Volume 10, Issue 1 (2024)
Pharm Biomed Res 2024, 10(1): 23-32 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ukwubile C A, Malgwi T S, Menkiti N D. Examining the Isolation of Bioactive Compounds, Antinociceptive, and Anti-inflammatory Activities of Strychnos Spinosa Lam. (Loganiaceae) Stembark Extract. Pharm Biomed Res 2024; 10 (1) :23-32
URL: http://pbr.mazums.ac.ir/article-1-530-en.html
URL: http://pbr.mazums.ac.ir/article-1-530-en.html
1- Department of Pharmacognosy, Faculty of Pharmacy, University of Maiduguri, Maiduguri, Nigeria.
2- Department of Chemistry, Faculty of Science, Ahmadu Bello University, Zaria, Nigeria.
2- Department of Chemistry, Faculty of Science, Ahmadu Bello University, Zaria, Nigeria.
Keywords: Bioactive compounds, Column chromatography, Strychnos spinosa, Antinociceptive, Anti-inflammatory
Full-Text [PDF 806 kb]
(601 Downloads)
| Abstract (HTML) (1448 Views)
Full-Text: (468 Views)
Introduction
Recent trends in natural products research have shown that medicinal plants and herbs contain arrays of bioactive phytocompounds that have been used to manage and treat various diseases for centuries. These plant-derived compounds have become a potential source of new drug discovery because they are essential for drug development [1]. The knowledge of using these plants is passed from one generation to the next by the indigenous traditional medical practitioners. Plants have been used to treat and manage diverse ailments, such as ulcers, pains, inflammation, cancers, fever, diabetes, hypertension, etc. due to their accessibility, low cost, low toxicity, and easy preparation methods [2].
Pains and inflammations are separate health problems; however, they are associated with each other issues. Pain is a sensory and emotionally unpleasant condition that is related to partial or actual damage to the tissue, while inflammation is an immunological response by tissue due to injury, which is often characterized by the gathering of white blood cells (WBC) antibodies, swelling, or inflammation and accumulation of fluids in the tissue. Pains and inflammations can be acute or chronic. For instance, sharp pains are usually due to injuries to tissues, diseases, or inflammations, while chronic pains are believed to be the disease itself [3, 4]. These two health conditions have been reported to be significant health challenges worldwide, resulting in excessive fatigue, reduction in quality of life, tissue degeneration, and deaths.
At present, there are several conventional drugs collectively, such as non-steroidal anti-inflammatory drugs (NSAIDS) and analgesics, that are used to treat inflammations and pains, respectively; however, these drugs are costly, produce undesirable side effects in the body, and some, like paracetamol, have been classified recently as proton pump inhibitors (PPIs) [5, 6]. Because of these shortcomings, the treatment of pains and inflammations using medicinal plants has received significant attention from natural product researchers and pharmacologists, and one such plant is Strychnos spinosa.
S. Spinosa is an evergreen thorny tree or shrub about 4 to 5 m in height. It sometimes grows up to 10 m high. This tree is found in woodlands, bushes, tropical forests, and elevated plains. The plant is commonly called spiny monkey orange, green monkey orange, or Kaffir orange. It is also called various native names in Nigeria, like Kokiya (Hausa), Ko’kiya (Kanuri), and Atako (Yoruba). The unripe fruits are poisonous when eaten. The plant is distributed in some countries in Africa, such as the Eastern Cape to Kwazulu-Natal in South Africa, Senegal, northern Nigeria, Mozambique, Namibia, and Zimbabwe, in addition to north African countries, like Morocco, as well as other parts of the world like South Florida and Israel. In traditional medicine, the leaves, stembarks, fruits, seeds, and roots have been used to treat various health challenges, like ulcers, wounds, headaches, snakebites, diabetes, cancers, stomachaches, fevers, etc. [7, 8, 9, 10]. Many phytoconstituents, such as alkaloids, phytosterols, triterpenes, essential oils, and flavonoids, have been isolated from plants [11].
Despite the uses of this plant for treating many diseases in traditional medicine in Africa, there is not enough research on the bioactive compounds present in the stembarks and their ability to reduce pain and inflammation. This study isolates bioactive compounds and evaluates the antinociceptive and anti-inflammatory activities of the methanol stembark extract of S. Spinosa using standard experimental procedures.
Materials and Methods
Study area
The study was conducted from January to April 2022 in the Department of Pharmacognosy, Faculty of Pharmacy, University of Maiduguri, Nigeria, Department of Chemistry, University of Ghana, Accra, Ghana, Central Research Laboratory, University of Lagos, Nigeria, and Department of Chemistry, King Abdul-Aziz University Jeddah, Saudi Arabia.
Plant and extraction
Fresh stembarks of S. spinosa were collected in the morning hour at a forest in Bali, Taraba State, Nigeria. It was identified at the herbarium of the Department of Pharmacognosy, University of Maiduguri, where a voucher number UMM/FP/LON/001 was deposited for the plant. They were washed with water to remove any unwanted dirt, air-dried under shade for two weeks, reduced into a fine powder using a wooden pestle and mortar, then cold-macerated using absolute methanol and kept at room temperature for 72 h. It was then filtered and evaporated using a rotary evaporator at 45oC temperature. The dark-greenish extract was weighed (percentage yield=16.24) and kept in the refrigerator for further use.
Preliminary phytochemical screening
The methanol stembark extract of S. spinosa was carried out to determine the presence of some metabolites, such as alkaloids, saponins, phenols, flavonoids, etc. using the methods described by Trease and Evans as well as Sofowora [12, 13].
Isolation and structural elucidation of bioactive compounds
The methanol stembark extract weighing 8 g was subjected to silica gel column chromatography using hexane: ethyl acetate (70:30) by gradient elution. A total of 50 fractions were collected and grouped into four sub-fractions (F1 to F4) based on their profiles on thin-layer chromatography plates. Fractions F1-F4 were further purified repeatedly on short columns using Sephadex® LH-20 (Merck, USA) and varying solvent systems, like F1 (hexane: Methanol [80:20]), F2 (hexane: Methanol [70:30]), F3 (hexane: Methanol [60:40]), and F4 (hexane: Methanol [50:50]). The purities of these compounds were monitored by obtaining a single spot on the thin layer chromatography (TLC) plates and sharp peaks and constant retention time from the high-liquid performance chromatography apparatus [14, 15]. Fractions were then recrystallized to yield various compounds as follows: F1 (compound A; 10 mg), F2 (compound B; 94 mg), F3 (compound C;100.3 mg), and F4 (compound D; 37 mg). The structures of these compounds were then elucidated as follows.
Fourier transform infrared spectroscopy
The compounds were carried out using an α II compact Fourier transform infrared (FTIR) spectroscopy spectrometer (Bruker, USA). The compounds were each scanned at 4000-500 wavelength cm-1 to show the various functional groups.
Gas chromatography/mass spectrometry (GC-MS)
The compounds were analyzed using a 7890A gas chromatography coupled to a 5975 triple-axis mass spectrometry detector (Agilent Technologies, US). The operating conditions are 50°C initial column temperature, final temperature was 200°C, 1 µL of sample was injected, gas flow rate was 1 mL/min, and MS spectra were taken at 60 eV. The compounds were identified using the National Institue for Science and Technology Structural Library [14, 15, 16, 17].
Liquid chromatography/mass spectrometry
The compounds were carried out using the quadrupole time of flight (TOF) 6530 Q-TOF 1.1. liquid chromatography/mass spectrometry instrument (Agilent Technologies, US). The method was followed as previously described by Steinmann and Ganreza [18]. The mass spectrometry parameters were optimized for each compound to ensure the most favorable conditions, like ionization, ionic transfers, and maximum signals for precursors and fragments [18].
Nuclear magnetic resonance spectroscopy
In this analysis, 1D (1H & 13C) nuclear magnetic resonance (NMR) spectroscopy was carried out on each of the compounds using the Bruker Avance III HD high resolution 850 MHz NMR spectrometer (Bruker, USA) at the Department of Chemistry, King Abdul-Aziz University Jeddah, Saudi Arabia. Deuterated chloroform (CDCl3) was used as the NMR solvent. Chemical shifts δ are reported in part per million (ppm) using CHCl3/CDCl3 (δH=7.25, δC=77.0 ppm) as an internal standard concerning tetramethylsilane. The coupling constants were denoted by J (expressed in Hz). The 2D NMR spectroscopies (correlation spectroscopy [COSY], heteronuclear single quantum correlation [HSQC] and heteronuclear multiple bonds correlation [HMBC]) were used to assign 1H and 13C NMR peaks [19]. KingDraw software was used to draw the structures of the corresponding compounds.
Experimental animals
Swiss albino mice weighing 15-25 g were purchased from the PJ Rat Farm Jos and the Department of Pharmacology, University of Jos, Nigeria. The mice were kept in aluminum cages with free access to water and food under average laboratory temperature for one week. The ethical protocol for using the mice was approved by the Research Ethics Committee of the University of Jos, Nigeria (approval number: UJ/FPS/ F17-00379).
Evaluation of antinociceptive activity
Hot plate and tail immersion methods were used to determine the ability of the plant extracts (crude and pure compounds) to acetic acid-induced writhing.
1. Acetic acid-induced writhing in mice
Mice were distributed into five groups of five mice per group. Group I received 10 mL of distilled water, and group II received 100 mg/kg of aspirin standard drug. In contrast, groups III, IV, and V received extract doses of 200, 400, and 800 mg/kg body weight (intraportal [IP]), respectively, administered to the animals. The number of abdominal writhing in mice induced by 0.6% acid (Sigma Aldrich, St Louis Mo, USA) was counted in 10, 20, 30, 40, 50, and 60 min with the help of a magnifying lens (Thomas Scientific, USA). The percentage of inhibition of writhing (a sign of analgesia) was calculated using the Equation 1 [20]:
1. % inhibition=[(Nc – Nt)/Nc]×100 (i)
Where Nc=number of writhing in the control group and Nt=number of writhing in the treated group.
Hot plate method
In this method, the animals from groups III, IV, and V were administered 200, 400, and 800 mg/kg S. spinosa stem bark extract, while groups I and II were given distilled water and pentazocine (IP) and were individually placed on a hot plate with temperature maintained at 55°C. In each group, the basal reaction times were noted by observing the licking of the hind paw or jumping responses in mice from the hot plate. The reaction times of the mice on the hot plate were recorded at 0, 15, 30, 45, and 60 min after extract administration. Exactly 17.5 mg/kg (IP) pentazocine (Ranbaxy Nig. Ltd.) was the standard drug used as the positive control group. A cut-off time of 15 s was observed to avoid damage to the paws [20, 21].
Tail immersion method
The mice were administered 200, 400, and 800 mg/kg S. spinosa stembark extract (IP) and had their distal 3 cm ends of the tails immersed in a water bath filled with hot water kept at 55°C. The time taken by the mice to withdraw their tails from the hot water was recorded as reaction times at intervals of 0, 15, 30, 45, and 60 min [20, 21]. The standard drug was pentazocine; 17.5 mg/kg (Ranbaxy Nig. Ltd.).
Anti-inflammatory evaluation
A carrageenan-induced paw edema model was used to evaluate the anti-inflammatory activity of the extracts. Briefly, the mice were divided into five groups of five mice per group. Each group (I-V) was administered (IP) 10 mL distilled water, 100 diclofenac sodium (Formulary, Nigeria), and 200, 400, and 800 mg/kg doses of plant extract, respectively. After 1 h of treatment, 0.1 mL 1% of carrageenan was injected to induce paw edema into the left hind paw immediately below the plantar of the aponeurosis. The edema volumes were measured using a vernier caliper at 1, 2, 3,4, and 6 h after carrageenan injection [22-26]. The percentage of inhibition of inflammation was determined from the Equation 2:
2. % inhibition=(1−Vt/Vc)100(ii)
Where Vt denotes the mean values of paw edema in the treated groups, and Vt represents the mean values of paw edema in the control group.
Statistical analysis
The data were expressed as Mean±SD. The one-way analysis of variance method was used to test the statistically significant differences between treated groups, followed by the Dunnett post-hoc test. The P<0.05 was considered important. All analyses were performed using the SPSS software, version 22 (IBM, SPSS Inc, US).
Results
Preliminary phytochemical screening
The phytochemical screening of the methanol stembark extract of S. spinosa revealed the presence of alkaloids, triterpenes, flavonoids, saponins, and sterols.
Isolation and structural elucidation of compounds
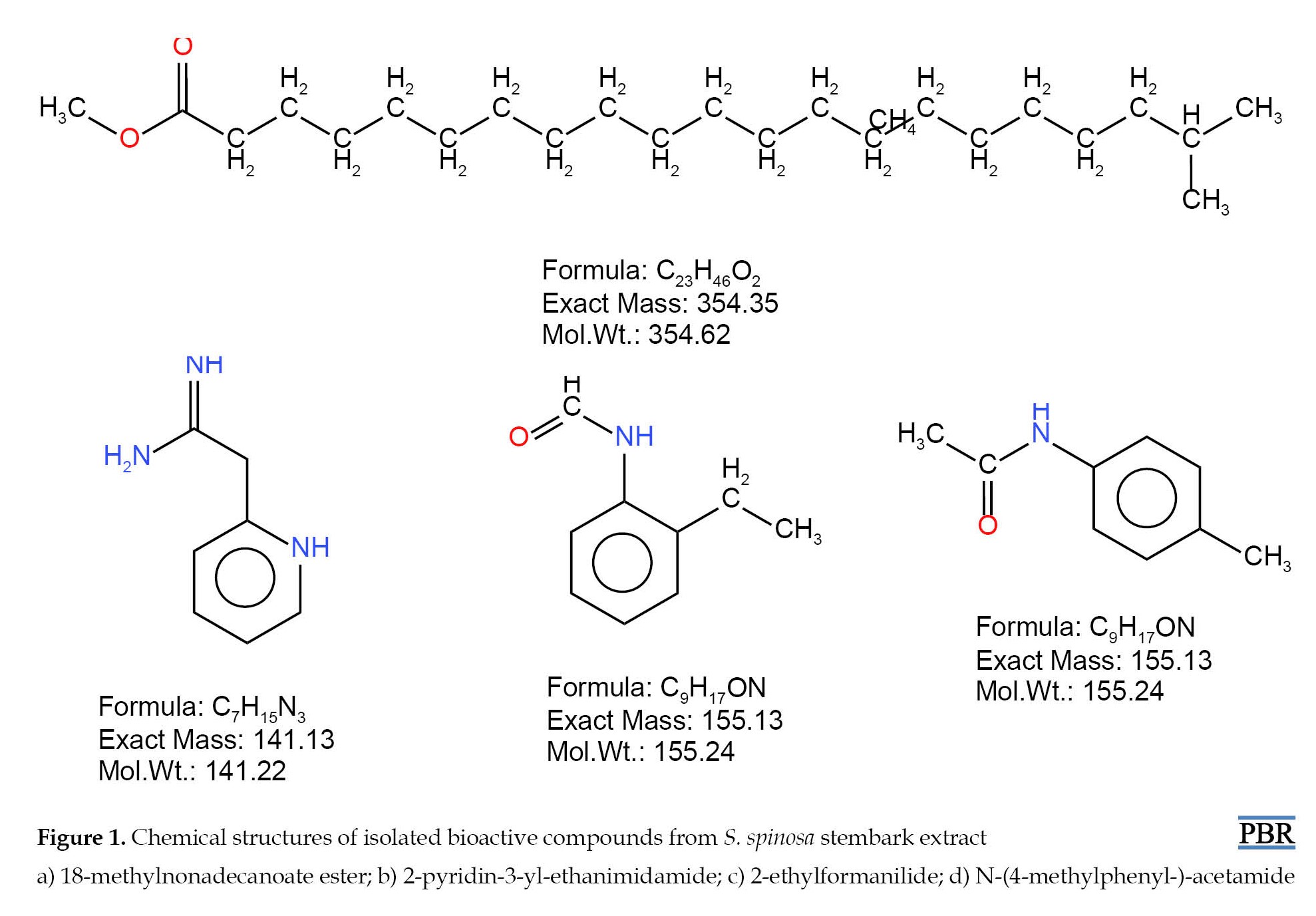
The isolated compounds (A, B, C, and D) showed various characteristic fingerprints, as described below (Figure 1). Compound A: 18-methylnonadecanoate ester C23H46O2 white powder, weight=10 mg, Rf=0.52, FTIR=3779.03 to 596.88 wavelength cm-1 (R-COOH of carboxylic acid at 3000-2500 wavelength cm-1 absorbance, CH2 aliphatic group at 2000-1500 wavelength cm-1, and CH3 stretching at 1490-1000 wavelength cm-1), EI-MS m/z 326, base ion peak=74, RT=24.5 min, peak area=0.20%, liquid chromatography-mass spectrometry (LCMS); m/z 360.3, 1H-NMR (850 MHz, CDCl3, ppm); five proton signals with CH3 group at 0.80 ppm (5H, d, J=2.4 Hz), CH2 group at 1.26 ppm (4H, d, J=11.5 Hz), CH2 group at 1.61 ppm (3H, s, J=16 Hz), CH2 group at 2.29 ppm (2H, s, J=10 Hz) and a CH3 group at 3.66 ppm (1H, s, J=10 Hz); 13C-NMR (850 MHz, CDCl3, ppm); 23 carbon atoms which mainly CH2 (methylene carbons) from 22.77 to 174.72 ppm.
Compound B: 2-pyridin-3-yl-ethanimidamide C7H15N3; white crystalline solid, weight=4.82 mg, Rf=0.56, FTIR=3500 to 1500 wavelength cm-1 (absorbance at 3320, 3340 and 3200 wavelength cm-1 represents amine groups (NH2), 1720 wavelength cm-1 is the region for carbonyl of ketone (-CO-), 2900 wavelength cm-1 is the region for aliphatic CH group), EI-MS m/z 135, base ion peak=43, RT=15.02 min, peak area=12.31%, LCMS; m/z 137.1, 1H-NMR (850 MHz; CDCl3, ppm); six proton signals, 1.34, 1.35, 1.37 ppm due to protons of -CH2-CH2-CO- (1H, t, J=0.25 Hz), 5.01 ppm of NH group (2H, s, J=0.48 Hz), 5.36 ppm of thiol (-SH) group (3H, s, J=1.84 Hz) and 7.41-7.85 ppm of phenyl rings (4H, s, J=2.24 Hz); 13C-NMR (850 MHz, CDCl3, ppm); seven carbon atoms mainly CH2 groups with one carbonyl group at 55 to 67.65 ppm and amide group at 76.90, 77.05 and 77.20 ppm.
Compound C: 2-ethylformanilide C9H17ON; green oily substance, weight=8.16 mg, Rf=0.55, FTIR=4000 to 1500 wavelength cm-1 (4000-3300 wavelength cm-1 of H-C=O group and 3200-3000 wavelength cm-1 showing NH group absorbance), EI-MS m/z 149.19, base ion peaks=106 & 120, RT=20.01 min, peak area=22.05%, LCMS; m/z 149.88, 1H-NMR (850 MHz; CDCl3, ppm); five proton signals, 2.82 and 2.94 ppm are signals due to CH3 protons (1H, dd, J=1.92 Hz), 7.41 ppm are region of NH (amide) group (2H, s, J=1.92 Hz), 8.03 ppm of -COOH (carboxylic group) (3H, s, J=0.77 Hz) and 8.16 ppm of C=O group (4H, s, J=1.24 Hz); 13C-NMR (850 MHz, CDCl3, ppm); nine carbon atoms mainly CH2 groups with one carbonyl group at 37.05 ppm and an amide group at 47.60 ppm and carbonyl group at 47.94 ppm.
Compound D: N-(4-methylphenyl-)-acetamide C9H17NO; colourless hygroscopic solid, weight=6.24 mg, Rf=0.54, FTIR=3000 to 1000 wavelength cm-1 (peak at 3500 wavelengths cm-1 showed absorbance of the carboxylic group while the peak at 3000 wavelengths cm-1 represents NH group attached to -COOH group), EI-MS m/z 149.18, base ion peaks=107, RT=19.98 min, peak area=23.02%, LCMS; m/z 149.28, 1H-NMR (850 MHz; CDCl3, ppm); four proton signals, 3.57 ppm showing CH2 group attached to -C=O group (1H, s, J=2.39 Hz), 5.5-5.9 ppm showing NH (amide) groups (2H, 3H, dd, J=11.47, 5.8 Hz) and 7.52-7.07 ppm showing CH of aromatic group (4H, m, J=0.97 Hz), 13C-NMR (850 MHz, CDCl3, ppm); nine carbon atoms mainly CH2 groups with one carbonyl group, an amide group and an ethoxy group at 22.73, 33.08 and 33.79 ppm, respectively.
Antinociceptive activity
Acetic acid-induced writhing in mice
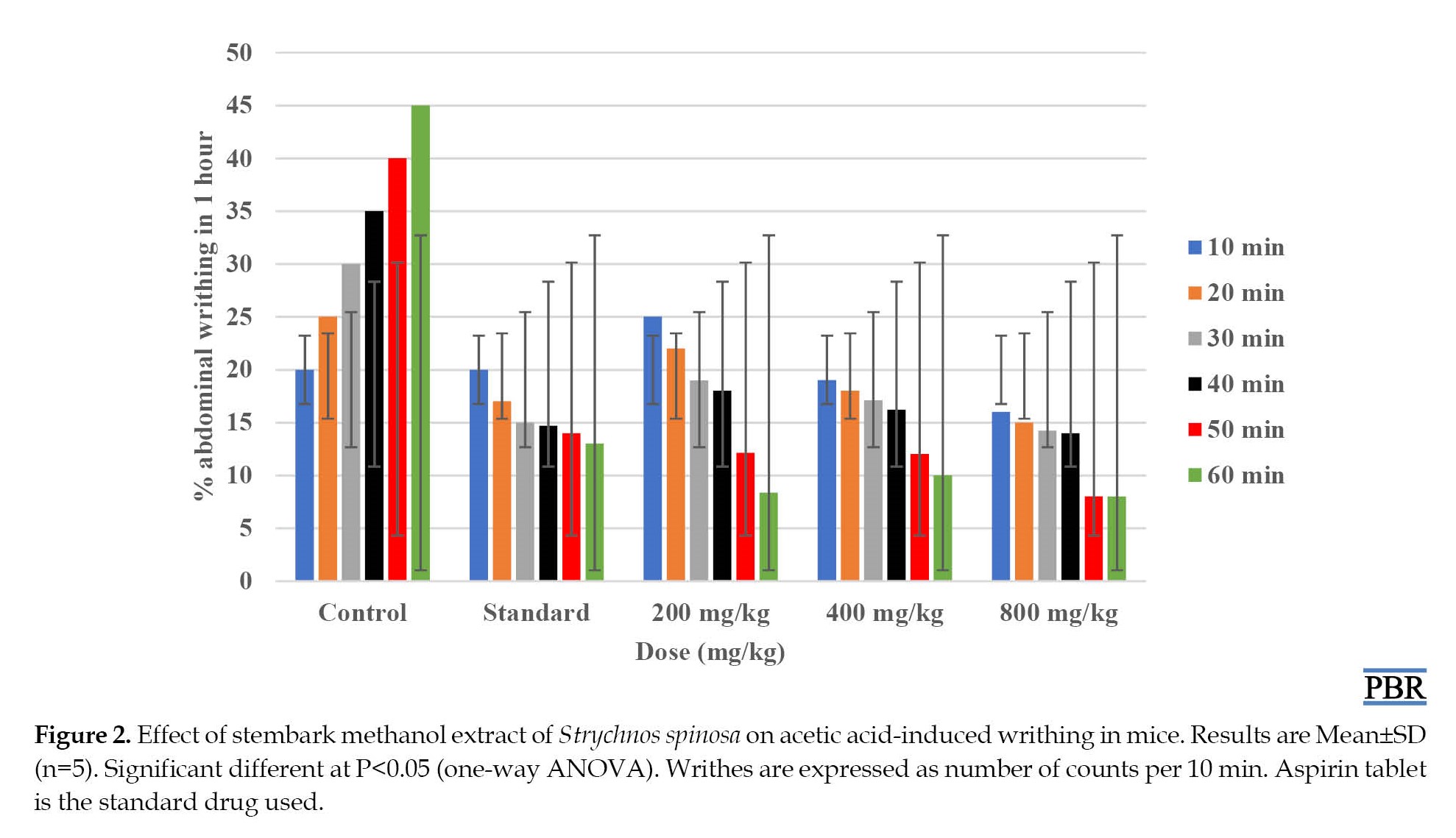
The stem bark methanol extract of S. spinosa (200, 400, and 800 mg/kg, IP) significantly (P<0.05) reduced the number of abdominal writhing in mice in a dose-dependent fashion in 60 min when compared to the control group (Figure 2).
Hot plate-induced nociception in mice
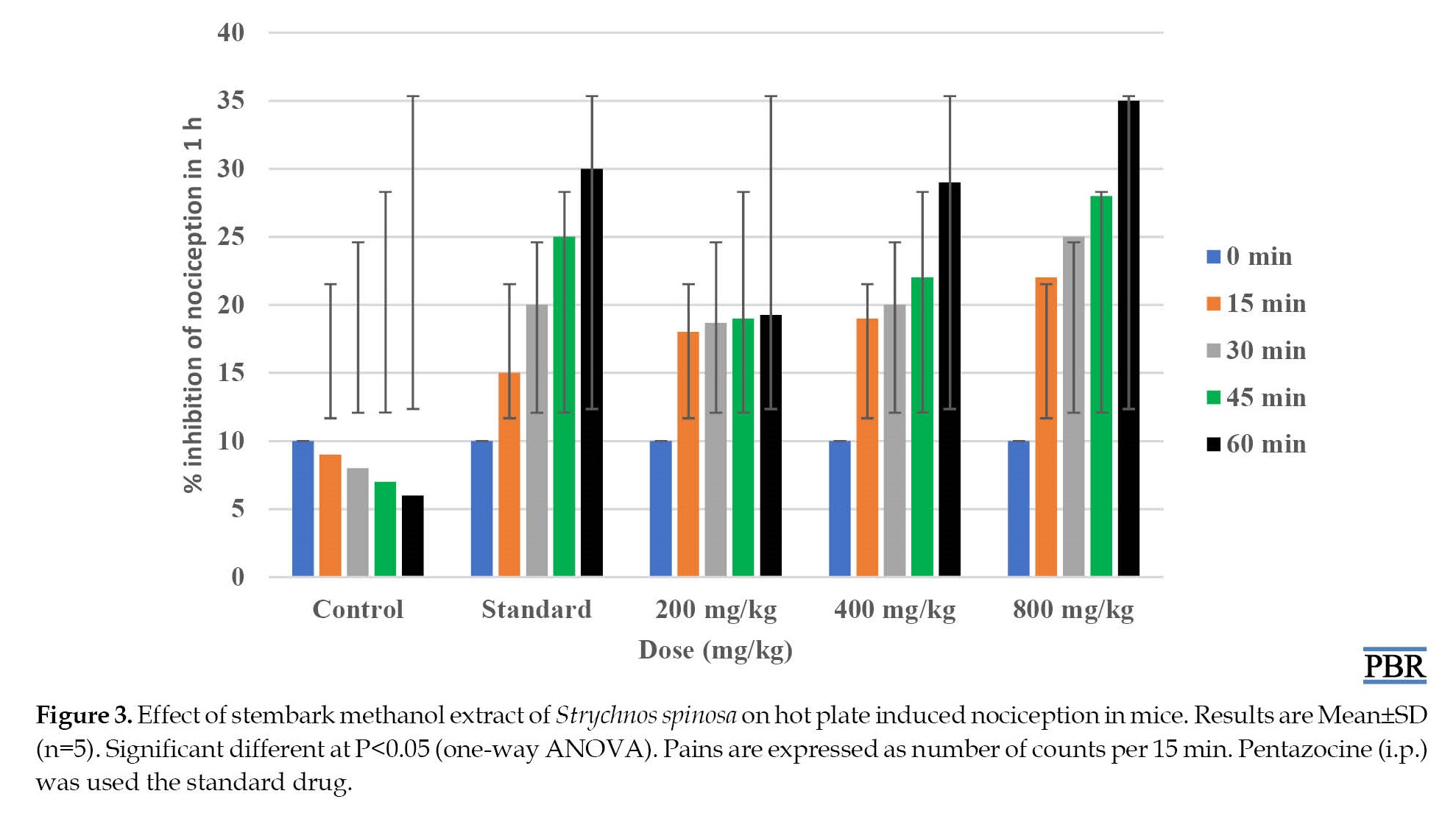
The results showed that S. spinosa stembark extract at the highest dose of 800 mg/kg, IP delayed significantly (P<0.05) the reaction times to pain by the mice on a hot plate for over 60 min period of study. This result was comparable to the standard drug pentazocine, which produced a similar effect in the mice (Figure 3).
Tail immersion nociception in mice
In tail immersion-induced pain, particularly at 400 and 800 mg/kg doses, the extract significantly prolonged the latency period (P<0.05) in pain response. The ability of the S. spinosa stem bark extract to prolong the reaction time to pain suggested that the extract was bestowed with central antinociceptive activity in the mice (Figure 4).
Carrageenan-induced paw-edema in mice
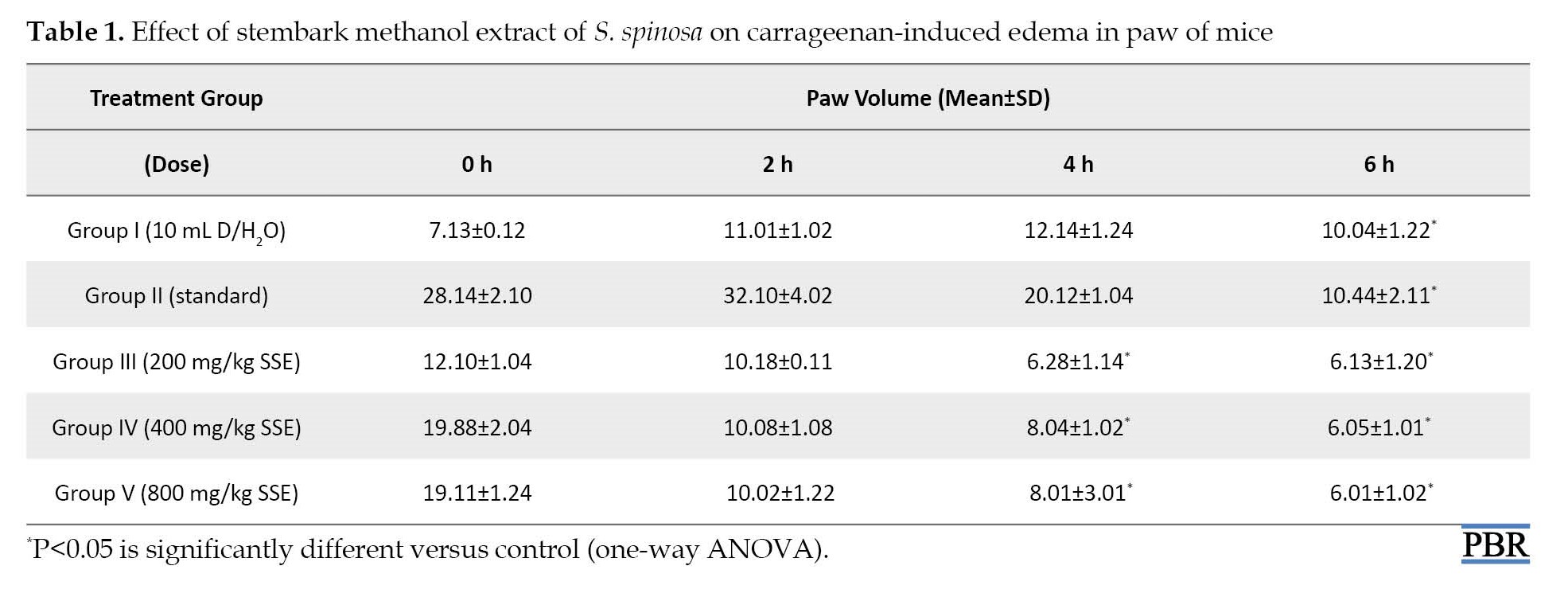
The result showed that the extract at 800 mg/kg (IP) dose significantly reduced carrageenan-induced edema in mice from 1 h up to the 6 h period of the study. From the result, the extract exerts a dose-dependent reduction in the volume of the paw-edema in mice. This result was comparable to the standard drug diclofenac sodium (Table 1).
Discussion
Medicinal plant parts, such as leaves, stembarks, fruits, seeds, roots, and latex in the healthcare delivery system, such as foods and food supplements, have been increasing for decades globally [27]. For instance, the decoction of S. spinosa stembarks has been used in traditional medicine in Nigeria for various diseases, such as pains, inflammation, wounds, fevers, and diabetes. Despite the overwhelming uses of the plant, there are no proven scientific experiments to ascertain these claims. In the current study, the phytochemical contents of the stembark crude methanol extract revealed the presence of alkaloids, triterpenes, flavonoids, saponins, steroids, terpenoids, and flavonoids. These secondary metabolites play essential biological roles in the body as anticancer, antidiabetics, analgesics, antipyretics, and anti-inflammation. For example, alkaloids show a wide array of pharmacological actions like analgesics, such as local anesthesia, cardiac stimulants, respiratory stimulants and relaxants, vasoconstrictors, muscular relaxants, anticancer, hypertensive, and hypotensive agents. Flavonoids are anti-inflammatory and antiallergic agents, antithrombotic, vasoprotective, antitumor, and gastric mucosa protectors. Similarly, as do antidotes in metal poisons and alkaloids, tannin-containing phytomedicine acts as an antidiarrheal. Some saponins have antitumor, anti-inflammatory, molluscicidal, spermicidal, sedative, expectorant, and analgesic effects [28-31]. These metabolites may have played similar roles in the current study. These bioactive compounds obtained from the present study were isolated from the ethyl acetate most active fraction from the liquid-liquid partition and bio-monitored. The characterization of bioactive compounds from plants is usually done using spectroscopic techniques, such as NMR, mass spectrometry, liquid chromatography-mass spectrometry, and FTIR. In this study, isolated bioactive compounds played vital roles and were characterized by these techniques. For example, 2-pyridin-3-yl-ethanimidamide and their derivatives are used as pharmaceuticals as Raf kinase inhibitors for the treatment of pains, neurotraumatic diseases, cancer, chronic neurodegeneration, migraine, and cardiac hypertrophy [32]. These compounds may play critical roles in the antinociception and anti-inflammation mechanisms of the plant.
The acetic acid-induced abdominal writhing assay in mice has been used for a long time as an experimental model for the testing of antinociceptive drugs [33]. This assay is a non-selective experimental model for the antinociceptive activity of plant extracts because it is an injection of acetic acid intraperitoneally, which activates the release of many mediators such as bradykinins, prostaglandins, like PGI2, as well as cytokines which are pro-inflammatory examples tumor necrosis factor-α (TNF-α), Interleukin (IL)-6, IL-8, and IL-1 [34]. The study showed that the methanol stem bark extracts significantly decreased the number of abdominal writhing in mice in a dose-dependent manner. The results obtained from the study were comparable to the control group (P<0.05). Our results showed that stem bark methanol extract possessed potential antinociceptive effects in mice at the doses (Figures 2, 3, and 4).
The involvement of the central nervous system in the antinociceptive activity of S. spinosa, hot plate, and tail immersion assays were carried out. The study showed an increase in latency on a hot plate, which is evidence of the involvement of the central nervous system, where most analgesic drugs act [35]. In this study, the extract must have achieved antinociceptive effects by stimulating the neurotransmitters to activate the nociceptor nerves from the spinal cord [36]. The antinociceptive effect of the plant extract was further affirmed by the dose-dependent increase in reaction time in tail withdrawing by the mice in the tail immersion test. The latency and reaction times increase in this current study were comparable to the controls (P<0.05).
The carrageenan-induced paw-edema mice model is used to assess plant extracts or compounds’ anti-inflammatory activity. The method has been described as highly reproducible. According to this study, S. spinosa methanol stembark extract exhibited a dose-dependent reduction in paw edema volume after 6 h (Table 1). The ability of the extract to reduce paw edema was due to the action of some inflammatory mediators, like serotonin, histamine, and bradykinin, which facilitated the release of prostaglandins prostaglandins A (PGAs) and nitric oxide to block histamine receptors [36]. Thus, the study showed the extract inhibited the paw-edema induced by carrageenan by blocking histamine or other pro-inflammatory enzymes, thereby causing blockage of sustained release of inflammatory promoters, such as prostaglandins PGAs and subsequent release of peroxidases. The reduction of paw edema in the mice was significant (P<0.05) compared to the control.
Conclusion
Our study showed that the crude methanol stembark extract of S. spinosa possessed significant antinociceptive and anti-inflammatory activities, which are possibly related to the activation of pain receptors and blockage of the release of pro-inflammatory mediators, such as histamines, neutrophils, and prostaglandins. The observed antinociceptive and anti-inflammatory activities of the extract were due to some phytochemical contents of the stembark and the isolated bioactive compounds. However, the specific roles of these compounds need to be investigated further, as well as the extract’s mechanism of action.
Ethical Considerations
Compliance with ethical guidelines
This research was approved by Research and Ethical Committee of University of Jos, Nigeria (Code: UJ/FPS/ F17-00379).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization and Supervision: Cletus Anes Ukwubile; Methodology: Cletus Anes Ukwubile and Pharm Troy Salvia Malgwi; Investigation, Writing – original draft, and Writing – review & editing: All authors; Data collection: All authors; Data analysis: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank King Abdulaziz University Jeddah, Republic of Saudi Arabia NMR Centre, and University of Lagos Nigeria, Central Laboratory for spectroscopic analysis.
References
Recent trends in natural products research have shown that medicinal plants and herbs contain arrays of bioactive phytocompounds that have been used to manage and treat various diseases for centuries. These plant-derived compounds have become a potential source of new drug discovery because they are essential for drug development [1]. The knowledge of using these plants is passed from one generation to the next by the indigenous traditional medical practitioners. Plants have been used to treat and manage diverse ailments, such as ulcers, pains, inflammation, cancers, fever, diabetes, hypertension, etc. due to their accessibility, low cost, low toxicity, and easy preparation methods [2].
Pains and inflammations are separate health problems; however, they are associated with each other issues. Pain is a sensory and emotionally unpleasant condition that is related to partial or actual damage to the tissue, while inflammation is an immunological response by tissue due to injury, which is often characterized by the gathering of white blood cells (WBC) antibodies, swelling, or inflammation and accumulation of fluids in the tissue. Pains and inflammations can be acute or chronic. For instance, sharp pains are usually due to injuries to tissues, diseases, or inflammations, while chronic pains are believed to be the disease itself [3, 4]. These two health conditions have been reported to be significant health challenges worldwide, resulting in excessive fatigue, reduction in quality of life, tissue degeneration, and deaths.
At present, there are several conventional drugs collectively, such as non-steroidal anti-inflammatory drugs (NSAIDS) and analgesics, that are used to treat inflammations and pains, respectively; however, these drugs are costly, produce undesirable side effects in the body, and some, like paracetamol, have been classified recently as proton pump inhibitors (PPIs) [5, 6]. Because of these shortcomings, the treatment of pains and inflammations using medicinal plants has received significant attention from natural product researchers and pharmacologists, and one such plant is Strychnos spinosa.
S. Spinosa is an evergreen thorny tree or shrub about 4 to 5 m in height. It sometimes grows up to 10 m high. This tree is found in woodlands, bushes, tropical forests, and elevated plains. The plant is commonly called spiny monkey orange, green monkey orange, or Kaffir orange. It is also called various native names in Nigeria, like Kokiya (Hausa), Ko’kiya (Kanuri), and Atako (Yoruba). The unripe fruits are poisonous when eaten. The plant is distributed in some countries in Africa, such as the Eastern Cape to Kwazulu-Natal in South Africa, Senegal, northern Nigeria, Mozambique, Namibia, and Zimbabwe, in addition to north African countries, like Morocco, as well as other parts of the world like South Florida and Israel. In traditional medicine, the leaves, stembarks, fruits, seeds, and roots have been used to treat various health challenges, like ulcers, wounds, headaches, snakebites, diabetes, cancers, stomachaches, fevers, etc. [7, 8, 9, 10]. Many phytoconstituents, such as alkaloids, phytosterols, triterpenes, essential oils, and flavonoids, have been isolated from plants [11].
Despite the uses of this plant for treating many diseases in traditional medicine in Africa, there is not enough research on the bioactive compounds present in the stembarks and their ability to reduce pain and inflammation. This study isolates bioactive compounds and evaluates the antinociceptive and anti-inflammatory activities of the methanol stembark extract of S. Spinosa using standard experimental procedures.
Materials and Methods
Study area
The study was conducted from January to April 2022 in the Department of Pharmacognosy, Faculty of Pharmacy, University of Maiduguri, Nigeria, Department of Chemistry, University of Ghana, Accra, Ghana, Central Research Laboratory, University of Lagos, Nigeria, and Department of Chemistry, King Abdul-Aziz University Jeddah, Saudi Arabia.
Plant and extraction
Fresh stembarks of S. spinosa were collected in the morning hour at a forest in Bali, Taraba State, Nigeria. It was identified at the herbarium of the Department of Pharmacognosy, University of Maiduguri, where a voucher number UMM/FP/LON/001 was deposited for the plant. They were washed with water to remove any unwanted dirt, air-dried under shade for two weeks, reduced into a fine powder using a wooden pestle and mortar, then cold-macerated using absolute methanol and kept at room temperature for 72 h. It was then filtered and evaporated using a rotary evaporator at 45oC temperature. The dark-greenish extract was weighed (percentage yield=16.24) and kept in the refrigerator for further use.
Preliminary phytochemical screening
The methanol stembark extract of S. spinosa was carried out to determine the presence of some metabolites, such as alkaloids, saponins, phenols, flavonoids, etc. using the methods described by Trease and Evans as well as Sofowora [12, 13].
Isolation and structural elucidation of bioactive compounds
The methanol stembark extract weighing 8 g was subjected to silica gel column chromatography using hexane: ethyl acetate (70:30) by gradient elution. A total of 50 fractions were collected and grouped into four sub-fractions (F1 to F4) based on their profiles on thin-layer chromatography plates. Fractions F1-F4 were further purified repeatedly on short columns using Sephadex® LH-20 (Merck, USA) and varying solvent systems, like F1 (hexane: Methanol [80:20]), F2 (hexane: Methanol [70:30]), F3 (hexane: Methanol [60:40]), and F4 (hexane: Methanol [50:50]). The purities of these compounds were monitored by obtaining a single spot on the thin layer chromatography (TLC) plates and sharp peaks and constant retention time from the high-liquid performance chromatography apparatus [14, 15]. Fractions were then recrystallized to yield various compounds as follows: F1 (compound A; 10 mg), F2 (compound B; 94 mg), F3 (compound C;100.3 mg), and F4 (compound D; 37 mg). The structures of these compounds were then elucidated as follows.
Fourier transform infrared spectroscopy
The compounds were carried out using an α II compact Fourier transform infrared (FTIR) spectroscopy spectrometer (Bruker, USA). The compounds were each scanned at 4000-500 wavelength cm-1 to show the various functional groups.
Gas chromatography/mass spectrometry (GC-MS)
The compounds were analyzed using a 7890A gas chromatography coupled to a 5975 triple-axis mass spectrometry detector (Agilent Technologies, US). The operating conditions are 50°C initial column temperature, final temperature was 200°C, 1 µL of sample was injected, gas flow rate was 1 mL/min, and MS spectra were taken at 60 eV. The compounds were identified using the National Institue for Science and Technology Structural Library [14, 15, 16, 17].
Liquid chromatography/mass spectrometry
The compounds were carried out using the quadrupole time of flight (TOF) 6530 Q-TOF 1.1. liquid chromatography/mass spectrometry instrument (Agilent Technologies, US). The method was followed as previously described by Steinmann and Ganreza [18]. The mass spectrometry parameters were optimized for each compound to ensure the most favorable conditions, like ionization, ionic transfers, and maximum signals for precursors and fragments [18].
Nuclear magnetic resonance spectroscopy
In this analysis, 1D (1H & 13C) nuclear magnetic resonance (NMR) spectroscopy was carried out on each of the compounds using the Bruker Avance III HD high resolution 850 MHz NMR spectrometer (Bruker, USA) at the Department of Chemistry, King Abdul-Aziz University Jeddah, Saudi Arabia. Deuterated chloroform (CDCl3) was used as the NMR solvent. Chemical shifts δ are reported in part per million (ppm) using CHCl3/CDCl3 (δH=7.25, δC=77.0 ppm) as an internal standard concerning tetramethylsilane. The coupling constants were denoted by J (expressed in Hz). The 2D NMR spectroscopies (correlation spectroscopy [COSY], heteronuclear single quantum correlation [HSQC] and heteronuclear multiple bonds correlation [HMBC]) were used to assign 1H and 13C NMR peaks [19]. KingDraw software was used to draw the structures of the corresponding compounds.
Experimental animals
Swiss albino mice weighing 15-25 g were purchased from the PJ Rat Farm Jos and the Department of Pharmacology, University of Jos, Nigeria. The mice were kept in aluminum cages with free access to water and food under average laboratory temperature for one week. The ethical protocol for using the mice was approved by the Research Ethics Committee of the University of Jos, Nigeria (approval number: UJ/FPS/ F17-00379).
Evaluation of antinociceptive activity
Hot plate and tail immersion methods were used to determine the ability of the plant extracts (crude and pure compounds) to acetic acid-induced writhing.
1. Acetic acid-induced writhing in mice
Mice were distributed into five groups of five mice per group. Group I received 10 mL of distilled water, and group II received 100 mg/kg of aspirin standard drug. In contrast, groups III, IV, and V received extract doses of 200, 400, and 800 mg/kg body weight (intraportal [IP]), respectively, administered to the animals. The number of abdominal writhing in mice induced by 0.6% acid (Sigma Aldrich, St Louis Mo, USA) was counted in 10, 20, 30, 40, 50, and 60 min with the help of a magnifying lens (Thomas Scientific, USA). The percentage of inhibition of writhing (a sign of analgesia) was calculated using the Equation 1 [20]:
1. % inhibition=[(Nc – Nt)/Nc]×100 (i)
Where Nc=number of writhing in the control group and Nt=number of writhing in the treated group.
Hot plate method
In this method, the animals from groups III, IV, and V were administered 200, 400, and 800 mg/kg S. spinosa stem bark extract, while groups I and II were given distilled water and pentazocine (IP) and were individually placed on a hot plate with temperature maintained at 55°C. In each group, the basal reaction times were noted by observing the licking of the hind paw or jumping responses in mice from the hot plate. The reaction times of the mice on the hot plate were recorded at 0, 15, 30, 45, and 60 min after extract administration. Exactly 17.5 mg/kg (IP) pentazocine (Ranbaxy Nig. Ltd.) was the standard drug used as the positive control group. A cut-off time of 15 s was observed to avoid damage to the paws [20, 21].
Tail immersion method
The mice were administered 200, 400, and 800 mg/kg S. spinosa stembark extract (IP) and had their distal 3 cm ends of the tails immersed in a water bath filled with hot water kept at 55°C. The time taken by the mice to withdraw their tails from the hot water was recorded as reaction times at intervals of 0, 15, 30, 45, and 60 min [20, 21]. The standard drug was pentazocine; 17.5 mg/kg (Ranbaxy Nig. Ltd.).
Anti-inflammatory evaluation
A carrageenan-induced paw edema model was used to evaluate the anti-inflammatory activity of the extracts. Briefly, the mice were divided into five groups of five mice per group. Each group (I-V) was administered (IP) 10 mL distilled water, 100 diclofenac sodium (Formulary, Nigeria), and 200, 400, and 800 mg/kg doses of plant extract, respectively. After 1 h of treatment, 0.1 mL 1% of carrageenan was injected to induce paw edema into the left hind paw immediately below the plantar of the aponeurosis. The edema volumes were measured using a vernier caliper at 1, 2, 3,4, and 6 h after carrageenan injection [22-26]. The percentage of inhibition of inflammation was determined from the Equation 2:
2. % inhibition=(1−Vt/Vc)100(ii)
Where Vt denotes the mean values of paw edema in the treated groups, and Vt represents the mean values of paw edema in the control group.
Statistical analysis
The data were expressed as Mean±SD. The one-way analysis of variance method was used to test the statistically significant differences between treated groups, followed by the Dunnett post-hoc test. The P<0.05 was considered important. All analyses were performed using the SPSS software, version 22 (IBM, SPSS Inc, US).
Results
Preliminary phytochemical screening
The phytochemical screening of the methanol stembark extract of S. spinosa revealed the presence of alkaloids, triterpenes, flavonoids, saponins, and sterols.
Isolation and structural elucidation of compounds
The isolated compounds (A, B, C, and D) showed various characteristic fingerprints, as described below (Figure 1). Compound A: 18-methylnonadecanoate ester C23H46O2 white powder, weight=10 mg, Rf=0.52, FTIR=3779.03 to 596.88 wavelength cm-1 (R-COOH of carboxylic acid at 3000-2500 wavelength cm-1 absorbance, CH2 aliphatic group at 2000-1500 wavelength cm-1, and CH3 stretching at 1490-1000 wavelength cm-1), EI-MS m/z 326, base ion peak=74, RT=24.5 min, peak area=0.20%, liquid chromatography-mass spectrometry (LCMS); m/z 360.3, 1H-NMR (850 MHz, CDCl3, ppm); five proton signals with CH3 group at 0.80 ppm (5H, d, J=2.4 Hz), CH2 group at 1.26 ppm (4H, d, J=11.5 Hz), CH2 group at 1.61 ppm (3H, s, J=16 Hz), CH2 group at 2.29 ppm (2H, s, J=10 Hz) and a CH3 group at 3.66 ppm (1H, s, J=10 Hz); 13C-NMR (850 MHz, CDCl3, ppm); 23 carbon atoms which mainly CH2 (methylene carbons) from 22.77 to 174.72 ppm.
Compound B: 2-pyridin-3-yl-ethanimidamide C7H15N3; white crystalline solid, weight=4.82 mg, Rf=0.56, FTIR=3500 to 1500 wavelength cm-1 (absorbance at 3320, 3340 and 3200 wavelength cm-1 represents amine groups (NH2), 1720 wavelength cm-1 is the region for carbonyl of ketone (-CO-), 2900 wavelength cm-1 is the region for aliphatic CH group), EI-MS m/z 135, base ion peak=43, RT=15.02 min, peak area=12.31%, LCMS; m/z 137.1, 1H-NMR (850 MHz; CDCl3, ppm); six proton signals, 1.34, 1.35, 1.37 ppm due to protons of -CH2-CH2-CO- (1H, t, J=0.25 Hz), 5.01 ppm of NH group (2H, s, J=0.48 Hz), 5.36 ppm of thiol (-SH) group (3H, s, J=1.84 Hz) and 7.41-7.85 ppm of phenyl rings (4H, s, J=2.24 Hz); 13C-NMR (850 MHz, CDCl3, ppm); seven carbon atoms mainly CH2 groups with one carbonyl group at 55 to 67.65 ppm and amide group at 76.90, 77.05 and 77.20 ppm.
Compound C: 2-ethylformanilide C9H17ON; green oily substance, weight=8.16 mg, Rf=0.55, FTIR=4000 to 1500 wavelength cm-1 (4000-3300 wavelength cm-1 of H-C=O group and 3200-3000 wavelength cm-1 showing NH group absorbance), EI-MS m/z 149.19, base ion peaks=106 & 120, RT=20.01 min, peak area=22.05%, LCMS; m/z 149.88, 1H-NMR (850 MHz; CDCl3, ppm); five proton signals, 2.82 and 2.94 ppm are signals due to CH3 protons (1H, dd, J=1.92 Hz), 7.41 ppm are region of NH (amide) group (2H, s, J=1.92 Hz), 8.03 ppm of -COOH (carboxylic group) (3H, s, J=0.77 Hz) and 8.16 ppm of C=O group (4H, s, J=1.24 Hz); 13C-NMR (850 MHz, CDCl3, ppm); nine carbon atoms mainly CH2 groups with one carbonyl group at 37.05 ppm and an amide group at 47.60 ppm and carbonyl group at 47.94 ppm.
Compound D: N-(4-methylphenyl-)-acetamide C9H17NO; colourless hygroscopic solid, weight=6.24 mg, Rf=0.54, FTIR=3000 to 1000 wavelength cm-1 (peak at 3500 wavelengths cm-1 showed absorbance of the carboxylic group while the peak at 3000 wavelengths cm-1 represents NH group attached to -COOH group), EI-MS m/z 149.18, base ion peaks=107, RT=19.98 min, peak area=23.02%, LCMS; m/z 149.28, 1H-NMR (850 MHz; CDCl3, ppm); four proton signals, 3.57 ppm showing CH2 group attached to -C=O group (1H, s, J=2.39 Hz), 5.5-5.9 ppm showing NH (amide) groups (2H, 3H, dd, J=11.47, 5.8 Hz) and 7.52-7.07 ppm showing CH of aromatic group (4H, m, J=0.97 Hz), 13C-NMR (850 MHz, CDCl3, ppm); nine carbon atoms mainly CH2 groups with one carbonyl group, an amide group and an ethoxy group at 22.73, 33.08 and 33.79 ppm, respectively.
Antinociceptive activity
Acetic acid-induced writhing in mice
The stem bark methanol extract of S. spinosa (200, 400, and 800 mg/kg, IP) significantly (P<0.05) reduced the number of abdominal writhing in mice in a dose-dependent fashion in 60 min when compared to the control group (Figure 2).
Hot plate-induced nociception in mice
The results showed that S. spinosa stembark extract at the highest dose of 800 mg/kg, IP delayed significantly (P<0.05) the reaction times to pain by the mice on a hot plate for over 60 min period of study. This result was comparable to the standard drug pentazocine, which produced a similar effect in the mice (Figure 3).
Tail immersion nociception in mice
In tail immersion-induced pain, particularly at 400 and 800 mg/kg doses, the extract significantly prolonged the latency period (P<0.05) in pain response. The ability of the S. spinosa stem bark extract to prolong the reaction time to pain suggested that the extract was bestowed with central antinociceptive activity in the mice (Figure 4).
Carrageenan-induced paw-edema in mice
The result showed that the extract at 800 mg/kg (IP) dose significantly reduced carrageenan-induced edema in mice from 1 h up to the 6 h period of the study. From the result, the extract exerts a dose-dependent reduction in the volume of the paw-edema in mice. This result was comparable to the standard drug diclofenac sodium (Table 1).

Discussion
Medicinal plant parts, such as leaves, stembarks, fruits, seeds, roots, and latex in the healthcare delivery system, such as foods and food supplements, have been increasing for decades globally [27]. For instance, the decoction of S. spinosa stembarks has been used in traditional medicine in Nigeria for various diseases, such as pains, inflammation, wounds, fevers, and diabetes. Despite the overwhelming uses of the plant, there are no proven scientific experiments to ascertain these claims. In the current study, the phytochemical contents of the stembark crude methanol extract revealed the presence of alkaloids, triterpenes, flavonoids, saponins, steroids, terpenoids, and flavonoids. These secondary metabolites play essential biological roles in the body as anticancer, antidiabetics, analgesics, antipyretics, and anti-inflammation. For example, alkaloids show a wide array of pharmacological actions like analgesics, such as local anesthesia, cardiac stimulants, respiratory stimulants and relaxants, vasoconstrictors, muscular relaxants, anticancer, hypertensive, and hypotensive agents. Flavonoids are anti-inflammatory and antiallergic agents, antithrombotic, vasoprotective, antitumor, and gastric mucosa protectors. Similarly, as do antidotes in metal poisons and alkaloids, tannin-containing phytomedicine acts as an antidiarrheal. Some saponins have antitumor, anti-inflammatory, molluscicidal, spermicidal, sedative, expectorant, and analgesic effects [28-31]. These metabolites may have played similar roles in the current study. These bioactive compounds obtained from the present study were isolated from the ethyl acetate most active fraction from the liquid-liquid partition and bio-monitored. The characterization of bioactive compounds from plants is usually done using spectroscopic techniques, such as NMR, mass spectrometry, liquid chromatography-mass spectrometry, and FTIR. In this study, isolated bioactive compounds played vital roles and were characterized by these techniques. For example, 2-pyridin-3-yl-ethanimidamide and their derivatives are used as pharmaceuticals as Raf kinase inhibitors for the treatment of pains, neurotraumatic diseases, cancer, chronic neurodegeneration, migraine, and cardiac hypertrophy [32]. These compounds may play critical roles in the antinociception and anti-inflammation mechanisms of the plant.
The acetic acid-induced abdominal writhing assay in mice has been used for a long time as an experimental model for the testing of antinociceptive drugs [33]. This assay is a non-selective experimental model for the antinociceptive activity of plant extracts because it is an injection of acetic acid intraperitoneally, which activates the release of many mediators such as bradykinins, prostaglandins, like PGI2, as well as cytokines which are pro-inflammatory examples tumor necrosis factor-α (TNF-α), Interleukin (IL)-6, IL-8, and IL-1 [34]. The study showed that the methanol stem bark extracts significantly decreased the number of abdominal writhing in mice in a dose-dependent manner. The results obtained from the study were comparable to the control group (P<0.05). Our results showed that stem bark methanol extract possessed potential antinociceptive effects in mice at the doses (Figures 2, 3, and 4).
The involvement of the central nervous system in the antinociceptive activity of S. spinosa, hot plate, and tail immersion assays were carried out. The study showed an increase in latency on a hot plate, which is evidence of the involvement of the central nervous system, where most analgesic drugs act [35]. In this study, the extract must have achieved antinociceptive effects by stimulating the neurotransmitters to activate the nociceptor nerves from the spinal cord [36]. The antinociceptive effect of the plant extract was further affirmed by the dose-dependent increase in reaction time in tail withdrawing by the mice in the tail immersion test. The latency and reaction times increase in this current study were comparable to the controls (P<0.05).
The carrageenan-induced paw-edema mice model is used to assess plant extracts or compounds’ anti-inflammatory activity. The method has been described as highly reproducible. According to this study, S. spinosa methanol stembark extract exhibited a dose-dependent reduction in paw edema volume after 6 h (Table 1). The ability of the extract to reduce paw edema was due to the action of some inflammatory mediators, like serotonin, histamine, and bradykinin, which facilitated the release of prostaglandins prostaglandins A (PGAs) and nitric oxide to block histamine receptors [36]. Thus, the study showed the extract inhibited the paw-edema induced by carrageenan by blocking histamine or other pro-inflammatory enzymes, thereby causing blockage of sustained release of inflammatory promoters, such as prostaglandins PGAs and subsequent release of peroxidases. The reduction of paw edema in the mice was significant (P<0.05) compared to the control.
Conclusion
Our study showed that the crude methanol stembark extract of S. spinosa possessed significant antinociceptive and anti-inflammatory activities, which are possibly related to the activation of pain receptors and blockage of the release of pro-inflammatory mediators, such as histamines, neutrophils, and prostaglandins. The observed antinociceptive and anti-inflammatory activities of the extract were due to some phytochemical contents of the stembark and the isolated bioactive compounds. However, the specific roles of these compounds need to be investigated further, as well as the extract’s mechanism of action.
Ethical Considerations
Compliance with ethical guidelines
This research was approved by Research and Ethical Committee of University of Jos, Nigeria (Code: UJ/FPS/ F17-00379).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization and Supervision: Cletus Anes Ukwubile; Methodology: Cletus Anes Ukwubile and Pharm Troy Salvia Malgwi; Investigation, Writing – original draft, and Writing – review & editing: All authors; Data collection: All authors; Data analysis: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank King Abdulaziz University Jeddah, Republic of Saudi Arabia NMR Centre, and University of Lagos Nigeria, Central Laboratory for spectroscopic analysis.
References
- Kaloudas D, Penchovsky R. Plant-derived compounds and their potential role in drug development. In: Management Association, editor. Research anthology on recent advancements in ethnopharmacology and nutraceuticals. Hershey: IGI Global; 2022. [DOI:10.4018/978-1-6684-3546-5.ch026]
- Atanasov AG, Zotchev SB, Dirsch VM; International Natural Product Sciences Taskforce; Supuran CT. Natural products in drug discovery: Advances and opportunities. Nat Rev Drug Discov. 2021; 20(3):200-16. [DOI:10.1038/s41573-020-00114-z] [PMID] [PMCID]
- Ghlichloo I, Gerriets V. Nonsteroidal anti-inflammatory drugs (NSAIDs). Treasure Island: StatPearls; 2022. [Link]
- Zacher J, Altman R, Bellamy N, Brühlmann P, Da Silva J, Huskisson E, et al. Topical diclofenac and its role in pain and inflammation: An evidence-based review. Curr Med Res Opin. 2008; 24(4):925-50. [DOI:10.1185/030079908X273066] [PMID]
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: The importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015; 22(5):388-407. [DOI:10.1097/MJT.0b013e3182459abd] [PMID]
- Sriuttha P, Sirichanchuen B, Permsuwan U. Hepatotoxicity of nonsteroidal anti-inflammatory drugs: A systematic review of randomized controlled trials. Int J Hepatol. 2018 Jan 15; 2018:5253623. [DOI:10.1155/2018/5253623] [PMID] [PMCID]
- Isa AI, Awouafack MD, Dzoyem JP, Aliyu M, Magaji RA, Ayo JO, et al. Some strychnos spinosa (loganiaceae) leaf extracts and fractions have good antimicrobial activities and low cytotoxicities. BMC Complement Altern Med. 2014; 14:456. [DOI:10.1186/1472-6882-14-456] [PMID] [PMCID]
- Burkill HM. Tropical Plants Database. Ken Fern. tropical.theferns.info. 1984.
- Adinortey MB, Sarfo JK, Adinortey CA, Ofori EG, Kwarteng J, Afrifa J. Inhibitory effects of launaea taraxacifolia and Strychnos spinosa leaves extract on an isolated digestive enzyme linked to type 2 diabetes mellitus. J Biol Life Sci. 2018; 9(2):52-64. [DOI:10.5296/jbls.v9i2.12649]
- Chinsembu KC, Syakalima M, Semenya SS. Ethnomedicinal plants used by traditional healers in the management of HIV/AIDS opportunistic diseases in Lusaka, Zambia. S Afr J Bot. 2019; 122:369-84. [DOI:10.1016/j.sajb.2018.09.007]
- Hoet S, Stévigny C, Hérent MF, Quetin-Leclercq J. Antitrypanosomal compounds from the leaf essential oil of Strychnos spinosa. Planta Med. 2006; 72(5):480-2. [DOI:10.1055/s-2005-916255] [PMID]
- Evans WC. Trease and evans pharmacognosy. Amsterdam: Elsevier Ltd; 2009. [Link]
- Sofowora A. Research on medicinal plants and traditional medicine in Africa. J Altern Complement Med. 1996; 2(3):365-72. [DOI:10.1089/acm.1996.2.365] [PMID]
- Konappa N, Udayashankar AC, Krishnamurthy S, Pradeep CK, Chowdappa S, Jogaiah S. GC-MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci Rep. 2020; 10(1):16438. [DOI:10.1038/s41598-020-73442-0] [PMID] [PMCID]
- Keskes H, Belhadj S, Jlail L, El Feki A, Damak M, Sayadi S, et al. LC-MS-MS and GC-MS analyses of biologically active extracts and fractions from Tunisian juniperus phoenice leaves. Pharm Biol. 2017; 55(1):88-95. [DOI:10.1080/13880209.2016.1230139] [PMID] [PMCID]
- Juszczak AM, Zovko-Končić M, Tomczyk M. Recent trends in the application of chromatographic techniques in the analysis of luteolin and its derivatives. Biomolecules. 2019; 9(11):731. [DOI:10.3390/biom9110731] [PMID] [PMCID]
- Fan S, Chang J, Zong Y, Hu G, Jia J. GC-MS analysis of the composition of the essential oil from dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018; 23(3):576. [DOI:10.3390/molecules23030576] [PMID] [PMCID]
- Steinmann D, Ganzera M. Recent advances on HPLC/MS in medicinal plant analysis. J Pharm Biomed Anal. 2011; 55(4):744-57. [DOI:10.1016/j.jpba.2010.11.015] [PMID]
- Mishig D, Gruner M, Lübken T, Ganbaatar C, Regdel D, Knölker HJ. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the mongolian medicinal plant caryopteris mongolica bunge. Sci Rep. 2021; 11(1):13740. [DOI:10.1038/s41598-021-93010-4] [PMID] [PMCID]
- Amabeoku GJ, Kabatende J. Antinociceptive and anti-inflammatory activities of leaf methanol extract of cotyledon orbiculata L. (crassulaceae). Adv Pharmacol Sci. 2012; 2012:862625. [DOI:10.1155/2012/862625] [PMID] [PMCID]
- Zapata-Morales JR, Alonso-Castro AJ, Domínguez F, Carranza-Álvarez C, Castellanos LM, Martínez-Medina RM, et al. Antinociceptive activity of an ethanol extract of justicia spicigera. Drug Dev Res. 2016; 77(4):180-6. [DOI:10.1002/ddr.21307] [PMID]
- Borquaye LS, Darko G, Laryea MK, Roberts V, Boateng R, Gasu EN, et al. Anti-inflammatory activities of extracts from Oliva sp., Patella rustica, and Littorina littorea collected from Ghana’s coastal shorelines. Cogent Biology. 2017; 3(1):1364063. [DOI:10.1080/23312025.2017.1364063]
- Ofori-Baah S, Borquaye LS, Zang T. Ethanolic leaf extract from Strophanthus gratus (Hook.) Franch. (Apocynaceae) exhibits anti-inflammatory and antioxidant activities. Cogent Biol. 2019; 5(1):1710431. [DOI:10.1080/23312025.2019.1710431]
- Anyasor GN, Okanlawon AA, Ogunbiyi B. Evaluation of anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clin Phytosci. 2019; 5:49. [DOI:10.1186/s40816-019-0137-8]
- Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017; 1863(2):585-97.[DOI:10.1016/j.bbadis.2016.11.005] [PMID]
- Ikwegbue PC, Masamba P, Oyinloye BE, Kappo AP. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals (Basel). 2017; 11(1):2. [DOI:10.3390/ph11010002] [PMID] [PMCID]
- Youssef Moustafa AM, Khodair AI, Saleh MA. Isolation, structural elucidation of flavonoid constituents from Leptadenia pyrotechnica and evaluation of their toxicity and antitumor activity. Pharm Biol. 2009; 47(6):539-52. [DOI:10.1080/13880200902875065]
- Hussein RA, El-Anssary AA. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herbal Med. 2019; 1(3):11-30. [DOI:10.5772/intechopen.76139]
- Montanher AB, Zucolotto SM, Schenkel EP, Fröde TS. Evidence of anti-inflammatory effects of Passiflora edulis in an inflammation model. J Ethnopharmacol. 2007; 109(2):281-8. [DOI:10.1016/j.jep.2006.07.031] [PMID]
- Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010; 69(3):273-8. [DOI:10.1017/S002966511000162X] [PMID]
- Güçlü-Ustündağ O, Mazza G. Saponins: Properties, applications and processing. Crit Rev Food Sci Nutr. 2007; 47(3):231-58. [DOI:10.1080/10408390600698197] [PMID]
- Martins MA, de Castro Bastos L, Tonussi CR. Formalin injection into knee joints of rats: Pharmacologic characterization of a deep somatic nociceptive model. J Pain. 2006; 7(2):100-7. [DOI:10.1016/j.jpain.2005.09.002] [PMID]
- Nemirovsky A, Chen L, Zelman V, Jurna I. The antinociceptive effect of the combination of spinal morphine with systemic morphine or buprenorphine. Anesth Analg. 2001; 93(1):197-203. [DOI:10.1097/00000539-200107000-00039] [PMID]
- Pinheiro BG, Silva AS, Souza GE, Figueiredo JG, Cunha FQ, Lahlou S, et al. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J Ethnopharmacol. 2011; 138(2):479-86. [DOI:10.1016/j.jep.2011.09.037] [PMID]
- Rujjanawate C, Kanjanapothi D, Panthong A. Pharmacological effect and toxicity of alkaloids from Gelsemium elegans Benth. J Ethnopharmacol. 2003; 89(1):91-5. [DOI:10.1016/S0378-8741(03)00267-8] [PMID]
- Fischer LG, Santos D, Serafin C, Malheiros A, Delle Monache F, Delle Monache G, et al. Further antinociceptive properties of extracts and phenolic compounds from Plinia glomerata (Myrtaceae) leaves. Biol Pharm Bull. 2008; 31(2):235-9. [DOI:10.1248/bpb.31.235] [PMID]
Type of Study: Original Research |
Subject:
Pharmacognosy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |