Volume 9, Issue 2 (2023)
Pharm Biomed Res 2023, 9(2): 147-152 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sheidaei S, Ghasemi M, Kavoosi E, Abedian Kenari F. Procalcitonin Serum Level in the Admitted COVID-19 Patients. Pharm Biomed Res 2023; 9 (2) :147-152
URL: http://pbr.mazums.ac.ir/article-1-511-en.html
URL: http://pbr.mazums.ac.ir/article-1-511-en.html
1- Department of Pathology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Immunogenetics Research Center, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Medical Student, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Immunogenetics Research Center, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Medical Student, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 518 kb]
(686 Downloads)
| Abstract (HTML) (1404 Views)
Full-Text: (722 Views)
Introduction
Procalcitonin (PCT) is a precursor peptide of the hormone calcitonin, produced by many body cells. It is produced especially in response to bacterial infections, sepsis, and tissue damage. This marker differentiates bacterial infection from other non-infectious inflammatory conditions, viral infections, and microbial contamination of the sample [1, 2, 3]. Therefore, one of the important benefits of PCT measurements is speeding up the diagnosis of bacterial infection. On the other hand, awareness of the need for antibiotic treatment in patients with fever reduces antibiotic overuse in epidemics in intensive care units or emergency departments. In other words, it prevents the possible side effects of antibiotic abuse in patients [1, 2, 4].
Coronavirus disease 2019 (COVID-19) due to the infection with an emerging RNA virus called acute respiratory virus (SARS-CoV-2) leads to a global outbreak with unfortunate physical, psychological, and social consequences and high mortality for humans [5]. Extensive studies are required to fully identify biological features, clinical signs, diagnostic tips, ways of prevention, therapeutic interventions, and access to markers determining the prognosis and severity of this emerging disease [6, 7, 8, 9]. PCT is a useful blood marker for developing antibiotic initiation guidelines introduced while treating bacterial pneumonia and acute respiratory distress syndrome (ARDS) [4, 10]. To determine the extent of changes in blood levels of this acute phase and estimate the frequency of bacterial superinfection and prescribe antibiotics in people with involvement, especially the lower respiratory tract or ARDS, as well as the limited number of studies on biomarkers of this disease and the reported variations of severity, manifestations, and outcome of the disease in different geographical areas [11, 12, 13, 14, 15, 16], we aimed to measure the serum PCT levels in COVID-19 patients admitted to hospitals in northern Iran.
Materials and Methods
Study design and population
This cross-sectional descriptive-analytical study was conducted in Boo-Ali Sina and Imam Khomeini hospitals, Sari City, Mazandaran Province, the north of Iran, from March 5, 2020, to June 4, 2020. The study population consisted of all patients diagnosed with COVID-19 who have been admitted to general or special units of these hospitals during the pandemic of COVID-19. Patients with a history of hemodialysis, amphetamine use, renal failure, rheumatoid arthritis, or uncontrolled lupus erythematosus were excluded from the study. This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.7681).
Data collection
A questionnaire consisting of demographic characteristics such as gender and age was filled out for every patient. Results of some laboratory and clinical manifestations of the disorder were extracted from their clinical records.
Diagnosis of COVID-19 was based on clinical manifestations and laboratory and molecular tests. Blood samples were collected with EDTA anticoagulant, and a cell blood count (CBC) test was done with a Sysmex machine, XS 500i (Sysmex Corporation Kobe, Japan). All patients were tested positive for SARS-CoV-2 using real-time polymerase chain reaction (RT-PCR). Samples from nose and throat swabs were collected in a virus transport medium. Samples were extracted, and PCR amplification was performed on the Applied Biosystems (ABI) 7500 using the VIASURE SAR-CoV-2 RT-PCR kit. Moreover, blood samples taken at the admission time were centrifuged for 10 minutes, then sera were separated. Eventually, serum PCT levels were measured by VIDAS instrument and PCT kit produced by BIOMERIEUX France (Lot:1007989680). The reference range of the serum PCT was less than 0.05 ng/mL. According to the manufacturing company’s advice, serum PCT level less than 0.25 ng/mL indicates no risk of severe sepsis/septic shock, levels more than or equal to 0.5 indicates high risk, and levels within this range point to low risk for developing sepsis. The patients were followed for one month after admission date by means of repeat RT-PCR test results, course of the patient symptoms, discharge or need to more hospitalization, regression of chest CT-scan or chest x-ray signs, and need to home care.
Data analysis
Statistical analysis of results was performed using SPSS software, version 24. Quantitative variables were expressed as mean and standard deviation (mean±SD). Qualitative variables were expressed as frequency and percentage. The Kolmorov-Smirnov test was used for the survey of the normality of data. The Student t-test or non-parametric equivalent Mann-Whitney U test was used to compare the means of the two groups. The Pearson correlation coefficient test and Spearman’s rank correlation coefficient were used to examine the significant correlations between quantitative parameters and non-parametric data. P<0.05 was considered statistically significant.
Results
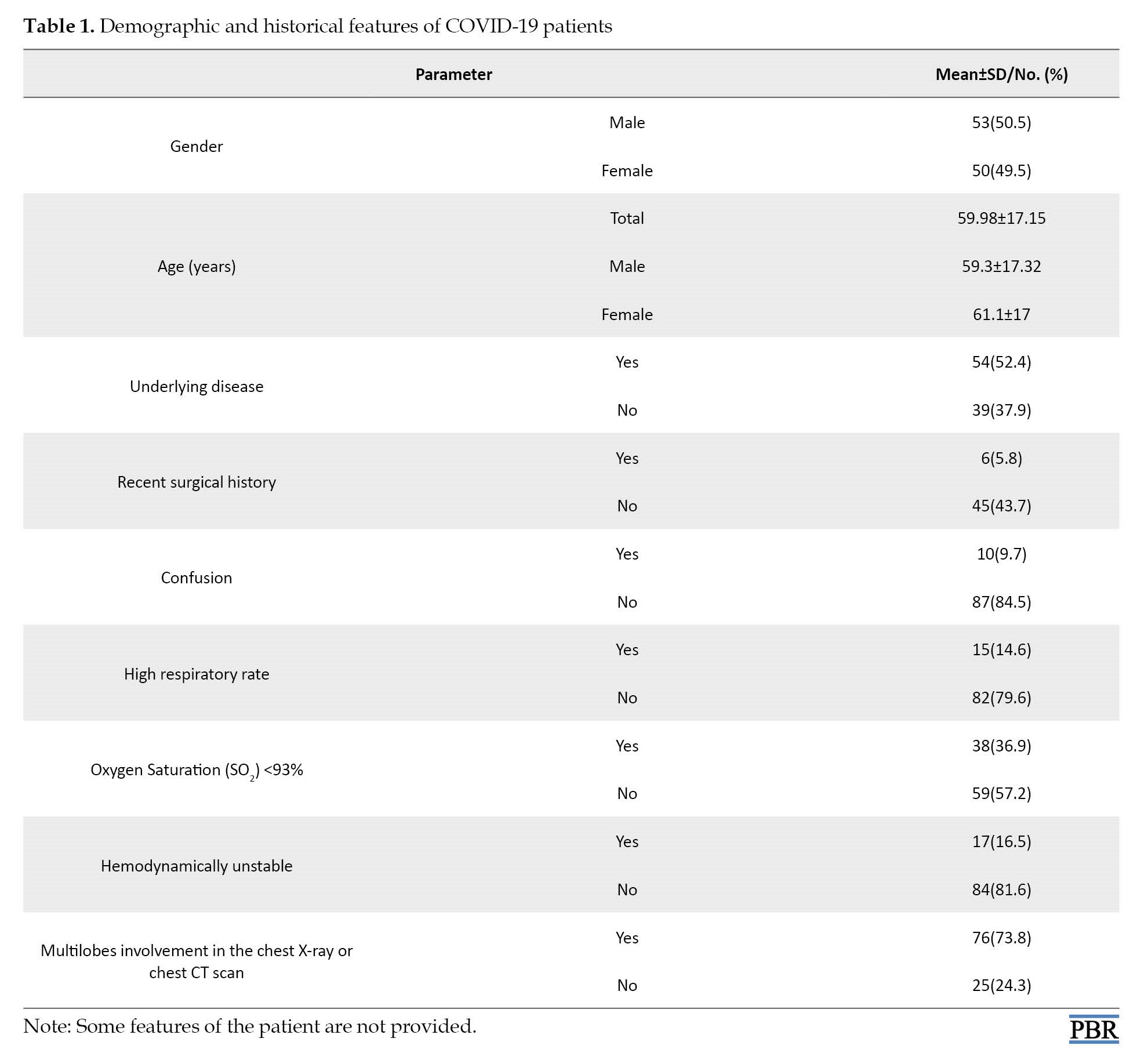
A total of 103 COVID-19 patients (53 males and 50 females) were included in the study. Finally, out of these patients, 55 patients (53.4%) improved, 23 patients (23.3%) were admitted, and 19 patients (18.4%) died. Demographic and historical features of COVID-19 patients are presented in Table 1.
The serum PCT levels were raised in 65 examined patients (63.1%), and the mean of the measurements was 0.18±0.024 ng/mL. The mean serum PCT levels
were 0.19±0.024 and 0.17±0.024 ng/mL in the men and women, respectively; the difference was negligible (P=0.895).
Forty-three (41.7%) of our patients were admitted to ICU units. The mean serum PCT levels did not show a significant difference in patients admitted to the ICU (0.18±0.019 ng/mL) compared to non-ICU patients (0.19±0.027 ng/mL) (P=0.389).
There was a significant relationship between the age of patients and serum PCT levels (P=0.025) and short-term prognosis (P=0.044). Table 2 presents the correlation between specific parameters and serum PCT levels in COVID-19 patients.

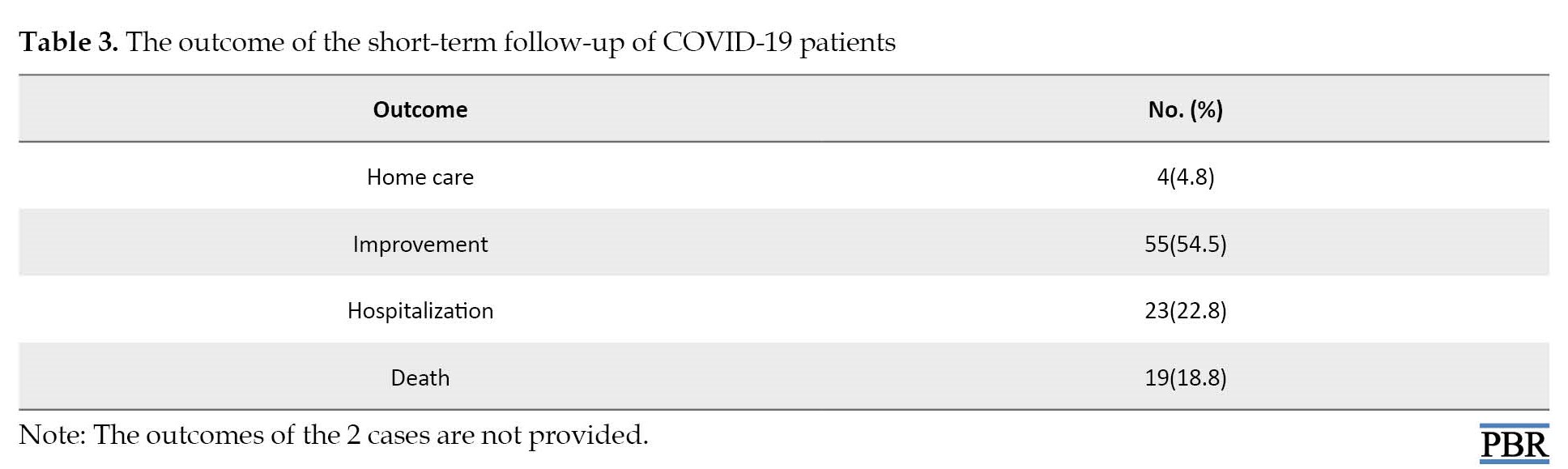
During our research period, we had 19 deaths. The outcome of the short-term follow-up of our patients is resented in Table 3.
There was a significant relationship between serum PCT levels and short-term prognosis (P=0.044).
Discussion
Studies have shown that high PCT level is associated with the severity of many diseases, such as bacterial endocarditis, pancreatitis, pyelonephritis and enterocolitis, and even appendicitis. PCT is a useful blood marker for developing antibiotic initiation guidelines introduced while treating bacterial pneumonia and acute respiratory distress syndrome (ARDS) [1, 2, 3]. Our study showed that the mean serum PCT level in patients with COVID-19 was 0.18±0.024 ng/mL. A statistically significant relationship existed between serum PCT level and age (P=0.025). It was shown that serum PCT levels are raised with the increasing age of patients.
A statistically significant relationship existed between serum PCT level and short-term prognosis (P=0.044). This finding was similar to Fang Liu et al. findings. They evaluated the prognostic value of PCT on 140 patients with COVID-19 at admission in two groups from the grade of clinical manifestation: SG (Severe group) and MG (Medium group). The two groups had no significant difference regarding the male-to-female ratio. Out of 140 patients, PCT increased in 8 patients (7.7%). The proportion of patients with increased levels of PCT in SG was significantly higher than in MG (P=0.001). They observed an increase in PCT in only 8 patients (5.7%) with a severe form of the disease and suggested that PCT efficacy in the management process matters [17]. Feu Zhou et al. supported our results. They showed that out of 168 patients for whom the PCT test was performed, 70% of cases were normal, and 30% were increased and had a significant relationship with disease severity and mortality [18].
Also, Lin C et al., in line with our findings, by investigating meta-analysis review studies, described the role of PCT in the short-term prognosis of monitoring. They concluded that discontinuing antibiotic therapy based on serum PCT in ICU patients reduces mortality. PCT treatment significantly reduced antibiotic use compared to standard therapy without increasing clinical failure and mortality [10].
Heesom et al. supported our results. They reported a significant difference in the length of ICU stay. Patients with high PCT levels require invasive ventilation compared to those with low PCT [19]. Lippi G et al., by conclusion from the available literature, reported that serum PCT levels increased in patients with a severe form of COVID-19 infection and bacterial superinfection [9].
According to our results, serum PCT level does not significantly increase in patients with COVID-19 at admission; the mean serum PCT levels was 0.18±0.024 ng/mL in our patients, which correlates to observations of other studies revealed a low level of serum PCT (<0.1−<0.5 µg/L) in viral respiratory tract infection without concurrent bacterial infection or systemic inflammatory reactions [1, 4, 8, 9, 10].
Conclusion
The short-term prognosis of the disease was significantly related to serum PCT levels, which indicates that high serum PCT level worsens the short-term prognosis of the disease. So, serum PCT levels at admission time may help determine disease severity and predict disease prognosis in COVID-19 patients.
Limitations of our study were low sample size, lack of a classification of our patients in terms of severity of clinical manifestation, and no serial measurement of serum PCT level. So, we suggest that in addition to overcoming these limitations, the administration of antibiotics be designated by measuring the serum PCT levels in patients with COVID-19 at admission.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Ethics Committee at Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.7681).
Funding
This study was supported by the Vice-Chancellor of Research and Technology of Mazandaran University of Medical Sciences (Grant No.: 1399.7681).
Authors' contributions
Conceptualization: Maryam Ghasemi, Somayeh Sheidaei, and Fatemeh Abedian Kenari; Collection of data: Elahe Kavoosi; Designing -writing, analysis of data and revision of paper: Maryam Ghasemi, Fatemeh Abedian Kenari, Somayeh Sheidaei, and Elahe Kavoosi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank the Vice-Chancellor of Research and Technology of Mazandaran University of Medical Sciences for supporting this project.
References
Procalcitonin (PCT) is a precursor peptide of the hormone calcitonin, produced by many body cells. It is produced especially in response to bacterial infections, sepsis, and tissue damage. This marker differentiates bacterial infection from other non-infectious inflammatory conditions, viral infections, and microbial contamination of the sample [1, 2, 3]. Therefore, one of the important benefits of PCT measurements is speeding up the diagnosis of bacterial infection. On the other hand, awareness of the need for antibiotic treatment in patients with fever reduces antibiotic overuse in epidemics in intensive care units or emergency departments. In other words, it prevents the possible side effects of antibiotic abuse in patients [1, 2, 4].
Coronavirus disease 2019 (COVID-19) due to the infection with an emerging RNA virus called acute respiratory virus (SARS-CoV-2) leads to a global outbreak with unfortunate physical, psychological, and social consequences and high mortality for humans [5]. Extensive studies are required to fully identify biological features, clinical signs, diagnostic tips, ways of prevention, therapeutic interventions, and access to markers determining the prognosis and severity of this emerging disease [6, 7, 8, 9]. PCT is a useful blood marker for developing antibiotic initiation guidelines introduced while treating bacterial pneumonia and acute respiratory distress syndrome (ARDS) [4, 10]. To determine the extent of changes in blood levels of this acute phase and estimate the frequency of bacterial superinfection and prescribe antibiotics in people with involvement, especially the lower respiratory tract or ARDS, as well as the limited number of studies on biomarkers of this disease and the reported variations of severity, manifestations, and outcome of the disease in different geographical areas [11, 12, 13, 14, 15, 16], we aimed to measure the serum PCT levels in COVID-19 patients admitted to hospitals in northern Iran.
Materials and Methods
Study design and population
This cross-sectional descriptive-analytical study was conducted in Boo-Ali Sina and Imam Khomeini hospitals, Sari City, Mazandaran Province, the north of Iran, from March 5, 2020, to June 4, 2020. The study population consisted of all patients diagnosed with COVID-19 who have been admitted to general or special units of these hospitals during the pandemic of COVID-19. Patients with a history of hemodialysis, amphetamine use, renal failure, rheumatoid arthritis, or uncontrolled lupus erythematosus were excluded from the study. This study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.7681).
Data collection
A questionnaire consisting of demographic characteristics such as gender and age was filled out for every patient. Results of some laboratory and clinical manifestations of the disorder were extracted from their clinical records.
Diagnosis of COVID-19 was based on clinical manifestations and laboratory and molecular tests. Blood samples were collected with EDTA anticoagulant, and a cell blood count (CBC) test was done with a Sysmex machine, XS 500i (Sysmex Corporation Kobe, Japan). All patients were tested positive for SARS-CoV-2 using real-time polymerase chain reaction (RT-PCR). Samples from nose and throat swabs were collected in a virus transport medium. Samples were extracted, and PCR amplification was performed on the Applied Biosystems (ABI) 7500 using the VIASURE SAR-CoV-2 RT-PCR kit. Moreover, blood samples taken at the admission time were centrifuged for 10 minutes, then sera were separated. Eventually, serum PCT levels were measured by VIDAS instrument and PCT kit produced by BIOMERIEUX France (Lot:1007989680). The reference range of the serum PCT was less than 0.05 ng/mL. According to the manufacturing company’s advice, serum PCT level less than 0.25 ng/mL indicates no risk of severe sepsis/septic shock, levels more than or equal to 0.5 indicates high risk, and levels within this range point to low risk for developing sepsis. The patients were followed for one month after admission date by means of repeat RT-PCR test results, course of the patient symptoms, discharge or need to more hospitalization, regression of chest CT-scan or chest x-ray signs, and need to home care.
Data analysis
Statistical analysis of results was performed using SPSS software, version 24. Quantitative variables were expressed as mean and standard deviation (mean±SD). Qualitative variables were expressed as frequency and percentage. The Kolmorov-Smirnov test was used for the survey of the normality of data. The Student t-test or non-parametric equivalent Mann-Whitney U test was used to compare the means of the two groups. The Pearson correlation coefficient test and Spearman’s rank correlation coefficient were used to examine the significant correlations between quantitative parameters and non-parametric data. P<0.05 was considered statistically significant.
Results
A total of 103 COVID-19 patients (53 males and 50 females) were included in the study. Finally, out of these patients, 55 patients (53.4%) improved, 23 patients (23.3%) were admitted, and 19 patients (18.4%) died. Demographic and historical features of COVID-19 patients are presented in Table 1.

The serum PCT levels were raised in 65 examined patients (63.1%), and the mean of the measurements was 0.18±0.024 ng/mL. The mean serum PCT levels
were 0.19±0.024 and 0.17±0.024 ng/mL in the men and women, respectively; the difference was negligible (P=0.895).
Forty-three (41.7%) of our patients were admitted to ICU units. The mean serum PCT levels did not show a significant difference in patients admitted to the ICU (0.18±0.019 ng/mL) compared to non-ICU patients (0.19±0.027 ng/mL) (P=0.389).
There was a significant relationship between the age of patients and serum PCT levels (P=0.025) and short-term prognosis (P=0.044). Table 2 presents the correlation between specific parameters and serum PCT levels in COVID-19 patients.

During our research period, we had 19 deaths. The outcome of the short-term follow-up of our patients is resented in Table 3.

There was a significant relationship between serum PCT levels and short-term prognosis (P=0.044).
Discussion
Studies have shown that high PCT level is associated with the severity of many diseases, such as bacterial endocarditis, pancreatitis, pyelonephritis and enterocolitis, and even appendicitis. PCT is a useful blood marker for developing antibiotic initiation guidelines introduced while treating bacterial pneumonia and acute respiratory distress syndrome (ARDS) [1, 2, 3]. Our study showed that the mean serum PCT level in patients with COVID-19 was 0.18±0.024 ng/mL. A statistically significant relationship existed between serum PCT level and age (P=0.025). It was shown that serum PCT levels are raised with the increasing age of patients.
A statistically significant relationship existed between serum PCT level and short-term prognosis (P=0.044). This finding was similar to Fang Liu et al. findings. They evaluated the prognostic value of PCT on 140 patients with COVID-19 at admission in two groups from the grade of clinical manifestation: SG (Severe group) and MG (Medium group). The two groups had no significant difference regarding the male-to-female ratio. Out of 140 patients, PCT increased in 8 patients (7.7%). The proportion of patients with increased levels of PCT in SG was significantly higher than in MG (P=0.001). They observed an increase in PCT in only 8 patients (5.7%) with a severe form of the disease and suggested that PCT efficacy in the management process matters [17]. Feu Zhou et al. supported our results. They showed that out of 168 patients for whom the PCT test was performed, 70% of cases were normal, and 30% were increased and had a significant relationship with disease severity and mortality [18].
Also, Lin C et al., in line with our findings, by investigating meta-analysis review studies, described the role of PCT in the short-term prognosis of monitoring. They concluded that discontinuing antibiotic therapy based on serum PCT in ICU patients reduces mortality. PCT treatment significantly reduced antibiotic use compared to standard therapy without increasing clinical failure and mortality [10].
Heesom et al. supported our results. They reported a significant difference in the length of ICU stay. Patients with high PCT levels require invasive ventilation compared to those with low PCT [19]. Lippi G et al., by conclusion from the available literature, reported that serum PCT levels increased in patients with a severe form of COVID-19 infection and bacterial superinfection [9].
According to our results, serum PCT level does not significantly increase in patients with COVID-19 at admission; the mean serum PCT levels was 0.18±0.024 ng/mL in our patients, which correlates to observations of other studies revealed a low level of serum PCT (<0.1−<0.5 µg/L) in viral respiratory tract infection without concurrent bacterial infection or systemic inflammatory reactions [1, 4, 8, 9, 10].
Conclusion
The short-term prognosis of the disease was significantly related to serum PCT levels, which indicates that high serum PCT level worsens the short-term prognosis of the disease. So, serum PCT levels at admission time may help determine disease severity and predict disease prognosis in COVID-19 patients.
Limitations of our study were low sample size, lack of a classification of our patients in terms of severity of clinical manifestation, and no serial measurement of serum PCT level. So, we suggest that in addition to overcoming these limitations, the administration of antibiotics be designated by measuring the serum PCT levels in patients with COVID-19 at admission.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Ethics Committee at Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.7681).
Funding
This study was supported by the Vice-Chancellor of Research and Technology of Mazandaran University of Medical Sciences (Grant No.: 1399.7681).
Authors' contributions
Conceptualization: Maryam Ghasemi, Somayeh Sheidaei, and Fatemeh Abedian Kenari; Collection of data: Elahe Kavoosi; Designing -writing, analysis of data and revision of paper: Maryam Ghasemi, Fatemeh Abedian Kenari, Somayeh Sheidaei, and Elahe Kavoosi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank the Vice-Chancellor of Research and Technology of Mazandaran University of Medical Sciences for supporting this project.
References
- Vijayan AL, Vanimaya, Ravindran S, Saikant R, Lakshmi S, Kartik R, et al. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy.J Intensive Care. 2017; 5:51. [PMID] [PMCID]
- Knudsen JB, Fuursted K, Petersen E, Wierup P, Mølgaard H, Poulsen SH, et al. Procalcitonin in 759 patients clinically suspected of infective endocarditis. Am J Med. 2010; 123(12):1121-7. [DOI:10.1016/j.amjmed.2010.07.018] [PMID]
- van Nieuwkoop C, Bonten TN, van't Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, et al. Procalcitonin reflects bacteremia and bacterial load inurosepsis syndrome: A prospective observational study. Crit Care. 2010; 14(6):R206. [PMID] [PMCID]
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: Past, present and future. BMC Med. 2011; 9:107. [PMID] [PMCID]
- Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): A review of clinical features, diagnosis, and treatment. Cureus. 2020; 12(3):e7355. [DOI:10.7759/cureus.7355]
- No Author. Pneumonia:Procalcitonin (PCT) for risk assessment and rule-out of bacterial. 2020.
- Bsuh.nhs. BritisEngland: Guidelines on the use of Procalcitoninin COVID-19. 2020.
- Responsebio. Canada: PCT and D-dimer in patients with COVID-19. Vancouver: Responsebio; 2020.
- Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis.Clin Chim Acta. 2020; 505:190-1. [DOI:10.1016/j.cca.2020.03.004] [PMID] [PMCID]
- Lin C, Pang Q. Meta-analysis and systematic review of procalcitonin-guided treatment in acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018; 12(1):10-5. [DOI:10.1111/crj.12519] [PMID]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395(10223):507-13. [DOI:10.1016/S0140-6736(20)30211-7] [PMID]
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically Ill patients in the seattle region -case series. N Engl J Med. 2020; 382(21):2012-22. [PMID] [PMCID]
- Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020; 382(24):2302-15. [PMID] [PMCID]
- Kovich H. Rural matters -coronavirus and the Navajo Nation. N Engl J Med. 2020; 383(2):105-7. [DOI:10.1056/NEJMp2012114] [PMID]
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020; 382(24):2372-4. [DOI:10.1056/NEJMc2010419] [PMID] [PMCID]
- Haug C. French pandemic resistance. N Engl J Med. 2020; 382(19):e51. [PMID] [PMCID]
- Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020; 127:104370. [DOI:10.1016/j.jcv.2020.104370] [PMID] [PMCID]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395(10229):1054-62. [DOI:10.1016/S0140-6736(20)30566-3] [PMID]
- Heesom L, Rehnberg L, Nasim-Mohi M, Jackson AIR, Celinski M, Dushianthan A, et al. Procalcitonin as an antibiotic stewardship tool in COVID-19 patients in the intensive care unit. J Glob Antimicrob Resist. 2020; 22:782-4. [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Laboratory
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |










