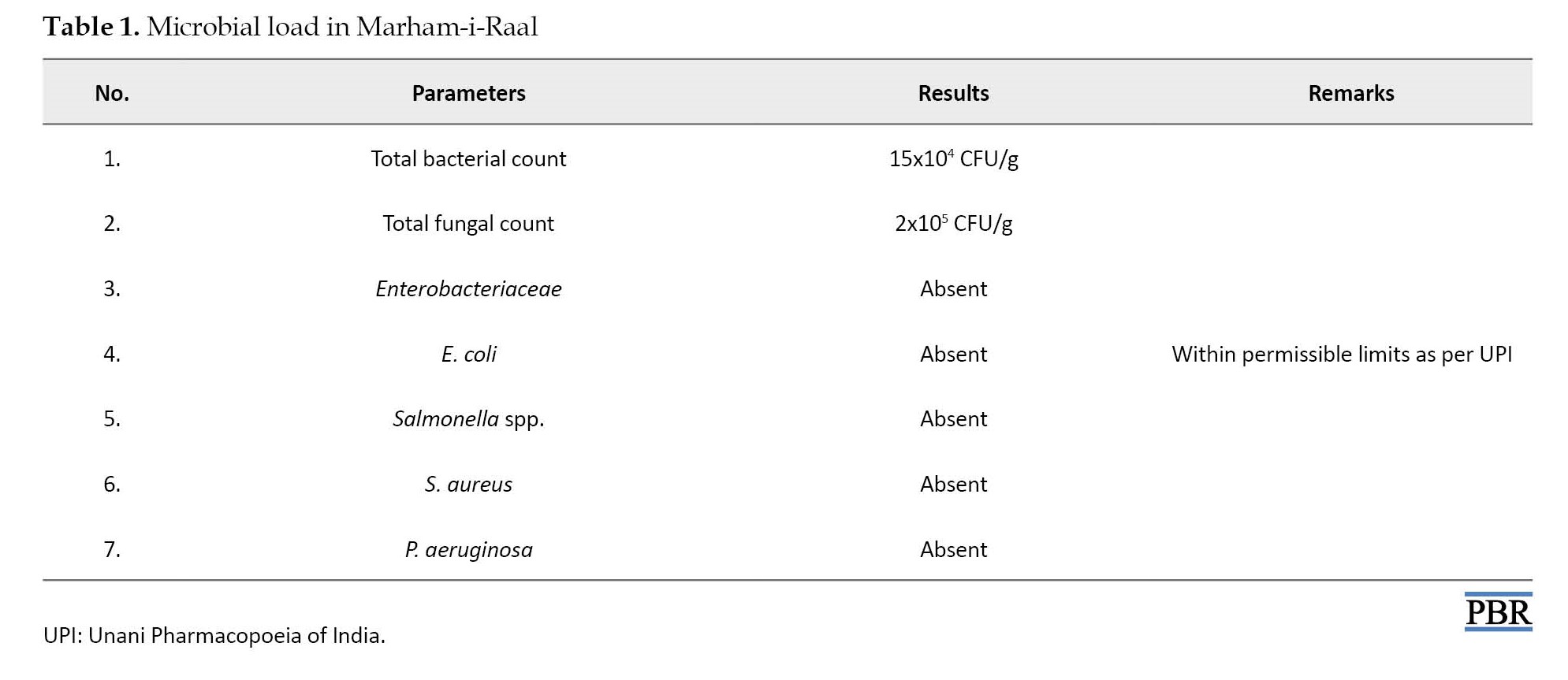
The results regarding the microbial load are illustrated in Table 1.
Hight-performance thin layer chromatography fingerprint profile
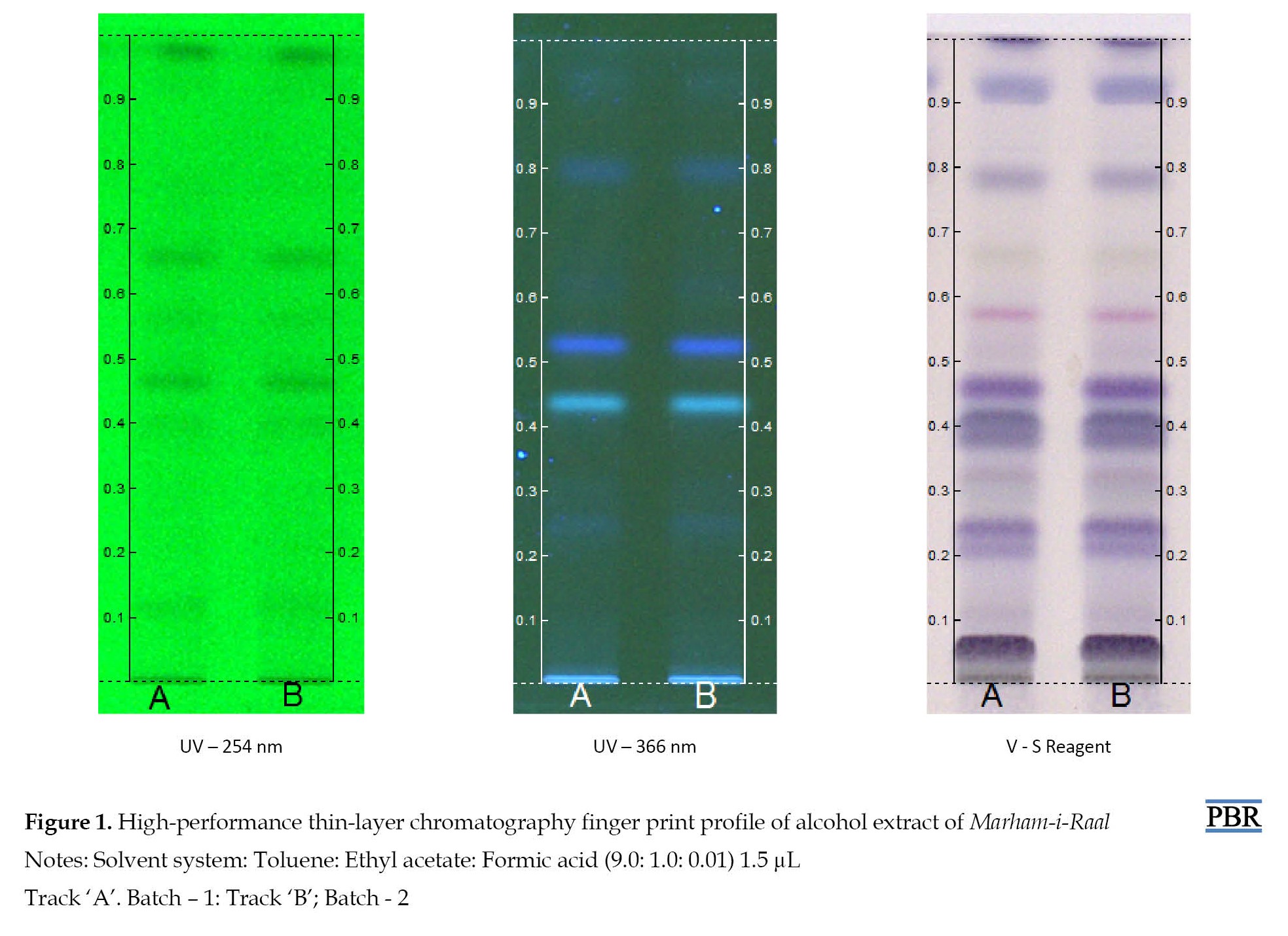
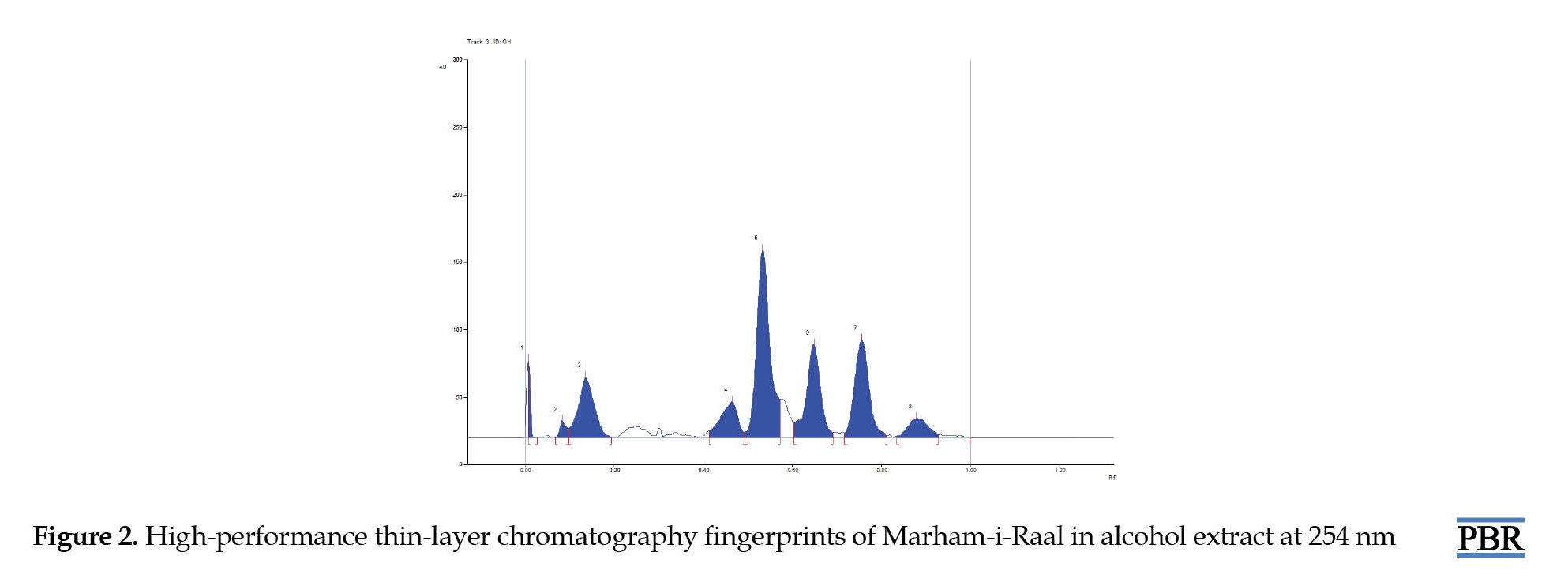
The HPTLC chromatogram of the alcoholic extract of Marham-i-Raal under UV-254 nm (absorbance mode) has observed 8 spots, amongst which the Rf value of 0.50 contributes to the highest area percentage, which denotes the presence of a prevalent compound of interest in the formulation, while the Rf of 0.72 ranked the second highest area percentage (Figures 1, 2 and 3).
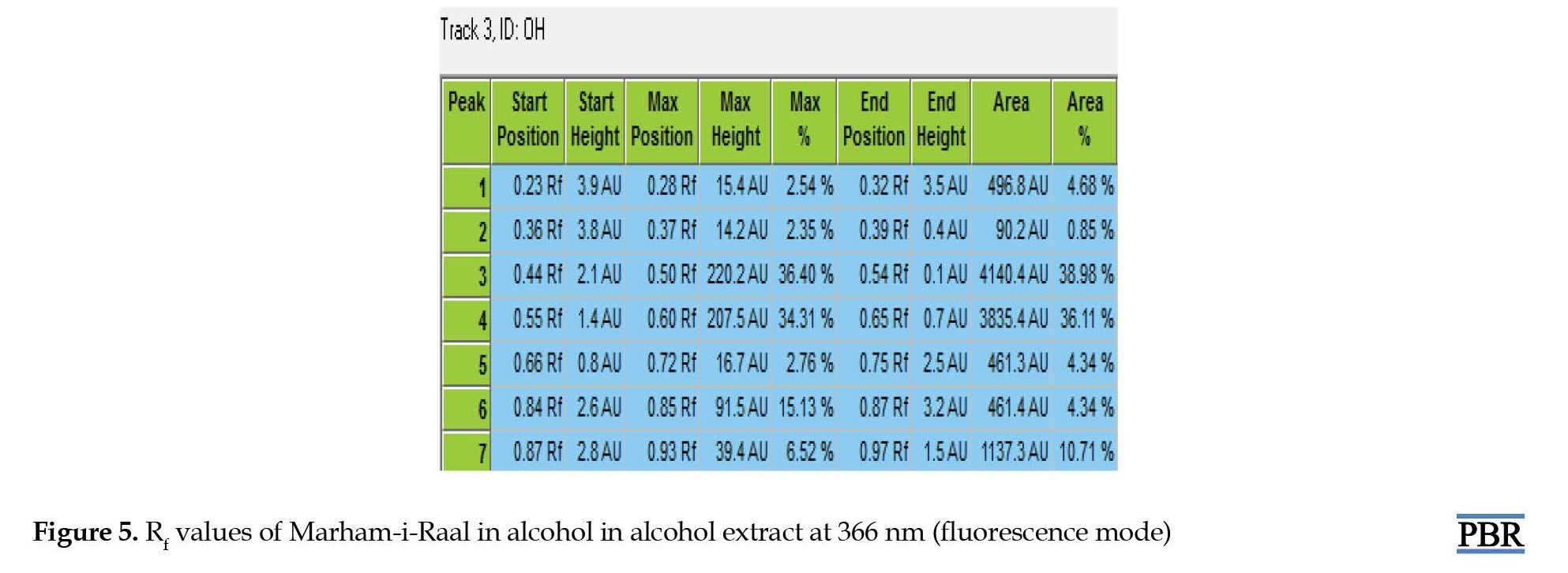
Similarly, the HPTLC chromatogram of the alcoholic extract of Marham-i-Raal under UV-366 nm (florescence mode) has shown 7 spots, amongst which the Rf value of 0.44 was found to be the dominant component, constituting about 38.98% as compared to the other six spots (Figures 1, 4 and 5).
Antimicrobial activity
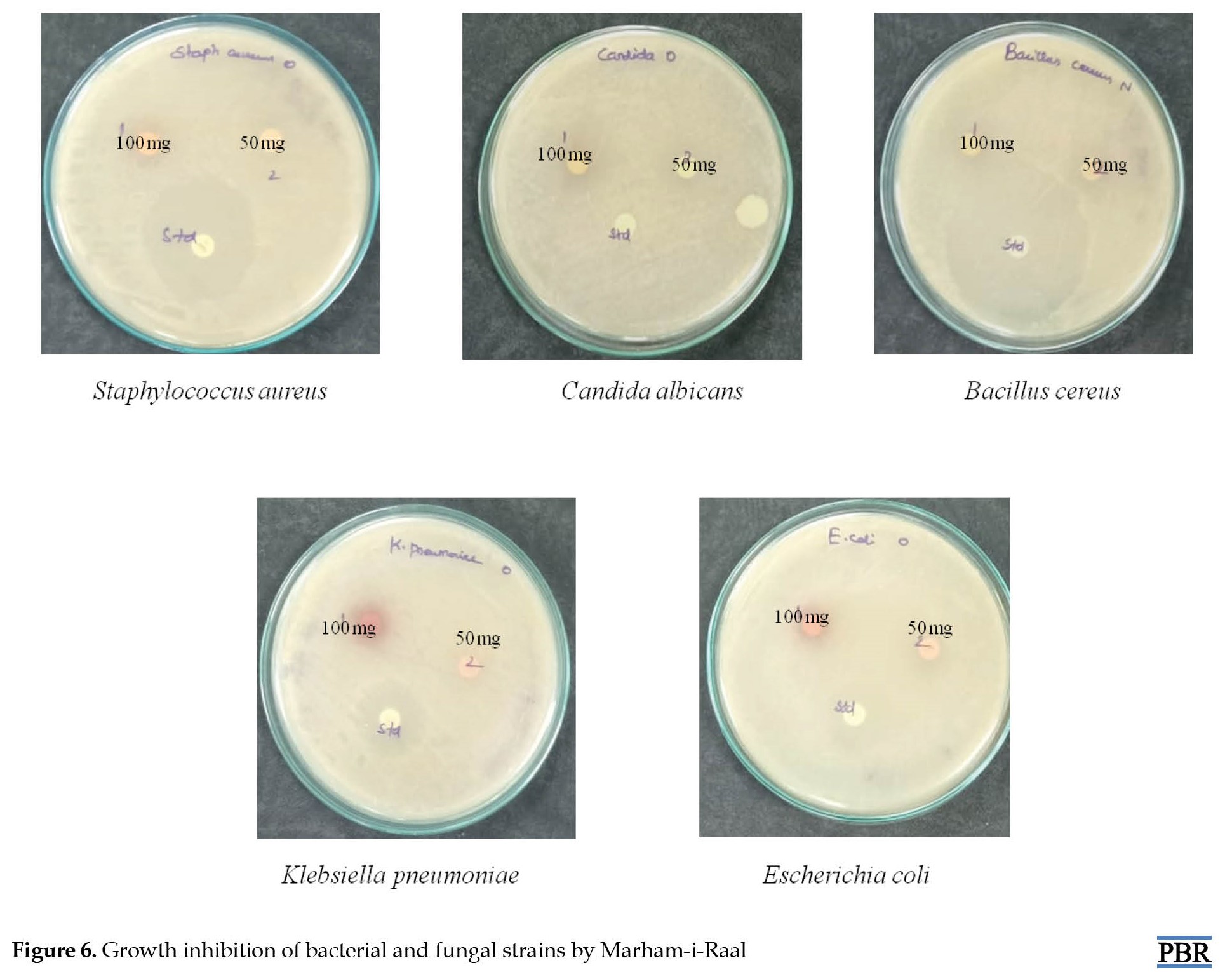
The results of the antimicrobial activity of Marham-i-Raal are provided in Table 2 and Figure 6.

Antioxidant activity
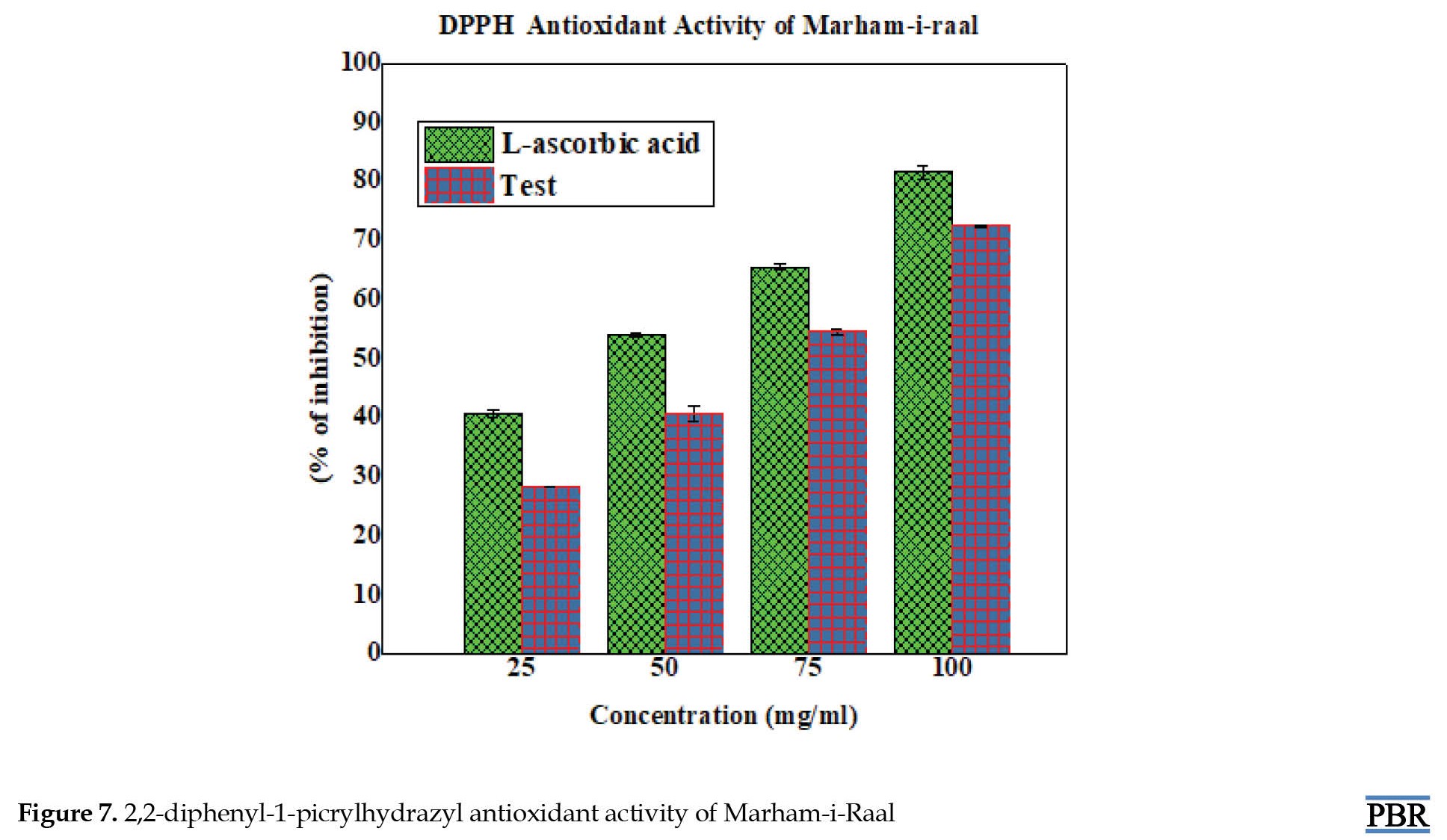
The 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of Marham-i-Raal was compared to that of the synthetic antioxidant standard L-ascorbic acid. The maximum inhibition was obtained in a 100 mg/mL concentration, and the percentage of inhibition was 72.84, with an IC50 value of 64.14 mg/mL (Figure 7). The ABTS scavenging activity of the formulation showed promising results at a 100 µg/mL concentration with 69.72% inhibition and an IC50 value of 57.74 mg/mL (Figure 8).
Discussion
Quality assurance is essential to ensure the quality of herbal products in all traditional systems of medicine. In this regard, various modern techniques, including macroscopic, microscopic, physicochemical, phytochemical, chromatographic fingerprinting, biological evaluations, etc. are investigated [16]. The present study was carried out to evaluate different quality control parameters of Marham-i-Raal to ensure its quality since no monograph on this pharmacopoeial has been published in the Unani Pharmacopoeia of India, part II. As per the WHO guidelines published in 2011, herbal products should not be contaminated with insects, moulds, animal excreta, soil, dust, sand, stones, etc. [8]. The study has reported that no foreign matter was detected in any ingredients of the formulation or in the preparation itself, showing the tested drug’s purity. The presence of excess water content in herbal products may cause the growth of microorganisms [8]. The amount of water in herbal materials should be between 10% and 20% to avoid decomposition [17]. The present study has reported the presence of moisture content in Marham-i-Raal to be within an acceptable limit, indicating its good storage, handling, and manufacturing. The pH of the formulation tested in this study was slightly acidic. The pH of the skin surface should be acidic, which plays a pivotal role in maintaining the homeostasis and barrier permeability of the stratum corneum. Thus, it is recommended that topical drugs’ pH be acidic between the range of 4-6 [18]. Inorganic salts in a drug, either naturally or adulterated, may be checked through the ash value parameter [19]. The entire amount of material left over after ignition is called the total ash value, which may contain physiological or non-physiological ash. The amount of silica, including sand and siliceous earth, is measured through the acid-insoluble ash value parameter [20]. In the present study, the total ash and acid-insoluble ash values were within the normal range. The plants are considered to have a greater affinity to accumulate heavy metals [21]. Lead, cadmium, arsenic, and mercury are regarded as the most toxic heavy metals, which may cause cancer in humans [22]. Lead poisoning leads to high blood pressure and damage to the brain and kidneys.
Cadmium is harmful to the respiratory system and leads to cardiac as well as kidney problems [23]. The heavy metal analysis report of Marham-i-Raal revealed that lead was not detected in it, whereas cadmium was within the permissible limit [7]. The total microbial count and fungal count were found within the permissible limits in the test sample. Specific microbes, such as Salmonella spp., Enterobacteriaceae, E. coli, Pseudomonas aeruginosa, and S. aureus were absent. The HPTLC fingerprint profile of the alcoholic extract of Marham-i-Raal showed the presence of various phytoconstituents. This is the first such characterization report of Marham-i-Raal by the HPTLC method. Hence, the obtained HPTLC fingerprinting data may be helpful in the authentication of this compound formulation. Furthermore, quantification of these active constituents by using biomarkers may be carried out in the future for further exploration. The antimicrobial reports of Marham-i-Raal revealed that it was potentially effective in suppressing the growth of gram-positive bacteria and a fungal strain with variable potency in two different concentrations. Studies have reported that the linalool present in C. camphora is highly effective against C. albicans and S. aureus. Likewise, terpenes found in Acacia catechu have been reported to have antimicrobial activity against S. aureus, Bacillus spp., and C. albicans. The monoterpene compounds found in Vateria indica are also responsible for suppressing the growth of several microorganisms [24]. The antioxidant study has shown that the Unani formulation possesses significant antioxidant effects at higher concentrations, almost similar to standard drugs. The antimicrobial and antioxidant properties of Marham-i-Raal found in the present study have validated the claims of Unani medicine for its use as an antiseptic and healing agent.
Conclusions
The standards developed for the quality control of Marham-i-Raal may be used as standards in future studies. It is also recommended that this pharmacopoeial ointment be topically may to treat wounds and ulcers.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Supervision, Investigation, Data Collection, Data Analysis and Writing- original draft: Athar Parvez Ansari and Mary Shamya Arokiarajan; Writing - review & editing: Wasim Akram Syed Akbar, N. Zaheer Ahmed, and Noman Anwar.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to the Head of the Institute and staff of the Drug Standardization Research Unit, Regional Research Institute of Unani Medicine, Chennai, for providing the facilities required for this research work.
References
Currently, the global propensity for using synthetic drugs has been shifted toward herbal medicines, which is also referred to as the return to nature. Botanicals are considered a rich source of medicinal properties for the management of many ailments of the body. The renaissance of complementary and alternative medical systems, including herbs, has deliberated to raise the demand and trade of herbal medicines, which has turned down their quality, mainly because of deficient quality control parameters and regulations for herbal drugs. The World Health Organization (WHO) has stressed the necessity of checking the quality of medicinal products used in complementary and alternative medical systems by applying modern methodologies and standards. To conquer the deficiencies found in the pharmacopoeial monographs, other important parameters to explore the quality and purity of herbal products may also be used [1]. The Unani system of medicine is one of the oldest systems proven to have productive treatment strategies. In Unani medicine, several systemic and topical dosage forms are used to treat various diseases [2]. Marham-i-Raal, a pharmacopoeial formulation, acts as dāfi‘-i-ta‘affun (antiseptic) and mudammil (healing agent) and is topically used in the treatment of qurūh-i-ātshak (syphilitic ulcers), qurūh al-anf (nasal ulcers), nāsūr (fistula) [3], qurūh (ulcers), jurūh (wounds) [4], etc. This ointment helps remove the slough materials and necrosed tissues from the site of the wound and produces granulation tissues, which help heal wounds. It also augments the collagen concentration along with diminishing fibrous tissues at the site of wounds, which causes increased epithelialization and contraction of the wound [5]. This pharmacopoeial formulation contains four plant-derived drugs, including Kafoor (Cinnamomum camphora [Linn.] Nees & Eberm.), Raal (Vateria indica L.), Kath Safaid (Acacia catechu [Linn. f.] Willd.), and Roghan Kunjad (Sesamum indicum L. oil) and one animal-derived drug, i.e. Mom Safaid (Beeswax) [3]. The standardization of Unani formulations is the current need for their worldwide acceptance [6]. The quality control of Marham-i-Raal has not yet been carried out in Unani medicine. Hence, its standardization was carried out using physicochemical evaluation, heavy metal analysis, microbial load, and high-performance thin-layer chromatography (HPTLC). Moreover, the antimicrobial and antioxidant activities of Marham-i-Raal were also carried out to explore its antimicrobial and antioxidant properties scientifically.
Materials and Methods
Source of data collection
The data were collected from the GMP-certified Pharmacy and Drug Testing Laboratory approved by the State Drug Licensing Authority, Tamil Nadu, i.e. Drug Standardization Research Unit and Microbiology Lab of the Regional Research Institute of Unani Medicine, Chennai, Central Council for Research in Unani Medicine, New Delhi, Ministry of Ayush, Government of India. The study was carried out in January 2023.
Collection of materials and chemicals
The ingredients of Marham-i-Raal were procured from an authorized drug supplier in Chennai, Tamil Nadu, and authenticated by Mageswari, pharmacognosist, Department of Pharmacognosy, Regional Research Institute of Unani Medicine, Chennai. The chemicals and reagents used in the quality control evaluation of Marham-i-Raal were obtained from authorized and registered suppliers in Chennai.
Preparation of Marham-i-Raal
The ointment was prepared as per the composition of ingredients and method described in the National Formulary of Unani Medicine, Part I [3] at the GMP-certified pharmacy of Regional Research Institute of Unani Medicine, Chennai. The botanical ingredients, such as Kafoor (Cinnamomum camphora), Raal (Vateria indica), and Kath Safaid (Acacia catechu), were ground separately by using a pulverizer. The powdered drug was then sieved using the sieve number 100. After sieving the powder, individual ingredients were uniformly mixed using a double-cone blender. The given ratio of Mom Safaid (beeswax) was then added to Roghan Kunjad (Sesamum indicum L. oil) and heated over a low flame. The powdered drug was mixed in the base and well-mixed using a planetary mixer machine. The prepared ointment was stored in a tightly sealed container [3]. The prepared formulation of 10 g contains Kafoor (2 g), Raal (2 g), Kath Safaid (2 g), Mom (2 g), and Roghan Kunjad (2 mL).
Quality control
The quality control parameters, including organoleptic characters, foreign matter, weight loss on drying, pH, ash values, microbial load, aflatoxins, heavy metals, and high-performance thin-layer chromatography, were carried out using standard methods.
Foreign matter
The foreign matters present in the individual ingredients and the formulation were checked in daylight with the naked eye [6].
Loss of weight on drying at 105°C
The weight loss on drying at 105°C is designed to measure the water in a dried sample under specified conditions. An accurately weighed 10 g of Marham-i-Raal was placed without preliminary drying after being accurately weighed in a tarred evaporated dish. This was dried at 105°C for 5 h and weighed. Drying and weighing were repeated at every 1-h interval until the weight was found to be constant. A constant weight was reached when two consecutive weights, after drying for 30 min and cooling for 30 min in a desiccator, showed not more than a 0.1 g difference evaluated based on the guidelines mentioned in the Unani Pharmacopoeia of India, part II, volume land WHO guidelines for quality control of herbal materials [7, 8].
pH of 5% solution
The pH of a 5% aqueous solution of the test drug was determined by following the standard methods described in the Unani pharmacopoeia of India (UPI) [7]. A weight of 5 g of the drug was dissolved in a measured 100 mL of water and filtered, and the pH of the filtrate was checked with a standardized glass electrode.
Ash values
The test sample’s total ash and acid insoluble ash values were also determined following the standard methods described in the UPI and WHO guidelines for quality control of herbal materials [7, 8].
Heavy metal analysis by atomic absorption spectrometer
The quantitative analysis of heavy metals, including cadmium and lead, was accomplished using an atomic absorption spectrometer (Thermo Scientific, iCE 3000 model) using SOLAAR, AA software, version 11.10.
Sample preparation for atomic absorption spectrometer
An amount of 0.5 g of the ointment was transferred into a TFM-PTFE Microwave digestion tube (50 mL), and 6 mL of concentrated HNO3 was added. The sample was then allowed to undergo hot digestion using the Anton Paar Microwave digestion system (Multi-wave GO Plus) from 0°C to 200°C for 30 min. After that, the pressure was released slowly from the TFM digestion tube, and it was allowed to release nitric acid fumes, followed by two portions of 5 mL of deionized water. The digested sample was transferred into a 100 mL volumetric flask using Whatman (No. 1) filter paper. The sample was made into 100 mL by adding deionized water. We have modified and adopted the procedures mentioned in the following references [9] for microwave digestion.
Microbial load
The microbial limit tests for the bacterial count, total fungal count, and specific microorganisms were carried out using the standard methods mentioned in the Unani Pharmacopoeia of India, part II, volume I [7].
High-performance thin layer chromatography
A total of 5 µL of the alcohol extract of the formulation was applied on an aluminum plate pre-coated with silica gel 60 F254 HPTLC plate (5×10 cm, E. Merck) by employing a CAMAG ATS4 sample applicator. The plates were developed up to a distance of 8 cm in the CAMAG twin-through glass chamber (10×10), using 10 mL of the developing system (toluene: Ethyl acetate: Formic acid; 9.0:1.0:0.01) as the mobile phase, previously saturated with solvent for 15 min at 26°C, and dried at room temperature. The mobile phase was chosen after testing different solvent systems of varying polarity. Data was processed using Win CATS planar chromatography manager software and scanned under UV 254 and 366 nm [10].
Antimicrobial activity
Agar disc diffusion method
The antimicrobial activity of Marham-i-Raal was investigated by the disc diffusion method [11]. The test strains, including Staphylococcus aureus (ATCC 27853), Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 700603), Bacillus cereus (Clinical Cochin University), and C. albicans (Clinical Cochin University), were collected from the Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai. The plates were prepared with 20 to 25 mL of Muller-Hinton agar. One loopful of inoculum from each bacterial culture to be tested was spread on agar plates with a sterile swab moistened with the bacterial suspension. Subsequently, the sterile disc was dropped with 50 µL of test extract and allowed to diffuse at room temperature. The plates were incubated at 37°C for 24 h. After 24 h of incubation, the test determines the efficacy of the test drug in terms of the zone of inhibition of the organism [12].
Antioxidant activity
Diphenyl-1-picrylhydrazyl radical scavenging activity
The free radical scavenging capacity of Marham-i-Raal was evaluated based on the method described by Brand-Williams et al. (1995) [13] with slight modifications. An amount of 1.5 mL of 0.1 mM diphenyl-1-picrylhydrazyl solution in methanol was mixed with 1 mL of the Unani formulation solution of different concentrations, i.e. 25, 50, 75, and 100 μg/mL. Blank samples were prepared, and L-ascorbic acid was used as a standard. These tubes were thoroughly mixed and kept in the dark for 30 min, and their absorbance was measured at 517 nm using an ultraviolet-visible spectrophotometer [14]. The percentage of inhibition was calculated using the Equation 1:
1. % of inhibition=A(control)-A(test)×100/A(control)
2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid assay
The free radical scavenging activity of Marham-i-Raal was also determined by the 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) radical cation decolorization assay. The ABTS+ cation radical was produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate (1:1), stored in the dark at room temperature for 12-16 h before use. The ABTS+ solution was then diluted with methanol to obtain an absorbance of 0.700 at 734 nm. After adding 500 μL of the Unani formulation to 3.5 mL of diluted ABTS+solution, the absorbance was measured for 30 min after the initial mixing. An appropriate solvent blank was run in each assay. All the measurements were carried out at least three times. The percent inhibition of absorbance at 734 nm was calculated using the Equation 2:
2. ABTS+Scavenging effect (%)=((AB–AA)/AB)×100,
Where AB is the absorbance of ABTS radical+methanol, and AA is the absorbance of ABTS radical+sample extract/standard. Trolox was used as a standard substance [15].
Statistical analysis
The antimicrobial and antioxidant potentials of Marham-i-Raal were determined in duplicate in all trials. The Mean±SD are used to express the data. The data was statistically examined for standard deviation using MS Excel, and a graph was created using Origin Pro3.
Results
Quality control
Organoleptic characters
The organoleptic evaluation of Marham-i-Raal revealed that it was tasteless, reddish-brown in color, and had a characteristic odor.
Physicochemical standardization
No foreign matters were detected in any ingredients of the formulation or in the preparation itself. The moisture content in Marham-i-Raal was 11.15%. The pH of a 5% aqueous solution of the test drug was determined to be 6.7%. The total ash and acid-insoluble ash values were recorded at 3.96% and 1.55%, respectively.
Heavy metal analysis
The findings showed that lead was not found in Marham-i-Raal, whereas cadmium was 0.0077 mg/L.
Microbial load
The results regarding the microbial load are illustrated in Table 1.

Hight-performance thin layer chromatography fingerprint profile
The HPTLC chromatogram of the alcoholic extract of Marham-i-Raal under UV-254 nm (absorbance mode) has observed 8 spots, amongst which the Rf value of 0.50 contributes to the highest area percentage, which denotes the presence of a prevalent compound of interest in the formulation, while the Rf of 0.72 ranked the second highest area percentage (Figures 1, 2 and 3).
Similarly, the HPTLC chromatogram of the alcoholic extract of Marham-i-Raal under UV-366 nm (florescence mode) has shown 7 spots, amongst which the Rf value of 0.44 was found to be the dominant component, constituting about 38.98% as compared to the other six spots (Figures 1, 4 and 5).
Antimicrobial activity
The results of the antimicrobial activity of Marham-i-Raal are provided in Table 2 and Figure 6.

Antioxidant activity
The 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity of Marham-i-Raal was compared to that of the synthetic antioxidant standard L-ascorbic acid. The maximum inhibition was obtained in a 100 mg/mL concentration, and the percentage of inhibition was 72.84, with an IC50 value of 64.14 mg/mL (Figure 7). The ABTS scavenging activity of the formulation showed promising results at a 100 µg/mL concentration with 69.72% inhibition and an IC50 value of 57.74 mg/mL (Figure 8).
Discussion
Quality assurance is essential to ensure the quality of herbal products in all traditional systems of medicine. In this regard, various modern techniques, including macroscopic, microscopic, physicochemical, phytochemical, chromatographic fingerprinting, biological evaluations, etc. are investigated [16]. The present study was carried out to evaluate different quality control parameters of Marham-i-Raal to ensure its quality since no monograph on this pharmacopoeial has been published in the Unani Pharmacopoeia of India, part II. As per the WHO guidelines published in 2011, herbal products should not be contaminated with insects, moulds, animal excreta, soil, dust, sand, stones, etc. [8]. The study has reported that no foreign matter was detected in any ingredients of the formulation or in the preparation itself, showing the tested drug’s purity. The presence of excess water content in herbal products may cause the growth of microorganisms [8]. The amount of water in herbal materials should be between 10% and 20% to avoid decomposition [17]. The present study has reported the presence of moisture content in Marham-i-Raal to be within an acceptable limit, indicating its good storage, handling, and manufacturing. The pH of the formulation tested in this study was slightly acidic. The pH of the skin surface should be acidic, which plays a pivotal role in maintaining the homeostasis and barrier permeability of the stratum corneum. Thus, it is recommended that topical drugs’ pH be acidic between the range of 4-6 [18]. Inorganic salts in a drug, either naturally or adulterated, may be checked through the ash value parameter [19]. The entire amount of material left over after ignition is called the total ash value, which may contain physiological or non-physiological ash. The amount of silica, including sand and siliceous earth, is measured through the acid-insoluble ash value parameter [20]. In the present study, the total ash and acid-insoluble ash values were within the normal range. The plants are considered to have a greater affinity to accumulate heavy metals [21]. Lead, cadmium, arsenic, and mercury are regarded as the most toxic heavy metals, which may cause cancer in humans [22]. Lead poisoning leads to high blood pressure and damage to the brain and kidneys.
Cadmium is harmful to the respiratory system and leads to cardiac as well as kidney problems [23]. The heavy metal analysis report of Marham-i-Raal revealed that lead was not detected in it, whereas cadmium was within the permissible limit [7]. The total microbial count and fungal count were found within the permissible limits in the test sample. Specific microbes, such as Salmonella spp., Enterobacteriaceae, E. coli, Pseudomonas aeruginosa, and S. aureus were absent. The HPTLC fingerprint profile of the alcoholic extract of Marham-i-Raal showed the presence of various phytoconstituents. This is the first such characterization report of Marham-i-Raal by the HPTLC method. Hence, the obtained HPTLC fingerprinting data may be helpful in the authentication of this compound formulation. Furthermore, quantification of these active constituents by using biomarkers may be carried out in the future for further exploration. The antimicrobial reports of Marham-i-Raal revealed that it was potentially effective in suppressing the growth of gram-positive bacteria and a fungal strain with variable potency in two different concentrations. Studies have reported that the linalool present in C. camphora is highly effective against C. albicans and S. aureus. Likewise, terpenes found in Acacia catechu have been reported to have antimicrobial activity against S. aureus, Bacillus spp., and C. albicans. The monoterpene compounds found in Vateria indica are also responsible for suppressing the growth of several microorganisms [24]. The antioxidant study has shown that the Unani formulation possesses significant antioxidant effects at higher concentrations, almost similar to standard drugs. The antimicrobial and antioxidant properties of Marham-i-Raal found in the present study have validated the claims of Unani medicine for its use as an antiseptic and healing agent.
Conclusions
The standards developed for the quality control of Marham-i-Raal may be used as standards in future studies. It is also recommended that this pharmacopoeial ointment be topically may to treat wounds and ulcers.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Supervision, Investigation, Data Collection, Data Analysis and Writing- original draft: Athar Parvez Ansari and Mary Shamya Arokiarajan; Writing - review & editing: Wasim Akram Syed Akbar, N. Zaheer Ahmed, and Noman Anwar.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to the Head of the Institute and staff of the Drug Standardization Research Unit, Regional Research Institute of Unani Medicine, Chennai, for providing the facilities required for this research work.
References
- Rasheed NM, Gupta VC. Standardization of a compound unani herbal formulation "qurs-e-luk" with modern techniques. Pharmacognosy Res. 2010; 2(4):237-41. [DOI:10.4103/0974-8490.69115] [PMID] [PMCID]
- Sultana A, Joonus AFM, Rahman K. Effect of marham-i-raal on episiotomy wound healing: A single-arm pre-and post-treatment study. Cellmed. 2021; 11(4):17.1-4. [DOI:10.5667/CellMed.2021.0017]
- Anonymous. National formulary of unani medicine. New Delhi: Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH), Ministry of Health & Family Welfare, Government of India; 2006. [Link]
- Kabiruddin HM. Bayaz-e-kabeer. Hyderabad: Hikmat Book Depo, Hyderabad; 2004. [Link]
- Ahmad W, Alam SS, Aquil Z. Herbo-medicinal formulation; marham-e-raal: A potent ointment for acute and chronic wounds-A review. Turk J Plast Surg. 2019; 27(2):77-83. [DOI:10.4103/tjps.tjps_48_18]
- Ali W, Shaikh H, Ansari A, Khanam S. Standardization of unani antidiabetic tablet - qurse tabasheer. Pharmacognosy Res. 2016; 8(2):147-52. [DOI:10.4103/0974-8490.175611] [PMID] [PMCID]
- Anonymous. The unani pharmacopoeia of India. New Delhi: Pharmacopoeia Commission for Indian Medicine & Homoeopathy Ghaziabad; 2009. [Link]
- World Health Organization (WHO). Quality control methods for herbal materials. Geneva: World Health Organization; 2011. [Link]
- Turek A, Wieczorek K, Wolf WM. Digestion procedure and determination of heavy metals in sewage sludge—An analytical problem. Sustainability. 2019; 11(6):1753. [DOI:10.3390/su11061753]
- Vadivel V, Ravichandran N, Rajalakshmi P, Brindha P, Gopal A, Kumaravelu C. Microscopic, phytochemical, HPTLC, GC-MS and NIRS methods to differentiate herbal adulterants: Pepper and papaya seeds. J Herbal Med. 2018; 1(11):36-45. [DOI:10.1016/j.hermed.2018.01.004]
- Genskowsky E, Puente LA, Perez-Alvarez JA, Fernandez-Lopez J, Munoz LA, Viuda-Martos M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (aristotelia chilensis). LWT Food Sci Technol. 2015; 64(2):1057-62. [DOI:10.1016/j.lwt.2015.07.026]
- Al-Zahrani SH. Bacteria isolated from contact and non-contact lens and antibiotic susceptibility patterns of isolated Pseudomonas aeruginosa. Afr J Microbiol Res. 2012; 6(47):7350-6. [DOI:10.5897/AJMR12.1134]
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995; 28(1):25-30. [DOI:10.1016/S0023-6438(95)80008-5]
- Srinivasan R, Chandrasekar MJ, Nanjan MJ, Suresh B. Antioxidant activity of Caesalpinia digyna root. J Ethnopharmacol. 2007; 113(2):284-91. [DOI:10.1016/j.jep.2007.06.006] [PMID]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005; 53(10):4290-302. [DOI:10.1021/jf0502698] [PMID]
- Shailajan S, Menon SN, Tiwari BR, Singh AS. Standardization of shadbindu taila: An ayurvedic oil based medicine. Ayu. 2013; 34(1):103-7. [DOI:10.4103/0974-8520.115442] [PMID] [PMCID]
- Sumbul S, Ahmad MA, Asif M, Akhtar M, Saud I. Physicochemical and phytochemical standardization of berries of myrtus communis linn. J Pharm Bioallied Sci. 2012; 4(4):322-6. [DOI:10.4103/0975-7406.103266] [PMID] [PMCID]
- Lukic M, Pantelic I, Savic SD. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics. 2021; 8(3):69. [DOI:10.3390/cosmetics8030069]
- Islam MN, Rub MA, Islam MR, Goni MA, Rana S, Kumar D, et al. Physico-chemical study of the effects of electrolytes and hydrotropes on the clouding development of TX-100 and ceftriaxone sodium drug mixture. J Mol Liq. 2023; 379:121601. [DOI:10.1016/j.molliq.2023.121601]
- Evans WC. Trease and evans’ pharmacognosy. Edinburgh: Elsevier Health Sciences; 2009. [Link]
- Li C, Yang G, Liu Z, Cai J. Overview of phytoremediation technology for heavy metal contaminated soil. E3S Web Conf. 2022; 350:01006. [DOI:10.1051/e3sconf/202235001006]
- Teschke R. Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int J Mol Sci. 2022; 23(20):12213. [DOI:10.3390/ijms232012213] [PMID] [PMCID]
- Mustapha B, Kubmarawa D, Shagal MH, Hayatudeen A. Heavy metal profiles of medicinal plants found within the vicinity of quarry site in Demsa, Adamawa State, Nigeria. Br J Appl Sci Technol. 2016; 13(1):1-6. [DOI:10.9734/BJAST/2016/21520]
- Ahmad W, Alam SS, Aquil Z. Herbo-medicinal formulation; Marham-i-Raal: A potent ointment for acute and chronic wounds-A review. Turk J Plast Surg. 2019; 27(2):77-83. [Link]
Type of Study: Original Research |
Subject:
Traditional Medicine
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |