Volume 9, Issue 2 (2023)
Pharm Biomed Res 2023, 9(2): 115-124 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Osarieme Imade R, Adesina Ayinde B, Alam A. GC-MS Analysis and In Vitro Cytotoxic Effects of Ocimum gratissimum (Lamiaceae) Volatile Oil and Thymol on Cancer Cells. Pharm Biomed Res 2023; 9 (2) :115-124
URL: http://pbr.mazums.ac.ir/article-1-501-en.html
URL: http://pbr.mazums.ac.ir/article-1-501-en.html
1- Department of Pharmacognosy, Faculty of Pharmacy, University of Benin, Benin City, Nigeria.
2- International Centre for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan.
2- International Centre for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan.
Full-Text [PDF 1239 kb]
(874 Downloads)
| Abstract (HTML) (1687 Views)
Full-Text: (1014 Views)
Introduction
Cancer is one of the major causes of death. Over a hundred forms of cancer have been discovered, with breast and cervical cancers being the most common in women [1]. The low success of clinical therapies, as shown by high morbidity and mortality rates, justifies the search for new and better treatment methods. In southeast Asian countries, herbs are used as spices, and help reduce the incidence of colon, prostate, breast, and other cancers [2]. They are widely used in alternative medicine to treat gastrointestinal and respiratory illnesses and have carminative, digestive, galactagogue, and diuretic effects [3]. Their use in cosmetic and pharmaceutical products is well known [4].
Essential oils have been shown to have an anti-cancer effect against various cancer cell lines. Hypericum hircinum, Thymus vulgaris, and Solanum erianthum leaf essential oils have demonstrated antiproliferative activity against human prostatic adenocarcinoma (PC3 cell line) [5, 6]. Xylopia frutescens leaf essential oil [2] also demonstrated anti-cancer effects in vitro and in vivo on the bronchioalveolar lung carcinoma cell line (NCI-H358M).
Ocimum gratissimum L. (Lamiaceae) is an aromatic perennial erect herb with many branches that grows to a maximum height of 1 m and has a woody base. It is native to South Africa, Asia, and Brazil [7]. The plant is frequently employed in traditional medicine, primarily in the form of teas and infusions of its leaves to treat headaches, stress, colds, pruritus, and abdominal discomfort [8, 9]. Additionally, there are reports of using O. gratissimum to treat skin lesions, pneumonia, conjunctivitis, and upper respiratory tract infections [10].
Numerous scientific investigations have revealed some of the plant’s properties, including anti-diabetic, anti-oxidant, anxiolytic, sedative, anti-inflammatory, hepatoprotective, anti-cancer, gastroprotective, and hypolipidemic [11-19]. Much research on various parts of the plant has confirmed its anti-bacterial properties [20]. O. gratissimum crude extract and its hydrophobic and hydrophilic fractions differentially suppress the progression of MCF10ADCIS.com xenografts and tumor growth and inhibit breast cancer cell growth in vivo [21].
The literature search reveals different constituents in the oil obtained from separate locations. Joshi [22] reported that the oil contained eugenol as the major constituent (57%) alongside β-caryophyllene, bulnesene, and others. Koba et al. [23] further documented the oil to possess mostly thymol, p-cymene, and γ-terpinene.
In light of these facts, this work was carried out to examine the chemical profile of O. gratissimum essential oil harvested from the southern part of Nigeria and to assess the cytotoxic activity of the oil and its major constituent against breast (AU565) and cervical (HeLa) cancer cell lines.
Materials and Methods
Collection and identification of plant material
Fresh leaves of the Ocimum gratissimum plant were collected in December 2016 in Benin City, Edo State, Nigeria. The plant was identified by Dr Henry Akinnibosun of the Plant Biology and Biotechnology Department, Faculty of Life Sciences, University of Benin, Benin City, Nigeria. A voucher number UBH O34 was assigned to it.
Extraction of volatile oil
With the help of a Clevenger apparatus, the leaves (approximately 1 kg) were hydro-distilled in batches to recover the volatile oil. The oil was placed in the freezer at -18⁰C for any water that may have accompanied it from collection to freezing. Subsequently, it was transferred to a dry vial with a pipette and kept in the refrigerator at 4⁰C until further use.
Column chromatography of O. gratissimum volatile oil
Silica gel (200–400 mesh) was used to triturate the volatile oil (15 mL), which was then subjected to column chromatography. For gradient elution, 200 mL of C6H14 (100%) and C6H14- CH3COOC2H5 were used (99:1, 98:2, 97:3, 96:4, 95:5, 94:6, 93:7, 92:8, 91:9, 90:10). Fractions (118) were collected, bulked in accordance with respective TLC (thin-layer chromatography) profiles, and assigned the following codes: F1 (5–12), F2 (13–28), F3 (29–40), F4 (4–63), F5 (62–90), and F6 (91-118).
As a result of the high yield and conspicuous nature, fraction F1 (5 mL) was subjected to preparative TLC analysis on silica gel using C6H14- CH3COOC2H5 (9.7:0.3). Two sub-fractions were obtained and coded as SF1 and SF2.
Source and identification of Raniceps raninus tadpoles
From within the University of Benin, tadpoles (5–6 days old) were gathered from toad colonies in small water communities. They were confirmed to be Raniceps raninus tadpoles by Professor M. Aisien, an Animal Parasitologist at the Department of Animal and Environmental Biology, Faculty of Science, University of Benin, Benin City, Nigeria.
Determination of the essential oil’s cytotoxic effects on tadpoles
Following the method stated in a study [24], 10 tadpoles were placed in a 50-mL beaker containing 15 mL water from the tadpoles’ colonies. This was made up to 49 mL with distilled water, and then 1 mL of the different concentrations of the oil (0.5, 1, and 2 mg/mL) was added to make up to 50 mL. The final concentrations were 10, 20, and 40 μg/mL. For each concentration, the experiment was carried out in triplicates. The tadpole mortality rate was observed for 24 hours.
Brine shrimps lethality bioassay (Artemia salina)
Brine shrimp lethality bioassay [25] was applied to determine cytotoxic activity. Then, 2 mL of acetone was used to dissolve the volatile oil, and from this solution, concentrations of 10, 100, and 1000 μg/mL were obtained in triplicates. The solvent was allowed to evaporate overnight. Using a Pasteur pipette, 10 larvae were introduced to each vial after 48 h of nauplii maturation and hatching. Seawater (38 g/L, pH 7.4) was used to make up the volume to 5 mL, and the vials were incubated at 250°C–270°C for 24 hours while being illuminated. The negative and positive controls were seawater and etoposide, respectively.
Preparation of the guinea corn (Sorghum bicolor)
Absolute alcohol was used to rinse the Sorghum bicolor seeds purchased from a market in Benin City, Nigeria. A quick viability test was conducted to remove the preservative by adding about 100 mL of water and immediately decanting, thus removing the floating compromised seeds. The submerged viable seeds were dried on filter papers before use.
Determination of growth inhibitory effects of the essential oil on Sorghum bicolor seeds
About 10 mL of 1, 2, 5, 10, 20, and 30 mg/mL of O. gratissimum oil prepared by dissolution with 2% Tween 80 in water, were poured into 9 cm wide glass Petri dishes lined with cotton wool and filter paper (Whatman No 1). Twenty viable seeds were dispersed on each plate and incubated in the dark. At 24, 48, 72, and 96 h, each seed’s radicle length (mm) was measured. Then, 10 mL of distilled water with 2% Tween 80 was used to treat the control seedlings. The test was performed in triplicates [24]. Chromatographic fractions F1, F2, SF1, and SF2 were also subjected to a similar procedure at 1 mg/mL concentration.
Assessment of cytotoxic activity of O. gratissimum oil on cancer cell lines
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure the cytotoxic activity. Human breast cancer (AU565) and cervical cancer (HeLa) cell lines were utilized. They were obtained from the International Center for Chemical and Biological Sciences (ICCBS) molecular bank at the University of Karachi in Pakistan. A density of 10000 cells/well/100 μL in 96-well plates was used, and they were incubated for 24 h in a complete medium at 37°C and 5% CO2. Next, 50 μg/mL of the oil and fractions were used to treat the cells in triplicates (F1, F2, F3, F4, and SF2). The standard utilized was doxorubicin (50 μM). Incubation was for 48 h at 37°C and in a humidified atmosphere of 5% CO2. Before the medium was taken out, a fresh medium was added containing 200 μL of MTT dye (0.5 mM) and then incubated for an additional 3–4 hours. The formazan crystals produced were dissolved in 100 μL of DMSO, and absorbance was measured at 570 nm [26, 27]. IC50 was calculated by EZ-Fit software.
Gas chromatography-mass spectrometry analysis
The oil and fraction SF2 were analyzed using gas chromatography-mass spectrometry (GC-MS). The Agilent technologies 7000 GC/MS triple quadrupole mass spectrometer was used to record the gas chromatogram, and it used an OPTIMA-5-ZB-5 column with measurements of 30 m x 250 m x 0.25 m. Electron ionization (EI) with an ionization energy of 70 eV was employed for GC-MS detection. Helium (99.999%) served as the carrier gas, flowing at a constant rate of 1.129 mL/min with an injection volume of 2 L (split ratio 15:1). It was 250°C at the ion source. The oven was programmed to start at 50°C (isothermal for 15 min), then decrease to 8°C/min, rise to 180°C for 15 min., then 15°C/min, and ultimately reach 290°C for 5 min. Runtime was 58.58 min in total. While NIST library match was utilized to identify the compounds, ChemStation software was used to handle the mass spectra and chromatograms.
Statistical analysis
To analyze the data, a 1-way analysis of variance (ANOVA) was performed using SPSS software, version 21, and the results were expressed as Mean±SEM. In every instance, differences were deemed significant at P< 0.05.
Results
Cytotoxic effects of the volatile oil on tadpoles
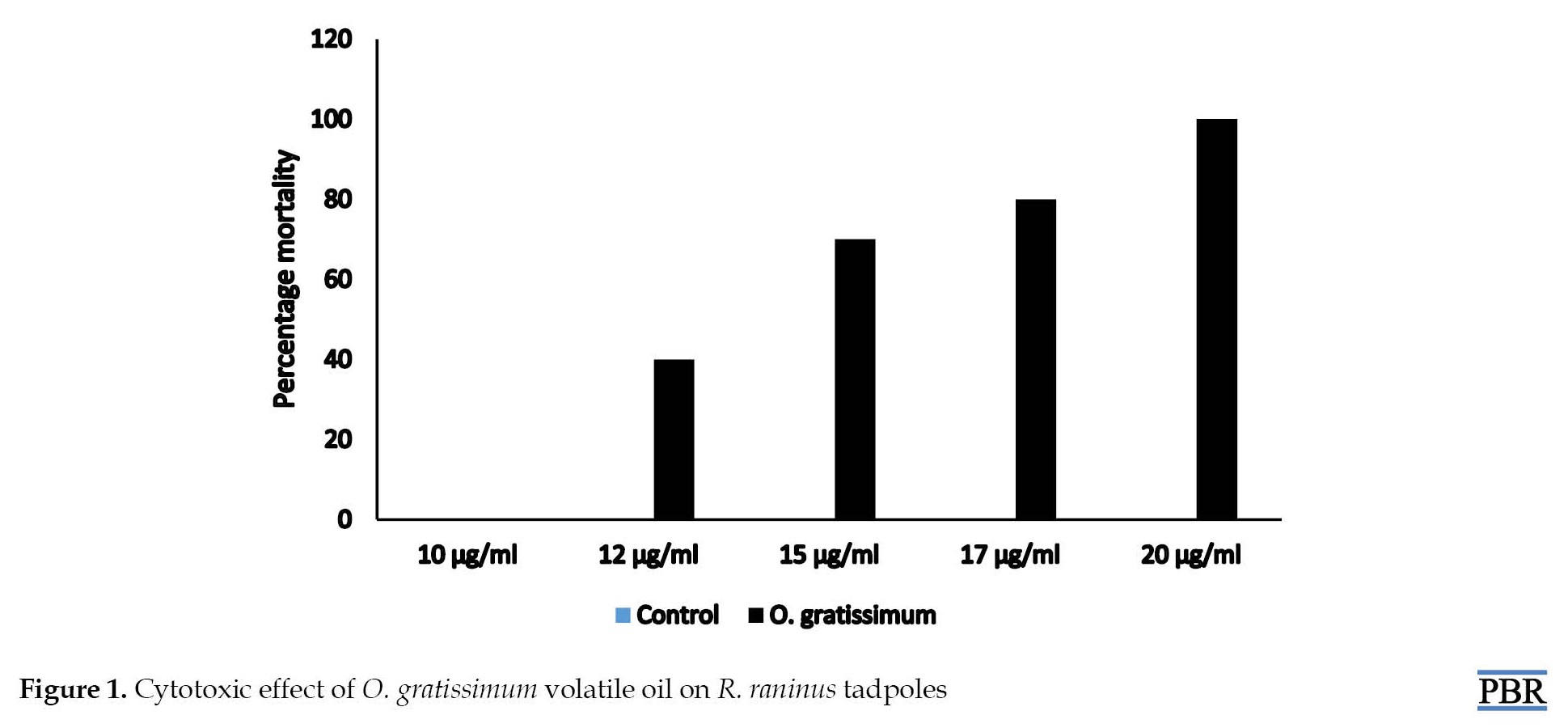
A concentration-dependent activity was observed in the tadpole mortality test. O. gratissimum oil showed no mortality at 10 μg/mL, while 70% and 100% mortality were seen at 15 and 20 μg/mL, respectively (Figure 1). An LC50 of 13.74 μg/mL was obtained.
Cytotoxic effects of the oil against Artemia salina Nauplii
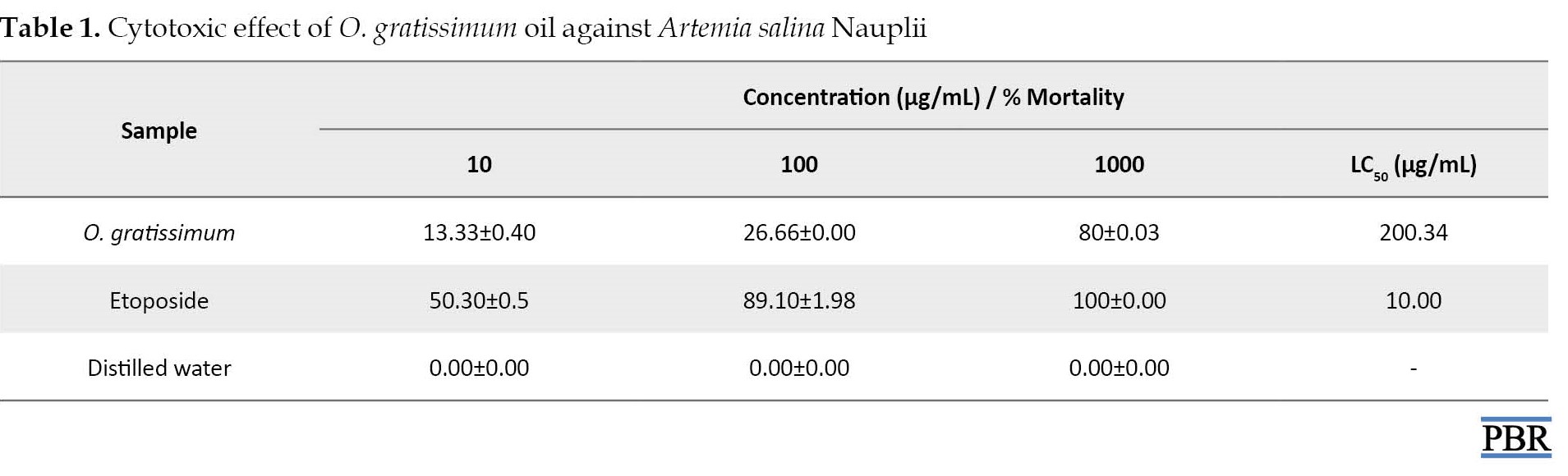
A concentration-dependent response was observed when the shrimps came in contact with the volatile oil. About 13.33 % mortality was observed at 10 μg/mL, which was increased to 80% at 1000 μg/mL with LC50 of 200.34 μg/mL (Table 1).
Growth inhibitory effects of the essential oil and fractions on radicle length of S. bicolor seeds
The volatile oil was found to greatly suppress radicle growth in a concentration-dependent manner. A significant difference (P<0.05) compared to negative control was observed at 5-30 mg/mL concentrations for 24-96 h. From 5 mg/ml at 48 h and 2 mg/mL at 72 h, the percentage reductions in radicle growth were highly significant (P<0.001). Also, 10 mg/mL of the oil completely inhibited the growth of the radicles at 96 h. An average length of 9.54±1.73 mm in the controls simultaneously was reduced to 0.77±0.02 mm in seeds treated with 5 mg/mL concentration showing a 91.93% reduction (Figure 2). The growth of the radicles was greatly retarded by the column fractions at 1 mg/mL concentration. From 72 to 96 h, the radicle growth reductions for fractions F1, F2, and SF2 were highly significant compared to the control (P<0.001). At 96 h, F1 and F2 gave 81.26% and 86.46% growth inhibitions, respectively. At the same concentration and time, SF1 and SF2 (obtained from F1 through preparative TLC) produced growth inhibitions of 28.73% and 92.03%, respectively (Figure 3). The fractions and subfractions remarkably inhibited the growth of the radicles, with SF2 showing the highest inhibitory effect (P<0.001) compared to the negative control.
Inhibitory effects of O. gratissimum volatile oil and fractions on the cell lines
The oil demonstrated moderate inhibition against breast cancer, +17.82%, but no activity was observed with the cervical cancer cell line (HeLa). However, the inhibitory actions of the column fractions varied, with F4 producing the highest inhibition of +34.42%. A significant difference in activity was observed between the oil and SF2 on both HeLa and AU565 cells (P<0.001). SF2 gave 85.07% inhibition on AU565 cells and an IC50 of 15.96 ± 1.61, while an inhibition of 29% was observed against HeLa cells (Figure 4). Doxorubicin gave 97.89% inhibition with an IC50 of 0.085±0.03 μM with AU565 cells.
GC-MS analysis of O. gratissimum essential oil and fraction SF2
Thymol (22.49%), p-cymene (13.47%), and γ-terpinene (11.5%) were present in the highest quantities in O. gratissimum oil. β-Myrcene, carvacrol, eugenol, and β-selinene were also present with a relative abundance of 4.58%, 2.6%, 0.28%, and 4.15%, respectively. A total of 25 components were obtained (Figure 5, Table 2).

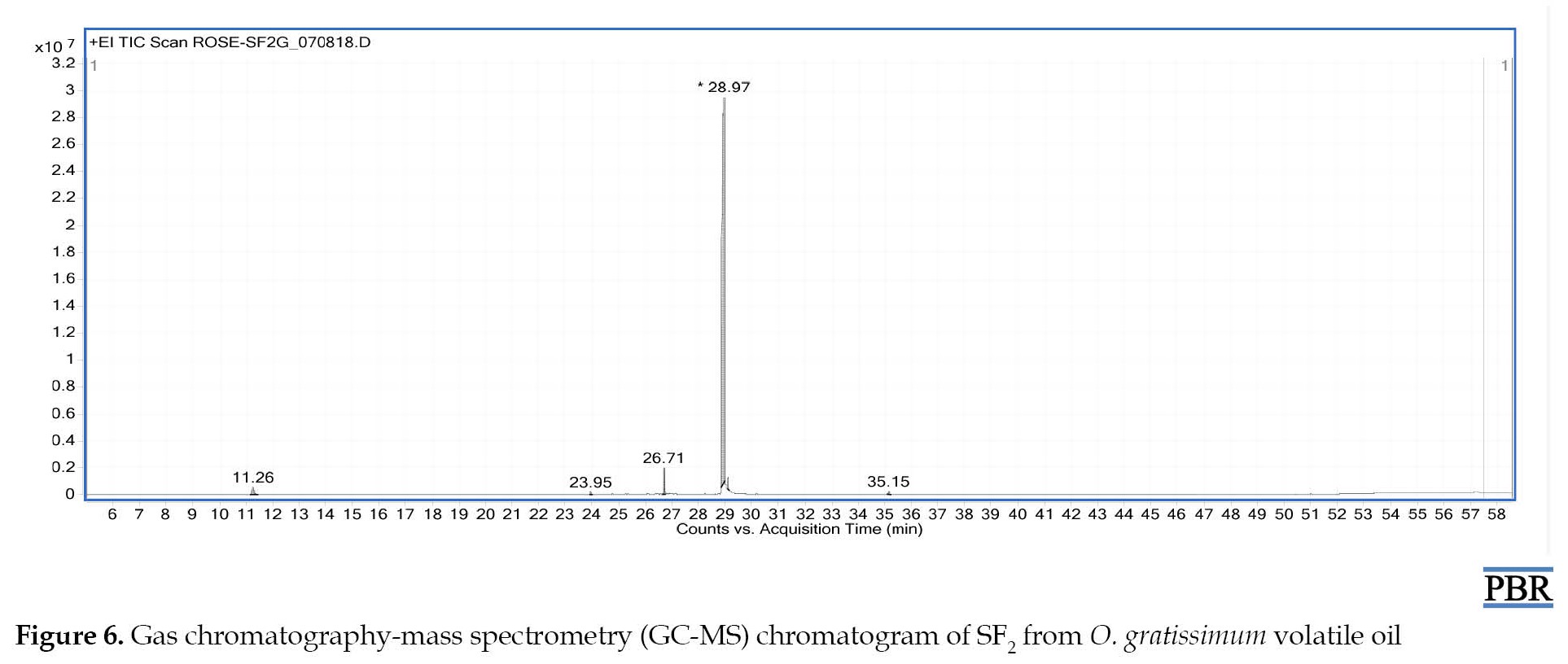
Thymol was the major identified component in preparative TLC fraction SF2 from O. gratissimum essential oil, with a relative abundance of 94.31% (Figure 6). Discussion
Cytotoxicity against simple organisms like the tadpoles of R. raninus and nauplii of A. salina is predictive of an extract’s ability to kill cancer cells in vivo [25, 28].
The meristematic tissues of seeds naturally proliferate under favorable conditions resulting in the elongation of the radicles. Growth inhibition of the seed radicles by an extract can also be used to predict its potential antiproliferative effect against cancer cell lines in cell culture. Seeds of S. bicolor were used in this study due to their availability, cost-effectiveness, and small size. They are reliable as up to 90% can germinate within 24 hours [24]. Radicle growth inhibition is tied to the suppression of cell division [29], thereby affecting its elongation [30]. The inhibition of the radicles could also be explained by changes in osmotic potential which prevents the development of turgor pressure in the seed cells, which is one of the essential elements for radicle development to begin during seed germination [31]. O. gratissimum oil and fractions retarded the growth of the radicles to varying degrees. The extent of sensitivity could be related to the biochemical resistance of the cells as a response to the constituents of the oil and fractions.
The cytotoxic potential of O. gratissimum oil was further observed in the brine shrimp lethality bioassay with LC50 of 200.34 μg/mL, which indicates its potential anticancer effects [32].
In vivo lethality in simple organisms can be used to analyze fractions, and this could lead to the separation and purification of biologically active components [25]. The growth inhibitory test with S. bicolor radicles correlated well in this regard as the active subfraction (SF2) with growth inhibition of 92.03% at 72 h also produced 85.07% inhibition against the AU565 cell line.
Essential oils generally contain terpenes, especially monoterpenes and sesquiterpenes [33]. Thus, in conducting studies on these oils, the chemical characterization and the possibility of linking the major components with particular biological properties come into play.
Thymol, the major component isolated from this oil, has previously been noted to have a cytotoxic effect on human epidermic cell line HaCat, HL-60 (acute promyelocytic leukemia) cells, intestinal (Caco-2) cell line, AGS cells, oral squamous carcinoma (Cal27) and cervical cancer (HeLa) cells derived from the mouse xenografts [34-37]. Its activity against AU565 breast cancer cells is reported here for the first time. Several reports state that thymol exerts its activity through various mechanisms, such as inhibiting cell development, triggering apoptosis, and creating intracellular reactive oxygen species. All these effects were observed in different experimental mode [36].
Biological activities observed with volatile oils have been attributed to the quality and quantity of their individual components, especially the major ones they possess [37], and variations have been observed in the chemical compositions of these oils harvested from different locations.
In this study, thymol (22.49 %) was of the highest abundance in O. gratissimum oil (Table 2), but some earlier reports stated eugenol [22, 38] and caryophyllene [15] as being the most abundant. The variability in the compositions of the oils could be due to climatic and geographic factors, including the degree of maturity at the time of collection [39].
Conclusion
In this study, O. gratissimum volatile oil had moderate cytotoxic effects on the AU565 cell line with no inhibition observed with HeLa cells, while thymol, its major component, showed remarkable potency with more inhibition against AU565 cells. Further investigation of the oil and its main component against other cancer cell lines is required.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This work was supported by NAMST-ICCBS Postgraduate Fellowship Award to Rose Imade (No.: NAM – 05/69/2017).
Authors' contributions
Conceptualization and Supervision: Buniyamin Ayinde; Methodology, investigation, data collection, and analysis: Rose Imade and Anam Alam; Writing the article: Rose Imade; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We wish to acknowledge the staff of the Molecular Bank at the International Center for Chemical and Biological Sciences, University of Karachi, Pakistan, for their assistance.
References
Cancer is one of the major causes of death. Over a hundred forms of cancer have been discovered, with breast and cervical cancers being the most common in women [1]. The low success of clinical therapies, as shown by high morbidity and mortality rates, justifies the search for new and better treatment methods. In southeast Asian countries, herbs are used as spices, and help reduce the incidence of colon, prostate, breast, and other cancers [2]. They are widely used in alternative medicine to treat gastrointestinal and respiratory illnesses and have carminative, digestive, galactagogue, and diuretic effects [3]. Their use in cosmetic and pharmaceutical products is well known [4].
Essential oils have been shown to have an anti-cancer effect against various cancer cell lines. Hypericum hircinum, Thymus vulgaris, and Solanum erianthum leaf essential oils have demonstrated antiproliferative activity against human prostatic adenocarcinoma (PC3 cell line) [5, 6]. Xylopia frutescens leaf essential oil [2] also demonstrated anti-cancer effects in vitro and in vivo on the bronchioalveolar lung carcinoma cell line (NCI-H358M).
Ocimum gratissimum L. (Lamiaceae) is an aromatic perennial erect herb with many branches that grows to a maximum height of 1 m and has a woody base. It is native to South Africa, Asia, and Brazil [7]. The plant is frequently employed in traditional medicine, primarily in the form of teas and infusions of its leaves to treat headaches, stress, colds, pruritus, and abdominal discomfort [8, 9]. Additionally, there are reports of using O. gratissimum to treat skin lesions, pneumonia, conjunctivitis, and upper respiratory tract infections [10].
Numerous scientific investigations have revealed some of the plant’s properties, including anti-diabetic, anti-oxidant, anxiolytic, sedative, anti-inflammatory, hepatoprotective, anti-cancer, gastroprotective, and hypolipidemic [11-19]. Much research on various parts of the plant has confirmed its anti-bacterial properties [20]. O. gratissimum crude extract and its hydrophobic and hydrophilic fractions differentially suppress the progression of MCF10ADCIS.com xenografts and tumor growth and inhibit breast cancer cell growth in vivo [21].
The literature search reveals different constituents in the oil obtained from separate locations. Joshi [22] reported that the oil contained eugenol as the major constituent (57%) alongside β-caryophyllene, bulnesene, and others. Koba et al. [23] further documented the oil to possess mostly thymol, p-cymene, and γ-terpinene.
In light of these facts, this work was carried out to examine the chemical profile of O. gratissimum essential oil harvested from the southern part of Nigeria and to assess the cytotoxic activity of the oil and its major constituent against breast (AU565) and cervical (HeLa) cancer cell lines.
Materials and Methods
Collection and identification of plant material
Fresh leaves of the Ocimum gratissimum plant were collected in December 2016 in Benin City, Edo State, Nigeria. The plant was identified by Dr Henry Akinnibosun of the Plant Biology and Biotechnology Department, Faculty of Life Sciences, University of Benin, Benin City, Nigeria. A voucher number UBH O34 was assigned to it.
Extraction of volatile oil
With the help of a Clevenger apparatus, the leaves (approximately 1 kg) were hydro-distilled in batches to recover the volatile oil. The oil was placed in the freezer at -18⁰C for any water that may have accompanied it from collection to freezing. Subsequently, it was transferred to a dry vial with a pipette and kept in the refrigerator at 4⁰C until further use.
Column chromatography of O. gratissimum volatile oil
Silica gel (200–400 mesh) was used to triturate the volatile oil (15 mL), which was then subjected to column chromatography. For gradient elution, 200 mL of C6H14 (100%) and C6H14- CH3COOC2H5 were used (99:1, 98:2, 97:3, 96:4, 95:5, 94:6, 93:7, 92:8, 91:9, 90:10). Fractions (118) were collected, bulked in accordance with respective TLC (thin-layer chromatography) profiles, and assigned the following codes: F1 (5–12), F2 (13–28), F3 (29–40), F4 (4–63), F5 (62–90), and F6 (91-118).
As a result of the high yield and conspicuous nature, fraction F1 (5 mL) was subjected to preparative TLC analysis on silica gel using C6H14- CH3COOC2H5 (9.7:0.3). Two sub-fractions were obtained and coded as SF1 and SF2.
Source and identification of Raniceps raninus tadpoles
From within the University of Benin, tadpoles (5–6 days old) were gathered from toad colonies in small water communities. They were confirmed to be Raniceps raninus tadpoles by Professor M. Aisien, an Animal Parasitologist at the Department of Animal and Environmental Biology, Faculty of Science, University of Benin, Benin City, Nigeria.
Determination of the essential oil’s cytotoxic effects on tadpoles
Following the method stated in a study [24], 10 tadpoles were placed in a 50-mL beaker containing 15 mL water from the tadpoles’ colonies. This was made up to 49 mL with distilled water, and then 1 mL of the different concentrations of the oil (0.5, 1, and 2 mg/mL) was added to make up to 50 mL. The final concentrations were 10, 20, and 40 μg/mL. For each concentration, the experiment was carried out in triplicates. The tadpole mortality rate was observed for 24 hours.
Brine shrimps lethality bioassay (Artemia salina)
Brine shrimp lethality bioassay [25] was applied to determine cytotoxic activity. Then, 2 mL of acetone was used to dissolve the volatile oil, and from this solution, concentrations of 10, 100, and 1000 μg/mL were obtained in triplicates. The solvent was allowed to evaporate overnight. Using a Pasteur pipette, 10 larvae were introduced to each vial after 48 h of nauplii maturation and hatching. Seawater (38 g/L, pH 7.4) was used to make up the volume to 5 mL, and the vials were incubated at 250°C–270°C for 24 hours while being illuminated. The negative and positive controls were seawater and etoposide, respectively.
Preparation of the guinea corn (Sorghum bicolor)
Absolute alcohol was used to rinse the Sorghum bicolor seeds purchased from a market in Benin City, Nigeria. A quick viability test was conducted to remove the preservative by adding about 100 mL of water and immediately decanting, thus removing the floating compromised seeds. The submerged viable seeds were dried on filter papers before use.
Determination of growth inhibitory effects of the essential oil on Sorghum bicolor seeds
About 10 mL of 1, 2, 5, 10, 20, and 30 mg/mL of O. gratissimum oil prepared by dissolution with 2% Tween 80 in water, were poured into 9 cm wide glass Petri dishes lined with cotton wool and filter paper (Whatman No 1). Twenty viable seeds were dispersed on each plate and incubated in the dark. At 24, 48, 72, and 96 h, each seed’s radicle length (mm) was measured. Then, 10 mL of distilled water with 2% Tween 80 was used to treat the control seedlings. The test was performed in triplicates [24]. Chromatographic fractions F1, F2, SF1, and SF2 were also subjected to a similar procedure at 1 mg/mL concentration.
Assessment of cytotoxic activity of O. gratissimum oil on cancer cell lines
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure the cytotoxic activity. Human breast cancer (AU565) and cervical cancer (HeLa) cell lines were utilized. They were obtained from the International Center for Chemical and Biological Sciences (ICCBS) molecular bank at the University of Karachi in Pakistan. A density of 10000 cells/well/100 μL in 96-well plates was used, and they were incubated for 24 h in a complete medium at 37°C and 5% CO2. Next, 50 μg/mL of the oil and fractions were used to treat the cells in triplicates (F1, F2, F3, F4, and SF2). The standard utilized was doxorubicin (50 μM). Incubation was for 48 h at 37°C and in a humidified atmosphere of 5% CO2. Before the medium was taken out, a fresh medium was added containing 200 μL of MTT dye (0.5 mM) and then incubated for an additional 3–4 hours. The formazan crystals produced were dissolved in 100 μL of DMSO, and absorbance was measured at 570 nm [26, 27]. IC50 was calculated by EZ-Fit software.
Gas chromatography-mass spectrometry analysis
The oil and fraction SF2 were analyzed using gas chromatography-mass spectrometry (GC-MS). The Agilent technologies 7000 GC/MS triple quadrupole mass spectrometer was used to record the gas chromatogram, and it used an OPTIMA-5-ZB-5 column with measurements of 30 m x 250 m x 0.25 m. Electron ionization (EI) with an ionization energy of 70 eV was employed for GC-MS detection. Helium (99.999%) served as the carrier gas, flowing at a constant rate of 1.129 mL/min with an injection volume of 2 L (split ratio 15:1). It was 250°C at the ion source. The oven was programmed to start at 50°C (isothermal for 15 min), then decrease to 8°C/min, rise to 180°C for 15 min., then 15°C/min, and ultimately reach 290°C for 5 min. Runtime was 58.58 min in total. While NIST library match was utilized to identify the compounds, ChemStation software was used to handle the mass spectra and chromatograms.
Statistical analysis
To analyze the data, a 1-way analysis of variance (ANOVA) was performed using SPSS software, version 21, and the results were expressed as Mean±SEM. In every instance, differences were deemed significant at P< 0.05.
Results
Cytotoxic effects of the volatile oil on tadpoles
A concentration-dependent activity was observed in the tadpole mortality test. O. gratissimum oil showed no mortality at 10 μg/mL, while 70% and 100% mortality were seen at 15 and 20 μg/mL, respectively (Figure 1). An LC50 of 13.74 μg/mL was obtained.
Cytotoxic effects of the oil against Artemia salina Nauplii
A concentration-dependent response was observed when the shrimps came in contact with the volatile oil. About 13.33 % mortality was observed at 10 μg/mL, which was increased to 80% at 1000 μg/mL with LC50 of 200.34 μg/mL (Table 1).

Growth inhibitory effects of the essential oil and fractions on radicle length of S. bicolor seeds
The volatile oil was found to greatly suppress radicle growth in a concentration-dependent manner. A significant difference (P<0.05) compared to negative control was observed at 5-30 mg/mL concentrations for 24-96 h. From 5 mg/ml at 48 h and 2 mg/mL at 72 h, the percentage reductions in radicle growth were highly significant (P<0.001). Also, 10 mg/mL of the oil completely inhibited the growth of the radicles at 96 h. An average length of 9.54±1.73 mm in the controls simultaneously was reduced to 0.77±0.02 mm in seeds treated with 5 mg/mL concentration showing a 91.93% reduction (Figure 2). The growth of the radicles was greatly retarded by the column fractions at 1 mg/mL concentration. From 72 to 96 h, the radicle growth reductions for fractions F1, F2, and SF2 were highly significant compared to the control (P<0.001). At 96 h, F1 and F2 gave 81.26% and 86.46% growth inhibitions, respectively. At the same concentration and time, SF1 and SF2 (obtained from F1 through preparative TLC) produced growth inhibitions of 28.73% and 92.03%, respectively (Figure 3). The fractions and subfractions remarkably inhibited the growth of the radicles, with SF2 showing the highest inhibitory effect (P<0.001) compared to the negative control.
Inhibitory effects of O. gratissimum volatile oil and fractions on the cell lines
The oil demonstrated moderate inhibition against breast cancer, +17.82%, but no activity was observed with the cervical cancer cell line (HeLa). However, the inhibitory actions of the column fractions varied, with F4 producing the highest inhibition of +34.42%. A significant difference in activity was observed between the oil and SF2 on both HeLa and AU565 cells (P<0.001). SF2 gave 85.07% inhibition on AU565 cells and an IC50 of 15.96 ± 1.61, while an inhibition of 29% was observed against HeLa cells (Figure 4). Doxorubicin gave 97.89% inhibition with an IC50 of 0.085±0.03 μM with AU565 cells.
GC-MS analysis of O. gratissimum essential oil and fraction SF2
Thymol (22.49%), p-cymene (13.47%), and γ-terpinene (11.5%) were present in the highest quantities in O. gratissimum oil. β-Myrcene, carvacrol, eugenol, and β-selinene were also present with a relative abundance of 4.58%, 2.6%, 0.28%, and 4.15%, respectively. A total of 25 components were obtained (Figure 5, Table 2).

Thymol was the major identified component in preparative TLC fraction SF2 from O. gratissimum essential oil, with a relative abundance of 94.31% (Figure 6). Discussion
Cytotoxicity against simple organisms like the tadpoles of R. raninus and nauplii of A. salina is predictive of an extract’s ability to kill cancer cells in vivo [25, 28].
The meristematic tissues of seeds naturally proliferate under favorable conditions resulting in the elongation of the radicles. Growth inhibition of the seed radicles by an extract can also be used to predict its potential antiproliferative effect against cancer cell lines in cell culture. Seeds of S. bicolor were used in this study due to their availability, cost-effectiveness, and small size. They are reliable as up to 90% can germinate within 24 hours [24]. Radicle growth inhibition is tied to the suppression of cell division [29], thereby affecting its elongation [30]. The inhibition of the radicles could also be explained by changes in osmotic potential which prevents the development of turgor pressure in the seed cells, which is one of the essential elements for radicle development to begin during seed germination [31]. O. gratissimum oil and fractions retarded the growth of the radicles to varying degrees. The extent of sensitivity could be related to the biochemical resistance of the cells as a response to the constituents of the oil and fractions.
The cytotoxic potential of O. gratissimum oil was further observed in the brine shrimp lethality bioassay with LC50 of 200.34 μg/mL, which indicates its potential anticancer effects [32].
In vivo lethality in simple organisms can be used to analyze fractions, and this could lead to the separation and purification of biologically active components [25]. The growth inhibitory test with S. bicolor radicles correlated well in this regard as the active subfraction (SF2) with growth inhibition of 92.03% at 72 h also produced 85.07% inhibition against the AU565 cell line.
Essential oils generally contain terpenes, especially monoterpenes and sesquiterpenes [33]. Thus, in conducting studies on these oils, the chemical characterization and the possibility of linking the major components with particular biological properties come into play.
Thymol, the major component isolated from this oil, has previously been noted to have a cytotoxic effect on human epidermic cell line HaCat, HL-60 (acute promyelocytic leukemia) cells, intestinal (Caco-2) cell line, AGS cells, oral squamous carcinoma (Cal27) and cervical cancer (HeLa) cells derived from the mouse xenografts [34-37]. Its activity against AU565 breast cancer cells is reported here for the first time. Several reports state that thymol exerts its activity through various mechanisms, such as inhibiting cell development, triggering apoptosis, and creating intracellular reactive oxygen species. All these effects were observed in different experimental mode [36].
Biological activities observed with volatile oils have been attributed to the quality and quantity of their individual components, especially the major ones they possess [37], and variations have been observed in the chemical compositions of these oils harvested from different locations.
In this study, thymol (22.49 %) was of the highest abundance in O. gratissimum oil (Table 2), but some earlier reports stated eugenol [22, 38] and caryophyllene [15] as being the most abundant. The variability in the compositions of the oils could be due to climatic and geographic factors, including the degree of maturity at the time of collection [39].
Conclusion
In this study, O. gratissimum volatile oil had moderate cytotoxic effects on the AU565 cell line with no inhibition observed with HeLa cells, while thymol, its major component, showed remarkable potency with more inhibition against AU565 cells. Further investigation of the oil and its main component against other cancer cell lines is required.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This work was supported by NAMST-ICCBS Postgraduate Fellowship Award to Rose Imade (No.: NAM – 05/69/2017).
Authors' contributions
Conceptualization and Supervision: Buniyamin Ayinde; Methodology, investigation, data collection, and analysis: Rose Imade and Anam Alam; Writing the article: Rose Imade; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We wish to acknowledge the staff of the Molecular Bank at the International Center for Chemical and Biological Sciences, University of Karachi, Pakistan, for their assistance.
References
- Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, et al. Cancer incidence in Nigeria: A report from population-based cancer registries. Cancer Epidemiol. 2012; 36(5):e271-8. [DOI:10.1016/j.canep.2012.04.007] [PMID] [PMCID]
- Ferraz RP, Cardoso GM, da Silva TB, Fontes JE, Prata AP, Carvalho AA, et al. Antitumor properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013; 141(1):196-200. [DOI:10.1016/j.foodchem.2013.02.114] [PMID]
- Silva RMG, Carvalho ACM, Matiolli LS, Figueiredo CCM, Gomes AC, Ferreira PC, et al. Genotoxicity and antioxidant activity of spices and herbs used in Brazilian cuisine. Biosci J. 2018; 34(3):727-43. [DOI:10.14393/BJ-v34n3a2018-39847]
- Hamedi A, Bayat M, Asemani Y, Amirghofran Z. A review of potential anti-cancer properties of some selected medicinal plants grown in Iran. J Herb Med. 2022; 33:100557. [Link]
- Zu Y, Yu H, Liang L, Fu Y, Efferth T, Liu X, et al. Activities of ten essential oils towards propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells.Molecules. 2010; 15(5):3200-10. [DOI:10.3390/molecules15053200] [PMID] [PMCID]
- Quassinti L, Lupidi G, Maggi F, Sagratini G, Papa F, Vittori S, et al. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat Prod Res. 2013; 27(10):862-8. [PMID]
- Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol. 2004; 35(4):275-80. [DOI:10.1590/S1517-83822004000300001]
- Matasyoh LG, Matasyoh JC, Wachira FN, Kinyua MG, Muigai AWT, Mukiama TK. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. Afr J Biotechnol. 2007; 6(6):760-5. [Link]
- de Albuquerque UP, Muniz de Medeiros P, de Almeida AL, Monteiro JM, Machado de Freitas Lins Neto E, Gomes de Melo J, et al. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J Ethnopharmacol. 2007; 114(3):325-54. [DOI:10.1016/j.jep.2007.08.017] [PMID]
- Singh V, Kahol A, Singh IP, Saraf I, Shri R. Evaluation of anti-amnesic effect of extracts of selected Ocimum species using invitro and invivo models. J Ethnopharmacol. 2016; 193:490- 9. [DOI:10.1016/j.jep.2016.10.026] [PMID]
- Akpan OU, Bassey RB, Agba BS, Edegha IA. Elevation of serum pancreatic amylase and distortion of pancreatic cyto-architecture in type 1 diabetes mellitus rats treated with Ocimum gratissimum. Niger Med J. 2014; 55(1):34-8. [DOI:10.4103/0300-1652.128157] [PMID] [PMCID]
- Ojo OA, Oloyede O, Tugbobo O, Olarewaju O, Ojo A. Antioxidant and inhibitory effect of scent leaf (Ocimum gratissimum) on Fe2+ and sodium nitroprusside induced lipid peroxidation in rat brain in vitro. Adv Biomed Res. 2014; 8(1):08-17. [Link]
- Venuprasad M, Kandikattu HK, Razack S, Khanum F. Phytochemical analysis of Ocimum gratissimum by LC-ESI-MS/MS and its antioxidant and anxiolytic effects. South Afr J Bot. 2014; 92:151-8. [DOI:10.1016/j.sajb.2014.02.010]
- Tankam JM, Ito M. Sedative, anxiolytic and antidepressant-like effects of inhalation of the essential oil of Ocimum gratissimum L. from Cameroon in mice. J Pharmacogn Phytochem. 2014; 2(5):1-9. [Link]
- Okoye FBC, Obonga WO, Onyegbule FA, Ndu OO, Ihekwereme CP. Chemical composition and anti-inflammatory activity of essential oils from the leaves of Ocimum basilicum L. and Ocimum gratissimum L. (Lamiaceae). Int J Pharm Sci Res. 2014; 5(6):2174-80. [Link]
- Awogbindin IO, Tade OG, Metibemu SD, Olorunsogo OO, Farombi O. Assessment of flavonoid content, free radical scavenging and hepatoprotective activities of Ocimum gratissimum and Spondias mombin in rats treated with dimethylnitrosamine. Arch Bas AppMed. 2014; 2(1):45-54. [Link]
- Lin CC, Chao PY, Shen CY, Shu JJ, Yen SK, Huang CY, et al. Novel target genes responsive to apoptotic activity by Ocimum gratissimum in human osteosarcoma cells. Am J Chin Med. 2014; 42(3):743-67. [DOI:10.1142/S0192415X14500487] [PMID]
- Gege-Adebayo GI, Igbokwe VU, Shafe MO, Akintayo CO, Mbaka DI. Anti-ulcer effect of Ocimum gratissimum on indomethacin induced ulcer and percentage of superoxide dismutase on wistar rats. J Med Med Sci. 2013; 4(1):8-12. [Link]
- Ekoh SN, Akubugwo EI, Ude VC, Nzubechukwu E. Anti-hyperglycemic and anti-hyperlipidemic effect of spices (Thymus vulgaris, Murraya koenigii, Ocimum gratissimum and Piper guineense) in alloxan-induced diabetic rats. Int J Biosci. 2014; 4(2):179-87. [Link]
- Chiemeka O, Onyeke C, Osibe D, Ugwuja F, Okoro A, Onyegirim P. Antimicrobial activity of Ocimum gratissimum L. and Carica papaya L. against postharvest pathogens of avocado pear (Persea americana Mill.). J Plant Pathol. 2020; 102:319-25. [Link]
- Nangia-Makker P, Raz T, Tait L, Shekhar MP, Li H, Balan V, et al. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol Ther. 2013; 14(5):417-27. [PMID] [PMCID]
- Joshi RK. GC-MS Analysis of the Essential Oil of Ocimum gratissimum L. Growing Desolately in South India. Acta Chromatogr. 2017; 29(1):111-9. [DOI:10.1556/1326.2017.29.1.10]
- Koba K, Sanda K, Guyon C, Raynaud C, Millet J, Chaumont JP, et al. Chemical composition and in vitro cytotoxic activity of essential oils from two tropical Lamiaceae: Aeollanthus pubescens Benth. and Ocimum gratissimum L. J Essent Oil-Bear Plants. 2007; 10(1):60-9. [DOI:10.1080/0972060X.2007.10643520]
- Ikpefan EO, Ukwubile CA, Nwankwo LU. Cytotoxic, phytotoxic and insecticidal assessment of the crude extract and fractions of leaves of Conyza sumatrensis (Retz.) E. Walker Asteraceae. Niger J Pharm Appl Sci Res. 2020; 9(3):52-8. [Link]
- Khatun R, Hasnin I, Haque A, Rahman MA. Comparative cytotoxicity of selected mikania species using brine shrimp lethality bioassay and sulforhodamine B (SRB) assay. Bangladesh Pharm J. 2021; 24(1):11-6. [DOI:10.3329/bpj.v24i1.51630]
- Puig T, Aguilar H, Cufí S, Oliveras G, Turrado C, Ortega-Gutiérrez S, et al. A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res. 2011; 13(6):R131. [PMID] [PMCID]
- Kritsanawong S, Innajak S, Imoto M, Watanapokasin R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int J Oncol. 2016; 48(5):2155-65. [DOI:10.3892/ijo.2016.3399] [PMID]
- Babajide JO, Mabusela WT, Green I, Ameer F, Weitz F, Iwuoha EI. Phytochemical screening and biological activity studies of five South African indigenous medicinal plants. J Med Plant Res. 2010; 2(18):1924-32. [Link]
- Ray-Gallet D, Quivy JP, Scamps C, Martini EM, Lipinski M, Almouzni G. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Mol Cell. 2002; 9(5):1091-100. [PMID]
- Fusconi A, Repetto O, Bona E, Massa N, Gallo C, Dumas-Gaudot E, et al. Effects of cadmium on meristem activity and nucleus ploidy on roots of Pisum sativum L. cv. Frisson seedlings. Environ Exp Bot. 2006; 58(1-3):253-60. [DOI:10.1016/j.envexpbot.2005.09.008]
- Akhalkatsi M, Lösch R. Changes in water relations, solute leakage and growth characters during seed germination and seedling development in Trigonella coerulea (Fabaceae). J Appl Bot Qual. 2001; 75(3-4):144-51. [Link]
- Meyer BN, Ferrigni NR, Putnam JT, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituent. Planta Med. 1982; 45(5):31-4. [DOI:10.1055/s-2007-971236]
- Daferera DJ, Tarantilis PA, Polissiou MG. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. J Agric Food Chem. 2002; 50(20):5503-7. [PMID]
- Sharma M., Grewal K, Jandrotia R, Batish DR, Singh HP, Kohli RK. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed Pharmacother. 2020; 146:112511. [DOI:https://doi.org/10.1016/j.biopha.2021.112514]
- Deb DD, Parimala G, Saravana Devi S, Chakraborty T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol Interact. 2011; 193(1):97-106. [DOI:10.1016/j.cbi.2011.05.009] [PMID]
- Llana-Ruiz-Cabello M, Gutiérrez-Praena D, Pichardo S, Moreno FJ, Bermúdez JM, Aucejo S, et al. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line Caco-2. Food Chem Toxicol. 2014; 64:281- 90. [DOI:10.1016/j.fct.2013.12.005] [PMID]
- De La Chapa JJ, Singha PK, Lee DR, Gonzales CB. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J Oral Pathol Med. 2018; 47(7):674- 82. [DOI:10.1111/jop.12735] [PMID] [PMCID]
- Padalia RC, Verma RS. Comparative volatile oil composition of four Ocimum species from northern India. Nat Prod Res. 2011; 25(6):569-75. [PMID]
- Barra A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat Prod Commun. 2009; 4(8):1147-54. [DOI:10.1177/1934578X0900400827]
Type of Study: Original Research |
Subject:
Pharmacognosy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |