Volume 9, Issue 2 (2023)
Pharm Biomed Res 2023, 9(2): 77-84 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saberi-Hasanabadi P, Malekshah O M, Mohammadi H. The Exposure and Hazards of Zinc Oxide Nanoparticles: In Vitro and In Vivo Studies. Pharm Biomed Res 2023; 9 (2) :77-84
URL: http://pbr.mazums.ac.ir/article-1-484-en.html
URL: http://pbr.mazums.ac.ir/article-1-484-en.html
1- Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Pharmaceutics, Rutgers, The State University of New Jersey, Piscataway, United States.
3- Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Pharmaceutics, Rutgers, The State University of New Jersey, Piscataway, United States.
3- Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 1230 kb]
(670 Downloads)
| Abstract (HTML) (799 Views)
Full-Text: (485 Views)
Introduction
Today, with the increasing development of nanotechnology, the environmental accumulation of nanomaterials and the exposure of humans to these nanoparticles are unavoidable [1]. Safety evaluation or toxicity assessment of various synthesized nanoparticles have been reported in different studies [2, 3, 4, 5, 6]. With the gradual increase in the use of zinc oxide nanoparticles in biomedical fields, its subsequent release in the environment and direct exposure to the human body is inevitable. Despite the widespread use of zinc oxide nanoparticles, the safety of this nanomaterial for humans is still unclear. The reported side effects of zinc-based nanoparticles have cast doubts about their use [7]. The current state of knowledge concerning the application of zinc oxide nanoparticles in the biomedical field is still in the preliminary stage. Despite numerous studies on zinc toxicity, there is a lack of knowledge on the characteristics and effects of zinc-based nanoparticles [1, 7]. Due to their small size and larger surface area, zinc oxide nanoparticles have a higher penetration capacity into the human body than metallic zinc [7]. These compounds can have desirable biological activities, such as carrying capacity for biomedical treatment, penetrating cellular barriers to drug delivery, and undesirable side effects, such as cytotoxicity, induction of oxidative stress, cell dysfunction, or a combination of both [8].
Numerous studies have reported the toxicity, free radical production, and induction of programmed cell death due to the high amount of zinc nanoparticles in environments and living organisms [8, 9]. The two main ways of adsorption of the nanoparticles in living cells are active adsorption by endocytosis and passive adsorption through diffusion. The process of cell uptake, the site of accumulation within the cell, and the ability of nanoparticles to produce toxic effects depend on their size, chemical properties, electric charge, crystallization, shape, and solubility [10].
The toxicity of these nanoparticles can be evaluated through in vitro studies using cells or cell lines or in vivo exposure to experimental animal models [11, 12]. Laboratory studies are usually the first step in measuring the toxicity of nanoparticles. However, cell culture cannot fully assess the complexity and relationships between components of biological matrices in an organism since this category can record events difficult to investigate in laboratory research scales. Therefore, more in vivo studies are needed.
This review aims to provide a critical summary of recent scientific literature on the potentially hazardous effects of zinc oxide nanoparticles and information on the possible mechanisms of these nanoparticle-induced toxicity in vitro and in vivo models.
Evidence Acquisition
To gather relevant information, a review of published studies reporting mechanisms of action of zinc oxide nanoparticles-induced toxicity in vitro and in vivo models in Scopus, PubMed, and Google Scholar databases was undertaken. Keywords like “Zinc oxide nanoparticles”, “toxicity”, and “in vitro and in vivo experiments” were used. In this review, the most important published literature of the last 10 years (2011 to 2021), related to in vivo and in vitro evaluations of organ toxicity to zinc oxide nanoparticles using animal models, were collected, analyzed, and discussed. In addition, the information related to the possible mechanisms of toxicity caused by zinc oxide nanoparticles, along with the influencing variables in this process, was summarized.
Zinc Oxide Nanoparticles
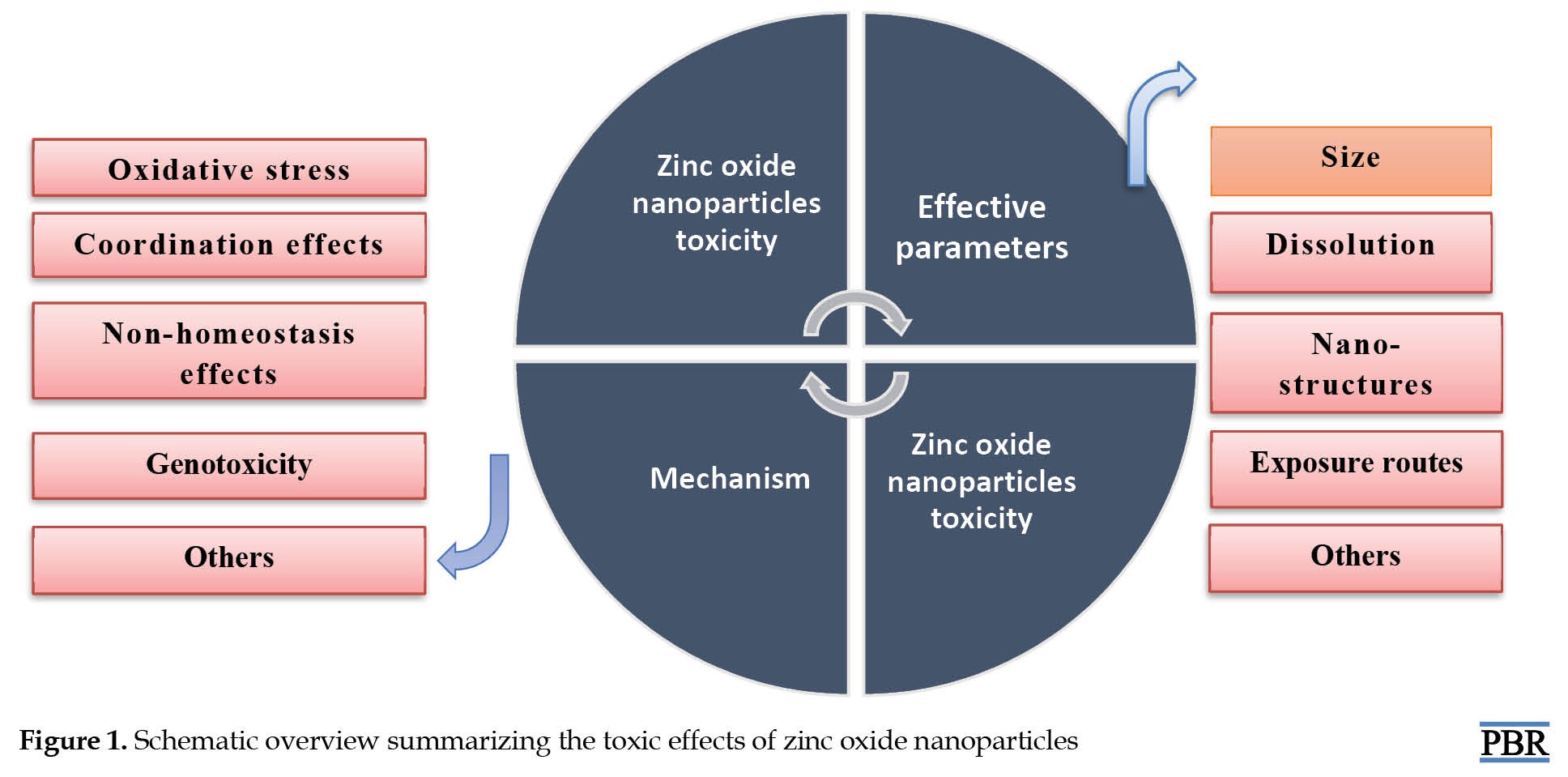
Among the metal oxide nanoparticles, zinc oxide nanoparticles have received considerable attention largely due to their various uses in the biomedical field—their enormous potential in disease diagnostics and monitoring. However, the increasing use of zinc nanoparticles has led to their release into the environment, and the toxicity of these compounds on organisms has become a concern in the biomedical research community [13]. The research conducted in this field shows that releasing zinc oxide nanoparticles may negatively affect organisms and the environment [13] (Figure 1). Toxic Mechanisms of Zinc Oxide Nanoparticles
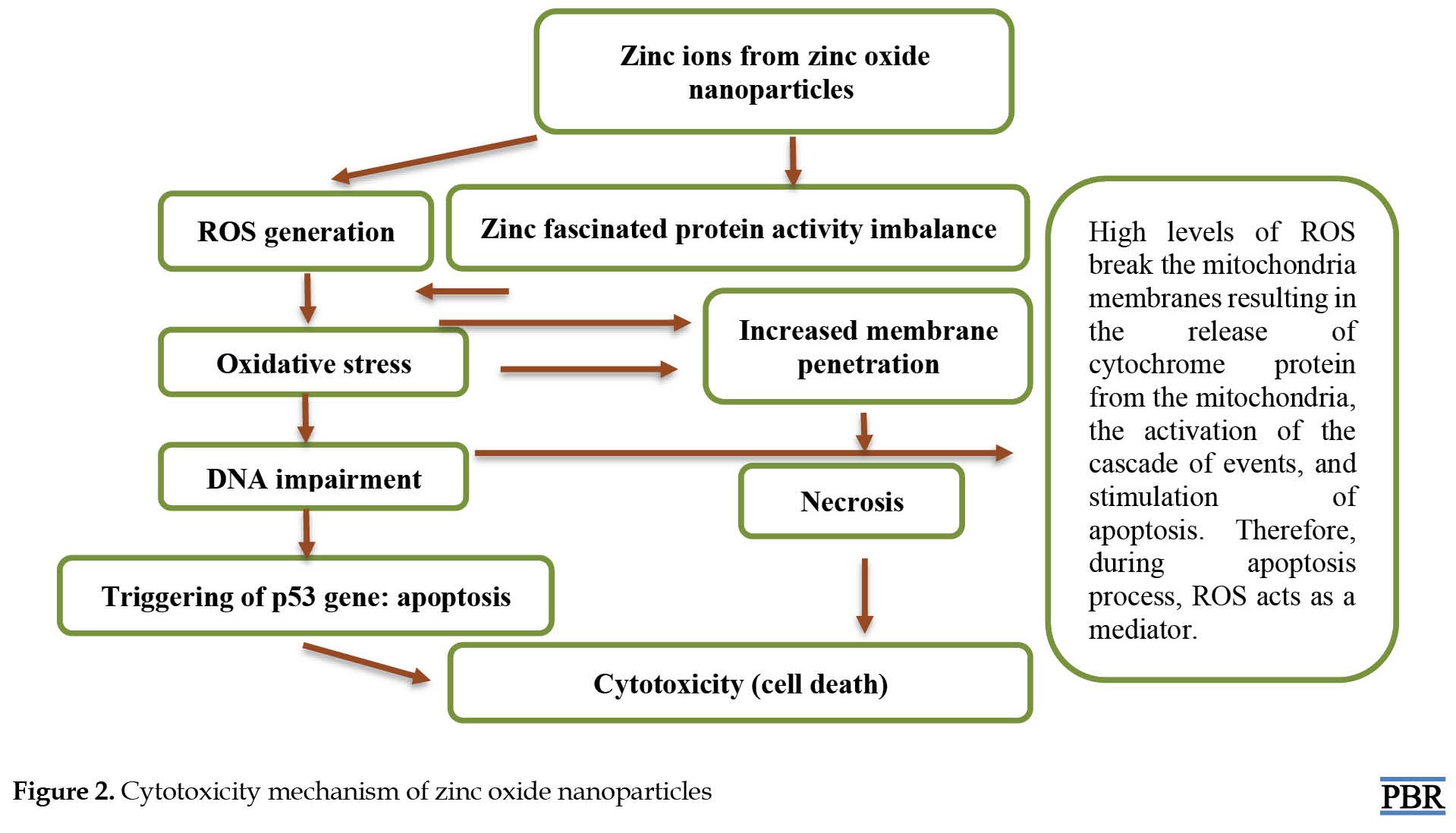
Zinc oxide nanoparticles are among the most important and multifunctional compounds. The ability of zinc oxide nanoparticles to release zinc is an important parameter from a risk assessment perspective [14]. Recent studies have shown that zinc oxide nanoparticles can be toxic to humans due to oxidative stress [14, 15], but the details and the full picture of their interplay have remained obscure. Figure 2 shows the possible role of zinc oxide nanoparticles in causing toxicity through reactive oxygen species (ROS) in biological organs. Mitochondria produce ROS as a by-product of metabolism, and they react as transport molecules in many important cellular activities such as gene transcription, signal transduction, and the immune response [11]. Furthermore, ROS has been suggested as an underlying reason for proliferative effects observed in human leukemia cells at low zinc concentrations [10]. Oxidative stress is a disorder of the oxidant-antioxidant balance, which disrupts normal cellular functions and leads to cytotoxicity or DNA damage [7]. Oxidant-antioxidant imbalance can result from a lack of antioxidant capacity due to impaired production and distribution of reactive oxygen species or their excess in relation to other factors. These events may cause oxidative damage to DNA, increasing the chromosomal aberrations associated with cell deformation [7]. Oxidative stress and its damage indicate an association between different forms of chronic liver damage [8]. In a study, the toxic effects of zinc-based nanoparticles in adult mice showed that high concentrations of these nanoparticles cause the cells to use different enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione S-transferase to remove ROS [9]. Superoxide dismutase can convert internal oxygen radicals into H2O2. In addition, catalase and glutathione peroxidase enzymes can reduce oxygenated water to water and oxygen. Therefore, superoxide dismutase, catalase, and glutathione peroxidase can keep oxygen levels low and prevent cell poisoning [9]. The excessive generation of various deleterious ROS like hydrogen peroxide, hydroxyl radical species, nitric oxide or superoxide anion as a result of zinc nanoparticles exposure may result in oxidative damages on DNA, RNA, proteins, and so on.
In general, zinc-based nanoparticles were found to induce oxidative DNA damage, inflammation, cell degeneration, cell cycle arrest, cytogenetic alterations, and apoptosis. Cytotoxicity studies have shown that zinc-based nanoparticles have dose- and time-dependent cytotoxic potential, genotoxicity, and carcinogenicity [9, 10].
In vitro toxicity studies
The in vitro studies on the toxicity of zinc oxide nanoparticles confirmed the induction of diverse adverse effects in pulmonary-blood pathways and central nervous system-derived cell toxicities [14]. This process can be related to excessive dissolved ionic Zn2+ in the culture medium or inside cells, leading to oxidative stress, cytotoxicity, and mitochondrial dysfunction [15, 16]. In this line, Kao et al. investigated an olfactory bulb-brain translocation pathway for zinc oxide nanoparticles in rodent cells in vitro and in vivo conditions. They concluded that an olfactory bulb-brain translocation pathway for airborne zinc oxide nanoparticles exists in rats and that endocytosis is required for the interneuron translocation of these particles [15]. Coating zinc oxide nanoparticles with polymeric compounds may reduce cytotoxicity effects and ROS generation in WIL2-NS cell lines in humans [15]. Further studies on in vitro exposure to zinc oxide nanoparticles are summarized in Table 1.

In many references, zinc oxide nanoparticles are also considered the most toxic nanoparticles with the lowest LD50 value among the other metal oxide nanoparticles. In this line, Cao et al. evaluated zinc oxide nanomaterials’ developmental and neurotoxic effects with different shapes/sizes compared to zebrafish and SH-SY5Y cells. They found the LD50 of treated zebrafish larvae with zinc oxide nanoparticles was 11 μg/mL for 144 h [10]. The LD50 values were calculated to compare the toxicity of three zinc oxide nanomaterials. These values for short and long zinc oxide nanorods were estimated at 15.4 μg/mL and 17 μg/mL, respectively. In another study, Ng et al. investigated the acute toxicological effects of zinc oxide nanoparticles in mice after intratracheal instillation. Their results also showed that the LD50 in the intratracheal instillation of zinc oxide nanoparticles (48 nm) was 493.85 μg/kg body weight [11].
In vivo toxicity studies
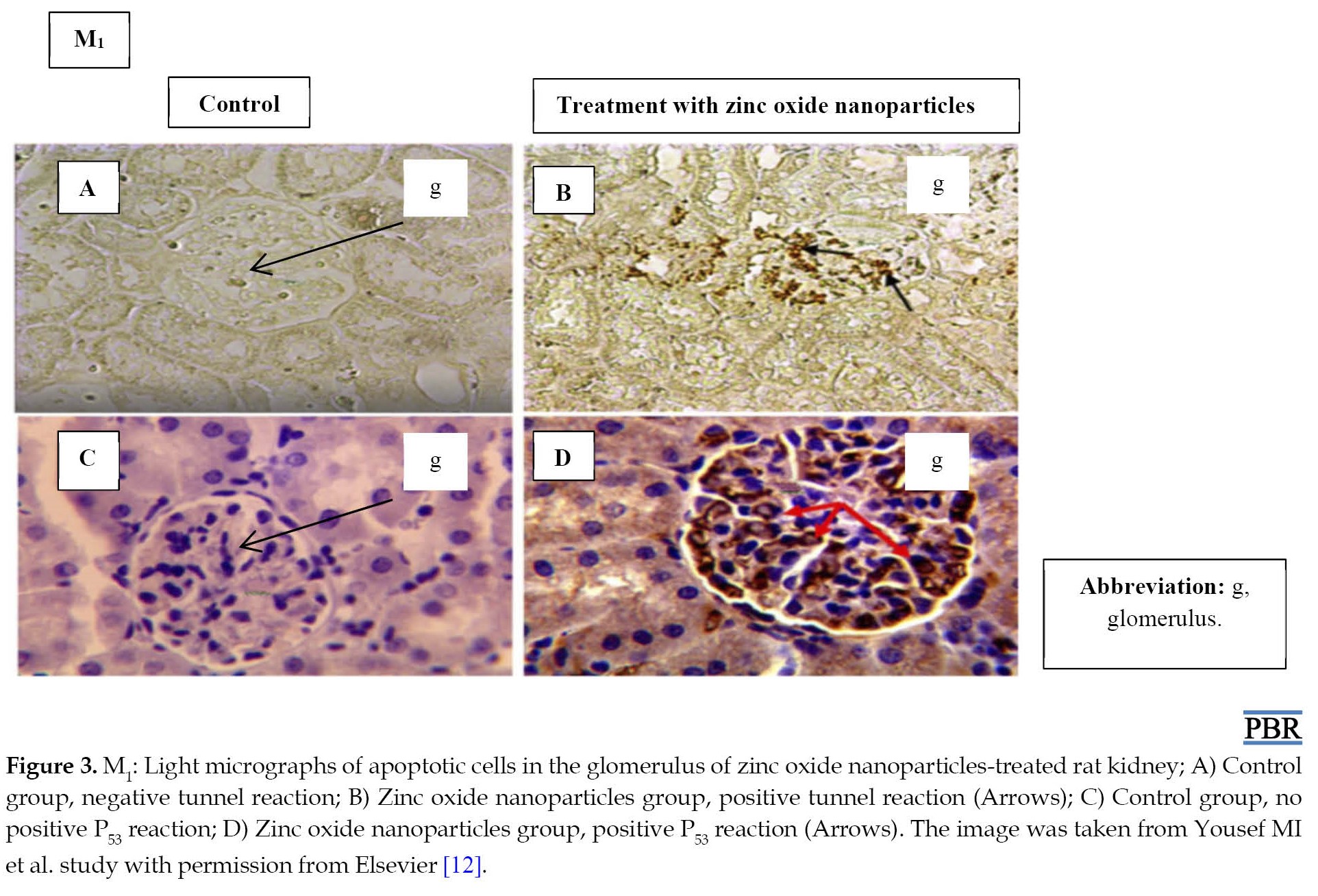
Toxicology results after exposure to zinc oxide nanoparticles are different and depend on various parameters [17, 18]. Particle penetration is mainly determined by their physicochemical properties, especially particle size. The zinc oxide nanoparticles toxicity increases malondialdehyde and glutathione S-transferase activity and decreases total antioxidant levels in the blood serum and liver of treated rats. Finally, this process leads to the formation of free radicals and an increase in the rate of oxidative stress [10]. Therefore, the induction of enzymatic antioxidant defense after zinc oxide nanoparticles exposure can be a successful adaptive response. It is a compensatory mechanism that enables the cell to overcome the damage. Other inflammatory responses and immune system activation are also commonly suggested in biochemical observations after exposure to zinc oxide nanoparticles. Detrimental health effects were also observed through intraperitoneally administrated zinc oxide nanoparticles in rats. Most symptoms were nonspecific toxicity and caused more nerve damage in the caudate nucleus and hippocampus [11]. The treated rats with zinc oxide nanoparticles also showed different tissue changes, including vacuolation and pyknosis in renal tubular epithelial cells and glomerular damage. Zinc oxide nanoparticles caused ultrastructural changes in the proximal convoluted tubule of renal tubules and certain glomerular cells. These particles significantly increased cell apoptosis in rat kidneys (Figure 3) [12]. In this study, the size of zinc oxide nanoparticles was about 20 nm in diameter, which could facilitate their entrance into cells. Zinc oxide nanoparticles with a size less than 20 nm have been shown to accumulate in the liver and spleen of rats, while zinc oxide nanoparticles of larger size accumulate in the kidney [12]. In a study, Amara et al. investigated behavioral performances and the brain contents of some monoamine neurotransmitters with zinc oxide nanoparticles exposure. The results revealed that acute intravenous injection of zinc oxide nanoparticles does not affect neurotransmitter contents, locomotor activity, or spatial working memory in adult rats [17].
Pasupuleti et al. evaluated the toxicity effects of zinc oxide nanoparticles through the oral route [18]. Their experiments aimed to determine the importance of the affected dose (5, 50, 300, 1000, and 2000 mg/kg body weight) by comparing zinc oxide’s acute oral toxicological potential in dimensions (20 nm) to its micro size. In the nano-dimensions of zinc oxide, microscopic lesions in the liver, pancreas, heart, and stomach were more frequent in lower and higher doses. However, the incidence of the above lesions was higher in mice treated with higher doses of zinc oxide in micro dimensions. In this study, nano-sized zinc oxide showed more toxicity in lower doses. Therefore, they concluded that future nano-scale toxicological research should focus on the importance of dose exposure criteria [18]. The other effects of in vivo exposure to zinc oxide nanoparticles are summarized in Table 1.
Discussion
One of the main risks of application and exposure to zinc oxide nanoparticles is the dissolution and release of Zn2+ ions that are toxic to cells [18]. Among the effective cytotoxic effects of zinc oxide nanoparticles, we can mention the increase in ROS production along with lipid peroxidation, genotoxicity, and induction of inflammatory pathways [11]. The review of the published articles in this field shows that increased oxidative stress is associated with decreased antioxidant activity in the studied models. This problem is justified by lower levels of glutathione and total antioxidant capacity and inhibiting the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase [12, 19, 20]. Zinc oxide nanoparticles have been reported to interact with mammalian intracellular enzymes. These nanoparticles may disrupt these antioxidants’ defense mechanisms through ROS production. The development of this process leads to inflammatory response, disruption, and destruction of mitochondria. Finally, we will see apoptosis in human liver cells, lung epithelial cells, and even some cancer cells [22, 23, 27]. In a controlled environment (in vitro conditions), inflammatory status has been confirmed in many studies by higher circulatory levels of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 in the treated rats with zinc oxide nanoparticles at both mRNA and protein levels. Identifying zinc oxide nanoparticles as external particles by immune cells may produce ROS, reactive nitrogen species (RNS), signaling pathway disturbance, and altered cytokine levels [23, 27, 28].
Zinc oxide nanoparticle toxicity is affected by different factors, including the routes of exposure, physio-chemical properties, and environmental conditions, making comparing the data of various studies difficult. Environmental conditions in any organ may affect the toxicity potential of nanoparticles. For instance, the acidic environment in lung lining fluid may dissolve zinc oxide (1 and 5 mg/kg of body weight), leading to the transient increase in the concentration of Zn2+ ions and local toxicity. Their solubility determines zinc oxide nanoparticles’ toxicity. In lung injury, the main cause of zinc oxide nanoparticle-induced toxicity is pH-dependent dissolution inside phagosomes [12, 22].
In vivo conditions, physicochemical properties, nanofabrication, and biological factors (such as species, age, gender, and size of the tested animals) seem to be common factors that govern zinc oxide nanoparticles’ toxicity [9, 11]. Regarding the complexity and diversity of reactions related to human biological systems, the generalization of animal model results to humans is often inconclusive. Concentrations used or determined in animal models do not necessarily imply the negative effects of exposure to zinc oxide nanoparticles in humans. Very low mass concentrations of these nanoparticles are needed in diagnostic interventions in humans. Therefore, future studies should rely on using real concentrations of these nanoparticles in the environment to evaluate their toxicity in living organism models. Parameters such as exposure routes, physicochemical properties, and environmental conditions should also be considered in these studies. The lack of sufficient understanding of the mechanism of toxicity caused by zinc oxide nanoparticles is one of the main obstacles to their use in various applications. More attention should be paid to the efforts made in understanding the mechanism of toxicity caused by zinc oxide nanoparticles and its control methods.
Conclusion
It is necessary to continue monitoring the process of release and exposure of Zn-based nanoparticles and their harmful effects on living systems. Zinc oxide nanoparticles currently have a wide range of applications in cancer treatment, gene-drug delivery, and biological materials for tissue engineering due to wide band-gap semiconductors, which can readily absorb UV rays. With their unique properties, zinc oxide nanoparticles can damage cancer cells using the mechanism of selective cytotoxicity through oxidative stress via ROS generation. Oxidative damage caused by free radicals during the toxicity of zinc nanoparticles leads to a cascade effect. Ultimately, this effect will have adverse consequences in both in vitro and in vivo conditions (typically liver, lung, reproductive tract, kidney, central nervous system function, dysregulation, and reduced mitochondrial respiration). The main mechanisms during zinc-based nanoparticle toxicity are ultrastructural, ROS, oxidant, antioxidant enzymes, and cell apoptosis-related factors in different model systems (in vitro/in vivo). More attention should be paid to the penetration mechanism, the dose used, and the shape and dimensions of nanoparticles in the body’s cell lines. Along with these cases, future studies should also focus on understanding the penetration mechanism of zinc oxide nanoparticles in combination with other materials and their penetration routes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this review.
Funding
The study was self-funded.
Authors' contributions
All authors equally contributed to preparing this review.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The author thanks the anonymous reviewers in the Pharmaceutical and Biomedical Research periodical for their helpful comments.
References
Today, with the increasing development of nanotechnology, the environmental accumulation of nanomaterials and the exposure of humans to these nanoparticles are unavoidable [1]. Safety evaluation or toxicity assessment of various synthesized nanoparticles have been reported in different studies [2, 3, 4, 5, 6]. With the gradual increase in the use of zinc oxide nanoparticles in biomedical fields, its subsequent release in the environment and direct exposure to the human body is inevitable. Despite the widespread use of zinc oxide nanoparticles, the safety of this nanomaterial for humans is still unclear. The reported side effects of zinc-based nanoparticles have cast doubts about their use [7]. The current state of knowledge concerning the application of zinc oxide nanoparticles in the biomedical field is still in the preliminary stage. Despite numerous studies on zinc toxicity, there is a lack of knowledge on the characteristics and effects of zinc-based nanoparticles [1, 7]. Due to their small size and larger surface area, zinc oxide nanoparticles have a higher penetration capacity into the human body than metallic zinc [7]. These compounds can have desirable biological activities, such as carrying capacity for biomedical treatment, penetrating cellular barriers to drug delivery, and undesirable side effects, such as cytotoxicity, induction of oxidative stress, cell dysfunction, or a combination of both [8].
Numerous studies have reported the toxicity, free radical production, and induction of programmed cell death due to the high amount of zinc nanoparticles in environments and living organisms [8, 9]. The two main ways of adsorption of the nanoparticles in living cells are active adsorption by endocytosis and passive adsorption through diffusion. The process of cell uptake, the site of accumulation within the cell, and the ability of nanoparticles to produce toxic effects depend on their size, chemical properties, electric charge, crystallization, shape, and solubility [10].
The toxicity of these nanoparticles can be evaluated through in vitro studies using cells or cell lines or in vivo exposure to experimental animal models [11, 12]. Laboratory studies are usually the first step in measuring the toxicity of nanoparticles. However, cell culture cannot fully assess the complexity and relationships between components of biological matrices in an organism since this category can record events difficult to investigate in laboratory research scales. Therefore, more in vivo studies are needed.
This review aims to provide a critical summary of recent scientific literature on the potentially hazardous effects of zinc oxide nanoparticles and information on the possible mechanisms of these nanoparticle-induced toxicity in vitro and in vivo models.
Evidence Acquisition
To gather relevant information, a review of published studies reporting mechanisms of action of zinc oxide nanoparticles-induced toxicity in vitro and in vivo models in Scopus, PubMed, and Google Scholar databases was undertaken. Keywords like “Zinc oxide nanoparticles”, “toxicity”, and “in vitro and in vivo experiments” were used. In this review, the most important published literature of the last 10 years (2011 to 2021), related to in vivo and in vitro evaluations of organ toxicity to zinc oxide nanoparticles using animal models, were collected, analyzed, and discussed. In addition, the information related to the possible mechanisms of toxicity caused by zinc oxide nanoparticles, along with the influencing variables in this process, was summarized.
Zinc Oxide Nanoparticles
Among the metal oxide nanoparticles, zinc oxide nanoparticles have received considerable attention largely due to their various uses in the biomedical field—their enormous potential in disease diagnostics and monitoring. However, the increasing use of zinc nanoparticles has led to their release into the environment, and the toxicity of these compounds on organisms has become a concern in the biomedical research community [13]. The research conducted in this field shows that releasing zinc oxide nanoparticles may negatively affect organisms and the environment [13] (Figure 1). Toxic Mechanisms of Zinc Oxide Nanoparticles
Zinc oxide nanoparticles are among the most important and multifunctional compounds. The ability of zinc oxide nanoparticles to release zinc is an important parameter from a risk assessment perspective [14]. Recent studies have shown that zinc oxide nanoparticles can be toxic to humans due to oxidative stress [14, 15], but the details and the full picture of their interplay have remained obscure. Figure 2 shows the possible role of zinc oxide nanoparticles in causing toxicity through reactive oxygen species (ROS) in biological organs. Mitochondria produce ROS as a by-product of metabolism, and they react as transport molecules in many important cellular activities such as gene transcription, signal transduction, and the immune response [11]. Furthermore, ROS has been suggested as an underlying reason for proliferative effects observed in human leukemia cells at low zinc concentrations [10]. Oxidative stress is a disorder of the oxidant-antioxidant balance, which disrupts normal cellular functions and leads to cytotoxicity or DNA damage [7]. Oxidant-antioxidant imbalance can result from a lack of antioxidant capacity due to impaired production and distribution of reactive oxygen species or their excess in relation to other factors. These events may cause oxidative damage to DNA, increasing the chromosomal aberrations associated with cell deformation [7]. Oxidative stress and its damage indicate an association between different forms of chronic liver damage [8]. In a study, the toxic effects of zinc-based nanoparticles in adult mice showed that high concentrations of these nanoparticles cause the cells to use different enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione S-transferase to remove ROS [9]. Superoxide dismutase can convert internal oxygen radicals into H2O2. In addition, catalase and glutathione peroxidase enzymes can reduce oxygenated water to water and oxygen. Therefore, superoxide dismutase, catalase, and glutathione peroxidase can keep oxygen levels low and prevent cell poisoning [9]. The excessive generation of various deleterious ROS like hydrogen peroxide, hydroxyl radical species, nitric oxide or superoxide anion as a result of zinc nanoparticles exposure may result in oxidative damages on DNA, RNA, proteins, and so on.
In general, zinc-based nanoparticles were found to induce oxidative DNA damage, inflammation, cell degeneration, cell cycle arrest, cytogenetic alterations, and apoptosis. Cytotoxicity studies have shown that zinc-based nanoparticles have dose- and time-dependent cytotoxic potential, genotoxicity, and carcinogenicity [9, 10].
In vitro toxicity studies
The in vitro studies on the toxicity of zinc oxide nanoparticles confirmed the induction of diverse adverse effects in pulmonary-blood pathways and central nervous system-derived cell toxicities [14]. This process can be related to excessive dissolved ionic Zn2+ in the culture medium or inside cells, leading to oxidative stress, cytotoxicity, and mitochondrial dysfunction [15, 16]. In this line, Kao et al. investigated an olfactory bulb-brain translocation pathway for zinc oxide nanoparticles in rodent cells in vitro and in vivo conditions. They concluded that an olfactory bulb-brain translocation pathway for airborne zinc oxide nanoparticles exists in rats and that endocytosis is required for the interneuron translocation of these particles [15]. Coating zinc oxide nanoparticles with polymeric compounds may reduce cytotoxicity effects and ROS generation in WIL2-NS cell lines in humans [15]. Further studies on in vitro exposure to zinc oxide nanoparticles are summarized in Table 1.

In many references, zinc oxide nanoparticles are also considered the most toxic nanoparticles with the lowest LD50 value among the other metal oxide nanoparticles. In this line, Cao et al. evaluated zinc oxide nanomaterials’ developmental and neurotoxic effects with different shapes/sizes compared to zebrafish and SH-SY5Y cells. They found the LD50 of treated zebrafish larvae with zinc oxide nanoparticles was 11 μg/mL for 144 h [10]. The LD50 values were calculated to compare the toxicity of three zinc oxide nanomaterials. These values for short and long zinc oxide nanorods were estimated at 15.4 μg/mL and 17 μg/mL, respectively. In another study, Ng et al. investigated the acute toxicological effects of zinc oxide nanoparticles in mice after intratracheal instillation. Their results also showed that the LD50 in the intratracheal instillation of zinc oxide nanoparticles (48 nm) was 493.85 μg/kg body weight [11].
In vivo toxicity studies
Toxicology results after exposure to zinc oxide nanoparticles are different and depend on various parameters [17, 18]. Particle penetration is mainly determined by their physicochemical properties, especially particle size. The zinc oxide nanoparticles toxicity increases malondialdehyde and glutathione S-transferase activity and decreases total antioxidant levels in the blood serum and liver of treated rats. Finally, this process leads to the formation of free radicals and an increase in the rate of oxidative stress [10]. Therefore, the induction of enzymatic antioxidant defense after zinc oxide nanoparticles exposure can be a successful adaptive response. It is a compensatory mechanism that enables the cell to overcome the damage. Other inflammatory responses and immune system activation are also commonly suggested in biochemical observations after exposure to zinc oxide nanoparticles. Detrimental health effects were also observed through intraperitoneally administrated zinc oxide nanoparticles in rats. Most symptoms were nonspecific toxicity and caused more nerve damage in the caudate nucleus and hippocampus [11]. The treated rats with zinc oxide nanoparticles also showed different tissue changes, including vacuolation and pyknosis in renal tubular epithelial cells and glomerular damage. Zinc oxide nanoparticles caused ultrastructural changes in the proximal convoluted tubule of renal tubules and certain glomerular cells. These particles significantly increased cell apoptosis in rat kidneys (Figure 3) [12]. In this study, the size of zinc oxide nanoparticles was about 20 nm in diameter, which could facilitate their entrance into cells. Zinc oxide nanoparticles with a size less than 20 nm have been shown to accumulate in the liver and spleen of rats, while zinc oxide nanoparticles of larger size accumulate in the kidney [12]. In a study, Amara et al. investigated behavioral performances and the brain contents of some monoamine neurotransmitters with zinc oxide nanoparticles exposure. The results revealed that acute intravenous injection of zinc oxide nanoparticles does not affect neurotransmitter contents, locomotor activity, or spatial working memory in adult rats [17].
Pasupuleti et al. evaluated the toxicity effects of zinc oxide nanoparticles through the oral route [18]. Their experiments aimed to determine the importance of the affected dose (5, 50, 300, 1000, and 2000 mg/kg body weight) by comparing zinc oxide’s acute oral toxicological potential in dimensions (20 nm) to its micro size. In the nano-dimensions of zinc oxide, microscopic lesions in the liver, pancreas, heart, and stomach were more frequent in lower and higher doses. However, the incidence of the above lesions was higher in mice treated with higher doses of zinc oxide in micro dimensions. In this study, nano-sized zinc oxide showed more toxicity in lower doses. Therefore, they concluded that future nano-scale toxicological research should focus on the importance of dose exposure criteria [18]. The other effects of in vivo exposure to zinc oxide nanoparticles are summarized in Table 1.
Discussion
One of the main risks of application and exposure to zinc oxide nanoparticles is the dissolution and release of Zn2+ ions that are toxic to cells [18]. Among the effective cytotoxic effects of zinc oxide nanoparticles, we can mention the increase in ROS production along with lipid peroxidation, genotoxicity, and induction of inflammatory pathways [11]. The review of the published articles in this field shows that increased oxidative stress is associated with decreased antioxidant activity in the studied models. This problem is justified by lower levels of glutathione and total antioxidant capacity and inhibiting the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase [12, 19, 20]. Zinc oxide nanoparticles have been reported to interact with mammalian intracellular enzymes. These nanoparticles may disrupt these antioxidants’ defense mechanisms through ROS production. The development of this process leads to inflammatory response, disruption, and destruction of mitochondria. Finally, we will see apoptosis in human liver cells, lung epithelial cells, and even some cancer cells [22, 23, 27]. In a controlled environment (in vitro conditions), inflammatory status has been confirmed in many studies by higher circulatory levels of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 in the treated rats with zinc oxide nanoparticles at both mRNA and protein levels. Identifying zinc oxide nanoparticles as external particles by immune cells may produce ROS, reactive nitrogen species (RNS), signaling pathway disturbance, and altered cytokine levels [23, 27, 28].
Zinc oxide nanoparticle toxicity is affected by different factors, including the routes of exposure, physio-chemical properties, and environmental conditions, making comparing the data of various studies difficult. Environmental conditions in any organ may affect the toxicity potential of nanoparticles. For instance, the acidic environment in lung lining fluid may dissolve zinc oxide (1 and 5 mg/kg of body weight), leading to the transient increase in the concentration of Zn2+ ions and local toxicity. Their solubility determines zinc oxide nanoparticles’ toxicity. In lung injury, the main cause of zinc oxide nanoparticle-induced toxicity is pH-dependent dissolution inside phagosomes [12, 22].
In vivo conditions, physicochemical properties, nanofabrication, and biological factors (such as species, age, gender, and size of the tested animals) seem to be common factors that govern zinc oxide nanoparticles’ toxicity [9, 11]. Regarding the complexity and diversity of reactions related to human biological systems, the generalization of animal model results to humans is often inconclusive. Concentrations used or determined in animal models do not necessarily imply the negative effects of exposure to zinc oxide nanoparticles in humans. Very low mass concentrations of these nanoparticles are needed in diagnostic interventions in humans. Therefore, future studies should rely on using real concentrations of these nanoparticles in the environment to evaluate their toxicity in living organism models. Parameters such as exposure routes, physicochemical properties, and environmental conditions should also be considered in these studies. The lack of sufficient understanding of the mechanism of toxicity caused by zinc oxide nanoparticles is one of the main obstacles to their use in various applications. More attention should be paid to the efforts made in understanding the mechanism of toxicity caused by zinc oxide nanoparticles and its control methods.
Conclusion
It is necessary to continue monitoring the process of release and exposure of Zn-based nanoparticles and their harmful effects on living systems. Zinc oxide nanoparticles currently have a wide range of applications in cancer treatment, gene-drug delivery, and biological materials for tissue engineering due to wide band-gap semiconductors, which can readily absorb UV rays. With their unique properties, zinc oxide nanoparticles can damage cancer cells using the mechanism of selective cytotoxicity through oxidative stress via ROS generation. Oxidative damage caused by free radicals during the toxicity of zinc nanoparticles leads to a cascade effect. Ultimately, this effect will have adverse consequences in both in vitro and in vivo conditions (typically liver, lung, reproductive tract, kidney, central nervous system function, dysregulation, and reduced mitochondrial respiration). The main mechanisms during zinc-based nanoparticle toxicity are ultrastructural, ROS, oxidant, antioxidant enzymes, and cell apoptosis-related factors in different model systems (in vitro/in vivo). More attention should be paid to the penetration mechanism, the dose used, and the shape and dimensions of nanoparticles in the body’s cell lines. Along with these cases, future studies should also focus on understanding the penetration mechanism of zinc oxide nanoparticles in combination with other materials and their penetration routes.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this review.
Funding
The study was self-funded.
Authors' contributions
All authors equally contributed to preparing this review.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The author thanks the anonymous reviewers in the Pharmaceutical and Biomedical Research periodical for their helpful comments.
References
- Sturikova H, Krystofova O, Huska D, Adam V. Zinc, zinc nanoparticles and plants. J Hazard Mater. 2018; 349:101-10. [DOI:10.1016/j.jhazmat.2018.01.040] [PMID]
- Tavakoli S, Sameni F, Ebrahimzadeh MA, Biparva P, Mohammadi H, Zahedi A, et al. Safety evaluation of nano iron zero valente green synthesized: A comparative study. Nanomedicine Res J. 2020; 5(2):160-70. [Link]
- Maghsoodloo S, Ebrahimzadeh MA, Tavakoli S, Mohammadi H, Biparva P, Rafiei A, et al. Green synthesis of multifunctional silver nanoparticles using quercetin and their therapeutic potential. Nanomedicine Res J. 2020; 5(2):171-81. [Link]
- Hamzeh M, Hosseinimehr SJ, Karimpour A, Mohammadi HR, Khalatbary AR,Talebpour Amiri F. Cerium oxide nanoparticles protect cyclophosphamide-induced testicular toxicity in mice. Int J Prev Med. 2019; 10:5. [DOI:10.4103/ijpvm.IJPVM_184_18] [PMID] [PMCID]
- Ebrahimzadeh MA, Biparva P, Mohammadi H, Tavakoli S, Rafiei A, Kardan M, et al. Highly concentrated multifunctional silver nanoparticle fabrication through green reduction of silver ions in terms of mechanics and therapeutic potentials. Anticancer Agents Med Chem. 2019; 19(17):2140-53. [DOI:10.2174/1871520619666191021115609] [PMID]
- Mohammadi H, Nekobahr E, Akhtari J, Saeedi M, Akbari J, Fathi F. Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol Rep. 2021; 8:331-6. [DOI:10.1016/j.toxrep.2021.01.012] [PMID] [PMCID]
- Sharma V, Anderson D, Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis. 2012; 17(8):852-70. [DOI:10.1007/s10495-012-0705-6] [PMID]
- Zhang J, Qin X, Wang B, Xu G, Qin Z, Wang J, et al. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis. 2017; 8(7):e2954. [DOI:10.1038/cddis.2017.337] [PMID] [PMCID]
- Ali A, Phull AR, Zia M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol Rev. 2018; 7(5):413-41. [DOI:10.1515/ntrev-2018-0067]
- Cao Y, Gong Y, Liao W, Luo Y, Wu C, Wang M, et al. A review of cardiovascular toxicity of TiO2, ZnO and Ag nanoparticles (NPs). Biometals. 2018; 31(4):457-76. [DOI:10.1007/s10534-018-0113-7] [PMID]
- Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, et al. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomedicine. 2017; 12:1621-37. [DOI:10.2147/IJN.S124403] [PMID] [PMCID]
- Yousef MI, Mutar TF, Kamel MAE. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep. 2019; 6:336-46. [DOI:10.1016/j.toxrep.2019.04.003] [PMID] [PMCID]
- Chang YN, Zhang M, Xia L, Zhang J, Xing G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials. 2012; 5(12):2850-71. [DOI:10.3390/ma5122850] [PMCID]
- Jin M, Li N, Sheng W, Ji X, Liang X, Kong B, et al. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ Int. 2021; 146:106179. [DOI:10.1016/j.envint.2020.106179] [PMID]
- Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012; 48(2):464-71. [DOI:10.1007/s12031-012-9756-y] [PMID]
- Abuelsamen A, Mahmud S, Mohd Kaus NH, Farhat OF, Mohammad SM, Al-Suede FSR, et al. Novel Pluronic F-127-coated ZnO nanoparticles: Synthesis, characterization, and their in-vitro cytotoxicity evaluation. Polym Adv Technol. 2021; 32(6):2541-51. [DOI:10.1002/pat.5285]
- Amara S, Ben-Slama I, Mrad I, Rihane N, Jeljeli M, El-Mir L, et al. Acute exposure to zinc oxide nanoparticles does not affect the cognitive capacity and neurotransmitters levels in adult rats. Nanotoxicology. 2014; 8(sup1):208-15. [DOI:10.3109/17435390.2013.879342] [PMID]
- Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya BM. Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health. 2012; 28(8):675-86. [DOI:10.1177/0748233711420473] [PMID]
- Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro. 2013; 27(2):731-8. [DOI:10.1016/j.tiv.2012.12.001] [PMID]
- Saber M, Hayaei-Tehrani RS, Mokhtari S, Hoorzad P, Esfandiari F. In vitro cytotoxicity of zinc oxide nanoparticles in mouse ovarian germ cells. Toxicol In Vitro. 2021; 70:105032. [DOI:10.1016/j.tiv.2020.105032] [PMID]
- Qiao Y, Liang X, Yan Y, Lu Y, Zhang D, Yao W, et al. Identification of exosomal miRNAs in rats with pulmonary neutrophilic inflammation induced by zinc oxide nanoparticles. Front Physiol. 2018; 9:217. [DOI:10.3389/fphys.2018.00217] [PMID] [PMCID]
- Yoo J, Seo GB, Yoon BI, Lim YM, Kim P, Kim HM, et al. Evaluation of recovery from acute lung injury induced by intratracheal instillation of zinc oxide nanoparticles. Appl Ecol Environ Res. 2018; 16(3):3145-57. [DOI:10.15666/aeer/1603_31453157]
- Amara S, Slama IB, Omri K, El Ghoul J, El Mir L, Rhouma KB, et al. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol Ind Health. 2015; 31(12):1202-9. [DOI:10.1177/0748233713491802] [PMID]
- de Souza JM, Mendes BO, Guimarães ATB, Rodrigues ASL, Chagas TQ, Rocha TL, et al. Zinc oxide nanoparticles in predicted environmentally relevant concentrations leading to behavioral impairments in male swiss mice. Sci Total Environ. 2018; 613-614:653-62. [DOI:10.1016/j.scitotenv.2017.09.051] [PMID]
- Hao L, Chen L. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf. 2012; 80:103-10. [DOI:10.1016/j.ecoenv.2012.02.017] [PMID]
- Kteeba SM, El-Ghobashy AE, El-Adawi HI, El-Rayis OA, Sreevidya VS, Guo L, et al. Exposure to ZnO nanoparticles alters neuronal and vascular development in zebrafish: Acute and transgenerational effects mitigated with dissolved organic matter. Environ Pollut. 2018; 242(Pt A):433-48. [DOI:10.1016/j.envpol.2018.06.030] [PMID]
- Saptarshi SR, Feltis BN, Wright PF, Lopata AL. Investigating the immunomodulatory nature of zinc oxide nanoparticles at sub-cytotoxic levels in vitro and after intranasal instillation in vivo. J Nanobiotechnology. 2015; 13:6. [DOI:10.1186/s12951-015-0067-7] [PMID] [PMCID]
- Ghandadi M, Valadan R, Mohammadi H, Akhtari J, Khodashenas S, Ashari S. Wnt-β-catenin signaling pathway, the Achilles’ Heels of Cancer Multidrug Resistance. Curr Pharm Des. 2019; 25(39):4192-207. [DOI:10.2174/1381612825666191112142943] [PMID]
Type of Study: Review article |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |