Volume 9, Issue 2 (2023)
Pharm Biomed Res 2023, 9(2): 133-146 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kousar Z, Manzoor S, Naz S, Shahid M S. Extraction, Fractionation, Phytochemical Profile, Antioxidant, and Antimicrobial Activities of Cassia absus L. and Citrus medica L.. Pharm Biomed Res 2023; 9 (2) :133-146
URL: http://pbr.mazums.ac.ir/article-1-502-en.html
URL: http://pbr.mazums.ac.ir/article-1-502-en.html
1- Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, Pakistan.
2- Division of Science and Technology, University of Education Lahore, Multan Campus 60700, Pakistan.
3- Department of Plant Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Al-Khoud 123, Muscat, Oman.
2- Division of Science and Technology, University of Education Lahore, Multan Campus 60700, Pakistan.
3- Department of Plant Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Al-Khoud 123, Muscat, Oman.
Keywords: Phytochemical, Phytonutrients, Plant-derived compounds, Citrus medica L., Cassia absus L., Antioxidant activity, Antimicrobial activity
Full-Text [PDF 1554 kb]
(1207 Downloads)
| Abstract (HTML) (1525 Views)
absus leaves, showing the maximum amount with methanol followed by ethyl acetate29, 30]. Moreover, it was reported earlier that phenolic content depends on the nature of plant material, specific part of the plant, the physiology of the plant, as well as the application of solvent polarity being used [31, 32, 33, 34].
Total flavonoid contents
According to our observation, the total flavonoid contents per 100 g extract of C. absus and C. medica fractions were 11.50-32.40 g and 24.91-36.36 g. It was shown that the higher yield with ethanol fractions in both C. absus (32.4/100 g) and C. medica (36.36 g/100 g) leaves were obtained, and the lowest was achieved by ethyl acetate fractions (Figure 4B). The total amount of flavonoids is expressed as catechin (mg/g) equivalents, and a significant deviation (P<0.05) was observed in TPC values of different fractions of solvent.
Estimation of reducing power
Reducing power is linked with antioxidant properties and may preserve as an important meditation of the antioxidant activity. A concentration range (2-8 mg/100 mL) was adopted to measure reducing power. Various sample fractions demonstrate high reducing power at 8 mg/100 mL concentration sequenced from 0.530-0.970 mg/100 mL (Figure 5A) and 0.560-1.140 mg/100 mL and 0.560-1.140 mg/100 mL (Figure 5B), respectively.
Results show the direct relationship between concentration and reducing potential [35], so the polar fractions (ethanol, acetic acid, methanol) provide inclined reducing potential instead of other respective fractions. In C. absus, the order of decreasing power was ethyl acetate DPPH radical scavenging assay
A significant variation was observed in the DPPH free radical inhibition (Figure 6A), where the polar extracts fractions showed maximum inhibition in both the plant extracts such as 64.28%-67.01% and 59.47%-74.74% whereas, n-hexane extracts fractions showed weak radical scavenging activity. The free radical inhibition might be declined due to polarity reduction or reduced phenolic compounds [17].
Inhibition of linoleic acid per oxidation
The inhibition percentage age of linoleic acid following 360 h incubation was observed. The sample fractions illustrate the linoleic acid peroxidation of 27.21% for non-polar and 13.730%-18.930% for polar fractions of C. absus extract while 17.693% and 28.88%-49.60% for a non-polar and polar fraction of C. medica plant extract (Figure 6B). The effect of polarity (P<0.005) at the oxidation of linoleic acid was significant. The findings of this study confirmed that C. absus and C. medica plant extracts showed a higher inhibition percentage of linoleic acid in methanol fractions. This condition further proves that polarity directly affects antioxidant activity as the decline in polarity resulted in the decline of antioxidant activity that may be responsible for fewer phenolics that cause this activity [36].
Assessment of antimicrobial activity
Fractions of C. absus and C. medica extracts were exploited to access antimicrobial activity (Figure 7).
The inhibition zone was measured with the help of a vernier caliper (mm), and the results demonstrated different inhibition zones for different fractions. These results were confirmed using 4 different bacterial and fungal strains to access antimicrobial activity determination. The data presented in Table 1 is the mean of three experiments that show methanol fractions as a prolific channel for maximum inhibition of microorganisms.
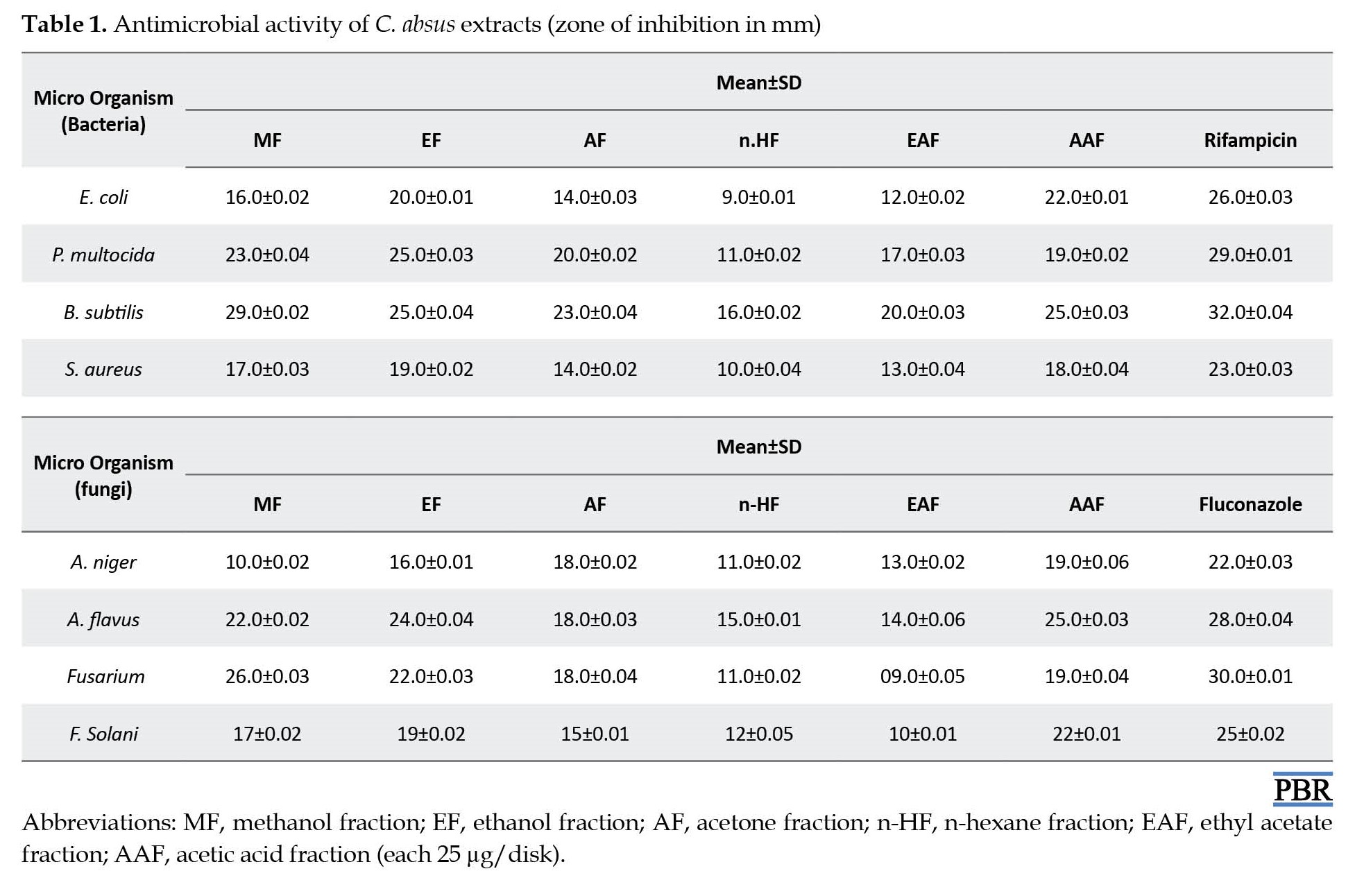
In the antibacterial assay, methanol fraction has a wide inhibition zone (29.0±0.02 mm) over the Bacillus subtilis bacterial strain, and nHF reduced zone of inhibition (9.0±0.01 mm) against the Escherichia coli bacterial strain in antifungal case methanol fraction exposed the wide zone of inhibition (26.0±0.03 mm) against fungus Fusarium and EA fraction lessen the inhibition zone (09.0±0.05 mm) despite the presence of same strains. Results demonstrate that methanol fractions provided an excellent result. Malpani reported in 2013 the antibacterial and antifungal assay of C. absus, whose results confirmed that the isolated active acidic ingredient was more effective than the whole extract of leaves, which showed moderate activity. The results obtained were assessed on their comparison with the activities of standard antibacterial agents like ampicillin and vancomycin as a control [37, 38, 39].
Discussion
Phytonutrients from traditional medicines and chemicals have recently gained extensive consideration as a targeted remedy to cure several health problems. Subsequently, more bioactive compounds from plants with medicinal characteristics are found. The antioxidants harvested from different plant species have been beneficial in curing human diseases in the recent decade. These traditional phytochemicals have been offered to treat and prevent certain diseases [25, 38]. The prepared crude plant extracts were achieved from the leaves of Cassia absus L. and Citrus medica L. for bioactive phytonutrients. The findings of this study suggest that these plant-based molecules are a rich source of biologically active compounds. This excessive concentration of these plant-derived compounds may be a part of plant defense systems. These identified plant-based chemicals might have some important biological significance. These phytochemicals present in the crude extracts of Cassia absus L. and Citrus medica L. host have not been studied earlier. Furthermore, the extracts from Cassia absus L. and Citrus medica L., including acetic acid, ethanol, and methanol, show antioxidant activity by scavenging free radicals in DPPH assay, reducing power test, and linoleic acid scavenging assay and can be used as the source of unique medicines with significant potential to prevent many illnesses. Besides the phytochemicals and antioxidant activity, C. absus and C. medica plants sample fractions offer antimicrobial activity, where HPLC is utilized to investigate both plants’ composition. Based on the findings, the investigated bioactive compounds in C. absus and C. medica might be consumed to synthesize different antimicrobial and antioxidant drugs for the sake of human beings.
The plant-based compounds here further confirm the availability of the phytochemicals cited in the literature, which belong to different plant families [40]. The antibacterial activity was also tested, and the study further confirmed the antibacterial activity inclined in methanol fraction against bacteria S. aureus strain (18.23±0.01 mm) unlike other fractions, which decrease (14.88±0.01 and 13.88±0.07 mm) the inhibition zone (Table 2).
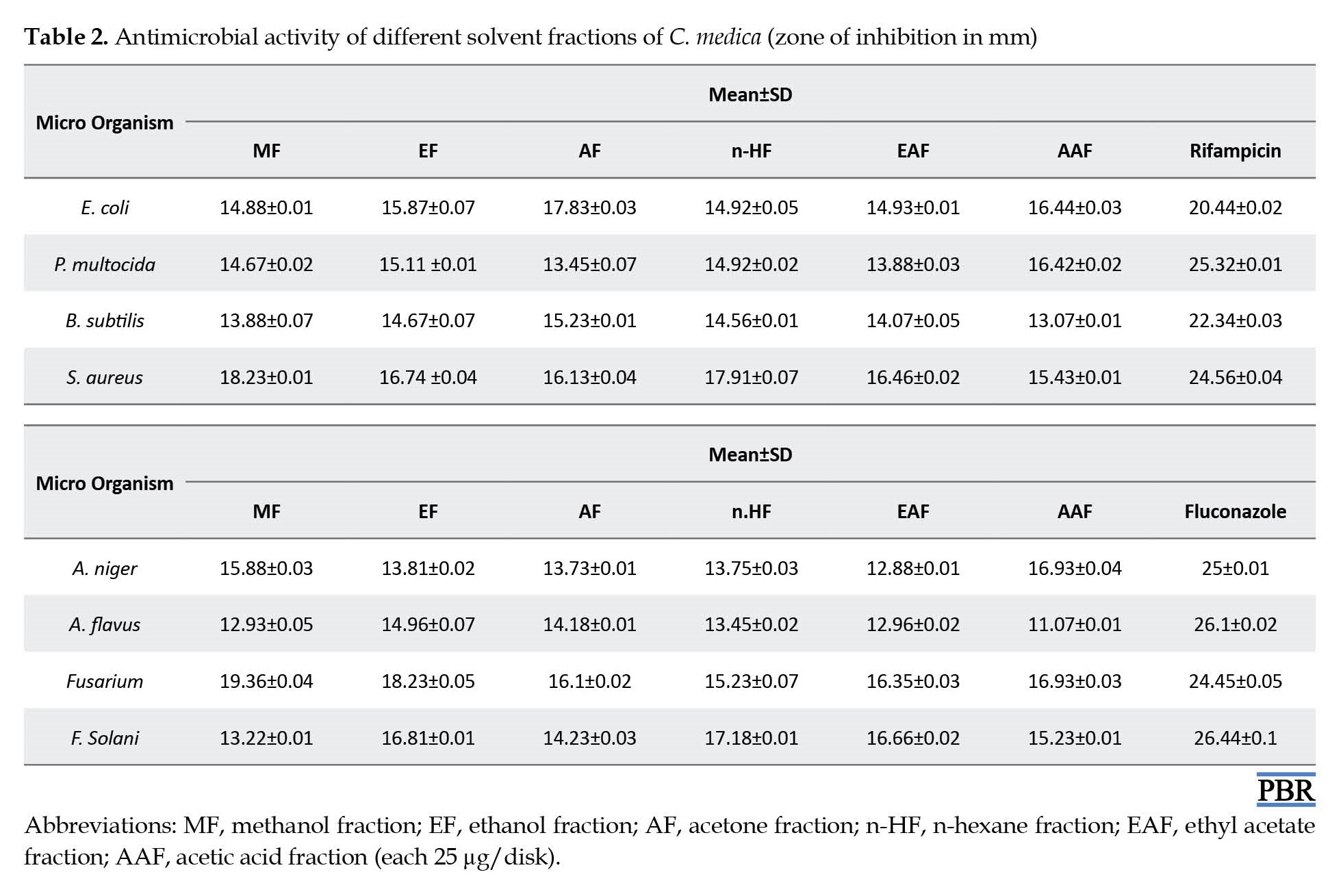
Likewise, in the antifungal activity, wide inhibition was given by methanol fraction against Fusarium fungus (19.36±0.04 mm) while exhibiting less inhibition zone against others (12.93±0.05 and 15.88±0.03 mm). This condition resulted in the methanol fractions providing excellent results in this study [41] reported. Leaf and root extracts and fruit juice showed varied antibacterial activity against one or more tested bacteria. Also, antifungal activity was shown by only root extract and fruit juice, while Candida albicans was resistant to all tested plant samples. Moreover, among all tested plant samples, leaf and peel extracts have shown less antimicrobial activity [40-42].
Conclusion
This study aimed to identify different compounds present in the leaf extract of C. absus L. and C. medica L. plants. The results obtained by the HPLC measurements exhibited different compounds from C. absus L. and C. medica L. extracts. The study confirmed that C. absus L. and C. medica L. are rich sources of various bioactive compounds with extensive applications in pharmaceutical companies. Based on these research findings, the investigated bioactive compounds in C. absus and C. medica might be consumed to synthesize different antimicrobial and antioxidant drugs for the sake of human beings.
Ethical Considerations
Compliance with ethical guidelines
All ethical issues related to this study were considered.
Funding
This study did not receive funds from any public/commercial/non-profit organization.
Authors' contributions
Conceived idea and designed work: Surryia Manzoor and Saima Naz; Lab work, performed analysis, and wrote the initial draft of the manuscript: Zartasha Kousar; Analyzed, proofread, and critically improved the manuscript: Surryia Manzoor, Saima Naz, and Muhammad Shafiq Shahid; Approved the final draft of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank our colleagues at the University of Education Lahore, Multan campus, and the Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, for their esteemed help completing this work.
References
Full-Text: (1557 Views)
Introduction
Since human domestication, plants have been exploited for various reasons, such as food, feed, fiber, and medicine. Each plant and its parts (roots, leaves, fruits, bark) have unique chemicals that provide different medicinal properties-for instance, phenolic compounds contribute to antioxidant activity. Every plant owns special biochemical materials and their therapeutic effects [1]. For example, free radicals are produced due to the higher reactivity of oxygen, which is responsible for many disorders [2]. Such oxidative stress of free radicals on body tissues plays a significant role in diverse pathological conditions, such as aging and infection at the cellular stage and in medicinal and biological systems [3, 4]. Different enzymatic and non-enzymatic reactions are responsible for the production of oxygen free radicals as well as during normal physiological phenomena [5]. The pathological condition results in the overproduction of cells that cause damage [6]. Antioxidants are known as radical scavengers that defend the body over free radicals, and their abundance in nature encouraged scientists to substitute synthetic antioxidants to avoid their conflicting effects. Microorganisms are responsible for food spoilage, thus reducing its shelf life and causing various diseases. For example, Staphylococcus aureus is responsible for toxic shock, food poisoning, and osteomyelitis [4, 7, 8].
Cassia absus L. (Chaksu) is a vertical annual plant belonging to the Leguminosae family. C. absus is commonly found in south and tropical Asia, Africa, Australia, and India, with lengths up to 12–25 inches [9]. Chemical studies have proved that the leaves and roots of C. absus are rich in biochemicals, such as chaksine, rutin, quercetin, chrysophanol, riboflavin, aloe-emodin, and isochaksine [10, 11]. Seeds are augmented with saponins, glycosides, flavonoids, tannins, terpenoids, gums, amino acids, resins, oleic acid, steroids, and linoleic acid. C. absus can scavenge free radicals and is an essential source of antioxidants and phytochemicals. The bitter and styptic nature of leaves helps to cure dermis infections, constipation, cancer, respiratory ailment, and hemorrhoids [12, 13].
Citrus medica L. (family: Rutaceae), also recognized as Bara Nimbu, is a perennial bush or small tree with a height of about 3.6 m and found in warm moist regions in Asia and more common in Northern Punjab, Pakistan [14]. It contains glucose and citric acid in excess. The leaves contain apigenin, rutin, quercetin, isolimonene, and erucylamide. The floral part is abundant with highly valuable compounds, such as hesperetin, hesperidin, rutin, diosmin, quercetin, and apigenin. Fruit contains diosmin, hesperidin, and apigenin [15]. Phytoestrogens like resveratrol, lignans, isoflavones, and 8-prehylnarinagenin are commonly found in seeds. Its waste contains highly valuable compounds used in food additives, food supplements, energy drinks, and relieving agents in respiratory disease by reducing viral attacks and emitting SARS-CoV-2 in the nasal route, causing intestinal troubles and sea sickness [16].
However, no reports exist on the antimicrobial and antioxidant activities of C. absus and C. medica plants. Thus, this study was designed to assess the phenolic profiling, partition fractionation, and antimicrobial and antioxidant activity of extracts of C. absus and C. medica leaves in various polar to less polar solvents and to confine their relative action. Particular emphasis was on looking at different extract fractions with viable antimicrobial activity.
Material and Methods
Study materials
The majority of chemicals/reagents such as Folin-Ciocalteu (FC) reagent, sodium carbonate anhydrous, gallic acid, sodium nitrite, sodium hydroxide, aluminum chloride anhydrous, 2,2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid, ferric chloride anhydrous, ascorbic acid, catechin HPLC-grade methanol, ethanol, and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA). In contrast, sulfuric acid and potassium-hexacyanoferrate were obtained from Merck (Darmstadt, Germany). Anhydrous monobasic potassium phosphate was purchased from Fisher Chemicals (Fair Lawn, NJ, USA).
Collection and identification of plants
In a garden located in the suburb of Burewala (coordinates 30°4'4''N 72°38'19''E), southwest of Pakistan, fresh newly emerged leaves of C. absus and C. medica were harvested from September to October 2017. The plant materials were carried out to the laboratory at the Department of Botany for further analysis. Dr Zafar Ullah Zafar, a taxonomist from Bahauddin Zakariya University (BZU), Pakistan, further identified and authenticated the harvested plant materials.
Extraction and preparation of extracts fractions
Plant samples were washed with distilled water to remove dust and foreign particles, broken down into small chunks, and cleaned specimens were air dried at room temperature under shade [17].
Plant leaves were then crushed and pulverized into a fine powder. Afterward, dried and fine powder (70 g) plant material was wrapped in a porous bag in the Soxhlet extraction procedure with the addition of 500 mL methanol solvent for about 4 h. The extract was concentrated through a rotary evaporator at 45°C (reduced pressure) (EYELIA, SB-651, and Rikakikai Co. Ltd. Tokyo, Japan), and the solvent was evaporated [2]. The suspension was filtered through a Büchner funnel, and the residue was washed twice with 100 mL of the same solvent. The filtrates were combined, and the solvent was evaporated under reduced pressure. The ethanol extract (5 g) was re-extracted with ethyl acetate (45 mL) under occasional shaking for 4 h at an ambient temperature in a conical flask. The mixture was filtered, and the solvent was evaporated under reduced pressure. Both extracts were transferred into a conical flask filled with argon and stored in a freezer at -20°C. During the extraction and sample preparation, laboratory glass was covered with aluminum foil to protect the extract against light. The percentage yield was calculated from dried extracts, kept at -40°C, and utilized for further analysis. Generally, water, methanol, and ethanol are commonly used in polar compounds, while hexane and dichloromethane are in nonpolar compound extraction. The methanol extracts from both plants was exposed to methanol fractions (Figure 1) (50%) [18].
Since human domestication, plants have been exploited for various reasons, such as food, feed, fiber, and medicine. Each plant and its parts (roots, leaves, fruits, bark) have unique chemicals that provide different medicinal properties-for instance, phenolic compounds contribute to antioxidant activity. Every plant owns special biochemical materials and their therapeutic effects [1]. For example, free radicals are produced due to the higher reactivity of oxygen, which is responsible for many disorders [2]. Such oxidative stress of free radicals on body tissues plays a significant role in diverse pathological conditions, such as aging and infection at the cellular stage and in medicinal and biological systems [3, 4]. Different enzymatic and non-enzymatic reactions are responsible for the production of oxygen free radicals as well as during normal physiological phenomena [5]. The pathological condition results in the overproduction of cells that cause damage [6]. Antioxidants are known as radical scavengers that defend the body over free radicals, and their abundance in nature encouraged scientists to substitute synthetic antioxidants to avoid their conflicting effects. Microorganisms are responsible for food spoilage, thus reducing its shelf life and causing various diseases. For example, Staphylococcus aureus is responsible for toxic shock, food poisoning, and osteomyelitis [4, 7, 8].
Cassia absus L. (Chaksu) is a vertical annual plant belonging to the Leguminosae family. C. absus is commonly found in south and tropical Asia, Africa, Australia, and India, with lengths up to 12–25 inches [9]. Chemical studies have proved that the leaves and roots of C. absus are rich in biochemicals, such as chaksine, rutin, quercetin, chrysophanol, riboflavin, aloe-emodin, and isochaksine [10, 11]. Seeds are augmented with saponins, glycosides, flavonoids, tannins, terpenoids, gums, amino acids, resins, oleic acid, steroids, and linoleic acid. C. absus can scavenge free radicals and is an essential source of antioxidants and phytochemicals. The bitter and styptic nature of leaves helps to cure dermis infections, constipation, cancer, respiratory ailment, and hemorrhoids [12, 13].
Citrus medica L. (family: Rutaceae), also recognized as Bara Nimbu, is a perennial bush or small tree with a height of about 3.6 m and found in warm moist regions in Asia and more common in Northern Punjab, Pakistan [14]. It contains glucose and citric acid in excess. The leaves contain apigenin, rutin, quercetin, isolimonene, and erucylamide. The floral part is abundant with highly valuable compounds, such as hesperetin, hesperidin, rutin, diosmin, quercetin, and apigenin. Fruit contains diosmin, hesperidin, and apigenin [15]. Phytoestrogens like resveratrol, lignans, isoflavones, and 8-prehylnarinagenin are commonly found in seeds. Its waste contains highly valuable compounds used in food additives, food supplements, energy drinks, and relieving agents in respiratory disease by reducing viral attacks and emitting SARS-CoV-2 in the nasal route, causing intestinal troubles and sea sickness [16].
However, no reports exist on the antimicrobial and antioxidant activities of C. absus and C. medica plants. Thus, this study was designed to assess the phenolic profiling, partition fractionation, and antimicrobial and antioxidant activity of extracts of C. absus and C. medica leaves in various polar to less polar solvents and to confine their relative action. Particular emphasis was on looking at different extract fractions with viable antimicrobial activity.
Material and Methods
Study materials
The majority of chemicals/reagents such as Folin-Ciocalteu (FC) reagent, sodium carbonate anhydrous, gallic acid, sodium nitrite, sodium hydroxide, aluminum chloride anhydrous, 2,2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid, ferric chloride anhydrous, ascorbic acid, catechin HPLC-grade methanol, ethanol, and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA). In contrast, sulfuric acid and potassium-hexacyanoferrate were obtained from Merck (Darmstadt, Germany). Anhydrous monobasic potassium phosphate was purchased from Fisher Chemicals (Fair Lawn, NJ, USA).
Collection and identification of plants
In a garden located in the suburb of Burewala (coordinates 30°4'4''N 72°38'19''E), southwest of Pakistan, fresh newly emerged leaves of C. absus and C. medica were harvested from September to October 2017. The plant materials were carried out to the laboratory at the Department of Botany for further analysis. Dr Zafar Ullah Zafar, a taxonomist from Bahauddin Zakariya University (BZU), Pakistan, further identified and authenticated the harvested plant materials.
Extraction and preparation of extracts fractions
Plant samples were washed with distilled water to remove dust and foreign particles, broken down into small chunks, and cleaned specimens were air dried at room temperature under shade [17].
Plant leaves were then crushed and pulverized into a fine powder. Afterward, dried and fine powder (70 g) plant material was wrapped in a porous bag in the Soxhlet extraction procedure with the addition of 500 mL methanol solvent for about 4 h. The extract was concentrated through a rotary evaporator at 45°C (reduced pressure) (EYELIA, SB-651, and Rikakikai Co. Ltd. Tokyo, Japan), and the solvent was evaporated [2]. The suspension was filtered through a Büchner funnel, and the residue was washed twice with 100 mL of the same solvent. The filtrates were combined, and the solvent was evaporated under reduced pressure. The ethanol extract (5 g) was re-extracted with ethyl acetate (45 mL) under occasional shaking for 4 h at an ambient temperature in a conical flask. The mixture was filtered, and the solvent was evaporated under reduced pressure. Both extracts were transferred into a conical flask filled with argon and stored in a freezer at -20°C. During the extraction and sample preparation, laboratory glass was covered with aluminum foil to protect the extract against light. The percentage yield was calculated from dried extracts, kept at -40°C, and utilized for further analysis. Generally, water, methanol, and ethanol are commonly used in polar compounds, while hexane and dichloromethane are in nonpolar compound extraction. The methanol extracts from both plants was exposed to methanol fractions (Figure 1) (50%) [18].
Assessment of antioxidant activity
Determination of total phenolic contents (TPC)
The Folin-Ciocalteu procedure was used to determine the total phenolic contents of the C. absus and C. medica extracts as described previously [19], with slight modification. Briefly, 200 µL of crude extracts (1 mg/mL) was made up of 3 mL volume with deionized H2O, stirred rigorously with 0.5 mL of the Folin-Ciocalteu reagent (diluted 1:10 with deionized H2O) for about 3-5 min, followed by neutralized with 1.5 mL of sodium carbonate 20% (w/v). The reaction mixture was homogenized and incubated at room temperature for half an hour with sporadic shaking for advancement. The absorbance was measured at 755 nm wavelength using a double-beam UV/Vis Spectrophotometer-U-2001 (Hitachi Instruments Inc., Tokyo, Japan). The phenolic concentrations of C. absus and C. medica were determined by the calibration curve and the gallic acid. Also, the total phenolic content was counted within the 10-100 mg/L range (R2=0.9986). Their results were specified as g/100 g of plants’ dry matter equivalents to gallic acid (GAE), where three concordant values were captured for samples and averaged results were expressed as dry weight.
Determination of total flavonoid contents (TFC)
The total flavonoid content (TFC) of crude extracts of C. absus and C. medica was evaluated with the standard method [20], with slight alternation. Briefly, in 50 mL (1 mg/mL ethanol), methanol extracts were added to make 1 mL volume, dissolved with 4 mL of deionized H2O. Subsequently, after 5 min of incubation, 0.3 mL of 5% sodium nitrite (NaNO2) and then 10% aluminum chloride (AlCl3) solutions were added. The mixtures were made up to 10 mL with deionized H2O. The solution was mixed and kept at room temperature for 20-30 min, and absorbance was computed at λ 510 nm. The TFC was determined from a calibration curve, and the findings were demonstrated as rutin (mg) corresponding to dry weight (per g).
Estimation of reducing power
Reducing power was measured as reported previously [21]. In brief, 100 µL extract (100-500 ug/mL) was stirred with 2.5 mL of 200 m molar phosphate buffer (pH=6.6) add 2.5 mL of 1% potassium ferricyanide (C6FeK3N6) solution and incubated at 50°C for 25 min. After incubation solution was supplemented with 2.5 mL of 10% trichloroacetic acid (C2HCl3O2) and centrifuged at 10000 rpm for 10 min. Afterward, 5 mL of the supernatant was mixed with 5.0 mL deionized H2O and 1 mL of 0.1% ferric chloride (FeCl3), and the absorbance of the reaction mixtures was determined at 700 nm.
DPPH free radical scavenging assay
Free radical inhibition activity of different concentrations of C. absus and C. medica were calculated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging protocols with minor optimization. Approximately 4 mL extract was mixed with 2 mL of 0.2 mM DPPH methanolic solution, kept in the dark at ambient temperature for 30 min, and absorbance was measured at λ 517 nm with various time zones 0, 0.5, 1, to 10 min, respectively. The following equation calculated free radical inhibition by DPPH (Equation 1):
1. Inhibition %= [(Ac-As)/Ac]×100
, where Ac refers to control reaction absorbance (with all reagents except the sample), and As refers to sample absorbance.
Determination of linoleic acid inhibition
The inhibition of peroxidation of linoleic acid was measured as reported in a study [22]. Briefly, linoleic acid (0.13 mL) and ethanol and phosphate buffer (10 mL) (pH=7) were mixed with each plant extract in a 25-mL volumetric flask and made the volume up to the mark with dH2O and incubated at 40°C for 15 days. To determine the oxidation rate, the thiocyanate method was adopted. Absorbance was measured through a spectrophotometer at 500 nm after stirring for 3 min along with the negative (without extracts) and positive (butylated hydroxytoluene) controls. For calculating the inhibition percentage of acid, the below formula was applied (Equation 2):
2. 100- [(increased sample absorbance at 360 h/ increased control absorbance at 360 h) 100]
Determination of antimicrobial activity
As reported in some studies, the antimicrobial activity of plant extracts was calculated by the disk diffusion method [23, 24]. The disks (6 mm in diameter) were soaked with 50 μL extract and placed in the inoculated agar. Rifampicin and fluconazole were used as positive references for fungi and bacteria to compare the activity with standard antibiotics such as 30 μg/disk. Both sample and standard disks were assigned separately in Petri dishes. Afterward, they were incubated at 37°C for 24 h and at 25°C for 3 days. Finally, the inhibition zones (mm) were measured to assess antimicrobial activity.
Determination of phenolic compounds by HPLC and data analysis
High-performance liquid chromatography (HPLC) was applied for the analysis of the chemical composition of plant extracts (model LC-10A, Shimadzu, Kyoto, Japan). The sample was diluted, and 20 μL was injected into analytical Supelco (Supelco Inc., Supelco Park, Bellefonte, PA, USA) reverse phase ODS (C18) column (250×4.6 mm). Two mobile phases, i.e. water with acetic acid (94:6 v/v) and 100% acetonitrile, were utilized to separate phenolic components. Isocratic elution was achieved at normal temperature with a 1.0 mL/min flow rate for separation. Wavelength was set at 280 nm for detection. The phenolics (gallic acid, vanillic acid, benzoic acid, quercetin, ferulic acid, caffeic acid, p-coumaric acid, catechin, ellagic acid, and chlorogenic acid) were identified by Sigma Chemicals Co. (St Louis, MO, USA). The calibration curve was used for quantitative determination. All assays were performed in triplicate, and the data were expressed as Mean±SD. Multiple range test analysis of variance (ANOVA) was accomplished using statistical software with P<0.05.
Results
Percentage yield
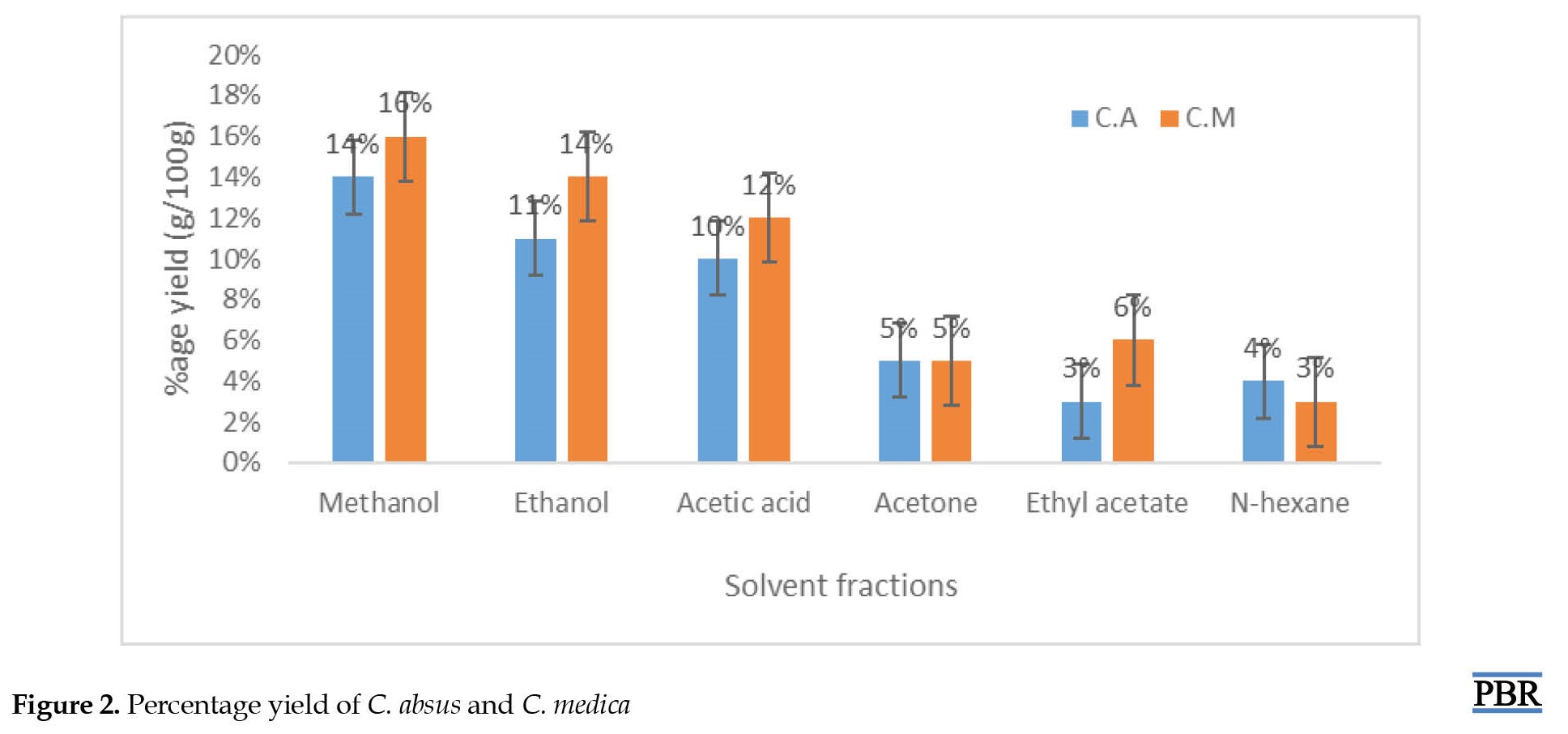
The nature of the solvent and season affect the extractive components of plants, so the bioactive components possess a high yield in polar solvents like methanol which is considered the best fit for extraction [25]. The yields of C. absus and C. medica depict that the polar solvents, i.e. acetic acid, ethanol, and methanol, provided high fractionations, such as 10%, 11%, 14% and 12%, 14%, and 16%, respectively, followed by lower polar fractions (5%) for both in acetone (3%) and in ethyl acetate (6%) (Figure 2).
Determination of total phenolic contents (TPC)
The Folin-Ciocalteu procedure was used to determine the total phenolic contents of the C. absus and C. medica extracts as described previously [19], with slight modification. Briefly, 200 µL of crude extracts (1 mg/mL) was made up of 3 mL volume with deionized H2O, stirred rigorously with 0.5 mL of the Folin-Ciocalteu reagent (diluted 1:10 with deionized H2O) for about 3-5 min, followed by neutralized with 1.5 mL of sodium carbonate 20% (w/v). The reaction mixture was homogenized and incubated at room temperature for half an hour with sporadic shaking for advancement. The absorbance was measured at 755 nm wavelength using a double-beam UV/Vis Spectrophotometer-U-2001 (Hitachi Instruments Inc., Tokyo, Japan). The phenolic concentrations of C. absus and C. medica were determined by the calibration curve and the gallic acid. Also, the total phenolic content was counted within the 10-100 mg/L range (R2=0.9986). Their results were specified as g/100 g of plants’ dry matter equivalents to gallic acid (GAE), where three concordant values were captured for samples and averaged results were expressed as dry weight.
Determination of total flavonoid contents (TFC)
The total flavonoid content (TFC) of crude extracts of C. absus and C. medica was evaluated with the standard method [20], with slight alternation. Briefly, in 50 mL (1 mg/mL ethanol), methanol extracts were added to make 1 mL volume, dissolved with 4 mL of deionized H2O. Subsequently, after 5 min of incubation, 0.3 mL of 5% sodium nitrite (NaNO2) and then 10% aluminum chloride (AlCl3) solutions were added. The mixtures were made up to 10 mL with deionized H2O. The solution was mixed and kept at room temperature for 20-30 min, and absorbance was computed at λ 510 nm. The TFC was determined from a calibration curve, and the findings were demonstrated as rutin (mg) corresponding to dry weight (per g).
Estimation of reducing power
Reducing power was measured as reported previously [21]. In brief, 100 µL extract (100-500 ug/mL) was stirred with 2.5 mL of 200 m molar phosphate buffer (pH=6.6) add 2.5 mL of 1% potassium ferricyanide (C6FeK3N6) solution and incubated at 50°C for 25 min. After incubation solution was supplemented with 2.5 mL of 10% trichloroacetic acid (C2HCl3O2) and centrifuged at 10000 rpm for 10 min. Afterward, 5 mL of the supernatant was mixed with 5.0 mL deionized H2O and 1 mL of 0.1% ferric chloride (FeCl3), and the absorbance of the reaction mixtures was determined at 700 nm.
DPPH free radical scavenging assay
Free radical inhibition activity of different concentrations of C. absus and C. medica were calculated by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging protocols with minor optimization. Approximately 4 mL extract was mixed with 2 mL of 0.2 mM DPPH methanolic solution, kept in the dark at ambient temperature for 30 min, and absorbance was measured at λ 517 nm with various time zones 0, 0.5, 1, to 10 min, respectively. The following equation calculated free radical inhibition by DPPH (Equation 1):
1. Inhibition %= [(Ac-As)/Ac]×100
, where Ac refers to control reaction absorbance (with all reagents except the sample), and As refers to sample absorbance.
Determination of linoleic acid inhibition
The inhibition of peroxidation of linoleic acid was measured as reported in a study [22]. Briefly, linoleic acid (0.13 mL) and ethanol and phosphate buffer (10 mL) (pH=7) were mixed with each plant extract in a 25-mL volumetric flask and made the volume up to the mark with dH2O and incubated at 40°C for 15 days. To determine the oxidation rate, the thiocyanate method was adopted. Absorbance was measured through a spectrophotometer at 500 nm after stirring for 3 min along with the negative (without extracts) and positive (butylated hydroxytoluene) controls. For calculating the inhibition percentage of acid, the below formula was applied (Equation 2):
2. 100- [(increased sample absorbance at 360 h/ increased control absorbance at 360 h) 100]
Determination of antimicrobial activity
As reported in some studies, the antimicrobial activity of plant extracts was calculated by the disk diffusion method [23, 24]. The disks (6 mm in diameter) were soaked with 50 μL extract and placed in the inoculated agar. Rifampicin and fluconazole were used as positive references for fungi and bacteria to compare the activity with standard antibiotics such as 30 μg/disk. Both sample and standard disks were assigned separately in Petri dishes. Afterward, they were incubated at 37°C for 24 h and at 25°C for 3 days. Finally, the inhibition zones (mm) were measured to assess antimicrobial activity.
Determination of phenolic compounds by HPLC and data analysis
High-performance liquid chromatography (HPLC) was applied for the analysis of the chemical composition of plant extracts (model LC-10A, Shimadzu, Kyoto, Japan). The sample was diluted, and 20 μL was injected into analytical Supelco (Supelco Inc., Supelco Park, Bellefonte, PA, USA) reverse phase ODS (C18) column (250×4.6 mm). Two mobile phases, i.e. water with acetic acid (94:6 v/v) and 100% acetonitrile, were utilized to separate phenolic components. Isocratic elution was achieved at normal temperature with a 1.0 mL/min flow rate for separation. Wavelength was set at 280 nm for detection. The phenolics (gallic acid, vanillic acid, benzoic acid, quercetin, ferulic acid, caffeic acid, p-coumaric acid, catechin, ellagic acid, and chlorogenic acid) were identified by Sigma Chemicals Co. (St Louis, MO, USA). The calibration curve was used for quantitative determination. All assays were performed in triplicate, and the data were expressed as Mean±SD. Multiple range test analysis of variance (ANOVA) was accomplished using statistical software with P<0.05.
Results
Percentage yield
The nature of the solvent and season affect the extractive components of plants, so the bioactive components possess a high yield in polar solvents like methanol which is considered the best fit for extraction [25]. The yields of C. absus and C. medica depict that the polar solvents, i.e. acetic acid, ethanol, and methanol, provided high fractionations, such as 10%, 11%, 14% and 12%, 14%, and 16%, respectively, followed by lower polar fractions (5%) for both in acetone (3%) and in ethyl acetate (6%) (Figure 2).
Less yield was produced by non-polar solvent, i.e. n-hexane (4% and 3%). It is revealed that compounds with sugar chains (glycosides, carbohydrates), phenolic compounds, and polar alkaloids are sufficiently reduced with polar solvents, as reported by Federica Menichini [26].
High-performance liquid chromatography
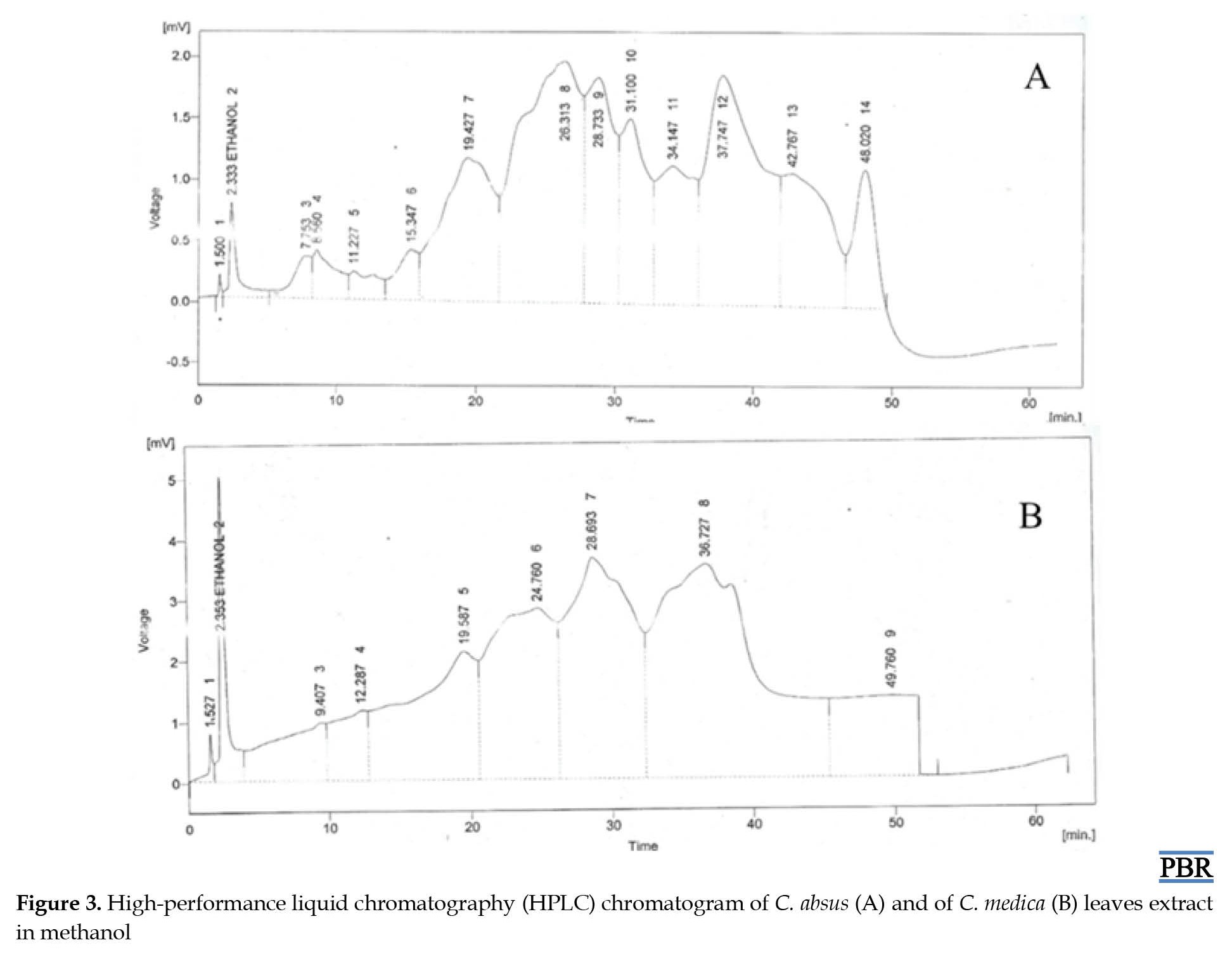
Partitioned fractions of both C. absus and C. medica show different HPLC fingerprints of flavonoids and phenolic compounds, i.e. ellagic acid, gallic acid, coumaric acid, vanillic acid, 4-hydroxy-3-methoxy benzoic acid, kaempferol, caffeic acid, quercetin, rutin, syringic acid, myricetin and cinnamic acid with different amounts. Figure 3A shows the quantity of major phenolics of methanol fraction, i.e.
High-performance liquid chromatography
Partitioned fractions of both C. absus and C. medica show different HPLC fingerprints of flavonoids and phenolic compounds, i.e. ellagic acid, gallic acid, coumaric acid, vanillic acid, 4-hydroxy-3-methoxy benzoic acid, kaempferol, caffeic acid, quercetin, rutin, syringic acid, myricetin and cinnamic acid with different amounts. Figure 3A shows the quantity of major phenolics of methanol fraction, i.e.
coumaric acid (172.11±0.04 µg/g), (112.21±0.01 µg/g), and (143.49±0.03 µg/g); gallic acid (192.03±0.02 µg/g), (141.51±0.03 µg/g), (175.15±0.02 µg/g); ellagic acid (159.18±0.01 µg/g), (110.31±0.04 µg/g), and (135.44±0.03 µg/g); cinnamic acid (108.27±0.02 µg/g), (97.24±0.03 µg/g), and (101.23±0.01 µg/g); and vanillic acid (133.17±0.02 µg/g), (101.24±0.03 µg/g), and (111.96±0.01 µg/g) µg/g. The quantity of minor phenolics (<100 µg/g) were caffeic acid (95.23±0.02 µg/g), (75.16±0.01 µg/g), and (83.54±0.03 µg/g); 4-hydroxy-3-methoxy benzoic acid (54.97±0.02 µg/g), (12.18±0.05 µg/g), and (36.26±0.03 µg/g) and syringic acid (76.35±0.01 µg/g), (34.34±0.02 µg/g), and (49.22±0.04 µg/g). Similarly, the major flavonoids of C. absus were quercetin (152.07±0.03 µg/g), (121.33±0.02 µg/g), and (134.23±0.01 µg/g); kaempferol (120.95±0.02 µg/g), (111.17±0.05 µg/g), and (118.87±0.01 µg/g); and rutin (134.03±0.01 µg/g), (128.12±0.04 µg/g), and (115.25±0.02 µg/g) by weight in methanol fractions. Minor flavonoids (<100 µg/g) were luteolin (75.05±0.01 µg/g), (43.44±0.06 µg/g), and (54.87±0.02 µg/g), and myricetin (98.4±0.02 µg/g), (83.13±0.03 µg/g), and (95.65±0.01 µg/g) in methanol fraction. Malpani M et al. reported that C. absus hold phenolic acid compounds, i.e. isochaksin, chaksine, and flavonoids, with various other compounds present in different quantities. Similarly, Figure 3B shows different flavonoids and phenolic compounds, such as gallic acid, coumarate, chlorogenic acid, caffeic acid, syringic acid, apigenin, ferulic acid, rutin, quercetin, kaempferol, catechin, and naringenin are present in different quantities. Gallic acid (175.02±0.01 µg/g), (161.14±0.04 µg/g), and (145.50±0.02 µg/g); ferulic acid (140.33±0.03 µg/g), (127.83±0.02 µg/g), and (107.30±0.01 µg/g) and chlorogenic acid (121.10±0.03 µg/g), (102.48±0.02 µg/g), and (81.21±0.02 µg/g) were major phenolics The minor phenolics were coumaric acid (71.18±0.01 µg/g), (57.31±0.04 µg/g), and (31.44±0.03 µg/g); caffeic acid (11.01±0.04 µg/g), (25.10±0.02 µg/g), and (10.63±0.01 µg/g); and syringic acid (34.03±0.03 µg/g), (22.12±0.04 µg/g), and (41.25±0.01 µg/g). The order of concentration was caffeic acid15, 27].
Total phenolic contents
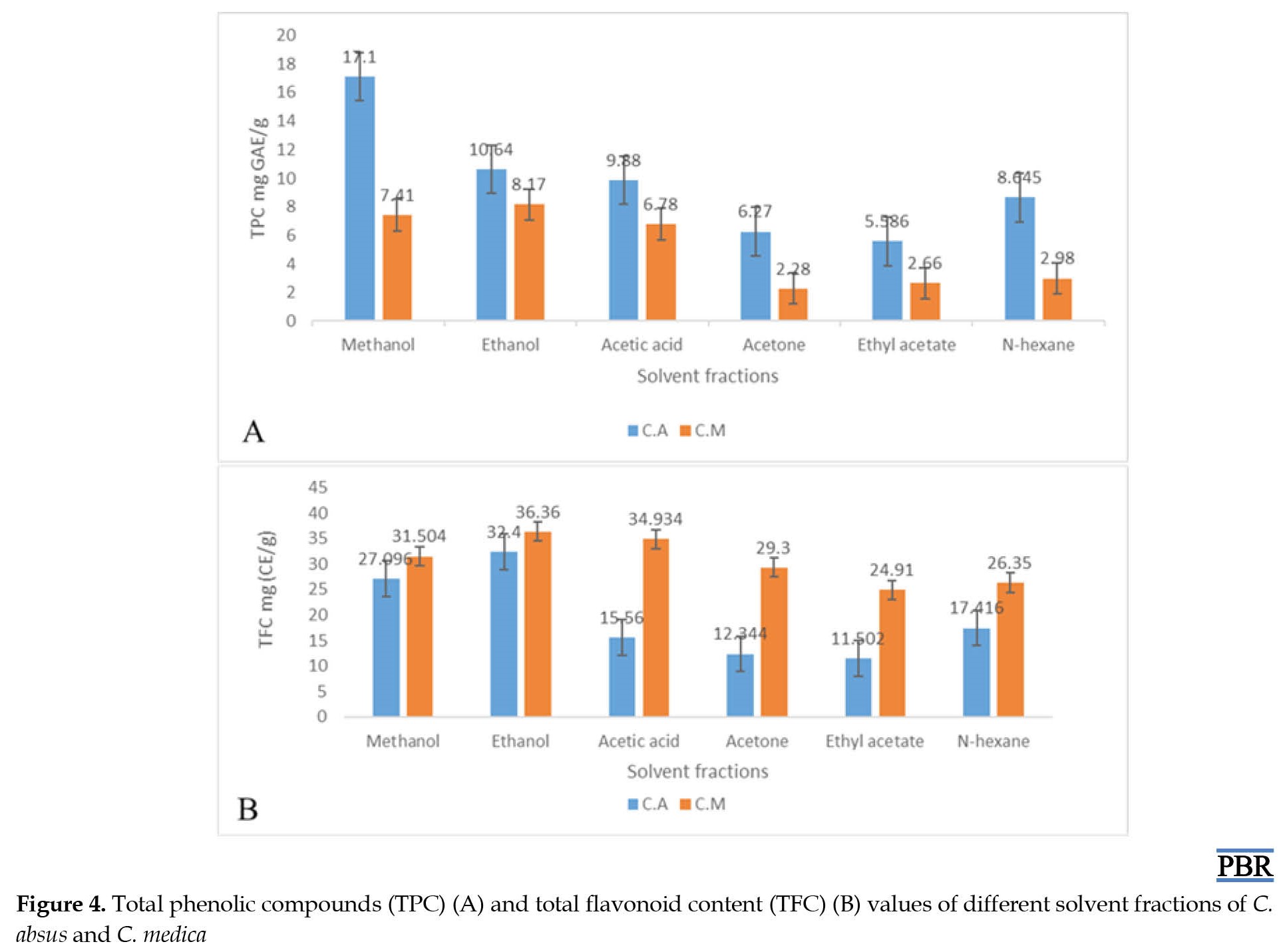
Total phenolic compounds (TPC) expressed as GAE, equivalents of gallic acid (mg/g), whose total amounts in C. absus and C. medica leaves extract were 5.58-17.1/100 g and 2.28-8.17/100 g, respectively. For their quantification, the Folin-Ciocalteu procedure was opted because of reproducibility and less interference. The amount of TPC in C. absus was 17.1 g per 100 g, higher than that of C. medica extracts [28]. Figure 4A shows the TPC quantity of C.
Total phenolic contents
Total phenolic compounds (TPC) expressed as GAE, equivalents of gallic acid (mg/g), whose total amounts in C. absus and C. medica leaves extract were 5.58-17.1/100 g and 2.28-8.17/100 g, respectively. For their quantification, the Folin-Ciocalteu procedure was opted because of reproducibility and less interference. The amount of TPC in C. absus was 17.1 g per 100 g, higher than that of C. medica extracts [28]. Figure 4A shows the TPC quantity of C.
Total flavonoid contents
According to our observation, the total flavonoid contents per 100 g extract of C. absus and C. medica fractions were 11.50-32.40 g and 24.91-36.36 g. It was shown that the higher yield with ethanol fractions in both C. absus (32.4/100 g) and C. medica (36.36 g/100 g) leaves were obtained, and the lowest was achieved by ethyl acetate fractions (Figure 4B). The total amount of flavonoids is expressed as catechin (mg/g) equivalents, and a significant deviation (P<0.05) was observed in TPC values of different fractions of solvent.
Estimation of reducing power
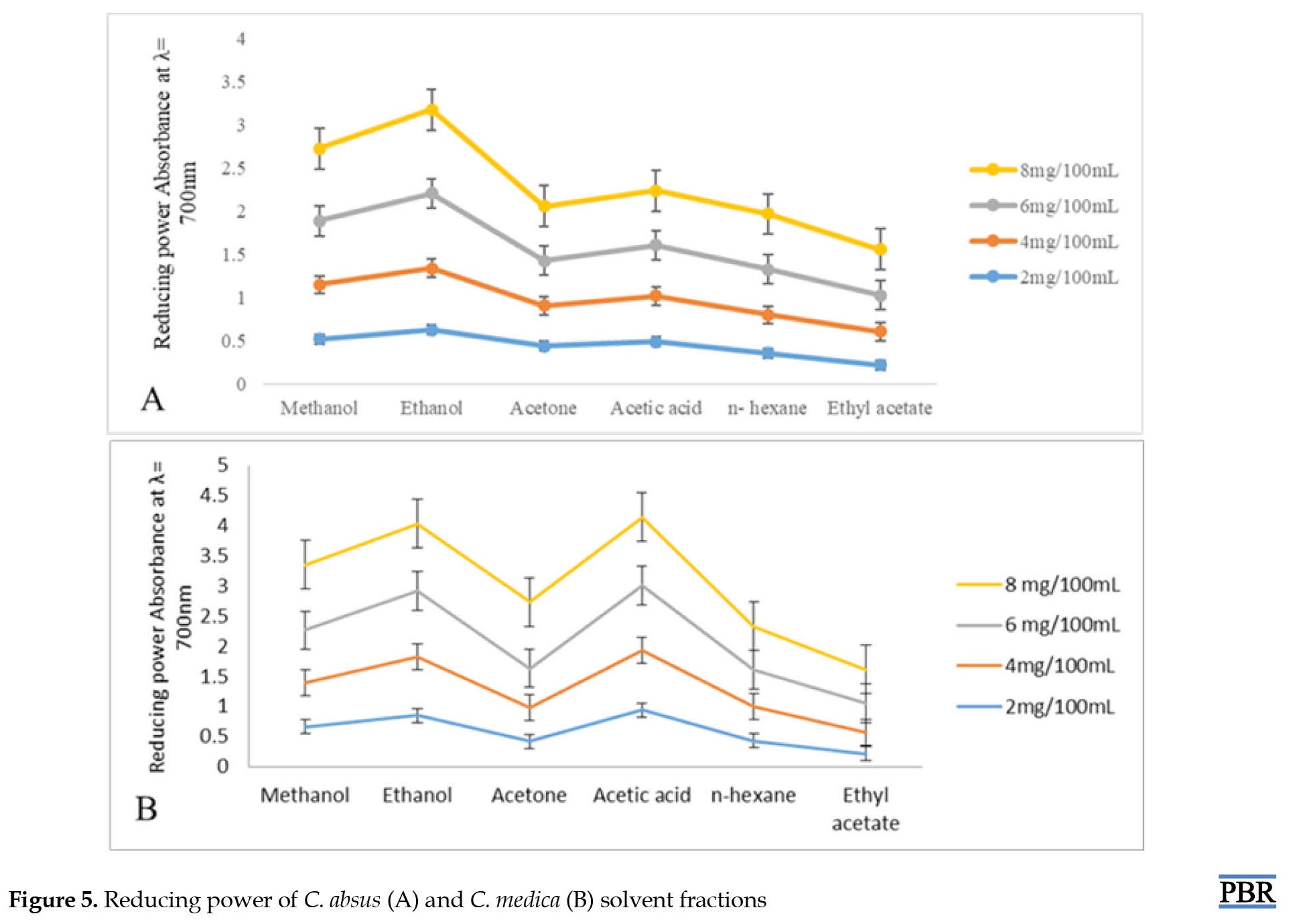
Reducing power is linked with antioxidant properties and may preserve as an important meditation of the antioxidant activity. A concentration range (2-8 mg/100 mL) was adopted to measure reducing power. Various sample fractions demonstrate high reducing power at 8 mg/100 mL concentration sequenced from 0.530-0.970 mg/100 mL (Figure 5A) and 0.560-1.140 mg/100 mL and 0.560-1.140 mg/100 mL (Figure 5B), respectively.
A significant variation was observed in the DPPH free radical inhibition (Figure 6A), where the polar extracts fractions showed maximum inhibition in both the plant extracts such as 64.28%-67.01% and 59.47%-74.74% whereas, n-hexane extracts fractions showed weak radical scavenging activity.
Inhibition of linoleic acid per oxidation
The inhibition percentage age of linoleic acid following 360 h incubation was observed. The sample fractions illustrate the linoleic acid peroxidation of 27.21% for non-polar and 13.730%-18.930% for polar fractions of C. absus extract while 17.693% and 28.88%-49.60% for a non-polar and polar fraction of C. medica plant extract (Figure 6B). The effect of polarity (P<0.005) at the oxidation of linoleic acid was significant. The findings of this study confirmed that C. absus and C. medica plant extracts showed a higher inhibition percentage of linoleic acid in methanol fractions. This condition further proves that polarity directly affects antioxidant activity as the decline in polarity resulted in the decline of antioxidant activity that may be responsible for fewer phenolics that cause this activity [36].
Assessment of antimicrobial activity
Fractions of C. absus and C. medica extracts were exploited to access antimicrobial activity (Figure 7).

In the antibacterial assay, methanol fraction has a wide inhibition zone (29.0±0.02 mm) over the Bacillus subtilis bacterial strain, and nHF reduced zone of inhibition (9.0±0.01 mm) against the Escherichia coli bacterial strain in antifungal case methanol fraction exposed the wide zone of inhibition (26.0±0.03 mm) against fungus Fusarium and EA fraction lessen the inhibition zone (09.0±0.05 mm) despite the presence of same strains. Results demonstrate that methanol fractions provided an excellent result. Malpani reported in 2013 the antibacterial and antifungal assay of C. absus, whose results confirmed that the isolated active acidic ingredient was more effective than the whole extract of leaves, which showed moderate activity. The results obtained were assessed on their comparison with the activities of standard antibacterial agents like ampicillin and vancomycin as a control [37, 38, 39].
Discussion
Phytonutrients from traditional medicines and chemicals have recently gained extensive consideration as a targeted remedy to cure several health problems. Subsequently, more bioactive compounds from plants with medicinal characteristics are found. The antioxidants harvested from different plant species have been beneficial in curing human diseases in the recent decade. These traditional phytochemicals have been offered to treat and prevent certain diseases [25, 38]. The prepared crude plant extracts were achieved from the leaves of Cassia absus L. and Citrus medica L. for bioactive phytonutrients. The findings of this study suggest that these plant-based molecules are a rich source of biologically active compounds. This excessive concentration of these plant-derived compounds may be a part of plant defense systems. These identified plant-based chemicals might have some important biological significance. These phytochemicals present in the crude extracts of Cassia absus L. and Citrus medica L. host have not been studied earlier. Furthermore, the extracts from Cassia absus L. and Citrus medica L., including acetic acid, ethanol, and methanol, show antioxidant activity by scavenging free radicals in DPPH assay, reducing power test, and linoleic acid scavenging assay and can be used as the source of unique medicines with significant potential to prevent many illnesses. Besides the phytochemicals and antioxidant activity, C. absus and C. medica plants sample fractions offer antimicrobial activity, where HPLC is utilized to investigate both plants’ composition. Based on the findings, the investigated bioactive compounds in C. absus and C. medica might be consumed to synthesize different antimicrobial and antioxidant drugs for the sake of human beings.
The plant-based compounds here further confirm the availability of the phytochemicals cited in the literature, which belong to different plant families [40]. The antibacterial activity was also tested, and the study further confirmed the antibacterial activity inclined in methanol fraction against bacteria S. aureus strain (18.23±0.01 mm) unlike other fractions, which decrease (14.88±0.01 and 13.88±0.07 mm) the inhibition zone (Table 2).

Likewise, in the antifungal activity, wide inhibition was given by methanol fraction against Fusarium fungus (19.36±0.04 mm) while exhibiting less inhibition zone against others (12.93±0.05 and 15.88±0.03 mm). This condition resulted in the methanol fractions providing excellent results in this study [41] reported. Leaf and root extracts and fruit juice showed varied antibacterial activity against one or more tested bacteria. Also, antifungal activity was shown by only root extract and fruit juice, while Candida albicans was resistant to all tested plant samples. Moreover, among all tested plant samples, leaf and peel extracts have shown less antimicrobial activity [40-42].
Conclusion
This study aimed to identify different compounds present in the leaf extract of C. absus L. and C. medica L. plants. The results obtained by the HPLC measurements exhibited different compounds from C. absus L. and C. medica L. extracts. The study confirmed that C. absus L. and C. medica L. are rich sources of various bioactive compounds with extensive applications in pharmaceutical companies. Based on these research findings, the investigated bioactive compounds in C. absus and C. medica might be consumed to synthesize different antimicrobial and antioxidant drugs for the sake of human beings.
Ethical Considerations
Compliance with ethical guidelines
All ethical issues related to this study were considered.
Funding
This study did not receive funds from any public/commercial/non-profit organization.
Authors' contributions
Conceived idea and designed work: Surryia Manzoor and Saima Naz; Lab work, performed analysis, and wrote the initial draft of the manuscript: Zartasha Kousar; Analyzed, proofread, and critically improved the manuscript: Surryia Manzoor, Saima Naz, and Muhammad Shafiq Shahid; Approved the final draft of the manuscript: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We thank our colleagues at the University of Education Lahore, Multan campus, and the Institute of Chemical Sciences, Bahauddin Zakariya University, Multan, for their esteemed help completing this work.
References
- Bouafia A, Laouini SE. Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: A review. Mini-Rev Org Chem. [Short Survey]. 2021; 18(6):725-34. [DOI:10.2174/1570193X17999200908091139]
- Mythri M, SanalDevK T, KottaiMuthu A. Evaluation of the antioxidant activity of various extracts of aerial parts of Cassia absus: An in-vitro techniques. Int J Res Pharm Sci. 2020; 11:1545-50. [DOI:10.26452/ijrps.v11i2.2031]
- Lai LS, Chou ST, Chao WW. Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem. 2001; 49(2):963-8. [DOI:10.1021/jf001146k] [PMID]
- Akbari B, Baghaei-Yazdi N, Bahmaie M, Mahdavi Abhari F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors. 2022; 48(3):611-33. [DOI:10.1002/biof.1831] [PMID]
- Arouma O. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998; 75(2):199-212. [PMID]
- Nagpal I, Abraham SK. Protective effects of tea polyphenols and β-carotene against γ-radiation induced mutation and oxidative stress in Drosophila melanogaster. Genes Environ. 2017; 39:24. [PMID] [PMCID]
- Li ZH, Cai M, Liu YS, Sun PL, Luo SL. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules. 2019; 24(8):1577. [DOI:10.3390/molecules24081577] [PMID] [PMCID]
- Souri E, Amin G, Farsam H, Jalalizadeh H, Barezi S. Screening of thirteen medicinal plant extracts for antioxidant activity. Iran J Pharm Res. 2022; 7(2):149-54. [DOI:10.22037/ijpr.2010.758]
- Kachwala Y. Screening Isolation and characterization of Hypoglycemic Plants for their Antioxidant activity [PhD dissertation]. Shodhganga: Narsee Monjee Institute of Management Studies; 2012. [Link]
- Danladi S, Alhassan AM, Sule MI, Musa AM, Yaro AH. Phytochemical constituents and pharmacological activities of Globimetula braunii (Loranthaceae): A review. Trop J Nat Prod Res. 2022; 6(9):1372-7. [Link]
- Srivastava R, Tiwari P. Medicinal plant used against cancer: A review. Asian J Pharm Res Dev. 2022; 10(4):pdf. [Link]
- Nancy P, Ashlesha V. Pharmacognostic and phytochemical studies of Cassia absus seeds extract. Int J Pharm Pharm Sci. 2015; 8(1):325-32. [Link]
- Gouthami K, Veeraraghavan V, Nagaraja P. In-silico characterization of phytochemicals identified from Vitex negundo (L) extract as potential therapy for Wnt-signaling proteins. Egypt J Med Hum Genet. 2022; 23(1):1-5. [DOI:10.1186/s43042-022-00219-7]
- Meena AK, Kandale A, Rao M, Panda P, Govind R. A review on citron-pharmacognosy, phytochemistry and medicinal uses. Int Res J Pharm. 2011; 2(1):14-9. [Link]
- Menichini F, Loizzo MR, Bonesi M, Conforti F, De Luca D, Statti GA, et al. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem Toxicol. 2011; 49(7):1549-55. [DOI:10.1016/j.fct.2011.03.048] [PMID]
- Singh P, Chauhan SS, Pandit S, Sinha M, Gupta S, Gupta A, et al. The dual role of phytochemicals on SARS-CoV-2 inhibition by targeting host and viral proteins. J Tradit Complement Med. 2022; 12(1):90-9. [DOI:10.1016/j.jtcme.2021.09.001] [PMID] [PMCID]
- Alamholo M, Amraie Y. Evaluation of susceptibility and resistance of human infectious bacteria and identification of bioactive compounds in pistacia atlantica, cassia absus, and quercus persica. Pharm Biomed Res. 2021; 7(2):105-14. [DOI:10.18502/pbr.v7i2.7363]
- Cho M, Kang IJ, Won MH, Lee HS, You S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J Med Food. 2010; 13(5):1232-9. [DOI:10.1089/jmf.2010.1124] [PMID]
- Chaovanalikit A, Wrolstad R. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J Food Sci. 2004; 69(1):FCT67-72. [DOI:10.1111/j.1365-2621.2004.tb17858.x]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002; 50(10):3010-4. [DOI:10.1021/jf0115589] [PMID]
- Yen GC, Duh PD, Chuang DY. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000; 70(4):437-41. [DOI:10.1016/S0308-8146(00)00108-4]
- Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 2005; 93(2):265-72. [DOI:10.1016/j.foodchem.2004.09.024]
- Flórez N, Conde E, Domínguez H. Microwave assisted water extraction of plant compounds. J Chem Technol Biotechnol. 2015; 90(4):590-607. [DOI:10.1002/jctb.4519]
- Gupta MK, Singh R, Rangan L. Phytochemical screening, antibacterial, anti-biofilm and quorum sensing inhibiting activity of Alpinia nigra leaf extract against infectious pathogen Pseudomonas aeruginosa PAO1. Food Control. 2023; 143. [DOI:10.1016/j.foodcont.2022.109327]
- Abdullah, Ahmad N, Tian W, Zengliu S, Zou Y, Farooq S, et al. Recent advances in the extraction, chemical composition, therapeutic potential, and delivery of cardamom phytochemicals. Front Nutr. 2022; 9:1024820. [PMID] [PMCID]
- Menichini F, Tundis R, Loizzo MR, Bonesi M, Liu B, Jones P, et al. C. medica cv Diamante peel chemical composition and influence on glucose homeostasis and metabolic parameters. Food Chem. 2011; 124(3):1083-9. [DOI:10.1016/j.foodchem.2010.07.083]
- Jit BP, Pattnaik S, Arya R, Dash R, Sahoo SS, Pradhan B, et al. Phytochemicals: A potential next generation agent for radioprotection. Phytomedicine. 2022; 106:154188. [DOI:10.1016/j.phymed.2022.154188] [PMID]
- Sultana B, Anwar F, Przybylski R. Antioxidant potential of corncob extracts for stabilization of corn oil subjected to microwave heating. Food Chem. 2007; 104(3):997-1005. [DOI:10.1016/j.foodchem.2006.12.061]
- Roidaki A, Zoumpoulakis P, Proestos C. Comparison of extraction methods for the determination of antioxidant activity in extracts of Hippophae rhamnoides L. and Lippia citriodora. The effect of seasonal collection. Austin J Nutr Food Sci. 2015; 3:1057.
- Kiprovski B, Mikulic-Petkovsek M, Slatnar A, Veberic R, Stampar F, Malencic D, et al. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015; 185:41-7. [DOI:10.1016/j.foodchem.2015.03.137] [PMID]
- Jakopic J, Veberic R, Štampar F. Extraction of phenolic compounds from green walnut fruits in different solvents. Acta Agric Slov. 2009; 93(1):11-5. [DOI:10.2478/v10014-009-0002-4]
- Jang HD, Chang KS, Huang YS, Hsu CL, Lee SH, Su MS. Principal phenolic phytochemicals and antioxidant activities of three Chinese medicinal plants. Food Chem. 2007; 103(3):749-56. [DOI:10.1016/j.foodchem.2006.09.026]
- Luximon-Ramma A, Bahorun T, Soobrattee MA, Aruoma OI. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J Agric Food Chem. 2002; 50(18):5042-7. [PMID]
- Dewi YSK, Lestari OA, Fadly D. Identification phytochemicals and antioxidant activities of various fractions of methanol extracts from bark of Kulim tree (Scorodocarpus borneensis Becc.). Syst Rev Pharm. 2020; 11(8):217-21. [Link]
- Kim YS, Hwang JW, Sung SH, Jeon YJ, Jeong JH, Jeon BT, et al. Antioxidant activity and protective effect of extract of Celosia cristata L. flower on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Food Chem. 2015; 168:572-9. [DOI:10.1016/j.foodchem.2014.07.106] [PMID]
- Iqbal D, Khan MS, Khan MS, Ahmad S, Hussain MS, Ali M. Bioactivity guided fractionation and hypolipidemic property of a novel HMG-CoA reductase inhibitor from Ficus virens Ait. Lipids Health Dis. 2015; 14:15. [PMID] [PMCID]
- Malpani M, Rajput PR. Antimicrobial study of whole extract, isolated ingredient, and newly synthesized analogue from leaves extract of Cassia absus plant. Int J Pharm Bio Sci A. 2013; 4(2):427-30. [Link]
- Noor N, Satapathy KB. Phytochemical screening and antibacterial potential of wild leafy vegetable-cayratia auriculata (Roxb.) gamble against selected enteric pathogens. Trop J Nat Prod Res. 2022; 6(9):1430-3. [Link]
- Verma D, Gupta S, Pant K, Pant B, Pandey C. Text mining for identification of anti-tubercular compounds present in plants of India and in-silico ADMET prediction of phytochemicals.AIP Conference Proceedings. 2022; 2481(1). [DOI:10.1063/5.0104492]
- Raghuvanshi D, Kumar S, Shukla MK, Kumar D, Kumar D, Verma R, et al. Assessment of phytochemicals, antioxidants and in-silico molecular dynamic simulation of plant derived potential inhibitory activity of Thalictrum foliolosum DC. and Cordia dichotoma G. Forst. against jaundice. Biomed Pharmacother. 2022; 156:113898. [PMID]
- Sah AN, Juyal V, Melkani AB. Antimicrobial activity of six different parts of the plant citrus medica linn. Pharm J. 2011; 3(21):80-3. [DOI:10.5530/pj.2011.21.15]
- ZahradNíkOVá L, Schmidt S, SékeLyOVá Z, Sekretár S. Fractionation and identification of some phenolics extracted from evening primrose seed meal. Czech J Food Sci. 2008; 26(1):58-64. [DOI:10.17221/1135-CJFS]
Type of Study: Original Research |
Subject:
Natural products
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |