Volume 10, Issue 2 (2024)
Pharm Biomed Res 2024, 10(2): 103-112 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi S, Beheshti F, Mohammadipour A. Effects of Tanacetum parthenium on Chromatin Quality, Sperm Parameters and Oxidative Stress in Mice. Pharm Biomed Res 2024; 10 (2) :103-112
URL: http://pbr.mazums.ac.ir/article-1-545-en.html
URL: http://pbr.mazums.ac.ir/article-1-545-en.html
1- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Neuroscience Research Center, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran.
2- Neuroscience Research Center, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran.
Full-Text [PDF 1101 kb]
(404 Downloads)
| Abstract (HTML) (1238 Views)
Full-Text: (404 Views)
Introduction
Infertility is one of the major problems in the countries of the world. According to the statistics, 15% to 18% of couples suffer from infertility worldwide [1, 2]. Nearly half of these infertility problems are related to male factors. Many factors, such as insecticides, obesity, drugs, toxic substances, and infection, lead to infertility in men [3]. The use of medicinal plants to treat infertility has a long history in different cultures, and it has increased in recent years because of lower side effects compared to chemical drugs [4]. The administration of medicinal herbs such as ginseng, ginger, Nigella sativa and saffron increases male fertility and improves semen parameters.
On the other hand, other natural products such as Ruta graveolens, Mentha pulegium and Achillea millefolium significantly reduce fertility and sperm quality [5, 6, 7]. Tanacetum parthenium is a medicinal plant from the Asteraceae family and the Asterales order found in America, Asia, Europe and Africa. In Iran, it is mostly grown on the river banks and forest areas, as well as in the cities of Kurdistan, Mazandaran, Gillan, Golestan, Khorasan, and Fars provinces. This plant has various compounds, including sesquiterpene lactone, especially parthenolide, germacronolide, guaianolide, flavonoid, phytoestrogen, phytosterol and camphor [8-11]. T. parthenium is widely used in the pharmaceutical, cosmetics, hygiene, and agriculture industries. Studies indicate that this plant has anti-rheumatic, anti-flatulent, antiseptic, anti-fungal, anti-parasitic, anti-inflammation and cytotoxic effects. It is used to treat asthma, indigestion, dizziness, migraine, and skin disease [12-15]. Considering that studies report that phytoestrogens and phytosterols of the plants can lead to an adverse effect on the male reproductive system, and based on our search, few documents exist on the administration of Tanacetum extract on testicular histology and sperm chromatin quality in the literature. Therefore, the purpose of research was to evaluate the effects of Tanacetum extract on sperm parameters, testicular tissue, sperm DNA integrity and free radical damage in adult male mice.
Materials and Methods
This experimental research was conducted on 18 adult BALB/c male mice (2-3 months). Mice were randomly divided into 3 groups: Control, TP1 and TP2. The control group received normal saline. TP term refers to T. parthenium. The TP1 group received 50 mg/kg of Tanacetum extract, and the TP2 group was gavaged 100 mg/kg of Tanacetum using a gavage needle once a day for 2 weeks. These doses were selected based on Soleimani’s study [16].
Tanacetum extraction
T. parthenium was collected by a botanist from Zoshk village and confirmed by the Herbarium of the Ferdowsi University of Mashhad (Code: 17895-FUMH). First, 100 g of the powder was mixed with a solvent (50% ethanol+50% distilled water). After shaking, it was passed through a Whatman filter paper (England) and after solvent removal, it was dissolved in normal saline [17].
Sperm analysis
Sperm analysis was performed according to the World Health Organization’s (WHO) guidelines for sperm analysis. The crushed epididymis was placed on a plate containing saline in a CO2 incubator. Then, using a Neubauer hemocytometer, the sperm count and morphology were evaluated [18, 19].
Histology examination
After the testis was isolated, it was placed in 10% formalin fixative (Merck, Germany). Following specimen processing, the tissue was cut into 5 µ slides. After staining, the slides were examined by an expert pathologist [20, 21].
Sperm chromatin quality
Smear sperms were fixed with methanol (Sigman, Germany) and stained with acridine orange solution (Merck, Germany). Then, they were examined with a fluorescent microscope. Red sperm heads were counted as damaged DNA, yellow sperm heads were considered intermediate damage of DNA and green sperm heads indicated healthy DNA [22].
Measurement of malondialdehyde (MDA) level
First, the thiobarbituric acid (TBA) reagent (Merck, Germany) was prepared, and then the testicular tissue was homogenized. The homogenous tissue was heated with TBA reagent, trichloroacetic acid and hydrogen chloride (Merck, Germany) at boiling temperature for 50 minutes. After centrifugation, the absorption was read at 535 nm. The concentration of MDA was expressed in nmol/g [23].
Measurement of thiol level
Tris EDTA buffer was added to homogenous tissue, and the absorbance was read. The absorption of the dithionitrobenzoic acid solution to the sample was measured. Also, the absorption of the dithionitrobenzoic acid solution was considered blank. Thiol concentration was calculated as µmol/g at the wavelength of 412 nm [24].
Measurement of catalase enzyme level
First, hydrogen peroxide (Merck, Germany) was added to the phosphate buffer (solution 2, 3). Homogenous testicular tissue was diluted 1:10 and was added to solution 3. After two minutes, the absorbance changes were read at a wavelength of 240 nm. Enzyme concentration was expressed in U/g [24].
Measurement of superoxide dismutase (SOD) enzyme level
To prepare the tissue homogenate, the testicular tissue was first weighed and then homogenized with 10 mM phosphate buffer saline with pH=4.7. Next, 65 µL of phosphate buffer saline, 30 µL of 25.1 mmol MTT and 75 µL of pyrogallol (Merck, Germany) were mixed with 10 µL of tissue homogenate and incubated for a minute at room temperature. Afterward, 75 µL of dimethyl sulfide oxide was added, and the optical absorption of the obtained solution was read at a wavelength of 570 nm using an ELISA device. It was expressed in U/g [25].
Statistical analysis
Data analysis was done using analysis of variance (ANOVA) and Tukey’s post hoc test in SPSS software, version 16.
Results
Sperm analysis
Figures 1 and 2 display the sperm analysis results in different experimental groups. The mean sperm number was 4.8±0.22 million/mL in the control group, while it was remarkably reduced in the TP1 and TP2 groups (4±0.21 vs 3.9±0.20 million/mL). The sperm count in the TP1 group (P=0.006) and TP2 group (P=0.004) indicated a significant decrease versus the control group. The abnormal morphology of sperm was 11% in the control group, 15% in the TP1 group, and 19% in the TP2 group. A significant decrease was observed in the percentage of normal sperm morphology in the TP1 group (P=0.006) and TP2 group (P=0.006) compared to the control group.
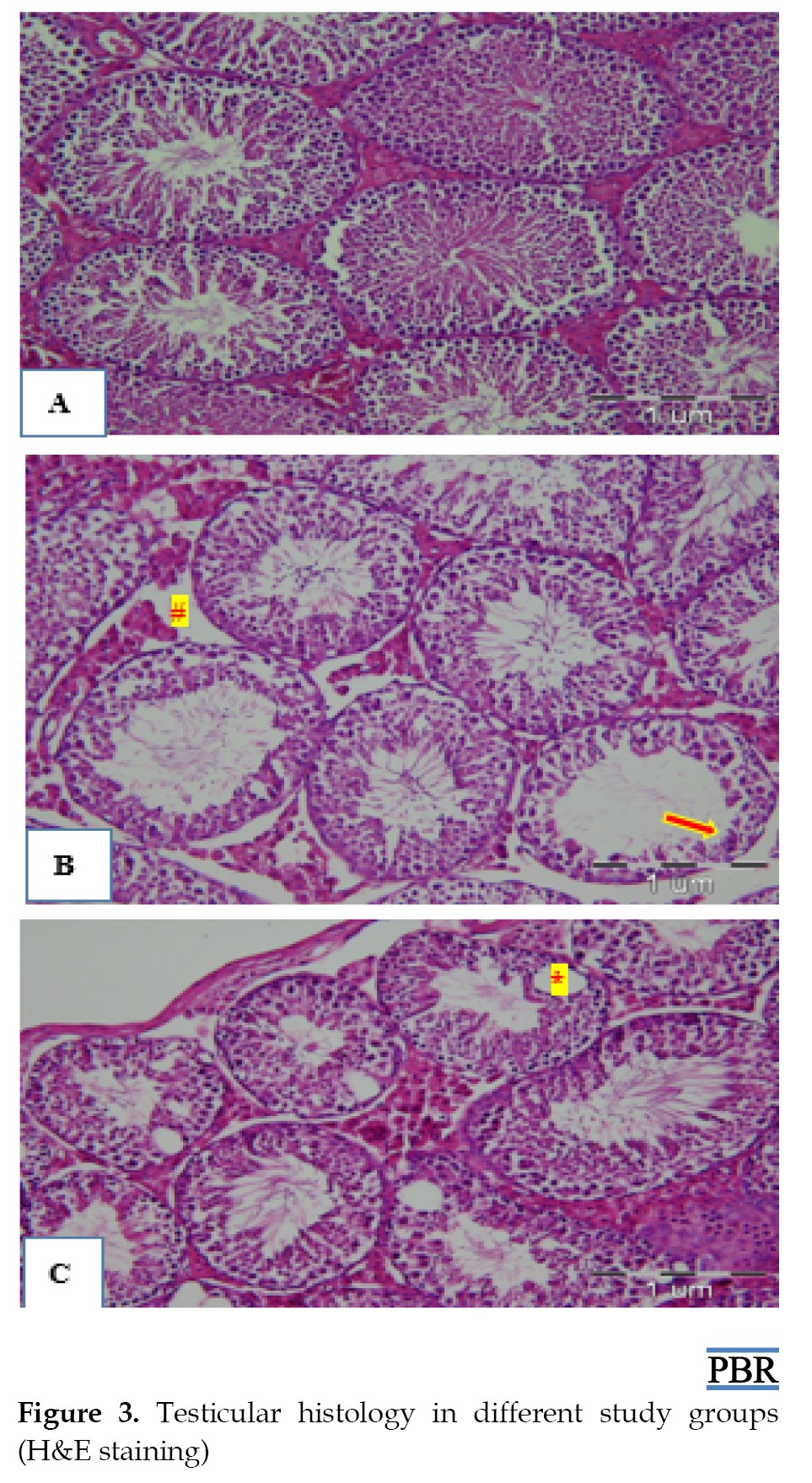
Testicular histology
Figure 3 displays the transverse section of the seminiferous tubules in different experimental groups. In the control group, seminiferous tubules were normal. A slight hyperplasia of Leydig cells and a reduction in the germinal epithelium thickness were observed in some tubules of the TP1 group. A remarkable decrease of the germinal epithelium thickness in some tubules and edema, congestion, and apoptotic vacuoles in the TP2 group were found.
Sperm chromatin quality
According to Figure 4, sperm with healthy DNA were observed in green color, and red sperm heads were counted as denatured DNA and yellow sperm heads were considered intermediate DNA damage. The percentage of sperms with damaged DNA in the control group was (2.40±4.3), while after receiving 50 mg/kg of T. parthenium, the sperm with damaged DNA remarkably increased (21.22±3.70). The sperm with denatured DNA in the group receiving 100 mg/kg of T. parthenium was 42.60±3.73, while it remarkably elevated versus the control group (P≤0.001).
MDA and thiol level in testicular tissue
The level of MDA in the control group was 6.85±1.35 nmol/g of tissue, in TP1, was 8.20±0.91 U/g of tissue, and in the TP2 group was 11.71±2.26 nmol/g of tissue (Figure 3). A significant difference was found in the level of MDA TP groups compared to the control group (P≤0.001). The level of thiol in the control group was 7.00±0.50 µmol/g tissue, in the TP1 group was 5.93±0.70 µmol/g tissue, and in the TP2 group was 4.52±1.01 µmol/g tissue (Figure 4). There was a significant difference in the level of thiol in groups receiving T. parthenium compared to the control group (P≤0.001).
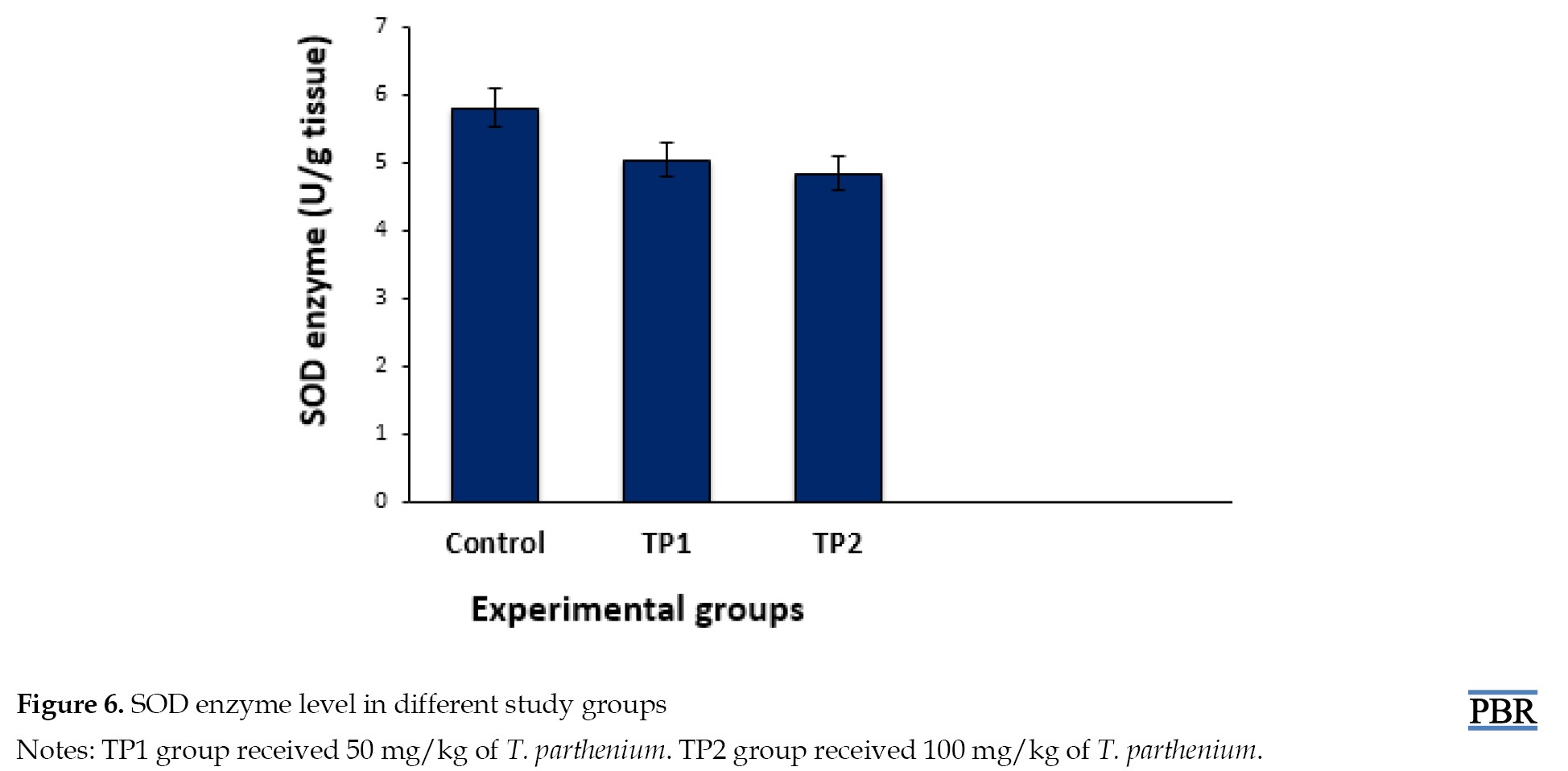
Catalase and SOD enzymes in testicular tissue
The catalase enzyme level was 0.37± 0.04 U/g in the control group, 0.27±0.04 U/g in the TP1 group, and 0.19±0.02 U/g in the TP2 group (Figure 5). A significant decrease in the catalase level was observed in the groups receiving T. parthenium versus the control group (P≤0.001). The SOD level in the groups receiving 50 mg/kg (P=0.73) and 100 mg/kg (P=0.82) T. parthenium decreased compared to the control group (Figure 6), but this reduction was not statistically significant (P>0.05).
Discussion
This study presents data about reduced sperm parameters and DNA integrity after administering Tanacetum extract in male mice. In addition, we observed a decrease in the thickness of the germinal epithelium and apoptotic vacuoles, as well as hyperplasia of Leydig cells in seminiferous tubules of the TP1 and TP2 groups. A remarkable increase in MDA level, which indicates oxidative stress, was observed in mice receiving T. parthenium. The level of antioxidant enzymes, a useful product for the body and neutralizing free radicals, was reduced compared to the control group. Studies indicate that administering medicinal herbs such as ginseng, ginger, N. sativa and saffron increases male fertility and improves semen parameters. On the other hand, other natural products such as R. graveolens, M. pulegium, A. millefolium and Foeniculum vulgare significantly reduce fertility and sperm quality [5, 6, 7].
Pareek et al. in a review article, reported that phytoestrogen compounds, phytosterols, camphor, and apigenin are present in T. parthenium [8]. Research indicates that phytoestrogens may damage the male reproductive system [16]. In addition, phytosterols inhibit the activity of the 5-alpha reductase enzyme, thereby reducing the dihydrotestosterone level, an active form of testosterone in the testicular tissue [26]. Phytosterol compounds reduce the sensitivity of tissues to androgens and, through activation of enzymes such as 5-alpha reductase and aromatase, reduce the level of testosterone hormone [27]. These compounds have a similar structure to cholesterol; lowering the cholesterol level reduces the precursor of testosterone hormone synthesis [28, 29]. It was observed that camphor in T. parthenium improves bacterial and fungal infections [30]. In addition, the reduction of cytochrome p450 enzyme level decreases some enzymes involved in the synthesis of testosterone hormone. Apigenin also inhibits important enzymes such as phosphatidyl inositol and aromatase, synthesizing testosterone hormone [31]. Consistent with our results, Soleimani et al. injected 50, 100 and 150 mg/kg of T. parthenium extract intraperitoneally into mice for 14 days. They reported that the hydroalcoholic extract of T. parthenium caused a remarkable reduction in testosterone, dihydrotestosterone, luteinizing hormone, and follicle-stimulating hormone, especially at a dose of 50 mg/kg [16]. Similar to this study, in our research, T. parthenium decreased sperm quality and germinal epithelium thickness. The reduction in hormones seems to be caused by the inhibitory effect of phytoestrogens in the T. parthenium on the hypothalamus-pituitary-gonadal axis.
On the other hand, Mazani et al. treated rats exposed to carbon tetrachloride with T. parthenium [32]. In this study, 7 groups were considered: Group 1 (control) fed pellet+water, group 2 (sham) fed olive oil+pellet, groups 3, 4 and 5 were pretreated with 40, 80 and 120 mg/kg of T. parthenium for 14 days, groups 6 and 7 were treated with T. parthenium after exposure to carbon tetrachloride. Antioxidant levels increased, and testicular histopathology improved in the groups pretreated with 80 and 120 mg/kg of Tanacetum. In Mazzini’s study, no group received only T. parthenium. Research indicates that phytoestrogens may cause a reduction in fertility. In addition, phytosterols inhibit the activity of the 5-alpha reductase enzyme and thereby reduce the dihydrotestosterone level, an active form of testosterone in the testicular tissue. Phytosterol compounds lessen the sensitivity of tissues to androgens and activating enzymes such as 5-alpha reductase and aromatase reduce the testosterone hormone level. In our study, it would have been better to investigate the effects of parthenolide as an active ingredient of T. parthenium, recommended to dear researchers for further research.
Conclusion
The results of this study showed that T. parthenium caused a significant decrease in sperm chromatin quality, MDA level, and germinal epithelium thickness at both doses. A reduction was found in the antioxidant enzyme level in the mice administrated with 50 mg/kg and 100 mg/kg of T. parthenium.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.MEDICAL.REC.1397.614).
Funding
This study was financially supported by the Research Vice-Chancellor of Mashhad University of Medical Sciences (Project No.: 970988).
Authors' contributions
Conceptualization and supervision: Shabnam Mohammadi; Methodology and data collection: Farimah Beheshti; Investigation: Abbas Mohammadipour; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Infertility is one of the major problems in the countries of the world. According to the statistics, 15% to 18% of couples suffer from infertility worldwide [1, 2]. Nearly half of these infertility problems are related to male factors. Many factors, such as insecticides, obesity, drugs, toxic substances, and infection, lead to infertility in men [3]. The use of medicinal plants to treat infertility has a long history in different cultures, and it has increased in recent years because of lower side effects compared to chemical drugs [4]. The administration of medicinal herbs such as ginseng, ginger, Nigella sativa and saffron increases male fertility and improves semen parameters.
On the other hand, other natural products such as Ruta graveolens, Mentha pulegium and Achillea millefolium significantly reduce fertility and sperm quality [5, 6, 7]. Tanacetum parthenium is a medicinal plant from the Asteraceae family and the Asterales order found in America, Asia, Europe and Africa. In Iran, it is mostly grown on the river banks and forest areas, as well as in the cities of Kurdistan, Mazandaran, Gillan, Golestan, Khorasan, and Fars provinces. This plant has various compounds, including sesquiterpene lactone, especially parthenolide, germacronolide, guaianolide, flavonoid, phytoestrogen, phytosterol and camphor [8-11]. T. parthenium is widely used in the pharmaceutical, cosmetics, hygiene, and agriculture industries. Studies indicate that this plant has anti-rheumatic, anti-flatulent, antiseptic, anti-fungal, anti-parasitic, anti-inflammation and cytotoxic effects. It is used to treat asthma, indigestion, dizziness, migraine, and skin disease [12-15]. Considering that studies report that phytoestrogens and phytosterols of the plants can lead to an adverse effect on the male reproductive system, and based on our search, few documents exist on the administration of Tanacetum extract on testicular histology and sperm chromatin quality in the literature. Therefore, the purpose of research was to evaluate the effects of Tanacetum extract on sperm parameters, testicular tissue, sperm DNA integrity and free radical damage in adult male mice.
Materials and Methods
This experimental research was conducted on 18 adult BALB/c male mice (2-3 months). Mice were randomly divided into 3 groups: Control, TP1 and TP2. The control group received normal saline. TP term refers to T. parthenium. The TP1 group received 50 mg/kg of Tanacetum extract, and the TP2 group was gavaged 100 mg/kg of Tanacetum using a gavage needle once a day for 2 weeks. These doses were selected based on Soleimani’s study [16].
Tanacetum extraction
T. parthenium was collected by a botanist from Zoshk village and confirmed by the Herbarium of the Ferdowsi University of Mashhad (Code: 17895-FUMH). First, 100 g of the powder was mixed with a solvent (50% ethanol+50% distilled water). After shaking, it was passed through a Whatman filter paper (England) and after solvent removal, it was dissolved in normal saline [17].
Sperm analysis
Sperm analysis was performed according to the World Health Organization’s (WHO) guidelines for sperm analysis. The crushed epididymis was placed on a plate containing saline in a CO2 incubator. Then, using a Neubauer hemocytometer, the sperm count and morphology were evaluated [18, 19].
Histology examination
After the testis was isolated, it was placed in 10% formalin fixative (Merck, Germany). Following specimen processing, the tissue was cut into 5 µ slides. After staining, the slides were examined by an expert pathologist [20, 21].
Sperm chromatin quality
Smear sperms were fixed with methanol (Sigman, Germany) and stained with acridine orange solution (Merck, Germany). Then, they were examined with a fluorescent microscope. Red sperm heads were counted as damaged DNA, yellow sperm heads were considered intermediate damage of DNA and green sperm heads indicated healthy DNA [22].
Measurement of malondialdehyde (MDA) level
First, the thiobarbituric acid (TBA) reagent (Merck, Germany) was prepared, and then the testicular tissue was homogenized. The homogenous tissue was heated with TBA reagent, trichloroacetic acid and hydrogen chloride (Merck, Germany) at boiling temperature for 50 minutes. After centrifugation, the absorption was read at 535 nm. The concentration of MDA was expressed in nmol/g [23].
Measurement of thiol level
Tris EDTA buffer was added to homogenous tissue, and the absorbance was read. The absorption of the dithionitrobenzoic acid solution to the sample was measured. Also, the absorption of the dithionitrobenzoic acid solution was considered blank. Thiol concentration was calculated as µmol/g at the wavelength of 412 nm [24].
Measurement of catalase enzyme level
First, hydrogen peroxide (Merck, Germany) was added to the phosphate buffer (solution 2, 3). Homogenous testicular tissue was diluted 1:10 and was added to solution 3. After two minutes, the absorbance changes were read at a wavelength of 240 nm. Enzyme concentration was expressed in U/g [24].
Measurement of superoxide dismutase (SOD) enzyme level
To prepare the tissue homogenate, the testicular tissue was first weighed and then homogenized with 10 mM phosphate buffer saline with pH=4.7. Next, 65 µL of phosphate buffer saline, 30 µL of 25.1 mmol MTT and 75 µL of pyrogallol (Merck, Germany) were mixed with 10 µL of tissue homogenate and incubated for a minute at room temperature. Afterward, 75 µL of dimethyl sulfide oxide was added, and the optical absorption of the obtained solution was read at a wavelength of 570 nm using an ELISA device. It was expressed in U/g [25].
Statistical analysis
Data analysis was done using analysis of variance (ANOVA) and Tukey’s post hoc test in SPSS software, version 16.
Results
Sperm analysis
Figures 1 and 2 display the sperm analysis results in different experimental groups. The mean sperm number was 4.8±0.22 million/mL in the control group, while it was remarkably reduced in the TP1 and TP2 groups (4±0.21 vs 3.9±0.20 million/mL). The sperm count in the TP1 group (P=0.006) and TP2 group (P=0.004) indicated a significant decrease versus the control group. The abnormal morphology of sperm was 11% in the control group, 15% in the TP1 group, and 19% in the TP2 group. A significant decrease was observed in the percentage of normal sperm morphology in the TP1 group (P=0.006) and TP2 group (P=0.006) compared to the control group.
Testicular histology
Figure 3 displays the transverse section of the seminiferous tubules in different experimental groups. In the control group, seminiferous tubules were normal. A slight hyperplasia of Leydig cells and a reduction in the germinal epithelium thickness were observed in some tubules of the TP1 group. A remarkable decrease of the germinal epithelium thickness in some tubules and edema, congestion, and apoptotic vacuoles in the TP2 group were found.
Sperm chromatin quality
According to Figure 4, sperm with healthy DNA were observed in green color, and red sperm heads were counted as denatured DNA and yellow sperm heads were considered intermediate DNA damage. The percentage of sperms with damaged DNA in the control group was (2.40±4.3), while after receiving 50 mg/kg of T. parthenium, the sperm with damaged DNA remarkably increased (21.22±3.70). The sperm with denatured DNA in the group receiving 100 mg/kg of T. parthenium was 42.60±3.73, while it remarkably elevated versus the control group (P≤0.001).
MDA and thiol level in testicular tissue
The level of MDA in the control group was 6.85±1.35 nmol/g of tissue, in TP1, was 8.20±0.91 U/g of tissue, and in the TP2 group was 11.71±2.26 nmol/g of tissue (Figure 3). A significant difference was found in the level of MDA TP groups compared to the control group (P≤0.001). The level of thiol in the control group was 7.00±0.50 µmol/g tissue, in the TP1 group was 5.93±0.70 µmol/g tissue, and in the TP2 group was 4.52±1.01 µmol/g tissue (Figure 4). There was a significant difference in the level of thiol in groups receiving T. parthenium compared to the control group (P≤0.001).
Catalase and SOD enzymes in testicular tissue
The catalase enzyme level was 0.37± 0.04 U/g in the control group, 0.27±0.04 U/g in the TP1 group, and 0.19±0.02 U/g in the TP2 group (Figure 5). A significant decrease in the catalase level was observed in the groups receiving T. parthenium versus the control group (P≤0.001). The SOD level in the groups receiving 50 mg/kg (P=0.73) and 100 mg/kg (P=0.82) T. parthenium decreased compared to the control group (Figure 6), but this reduction was not statistically significant (P>0.05).
Discussion
This study presents data about reduced sperm parameters and DNA integrity after administering Tanacetum extract in male mice. In addition, we observed a decrease in the thickness of the germinal epithelium and apoptotic vacuoles, as well as hyperplasia of Leydig cells in seminiferous tubules of the TP1 and TP2 groups. A remarkable increase in MDA level, which indicates oxidative stress, was observed in mice receiving T. parthenium. The level of antioxidant enzymes, a useful product for the body and neutralizing free radicals, was reduced compared to the control group. Studies indicate that administering medicinal herbs such as ginseng, ginger, N. sativa and saffron increases male fertility and improves semen parameters. On the other hand, other natural products such as R. graveolens, M. pulegium, A. millefolium and Foeniculum vulgare significantly reduce fertility and sperm quality [5, 6, 7].
Pareek et al. in a review article, reported that phytoestrogen compounds, phytosterols, camphor, and apigenin are present in T. parthenium [8]. Research indicates that phytoestrogens may damage the male reproductive system [16]. In addition, phytosterols inhibit the activity of the 5-alpha reductase enzyme, thereby reducing the dihydrotestosterone level, an active form of testosterone in the testicular tissue [26]. Phytosterol compounds reduce the sensitivity of tissues to androgens and, through activation of enzymes such as 5-alpha reductase and aromatase, reduce the level of testosterone hormone [27]. These compounds have a similar structure to cholesterol; lowering the cholesterol level reduces the precursor of testosterone hormone synthesis [28, 29]. It was observed that camphor in T. parthenium improves bacterial and fungal infections [30]. In addition, the reduction of cytochrome p450 enzyme level decreases some enzymes involved in the synthesis of testosterone hormone. Apigenin also inhibits important enzymes such as phosphatidyl inositol and aromatase, synthesizing testosterone hormone [31]. Consistent with our results, Soleimani et al. injected 50, 100 and 150 mg/kg of T. parthenium extract intraperitoneally into mice for 14 days. They reported that the hydroalcoholic extract of T. parthenium caused a remarkable reduction in testosterone, dihydrotestosterone, luteinizing hormone, and follicle-stimulating hormone, especially at a dose of 50 mg/kg [16]. Similar to this study, in our research, T. parthenium decreased sperm quality and germinal epithelium thickness. The reduction in hormones seems to be caused by the inhibitory effect of phytoestrogens in the T. parthenium on the hypothalamus-pituitary-gonadal axis.
On the other hand, Mazani et al. treated rats exposed to carbon tetrachloride with T. parthenium [32]. In this study, 7 groups were considered: Group 1 (control) fed pellet+water, group 2 (sham) fed olive oil+pellet, groups 3, 4 and 5 were pretreated with 40, 80 and 120 mg/kg of T. parthenium for 14 days, groups 6 and 7 were treated with T. parthenium after exposure to carbon tetrachloride. Antioxidant levels increased, and testicular histopathology improved in the groups pretreated with 80 and 120 mg/kg of Tanacetum. In Mazzini’s study, no group received only T. parthenium. Research indicates that phytoestrogens may cause a reduction in fertility. In addition, phytosterols inhibit the activity of the 5-alpha reductase enzyme and thereby reduce the dihydrotestosterone level, an active form of testosterone in the testicular tissue. Phytosterol compounds lessen the sensitivity of tissues to androgens and activating enzymes such as 5-alpha reductase and aromatase reduce the testosterone hormone level. In our study, it would have been better to investigate the effects of parthenolide as an active ingredient of T. parthenium, recommended to dear researchers for further research.
Conclusion
The results of this study showed that T. parthenium caused a significant decrease in sperm chromatin quality, MDA level, and germinal epithelium thickness at both doses. A reduction was found in the antioxidant enzyme level in the mice administrated with 50 mg/kg and 100 mg/kg of T. parthenium.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.MEDICAL.REC.1397.614).
Funding
This study was financially supported by the Research Vice-Chancellor of Mashhad University of Medical Sciences (Project No.: 970988).
Authors' contributions
Conceptualization and supervision: Shabnam Mohammadi; Methodology and data collection: Farimah Beheshti; Investigation: Abbas Mohammadipour; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Ashraf DM, Ali D, Azadeh DM. Effect of infertility on sexual function: A cross-sectional study. J Clin Diagn Res. 2015; 9(5):QC01-3. [PMID] [PMCID]
- Guz H, Ozkan A, Sarisoy G, Yanik F, Yanik A. Psychiatric symptoms in Turkish infertile women. J Psychosom Obstet Gynaecol. 2003; 24(4):267-71. [DOI:10.3109/01674820309074691] [PMID]
- Skakkebæk NE, Lindahl-Jacobsen R, Levine H, Andersson AM, Jørgensen N, Main KM, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol. 2022; 18(3):139-57. [DOI:10.1038/s41574-021-00598-8] [PMID]
- Jaradat N, Zaid AN. Herbal remedies used for the treatment of infertility in males and females by traditional healers in the rural areas of the West Bank/Palestine. BMC Complement Altern Med. 2019; 19(1):194. [DOI:10.1186/s12906-019-2617-2] [PMID] [PMCID]
- Mishra RK, Singh S, Singh SK. Natural products in regulation of male fertility. Indian J Med Res. 2018; 148(Suppl):S107-14. [DOI: 10.4103/ijmr.IJMR_1968_17]
- Daniyal M, Akram M. Antifertility activity of medicinal plants. J Chin Med Assoc. 2015; 78(7):382-8. [DOI:10.1016/j.jcma.2015.03.008] [PMID]
- Roozbeh N, Rostami S, Abdi F. [A review of medicinal herbs with rickets and anti-fertility in men (Persian)]. Iran J Obstet Gynecol Infertil. 2016; 19(13):18-32. [DOI:10.22038/IJOGI.2016.7278]
- Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev. 2011; 5(9):103-10. [DOI:10.4103/0973-7847.79105] [PMID] [PMCID]
- Di Cesare Mannelli L, Tenci B, Zanardelli M, Maidecchi A, Lugli A, Mattoli L, et al. Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine. 2015; 22(7-8):752-8. [DOI:10.1016/j.phymed.2015.05.006] [PMID]
- Mahmoodzadeh Y, Mazani M, Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol Rep. 2017; 4:455-62. [DOI:10.1016/j.toxrep.2017.08.003] [PMID] [PMCID]
- Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015; 4(4):CD002286. [DOI:10.1002/14651858.CD002286.pub3] [PMID] [PMCID]
- Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium L.): An update of a systematic review. Public Health Nutr. 2000; 3(4A):509-14. [DOI:10.1017/S1368980000000598] [PMID]
- Studzińska-Sroka E, Znajdek-Awizeń P, Gawron-Gzella A. [Studies on the antimigraine action of Feverfew (Tanacetum parthenium (L.) Sch. Bip.) (Polish)]. Wiad Lek. 2013;66(2 Pt 2):195-9. [PMID]
- Majdi M, Charnikhova T, Bouwmeester H. Genetical, developmental and spatial factors influencing parthenolide and its precursor costunolide in feverfew (Tanacetum parthenium L. Schulz Bip.) Ind Crop Prod. 2013; 47:270-6. [DOI:10.1016/j.indcrop.2013.03.021]
- Pavela R, Sajfrtová M, Sovová H, Bárnet M, Karban J. The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip. extractsobtained by supercritical fluid extraction and hydrodistillation. Ind Crop Prod. 2010; 31(3):449-54 [DOI:10.1016/j.indcrop.2010.01.003]
- Soleimani M, Ramezani M, Siadat F. [The effects of tanacetum parthenium hydroalchoholic extract on the sex hormones of NMRI mouse (Persian)]. J Anim Biol. 2016; 8(2):39-54. [Link]
- Vafaei A, Mohammadi S, Fazel A, Soukhtanloo M, Mohammadipour A, Beheshti F. Effects of carob (Ceratonia siliqua) on sperm quality, testicular structure, testosterone level and oxidative stress in busulfan-induced infertile mice. Pharm Sci. 2018; 24(2); 104-11. [DOI:10.15171/PS.2018.16]
- Mohammadi S, Gholamin M, Mohammadi M, Mansouri A, Mahmoodian R, Attari S, et al. Down-regulation of CatSper 1 and CatSper 2 genes by lead and mercury. Environ Toxicol Pharmacol. 2018; 59:82-6. [DOI:10.1016/j.etap.2018.03.007] [PMID]
- Boitrelle F, Shah R, Saleh R, Henkel R, Kandil H, Chung E, et al. The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life. 2021; 11(12):1368. [DOI:10.3390/life11121368] [PMID] [PMCID]
- Mohammadi S, Safari F, Seyedi Z, Seyed Hosseini E, Karimi F, Mohammadi M, et al. [Effect of different doses of N-acetyl cysteine on biochemical and histopathological parameters in kidney of formalin-treated (Persian)]. J Kerman Univ Med Sci. 2016; 23(5):607-17. [Link]
- Mohammadi S, Khakbaz M, Shoraka M, Vakil S, Moghimian M, Mohammadzadeh F, et al. Effects of different doses of manganese on lead poisoning in the kidney of adult male mice. Koomesh. 2016; 18(1):203-10. [Link]
- Norasteh H, Mohammadi S, Nikravesh MR, Boroumand-Noughabi S, Beheshti F. Effects of bene (pistacia atlantica) on histopathology of testis, sperm chromatin quality and stress oxidative in busulfan-induced infertile mice. Pharm Sci. 2019; 25(1):24-30. [DOI:10.15171/PS.2019.4]
- Attari SS, Mohammadi S, Ebrahimzadeh A, Hosseinzadeh H, Soukhtanloo M, Rajabzadeh A. Effects of thymoquinone on sperm parameters, apoptosis, testosterone level, and oxidative stress in a mouse model of D-galactose-induced aging. Pharm Sci. 2018; 24(3):180-6. [DOI:10.15171/PS.2018.26]
- Mohammadi S, Rahmani F, Hasanian SM, Beheshti F, Akbari Oryani M, Ebrahimzadeh A, et al. Effects of dioxin on testicular histopathology, sperm parameters, and CatSper2 gene and protein expression in Naval Medical Research Institute male mice. Andrologia. 2019; 51(11):e13411.[DOI:10.1111/and.13411]
- Yousefi M, Mohammadi S, Jalali M, Beheshti F. [Effect of different doses of curcumin on sperm parameters and oxidative stress in testis of D-Galactose induced aging mice model (Persian)]. J Babol Univ Med Sci. 2019; 21(1):53-60. [DOI:10.22088/jbums.21.1.53]
- Amory JK, Anawalt BD, Matsumoto AM, Page ST, Bremner WJ, Wang C, et al. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on bone mineral density, serum lipoproteins, hemoglobin, prostate specific antigen and sexual function in healthy young men. J Urol. 2008; 179(6):2333-8. [DOI:10.1016/j.juro.2008.01.145] [PMID] [PMCID]
- Khan U, Aslam M, Saeeds A. Effect of beta adrenergic antagonist on the production of testosterone by rat leydig cells. J Ayub Med Coll Abbottabad. 2004; 16(4):26-8. [Link]
- Sugano M, Morioka H, Ikeda I. A comparison of hypocholesterolemic activity of beta-sitosterol and beta-sitostanol in rats. J Nutr. 1977; 107(11):2011-9. [DOI:10.1093/jn/107.11.2011] [PMID]
- Bartke A, Musto N, Caldwell BV, Behrman HR. Effects of a cholesterol esterase inhibitor and of prostaglandin F2alpha on testis cholesterol and on plasma testosterone in mice. Prostaglandins. 1973; 3(1):97-104. [DOI: 10.1016/0090-6980(73)90141-x] [PMID]
- Chen S, Cho M, Karlsberg K, Zhou D, Yuan YC. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem. 2004; 279(46):48071-8. [DOI:10.1074/jbc.M406847200] [PMID]
- Jeong HJ, Shin YG, Kim IH, Pezzuto JM. Inhibition of aromatase activity by flavonoids. Arch Pharm Res. 1999; 22(3):309-12. [DOI:10.1007/BF02976369] [PMID]
- Mazani M, Banaei S, Rezagholizadeh L. Feverfew attenuates carbon tetrachloride-induced testicular damage in rats. J Herbmed Pharmacol. 2020; 9(1):42-7. [DOI:10.15171/jhp.2020.06]
Type of Study: Original Research |
Subject:
General
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |