Volume 9, Issue 4 (2023)
Pharm Biomed Res 2023, 9(4): 289-296 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nakhshab M, Soltani M, Ghaffari V, Farhadi R, Saeedi M, Rafati M. Investigating Different Effects of Divided Doses of Sucrose 24% Compared to Single Dose for Pain Prophylaxis Before Heel Stick in Term and Preterm Neonates. Pharm Biomed Res 2023; 9 (4) :289-296
URL: http://pbr.mazums.ac.ir/article-1-535-en.html
URL: http://pbr.mazums.ac.ir/article-1-535-en.html
Maryam Nakhshab1 

 , Mahkameh Soltani2
, Mahkameh Soltani2 

 , Vajiheh Ghaffari1
, Vajiheh Ghaffari1 

 , Roya Farhadi1
, Roya Farhadi1 

 , Majid Saeedi3
, Majid Saeedi3 

 , Mohammadreza Rafati *4
, Mohammadreza Rafati *4 




 , Mahkameh Soltani2
, Mahkameh Soltani2 

 , Vajiheh Ghaffari1
, Vajiheh Ghaffari1 

 , Roya Farhadi1
, Roya Farhadi1 

 , Majid Saeedi3
, Majid Saeedi3 

 , Mohammadreza Rafati *4
, Mohammadreza Rafati *4 


1- Department of Pediatrics, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Pharmaceutics, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Clinical Pharmacy, Pharmaceutical Sciences Research Center, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Pharmaceutics, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Clinical Pharmacy, Pharmaceutical Sciences Research Center, School of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 746 kb]
(817 Downloads)
| Abstract (HTML) (2585 Views)
Full-Text: (980 Views)
Introduction
Two-thirds of infants admitted to the hospital do not receive appropriate analgesic therapy suffering repeated invasive procedures. During 14 days of neonatal intensive care unit stay, an infant experiences a median of 75 painful procedures and ten painful procedures per day during hospitalization. Painful procedures were performed in 79.2% of them without specific preprocedural sedation [1]. Heel lance is one of the frequent procedures that neonates experience in intensive care units [2]. Increasing evidence of short-term and long-term adverse neurodevelopmental consequences [3, 4] and ethical guidelines emphasize that prevention and management of pain and stress should be considered an important strategy in neonatal care [5].
International clinical guidelines have introduced oral sucrose as an analgesic in painful neonatal procedures [6]. The claim is based on several randomized controlled clinical trial results that sucrose is an effective and safe analgesic agent to reduce procedural pain in preterm and term neonates [7]. To the best of our knowledge, studies have investigated oral sucrose’s analgesic effect from 1987 [8] to date [9]. Only one study investigated the relationship between the administration route and sucrose’s efficacy in patients [10]. Accordingly, the sense of taste is necessary to produce sucrose analgesia in infants exposed to mildly painful procedures. In humans, sucrose’s exact mechanism of action is not well-elucidated; however, this mechanism is related to the sweet taste response by opioid, endorphin, and possibly dopamine or acetylcholine pathways [11, 12]. Nociceptive pathways in immature neonates are active and functional at 25 weeks gestation [13]. The best results with sucrose to reduce pain indicators were obtained approximately 2 min before painful procedures [14, 15].
This study reasoned that a greater analgesic effect would be produced if the infant is exposed to sucrose in the oral region longer. To answer the question, “does sucrose have a better analgesic effect when used multiple times before the procedure?” This study compares the analgesic effect of a single vs divided dose of oral sucrose 24% during 2 min before heel stick in the neonatal intensive care unit.
Materials and Methods
This prospective, double-blind, randomized, controlled clinical trial was conducted in the neonatal intensive care unit and neonatal ward from January 2018 to September 2018 in a university-affiliated tertiary hospital Bu-Ali Sina, Sari City, Iran. We obtained informed consent from all enrolled neonates’ parents or legal guardians.
Study patients
This study included neonates in the age range of 1 to 28 days of both sexes who were admitted to the neonatal intensive care units and neonatal ward of Bu-Ali Sina Teaching Hospital, Sari City, Iran, and required heel lance. The exclusion criteria were carbohydrate intolerance due to short gut syndrome (unless approved by physician), metabolic or endocrine disease, inability to tolerate oral administration of the solution to the tongue, absent/deficient protective airway reflexes (gag, cough, swallow), central nervous system dysfunction, use of sedation/analgesics, neuromuscular blocking agents, or anesthetic agents, patient with suspected or confirmed necrotizing enterocolitis, oral surgery (unless approved by physician), nothing by mouth (unless physician approval), and neonates with congenital anomalies in their faces [16].
A random number table was used to assign each eligible neonate to one of the two study groups: Single dose and divided dose groups. The randomization was performed by an individual not involved in other aspects of the study. The assignments were contained in secured, opaque, serially numbered envelopes and opened immediately before receiving the sucrose solution. To calculate the sample size, we considered the results of previous studies [17] that assessed pain responses using methods similar to some indices of the premature infant pain profile-revised (PIPP-R) scale.
Study interventions
Expert nurses performed all heel stick procedures according to standard techniques. All neonates received oral sucrose 24% solution based on their weight, 0.3 mL/kg, either in a single dose of 120 s or three divided doses of 120, 90, and 60 s before heel lance.
All newborns were assessed for pain with the premature infant pain profile revised [18]. The PIPP-R scale is based on seven parameters: Gestational age, behavioral state, changes in maximum heart rate, changes in minimum arterial oxygen saturation, brow bulging, eye squeezing, and nasolabial furrowing. These indices are individually scored from 0 to 3, and the sum of these seven indices obtains the total score.
For more careful monitoring of the neonates’ faces and upper bodies, a videotape was taken from the beginning of the procedure until 30 s after the end. The changes in facial expression, heart rate, and oxygen saturation were recorded during the 30-s period immediately after initiating the procedures. No conversation revealed the receiving sucrose. Two neonatologists who were blind to the type of used regimens watched the videos and independently determined the PIPP-R scores for each patient. The patient was omitted if the baby’s scores had more than two units’ difference reported by two evaluators. The behavioral state was measured by observing the patient for 15 s before the procedure and was scored according to PIPP-R (active/awake, quiet/awake, active/asleep, and quiet/asleep). The changes in facial expressions were scored by measuring the time a particular facial change was present during the first 30 s after initiating the heel stick. The outcome pointed to the severity of the experienced pain among two groups of newborns by the mean PIPP-R score.
Study statistics
Descriptive results are expressed as counts and proportions for categorical variables and as Mean±SD for continuous variables. The student t-test was used to analyze continuous variables to compare the two groups, and the χ2 or the Fisher exact test was used for categorical variables. All analyses were performed using the SPSS software, version 22 (SPSS Inc), and a P≤0.05 was considered significant.
Results
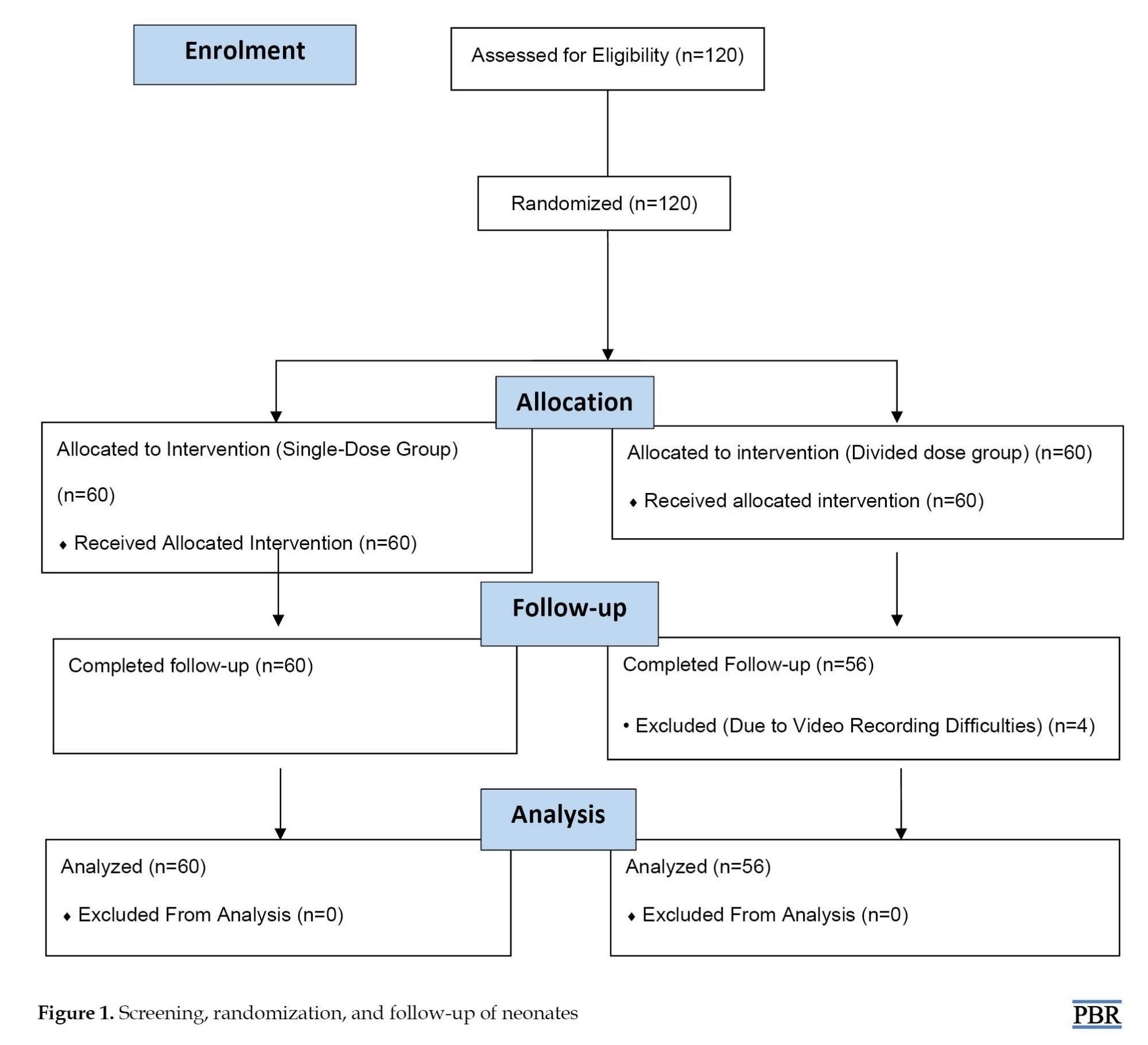
A total of 120 neonates were recruited and randomly assigned to receive either single or divided doses of sucrose 24% solution. Four patients were excluded due to video recording difficulties (Figure 1).
Two-thirds of infants admitted to the hospital do not receive appropriate analgesic therapy suffering repeated invasive procedures. During 14 days of neonatal intensive care unit stay, an infant experiences a median of 75 painful procedures and ten painful procedures per day during hospitalization. Painful procedures were performed in 79.2% of them without specific preprocedural sedation [1]. Heel lance is one of the frequent procedures that neonates experience in intensive care units [2]. Increasing evidence of short-term and long-term adverse neurodevelopmental consequences [3, 4] and ethical guidelines emphasize that prevention and management of pain and stress should be considered an important strategy in neonatal care [5].
International clinical guidelines have introduced oral sucrose as an analgesic in painful neonatal procedures [6]. The claim is based on several randomized controlled clinical trial results that sucrose is an effective and safe analgesic agent to reduce procedural pain in preterm and term neonates [7]. To the best of our knowledge, studies have investigated oral sucrose’s analgesic effect from 1987 [8] to date [9]. Only one study investigated the relationship between the administration route and sucrose’s efficacy in patients [10]. Accordingly, the sense of taste is necessary to produce sucrose analgesia in infants exposed to mildly painful procedures. In humans, sucrose’s exact mechanism of action is not well-elucidated; however, this mechanism is related to the sweet taste response by opioid, endorphin, and possibly dopamine or acetylcholine pathways [11, 12]. Nociceptive pathways in immature neonates are active and functional at 25 weeks gestation [13]. The best results with sucrose to reduce pain indicators were obtained approximately 2 min before painful procedures [14, 15].
This study reasoned that a greater analgesic effect would be produced if the infant is exposed to sucrose in the oral region longer. To answer the question, “does sucrose have a better analgesic effect when used multiple times before the procedure?” This study compares the analgesic effect of a single vs divided dose of oral sucrose 24% during 2 min before heel stick in the neonatal intensive care unit.
Materials and Methods
This prospective, double-blind, randomized, controlled clinical trial was conducted in the neonatal intensive care unit and neonatal ward from January 2018 to September 2018 in a university-affiliated tertiary hospital Bu-Ali Sina, Sari City, Iran. We obtained informed consent from all enrolled neonates’ parents or legal guardians.
Study patients
This study included neonates in the age range of 1 to 28 days of both sexes who were admitted to the neonatal intensive care units and neonatal ward of Bu-Ali Sina Teaching Hospital, Sari City, Iran, and required heel lance. The exclusion criteria were carbohydrate intolerance due to short gut syndrome (unless approved by physician), metabolic or endocrine disease, inability to tolerate oral administration of the solution to the tongue, absent/deficient protective airway reflexes (gag, cough, swallow), central nervous system dysfunction, use of sedation/analgesics, neuromuscular blocking agents, or anesthetic agents, patient with suspected or confirmed necrotizing enterocolitis, oral surgery (unless approved by physician), nothing by mouth (unless physician approval), and neonates with congenital anomalies in their faces [16].
A random number table was used to assign each eligible neonate to one of the two study groups: Single dose and divided dose groups. The randomization was performed by an individual not involved in other aspects of the study. The assignments were contained in secured, opaque, serially numbered envelopes and opened immediately before receiving the sucrose solution. To calculate the sample size, we considered the results of previous studies [17] that assessed pain responses using methods similar to some indices of the premature infant pain profile-revised (PIPP-R) scale.
Study interventions
Expert nurses performed all heel stick procedures according to standard techniques. All neonates received oral sucrose 24% solution based on their weight, 0.3 mL/kg, either in a single dose of 120 s or three divided doses of 120, 90, and 60 s before heel lance.
All newborns were assessed for pain with the premature infant pain profile revised [18]. The PIPP-R scale is based on seven parameters: Gestational age, behavioral state, changes in maximum heart rate, changes in minimum arterial oxygen saturation, brow bulging, eye squeezing, and nasolabial furrowing. These indices are individually scored from 0 to 3, and the sum of these seven indices obtains the total score.
For more careful monitoring of the neonates’ faces and upper bodies, a videotape was taken from the beginning of the procedure until 30 s after the end. The changes in facial expression, heart rate, and oxygen saturation were recorded during the 30-s period immediately after initiating the procedures. No conversation revealed the receiving sucrose. Two neonatologists who were blind to the type of used regimens watched the videos and independently determined the PIPP-R scores for each patient. The patient was omitted if the baby’s scores had more than two units’ difference reported by two evaluators. The behavioral state was measured by observing the patient for 15 s before the procedure and was scored according to PIPP-R (active/awake, quiet/awake, active/asleep, and quiet/asleep). The changes in facial expressions were scored by measuring the time a particular facial change was present during the first 30 s after initiating the heel stick. The outcome pointed to the severity of the experienced pain among two groups of newborns by the mean PIPP-R score.
Study statistics
Descriptive results are expressed as counts and proportions for categorical variables and as Mean±SD for continuous variables. The student t-test was used to analyze continuous variables to compare the two groups, and the χ2 or the Fisher exact test was used for categorical variables. All analyses were performed using the SPSS software, version 22 (SPSS Inc), and a P≤0.05 was considered significant.
Results
A total of 120 neonates were recruited and randomly assigned to receive either single or divided doses of sucrose 24% solution. Four patients were excluded due to video recording difficulties (Figure 1).
No significant differences were observed in the baseline characteristics of neonates enrolling in two groups, according to their gender, age, gestational age, weight, birth weight, cause of admission, heart rate, and oxygen saturation percentage (Table 1).
The behavioral state scores before starting the procedure showed no significant difference between the study groups (P=0.602). The physiological responses were not significantly different between the two groups (for heart rate changes, P=0.909, and for changes in oxygen saturation, P=0.87).
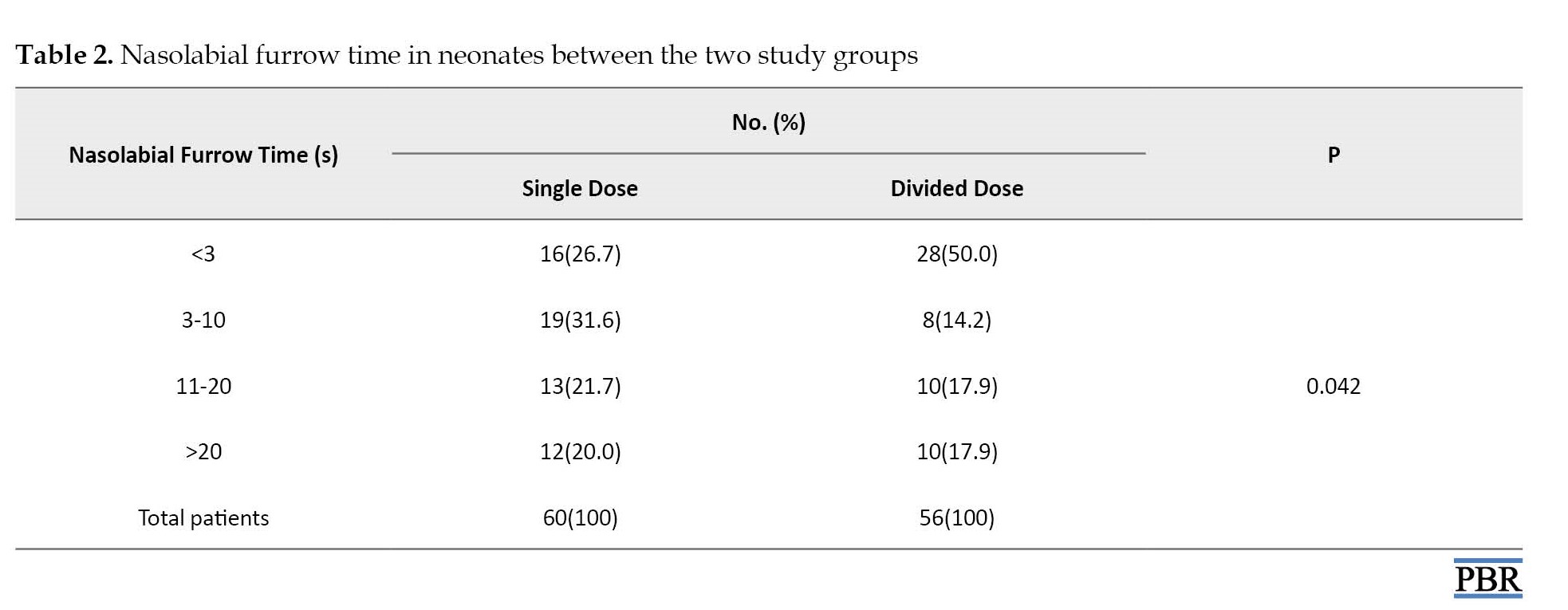
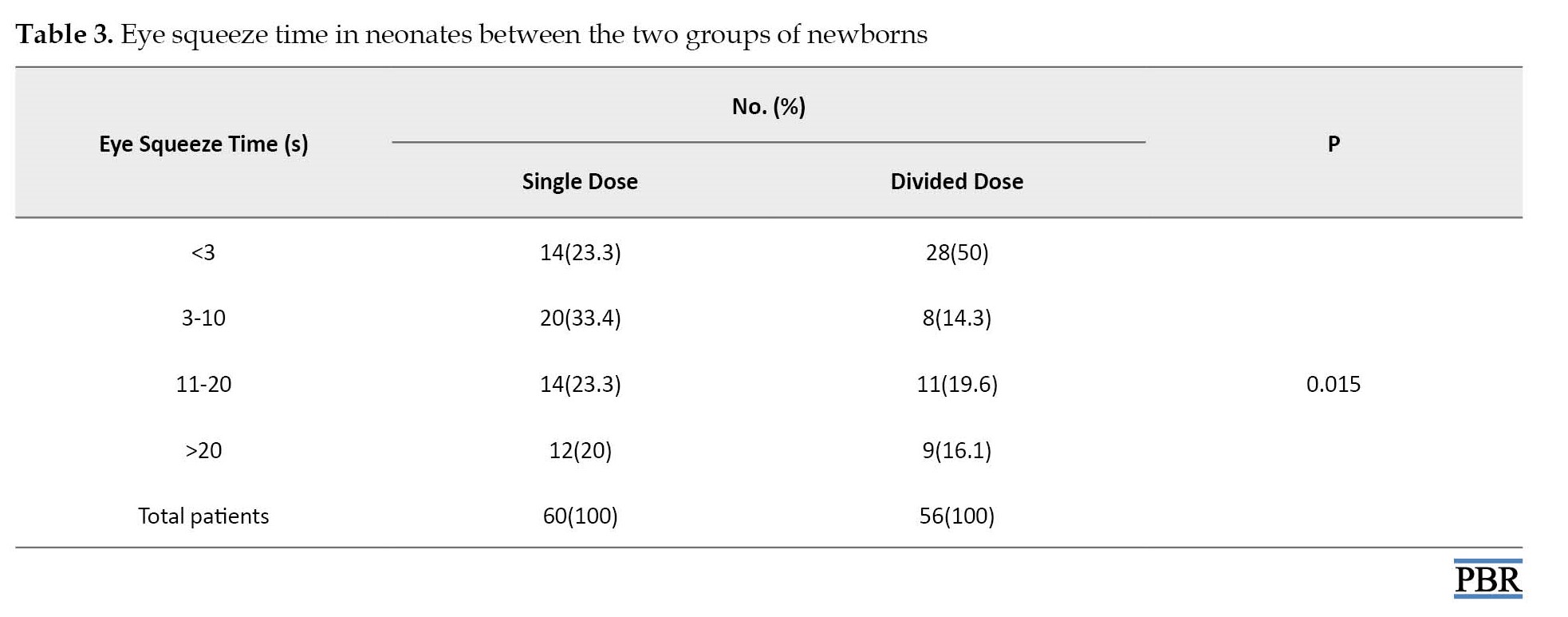
Contrary to physiologic responses, nasolabial furrow time was significantly improved in the divided dose group (P=0.042). Also, eye squeeze time showed better results in patients receiving divided doses of sucrose (P=0.015).
Although the number of patients who experienced eyebrow bulge time less than 3 s was fewer in neonates who received sucrose three-divided dose (12.9% vs 22.4%), there was no significant difference between the two groups (P=0.094).
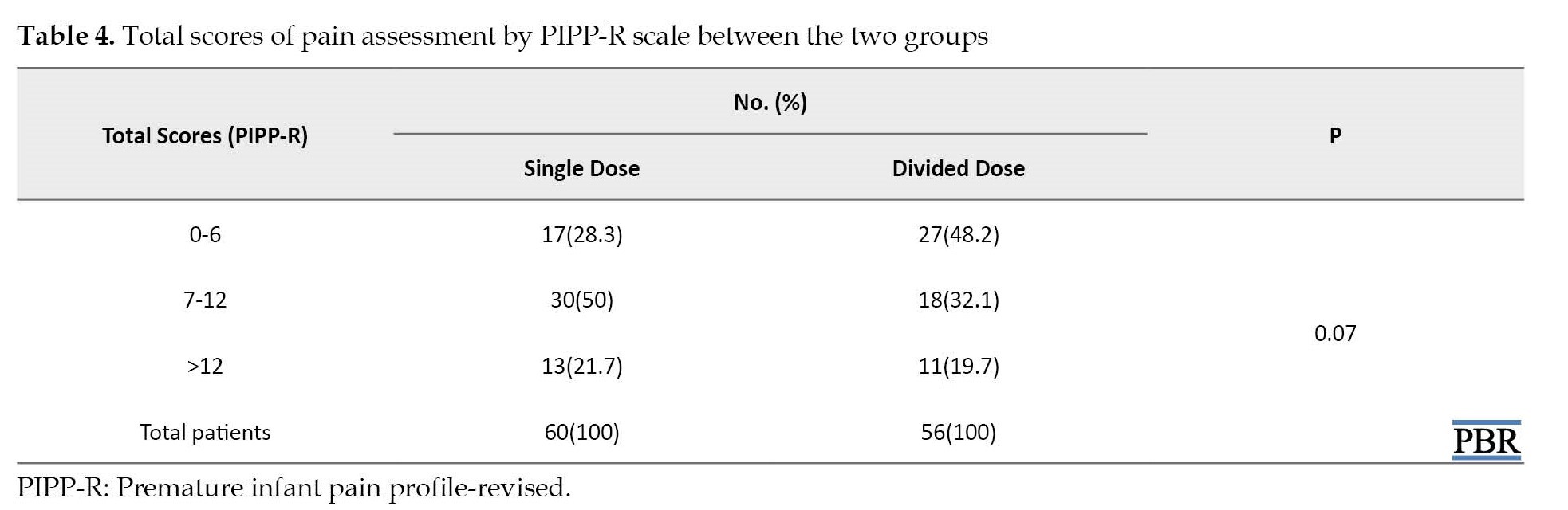
The mean PIPP-R score was 9.03±4.3 for neonates receiving single-dose oral sucrose, while the score for neonates receiving oral sucrose in 3-divided doses was 8.13±4.5 (P=0.27). However, a categorized comparison of the total PIPP-R score between the two groups showed a remarkable difference trend. Yet, it was not significant (P=0.07).
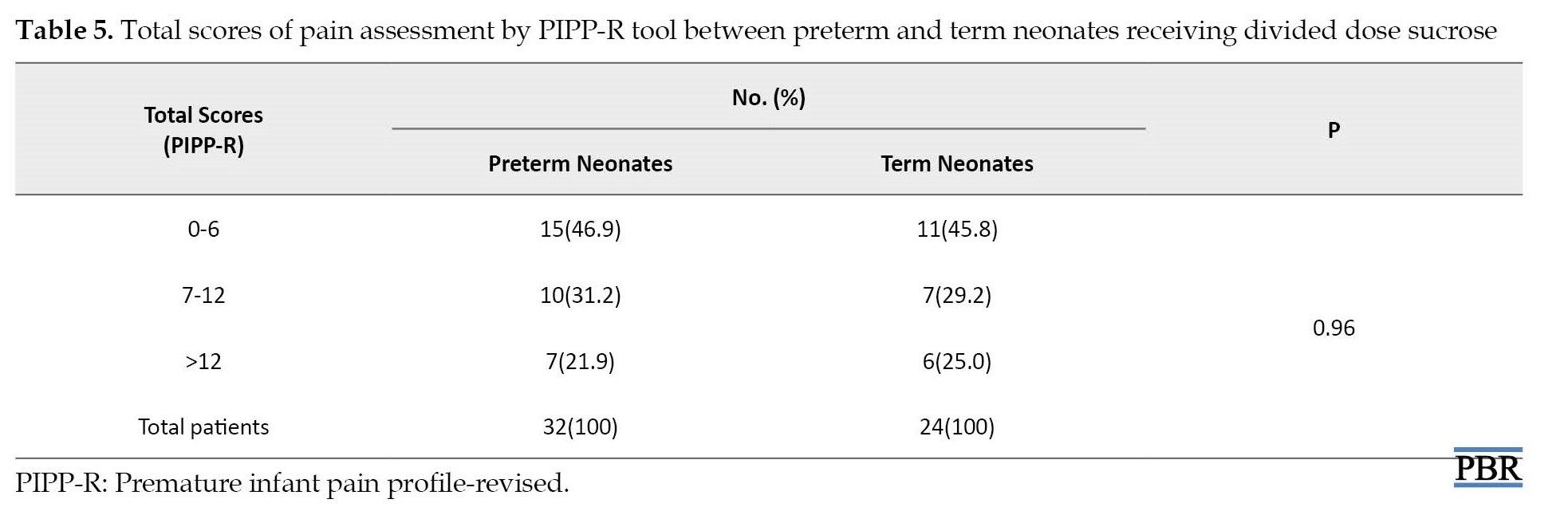
Pain assessment in preterm (<37 weeks gestation) and term neonates (≥37 weeks gestation) showed that divided doses of sucrose could similarly decrease the PIPP-R score in all neonates (P=0.96; Table 2).
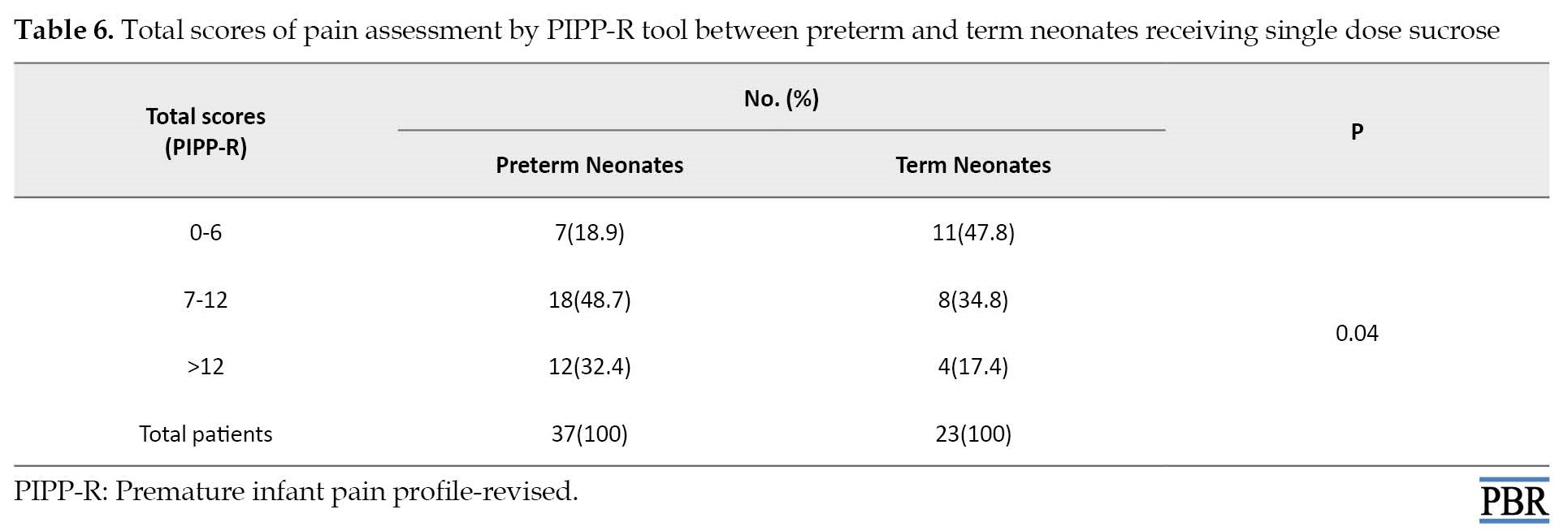
The mean PIPP-R score was 7.7±3.9 and 8.4±4.4 in preterm and term neonates, respectively (P=0.45), while the PIPP-R score was meaningfully reduced in term neonates receiving single dose sucrose in comparison with preterm neonates (P=0.04; Table 3).
The mean PIPP-R score was 10.1±4.0 and 8.2±4.1 in preterm and term neonates, respectively (P=0.01).
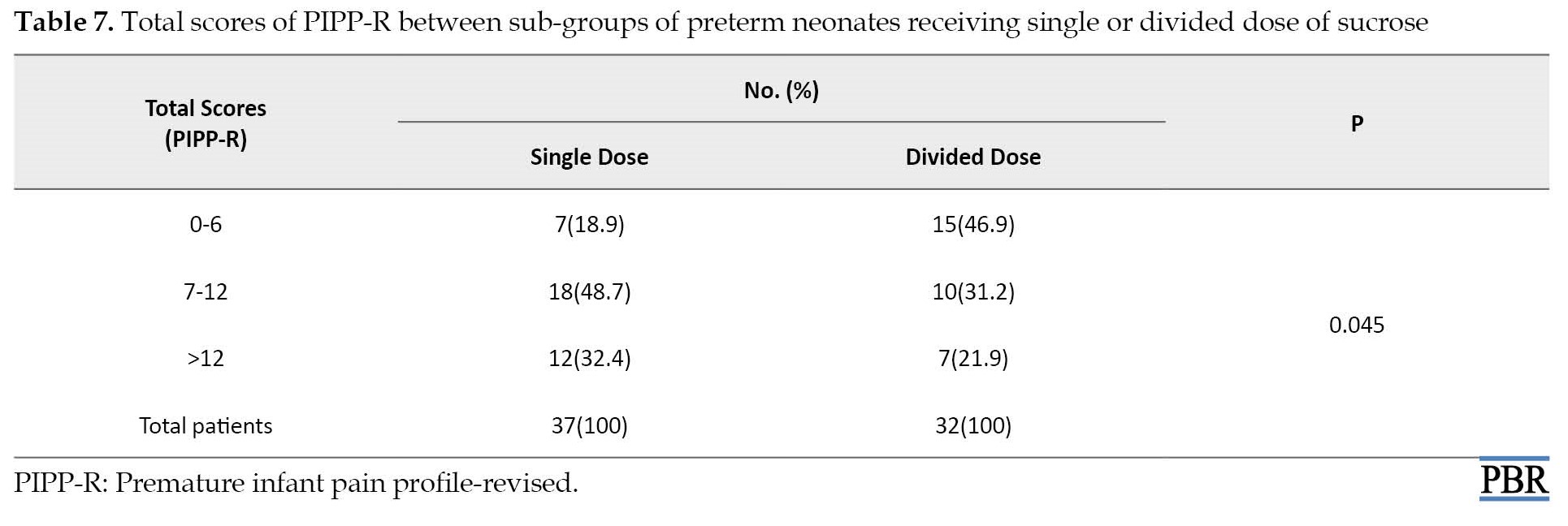
There was a significant difference in mean PIPP-R scores when only preterm neonates were compared as sub-groups in single and divided dose groups (10.1±4.1 and 7.7±3.9; P=0.016). The sucrose administration method’s effect in reducing pain (categorical PIPP-R score) in preterm neonates is provided in Tables 4, 5, 6 and 7.
Discussion
This study aimed to investigate whether oral administration affects the analgesic properties of sucrose. We compared PIPP-R scores between two groups of neonates who received oral sucrose 24% solution 0.3 mL/kg, either a single dose of 120 s or in three-divided doses of 120, 90, and 60 s before heel lance. Single-dose sucrose could significantly reduce PIPP-R scores only in term infants compared to preterm infants, while in the divided dose method, PIPP-R scores decreased equally in both full-term and pre-term infants. Accordingly, when the sweet taste lasts longer in the mouth, less pain is experienced by neonates. To the best of our knowledge, this is the first study that tests the method of divided-dose administration of sucrose solution.
Okan et al. monitored heart rate changes for 3 min after heel lance between neonates receiving sucrose 20%, glucose 20%, and sterile water. The results showed that the mean heart rate was significantly higher in the placebo group than in the sucrose and glucose groups during the first min [19]. Most research did not show a direct relationship between oral sucrose and a decrease in heart rate changes [20]. In this study, the changes in heart rate were not significantly different during 30 s after heel lance between the two groups.
Oxygen saturation is another physiologic parameter that influences the total PIPP-R score. In the present study, changes in oxygen saturation were not significantly different between the two groups (P=0.87). In a systematic review in 2013, among 44 articles that investigated the analgesic properties of sucrose, only six articles appraised the effect of sucrose on oxygen saturation [21]. Five studies reported that sucrose exerts no enterprising effect on oxygen saturation. In another study, oral sucrose may decrease oxygen saturation [22].
Based on a meta-analysis article published in 2017, oral sweet solutions decrease crying time in neonates [23]. Despite this meta-analysis, Mundol et al. recently reported that sucrose did not ameliorate neonates’ cries during neonatal immunization with bacille Calmette-Guerin [24]. In the present study, we evaluated brow bulge, eye squeeze, and nasolabial furrow time. Nasolabial furrow time was significantly shorter for neonates receiving sucrose in three-divided doses between two groups (P=0.042). Like nasolabial furrow time, eye squeeze time was meaningfully improved in neonates participating in a three-divided dose group (P=0.015). Around 46% of babies in the three divided dose group experienced brow bulge time of less than 3 s compared to 25% of neonates participating in the single dose group. However, the difference in brow bulge time was statistically significant (P=0.094).
Several studies have shown that oral sucrose can exert a soothing effect among the neonate population during minor invasive procedures and reduce the total score of the PIPP scale [9, 25, 26, 27]. According to these studies, we accept the analgesic effect of oral sucrose; therefore, there was no placebo group in the present study.
Our study showed that sucrose may act differently in preterm and term neonates. Sucrose administration in divided doses could equally reduce PIPP-R score in both term and preterm neonates; however, using sucrose as a single dose could significantly decrease pain scores only in term neonates. Although the nociceptive pathways have function as early as 25 weeks gestation, it seems there are some differences in maturity and development of this pathway in preterm and term neonates [28]. Because the peripheral and central nervous system in preterm neonates is immature, their response to pain is different [29]. Preterm shows a lower threshold and greater reflex responses to touch stimulations. In very preterm neonates, pain modulation in descending endogenous pathways is not mature [30]; hence, it would be anticipated that the response to pain and analgesic effect will be different in preterm and term neonates.
Conclusion
In contrast to the single dose method that could meaningfully decrease PIPP-R score only in term compared to preterm neonates, divided doses of sucrose could similarly reduce PIPP-R score after heel stick in both term and preterm infants. Sucrose administration in divided doses may be more effective in reducing pain in preterm infants and could be a better strategy for prophylaxis of pain in this group of neonates.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1395.2433) and was registered with the Iranian Registry of Clinical Trials (IRCT) (No.: IRCT20090813002342N8).
Funding
This paper was extracted from the PhD dissertation of Mahkameh Soltani, approved by Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, and was supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Mohammadreza Rafati and Maryam Nakhshab; Drug preparation: Majid Saeedi; Investigation, writing original draft, and editing: Mohammadreza Rafati, Maryam Nakhshab and Mahkameh Soltani; Data collection: Mahkameh Soltani, Vajiheh Ghaffari and Roya Farhadi; Data analysis: Mohammadreza Rafati and Maryam Nakhshab; Funding acquisition and resources Mohammadreza Rafati; Final approval: All authors.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors appreciate the NICU personnel at Bu-Ali Sina Hospital affiliated to Mazandaran University of Medical Sciences.
References

The behavioral state scores before starting the procedure showed no significant difference between the study groups (P=0.602). The physiological responses were not significantly different between the two groups (for heart rate changes, P=0.909, and for changes in oxygen saturation, P=0.87).
Contrary to physiologic responses, nasolabial furrow time was significantly improved in the divided dose group (P=0.042). Also, eye squeeze time showed better results in patients receiving divided doses of sucrose (P=0.015).
Although the number of patients who experienced eyebrow bulge time less than 3 s was fewer in neonates who received sucrose three-divided dose (12.9% vs 22.4%), there was no significant difference between the two groups (P=0.094).
The mean PIPP-R score was 9.03±4.3 for neonates receiving single-dose oral sucrose, while the score for neonates receiving oral sucrose in 3-divided doses was 8.13±4.5 (P=0.27). However, a categorized comparison of the total PIPP-R score between the two groups showed a remarkable difference trend. Yet, it was not significant (P=0.07).
Pain assessment in preterm (<37 weeks gestation) and term neonates (≥37 weeks gestation) showed that divided doses of sucrose could similarly decrease the PIPP-R score in all neonates (P=0.96; Table 2).

The mean PIPP-R score was 7.7±3.9 and 8.4±4.4 in preterm and term neonates, respectively (P=0.45), while the PIPP-R score was meaningfully reduced in term neonates receiving single dose sucrose in comparison with preterm neonates (P=0.04; Table 3).

The mean PIPP-R score was 10.1±4.0 and 8.2±4.1 in preterm and term neonates, respectively (P=0.01).
There was a significant difference in mean PIPP-R scores when only preterm neonates were compared as sub-groups in single and divided dose groups (10.1±4.1 and 7.7±3.9; P=0.016). The sucrose administration method’s effect in reducing pain (categorical PIPP-R score) in preterm neonates is provided in Tables 4, 5, 6 and 7.




Discussion
This study aimed to investigate whether oral administration affects the analgesic properties of sucrose. We compared PIPP-R scores between two groups of neonates who received oral sucrose 24% solution 0.3 mL/kg, either a single dose of 120 s or in three-divided doses of 120, 90, and 60 s before heel lance. Single-dose sucrose could significantly reduce PIPP-R scores only in term infants compared to preterm infants, while in the divided dose method, PIPP-R scores decreased equally in both full-term and pre-term infants. Accordingly, when the sweet taste lasts longer in the mouth, less pain is experienced by neonates. To the best of our knowledge, this is the first study that tests the method of divided-dose administration of sucrose solution.
Okan et al. monitored heart rate changes for 3 min after heel lance between neonates receiving sucrose 20%, glucose 20%, and sterile water. The results showed that the mean heart rate was significantly higher in the placebo group than in the sucrose and glucose groups during the first min [19]. Most research did not show a direct relationship between oral sucrose and a decrease in heart rate changes [20]. In this study, the changes in heart rate were not significantly different during 30 s after heel lance between the two groups.
Oxygen saturation is another physiologic parameter that influences the total PIPP-R score. In the present study, changes in oxygen saturation were not significantly different between the two groups (P=0.87). In a systematic review in 2013, among 44 articles that investigated the analgesic properties of sucrose, only six articles appraised the effect of sucrose on oxygen saturation [21]. Five studies reported that sucrose exerts no enterprising effect on oxygen saturation. In another study, oral sucrose may decrease oxygen saturation [22].
Based on a meta-analysis article published in 2017, oral sweet solutions decrease crying time in neonates [23]. Despite this meta-analysis, Mundol et al. recently reported that sucrose did not ameliorate neonates’ cries during neonatal immunization with bacille Calmette-Guerin [24]. In the present study, we evaluated brow bulge, eye squeeze, and nasolabial furrow time. Nasolabial furrow time was significantly shorter for neonates receiving sucrose in three-divided doses between two groups (P=0.042). Like nasolabial furrow time, eye squeeze time was meaningfully improved in neonates participating in a three-divided dose group (P=0.015). Around 46% of babies in the three divided dose group experienced brow bulge time of less than 3 s compared to 25% of neonates participating in the single dose group. However, the difference in brow bulge time was statistically significant (P=0.094).
Several studies have shown that oral sucrose can exert a soothing effect among the neonate population during minor invasive procedures and reduce the total score of the PIPP scale [9, 25, 26, 27]. According to these studies, we accept the analgesic effect of oral sucrose; therefore, there was no placebo group in the present study.
Our study showed that sucrose may act differently in preterm and term neonates. Sucrose administration in divided doses could equally reduce PIPP-R score in both term and preterm neonates; however, using sucrose as a single dose could significantly decrease pain scores only in term neonates. Although the nociceptive pathways have function as early as 25 weeks gestation, it seems there are some differences in maturity and development of this pathway in preterm and term neonates [28]. Because the peripheral and central nervous system in preterm neonates is immature, their response to pain is different [29]. Preterm shows a lower threshold and greater reflex responses to touch stimulations. In very preterm neonates, pain modulation in descending endogenous pathways is not mature [30]; hence, it would be anticipated that the response to pain and analgesic effect will be different in preterm and term neonates.
Conclusion
In contrast to the single dose method that could meaningfully decrease PIPP-R score only in term compared to preterm neonates, divided doses of sucrose could similarly reduce PIPP-R score after heel stick in both term and preterm infants. Sucrose administration in divided doses may be more effective in reducing pain in preterm infants and could be a better strategy for prophylaxis of pain in this group of neonates.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research Ethics Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1395.2433) and was registered with the Iranian Registry of Clinical Trials (IRCT) (No.: IRCT20090813002342N8).
Funding
This paper was extracted from the PhD dissertation of Mahkameh Soltani, approved by Department of Clinical Pharmacy, School of Pharmacy, Mazandaran University of Medical Sciences, and was supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Mohammadreza Rafati and Maryam Nakhshab; Drug preparation: Majid Saeedi; Investigation, writing original draft, and editing: Mohammadreza Rafati, Maryam Nakhshab and Mahkameh Soltani; Data collection: Mahkameh Soltani, Vajiheh Ghaffari and Roya Farhadi; Data analysis: Mohammadreza Rafati and Maryam Nakhshab; Funding acquisition and resources Mohammadreza Rafati; Final approval: All authors.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors appreciate the NICU personnel at Bu-Ali Sina Hospital affiliated to Mazandaran University of Medical Sciences.
References
- Carbajal R. Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008; 300(1):60-70. [DOI:10.1001/jama.300.1.60] [PMID]
- Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur J Pain. 2016; 20(4):489-98. [DOI:10.1002/ejp.757] [PMID]
- Lemons JA, Blackmon LR, Kanto J, MacDonald HM, Miller CA, Papile LA, et al. Prevention and management of pain and stress in the neonate. Pediatrics. 2000; 105(2):454-61. [Link]
- Anand KJ. Effects of perinatal pain and stress. In: Mayer EA, Saper CB, editor. Progress in brain research: Brain and Maths in Ibero-America. Amsterdam: Elsevier; 2000. [DOI:10.1016/S0079-6123(08)62134-2]
- American Academy of Pediatrics Committee on Fetus and Newborn; American Academy of Pediatrics Section on Surgery; Canadian Paediatric Society Fetus and Newborn Committee; Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: An update. Pediatrics. 2006; 118(5):2231-41. [PMID]
- Anand KJ; International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001; 155(2):173-80. [DOI:10.1001/archpedi.155.2.173] [PMID]
- Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2016; 7(7):CD001069. [DOI:10.1002/14651858.CD001069.pub5] [PMID]
- Blass E, Fitzgerald E, Kehoe P. Interactions between sucrose, pain and isolation distress. Pharmacol Biochem Behav. 1987; 26(3):483-9. [DOI:10.1016/0091-3057(87)90153-5] [PMID]
- Stevens B, Yamada J, Campbell-Yeo M, Gibbins S, Harrison D, Dionne K, et al. The minimally effective dose of sucrose for procedural pain relief in neonates: A randomized controlled trial. MC Pediatr. 2018; 18(1):85. [DOI:10.1186/s12887-018-1026-x] [PMID]
- Ramenghi LA, Evans DJ, Levene MI. “Sucrose analgesia”: Absorptive mechanism or taste perception? Arch Dis Child Fetal Neonatal Ed. 1999; 80(2):F146-7. [DOI:10.1136/fn.80.2.F146] [PMID]
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behav Neurosci. 1989; 103(6):1168-75. [DOI:10.1037/0735-7044.103.6.1168] [PMID]
- Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005; 133(1):231-43. [DOI:10.1016/j.neuroscience.2005.01.055] [PMID]
- Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: A randomised controlled trial. Lancet. 2010; 376(9748):1225-32. [DOI:10.1016/S0140-6736(10)61303-7] [PMID]
- Johnston CC, Stremler R, Horton L, Friedman A. Effect of repeated doses of sucrose during heel stick procedure in preterm neonates. Neonatology. 1999; 75(3):160-6. [DOI:10.1159/000014092] [PMID]
- Lefrak L, Burch K, Caravantes R, Knoerlein K, DeNolf N, Duncan J, et al. Sucrose analgesia: Identifying potentially better practices. Pediatrics. 2006; 118(Supplement_2):S197-202. [DOI:10.1542/peds.2006-0913R] [PMID]
- Asmerom Y, Slater L, Boskovic DS, Bahjri K, Holden MS, Phillips R, et al. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J Pediatr. 2013; 163(1):29-35. [DOI:10.1016/j.jpeds.2012.12.088] [PMID]
- Yamada J, Bueno M, Santos L, Haliburton S, Campbell-Yeo M, Stevens B. Sucrose analgesia for heel-lance procedures in neonates. Cochrane Database Syst Rev. 2023, 8(8):CD014806. [PMID]
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: Development and initial validation. Clin J Pain. 1996; 12(1):13-22. [DOI:10.1097/00002508-199603000-00004] [PMID]
- Okan F, Coban A, Ince Z, Yapici Z, Can G. Analgesia in preterm newborns: the comparative effects of sucrose and glucose. Eur J Pediatr. 2007; 166(10):1017-24. [DOI:10.1007/s00431-006-0373-z] [PMID]
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2004; 3(3). [DOI:10.1002/14651858.CD001069.pub2]
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2013; 1(1). [DOI:10.1002/14651858.CD001069.pub4]
- Grabska J, Walden P, Lerer T, Kelly C, Hussain N, Donovan T, et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy of prematurity? J Perinatol. 2005; 25(1):33-5. [DOI:10.1038/sj.jp.7211199] [PMID]
- Harrison D, Larocque C, Bueno M, Stokes Y, Turner L, Hutton B, et al. Sweet solutions to reduce procedural pain in neonates: a meta-analysis. Pediatrics. 2017; 139(1):e20160955. [DOI:10.1542/peds.2016-0955] [PMID]
- Mundol TH, Prabhu AS, Saldanha PR. 25% oral dextrose as analgesia during neonatal immunisation with BCG. Int J Contemp Pediatri. 2018; 5(2):416-9. [DOI:10.18203/2349-3291.ijcp20180527]
- Collados-Gómez L, Ferrera-Camacho P, Fernandez-Serrano E, Camacho-Vicente V, Flores-Herrero C, García-Pozo AM, et al. Randomised crossover trial showed that using breast milk or sucrose provided the same analgesic effect in preterm infants of at least 28 weeks. Acta Paediatr. 2018; 107(3):436-41. [DOI:10.1111/apa.14151] [PMID]
- Kumari S, Datta V, Rehan H. Comparison of the efficacy of oral 25% glucose with oral 24% sucrose for pain relief during heel lance in preterm neonates: A double blind randomized controlled trial. J Trop Pediatr. 2017; 63(1):30-5. [DOI:10.1093/tropej/fmw045] [PMID]
- Thakkar P, Arora K, Goyal K, Das RR, Javadekar B, Aiyer S, et al. To evaluate and compare the efficacy of combined sucrose and non-nutritive sucking for analgesia in newborns undergoing minor painful procedure: A randomized controlled trial. J Perinatol. 2016; 36(1):67-70. [PMID]
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, et al. Cortical pain responses in human infants. J Neurosci. 2006; 26(14):3662-6. [DOI:10.1523/JNEUROSCI.0348-06.2006] [PMID]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005; 6(7):507-20. [DOI:10.1038/nrn1701] [PMID]
- Grunau R. Neonatal pain in very preterm infants: Long-term effects on brain, neurodevelopment and pain reactivity Rambam Maimonides Med J. 2013; 4(4):e0025. [DOI:10.5041/RMMJ.10132] [PMID]
Type of Study: Original Research |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |