Volume 10, Issue 1 (2024)
Pharm Biomed Res 2024, 10(1): 73-78 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moghimi M, Nekoukar Z, Khosravi N. Acute Efavirenz Intoxication in a 16-year-old HIV Negative Girl: A Case Report and Literature Review. Pharm Biomed Res 2024; 10 (1) :73-78
URL: http://pbr.mazums.ac.ir/article-1-549-en.html
URL: http://pbr.mazums.ac.ir/article-1-549-en.html
Acute Efavirenz Intoxication in a 16-year-old HIV Negative Girl: A Case Report and Literature Review
1- Department of Clinical Pharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Emergency Medicine, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Emergency Medicine, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 461 kb]
(549 Downloads)
| Abstract (HTML) (1340 Views)
Full-Text: (400 Views)
Introduction
Intentional drug overdose (IDO) is one of the common causes of emergency room referrals in the adolescent population. While women are more likely to proceed with suicidal attempts by IDO, drug abuse poisonings are responsible for these cases in men [1]. According to one research by Meghan Gilley et al., acetaminophen, antidepressants, and non-steroidal anti-inflammatory drugs were the most common medications used for IDO [2]. Reports from the north of Iran indicate that opioids (especially tramadol) and benzodiazepines are the leading drugs involved in IDO [3]; however, IDO does not always happen with these agents, and uncommon prescribed drugs are sometimes responsible as well. Efavirenz (EFV), a non-nucleoside reverse transcriptase inhibitor used for the treatment of acquired immunodeficiency syndrome is available in a single form and in combination with other anti-HIV drugs [4]. The recommended dose is 600 mg daily and should be used in a combination regimen with a protease inhibitor or nucleoside reverse transcriptase inhibitor. The common combination formulations are EFV/emtricitabine/tenofovir (EFV/FTC/TDF) and EFV/lamivudine/tenofovir (EFV/3TC/TDF) [5]. EFV in patients receiving combinations with antiretroviral therapy can endanger several body organs, including the nervous system (dizziness, depression, insomnia, anxiety, pain, headache, drowsiness, and nervousness), skin (mild to moderate maculopapular rash), gastrointestinal (diarrhea, nausea, vomiting, increased gamma-glutamyltransferase, and amylase), endocrine and metabolic (increased serum cholesterol, high-density lipoprotein cholesterol, and triglycerides, hyperglycemia), hematologic (neutropenia), and hepatic systems (increased aspartate aminotransferase and alanine aminotransferase) [6]. In Iran, EFV is not a widely available drug and is only supplied in certain drugstores. To the best of our knowledge, this study presents the first case of EFV overdose in a 16-year-old HIV-negative female.
Case Presentation
A 16-year-old girl was brought to the emergency department 3.5 h after an intentional ingestion of 20 EFV tablets (600 mg) for a suicidal attempt. She was alert and her chief complaints were headaches, dizziness, nausea, and bloating. On arrival, vital signs were as follows: Blood pressure=100/60 mmHg, pulse rate=98 beats/min, temperature=37.2°C, respiratory rate=18/min, and O2 saturation=98% . The abdomen had no tenderness. The physical examination showed no abnormal points. This HIV-negative girl had no underlying disease and no history of drug use. In the emergency room, charcoal 1 g/kg and sorbitol powder 1 g/kg were ordered orally for her, and serum NaCl 0.9% 500 mL and ampule ondansetron were administered via intravenous line infusion. Her blood was checked for blood sugar, creatinine, and blood urea. The results are presented in Table 1.
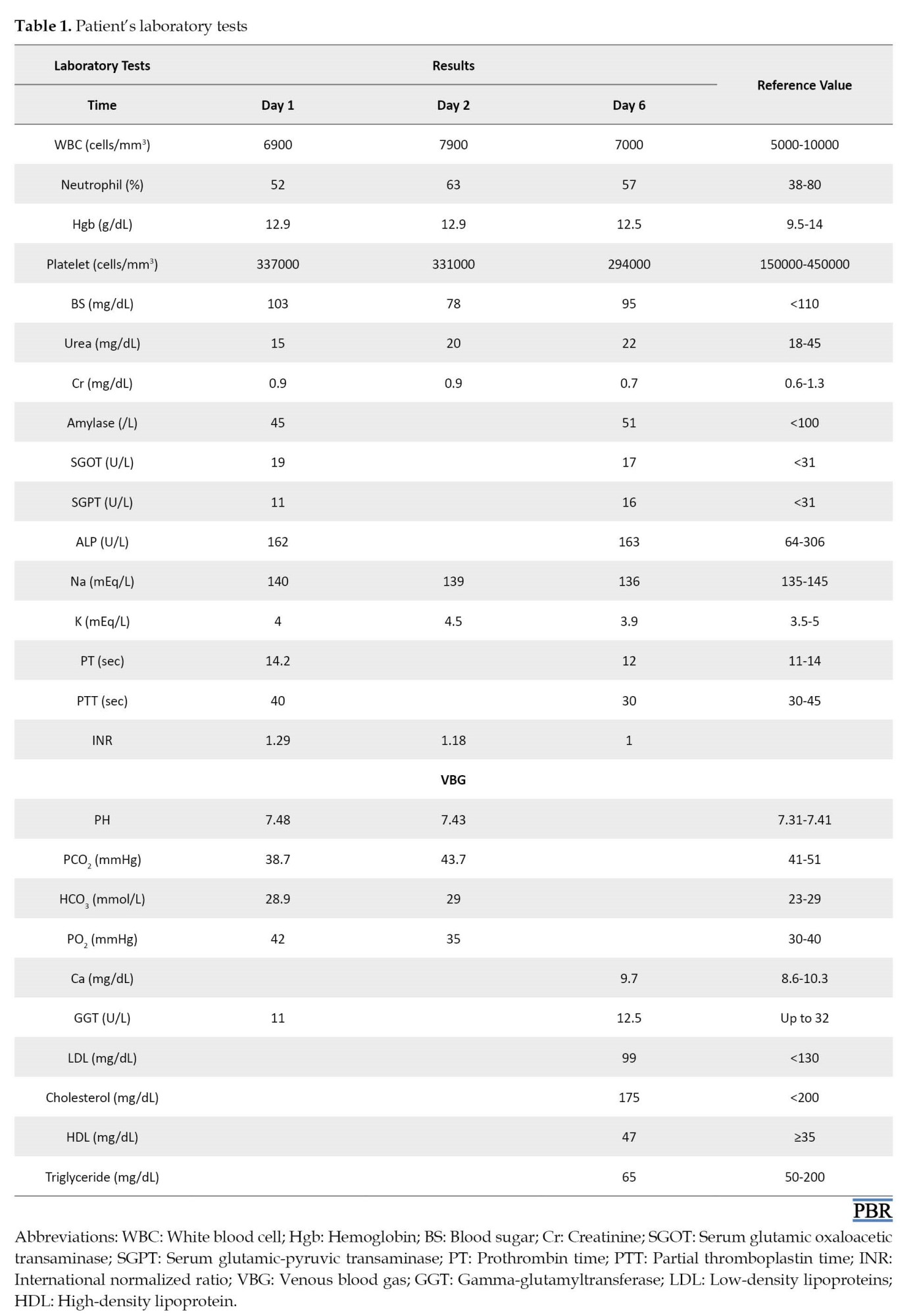
Complete blood counts were also ordered and she was admitted to the clinical toxicology ward. On the first day, she was hydrated with 2500 mL NaCl 0.9% every 24 h. Famotidine tablet 40 mg daily, ampule of ondansetron 4 mg PRN, blood sugar checking every 6 h, cardiac monitoring, pulse oximetry, and venous blood gas were also ordered. Acetaminophen tablet 500 mg was prescribed according to her headache.
Psychiatric consultation was requested based on the potential acute psychiatric side effects of EFV and also her suicidal ideation. Nausea and bloating were gradually resolved on the same day.
On the second day of hospitalization, she stated that the pain from insomnia last night and dizziness remained. Because EFV can increase gamma-glutamyltransferase and amylase, they also were checked (Table 1). On the next day, the patient had no headache, dizziness, or gastrointestinal disturbances. She was discharged from the hospital and advised to refer to the toxicological clinic with the prescribed laboratory tests. After 6 days of EFV overdose, the patient returned to the clinic. Physical examinations, including neurological and psychiatric, were normal. Also, all of the requested follow-up laboratory tests were in the normal range (Table 1).
Discussion
This is the first case report of acute intoxication with 12 g EFV alone in a young female. EFV, a non-nucleoside reverse transcriptase inhibitor, is used to treat HIV Type 1 infection or in combination with other nucleoside reverse transcriptase inhibitors or protease inhibitors. Our case intentionally consumed 20 tablets of EFV 600 mg as a single product, while she had no history of HIV infection. Rashmee Patil et al. reported acute liver failure in a 24-year-old Hispanic HIV-infected male who started the treatment regimen consisting of EFV/FTC/TDF two months before hospitalization. He suffered from nausea and right upper quadrant pain with a history of acute alcohol drinking a few days before admission. The authors derived that acute liver failure was associated with EFV/FTC/TDF other than alcohol and the case was recovered after stopping the medication [7]. Conversely, our case did not have previous exposure to EFV, and also no history of alcohol consumption or any other hepatotoxicity risk factors that predisposed her to liver damage. In another case series reported by Innocent Lule Segamwenge et al., four HIV-infected patients presented with remarkably increased liver aminotransferases and bilirubin led to the diagnosis of acute liver failure. All the cases were treated with the same EFV-based regimen, EFV/3TC/TDF, and recovered after discontinuation of it [8]. EFV-induced hepatotoxicity is known to be associated with chronic use with standard doses, as shown in the mentioned cases. As expected, liver function tests were normal in our case regarding the acute consumption, and also gastric irrigation in the emergency department. On the other hand, it is unknown whether a combination regimen with EFV has more likelihood of hepatotoxicity or not. However, it is recommended to monitor LFT routinely in patients receiving antiretroviral therapy [9].
Another serious concern of EFV consumption is neurotoxicity. EFV-related neurotoxicity is expected to happen within days of starting; however, its symptoms may present even after a single dose and are usually resolved after a month of therapy despite ongoing treatment [10]. One of the neuronal toxicity signs of EFV, ataxia, is related to the super-therapeutic concentration both in plasma and cerebrospinal fluid. Reports showed that the signs were improved after the discontinuation of EFV in both children and adults [11, 12, 13]. A case report of acute unintentional intoxication with 3 g of EFV in a 12-year-old HIV-negative boy showed neurotoxicity, including blurring vision, lower limb weakness, trouble walking, and tremor in upper limbs, which was gradually resolved [14]. Although the amount of EFV ingested was significantly lower in this case compared to our case, he suffered from more central nervous system toxicity which may be explained by a delay (5 days) from intoxication to admission and no prehospital treatments. In the other reported case by Talia Puzantian, a 47-year-old HIV-infected man revealed severe depression and suicidal ideation in the first few weeks of EFV addition to his treatment regimen which led to admission to the psychiatric ward and antidepressant therapy [15]. Our patient complained only of headache and dizziness which were resolved by supportive therapy. The mild signs and symptoms with no serious complications after acute ingestion of 16 g EFV may be related to high protein binding (>99%) and the small distribution volume of this drug. After 3 days and on the 6 and 30 days of follow-up she did not have any problem. Also, she did not show psychiatric symptoms on follow-up.
Conclusion
IDO is a common cause of suicidal attempts in adolescents. Although EFV is not a widely available drug, this study presented a 16-year-old non-HIV-infected girl with acute EFV intoxication. The neurotoxicity in our patient, including headache and dizziness, as the early-onset and dose-related EFV adverse effects, were successfully managed according to the early emergency department referral and gastric lavage. Another serious EFV-related adverse effect, hepatotoxicity, was not seen possibly due to the acute overdose. Finally, serious side effects of EFV were observed in HIV-infected patients who took this drug for a long time in combination with other antiretroviral drugs. Using a relatively high dose of EFV in an HIV-negative person does not expect such side effects. However, more studies are needed.
This study faced some limitations in measuring the EFV plasma concentration in which too high a concentration of this drug may increase the risk of toxicity, including neurologic toxicity. Also, measuring the drug plasma concentration could help to follow up on the patient’s status.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patient’s parents for the publication of this report. This study was conducted according to the Declaration of Helsinki principles. Also, the case report guidelines and methodology were followed in this study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Supervision: Navid Khosravi; Investigation, Writing original draft, and Writing review & editing: All authors; Data collection: All authors; Data analysis: Minoo Moghimi and Zahra Nekoukar.
Conflict of interest
The authors declared no conflict of interest.
References
Intentional drug overdose (IDO) is one of the common causes of emergency room referrals in the adolescent population. While women are more likely to proceed with suicidal attempts by IDO, drug abuse poisonings are responsible for these cases in men [1]. According to one research by Meghan Gilley et al., acetaminophen, antidepressants, and non-steroidal anti-inflammatory drugs were the most common medications used for IDO [2]. Reports from the north of Iran indicate that opioids (especially tramadol) and benzodiazepines are the leading drugs involved in IDO [3]; however, IDO does not always happen with these agents, and uncommon prescribed drugs are sometimes responsible as well. Efavirenz (EFV), a non-nucleoside reverse transcriptase inhibitor used for the treatment of acquired immunodeficiency syndrome is available in a single form and in combination with other anti-HIV drugs [4]. The recommended dose is 600 mg daily and should be used in a combination regimen with a protease inhibitor or nucleoside reverse transcriptase inhibitor. The common combination formulations are EFV/emtricitabine/tenofovir (EFV/FTC/TDF) and EFV/lamivudine/tenofovir (EFV/3TC/TDF) [5]. EFV in patients receiving combinations with antiretroviral therapy can endanger several body organs, including the nervous system (dizziness, depression, insomnia, anxiety, pain, headache, drowsiness, and nervousness), skin (mild to moderate maculopapular rash), gastrointestinal (diarrhea, nausea, vomiting, increased gamma-glutamyltransferase, and amylase), endocrine and metabolic (increased serum cholesterol, high-density lipoprotein cholesterol, and triglycerides, hyperglycemia), hematologic (neutropenia), and hepatic systems (increased aspartate aminotransferase and alanine aminotransferase) [6]. In Iran, EFV is not a widely available drug and is only supplied in certain drugstores. To the best of our knowledge, this study presents the first case of EFV overdose in a 16-year-old HIV-negative female.
Case Presentation
A 16-year-old girl was brought to the emergency department 3.5 h after an intentional ingestion of 20 EFV tablets (600 mg) for a suicidal attempt. She was alert and her chief complaints were headaches, dizziness, nausea, and bloating. On arrival, vital signs were as follows: Blood pressure=100/60 mmHg, pulse rate=98 beats/min, temperature=37.2°C, respiratory rate=18/min, and O2 saturation=98% . The abdomen had no tenderness. The physical examination showed no abnormal points. This HIV-negative girl had no underlying disease and no history of drug use. In the emergency room, charcoal 1 g/kg and sorbitol powder 1 g/kg were ordered orally for her, and serum NaCl 0.9% 500 mL and ampule ondansetron were administered via intravenous line infusion. Her blood was checked for blood sugar, creatinine, and blood urea. The results are presented in Table 1.

Complete blood counts were also ordered and she was admitted to the clinical toxicology ward. On the first day, she was hydrated with 2500 mL NaCl 0.9% every 24 h. Famotidine tablet 40 mg daily, ampule of ondansetron 4 mg PRN, blood sugar checking every 6 h, cardiac monitoring, pulse oximetry, and venous blood gas were also ordered. Acetaminophen tablet 500 mg was prescribed according to her headache.
Psychiatric consultation was requested based on the potential acute psychiatric side effects of EFV and also her suicidal ideation. Nausea and bloating were gradually resolved on the same day.
On the second day of hospitalization, she stated that the pain from insomnia last night and dizziness remained. Because EFV can increase gamma-glutamyltransferase and amylase, they also were checked (Table 1). On the next day, the patient had no headache, dizziness, or gastrointestinal disturbances. She was discharged from the hospital and advised to refer to the toxicological clinic with the prescribed laboratory tests. After 6 days of EFV overdose, the patient returned to the clinic. Physical examinations, including neurological and psychiatric, were normal. Also, all of the requested follow-up laboratory tests were in the normal range (Table 1).
Discussion
This is the first case report of acute intoxication with 12 g EFV alone in a young female. EFV, a non-nucleoside reverse transcriptase inhibitor, is used to treat HIV Type 1 infection or in combination with other nucleoside reverse transcriptase inhibitors or protease inhibitors. Our case intentionally consumed 20 tablets of EFV 600 mg as a single product, while she had no history of HIV infection. Rashmee Patil et al. reported acute liver failure in a 24-year-old Hispanic HIV-infected male who started the treatment regimen consisting of EFV/FTC/TDF two months before hospitalization. He suffered from nausea and right upper quadrant pain with a history of acute alcohol drinking a few days before admission. The authors derived that acute liver failure was associated with EFV/FTC/TDF other than alcohol and the case was recovered after stopping the medication [7]. Conversely, our case did not have previous exposure to EFV, and also no history of alcohol consumption or any other hepatotoxicity risk factors that predisposed her to liver damage. In another case series reported by Innocent Lule Segamwenge et al., four HIV-infected patients presented with remarkably increased liver aminotransferases and bilirubin led to the diagnosis of acute liver failure. All the cases were treated with the same EFV-based regimen, EFV/3TC/TDF, and recovered after discontinuation of it [8]. EFV-induced hepatotoxicity is known to be associated with chronic use with standard doses, as shown in the mentioned cases. As expected, liver function tests were normal in our case regarding the acute consumption, and also gastric irrigation in the emergency department. On the other hand, it is unknown whether a combination regimen with EFV has more likelihood of hepatotoxicity or not. However, it is recommended to monitor LFT routinely in patients receiving antiretroviral therapy [9].
Another serious concern of EFV consumption is neurotoxicity. EFV-related neurotoxicity is expected to happen within days of starting; however, its symptoms may present even after a single dose and are usually resolved after a month of therapy despite ongoing treatment [10]. One of the neuronal toxicity signs of EFV, ataxia, is related to the super-therapeutic concentration both in plasma and cerebrospinal fluid. Reports showed that the signs were improved after the discontinuation of EFV in both children and adults [11, 12, 13]. A case report of acute unintentional intoxication with 3 g of EFV in a 12-year-old HIV-negative boy showed neurotoxicity, including blurring vision, lower limb weakness, trouble walking, and tremor in upper limbs, which was gradually resolved [14]. Although the amount of EFV ingested was significantly lower in this case compared to our case, he suffered from more central nervous system toxicity which may be explained by a delay (5 days) from intoxication to admission and no prehospital treatments. In the other reported case by Talia Puzantian, a 47-year-old HIV-infected man revealed severe depression and suicidal ideation in the first few weeks of EFV addition to his treatment regimen which led to admission to the psychiatric ward and antidepressant therapy [15]. Our patient complained only of headache and dizziness which were resolved by supportive therapy. The mild signs and symptoms with no serious complications after acute ingestion of 16 g EFV may be related to high protein binding (>99%) and the small distribution volume of this drug. After 3 days and on the 6 and 30 days of follow-up she did not have any problem. Also, she did not show psychiatric symptoms on follow-up.
Conclusion
IDO is a common cause of suicidal attempts in adolescents. Although EFV is not a widely available drug, this study presented a 16-year-old non-HIV-infected girl with acute EFV intoxication. The neurotoxicity in our patient, including headache and dizziness, as the early-onset and dose-related EFV adverse effects, were successfully managed according to the early emergency department referral and gastric lavage. Another serious EFV-related adverse effect, hepatotoxicity, was not seen possibly due to the acute overdose. Finally, serious side effects of EFV were observed in HIV-infected patients who took this drug for a long time in combination with other antiretroviral drugs. Using a relatively high dose of EFV in an HIV-negative person does not expect such side effects. However, more studies are needed.
This study faced some limitations in measuring the EFV plasma concentration in which too high a concentration of this drug may increase the risk of toxicity, including neurologic toxicity. Also, measuring the drug plasma concentration could help to follow up on the patient’s status.
Ethical Considerations
Compliance with ethical guidelines
Written informed consent was obtained from the patient’s parents for the publication of this report. This study was conducted according to the Declaration of Helsinki principles. Also, the case report guidelines and methodology were followed in this study.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Supervision: Navid Khosravi; Investigation, Writing original draft, and Writing review & editing: All authors; Data collection: All authors; Data analysis: Minoo Moghimi and Zahra Nekoukar.
Conflict of interest
The authors declared no conflict of interest.
References
- Pringle K, Caupp S, Shi J, Wheeler KK, Spiller HA, Casavant MJ, et al. Analysis of intentional drug poisonings using Ohio poison control center data, 2002-2014. Clin Toxicol. 2017; 55(7):652-8. [DOI:10.1080/15563650.2017.1309050] [PMID]
- Gilley M, Sivilotti MLA, Juurlink DN, Macdonald E, Yao Z, Finkelstein Y. Trends of intentional drug overdose among youth: A population-based cohort study. Clin Toxicol. 2020; 58(7):711-5. [DOI:10.1080/15563650.2019.1687900] [PMID]
- Shokrzadeh M, Hoseinpoor R, Jafari D, Delaram A, Pouyan Sadr A, Deylami M, et al. A ten-year study of drug poisoning cases admitted to the intensive care unit of 5 Azar Hospital in Gorgan, Iran. J Clin Basic Res. 2019; 3(2):8-13. [DOI:10.29252/jcbr.3.2.8]
- National Center for Biotechnology Information. PubChem: Compound summary: Efavirenz [Internet]. 2022 [Updated 2022 January 10]. Available from: [Link]
- Drugs.com. Efavirenz (monograph) [Internet]. 2021 [Updated 2023 May 5] Available from: [Link]
- Fletcher CV, Bartlett JG, Sax PE, Mitty J. Overview of antiretroviral agents used to treat HIV [Internet]. 2021 [Updated 2023 February 2]. Available from: [Link]
- Patil R, Ona MA, Papafragkakis H, Carey J, Moshenyat Y, Alhaddad A, et al. Acute liver toxicity due to efavirenz/emtricitabine/tenofovir. Case Reports Hepatol. 2015; 2015:280353. [DOI:10.1155/2015/280353] [PMID] [PMCID]
- Segamwenge IL, Bernard MK. Acute liver failure among patients on efavirenz-based antiretroviral therapy. Case Reports Hepatol. 2018; 2018:1270716. [DOI:10.1155/2018/1270716] [PMID] [PMCID]
- Fink DL, Bloch E. Liver transplantation for acute liver failure due to efavirenz hepatotoxicity: The importance of routine monitoring. Int J STD AIDS. 2013; 24(10):831-3. [DOI:10.1177/0956462413483720] [PMID]
- Decloedt EH, Maartens G. Neuronal toxicity of efavirenz: A systematic review. Expert Opin Drug Saf. 2013 ; 12(6):841-6. [DOI:10.1517/14740338.2013.823396] [PMID]
- Brasher WP, Bvumbwe M, Kazembe PN. Infection or drug toxicity? Acute ataxia and encephalopathy after uncomplicated falciparum malaria and efavirenz dose adjustment. Malawi Med J. 2020; 32(4):229-31. [DOI:10.4314/mmj.v32i4.9] [PMID] [PMCID]
- Variava E, Sigauke FR, Norman J, Rakgokong M, Muchichwa P, Mochan A, et al. Brief report: Late efavirenz-induced ataxia and encephalopathy: A case series. J Acquir Immune Defic Syndr. 2017; 75(5):577-9. [DOI:10.1097/QAI.0000000000001451] [PMID] [PMCID]
- Hauptfleisch MP, Moore DP, Rodda JL. Efavirenz as a cause of ataxia in children. S Afr Med J. 2015; 105(11):897-8. [DOI:10.7196/SAMJ.2015.v105i11.9451] [PMID]
- Nazziwa R, Sekadde M, Kanyike F, Wobudeya E, Nabukeera-Barungi N. Efavirenz poisoning in a 12-year-old HIV negative African boy. Pan Afr Med J. 2012; 12(1):1-3. [Link]
- Puzantian T. Central nervous system adverse effects with efavirenz: Case report and review. Pharmacotherapy. 2002; 22(7):930-3. [DOI:10.1592/phco.22.11.930.33624] [PMID]
Type of Study: case report |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









