Volume 9, Issue 3 (2023)
Pharm Biomed Res 2023, 9(3): 183-200 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rahamouz-Haghighi S, Bagheri K, Sharafi A. Antibacterial Activities and Chemical Compounds of Plantago lanceolata (Ribwort Plantain) and Plantago major (Broadleaf Plantain) Leaf Extracts. Pharm Biomed Res 2023; 9 (3) :183-200
URL: http://pbr.mazums.ac.ir/article-1-520-en.html
URL: http://pbr.mazums.ac.ir/article-1-520-en.html
1- Department of Plant Production and Genetics, Faculty of Agriculture, University of Zanjan, Zanjan, Iran.
2- Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran.
2- Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran.
Keywords: Disc diffusion method, Phytochemical analysis, Microtiter broth dilution, Plantago lanceolata, Plantago major
Full-Text [PDF 1425 kb]
(1373 Downloads)
| Abstract (HTML) (3657 Views)
Full-Text: (2543 Views)
Introduction
Plantago, as a genus within the Plantaginaceae family, has about 275 species, with a global distribution. The aerial parts of Plantago species can be used as herbal medicine for treatment of some diseases related to the skin, respiratory, and digestive systems [1]. The benefits of these aerial parts for treatment of diabetes and cardiovascular diseases have been attributed to the presence of rutin, luteolin 7-o-glucoside, quercetin hexoside, and chlorogenic acid compounds [2]. On the other hand, ruminant microbiome functions are modulated due to their antimicrobial and antioxidant properties [3].
Plantago lanceolata L. and Plantago major L. are medicinal plants that are widely used without having significant side effects [4]. The biocompatibility and cytotoxic activity of different extracts of P. lanceolata and P. major roots and aerial parts have been assessed against human red blood cells and cancer cells using different techniques [5, 6]. The toxicity effects of crude extracts of these plants against Artemia salina in mice has also been investigated [4-6]. A study examined the biological activities of the root extracts of these plants by fractionating the crude extracts [7]. These plants contain aucubin and catalpol which are chemotaxonomic markers for examining the quality of extracts [8, 9]. These compounds have also been evaluated in different parts of these plants [10]. Since there is a need to improve the knowledge for the treatment of opportunistic bacterial infections, this in-vitro study aims to investigate the antimicrobial activities of different extracts of Plantago species against pathogenic bacteria. To confirm the antibacterial activity of the extracts, phytochemical compounds were also analyzed.
Materials and Methods
Samples
The plants were first obtained from Zanjan, Iran (36°41’15.5”N 48°24’02.2”E). Then, they were authenticated at the Department of Botany, University of Zanjan. All sections of plants were completely washed and separated. The leaves were then cut into small pieces and dried in the shade and at a room temperature for 10 days.
Extract preparation
The leaves of plants (250 g) were extracted by petroleum ether using the reflex method for 16 hours. The leaves of plants (250 g) were extracted by petroleum ether using the reflex method for 16 hours followed by methanol with the same duration time. The methanol extract was then separated using liquid-liquid extraction with ethyl acetate, n-butanol, and aqueous phases in a separatory funnel [11]. The aqueous extract was filtered using a filter paper to delete the herbal fibers. The extracts were concentrated using a rotary evaporator and then dried at room temperature for 10 days.
Pathogenic bacteria
The culture of three standard pathogenic bacteria, including gram-positive Bacillus cereus (ATCC 11778), gram-negative Salmonella paratyphi (ATCC 5702), and Proteus vulgaris (PTCC 1182) were prepared from the culture collection of Iranian Biological Resource Center. The mentioned bacteria were then cultured in Mueller-Hinton broth (MHB) at 37°C for 18 hours. Subsequently, 0.5 McFarland bacterial suspensions (1.5×108 CFU/mL) were prepared.
Antibacterial activity assessment
The disc diffusion method was employed to determine the antibacterial activity of P. lanceolata and P. major according to the guidelines of the National Committee for Clinical Laboratory Standards [12]. The paper discs (Whatman No. 2) with 6 mm in diameter were then impregnated with 5 µL of extract dissolved in Dimethyl sulfoxide (DMSO) to obtain the concentration of 100 mg/mL. The sterile blotting paper discs were soaked in the diluted extracts and left to fully dry. The dried discs were then applied for antibacterial tests using the disc diffusion method. The turbidity of inoculums was matched with the 0.5 McFarland turbidity standard. Subsequently, the inoculums were inoculated onto the Mueller-Hinton agar (MHA) plate with a sterile cotton swab to reach uniform microbial growth. Gentamicin (10 µg/mL) was used as the positive control, while the DMSO-soaked discs were considered as negative controls. The plates underwent 24 hours of incubation at 37°C. The antibacterial properties were then evaluated based on the inhibition zone diameter (mm).
Assessment of minimum inhibitory and bactericidal concentrations
The minimum inhibitory concentration (MIC) of extracts was determined by the broth microdilution method according to the guidelines of Clinical Laboratory Standards Institute guidelines [13, 14]. Growth inhibition assays were performed in the sterile 96-well plates at a final volume of 200 μL. The cell concentrations were estimated from the optical densities at a 600-nm wavelength. Then, 100 µL of mid-logarithmic-phase bacterial cultures (105 CFU/mL) in MHB were added to 100 μL of serially diluted extracts. The final concentration of extracts in each well was 0.5 to 4 mg/mL. The wells containing MHB with bacterial inoculum only used for the bacterial growth control and those with MHB alone were applied for the control of sterility. All samples were prepared in triplicate. Microplates were incubated at 37°C for 24 hours, and the bacterial cell growth was assessed by measuring the optical density of cultures at a 600-nm wavelength using an ELISA plate reader (Tecan Infinite M200, Austria).The MICs were defined as the lowest concentration that completely inhibits the bacterial growth. To determine the minimum bactericidal concentration (MBC) of the extracts, 100 μL of solutaion in the clear wells were inoculated on MHA plates and incubated at 37°C for 24 hours.
Phytochemical screening
The findings of gas chromatography-mass spectrometry (GC-MS) were analyzed using a GC-MS device (Agilent technologies 5975c, USA). The leaf extracts of P. lanceolata and P. major (1 μL) were injected into the GC-MS system equipped with a capillary column (30 m ×250 μm ×0.25 μm). Helium was applied at a flow rate of 1.0 mL/min. The temperature of the injector and the interface were kept at 350°C. The column temperature was first 50°C for 2 min and then increased to 230°C at a rate of 4°C/min for 2 min. The fragments were detected by comparing mass spectral fragmentation patterns in MS data, NIST08.L library [11]. To prepare the applying solutions (5 mg/mL), the dried extracts were diluted in methanol (HPLC grade) and then the extracts were filtered by a 0.22-μm sterile filter and kept in a vial at 4°C.
Statistical analysis
All graphs were drawn in Excel software 2016. The values were reported as Mean±SD. The mean values were compared using the Duncan test in SPSS software, version 21. P<0.05 was considered as statitically significant.
Results
Antibacterial activity
The leaf extracts of P. lanceolata and P. major showed varied inhibitory functions against gram-positive and gram-negative bacterial strains (Table 1). The dichloromethane extract of P. lanceolata and P. major leaves exhibited the highest inhibitory activity against S. paratyphi (18.83±0.3 mm and 20.00±1.5 mm). petroleum extract of P. lanceolata and ethyl acetate and butanol extracts of P. major showed very good inhibitory activity against B. cereus and P. vulgaris, respectively.

The MICs of P. lanceolata and P. major extracts showed good antibacterial activity against P. vulgaris and S. paratyphi (Table 2). These MIC values indicated the inhibition of bacterial proliferation and growth after treatment by using different extracts. The lowest MIC was related to the dichloromethane leaf extracts of two plants (500 µg/mL) against S. paratyphi. The dichloromethane leaf extracts had strong inhibitorory activity against S. paratyphi similar to that of gentamicin (Table 2). The petroleum ether extract of P. lanceolata leaf showed more effective activity against B. cereus with a MIC value of 1000 µg/mL (Table 2). The acceptable antibacterial activity of different extracts on the growth of bacteria after 24 hours indicates their significant antibacterial potential.

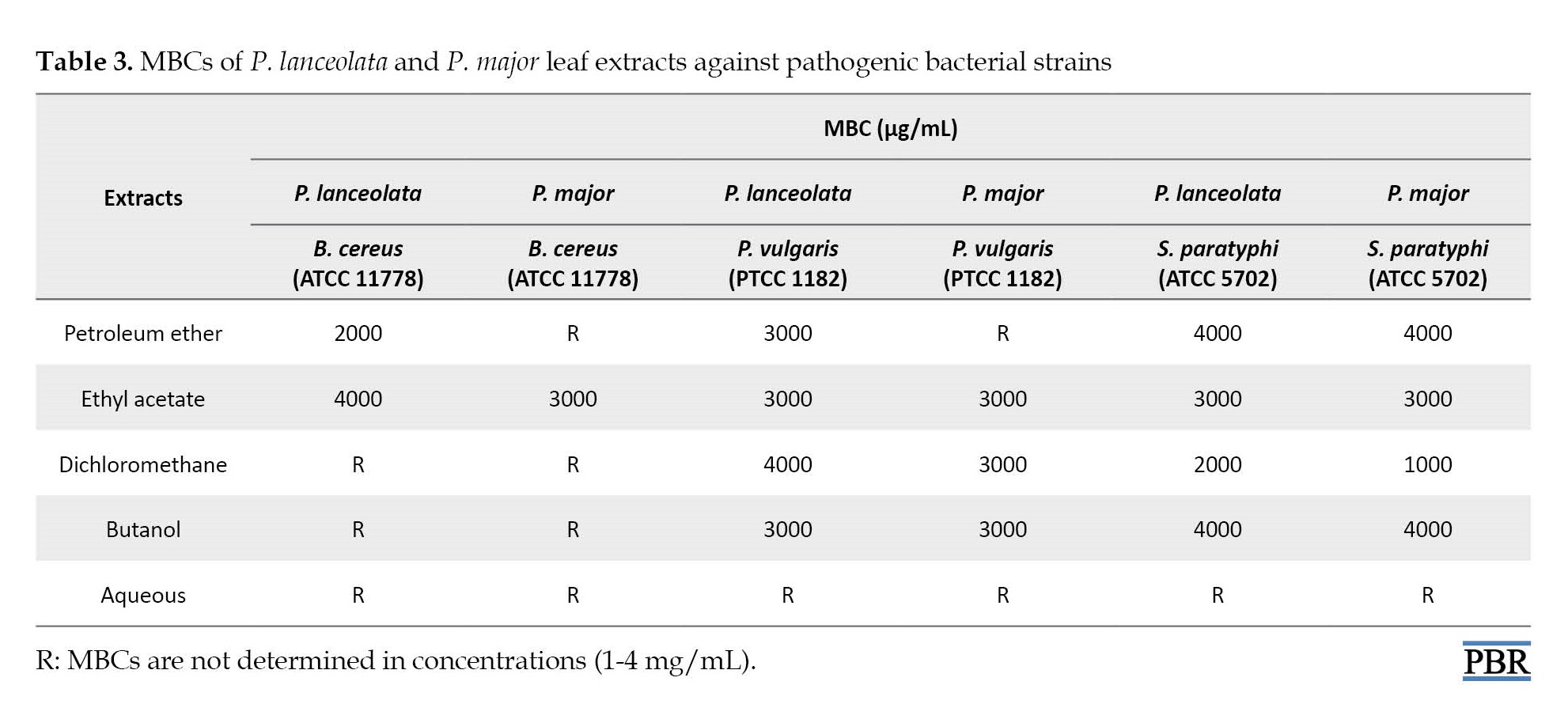
Table 3 shows the MBC values. The lowest MBCs (1000 µg/mL) was relatd to the dichloromethane leaf extract of P. major against S. paratyphi. On the other hand, B. cereus was the most resistant bacteria to dichloromethane, butanol, and aqueous extracts of two plants, because it did not show MBC values at 0.5-4 mg/mL concentrations. The aqueous extract showed the lowest antibacterial effect, since the MBC values were not determined for extract against tested bacteria.
Phytochemical analysis
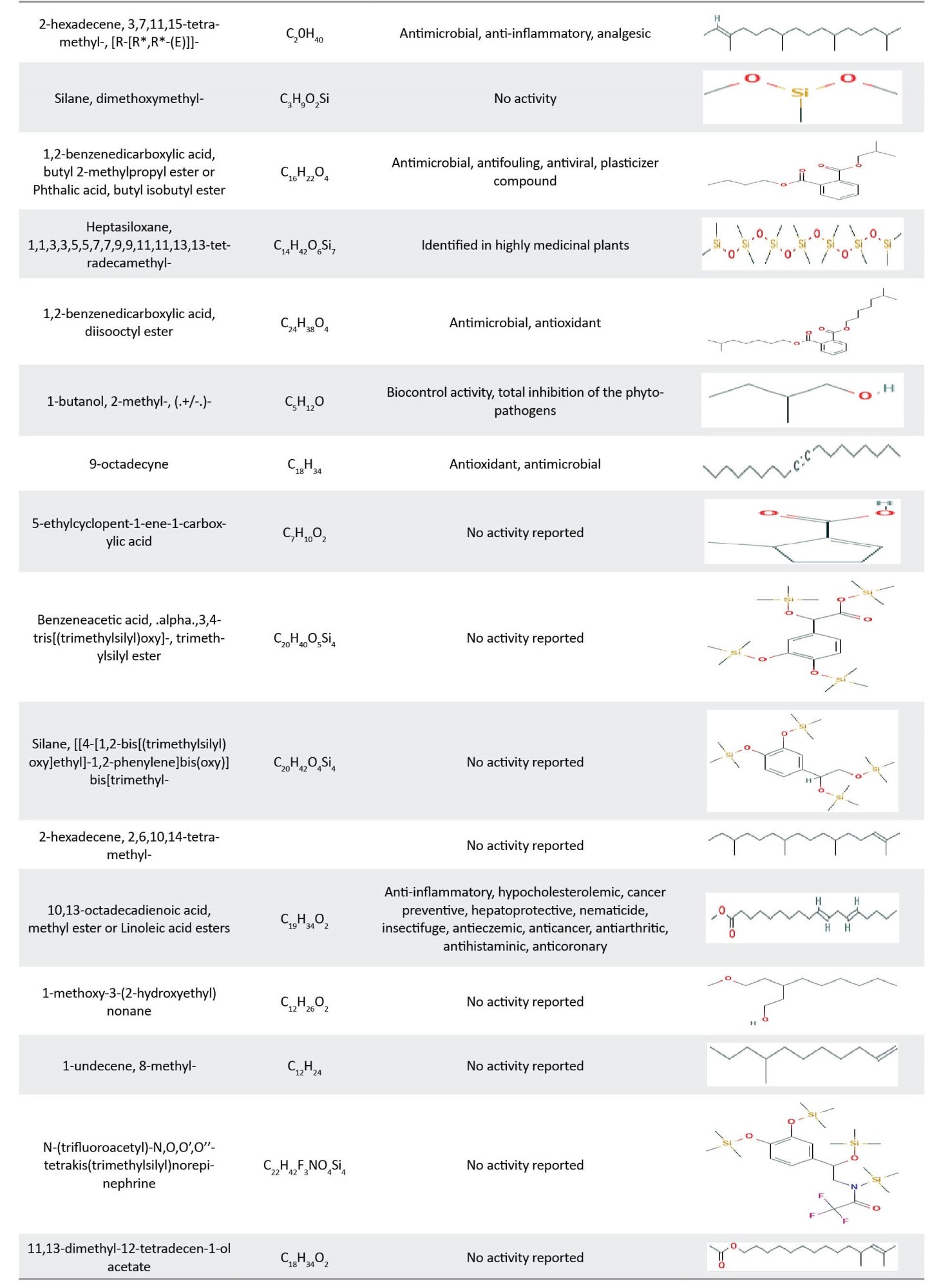
The petroleum ether solvent was applied to delete non-polar compounds. In this regard, the crude methanol extract was fractionated to make various extracts. Table 4 shows the main components of P. lanceolata leaf extracts. The main chemical compounds of petroleum ether, ethyl acetate, dichloromethane, n-butanol, and aqueous extracts were phthalates (43.76%), alcohols (32.69%), terpenoids (26.50%), Siloxanes (53.68% and 58.62%), respectively. As can be seen, the main component of petroleum ether extract was bis (2-ethylhexyl) phthalate (41.96%), while the main component of ethyl acetate extract was 1-methoxy-3-(2-hydroxyethyl) nonane (32.69%).

The major component of dichloromethane, extract was bicyclo [3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- (10.45%), while for the both of butanol and aqueous extracts, the main component was cycloheptasiloxane tetradecamethyl- (27.96%, and 31.33%, respectively) (Table 5). Cycloheptasiloxane, tetradecamethyl- and cyclohexasiloxane-, dodecamethyl- were common components in all fractions of P. lanceolata leaf (Table 5).

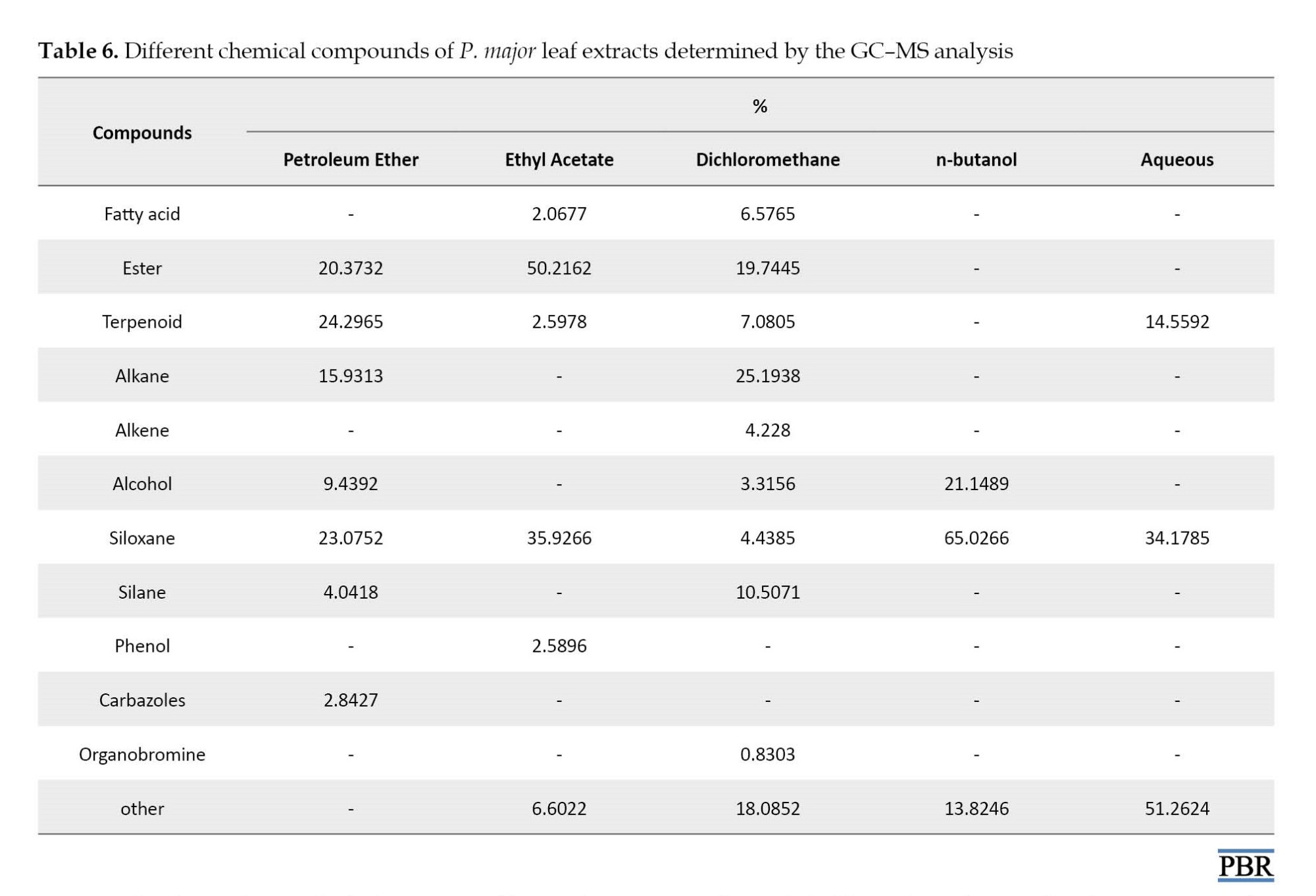
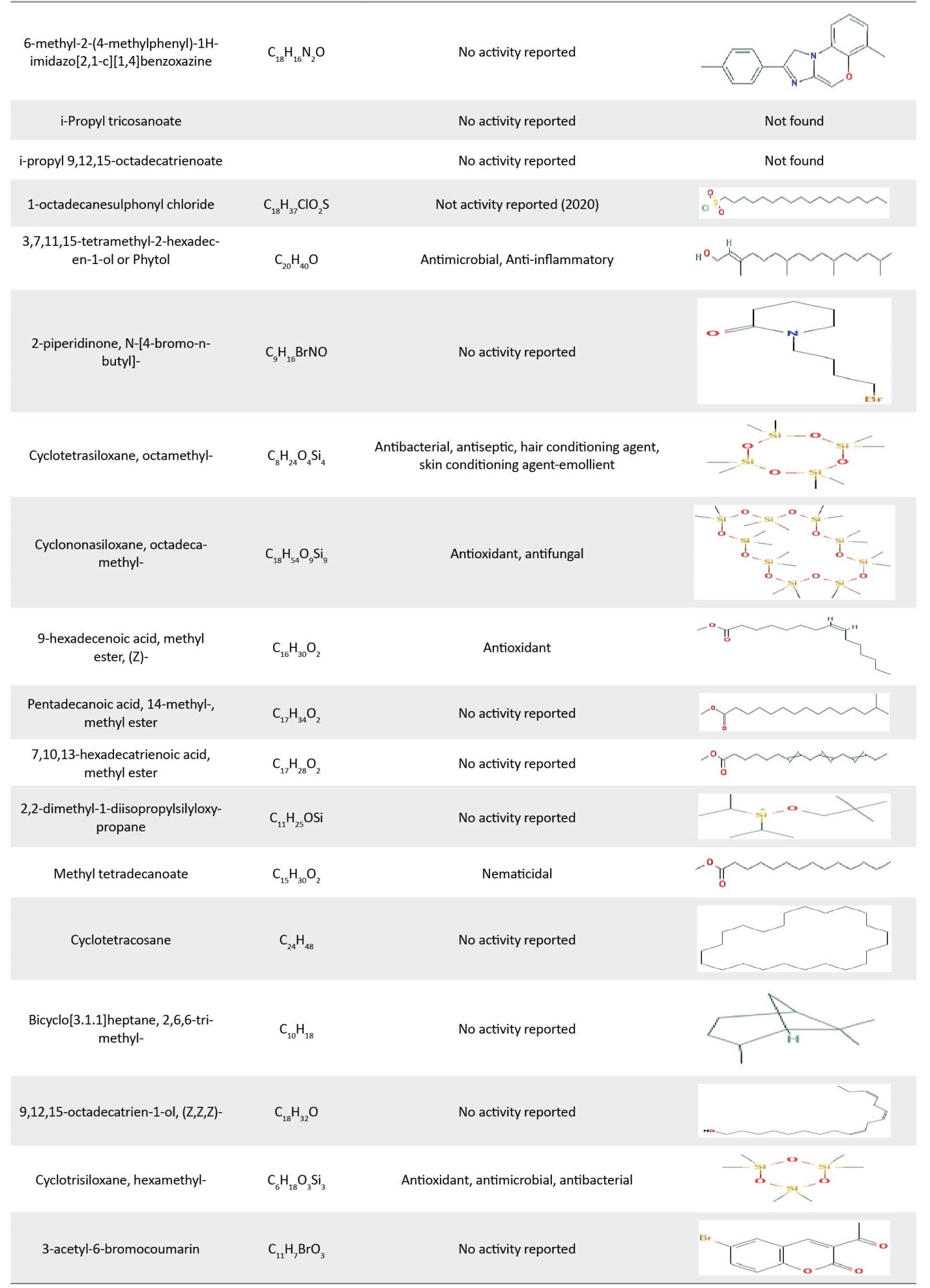
The components of P. major leaf extracts are shown in Table 6. The main chemical compounds of petroleum ether, ethyl acetate, dichloromethane, butanol, and aqueous extracts were terpenoid (24.29%), esters (50.21%), alkanes (25.19%), and Siloxanes (65.02%), and Siloxanes (34.17%).
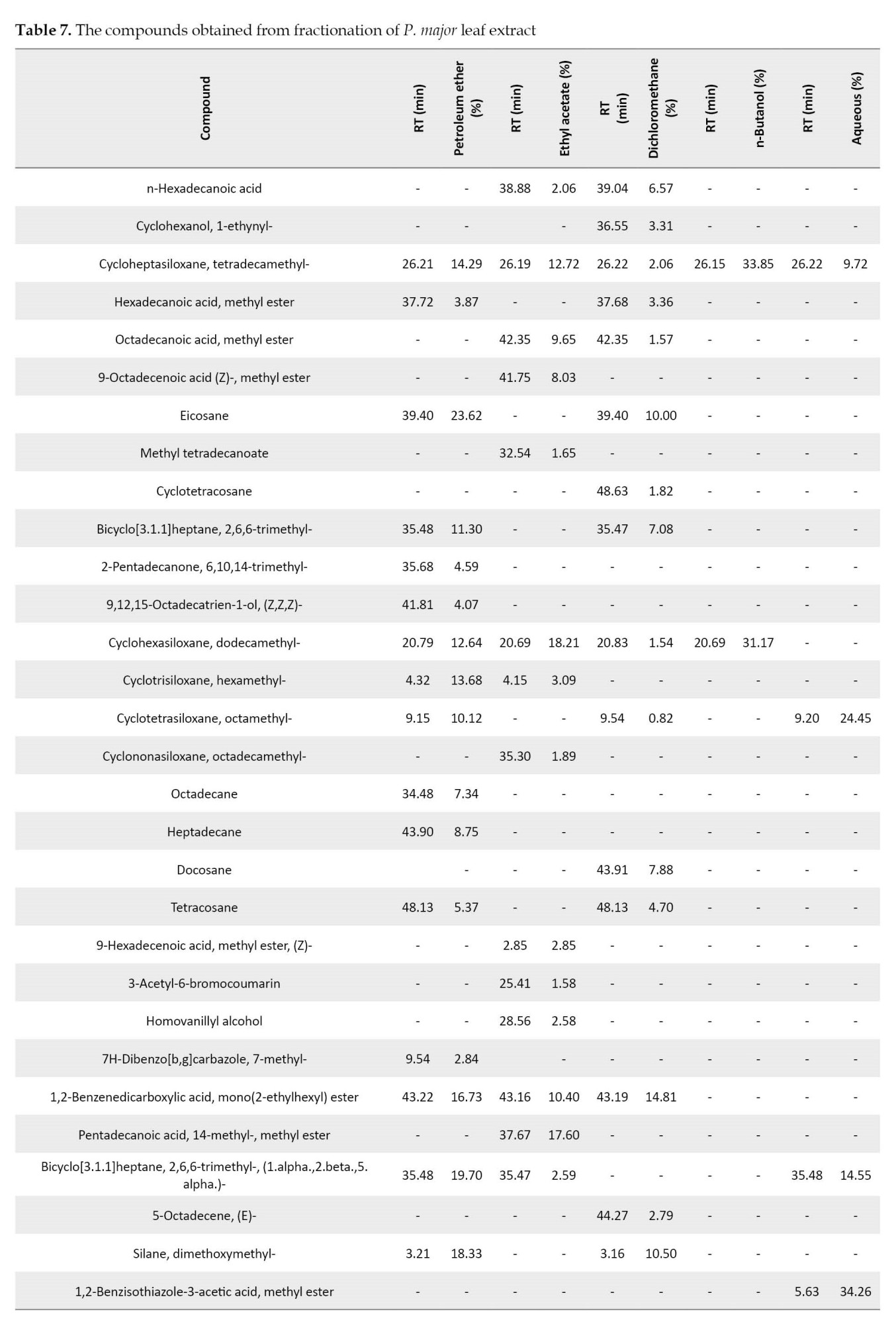
Eicosane (23.62%) and Cyclohexasiloxane, dodecamethyl- (18.21%) were the main compounds of petroleum ether and ethyl acetate extracts, respectively. The main components of dichloromethane, n-butanol, and aqueous extracts were 1-methyl-3-n-propyl-2-pyrazolin-5-one (18.08%), cycloheptasiloxane, tetradecamethyl- (33.85%), and 1,2-benzisothiazole-3-acetic acid, methyl ester (34.26%), respectively (Table 7). Cycloheptasiloxane, tetradecamethyl- was present in all fractions of P. major leaf. Cyclohexasiloxane, dodecamethyl was also observed in all fractions except for the aqueous extract.
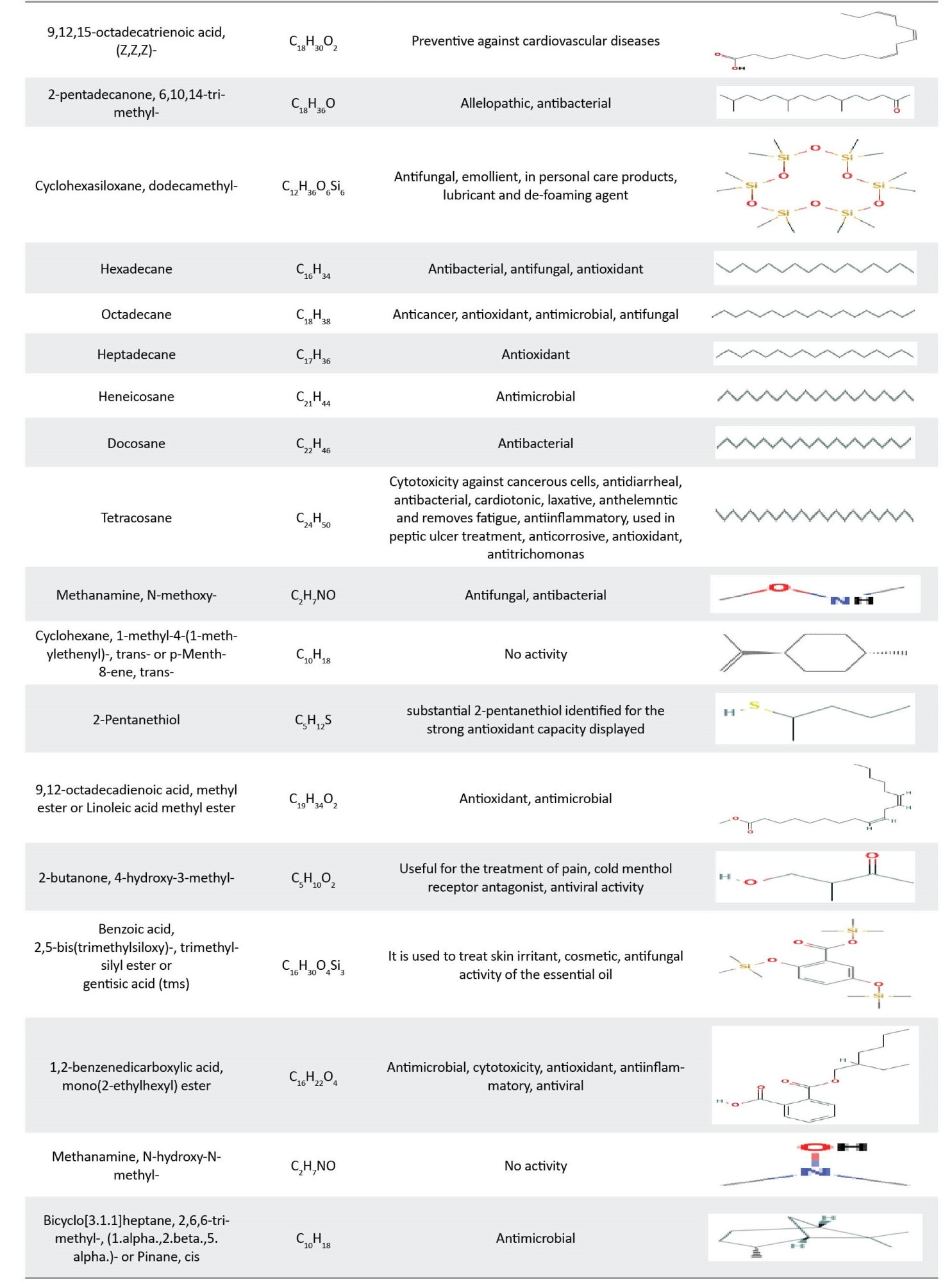
Both P. lanceolata and P. major extracts contain valuable compounds which exhibited numerous properties, such as anti-microbial, anti-viral, anti-inflammatory, and anti-cancer properties. The biological activities of identified compounds are reported in Table 8.


Discussion
P. lanceolata and P. major are two medicinal plants with extensive applications for treatment of various diseases. The antibacterial assessment showed that they could be classified as potent inhibitors. Studies have reported varying degrees of antibacterial activity for P. lanceolata extract against some human pathogens [15]. However, Karakas et al. reported no significant antibacterial activity for methanolic extract of P. lanceolata against gram-positive or gram-negative bacteria. Their aqueous extract offered poor or moderate antimicrobial activity against bacteria such as P. vulgaris [16]. The present study also showed the poor antibacterial activity of the aqueous extracts against the bacteria. However, the methanolic extracts of two plants showed good antibacterial activity against some pathogenic bacteria, including P. vulgaris [8, 9, 17]. In this regard, P. major ethyl acetate extract showed more inhibitory function against gram-positive and gram-negative bacteria compared to the aqueous extract [18]. The results of our study are consistent with the previous studies where ethyl acetate extracts showed a significant inhibitory effect on the bacteria. Abate et al. assessed the antibacterial activity of different extracts such as pure petroleum ether extract of P. lanceolata leaf against pathogenic bacteria [19]. In our study, the petroleum ether extract of P. lanceolata showed a suitable inhibitory effect against B. cereus. The difference in the antibacterial activities of extracts for different species of the same genus can be attributed to differences in the growth and harvesting locations, loss of compounds during extraction and extraction methods, and thus the presence of various secondary metabolites in them [20]. MICs of extracts are different in each study based on the density of bacterial suspension (CFU/mL) and tested concentrations.
A study reported that n-hexane leaf extract of Iraqi P. lanceoleta contains a high amount of hydrocarbons, fatty acids, steroids, terpenoids, and other compounds [21]. The various components of P. major leaf extracts found in the study by Jamileh et al. included 13.22% phytol, 10.48% benzofuranone, 10.26% penthynediol and 10.18% benzene propanoic acid in petroleum ether extract; 30.70% glycerin, 21.81% benzene, and 16.22% dibuthyl phthalate in ethyl acetate extract; 24.62% phtalic acid, 16.83% benzene propanoic acid, and 10.20% phenol in butanol extract; and 27.47% phenol, 14.53% diathiapentene, 14.13% napthalenone, and 12.02% glycerine in aqueous extract [11]. In the current study, 1.27% and 6.09% phytol were found in petroleum ether and dichloromethane extracts of P. lanceolata leaf, respectively. All these extracts had different chemical compositions due to the different polarities of the extraction solvents. P. lanceolata leaf essential oil is mainly composed of fatty acids (28%-52%), especially myristic acid, palmitic acid, linoleic acid, and linolenic acid [22]. In the present study, fatty acids (2%-39%) and esters (2%-74%) were found in all extracts, except for the aqueous leaf extracts. Fatty acids have been recognized as antimicrobial agents. Fatty acids like n-hexadecanoic acid, 9,12,15-octadecatrienoic acid, methyl ester, (Z,Z,Z)-; and 9,12-octadecadienoic acid (Z,Z)- were present in the most of extracts. These components have reported antimicrobial, and anti-inflammatory activities [23, 24]. The analysis of leaf extracts of Plantago species showed that they contain a considerable amount of alkanes such as docosane, eicosane, heneicosane, heptadecane, octadecane, and tetracosane whose antibacterial properties have been reported in previous studies [25-28]. Siloxanes that are present in the leaf extracts of plants are also known as antimicrobial phytochemicals. Hexadecane, which is present in the petroleum extract of P. lanceolata, has been reported to be effective against Pseudomonas aeruginosa [23].
Rahamouz-Haghighi et al. reported various components for the root extracts of P. lanceolata. For its ethyl acetate extract, the main component was 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester (60.93%). For the dichloromethane extract, the main component was 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester (60.64%); and for the butanol extract, the main component was 1-butanol, 2-methyl-, (.+/-.)- (17.85%) [7]. volatiles produced by Saccharomyces cerevisiae, Fialho et al. reported that the mycelium growth of the phytopathogen was inhibited almost 100% by the compound 2-methyl-1-butanol [29]. In the present study, 2-methyl-1-butanol was identified in the butanol extracts of two Plantago species that can be considered the main compound responsible for the antimicrobial activity. We could not determine whether di-(2-ethylhexyl) phthalate is synthesized by P. lanceolata, is absorbed from the atmosphere, or is absorbed by the roots; however, it seems that the compound, regardless of its origin, is probably present in the P. lanceolata leaf [30]. This compound has been reported in Euphorbia cyparissias, Euphorbia seguieriana [31], Aloe vera [32], Alchornea cordifolia [33], and Calotropis gigantea flowers [30]. According to the studies, this compound is not a contaminant, which was further confirmed by the GC-MS analysis. C. gigantea flowers can not be seen in plastic bags; therefore, they can be discounted as a source of di-(2-ethylhexyl) phthalate. Indeed, it may have taxonomic significance [30].
The antibacterial properties of P. lanceolata and P. major leaf extracts may be attributed to the presence of antibacterial compounds found in the GC/MS analysis, including palmitic acid [34], linolenic acid [24], gamma.-sitosterol [35], squalene [36], cycloheptasiloxane, tetradecamethyl- [37], hexadecanoic acid, methyl ester [38], phytol [39], 9,12-octadecadienoic acid, methyl ester [40], 1,2-benzenedicarboxylic acid, diisooctyl ester [41], 9-octadecyne [42], stearic acid [43], 9-octadecenoic acid (Z)-, methyl ester [44], cyclotetrasiloxane, octamethyl- [45], 2-methoxy-4-vinylphenol [24], pentadecanoic acid, ethyl ester [46], trans-vaccenic acid [40], Z-10-octadecen-1-ol acetate [47], 9,17-octadecadienal, (Z)- [48], phthalic acid, butyl undecyl ester [49], cyclotrisiloxane, hexamethyl- [50], octadecanoic acid [51], and other compounds.
Conclusions
Fractionation of the methanolic leaf extract of P. lanceolata and P. major can help better isolate active components from these plants, so that the antibacterial activity of the extracts can be carefully evaluated. We found numerous compounds in two Plantago species with antibacterial properties.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The paper was extracted from the PhD dissertation of Samaneh Rahamouz-Haghighi, approved by Department of Plant Production and Genetics, University of Zanjan (Grant No: A-12-848-35).
Authors' contributions
Conceptualization, project administration, investigation, formal analysis and writing the original draft: Samaneh Rahamouz-Haghighi; Funding acquisition and supervision: Khadijeh Bagheri and Ali Sharafi.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the School of Pharmacy of Zanjan University of Medical Sciences and University of Zanjan.
References
Plantago, as a genus within the Plantaginaceae family, has about 275 species, with a global distribution. The aerial parts of Plantago species can be used as herbal medicine for treatment of some diseases related to the skin, respiratory, and digestive systems [1]. The benefits of these aerial parts for treatment of diabetes and cardiovascular diseases have been attributed to the presence of rutin, luteolin 7-o-glucoside, quercetin hexoside, and chlorogenic acid compounds [2]. On the other hand, ruminant microbiome functions are modulated due to their antimicrobial and antioxidant properties [3].
Plantago lanceolata L. and Plantago major L. are medicinal plants that are widely used without having significant side effects [4]. The biocompatibility and cytotoxic activity of different extracts of P. lanceolata and P. major roots and aerial parts have been assessed against human red blood cells and cancer cells using different techniques [5, 6]. The toxicity effects of crude extracts of these plants against Artemia salina in mice has also been investigated [4-6]. A study examined the biological activities of the root extracts of these plants by fractionating the crude extracts [7]. These plants contain aucubin and catalpol which are chemotaxonomic markers for examining the quality of extracts [8, 9]. These compounds have also been evaluated in different parts of these plants [10]. Since there is a need to improve the knowledge for the treatment of opportunistic bacterial infections, this in-vitro study aims to investigate the antimicrobial activities of different extracts of Plantago species against pathogenic bacteria. To confirm the antibacterial activity of the extracts, phytochemical compounds were also analyzed.
Materials and Methods
Samples
The plants were first obtained from Zanjan, Iran (36°41’15.5”N 48°24’02.2”E). Then, they were authenticated at the Department of Botany, University of Zanjan. All sections of plants were completely washed and separated. The leaves were then cut into small pieces and dried in the shade and at a room temperature for 10 days.
Extract preparation
The leaves of plants (250 g) were extracted by petroleum ether using the reflex method for 16 hours. The leaves of plants (250 g) were extracted by petroleum ether using the reflex method for 16 hours followed by methanol with the same duration time. The methanol extract was then separated using liquid-liquid extraction with ethyl acetate, n-butanol, and aqueous phases in a separatory funnel [11]. The aqueous extract was filtered using a filter paper to delete the herbal fibers. The extracts were concentrated using a rotary evaporator and then dried at room temperature for 10 days.
Pathogenic bacteria
The culture of three standard pathogenic bacteria, including gram-positive Bacillus cereus (ATCC 11778), gram-negative Salmonella paratyphi (ATCC 5702), and Proteus vulgaris (PTCC 1182) were prepared from the culture collection of Iranian Biological Resource Center. The mentioned bacteria were then cultured in Mueller-Hinton broth (MHB) at 37°C for 18 hours. Subsequently, 0.5 McFarland bacterial suspensions (1.5×108 CFU/mL) were prepared.
Antibacterial activity assessment
The disc diffusion method was employed to determine the antibacterial activity of P. lanceolata and P. major according to the guidelines of the National Committee for Clinical Laboratory Standards [12]. The paper discs (Whatman No. 2) with 6 mm in diameter were then impregnated with 5 µL of extract dissolved in Dimethyl sulfoxide (DMSO) to obtain the concentration of 100 mg/mL. The sterile blotting paper discs were soaked in the diluted extracts and left to fully dry. The dried discs were then applied for antibacterial tests using the disc diffusion method. The turbidity of inoculums was matched with the 0.5 McFarland turbidity standard. Subsequently, the inoculums were inoculated onto the Mueller-Hinton agar (MHA) plate with a sterile cotton swab to reach uniform microbial growth. Gentamicin (10 µg/mL) was used as the positive control, while the DMSO-soaked discs were considered as negative controls. The plates underwent 24 hours of incubation at 37°C. The antibacterial properties were then evaluated based on the inhibition zone diameter (mm).
Assessment of minimum inhibitory and bactericidal concentrations
The minimum inhibitory concentration (MIC) of extracts was determined by the broth microdilution method according to the guidelines of Clinical Laboratory Standards Institute guidelines [13, 14]. Growth inhibition assays were performed in the sterile 96-well plates at a final volume of 200 μL. The cell concentrations were estimated from the optical densities at a 600-nm wavelength. Then, 100 µL of mid-logarithmic-phase bacterial cultures (105 CFU/mL) in MHB were added to 100 μL of serially diluted extracts. The final concentration of extracts in each well was 0.5 to 4 mg/mL. The wells containing MHB with bacterial inoculum only used for the bacterial growth control and those with MHB alone were applied for the control of sterility. All samples were prepared in triplicate. Microplates were incubated at 37°C for 24 hours, and the bacterial cell growth was assessed by measuring the optical density of cultures at a 600-nm wavelength using an ELISA plate reader (Tecan Infinite M200, Austria).The MICs were defined as the lowest concentration that completely inhibits the bacterial growth. To determine the minimum bactericidal concentration (MBC) of the extracts, 100 μL of solutaion in the clear wells were inoculated on MHA plates and incubated at 37°C for 24 hours.
Phytochemical screening
The findings of gas chromatography-mass spectrometry (GC-MS) were analyzed using a GC-MS device (Agilent technologies 5975c, USA). The leaf extracts of P. lanceolata and P. major (1 μL) were injected into the GC-MS system equipped with a capillary column (30 m ×250 μm ×0.25 μm). Helium was applied at a flow rate of 1.0 mL/min. The temperature of the injector and the interface were kept at 350°C. The column temperature was first 50°C for 2 min and then increased to 230°C at a rate of 4°C/min for 2 min. The fragments were detected by comparing mass spectral fragmentation patterns in MS data, NIST08.L library [11]. To prepare the applying solutions (5 mg/mL), the dried extracts were diluted in methanol (HPLC grade) and then the extracts were filtered by a 0.22-μm sterile filter and kept in a vial at 4°C.
Statistical analysis
All graphs were drawn in Excel software 2016. The values were reported as Mean±SD. The mean values were compared using the Duncan test in SPSS software, version 21. P<0.05 was considered as statitically significant.
Results
Antibacterial activity
The leaf extracts of P. lanceolata and P. major showed varied inhibitory functions against gram-positive and gram-negative bacterial strains (Table 1). The dichloromethane extract of P. lanceolata and P. major leaves exhibited the highest inhibitory activity against S. paratyphi (18.83±0.3 mm and 20.00±1.5 mm). petroleum extract of P. lanceolata and ethyl acetate and butanol extracts of P. major showed very good inhibitory activity against B. cereus and P. vulgaris, respectively.

The MICs of P. lanceolata and P. major extracts showed good antibacterial activity against P. vulgaris and S. paratyphi (Table 2). These MIC values indicated the inhibition of bacterial proliferation and growth after treatment by using different extracts. The lowest MIC was related to the dichloromethane leaf extracts of two plants (500 µg/mL) against S. paratyphi. The dichloromethane leaf extracts had strong inhibitorory activity against S. paratyphi similar to that of gentamicin (Table 2). The petroleum ether extract of P. lanceolata leaf showed more effective activity against B. cereus with a MIC value of 1000 µg/mL (Table 2). The acceptable antibacterial activity of different extracts on the growth of bacteria after 24 hours indicates their significant antibacterial potential.

Table 3 shows the MBC values. The lowest MBCs (1000 µg/mL) was relatd to the dichloromethane leaf extract of P. major against S. paratyphi. On the other hand, B. cereus was the most resistant bacteria to dichloromethane, butanol, and aqueous extracts of two plants, because it did not show MBC values at 0.5-4 mg/mL concentrations. The aqueous extract showed the lowest antibacterial effect, since the MBC values were not determined for extract against tested bacteria.

Phytochemical analysis
The petroleum ether solvent was applied to delete non-polar compounds. In this regard, the crude methanol extract was fractionated to make various extracts. Table 4 shows the main components of P. lanceolata leaf extracts. The main chemical compounds of petroleum ether, ethyl acetate, dichloromethane, n-butanol, and aqueous extracts were phthalates (43.76%), alcohols (32.69%), terpenoids (26.50%), Siloxanes (53.68% and 58.62%), respectively. As can be seen, the main component of petroleum ether extract was bis (2-ethylhexyl) phthalate (41.96%), while the main component of ethyl acetate extract was 1-methoxy-3-(2-hydroxyethyl) nonane (32.69%).

The major component of dichloromethane, extract was bicyclo [3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- (10.45%), while for the both of butanol and aqueous extracts, the main component was cycloheptasiloxane tetradecamethyl- (27.96%, and 31.33%, respectively) (Table 5). Cycloheptasiloxane, tetradecamethyl- and cyclohexasiloxane-, dodecamethyl- were common components in all fractions of P. lanceolata leaf (Table 5).

The components of P. major leaf extracts are shown in Table 6. The main chemical compounds of petroleum ether, ethyl acetate, dichloromethane, butanol, and aqueous extracts were terpenoid (24.29%), esters (50.21%), alkanes (25.19%), and Siloxanes (65.02%), and Siloxanes (34.17%).

Eicosane (23.62%) and Cyclohexasiloxane, dodecamethyl- (18.21%) were the main compounds of petroleum ether and ethyl acetate extracts, respectively. The main components of dichloromethane, n-butanol, and aqueous extracts were 1-methyl-3-n-propyl-2-pyrazolin-5-one (18.08%), cycloheptasiloxane, tetradecamethyl- (33.85%), and 1,2-benzisothiazole-3-acetic acid, methyl ester (34.26%), respectively (Table 7). Cycloheptasiloxane, tetradecamethyl- was present in all fractions of P. major leaf. Cyclohexasiloxane, dodecamethyl was also observed in all fractions except for the aqueous extract.

Both P. lanceolata and P. major extracts contain valuable compounds which exhibited numerous properties, such as anti-microbial, anti-viral, anti-inflammatory, and anti-cancer properties. The biological activities of identified compounds are reported in Table 8.





Discussion
P. lanceolata and P. major are two medicinal plants with extensive applications for treatment of various diseases. The antibacterial assessment showed that they could be classified as potent inhibitors. Studies have reported varying degrees of antibacterial activity for P. lanceolata extract against some human pathogens [15]. However, Karakas et al. reported no significant antibacterial activity for methanolic extract of P. lanceolata against gram-positive or gram-negative bacteria. Their aqueous extract offered poor or moderate antimicrobial activity against bacteria such as P. vulgaris [16]. The present study also showed the poor antibacterial activity of the aqueous extracts against the bacteria. However, the methanolic extracts of two plants showed good antibacterial activity against some pathogenic bacteria, including P. vulgaris [8, 9, 17]. In this regard, P. major ethyl acetate extract showed more inhibitory function against gram-positive and gram-negative bacteria compared to the aqueous extract [18]. The results of our study are consistent with the previous studies where ethyl acetate extracts showed a significant inhibitory effect on the bacteria. Abate et al. assessed the antibacterial activity of different extracts such as pure petroleum ether extract of P. lanceolata leaf against pathogenic bacteria [19]. In our study, the petroleum ether extract of P. lanceolata showed a suitable inhibitory effect against B. cereus. The difference in the antibacterial activities of extracts for different species of the same genus can be attributed to differences in the growth and harvesting locations, loss of compounds during extraction and extraction methods, and thus the presence of various secondary metabolites in them [20]. MICs of extracts are different in each study based on the density of bacterial suspension (CFU/mL) and tested concentrations.
A study reported that n-hexane leaf extract of Iraqi P. lanceoleta contains a high amount of hydrocarbons, fatty acids, steroids, terpenoids, and other compounds [21]. The various components of P. major leaf extracts found in the study by Jamileh et al. included 13.22% phytol, 10.48% benzofuranone, 10.26% penthynediol and 10.18% benzene propanoic acid in petroleum ether extract; 30.70% glycerin, 21.81% benzene, and 16.22% dibuthyl phthalate in ethyl acetate extract; 24.62% phtalic acid, 16.83% benzene propanoic acid, and 10.20% phenol in butanol extract; and 27.47% phenol, 14.53% diathiapentene, 14.13% napthalenone, and 12.02% glycerine in aqueous extract [11]. In the current study, 1.27% and 6.09% phytol were found in petroleum ether and dichloromethane extracts of P. lanceolata leaf, respectively. All these extracts had different chemical compositions due to the different polarities of the extraction solvents. P. lanceolata leaf essential oil is mainly composed of fatty acids (28%-52%), especially myristic acid, palmitic acid, linoleic acid, and linolenic acid [22]. In the present study, fatty acids (2%-39%) and esters (2%-74%) were found in all extracts, except for the aqueous leaf extracts. Fatty acids have been recognized as antimicrobial agents. Fatty acids like n-hexadecanoic acid, 9,12,15-octadecatrienoic acid, methyl ester, (Z,Z,Z)-; and 9,12-octadecadienoic acid (Z,Z)- were present in the most of extracts. These components have reported antimicrobial, and anti-inflammatory activities [23, 24]. The analysis of leaf extracts of Plantago species showed that they contain a considerable amount of alkanes such as docosane, eicosane, heneicosane, heptadecane, octadecane, and tetracosane whose antibacterial properties have been reported in previous studies [25-28]. Siloxanes that are present in the leaf extracts of plants are also known as antimicrobial phytochemicals. Hexadecane, which is present in the petroleum extract of P. lanceolata, has been reported to be effective against Pseudomonas aeruginosa [23].
Rahamouz-Haghighi et al. reported various components for the root extracts of P. lanceolata. For its ethyl acetate extract, the main component was 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester (60.93%). For the dichloromethane extract, the main component was 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester (60.64%); and for the butanol extract, the main component was 1-butanol, 2-methyl-, (.+/-.)- (17.85%) [7]. volatiles produced by Saccharomyces cerevisiae, Fialho et al. reported that the mycelium growth of the phytopathogen was inhibited almost 100% by the compound 2-methyl-1-butanol [29]. In the present study, 2-methyl-1-butanol was identified in the butanol extracts of two Plantago species that can be considered the main compound responsible for the antimicrobial activity. We could not determine whether di-(2-ethylhexyl) phthalate is synthesized by P. lanceolata, is absorbed from the atmosphere, or is absorbed by the roots; however, it seems that the compound, regardless of its origin, is probably present in the P. lanceolata leaf [30]. This compound has been reported in Euphorbia cyparissias, Euphorbia seguieriana [31], Aloe vera [32], Alchornea cordifolia [33], and Calotropis gigantea flowers [30]. According to the studies, this compound is not a contaminant, which was further confirmed by the GC-MS analysis. C. gigantea flowers can not be seen in plastic bags; therefore, they can be discounted as a source of di-(2-ethylhexyl) phthalate. Indeed, it may have taxonomic significance [30].
The antibacterial properties of P. lanceolata and P. major leaf extracts may be attributed to the presence of antibacterial compounds found in the GC/MS analysis, including palmitic acid [34], linolenic acid [24], gamma.-sitosterol [35], squalene [36], cycloheptasiloxane, tetradecamethyl- [37], hexadecanoic acid, methyl ester [38], phytol [39], 9,12-octadecadienoic acid, methyl ester [40], 1,2-benzenedicarboxylic acid, diisooctyl ester [41], 9-octadecyne [42], stearic acid [43], 9-octadecenoic acid (Z)-, methyl ester [44], cyclotetrasiloxane, octamethyl- [45], 2-methoxy-4-vinylphenol [24], pentadecanoic acid, ethyl ester [46], trans-vaccenic acid [40], Z-10-octadecen-1-ol acetate [47], 9,17-octadecadienal, (Z)- [48], phthalic acid, butyl undecyl ester [49], cyclotrisiloxane, hexamethyl- [50], octadecanoic acid [51], and other compounds.
Conclusions
Fractionation of the methanolic leaf extract of P. lanceolata and P. major can help better isolate active components from these plants, so that the antibacterial activity of the extracts can be carefully evaluated. We found numerous compounds in two Plantago species with antibacterial properties.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The paper was extracted from the PhD dissertation of Samaneh Rahamouz-Haghighi, approved by Department of Plant Production and Genetics, University of Zanjan (Grant No: A-12-848-35).
Authors' contributions
Conceptualization, project administration, investigation, formal analysis and writing the original draft: Samaneh Rahamouz-Haghighi; Funding acquisition and supervision: Khadijeh Bagheri and Ali Sharafi.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the School of Pharmacy of Zanjan University of Medical Sciences and University of Zanjan.
References
- Janković T, Zdunić G, Beara I, Balog K, Pljevljakušić D, Stešević D, et al. Comparative study of some polyphenols in plantago species. Biochem Syst Ecol. 2012; 42:69-74. [DOI:10.1016/j.bse.2012.02.013]
- Ozkan G, Kamiloglu S, Ozdal T, Boyacioglu D, Capanoglu E. Potential use of Turkish medicinal plants in the treatment of various diseases. Molecules. 2016; 21(3):257. [DOI:10.3390/molecules21030257] [PMID] [PMCID]
- Lan W, Yang C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci Total Environ. 2019; 654:1270-83. [DOI:10.1016/j.scitotenv.2018.11.180] [PMID]
- Rahamouz Haghighi S, Yazdinezhad A, Bagheri K, Sharafi A. Volatile constituents and toxicity of essential oils extracted from aerial parts of plantago lanceolata and plantago major growing in Iran. Pharm Biomed Res. 2022; 8(3):205-24. [DOI:10.18502/pbr.v8i3.11035]
- Rahamooz-Haghighi S, Bagheri K, Danafar H, Sharafi A. Anti-proliferative properties, biocompatibility, and chemical composition of different extracts of plantago major medicinal plant. Iran Biomed J. 2021; 25(2):106-16. [DOI:10.29252/ibj.25.2.106] [PMID] [PMCID]
- Rahamouz-Haghighi S, Bagheri K, Sharafi A, Tavakolizadeh M, Mohsen-Pour N. Phytochemical screening and cytotoxicity assessment of plantago lanceolata L. root extracts on colorectal cancer cell lines and brine shrimp larvae and determination of the median lethal dose in mice. S Afr J Bot. 2022; 149:740-7. [DOI:10.1016/j.sajb.2022.06.058]
- Rahamouz-Haghighi S, Bagheri K, Mohsen-Pour N, Sharafi A. In vitro evaluation of cytotoxicity and antibacterial activities of ribwort plantain (plantago lanceolata L.) root fractions and phytochemical analysis by gas chromatography-mass spectrometry. Arch Razi Inst. 2022; 77(6):2131-43. [DOI:10.22092/ARI.2022.358045.2143] [PMID] [PMCID]
- Rahamooz-Haghighi S, Bagheri K, Sharafi A, Danafar H. Establishment and elicitation of transgenic root culture of plantago lanceolata and evaluation of its anti-bacterial and cytotoxicity activity. Prep Biochem Biotechnol. 2021; 51(3):207-24. [DOI:10.1080/10826068.2020.1805757] [PMID]
- Rahamouz-Haghighi S, Bagheri K, Sharafi A. In vitro elicitation and detection of apigenin, catalpol and gallic acid in hairy root culture of plantago major L. and assessment of cytotoxicity and anti-bacterial activity of its methanolic extract. Nat Prod Res. 2023; 37(4):633-7. [DOI:10.1080/14786419.2022.2068543] [PMID]
- Rahamouz-Haghighi S, Bagheri K, Mohsen-Pour N, Sharafi A. Quantitative determination of apigenin, catalpol, and gallic acid in total extracts from different parts of plantago species by high-performance liquid chromatography. Avicenna J Pharm Res. 2021; 2(2):49-54. [DOI:10.34172/ajpr.2021.10]
- Jamilah J, Sharifa A, Sharifah NR. GC-MS analysis of various extracts from leaf of plantago major used as traditional medicine. World Appl Sci J. 2012; 17:67-70. [Link]
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests; approved standard: NCCLS document M2-A6. Wayne, PA: Clinical and Laboratory Standards Institute; 1997. [Link]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E.dis. 5.1. Clin Microbiol Infect. 2003; 9(8):1-7. [DOI:10.1046/j.1469-0691.2003.00790.x]
- Clinical and Laboratory Standards Institute). Performance standards for antimicrobial susceptibility testing: 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020. [Link]
- Satish S, Mohana D, Raghavendra M, BABU S, Raveesha K. Antibacterial evaluation of some Iranian medicinal plants against some human pathogenic bacteria. Asian J Microbiol Biotech Environ Sci. 2009; 11(4):735-8. [Link]
- Karakaş FP, Yildirim A, Türker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk J Biol. 2012; 36(6):641-52. [DOI:10.3906/biy-1203-16]
- Abd Razik BM, Hasan HA, Murtadha MK. The study of antibacterial activity of plantago major and ceratonia siliqua. Postgrad Med J. 2012; 11(1):130-5. [Link]
- Karima S, Farida S, Mihoub ZM. Antioxidant and antimicrobial activities of plantago major. Int J Pharm Pharm Sci. 2015; 7(5):58-64. [Link]
- Abate L, Abebe A, Mekonnen A. Studies on antioxidant and antibacterial activities of crude extracts of plantago lanceolata leaves. Chem Int. 2017; 3(3):277-87. [Link]
- Özkan O, Aydın H, Bağcıgil AF. [Salvia verticillata ve Phlomis pungens’ in in vitro antibakteriyel etkinliğinin değerlendirilmesi (Turkish)]. Kafkas Univ Vet Fak Derg. 2009; 15(4):587-90. [DOI:10.9775/kvfd.2009.073-A]
- Khalaf HAA, Mahdi MF, Abaas IS. Preliminary phytochemical and GC-MS analysis of chemical constituents of Iraqi Plantago lanceoleta L. Al Mustansiriyah J Pharm Sci. 2018; 18(2):114-21. [DOI:10.32947/ajps.v18i2.485]
- Bajer T, Janda V, Bajerová P, Kremr D, Eisner A, Ventura K. Chemical composition of essential oils from Plantago lanceolata L. leaves extracted by hydrodistillation. J Food Sci Technol. 2016; 53(3):1576-84. [DOI:10.1007/s13197-015-2083-x] [PMID] [PMCID]
- Alli KA, Ln M. Comparative evaluation of antimicrobial activities of root, stem and leaves of Holoptelea integrifolia against pathogenic bacteria. Asian J Microbiol Biotech Env Sc. 2014; 16(1):145-54. [link]
- Akpuaka A, Ekwenchi MM, Dashak DA, Dildar A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nat Sci. 2013; 11(5):141-7. [DOI:10.7537/marsnsj110513.21]
- Uma B, Parvathavarthini R. Antibacterial effect of Hexane Extract of sea urchin, temnopleurus alexandri (Bell, 1884). Int J Pharm Tech Res. 2010; 2(3):1677-80. [Link]
- Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of ceratonia siliqua essential oil with preservative effects against listeria inoculated in minced beef meat. Int J Food Microbiol. 2011; 148(1):66-72. [DOI:10.1016/j.ijfoodmicro.2011.04.028] [PMID]
- Faridha Begum I, Mohankumar R, Jeevan M, Ramani K. GC-MS analysis of bio-active molecules derived from paracoccus pantotrophus FMR19 and the antimicrobial activity against bacterial pathogens and MDROs. Indian J Microbiol. 2016; 56:426-32. [DOI:10.1007/s12088-016-0609-1] [PMID] [PMCID]
- Thirunavukkarasu K, Rajkumar P, Selvaraj S, Kumaresan S. GC-MS analysis of gymnema sylvestre leaves methanolic extract for antidiabetic and anticancer drug identification. J Chem Pharm Sci. 2016; 9(2):1011-13. [Link]
- Fialho MB, Moraes MH de, Tremocoldi AR, Pascholati SF. Potential of antimicrobial volatile organic compounds to control sclerotinia sclerotiorum in bean seeds. Pesqui Agropecu Bras. 2011; 46(2):137-42. [DOI:10.1590/S0100-204X2011000200004]
- Habib MR, Karim MR. Antimicrobial and cytotoxic activity of Di-(2-ethylhexyl) phthalate and anhydrosophoradiol-3-acetate isolated from calotropis gigantea (Linn.) flower. Mycobiology. 2009; 37(1):31-6. [PMID] [PMCID]
- Tóth-Soma LT, Gulyás S, Szegletes Z. Functional connection between intracellular and extracellular secretion in species of euphorbia genus. Acta Biol Hung. 1993; 44(4):433-43. [PMID]
- Lee KH, Kim JH, Lim DS, Kim CH. Antileukaemic and anti-mutagenic effects of Di-(2-ethylhexyl) phthalate isolated from aloe vera Linn. J Pharm Pharmacol. 2000; 52(5):593-8. [DOI:10.1211/0022357001774246] [PMID]
- Mavar-Manga H, Haddad M, Pieters L, Baccelli C, Penge A, Quetin-Leclercq J. Anti-inflammatory compounds from leaves and root bark of alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. J Ethnopharmacol. 2008; 115(1):25-9.[DOI:10.1016/j.jep.2007.08.043] [PMID]
- Sunita A, Ganesh K, Sonam M. Screening and evaluation of bioactive components of cenchrus ciliaris L. by GC-MS analysis. Int Res J Pharm. 2017; 8(6):69-76. [DOI:10.7897/2230-8407.08699]
- Raman BV, Samuel LA, Saradhi MP, Rao BN, Krishna NV, Sudhakar M, Radhakrishnan TM. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J Pharm Clin Res. 2012; 5(2):99-106. [Link]
- Rency RC, Vasantha K, Maruthasalam A. Identification of bioactive compounds from ethanolic leaf extracts of premna serratifolia L. using GC-MS. Biosci Discov. 2015; 6(2):96-101.
- Phuong TV, Lam PV, Diep CN. Bioactive compounds from marine streptomyces Sp. by gas chromatography-mass spectrometry. Pharm Chem J. 2018; 5(1):196-203. [Link]
- Chandrasekaran M, Senthilkumar A, Venkatesalu V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of sesuvium portulacastrum L. Eur Rev Med Pharmcol Sci. 2011; 15(7):775-80. [Link]
- Kumar D, Karthik M, Rajakumar R. GC-MS analysis of bioactive compounds from ethanolic leaves extract of eichhornia crassipes (mart) Solms. and their pharmacological activities. Pharma Innov J. 2018; 7(8):459-62. [Link]
- Rahman MM, Ahmad SH, Mohamed MT, Ab Rahman MZ. Antimicrobial compounds from leaf extracts of jatropha curcas, psidium guajava, and andrographis paniculata. Sci World J. 2014; 2014:635240. [DOI:10.1155/2014/635240] [PMID] [PMCID]
- Shettima AY, Karumi Y, Sodipo OA, Usman H, Tijjani MA. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of bioactive components of ethyl acetate root extract of guiera senegalensis JF Gmel. J App Pharm Sci. 2013; 3(3):146-50. [Link]
- Upgade A, Bhaskar A. Characterization and medicinal importance of phytoconstituents of C. papaya from Down south Indian region using gas chromatography and mass spectroscopy. Asian J Pharm Clin Res. 2013; 6(4):101-6. [Link]
- Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu MJ. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol. 2007; 38(4):739-42. [DOI:10.1590/S1517-83822007000400028]
- Ojinnaka CM, Nwachukwu KI, Ezediokpu MN. The chemical constituents and bioactivity of the seed (fruit) extracts of buchholzia coriacea engler (capparaceae). J Appl Sci Environ Manag. 2015; 19(4):795-801. [DOI:10.4314/jasem.v19i4.29]
- Zhen HS, Ge J, Liang J, Qiu Q, Song ZH, Zhong ZG, et al. [Analysis of the volatile oils chemical constituents of roots of Actinidia deliciosa (Chinese)]. Zhong Yao Cai. 2008; 31(5):677-8. [Link]
- Mujeeb F, Bajpai P, Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res Int. 2014; 2014:497606. [DOI:10.1155/2014/497606] [PMID] [PMCID]
- Subavathy P, Thilaga RD. GC-MS analysis of bioactive compounds from whole body tissue methanolic extract of cypraea arabica (L. 1758). World J Pharm Res. 2016; 5(3):800-6. [Link]
- Barathikannan K, Venkatadri B, Khusro A, Al-Dhabi NA, Agastian P, Arasu MV, et al. Chemical analysis of punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement Altern Med. 2016; 16:264. [DOI:10.1186/s12906-016-1237-3] [PMID] [PMCID]
- Al-Gara NI, Abu-Serag NA, Shaheed KA, Al Bahadly ZK. Analysis of bioactive phytochemical compound of (Cyperus alternifolius L.) by using gas chromatography-mass spectrometry. Paper presented at: The 4th International Conference on Agricultural Sciences. 17–18 November 2019; Kerbala City, Iraq. [DOI:10.1088/1757-899X/571/1/012047]
- Keskın D, Ceyhan N, Uğur A, Dbeys AD. Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea Europaea L.) leaves. J Food Agric Environ. 2012; 10(2):99-102. [DOI:10.1234/4.2012.2896]
- Manivannan P, Muralitharan G, Balaji NP. Prediction aided in vitro analysis of octa-decanoic acid from cyanobacterium lyngbya Sp. as a proapoptotic factor in eliciting anti-inflammatory properties. Bioinformation. 2017; 13(9):301-6. [DOI:10.6026/97320630013301] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Phyochemistry
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









