Volume 9, Issue 3 (2023)
Pharm Biomed Res 2023, 9(3): 243-258 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Faridi F, Bahador N, Shoja S, Abbasi S. Synthesis and Characterization of Ciprofloxacin-loaded Chitosan Nanoparticles for Eradication of Pseudomonas aeroginosa Biofilm. Pharm Biomed Res 2023; 9 (3) :243-258
URL: http://pbr.mazums.ac.ir/article-1-494-en.html
URL: http://pbr.mazums.ac.ir/article-1-494-en.html
1- Department of Microbiology, Faculty of Science, Agriculture and New Technologies, Shiraz Branch, Islamic Azad University, Shiraz, Iran.
2- Infectious and Tropical Diseases Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
3- Department of Pharmaceutics, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
2- Infectious and Tropical Diseases Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
3- Department of Pharmaceutics, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran.
Full-Text [PDF 2029 kb]
(950 Downloads)
| Abstract (HTML) (1910 Views)
Full-Text: (1031 Views)
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen and causes acute and chronic infections in patients with immunocompromised or other predisposing conditions. Chronic infections will last for years or perhaps decades, and during this period, the P. aeruginosa populations bear thousands of generations of growth while facing challenges together with antibiotic treatment [1]. Biofilm is a community of microorganisms, which is surrounded by a matrix of extracellular polymers. Bacteria entrenched in a biofilm are more resistant to antibiotics due to a decrease in antimicrobial diffusion, slower bacterial metabolic state, as well as easier exchange of resistance genes among cells [2].
Recent studies have indicated that antimicrobial resistance in microorganisms was closely associated with the formation of biofilms, which is related to concerning 75% of human microorganism infections. The resistance microorganism in biofilms raised 10–1000 times compared to their organism forms. The progress of biofilm-specific medicine to treat biofilm-related infections is urged [3]. The fluoroquinolone antibiotic drug, the sole antipseudomonal drug obtainable for oral administration, is extensively wont to treat biofilm infections caused by P. aeruginosa. However, resistance to the present antibiotic is well noninheritable through mutations [4]. The role of efflux pumps in the development and maintenance of biofilms varies between microorganism species. However, the mechanisms behind their contribution fall into four categories: The flow of molecules required for biofilm formation and regulation (molecules associated with the quorum system); indirect regulation of transcription factors related to biofilm formation; excretion of toxins (e.g. antibiotics) and waste metabolites; and facilitating aggregation by affecting adhesion to any cell and alternative surfaces. The expression of pumps in biofilms could increase resistance to antibiotics, leading to establishing the potential of efflux pump inhibitors (EPIs) as anti-biofilm agents [5]. Phe-Arg-β-naphthylamide (PAβN) is the most active and best-studied substance of P. aeruginosa rresistance-nodulation-division (RND) efflux pumps. This might facilitate mete out the fluoroquinolone, in a P. aeruginosa strain that over-expressed MexAB-OprM [6]. The emergence of antibiotic resistance is a major public health concern, which results from abuse and ill-use of antibiotics, and limits their utility. Therefore, researchers are looking for different therapeutic approaches to overcome microbial antibiotic resistance. Among these approaches, drugs referred to as ‘‘nonantibiotics” are accustomed to managing microbial infections [7]. Natural polysaccharides are excellent candidates as base ingredients for both edible films and coatings. Chitosan is one of the most abundant renewable polymers used for medical purposes [8]. It may be a modified biopolymer, derived by partial deacetylation of chitin. Chitosan consists of alternating units of (1→4)- linked N-acetyl glucosamine and glucosamine units. Chitosan finds multifarious applications because of its nontoxicity, biodegradability, and antimicrobial properties [9]. This led to studies on chitosan as a good antibiofilm agent for penetrating biofilms, particularly in medical devices. Therefore, chitosan is able to disrupt the microbial cell membrane. Thus, biofilm formation will be inhibited due to the disruption of the attachment of the bacteria’s cell membrane to the solid surfaces. By destroying the biofilm, the bacteria can then be susceptible to antibiotics, which can consequently result in bacterial cell death [10]. This study aimed at the synthesis and characterization of ciprofloxacin-loaded chitosan nanoparticles for the eradication of P. aeroginosa biofilm.
Materials and Methods
Materials
High molecular weight (HMW) chitosan, tripolyphosphate (TPP), histidine, N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), hydrochloric acid (HCl), Sodium hydroxide (NaOH), sodium nitrite (NaNO2), sodium chloride (NaCl), alanine, sodium dodecyl sulfate, sodium bicarbonate, and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ciprofloxacin was obtained from Floka (USA). Mueller Hinton agar (MHA) and Mueller-Hinton broth (MHB) were purchased from Merck (Germany). All other solvents and chemicals were of analytical grade.
Preparation and MW determination of low molecular weight (LMW) chitosan
Chitosan was selectively depolymerized following the method developed by Huang et al. Briefly, 100 mL of a 1% (w/v) chitosan (400000 g/mol) solution in 1% (v/v) acetic acid solution was prepared. Then, the dissolved chitosan was depolymerized at room temperature under stirring with 10 mL of NaNO2 solutions in water at 2.7 M (molarity), to obtain the desired final molecular weight. After 1 hour of reaction, precipitated chitosan was obtained by raising the pH to 9.0 with the addition of 4N NaOH. The white-yellowish solid was filtrated, washed thoroughly with acetone, and re-dissolved in a minimum volume of acetic acid 0.1 N. Purification was carried out by subsequent dialyzes against purified water (Sigma dialysis tubes, molecular weight cutoff: 1 kDa). The dialyzed product was lyophilized and the yellowish lyophilized product was then stored at 4°C until use [11].
Chitosan molecular weight (viscosity average) was calculated from the classical Mark-Houwink relationship: [η]=k·Mwa, where η is the intrinsic viscosity, Mw is the molecular weight, k=1.81×10-3cm3/g, and a=0.93 [12]. Polymer solutions of known concentrations were prepared in a solvent system consisting of 0.1 M acetic acid and 0.2 M sodium chloride in deionized water. The viscosity measurements were made by recording the efflux times of the solutions in Ubbelohde viscometers (0.5 mm) maintained in a constant-temperature bath at 25±1°C [13].
Synthesis of histidine-modified chitosan (HCS)
Chitosan (125 mg) was dissolved in 10 mL 1% acetic acid. Different concentrations of histidine (25% and 50%) were dissolved in distilled water and methanol and treated with NHS and EDC at a molar ratio of 3:1:1 (EDC:NHS:His) for 2 hours to activate the carboxyl groups of histidine. The activated amino acid solution was added to the chitosan solution, and the resultant mixtures were allowed to react at ambient temperature with stirring for 24 h. The products formed were dialyzed in membrane tubing (molecular weight cut off of 3500 Da) against distilled water for three days and then freeze-dried [14].
Preparation of nanoparticles
Nanoparticles were prepared using the ionic gelation method as described [15] previously with minor modifications. Solutions of LMW and HMW chitosan were prepared at a concentration of 1% (w/v) in different concentrations of acetic acid (v/v). In the next step, TPP was added to the chitosan solution at different ratios drop-by-drop under probe sonication for 10 min. The resultant NPs were collected by centrifugation at 9000 rpm for 30 minutes at 4°C, the supernatant was discarded, and then the NPs were dispersed in distilled water.
Different modes for the synthesis of chitosan, including the amount of TPP and acetic acid were investigated (Table 1).

Physico-chemical characterization
1H-NMR and FT-IR spectroscopy
To determine the structural integrity and deacetylation of the degree of LMW chitosan as well as the degree of histidine modification for HCS, 1H-NMR spectroscopy (400 MHz, Brooker) was carried out. Samples were prepared by dissolution of each polymer (2 mg) in D2O containing 10% trifluoroacetic acid. The degree of histidine modification was calculated by comparing the integration of the proton on the imidazole ring of His side chain with the integration of H2-D in the CS backbone using Equation 1:
1.
Nm=(Integration of m group×nH2-D×Nglucosamine)/(Integration of H2-D groups of glucosamine×nHm)
Where, N stands for the number of each modifying group, m for the modifying group (His), and nH for the number of protons in each group.
FT-IR spectroscopy was performed using ALPHA (Bruker, Germany) in KBr discs.
Characterization of nanoparticles
Measurement of the particle size and zeta potential
The particle size, polydispersity index (PDI), and zeta potential of nanoparticles were measured using dynamic light scattering with a Zeta sizer (particle sizing systems, Horiba, SZ-100, Japan). For this purpose, chitosan samples at the concentration range of 0.1-0.05 mg/mLwere dissolved in 1% v/v acetic acid solution, and TPP was used as a gelating agent. The effect of the concentration of chitosan and TPP as well as the stirring speed was studied. All the measurements were performed in triplicate and reported as the Mean±SD.
Morphological assessment
The morphology of nanoparticles was examined using the transmission electron microscope (TEM) (Temad Kala, Iran). For this purpose, we dispersed the powder in a solvent (acetic acid 0.1%).
Ciprofloxacin-loaded chitosan nanoparticles
The best chitosan nanoparticle in terms of size was used for loading. Ciprofloxacin 1 mL/mg was prepared in 0.1% (v/v) acetic acid. After that, 1 mg of chitosan was dissolved, and then, a specific volume of TPP solution was added while stirring at room temperature (6000 rpm) until faint turbidity was observed showing the formation of nanoparticles [16].
Loading efficiency (LE) and encapsulation efficiency (EE)
The LE of ciprofloxacin and EE of nanoparticles were determined according to the procedure reported by Cevher et al. (2006) [17]. After drug loading, nanoparticles were separated from the suspension by ultracentrifugation (Froilabo, France) at 15500 rpm and 4°C for 30 minutes. The amount of free ciprofloxacin in the supernatant was measured by UV-Vis spectrophotometer (Sco Tech, Germany) at 264 nm. The encapsulation efficiency (EE) was calculated by Equation 2 and Equation 3:

All analyses were carried out in triplicate [16].
In vitro drug release study
For this purpose, 2 mg of ciprofloxacin-loaded chitosan nanoparticles were suspended in separate tubes containing equal volumes of 0.2 mol/L PBS solutions (pH 7.4) and incubated while stirring at 37°C and 100 rpm. At appropriate time intervals (1, 2, 4, 8, 12, 24, 48, and 72 hours), one tube was removed and the sample was centrifuged at 15000 g and 14°C for 30 minutes. The amount of released drug from NPs was determined by measuring the absorbance of the supernatant within time intervals at 264 nm by a UV spectrophotometer (Multiskan GO, Thermo Scientific) using a standard curve [18].
Specimen collection
During the study period from Nov 2018 to Oct 2019, P. aeruginosa isolates were collected from clinical specimens of Shahid Mohammadi teaching hospital affiliated with the Hormozgan University of Medical Sciences (HUMS) at Bandar Abbas located in the south of Iran. Bacterial isolates were identified based on the biochemical tests, including gram staining, growth on MacConkey agar, citrate utilization, oxidation-fermentation, and oxidase test. Ciprofloxacin-resistant isolates were screened by 5 µg ciprofloxacin disk using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guideline [19].
Biofilm formation
The abilities to produce biofilm of all isolates were analyzed using a colorimetric microtiter method. In order to quantitatively determine biofilm formation capacity, bacterial colonies were grown overnight at 37°C in Tryptic Soy Broth (TSB) (Merck Darmstadt, Germany). The bacterial suspensions were diluted (1:100) in a new TSB medium and 150 μL of this was added to the sterile flat-bottomed 96-well polystyrene microplates followed by incubation for 24 hours at 37°C. The wells were washed with distilled water three times. After drying the wells at room temperature, they were stained with 125 μL of 0.1% crystal violet solution (CV) for 10-15 minutes. The CV was thrown away and the wells were washed three times to remove excess CV. Finally, the bounded CV was released by the addition of 125 μL of 30% acetic acid. A new sterile plate was inoculated with 125 μL of detection solution per well. The optical density (OD) absorbance of each well was measured at a wavelength of 550 nm using an ELISA reader (Biotek elx800). All the assays were repeated based on Equation 4 and Table 2 [20].

4. Optical density cut-off value (ODc)=Average OD of negative control+3×SD of negative control
MBIC and MBEC determinations
MBIC and MBEC experiments were performed using a modified version of the Calgary biofilm device method [21, 22]. In brief, 20 mL108 c.f.u. mL-1 bacterial suspensions were added to 180 mL1% TSB, placed into a sterile 96-well polystyrene flat-bottom microtitre plate, and incubated overnight at 37°C without shaking, to allow bacterial attachment. Non-adherent cells were removed by gentle washing three times with sterile saline solution (150 mL 0.9 % NaCl). The plates were air-dried for 15 minutes. Serial twofold dilutions of each antimicrobial agent ciprofloxacin, ciprofloxacin-PAβN, CS ciprofloxacin, and CS ciprofloxacin- PAβN in Mueller–Hinton broth (MHB) were added to the microplates followed by incubation at 35°C for 24 hours. MBIC was defined as the minimal antimicrobial concentration, at which there was no observable bacterial growth in wells containing adherent microcolonies, the minimal concentration that inhibited the release of planktonic bacteria from the biofilm. After MBIC measurement, the broth was removed and wells were washed three times with sterile saline solution (150 mL 0.9 % NaCl) and antimicrobial-free MHB was added, followed by incubation for 24 hours at 35°C. MBEC was defined as the minimal antimicrobial concentration, at which bacteria fail to regrow after antimicrobial exposure, i.e. the minimal concentration required for eradicating the biofilm. All determinations were performed in duplicate [23].
Results
One of the best practical methods for calculation of the MW of polymeric samples is the intrinsic viscosity measurements, using the Mark-Houwink-Sakurada equation (Equation 5). Based on the results, the y-intercepts of Ln ɳrel/c versus concentration curve, stands for intrinsic viscosity, were 0.572 and 0.066 for HMW and LMW samples, respectively. The viscosity-based average of MW of HMW and LMW samples were calculated as 478.84 and 47.79 KD, respectively.
5. [ɳ]=KMa
The structures of acetylated and deacetylated monomers of chitosan as well as the 1H-NMR spectra of LMW and HMW chitosan samples are presented in Figure 1 and Figure 2. In the highly deacetylated samples, H1-A (5 ppm<δ<5.5 ppm) is not visible in the spectrum and the use of the acetyl group peak (H-Ac) presents two advantages over the use of the H1 peak of acetylated monomer (H1-A). First, the H-Ac peak is three times more intense than the H1-A peak and second, the H-Ac peak is well resolved with a flat baseline on each side of the peak.


There are several equations that can be used to determine deacetylation and acetylation degree. In the current project, based on the Equation 6, degree of deacetylation of about 92% for depolymerized chitosan (LMW sample) and 95% for parent molecule (HMW sample) was calculated indicating that polymerization process did not affect the degree of deacetylation significantly.
6. DD=[1-(1/3 ICH3)/(1/6 IH2-H6)]×100
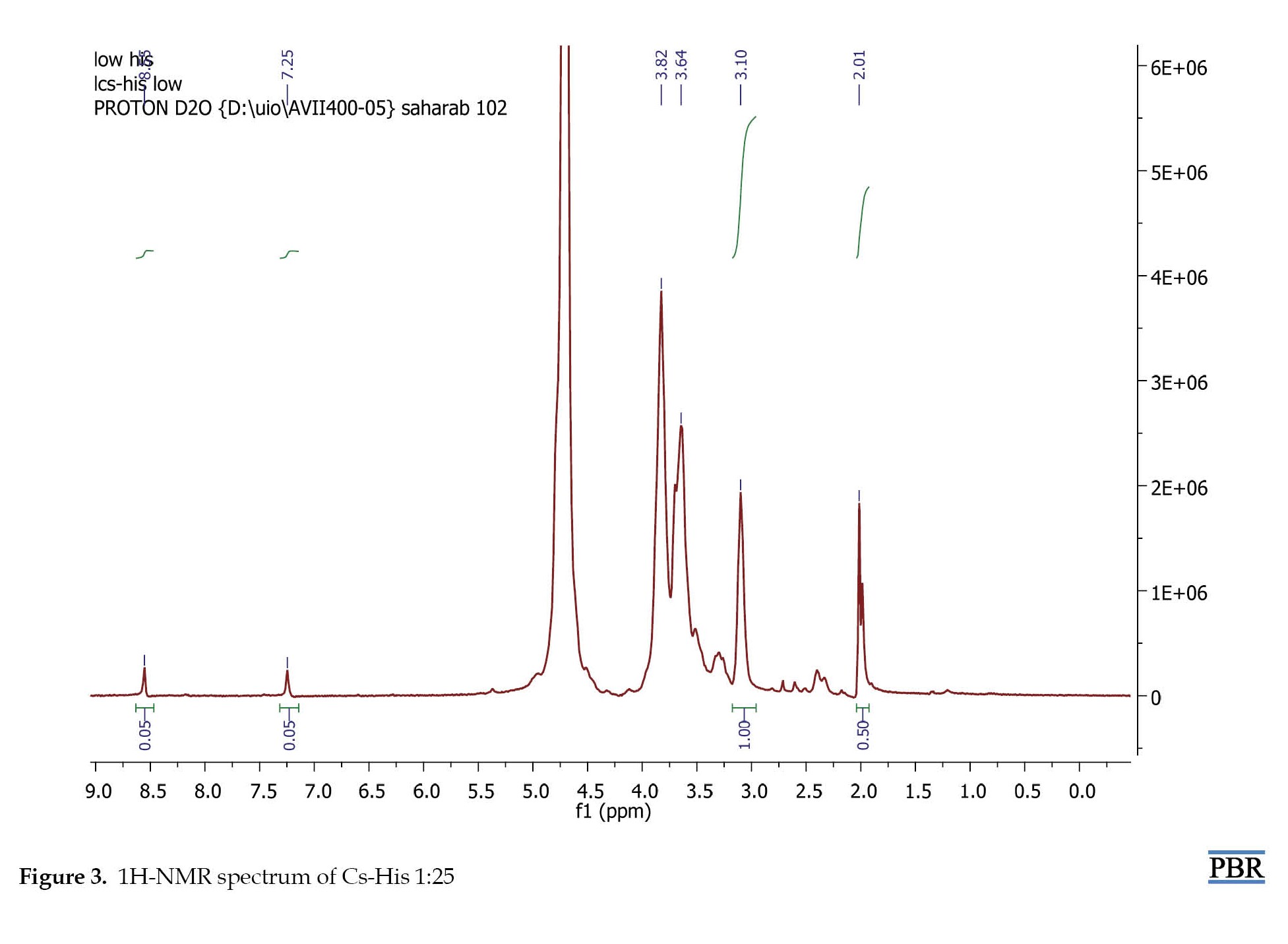
Histidine was conjugated to the chitosan backbone by the reaction of the carboxyl group on histidine to amine groups of chitosan through an NHS- and EDC-mediated reaction. In order to determine the degree of histidine modification, 1H-NMR spectroscopy was carried out. The spectra of His-CS samples are shown in Figure 3 and Figure 4. The imidazole group of histidine showed a characteristic peak at 7-9 ppm, which was used to determine the degree of modification according to equation 1. The results are shown in Table 3.
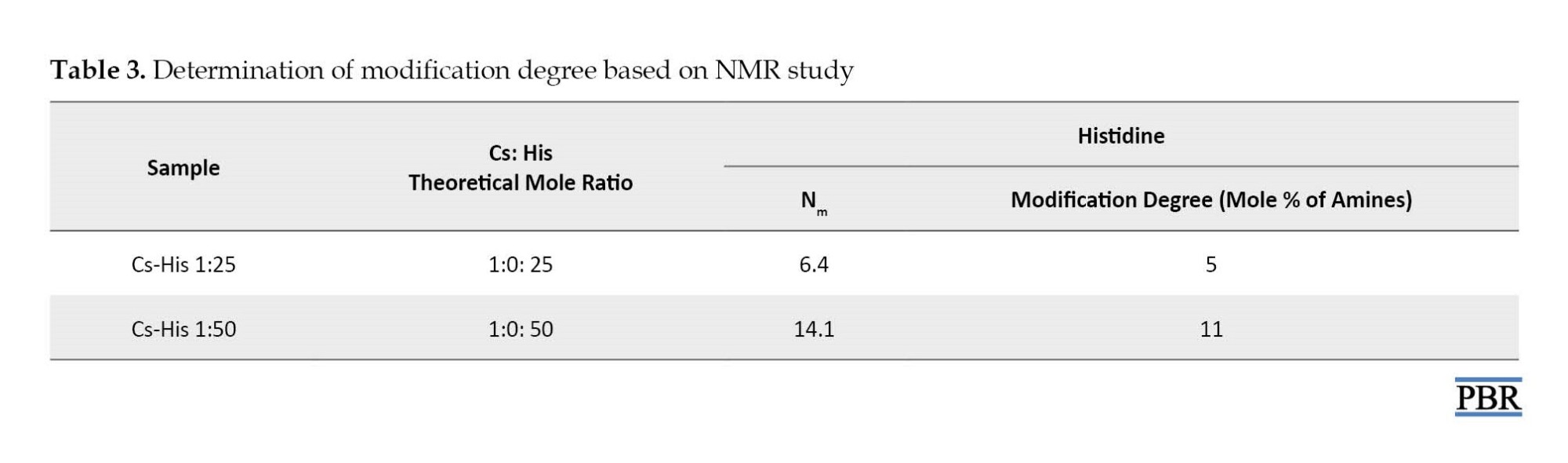
In the case of histidine modification, the degree of modification was about 24-28% for both Cs-His derivatives. The reason for the low percentage of modification could be the acidic pH of the reaction (4.5), in which most of the amine groups are protonated.

The average size of chitosan nanoparticles in an aqueous medium was measured by dynamic laser light scattering for the prepared samples. In this study, all compounds were less than 1000 nm in size. Formula 12 had a smaller mean particle size (164.7±11.24 nm) and was selected for further studies (Figure 5).

TEM has been used as a powerful technique to characterize the morphology and the internal structure of the nanoparticles. The results of the TEM study showed that the nanoparticles had an almost spherical shape, smooth surface, and an approximate size range of 75-200 nm (Figure 6).

Ciprofloxacin loading efficiency was 35.51% and encapsulation efficiency was 55.06%.
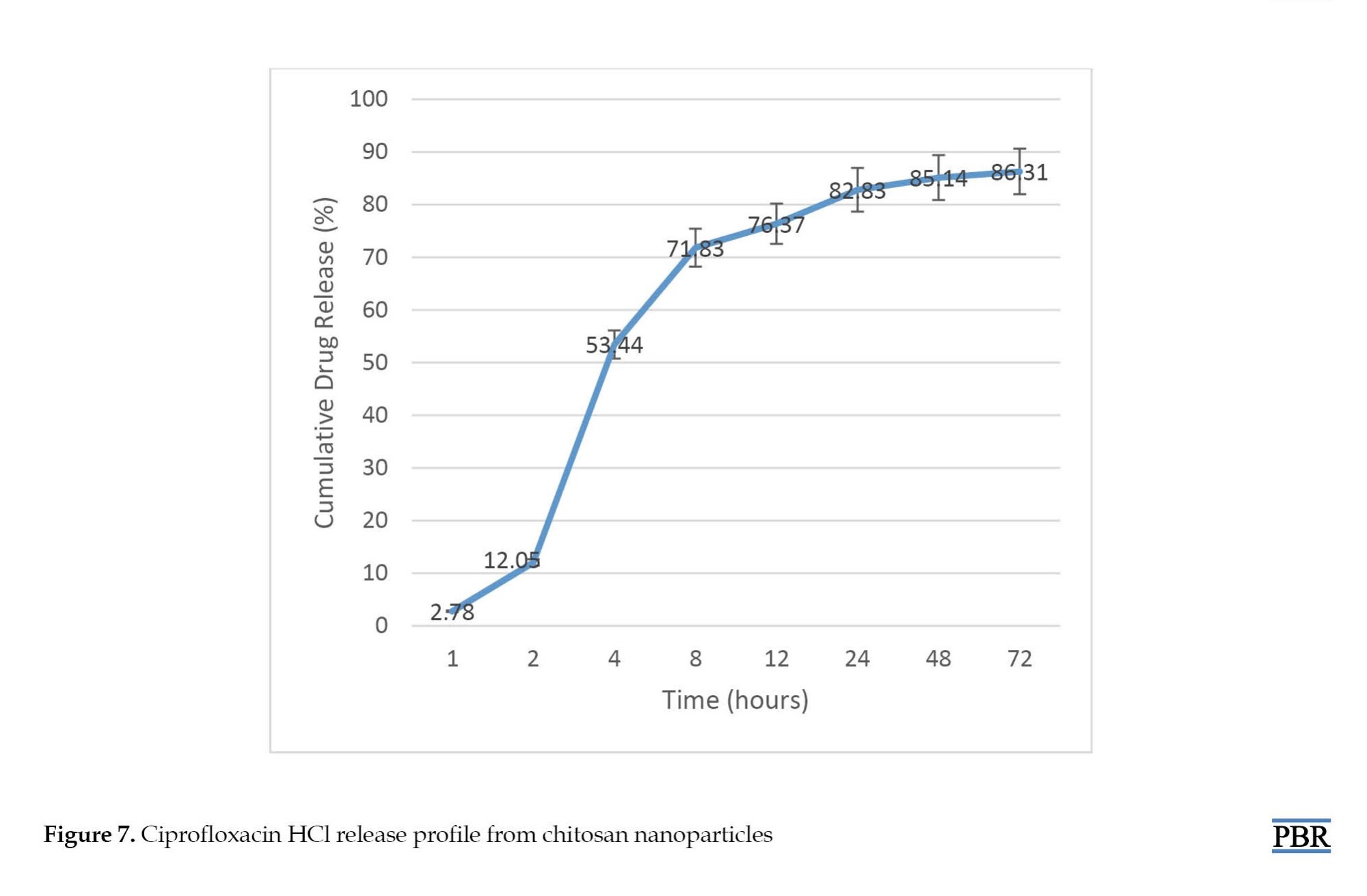
The release profile of ciprofloxacin from ciprofloxacin-loaded nanoparticles at pH 7.4 is displayed in Figure 7. After 4 hours, 53% of the drug was released from CS nanoparticles and more than 80% of the drug was released in 24 hours.
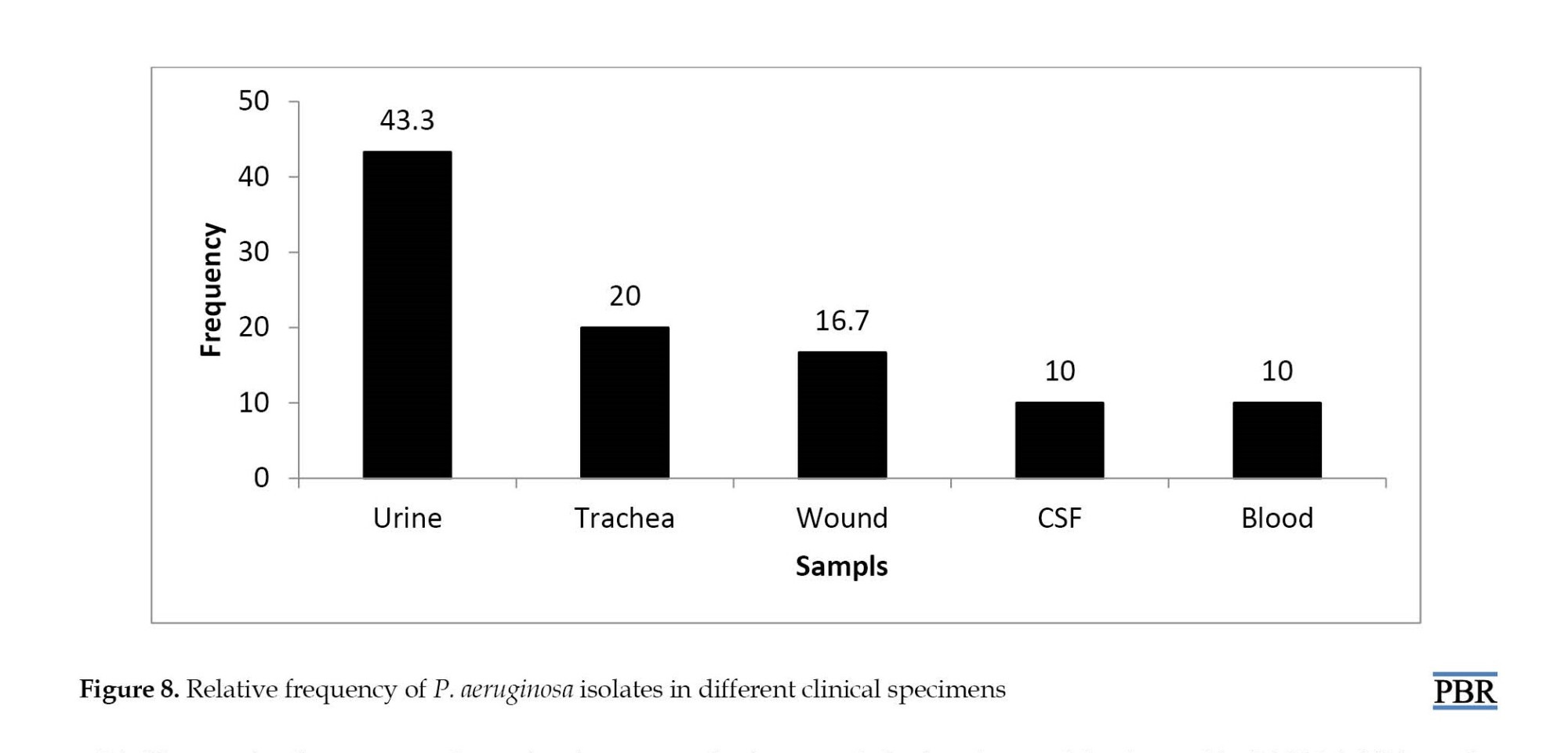
The collected bacteria were isolated from different clinical samples. The highest rate of bacterial isolation from urine culture was 43.3% and the lowest rate of blood infection and CSF was 10%. The relative abundance of P. aeruginosa isolates from different clinical specimens is shown in Figure 8. P. aeruginosa can produce different color pigments. In this study, four types of pigments and 20% of isolates did not contain color pigments.
Biofilm production was performed using a standard and quantitative method proposed by Christensen et al. The numerical results obtained from absorption at 570 nm by ELISA were stored in Excel software for quantitative calculation of biofilm production and calculated according to the following formula. Twenty-nine (96.7%) isolates were able to produce biofilm, while 30.1% of the samples had strong biofilm (Figure 9).

Based on the results of the MBIC test listed in Table 4, the effective concentrations for ciprofloxacin were in the range of 40-5120 μg/mL. However, with the addition of PAβN inhibitor, a decrease was observed in the MBIC range. The best effect was found in the antibiotic group ciprofloxacin and chitosan.
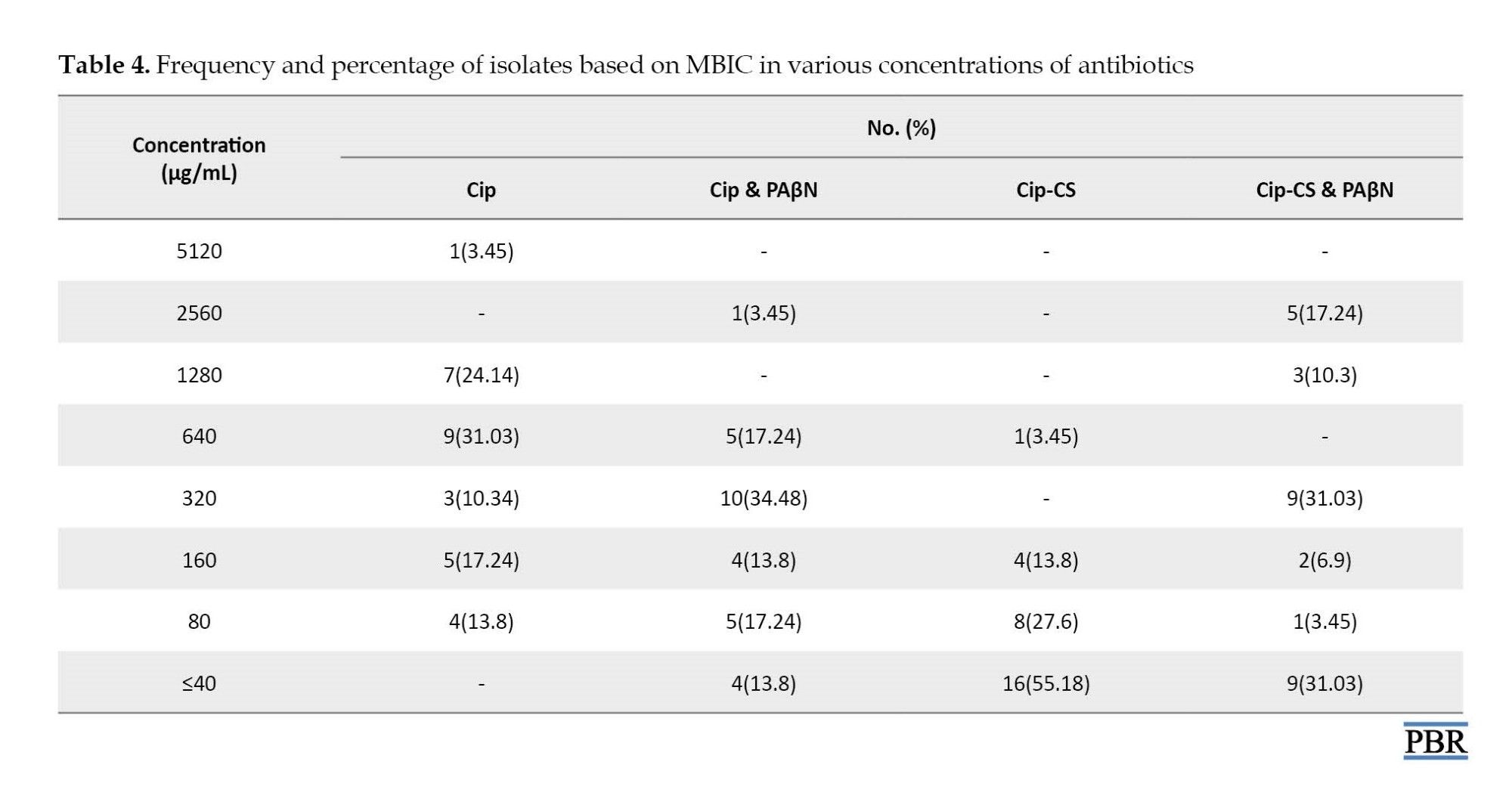
Table 5 shows the results of MBEC. The highest frequency was related to the antibiotic ciprofloxacin 2560 μg/mL, but in combination with PAβN inhibitor, the highest frequency was reported at a concentration of 640 μg/mL. For the chitosan-loaded drug, the highest frequency of MBEC results was recorded at a lower concentration of 40 μg/mL.

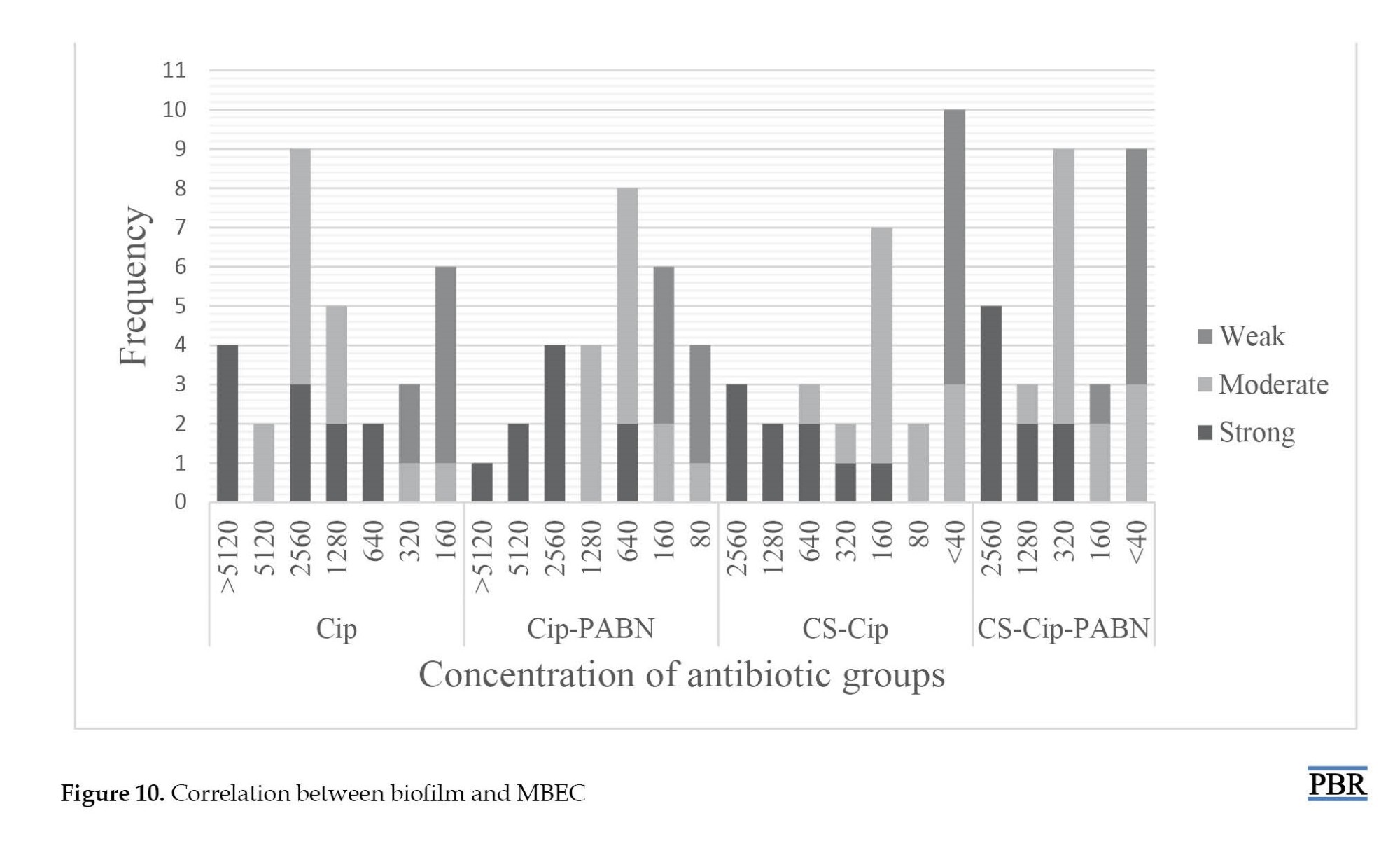
The ciprofloxacin loaded on chitosan with an efflux pump inhibitor showed the highest frequency at 320 μg/mL. MBEC test in strains with strong biofilm showed the highest frequency at a concentration of >5120 μg/mL and the lowest frequency at a concentration of 1280 μg/mL. MBEC and MBIC of samples 21 and 18 were similar to strong biofilms despite producing moderate biofilms at the high concentration range. MBEC results showed that the effect of ciprofloxacin loaded on chitosan on biofilm in 19(65.5%) isolates was in the range of 160 μg/mL and less. Also, after adding an inhibitor to this compound, 160(86.2%) MBEC isolates of 160 μg/mL or less were recorded. MBIC50 was recorded for the antibiotic ciprofloxacin (640 μg/mL). However, with the addition of PAβN inhibitor, a decrease was observed in the MBIC range; thus, MBIC50 was reduced to 320 μg/mL for the combination of ciprofloxacin antibiotic and efflux pump inhibitor. The best effect of the ciprofloxacin-loaded antibiotic group in chitosan with MBIC50 was at 40 μg/mL. Ciprofloxacin loaded with chitosan with an efflux pump inhibitor was less effective than the previous group but was better than ciprofloxacin alone with the inhibitor and the MBIC50 of this group was set at 80 μg/mL. MBEC50 different antibiotic groups of ciprofloxacin, ciprofloxacin and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor were calculated at 1280, 640, 160, and 320 μg/mL, respectively. As shown in Figure 10, the concentration in samples with strong biofilm was higher than in isolates with moderate or weak biofilm.
Discussion
Today, the ecological role of biofilms in the development of healthcare-related infections is well established and has a significant impact on medicine. The National Institutes of Health (NIH) estimates that bacterial biofilms are involved in 65% of microbial diseases and more than 80% of chronic infections [24]. The biofilm-associated infections are an enormous challenge in healthcare. The development of a unique drug delivery system targeting the bacterial cells within the biofilm and dispersing the biofilm structure is an urgent necessity. Recently, more studies have focused on the use of nanoparticles for the inhibition of biofilm formation and also the detachment of mature biofilms [25]. Today, combining nanodrugs with antibiotics is an effective delivery system that protects antibiotics against enzyme degradation and increases the effectiveness of the drug [26]. Chitosan nanoparticles are well established for drug delivery and have many advantages, including crossing biological barriers, large-scale non-degradation of molecules, controlled release of the drug to the desired site, and uptake through endocytosis due to their small size [9]. In 2019, Alqahtani et al. reported the efficiency of diclofenac encapsulation in chitosan as 29.3%, which due to the type of antibiotic, a different percentage was recorded compared to the present study [7]. Drug loading on chitosan was reported by 87.4% in the study by Kucukoglu et al., which was higher than the results of our study [27]. Porras-Gómez et al. reported that the efficiency of imipenem encapsulation in chitosan was 41.65%, which was close to the results of this study, but due to differences in the type of antibiotic, the results could be different [28]. In our study, the rapid release of ciprofloxacin in the first hour was about 2% but after 4 hours, it was approximately 53%, which did not correspond to the results of Al-Qattani et al. [7]. In the present study, approximately 82-86% of the drug was released after 24 to 72 hours. Kucukoglu et al. similar to our study, reported that chitosan was released after a maximum of 24 hours [27]. In the study by Porras-Gómez, the release of imipenem peaked after 24 hours and about 40% of the drug was released after 24 hours [28]. Only in the study by Sahi et al., penicillin was loaded into chitosan, and they reported initial drug release in the first 2 hours and the maximum release after 24 hours was about 88%, which was consistent with the results of our study [29]. On the contrary, Singh et al. reported the highest release of ciprofloxacin from chitosan after 8 hours, which was about 99% and is consistent with the results of our study [18]. Different rates of drug release in different studies depend on using different concentrations of TPP. Also, the more drug is loaded, the higher the drug release rate; thus, more continuous release can be eliminated by increasing the drug load. The particle size plays a crucial role in nanoparticle properties and therefore an essential task in the property characterization of nanoparticles is particle sizing. For example, the melting point of nanoparticles is decreased when the size is reached the nanometer scale [30]. Al-Qahtani et al. reported a particle size of about 295.33 nm, which was larger than the particle size synthesized in this study [7]. In a cultural study, a chitosan particle was loaded into ciprofloxacin with a size of 260 nm [31]. In the study by Porras-Gómez et al., the particle size of chitosan nanoparticles containing imipenem antibiotics was reported at 127.77 nm [28]. These results are close to the data of the present study. The average particle size synthesized in the study by Safhi et al. was about 360.4 nm, which is more than the particle size in this study [29]. Particle size was reported in the study by Singh et al. as 100 to 200 nm, which was consistent with our results [18]. According to our results, 96.7% of the P. aeruginosa isolates made biofilms, of which 30.1% were strong biofilm producers. In agreement with our results, Ghanbarzadeh et al. [32]and Jabalameli et al. [33] reported a biofilm rate of 92.4 and 96.9% for the isolates, respectively. Fluoroquinolones, like ciprofloxacin, are the primary alternative as antibiofilm antibiotics for treatment. Our results showed MBIC values of ciprofloxacin ranging from 80 μg/mL to >5120 μg/mL in P. aeruginosa strains, also the range for MBEC was 160 μg/mL to >5120 μg/mL. Over the last few years, various compounds are synthesized and investigated for their inhibitory activity against P. aeruginosa efflux pumps. PAβN, a broad-spectrum EPI, is shown to potentiate fluoroquinolones and levofloxacin, by out-competing the antibiotics for the pumps [5]. Several studies reported synergistic activities of PaβN and antibiotics against P. aeruginosa and biofilm growth. Quorum sensing operates the discharge of P. aeruginosa virulence factors and also the synthesis of biofilm throughout infection. PAβN significantly reduced quorum sensing signal in P. aeruginosa [34]. The mixture of ciprofloxacin and PAβN resulted in the largest decrease in biofilm mass (2 folds and more) compared with the ciprofloxacin alone. Chitosan could be a natural mucoadhesive polymer with antibacterial activity. We prepared nanoparticles of chitosan by ionic gelation technique containing ciprofloxacin to judge the possible changes within the efficiency of antibacterial agent. Our results revealed the importance of ciprofloxacin-loaded chitosan as they need strong antibiofilm activity against resistant bacteria, like P. aeruginosa. Ciprofloxacin+chitosan was also able to reduce MBIC by 2–4-fold in all isolates, compared to free ciprofloxacin. Additionally, they reduced MBEC in 28 of the tested isolates (96.55%). To our knowledge, there are no data on the antibiofilm activity of chitosan-loaded ciprofloxacin in combination with PAβN. According to previous studies, concentrations of MBIC for ciprofloxacin, ciprofloxacin+PaβN, and chitosan+ciprofloxacin were 640, 320 and <40 µg/mL, respectively. The vast majority of MBEC for ciprofloxacin, ciprofloxacin+PaβN, and chitosan+ciprofloxacin was 2560, 640, and 160 µg/mL, respectively, and the rate of MBEC for a combination of chitosan+ciprofloxacin+PAβN was <40 µg/mL. Our data revealed that MBIC for ciprofloxacin was less than 160 μg/mL in 31% of the strains. After adding the inhibitor to ciprofloxacin, the number of strains at this concentration reached 44.7%. Also, the MBIC of ciprofloxacin loaded in chitosan in 96.6% of strains was in the range of 160 μg/mL or lower concentrations. MBIC50 of different antibiotic groups of ciprofloxacin, ciprofloxacin, and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor were calculated at 640, 320, 40, and 80 μg/mL, respectively. Kashef et al. assessed the biofilm of S. aureus and showed a reduction of 2 to 4 folds in MBIC for ciprofloxacin loaded into the niosome [35]. MBEC50 of different antibiotic groups of ciprofloxacin, ciprofloxacin and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor was calculated at 1280, 640, 160, and 320 μg/mL, respectively. Collectively, the results of this study showed that the ciprofloxacin-loaded chitosan had the best effect on penetrating the biofilm and killing the bacteria trapped in the biofilm. A comparison of MIC50 and MBIC50 showed that 10 to 160 times more antibiotics are needed to inhibit biofilm. These ratios were also calculated to be 20 to 160 times higher for MBC50 and MBEC50 in different groups of antibiotics. Recently, assessment of the effect of chitosan-streptomycin on P. aeruginosa biofilm showed that it removed more than 70% of the biofilm mass, while streptomycin was only able to remove 22% of the cell mass [3]. In the MBEC study, it appears that the intramolecular hydrogen bond between the efflux pump inhibitor and the chitosan, followed by the formation of a large and heavy structure, disrupts the efflux of food in the biofilm channels. As a result, the ciprofloxacin loaded in chitosan with an efflux pump inhibitor had the best results.
Conclusion
The findings of this study provided more evidence for the significance of antibiotics-chitosan against resistant infections. But the precise mechanisms that lead to the synergistic result of chitosan and antibiotics-chitosan combos are largely unknown. Understanding these mechanisms is essential for overcoming treatment failure against resistant bacteria. Such information encourages additional studies with polysaccharide derivatives and alternative antimicrobial categories and in vivo animal experiments to validate these attention-grabbing findings before clinical tests will move forward.
Ethical Considerations
Compliance with ethical guidelines
The project was approved by the Research Center of the Shiraz Branch of Islamic Azad University (Code: 16330507962020).
Funding
This study was funded by Shiraz Branch of the Islamic Azad University.
Authors' contributions
All authors equally contributed in preparation of the article.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors acknowledged the Islamic Azad University of Shiraz Branch for providing the research resources.
References
Pseudomonas aeruginosa is an opportunistic pathogen and causes acute and chronic infections in patients with immunocompromised or other predisposing conditions. Chronic infections will last for years or perhaps decades, and during this period, the P. aeruginosa populations bear thousands of generations of growth while facing challenges together with antibiotic treatment [1]. Biofilm is a community of microorganisms, which is surrounded by a matrix of extracellular polymers. Bacteria entrenched in a biofilm are more resistant to antibiotics due to a decrease in antimicrobial diffusion, slower bacterial metabolic state, as well as easier exchange of resistance genes among cells [2].
Recent studies have indicated that antimicrobial resistance in microorganisms was closely associated with the formation of biofilms, which is related to concerning 75% of human microorganism infections. The resistance microorganism in biofilms raised 10–1000 times compared to their organism forms. The progress of biofilm-specific medicine to treat biofilm-related infections is urged [3]. The fluoroquinolone antibiotic drug, the sole antipseudomonal drug obtainable for oral administration, is extensively wont to treat biofilm infections caused by P. aeruginosa. However, resistance to the present antibiotic is well noninheritable through mutations [4]. The role of efflux pumps in the development and maintenance of biofilms varies between microorganism species. However, the mechanisms behind their contribution fall into four categories: The flow of molecules required for biofilm formation and regulation (molecules associated with the quorum system); indirect regulation of transcription factors related to biofilm formation; excretion of toxins (e.g. antibiotics) and waste metabolites; and facilitating aggregation by affecting adhesion to any cell and alternative surfaces. The expression of pumps in biofilms could increase resistance to antibiotics, leading to establishing the potential of efflux pump inhibitors (EPIs) as anti-biofilm agents [5]. Phe-Arg-β-naphthylamide (PAβN) is the most active and best-studied substance of P. aeruginosa rresistance-nodulation-division (RND) efflux pumps. This might facilitate mete out the fluoroquinolone, in a P. aeruginosa strain that over-expressed MexAB-OprM [6]. The emergence of antibiotic resistance is a major public health concern, which results from abuse and ill-use of antibiotics, and limits their utility. Therefore, researchers are looking for different therapeutic approaches to overcome microbial antibiotic resistance. Among these approaches, drugs referred to as ‘‘nonantibiotics” are accustomed to managing microbial infections [7]. Natural polysaccharides are excellent candidates as base ingredients for both edible films and coatings. Chitosan is one of the most abundant renewable polymers used for medical purposes [8]. It may be a modified biopolymer, derived by partial deacetylation of chitin. Chitosan consists of alternating units of (1→4)- linked N-acetyl glucosamine and glucosamine units. Chitosan finds multifarious applications because of its nontoxicity, biodegradability, and antimicrobial properties [9]. This led to studies on chitosan as a good antibiofilm agent for penetrating biofilms, particularly in medical devices. Therefore, chitosan is able to disrupt the microbial cell membrane. Thus, biofilm formation will be inhibited due to the disruption of the attachment of the bacteria’s cell membrane to the solid surfaces. By destroying the biofilm, the bacteria can then be susceptible to antibiotics, which can consequently result in bacterial cell death [10]. This study aimed at the synthesis and characterization of ciprofloxacin-loaded chitosan nanoparticles for the eradication of P. aeroginosa biofilm.
Materials and Methods
Materials
High molecular weight (HMW) chitosan, tripolyphosphate (TPP), histidine, N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), hydrochloric acid (HCl), Sodium hydroxide (NaOH), sodium nitrite (NaNO2), sodium chloride (NaCl), alanine, sodium dodecyl sulfate, sodium bicarbonate, and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ciprofloxacin was obtained from Floka (USA). Mueller Hinton agar (MHA) and Mueller-Hinton broth (MHB) were purchased from Merck (Germany). All other solvents and chemicals were of analytical grade.
Preparation and MW determination of low molecular weight (LMW) chitosan
Chitosan was selectively depolymerized following the method developed by Huang et al. Briefly, 100 mL of a 1% (w/v) chitosan (400000 g/mol) solution in 1% (v/v) acetic acid solution was prepared. Then, the dissolved chitosan was depolymerized at room temperature under stirring with 10 mL of NaNO2 solutions in water at 2.7 M (molarity), to obtain the desired final molecular weight. After 1 hour of reaction, precipitated chitosan was obtained by raising the pH to 9.0 with the addition of 4N NaOH. The white-yellowish solid was filtrated, washed thoroughly with acetone, and re-dissolved in a minimum volume of acetic acid 0.1 N. Purification was carried out by subsequent dialyzes against purified water (Sigma dialysis tubes, molecular weight cutoff: 1 kDa). The dialyzed product was lyophilized and the yellowish lyophilized product was then stored at 4°C until use [11].
Chitosan molecular weight (viscosity average) was calculated from the classical Mark-Houwink relationship: [η]=k·Mwa, where η is the intrinsic viscosity, Mw is the molecular weight, k=1.81×10-3cm3/g, and a=0.93 [12]. Polymer solutions of known concentrations were prepared in a solvent system consisting of 0.1 M acetic acid and 0.2 M sodium chloride in deionized water. The viscosity measurements were made by recording the efflux times of the solutions in Ubbelohde viscometers (0.5 mm) maintained in a constant-temperature bath at 25±1°C [13].
Synthesis of histidine-modified chitosan (HCS)
Chitosan (125 mg) was dissolved in 10 mL 1% acetic acid. Different concentrations of histidine (25% and 50%) were dissolved in distilled water and methanol and treated with NHS and EDC at a molar ratio of 3:1:1 (EDC:NHS:His) for 2 hours to activate the carboxyl groups of histidine. The activated amino acid solution was added to the chitosan solution, and the resultant mixtures were allowed to react at ambient temperature with stirring for 24 h. The products formed were dialyzed in membrane tubing (molecular weight cut off of 3500 Da) against distilled water for three days and then freeze-dried [14].
Preparation of nanoparticles
Nanoparticles were prepared using the ionic gelation method as described [15] previously with minor modifications. Solutions of LMW and HMW chitosan were prepared at a concentration of 1% (w/v) in different concentrations of acetic acid (v/v). In the next step, TPP was added to the chitosan solution at different ratios drop-by-drop under probe sonication for 10 min. The resultant NPs were collected by centrifugation at 9000 rpm for 30 minutes at 4°C, the supernatant was discarded, and then the NPs were dispersed in distilled water.
Different modes for the synthesis of chitosan, including the amount of TPP and acetic acid were investigated (Table 1).

Physico-chemical characterization
1H-NMR and FT-IR spectroscopy
To determine the structural integrity and deacetylation of the degree of LMW chitosan as well as the degree of histidine modification for HCS, 1H-NMR spectroscopy (400 MHz, Brooker) was carried out. Samples were prepared by dissolution of each polymer (2 mg) in D2O containing 10% trifluoroacetic acid. The degree of histidine modification was calculated by comparing the integration of the proton on the imidazole ring of His side chain with the integration of H2-D in the CS backbone using Equation 1:
1.
Nm=(Integration of m group×nH2-D×Nglucosamine)/(Integration of H2-D groups of glucosamine×nHm)
Where, N stands for the number of each modifying group, m for the modifying group (His), and nH for the number of protons in each group.
FT-IR spectroscopy was performed using ALPHA (Bruker, Germany) in KBr discs.
Characterization of nanoparticles
Measurement of the particle size and zeta potential
The particle size, polydispersity index (PDI), and zeta potential of nanoparticles were measured using dynamic light scattering with a Zeta sizer (particle sizing systems, Horiba, SZ-100, Japan). For this purpose, chitosan samples at the concentration range of 0.1-0.05 mg/mLwere dissolved in 1% v/v acetic acid solution, and TPP was used as a gelating agent. The effect of the concentration of chitosan and TPP as well as the stirring speed was studied. All the measurements were performed in triplicate and reported as the Mean±SD.
Morphological assessment
The morphology of nanoparticles was examined using the transmission electron microscope (TEM) (Temad Kala, Iran). For this purpose, we dispersed the powder in a solvent (acetic acid 0.1%).
Ciprofloxacin-loaded chitosan nanoparticles
The best chitosan nanoparticle in terms of size was used for loading. Ciprofloxacin 1 mL/mg was prepared in 0.1% (v/v) acetic acid. After that, 1 mg of chitosan was dissolved, and then, a specific volume of TPP solution was added while stirring at room temperature (6000 rpm) until faint turbidity was observed showing the formation of nanoparticles [16].
Loading efficiency (LE) and encapsulation efficiency (EE)
The LE of ciprofloxacin and EE of nanoparticles were determined according to the procedure reported by Cevher et al. (2006) [17]. After drug loading, nanoparticles were separated from the suspension by ultracentrifugation (Froilabo, France) at 15500 rpm and 4°C for 30 minutes. The amount of free ciprofloxacin in the supernatant was measured by UV-Vis spectrophotometer (Sco Tech, Germany) at 264 nm. The encapsulation efficiency (EE) was calculated by Equation 2 and Equation 3:

All analyses were carried out in triplicate [16].
In vitro drug release study
For this purpose, 2 mg of ciprofloxacin-loaded chitosan nanoparticles were suspended in separate tubes containing equal volumes of 0.2 mol/L PBS solutions (pH 7.4) and incubated while stirring at 37°C and 100 rpm. At appropriate time intervals (1, 2, 4, 8, 12, 24, 48, and 72 hours), one tube was removed and the sample was centrifuged at 15000 g and 14°C for 30 minutes. The amount of released drug from NPs was determined by measuring the absorbance of the supernatant within time intervals at 264 nm by a UV spectrophotometer (Multiskan GO, Thermo Scientific) using a standard curve [18].
Specimen collection
During the study period from Nov 2018 to Oct 2019, P. aeruginosa isolates were collected from clinical specimens of Shahid Mohammadi teaching hospital affiliated with the Hormozgan University of Medical Sciences (HUMS) at Bandar Abbas located in the south of Iran. Bacterial isolates were identified based on the biochemical tests, including gram staining, growth on MacConkey agar, citrate utilization, oxidation-fermentation, and oxidase test. Ciprofloxacin-resistant isolates were screened by 5 µg ciprofloxacin disk using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guideline [19].
Biofilm formation
The abilities to produce biofilm of all isolates were analyzed using a colorimetric microtiter method. In order to quantitatively determine biofilm formation capacity, bacterial colonies were grown overnight at 37°C in Tryptic Soy Broth (TSB) (Merck Darmstadt, Germany). The bacterial suspensions were diluted (1:100) in a new TSB medium and 150 μL of this was added to the sterile flat-bottomed 96-well polystyrene microplates followed by incubation for 24 hours at 37°C. The wells were washed with distilled water three times. After drying the wells at room temperature, they were stained with 125 μL of 0.1% crystal violet solution (CV) for 10-15 minutes. The CV was thrown away and the wells were washed three times to remove excess CV. Finally, the bounded CV was released by the addition of 125 μL of 30% acetic acid. A new sterile plate was inoculated with 125 μL of detection solution per well. The optical density (OD) absorbance of each well was measured at a wavelength of 550 nm using an ELISA reader (Biotek elx800). All the assays were repeated based on Equation 4 and Table 2 [20].

4. Optical density cut-off value (ODc)=Average OD of negative control+3×SD of negative control
MBIC and MBEC determinations
MBIC and MBEC experiments were performed using a modified version of the Calgary biofilm device method [21, 22]. In brief, 20 mL108 c.f.u. mL-1 bacterial suspensions were added to 180 mL1% TSB, placed into a sterile 96-well polystyrene flat-bottom microtitre plate, and incubated overnight at 37°C without shaking, to allow bacterial attachment. Non-adherent cells were removed by gentle washing three times with sterile saline solution (150 mL 0.9 % NaCl). The plates were air-dried for 15 minutes. Serial twofold dilutions of each antimicrobial agent ciprofloxacin, ciprofloxacin-PAβN, CS ciprofloxacin, and CS ciprofloxacin- PAβN in Mueller–Hinton broth (MHB) were added to the microplates followed by incubation at 35°C for 24 hours. MBIC was defined as the minimal antimicrobial concentration, at which there was no observable bacterial growth in wells containing adherent microcolonies, the minimal concentration that inhibited the release of planktonic bacteria from the biofilm. After MBIC measurement, the broth was removed and wells were washed three times with sterile saline solution (150 mL 0.9 % NaCl) and antimicrobial-free MHB was added, followed by incubation for 24 hours at 35°C. MBEC was defined as the minimal antimicrobial concentration, at which bacteria fail to regrow after antimicrobial exposure, i.e. the minimal concentration required for eradicating the biofilm. All determinations were performed in duplicate [23].
Results
One of the best practical methods for calculation of the MW of polymeric samples is the intrinsic viscosity measurements, using the Mark-Houwink-Sakurada equation (Equation 5). Based on the results, the y-intercepts of Ln ɳrel/c versus concentration curve, stands for intrinsic viscosity, were 0.572 and 0.066 for HMW and LMW samples, respectively. The viscosity-based average of MW of HMW and LMW samples were calculated as 478.84 and 47.79 KD, respectively.
5. [ɳ]=KMa
The structures of acetylated and deacetylated monomers of chitosan as well as the 1H-NMR spectra of LMW and HMW chitosan samples are presented in Figure 1 and Figure 2. In the highly deacetylated samples, H1-A (5 ppm<δ<5.5 ppm) is not visible in the spectrum and the use of the acetyl group peak (H-Ac) presents two advantages over the use of the H1 peak of acetylated monomer (H1-A). First, the H-Ac peak is three times more intense than the H1-A peak and second, the H-Ac peak is well resolved with a flat baseline on each side of the peak.


There are several equations that can be used to determine deacetylation and acetylation degree. In the current project, based on the Equation 6, degree of deacetylation of about 92% for depolymerized chitosan (LMW sample) and 95% for parent molecule (HMW sample) was calculated indicating that polymerization process did not affect the degree of deacetylation significantly.
6. DD=[1-(1/3 ICH3)/(1/6 IH2-H6)]×100
Histidine was conjugated to the chitosan backbone by the reaction of the carboxyl group on histidine to amine groups of chitosan through an NHS- and EDC-mediated reaction. In order to determine the degree of histidine modification, 1H-NMR spectroscopy was carried out. The spectra of His-CS samples are shown in Figure 3 and Figure 4. The imidazole group of histidine showed a characteristic peak at 7-9 ppm, which was used to determine the degree of modification according to equation 1. The results are shown in Table 3.

In the case of histidine modification, the degree of modification was about 24-28% for both Cs-His derivatives. The reason for the low percentage of modification could be the acidic pH of the reaction (4.5), in which most of the amine groups are protonated.


The average size of chitosan nanoparticles in an aqueous medium was measured by dynamic laser light scattering for the prepared samples. In this study, all compounds were less than 1000 nm in size. Formula 12 had a smaller mean particle size (164.7±11.24 nm) and was selected for further studies (Figure 5).

TEM has been used as a powerful technique to characterize the morphology and the internal structure of the nanoparticles. The results of the TEM study showed that the nanoparticles had an almost spherical shape, smooth surface, and an approximate size range of 75-200 nm (Figure 6).

Ciprofloxacin loading efficiency was 35.51% and encapsulation efficiency was 55.06%.
The release profile of ciprofloxacin from ciprofloxacin-loaded nanoparticles at pH 7.4 is displayed in Figure 7. After 4 hours, 53% of the drug was released from CS nanoparticles and more than 80% of the drug was released in 24 hours.

The collected bacteria were isolated from different clinical samples. The highest rate of bacterial isolation from urine culture was 43.3% and the lowest rate of blood infection and CSF was 10%. The relative abundance of P. aeruginosa isolates from different clinical specimens is shown in Figure 8. P. aeruginosa can produce different color pigments. In this study, four types of pigments and 20% of isolates did not contain color pigments.

Biofilm production was performed using a standard and quantitative method proposed by Christensen et al. The numerical results obtained from absorption at 570 nm by ELISA were stored in Excel software for quantitative calculation of biofilm production and calculated according to the following formula. Twenty-nine (96.7%) isolates were able to produce biofilm, while 30.1% of the samples had strong biofilm (Figure 9).

Based on the results of the MBIC test listed in Table 4, the effective concentrations for ciprofloxacin were in the range of 40-5120 μg/mL. However, with the addition of PAβN inhibitor, a decrease was observed in the MBIC range. The best effect was found in the antibiotic group ciprofloxacin and chitosan.

Table 5 shows the results of MBEC. The highest frequency was related to the antibiotic ciprofloxacin 2560 μg/mL, but in combination with PAβN inhibitor, the highest frequency was reported at a concentration of 640 μg/mL. For the chitosan-loaded drug, the highest frequency of MBEC results was recorded at a lower concentration of 40 μg/mL.

The ciprofloxacin loaded on chitosan with an efflux pump inhibitor showed the highest frequency at 320 μg/mL. MBEC test in strains with strong biofilm showed the highest frequency at a concentration of >5120 μg/mL and the lowest frequency at a concentration of 1280 μg/mL. MBEC and MBIC of samples 21 and 18 were similar to strong biofilms despite producing moderate biofilms at the high concentration range. MBEC results showed that the effect of ciprofloxacin loaded on chitosan on biofilm in 19(65.5%) isolates was in the range of 160 μg/mL and less. Also, after adding an inhibitor to this compound, 160(86.2%) MBEC isolates of 160 μg/mL or less were recorded. MBIC50 was recorded for the antibiotic ciprofloxacin (640 μg/mL). However, with the addition of PAβN inhibitor, a decrease was observed in the MBIC range; thus, MBIC50 was reduced to 320 μg/mL for the combination of ciprofloxacin antibiotic and efflux pump inhibitor. The best effect of the ciprofloxacin-loaded antibiotic group in chitosan with MBIC50 was at 40 μg/mL. Ciprofloxacin loaded with chitosan with an efflux pump inhibitor was less effective than the previous group but was better than ciprofloxacin alone with the inhibitor and the MBIC50 of this group was set at 80 μg/mL. MBEC50 different antibiotic groups of ciprofloxacin, ciprofloxacin and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor were calculated at 1280, 640, 160, and 320 μg/mL, respectively. As shown in Figure 10, the concentration in samples with strong biofilm was higher than in isolates with moderate or weak biofilm.

Discussion
Today, the ecological role of biofilms in the development of healthcare-related infections is well established and has a significant impact on medicine. The National Institutes of Health (NIH) estimates that bacterial biofilms are involved in 65% of microbial diseases and more than 80% of chronic infections [24]. The biofilm-associated infections are an enormous challenge in healthcare. The development of a unique drug delivery system targeting the bacterial cells within the biofilm and dispersing the biofilm structure is an urgent necessity. Recently, more studies have focused on the use of nanoparticles for the inhibition of biofilm formation and also the detachment of mature biofilms [25]. Today, combining nanodrugs with antibiotics is an effective delivery system that protects antibiotics against enzyme degradation and increases the effectiveness of the drug [26]. Chitosan nanoparticles are well established for drug delivery and have many advantages, including crossing biological barriers, large-scale non-degradation of molecules, controlled release of the drug to the desired site, and uptake through endocytosis due to their small size [9]. In 2019, Alqahtani et al. reported the efficiency of diclofenac encapsulation in chitosan as 29.3%, which due to the type of antibiotic, a different percentage was recorded compared to the present study [7]. Drug loading on chitosan was reported by 87.4% in the study by Kucukoglu et al., which was higher than the results of our study [27]. Porras-Gómez et al. reported that the efficiency of imipenem encapsulation in chitosan was 41.65%, which was close to the results of this study, but due to differences in the type of antibiotic, the results could be different [28]. In our study, the rapid release of ciprofloxacin in the first hour was about 2% but after 4 hours, it was approximately 53%, which did not correspond to the results of Al-Qattani et al. [7]. In the present study, approximately 82-86% of the drug was released after 24 to 72 hours. Kucukoglu et al. similar to our study, reported that chitosan was released after a maximum of 24 hours [27]. In the study by Porras-Gómez, the release of imipenem peaked after 24 hours and about 40% of the drug was released after 24 hours [28]. Only in the study by Sahi et al., penicillin was loaded into chitosan, and they reported initial drug release in the first 2 hours and the maximum release after 24 hours was about 88%, which was consistent with the results of our study [29]. On the contrary, Singh et al. reported the highest release of ciprofloxacin from chitosan after 8 hours, which was about 99% and is consistent with the results of our study [18]. Different rates of drug release in different studies depend on using different concentrations of TPP. Also, the more drug is loaded, the higher the drug release rate; thus, more continuous release can be eliminated by increasing the drug load. The particle size plays a crucial role in nanoparticle properties and therefore an essential task in the property characterization of nanoparticles is particle sizing. For example, the melting point of nanoparticles is decreased when the size is reached the nanometer scale [30]. Al-Qahtani et al. reported a particle size of about 295.33 nm, which was larger than the particle size synthesized in this study [7]. In a cultural study, a chitosan particle was loaded into ciprofloxacin with a size of 260 nm [31]. In the study by Porras-Gómez et al., the particle size of chitosan nanoparticles containing imipenem antibiotics was reported at 127.77 nm [28]. These results are close to the data of the present study. The average particle size synthesized in the study by Safhi et al. was about 360.4 nm, which is more than the particle size in this study [29]. Particle size was reported in the study by Singh et al. as 100 to 200 nm, which was consistent with our results [18]. According to our results, 96.7% of the P. aeruginosa isolates made biofilms, of which 30.1% were strong biofilm producers. In agreement with our results, Ghanbarzadeh et al. [32]and Jabalameli et al. [33] reported a biofilm rate of 92.4 and 96.9% for the isolates, respectively. Fluoroquinolones, like ciprofloxacin, are the primary alternative as antibiofilm antibiotics for treatment. Our results showed MBIC values of ciprofloxacin ranging from 80 μg/mL to >5120 μg/mL in P. aeruginosa strains, also the range for MBEC was 160 μg/mL to >5120 μg/mL. Over the last few years, various compounds are synthesized and investigated for their inhibitory activity against P. aeruginosa efflux pumps. PAβN, a broad-spectrum EPI, is shown to potentiate fluoroquinolones and levofloxacin, by out-competing the antibiotics for the pumps [5]. Several studies reported synergistic activities of PaβN and antibiotics against P. aeruginosa and biofilm growth. Quorum sensing operates the discharge of P. aeruginosa virulence factors and also the synthesis of biofilm throughout infection. PAβN significantly reduced quorum sensing signal in P. aeruginosa [34]. The mixture of ciprofloxacin and PAβN resulted in the largest decrease in biofilm mass (2 folds and more) compared with the ciprofloxacin alone. Chitosan could be a natural mucoadhesive polymer with antibacterial activity. We prepared nanoparticles of chitosan by ionic gelation technique containing ciprofloxacin to judge the possible changes within the efficiency of antibacterial agent. Our results revealed the importance of ciprofloxacin-loaded chitosan as they need strong antibiofilm activity against resistant bacteria, like P. aeruginosa. Ciprofloxacin+chitosan was also able to reduce MBIC by 2–4-fold in all isolates, compared to free ciprofloxacin. Additionally, they reduced MBEC in 28 of the tested isolates (96.55%). To our knowledge, there are no data on the antibiofilm activity of chitosan-loaded ciprofloxacin in combination with PAβN. According to previous studies, concentrations of MBIC for ciprofloxacin, ciprofloxacin+PaβN, and chitosan+ciprofloxacin were 640, 320 and <40 µg/mL, respectively. The vast majority of MBEC for ciprofloxacin, ciprofloxacin+PaβN, and chitosan+ciprofloxacin was 2560, 640, and 160 µg/mL, respectively, and the rate of MBEC for a combination of chitosan+ciprofloxacin+PAβN was <40 µg/mL. Our data revealed that MBIC for ciprofloxacin was less than 160 μg/mL in 31% of the strains. After adding the inhibitor to ciprofloxacin, the number of strains at this concentration reached 44.7%. Also, the MBIC of ciprofloxacin loaded in chitosan in 96.6% of strains was in the range of 160 μg/mL or lower concentrations. MBIC50 of different antibiotic groups of ciprofloxacin, ciprofloxacin, and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor were calculated at 640, 320, 40, and 80 μg/mL, respectively. Kashef et al. assessed the biofilm of S. aureus and showed a reduction of 2 to 4 folds in MBIC for ciprofloxacin loaded into the niosome [35]. MBEC50 of different antibiotic groups of ciprofloxacin, ciprofloxacin and efflux pump inhibitor, ciprofloxacin loaded in chitosan, and ciprofloxacin loaded in chitosan with efflux pump inhibitor was calculated at 1280, 640, 160, and 320 μg/mL, respectively. Collectively, the results of this study showed that the ciprofloxacin-loaded chitosan had the best effect on penetrating the biofilm and killing the bacteria trapped in the biofilm. A comparison of MIC50 and MBIC50 showed that 10 to 160 times more antibiotics are needed to inhibit biofilm. These ratios were also calculated to be 20 to 160 times higher for MBC50 and MBEC50 in different groups of antibiotics. Recently, assessment of the effect of chitosan-streptomycin on P. aeruginosa biofilm showed that it removed more than 70% of the biofilm mass, while streptomycin was only able to remove 22% of the cell mass [3]. In the MBEC study, it appears that the intramolecular hydrogen bond between the efflux pump inhibitor and the chitosan, followed by the formation of a large and heavy structure, disrupts the efflux of food in the biofilm channels. As a result, the ciprofloxacin loaded in chitosan with an efflux pump inhibitor had the best results.
Conclusion
The findings of this study provided more evidence for the significance of antibiotics-chitosan against resistant infections. But the precise mechanisms that lead to the synergistic result of chitosan and antibiotics-chitosan combos are largely unknown. Understanding these mechanisms is essential for overcoming treatment failure against resistant bacteria. Such information encourages additional studies with polysaccharide derivatives and alternative antimicrobial categories and in vivo animal experiments to validate these attention-grabbing findings before clinical tests will move forward.
Ethical Considerations
Compliance with ethical guidelines
The project was approved by the Research Center of the Shiraz Branch of Islamic Azad University (Code: 16330507962020).
Funding
This study was funded by Shiraz Branch of the Islamic Azad University.
Authors' contributions
All authors equally contributed in preparation of the article.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
The authors acknowledged the Islamic Azad University of Shiraz Branch for providing the research resources.
References
- Rehman A, Patrick WM, Lamont IL. Mechanisms of ciprofloxacin resistance in pseudomonas aeruginosa: New approaches to an old problem. J Med Microbiol. 2019; 68(1):1-10. [DOI:10.1099/jmm.0.000873] [PMID]
- Zamani H, Rahbar S, Garakoui SR, Afsah Sahebi A, Jafari H. Antibiofilm potential of lactobacillus plantarum spp. cell free supernatant (CFS) against multidrug resistant bacterial pathogens. Pharm Biomed Res. 2017; 3:39-44. [DOI:10.29252/pbr.3.2.39]
- Li R, Yuan X, Wei J, Zhang X, Cheng G, Wang ZA, et al. Synthesis and evaluation of a chitosan oligosaccharide-streptomycin conjugate against pseudomonas aeruginosa biofilms. Mar Drugs. 2019; 17(1):43. [DOI:10.3390/md17010043] [PMID] [PMCID]
- Ahmed MN, Porse A, Sommer MOA, Høiby N, Ciofu O. Evolution of antibiotic resistance in biofilm and planktonic pseudomonas aeruginosa populations exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 2018; 62(8):e00320-18. [DOI:10.1128/AAC.00320-18] [PMID] [PMCID]
- Reza A, Sutton JM, Rahman KM. Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in gram-negative (ESKAPEE) bacteria. Antibiotics. 2019; 8(4):229. [DOI:10.3390/antibiotics8040229] [PMID] [PMCID]
- Rampioni G, Pillai CR, Longo F, Bondì R, Baldelli V, Messina M, et al. Effect of efflux pump inhibition on pseudomonas aeruginosa transcriptome and virulence. Sci Rep. 2017; 7(1) :11392. [DOI:10.1038/s41598-017-11892-9] [PMID] [PMCID]
- Alqahtani FY, Aleanizy FS, Tahir EE, Alquadeib BT, Alsarra IA, Alanazi JS, et al. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm J. 2019; 27(1):82-7. [DOI:10.1016/j.jsps.2018.08.001] [PMID] [PMCID]
- Karami N, Kamkar A, Shahbazi Y, Misaghi A. Edible films based on chitosan-flaxseed mucilage: In vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm Biomed Res. 2019; 5(2):10-6. [DOI:10.18502/pbr.v5i2.1580]
- Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Let. 2018; 16:101-12. [DOI:10.1007/s10311-017-0670-y]
- Aurestila B, Villaver E, Tan E. Anti-biofilm activity of chitosan from crab and shrimp species indigenous to the philippines on established biofilms of pseudomonas aeruginosa and staphylococcus aureus. J Pharmacogn Nat Prod. 2018; 4:149. [DOI:10.4172/2472-0992.1000149]
- Xie H, Jia Z, Huang J, Zhang C. Preparation of low molecular weight chitosan by complex enzymes hydrolysis. Int J Chem. 2011; 3(2):180-6. [DOI:10.5539/ijc.v3n2p180]
- Roberts GAF. Structure of chitin and chitosan. In: Roberts GAF (editor). Chitin Chemistry. London: Palgrave; 1992. [DOI:10.1007/978-1-349-11545-7_1]
- Zheng X, Yin Y, Jiang W, Xing L, Pu J. Synthesis and characterization of low molecular weight chitosan. BioRes. 2015; 10:2338-49. [Link]
- Abbad S, Zhang Z, Waddad AY, Munyendo WL, Lv H, Zhou J. Chitosan-modified cationic amino acid nanoparticles as a novel oral delivery system for insulin. J Biomed Nanotechnol. 2015; 11(3):486-99. [DOI:10.1166/jbn.2015.1924] [PMID]
- Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Appl Polymere. 1997; 63(1):125-32. [DOI:10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4]
- Sobhani Z, Mohammadi Samani S, Montaseri H, Khezri E. Nanoparticles of chitosan loaded ciprofloxacin: Fabrication and antimicrobial activity. Adv Pharm Bull. 2017; 7(3):427-32. [DOI:10.15171/apb.2017.051] [PMID] [PMCID]
- Cevher E, Orhan Z, Mülazımoğlu L, Şensoy D, Alper M, Yıldız A, et al. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with prepared microspheres. Int J Pharm. 2006; 317:127-35. [DOI:10.1016/j.ijpharm.2006.03.014]
- Singh K, Mishra A, Singh A. Synthesis characterization and in vitro release study of ciprofloxacin-loaded chitosan nanoparticle. Bionanoscience. 2018; 8:229-36. [DOI:10.1007/s12668-017-0470-7]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. Wayne: Clinical and Laboratory Standards Institute; 2017. [Link]
- O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011; (47):2437. [DOI:10.3791/2437] [PMID] [PMCID]
- Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999; 37(6):1771-6. [DOI:10.1128/JCM.37.6.1771-1776.1999] [PMID]
- Cafiso V, Bertuccio T, Spina D, Purrello S, Stefani S. Tigecycline inhibition of a mature biofilm in clinical isolates of Staphylococcus aureus: Comparison with other drugs. FEMS Immunol Med Microbiol. 2010; 59(3):466-469. [DOI:10.1111/j.1574-695X.2010.00701.x] [PMID]
- Reiter KC, Villa B, Paim T, de Oliveira CF, d’Azevedo PA. Inhibition of biofilm maturation by linezolid in meticillin-resistant staphylococcus epidermidis clinical isolates: Comparison with other drugs. J Med Microbiol. 2013; 62(3):394-9. [DOI:10.1099/jmm.0.048678-0] [PMID]
- Olivares E, Badel-Berchoux S, Provot C, Prévost G, Bernardi T, Jehl F. Clinical impact of antibiotics for the treatment of pseudomonas aeruginosa biofilm infections. Front Microbiol. 2020; 10:2894. [DOI:10.3389/fmicb.2019.02894] [PMID] [PMCID]
- Tan Y, Ma S, Leonhard M, Moser D, Haselmann GM, Wang J, et al. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr Polym. 2018; 200:35-42. [DOI:10.1016/j.carbpol.2018.07.072] [PMID]
- Van Giau V, An SSA, Hulme J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des Devel Ther. 2019; 13:327-43. [DOI:10.2147/DDDT.S190577] [PMID] [PMCID]
- Kucukoglu V, Uzuner H, Kenar H, Karadenizli A. In vitro antibacterial activity of ciprofloxacin loaded chitosan microparticles and their effects on human lung epithelial cells. Int J Pharm. 2019; 569:118578. [DOI:10.1016/j.ijpharm.2019.118578] [PMID]
- Porras-Gómez M, Vega-Baudrit J, García F, Núñez-Corrales S, Madrigal-Carballo S. Evaluation of the synergistic effect of EDTA-functionalized chitosan nanoparticles on imipenem delivery in pseudomonas aeruginosa carbapenem-resistant strain AG1. J Biomater Nanobiotechnol. 2017; 9:64-78. [DOI:10.4236/jbnb.2018.91006]
- Safhi M, Sivakumar S, Jabeen A, Zakir F, Islam F, Barik B. Chitosan nanoparticles as a sustained delivery of penicillin G prepared by ionic gelation technique. J Pharm Res. 2014; 8:1352-4. [Link]
- Akbari B, Tavandashti MP, Zandrahimi M. Particle size characterization of nanoparticles-a practicalapproach. Iran J Mater Sci Eng. 2011; 8(2):48-56. [Link]
- Farhangi M, Kobarfard F, Mahboubi A, Vatanara A, Mortazavi SA. Preparation of an optimized ciprofloxacin-loaded chitosan nanomicelle with enhanced antibacterial activity. Drug Dev Ind Pharm. 2018; 44(8):1273-84. [DOI:10.1080/03639045.2018.1442847] [PMID]
- Ghanbarzadeh Corehtash Z, Khorshidi A, Firoozeh F, Akbari H, Mahmoudi Aznaveh A. Biofilm formation and virulence factors among pseudomonas aeruginosa isolated from burn patients. Jundishapur J Microbiol. 2015; 8(10):e22345. [DOI:10.5812/jjm.22345] [PMID] [PMCID]
- Jabalameli F, Mirsalehian A, Khoramian B, Aligholi M, Khoramrooz SS, Asadollahi P, et al. Evaluation of biofilm production and characterization of genes encoding type III secretion system among pseudomonas aeruginosa isolated from burn patients. Burns. 2012; 38(8):1192-7. [DOI:10.1016/j.burns.2012.07.030] [PMID]
- El-Shaer S, Shaaban M, Barwa R, Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. J Med Microbiol. 2016; 65(10):1194-204. [DOI:10.1099/jmm.0.000327] [PMID]
- Kashef MT, Saleh NM, Assar NH, Ramadan MA. The antimicrobial activity of ciprofloxacin-loaded niosomes against ciprofloxacin-resistant and biofilm-forming staphylococcus aureus. Infect Drug Resist. 2020; 13:1619-29. [DOI:10.2147/IDR.S249628] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
General
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









