Volume 9, Issue 3 (2023)
Pharm Biomed Res 2023, 9(3): 211-222 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ramezaninejad S, Namvar H R, Sohrabi M, Darvishnia D, Ahangar N, Alikhani A, et al . The Efficacy and Safety of Adding Chlorpromazine to Atazanavir/Ritonavir Regimen in the Treatment of Moderate COVID-19 Patients, a Randomized Double-blind Clinical Trial. Pharm Biomed Res 2023; 9 (3) :211-222
URL: http://pbr.mazums.ac.ir/article-1-480-en.html
URL: http://pbr.mazums.ac.ir/article-1-480-en.html
Sima Ramezaninejad1 

 , Hamid Reza Namvar1
, Hamid Reza Namvar1 

 , Masoumeh Sohrabi1
, Masoumeh Sohrabi1 

 , David Darvishnia2
, David Darvishnia2 

 , Nematollah Ahangar3
, Nematollah Ahangar3 

 , Ahmad Alikhani4
, Ahmad Alikhani4 

 , Hamideh Abbaspour5
, Hamideh Abbaspour5 

 , Reza Valadan6
, Reza Valadan6 

 , Zahra Akbari4
, Zahra Akbari4 

 , Jafar Akbari7
, Jafar Akbari7 

 , Roya Ghasemian *4
, Roya Ghasemian *4 

 , Ebrahim Salehifar7
, Ebrahim Salehifar7 




 , Hamid Reza Namvar1
, Hamid Reza Namvar1 

 , Masoumeh Sohrabi1
, Masoumeh Sohrabi1 

 , David Darvishnia2
, David Darvishnia2 

 , Nematollah Ahangar3
, Nematollah Ahangar3 

 , Ahmad Alikhani4
, Ahmad Alikhani4 

 , Hamideh Abbaspour5
, Hamideh Abbaspour5 

 , Reza Valadan6
, Reza Valadan6 

 , Zahra Akbari4
, Zahra Akbari4 

 , Jafar Akbari7
, Jafar Akbari7 

 , Roya Ghasemian *4
, Roya Ghasemian *4 

 , Ebrahim Salehifar7
, Ebrahim Salehifar7 


1- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
2- Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Pharmacology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Infectious Diseases, Antimicrobial Resistance Research Center, Communicable Disease Institute, Mazandaran University of Medical Sciences, Sari, Iran.
5- Department of Clinical of Pharmaceutics, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
6- Department of Immunology, Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Department of Clinical of Pharmaceutics, Pharmaceutical Sciences Research Center, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Pharmacology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Infectious Diseases, Antimicrobial Resistance Research Center, Communicable Disease Institute, Mazandaran University of Medical Sciences, Sari, Iran.
5- Department of Clinical of Pharmaceutics, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
6- Department of Immunology, Molecular and Cell Biology Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
7- Department of Clinical of Pharmaceutics, Pharmaceutical Sciences Research Center, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 926 kb]
(660 Downloads)
| Abstract (HTML) (1329 Views)
Full-Text: (388 Views)
Introduction
The coronavirus disease 2019 (COVID-19) causes severe acute respiratory syndrome (SARS) in humans. It is caused by a novel coronavirus and originated in Wuhan, China in December 2019 and became a pandemic in the 21st century [1, 2]. Globally on March 2022, there have been 469,212,705 confirmed cases of COVID-19, including 6,077,252 deaths, reported to the World Health Organization (WHO) [3]. It is the third and the most serious coronavirus epidemic after SARS-CoV-1 in 2003 and MERS-CoV in 2012. The virus contains a very large RNA virus genome and in cryo-electron tomography, it has been revealed that it is spherical with an envelope [4, 5]. The first stages of infection with coronavirus are entry into human cells and replicating its genome to initiate and spread the infection [6, 7]. COVID-19 progression can be divided into three phases: The early infection phase involving viral replication and mild symptoms, pulmonary phase involving adaptive immunity stimulation and predominance of respiratory symptoms, and the hyperinflammation phase leading to conditions, such as acute respiratory disease syndrome (ARDS) [8, 9].
The treatment of patients with mild to moderate COVID-19 is of utmost importance because about 14% of patients progress to severe COVID-19 in only one week [10, 11]. The median time to critical COVID-19 appearance may be as early as eight days from the beginning of symptoms [12] and the median time to death from the symptom’s onset is 16 days [13]. Therefore, it would be necessary to pharmacologically treat patients with mild to moderate COVID-19 who are at risk of progression to severe or critical disease.
Some antiviral drugs have shown efficacy against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in both in vitro and in vivo models [14, 15]. Atazanavir, an azapeptide oral protease inhibitor, was approved by the food and drug administration (FDA) in 2003 for HIV infection with a good safety profile [16].
In addition, Atazanavir decreases the levels of IL-6 and TNF-α in COVID-19-infected patients, an effect that was better than chloroquine, a compound recognized for its anti-viral and anti-inflammatory activities [17, 18]. Numerous studies have been initiated for out- and in-patients with COVID-19 to test atazanavir combined or not with other commercial drugs, such as ritonavir or dexamethasone; however, no definitive response has emerged from these studies.
Chlorpromazine synthesized in 1951 by Rhône Poulenc, has been used in psychiatry since 1952 when Jean Delay and Pierre Deniker, two psychiatrists at Sainte-Anne hospital, discovered its antipsychotic properties. According to Inoue et al., SARS-CoV (a coronavirus similar to SARS-CoV-2) uses clathrin-mediated endocytosis (CME) to enter the host cell [19]. Chlorpromazine, prescribed as an antipsychotic medication, inhibits the formation of clathrin-coated vesicles and can block CME [20]. On the other hand, chlorpromazine increases the intra-vesicular pH and because of its cationic amphiphilic properties, it can inhibit S protein activation [21]. According to animal studies, in mice, chlorpromazine increases the concentration of the anti-inflammatory cytokines, such as IL-10, and decreases the pro-inflammatory cytokines, like IL-6 and TNFα after administration of endotoxins [22]. Adverse effects of chlorpromazine are drowsiness, dry mouth, constipation, blurred vision, urine retention, orthostatic hypotension, and some uncommon adverse reactions, including QT prolongation and cardiac rhythm disorders [23-25]. These risks were limited by clinical daily monitoring at the hospital over the whole period of chlorpromazine treatment and the vital signs, laboratory tests, and also all complaints related to disease or chlorpromazine were recorded.
The aim of this study was to evaluate the role of chlorpromazine as an add-on therapy to the atazanavir/ritonavir regimen in the treatment of moderate COVID-19 patients. This hypothesis is mainly based on the activity of chlorpromazine against RNA viruses, like COVID-19 with established efficacy for in vitro studies [26-28].
Materials and Methods
Study design
This study was a pilot double-blind placebo-controlled randomized clinical trial. This study recruited patients from October 22, 2020, to January 7, 2021, at two educational hospitals of Mazandaran University of Medical Science, in the north of Iran. Written informed consent was obtained from all patients, or their legal representatives if they were unable to provide consent.
Sixty patients who had inclusion criteria entered the study after the goals of the study were explained. Patients were randomly assigned to one of the two parallel treatment groups using a block randomization method. To keep the blindness, a computer-based 5-digit random code was assigned to each patient.
In this study, 169 patients were studied, of whom 60 patients met the inclusion criteria and were randomized with a 1:1 ratio to receive chlorpromazine (Tehran-Shimi Pharmaceutical Company, Iran, 25 mg) three times a day or the placebo. Both groups received atazanavir /ritonavir 300 mg/100 mg (Mylan Pharmaceutical Pvt, India) once a day for up to 14 days in the hospital.
The placebo was made in the Department of Pharmaceutics of the School of Pharmacy using Avicel (microcrystalline cellulose).
Inclusion criteria
Patients who had all of these criteria were included: 1) The age of 18-70 years, 2) Being diagnosed with COVID-19 according to clinical symptoms, confirmed by reverse transcription polymerase chain reaction (RT-PCR) or CT scan of the lungs, 3) Intend to receive atazanavir/ritonavir as antiviral regimen, 4) The SpO2 of less than 94 mmHg (without oxygen), oral temperature >37.2 °C, respiratory rate (RR) >24/min, or having dyspnea.
Exclusion criteria
Patients who had each of these criteria were excluded: 1) Those who did not give informed consent; 2) Receiving antipsychotic medicines at present or during the last 14 days; 3) History of seizure, Parkinson’s disease, or dementia; 4) Liver dysfunction; 5) History of pheochromocytoma; 6) Allergy to phenothiazine drugs; 7) Existence of any major drug interactions between the patient’s routine medications and chlorpromazine; 8) Pregnancy or breastfeeding; 9) Previously treated for COVID-19 or enrolment in other trials.
Outcome measures
The primary outcome of the study was the improvement of SpO2.
Secondary outcomes were the day of hospitalization, the need for ICU admission, lymphocyte count, and the rate of conversion of RT-PCR positive test to negative result. Initial PCR was performed for all patients. The next PCR was performed on the fifth or discharge day, whichever was earlier, and if positive, it was repeated a week later.
The complete blood count (CBC) and biochemical tests, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), lactate dehydrogenase (LDH), C - reactive protein (CRP) as well as erythrocyte sedimentation rate (ESR) were conducted on days 1, 3, 5, 7, and 10. Oxygen saturation and respiratory rate (RR) were evaluated for all days of hospitalization. On all days of hospitalization, patients were evaluated for any potential side effects caused by chlorpromazine. If the side effects were bothersome or serious, patients were excluded from the study.
Statistical analysis
The normality of data was checked by Shapiro-Wilk Test. Independent sample t-test or Mann-Whitney U test was used for the comparison of continuous variables between the two groups. Wilcoxon matched-pair signed-rank test and chi-square test were used for the comparison of continuous variables and qualitative data before and after treatment, respectively. Repeated measures ANOVA was used to determine the changes of variables (e.g. laboratory data) on different days in each group.
The trend of changes in laboratory data and O2 saturation was compared by the generalized estimating equation (GEE) model. SPSS software, version 26.0 was applied for statistical analysis.
Result
Patients
A total of sixty subjects were enrolled in this study, including the chlorpromazine group (n=30) and placebo group (n=30). Thirteen patients (ten patients in the CLPZ group and three patients in the placebo group) did not continue the study (Figure 1).
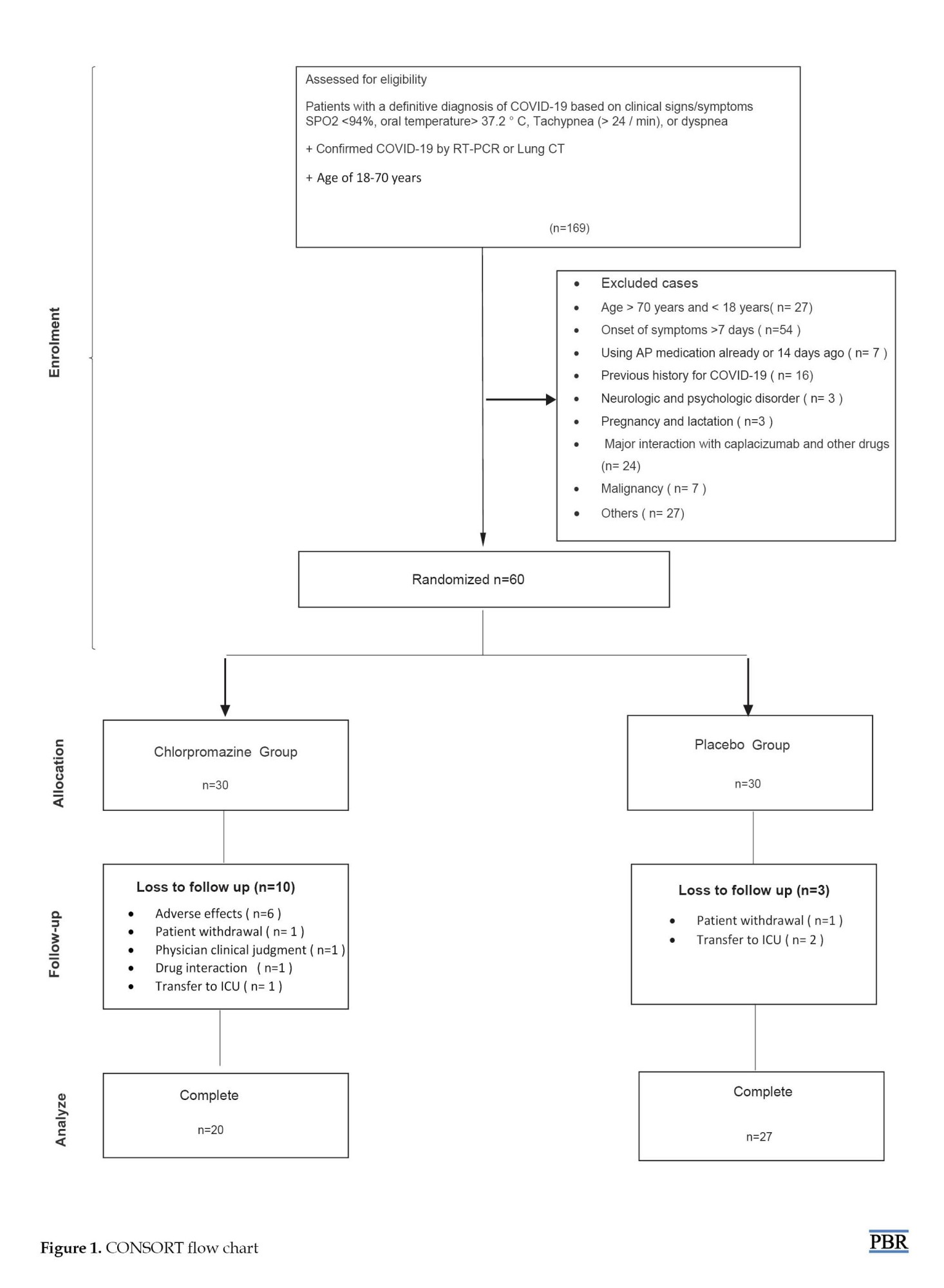
Table 1 shows the baseline demographic and clinical characteristics of the 47 patients who completed the study. The mean age of patients was 52±12.74 years, and 28% of the patients were men. The most common presenting symptoms were fever (defined as temperature ≥37.8) (61.7%), dyspnea (53.2%), elevated CRP (50.23%), and cough (38.3%). All patients had lung injury on the first day and the mean percentage of their lung involvements was 48.9±14.65.

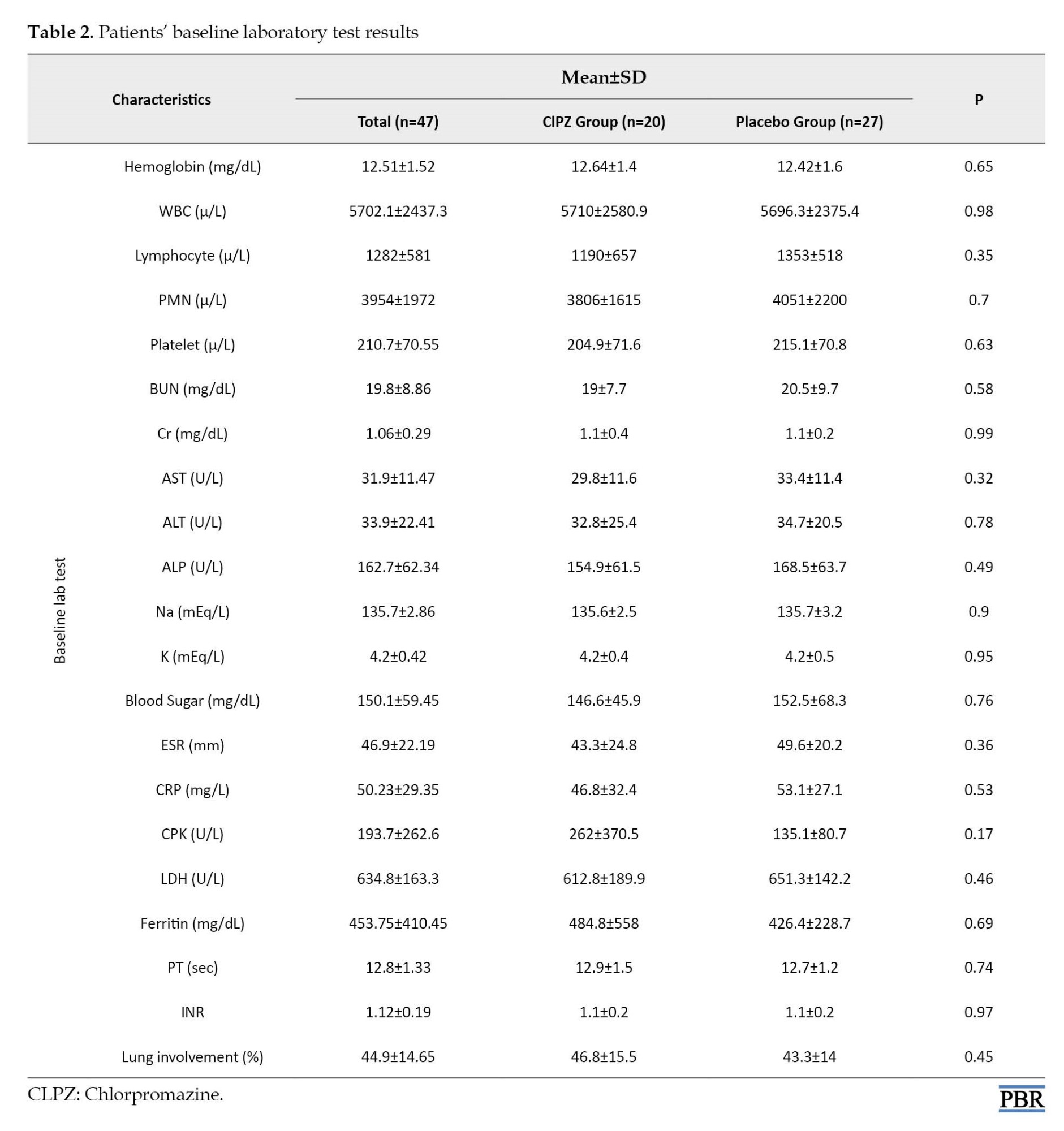
The WBC counts were 5702±2437 cells/µL. On admission, lymphopenia (lymphocyte count <1500 cells/µL) was found in 33(70.2%) patients. On admission, patients had mild to moderate pneumonia as documented by clinical manifestations and CT findings. There were no between-group differences in baseline laboratory test results (e.g. CRP, WBC, and lymphocyte counts) and CT scores for lung lesions (Table 2).
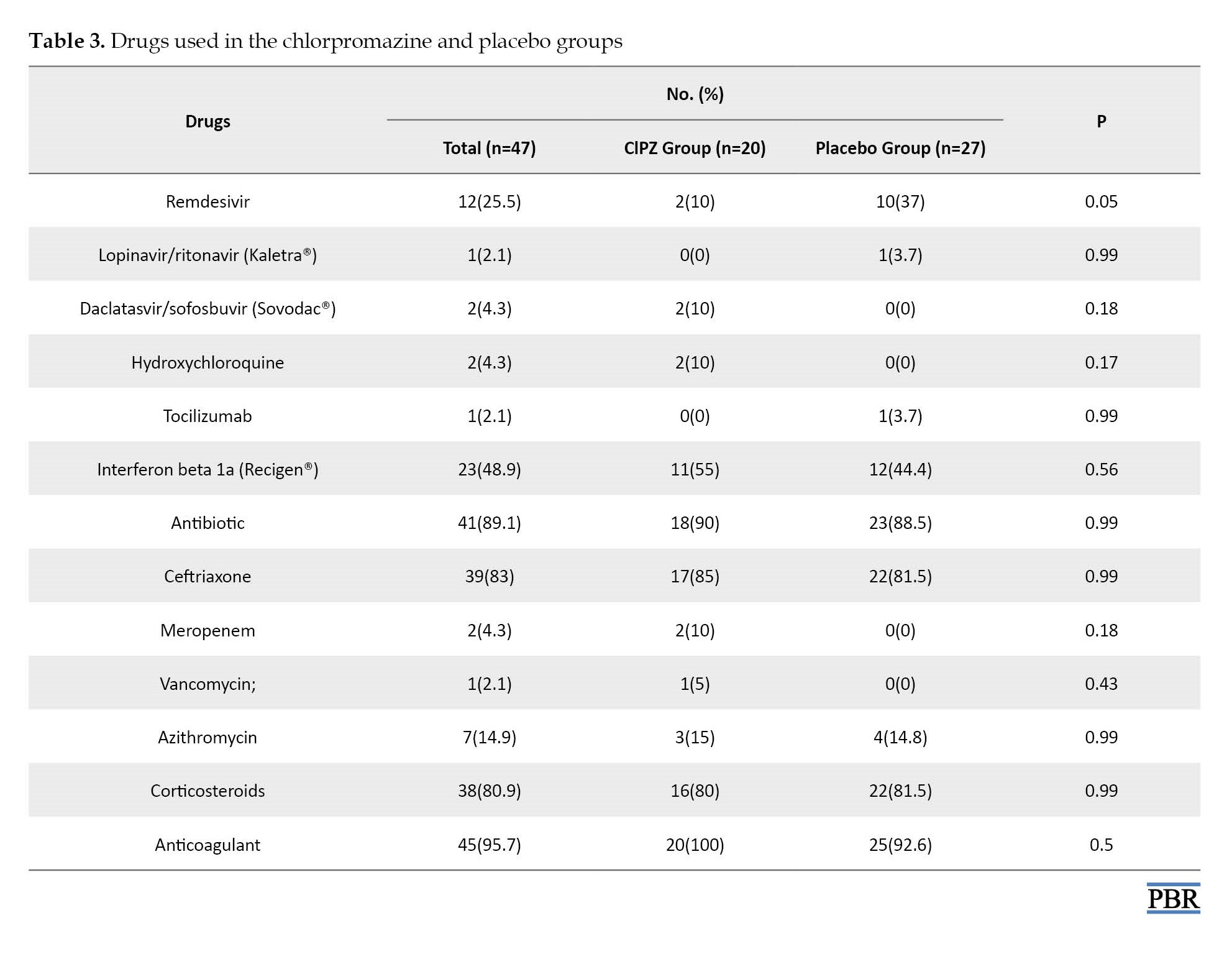
Regarding the incidence of adverse effects, xerostomia was seen in five cases (16.6%) in the chlorpromazine group and one case (6.7%) in the placebo group. Somnolence occurred in ten cases (33.3%) of the chlorpromazine group and two cases of the placebo group. Dizziness was seen in four cases (13.3%) in the chlorpromazine group. Six patients from the drug group were excluded from the study due to intolerance to side effects, one case was due to xerostomia, three cases were due to somnolence, and the other two cases were due to somnolence and dizziness. The mortality rate in the chlorpromazine group was one case (3.3%) and in the placebo group, was two cases (6.7%) (Table 3).
Primary outcomes
There was no significant difference in oxygen saturation in the chlorpromazine group compared to the placebo group during the hospital stay.
Secondary outcome
The duration of hospitalization in the chlorpromazine group (7.4±2.7) was almost 0.8 days less than the placebo group (8.2±3), but the difference was not significant (P=0.2). In the placebo group, an RT-PCR test was performed for all individuals; out of 22 people whose RT-RT-PCR test were positive (81.5%) on the day one of hospitalization, five (18.5%) patients had a positive PCR test on the seventh day, which represents a decrease of 77.3%. On the other hand, in the chlorpromazine group, an RT-PCR test was performed for all individuals; out of 14 people who had an RT-PCR positive test (70%) on the first day of hospitalization, two (10%) patients on the seventh day were RT-PCR-positive indicating an 85.7% decrease (Table 4).

On the 14th day of hospitalization, none of the remaining 16 patients in the chlorpromazine group had a positive test (100% reduction) and in the placebo group, out of 20 patients, only one test was positive (95% reduction). The mean lymphocytes counts were 1190±657 cells/µL and 1353±518 cells/µL in the chlorpromazine and placebo groups on the first days of admission whereas they decreased to 1069±580 cells/µL and 1034±634 cells/µL after chlorpromazine and placebo treatments, respectively. The mean CRP levels on the first day for the placebo and chlorpromazine groups were 53.1±27.1 mg/dL and 46.8±24.8 mg/dL, which decreased to 44±30.2 mg/dL and 40.3±32.5 mg/dL after the treatment period, respectively. The median time from the onset of symptoms to enrolling in the trial was 6.43±1.9 days. The mean severity of lung involvement according to CT scan was 44.9±14.65% and the mean oxygen saturation on admission to the hospitalization was 93.62±2.8. The mean of ESR, CRP, CPK, and LDH levels in patients showed no significant difference between the two groups (Table 5).


Discussion
This randomized clinical trial found that adding chlorpromazine to the antiviral regimen of patients with moderate COVID-19 was not significantly effective on the SpO2 or days of ICU or non-ICU stay.
Lymphocyte count is one of the most significant and consistent trends, suggesting that this indicator might reflect the disease progression, and lymphopenia is a predictor of prognosis in COVID-19 patients [26]. Patients with severe disease had more prominent laboratory abnormalities (including lymphopenia and elevated CRP) than those with non-severe disease [27]. Lymphopenia (lymphocytes <1500 per mm3) is the most common lab finding in COVID-19 and is found in as many as 70% of the patients on admission. All patients had high CRP levels with an average of 50.23±29.35 on the first day of hospitalization.
This is the second clinical trial using chlorpromazine in patients with COVID-19, which was performed as a double-blind randomized study in two hospitals, while another study in France on chlorpromazine was a single-blind study [28].
Multiple drugs with in vitro antiviral activity against SARS-CoV-2 and/or immunomodulatory effects have been suggested to be clinically beneficial. In our study, atazanavir-ritonavir was prescribed as an antiviral regimen based on the national guideline regarding the treatment of COVID-19. Lymphopenia was observed in 70% of patients on admission, while lymphocyte counts showed no significant increase after 3, 5, 7, and 10 days of treatments to the normal range (P=0.67).
Elevated CRP levels (≥6 mg/dL) as positive CRP was observed in all patients on admission, while chlorpromazine could not significantly reduce CRP levels after 3, 5, 7, 10 days of treatment to the negative range and there was no statistically significant difference between the two groups according to changes in CRP during the treatment.
In a study conducted in 2014 to investigate the inhibitory effect of chlorpromazine on the Huh 7 cell line, the drug completely inhibited the proliferation of all seven cell lines at a dose of 12 μM. On the other hand, this study showed that the addition of chlorpromazine 1 hour prior to cell line infection led to a 2-log reduction of virus progeny titers, and the addition of chlorpromazine 1 hour after cell line infection reduced the virus by half to one log [29].
In a group of patients receiving the chlorpromazine, no life-threatening side effects were observed, and the annoying side effect in the patients was dry mouth.
In the treatment of HIV patients, atazanavir is a potent, well-tolerated, and effective once-daily protease inhibitor with a low pill burden [30]. A 96-week analysis suggested that the long-term efficacy of atazanavir/ritonavir monotherapy was inferior as compared to atazanavir/ritonavir plus two nucleosides reverse transcriptase inhibitors (NRTIs). Monotherapy was also associated with a lower incidence of adverse events [31].
Remdesivir demonstrated clinical benefit in a placebo-controlled trial in patients with severe COVID-19. However, its effect in patients with moderate disease who were randomized to a 10-day course of remdesivir, showed no statistically significant difference in clinical status (P=0.18 by Wilcoxon rank sum test) compared to standard care 11 days after treatment, but in a 5-day course, it showed a statistically significant difference (odds ratio, 1.65; 95% CI, 1.09-2.48; P=0.02) [32]. In this trial, atazanavir was considered the standard antiviral treatment for patients with moderate involvement, and in the case of disease progression or inadequate response, it was changed to another drug, including remedesivir, at the discretion of the clinician. According to the results, in the chlorpromazine group, fewer people (two vs. ten in the placebo group) needed to change their antiviral regimen to remdesivir (P=0.05).
Our study was designed according to our country’s guidelines and hydroxychloroquine was ordered. Remdesivir was ordered for severe COVID-19 patients and availability of this drug was limited; thus, we used atazanavir as the primary antiviral.
Conclusion
Based on this study, adding chlorpromazine is not effective in the alleviation of clinical symptoms and modifying laboratory tests of moderate to severe COVID-19 in hospitalized patients that received atazanavir/ritunavir.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.617) and registered at the Iranian Clinical Trial Registration Database (IRCT) (No.: IRCT20200913048708N1).
Funding
This study was supported by the Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: David Darvishnia, Roya Ghasemian, Ahmad Alikhani and Jafar Akbari; Methodology: Nematollah Ahangar, Hamideh Abbaspour and Ebrahim Salehifar; Investigation, writing the paper, review & editing: All authors; Data analysis: Sima Ramezaninejad, Hamid Reza Namvar and Zahra Akbari.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
We thank all patients who participated in this trial and their families. We appreciate the healthcare personnel at Emam Komeini Hospital and Razi Hospital of the Mazandaran University of Medical Sciences who have given their lives in the care of patients with COVID-19.
References
The coronavirus disease 2019 (COVID-19) causes severe acute respiratory syndrome (SARS) in humans. It is caused by a novel coronavirus and originated in Wuhan, China in December 2019 and became a pandemic in the 21st century [1, 2]. Globally on March 2022, there have been 469,212,705 confirmed cases of COVID-19, including 6,077,252 deaths, reported to the World Health Organization (WHO) [3]. It is the third and the most serious coronavirus epidemic after SARS-CoV-1 in 2003 and MERS-CoV in 2012. The virus contains a very large RNA virus genome and in cryo-electron tomography, it has been revealed that it is spherical with an envelope [4, 5]. The first stages of infection with coronavirus are entry into human cells and replicating its genome to initiate and spread the infection [6, 7]. COVID-19 progression can be divided into three phases: The early infection phase involving viral replication and mild symptoms, pulmonary phase involving adaptive immunity stimulation and predominance of respiratory symptoms, and the hyperinflammation phase leading to conditions, such as acute respiratory disease syndrome (ARDS) [8, 9].
The treatment of patients with mild to moderate COVID-19 is of utmost importance because about 14% of patients progress to severe COVID-19 in only one week [10, 11]. The median time to critical COVID-19 appearance may be as early as eight days from the beginning of symptoms [12] and the median time to death from the symptom’s onset is 16 days [13]. Therefore, it would be necessary to pharmacologically treat patients with mild to moderate COVID-19 who are at risk of progression to severe or critical disease.
Some antiviral drugs have shown efficacy against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in both in vitro and in vivo models [14, 15]. Atazanavir, an azapeptide oral protease inhibitor, was approved by the food and drug administration (FDA) in 2003 for HIV infection with a good safety profile [16].
In addition, Atazanavir decreases the levels of IL-6 and TNF-α in COVID-19-infected patients, an effect that was better than chloroquine, a compound recognized for its anti-viral and anti-inflammatory activities [17, 18]. Numerous studies have been initiated for out- and in-patients with COVID-19 to test atazanavir combined or not with other commercial drugs, such as ritonavir or dexamethasone; however, no definitive response has emerged from these studies.
Chlorpromazine synthesized in 1951 by Rhône Poulenc, has been used in psychiatry since 1952 when Jean Delay and Pierre Deniker, two psychiatrists at Sainte-Anne hospital, discovered its antipsychotic properties. According to Inoue et al., SARS-CoV (a coronavirus similar to SARS-CoV-2) uses clathrin-mediated endocytosis (CME) to enter the host cell [19]. Chlorpromazine, prescribed as an antipsychotic medication, inhibits the formation of clathrin-coated vesicles and can block CME [20]. On the other hand, chlorpromazine increases the intra-vesicular pH and because of its cationic amphiphilic properties, it can inhibit S protein activation [21]. According to animal studies, in mice, chlorpromazine increases the concentration of the anti-inflammatory cytokines, such as IL-10, and decreases the pro-inflammatory cytokines, like IL-6 and TNFα after administration of endotoxins [22]. Adverse effects of chlorpromazine are drowsiness, dry mouth, constipation, blurred vision, urine retention, orthostatic hypotension, and some uncommon adverse reactions, including QT prolongation and cardiac rhythm disorders [23-25]. These risks were limited by clinical daily monitoring at the hospital over the whole period of chlorpromazine treatment and the vital signs, laboratory tests, and also all complaints related to disease or chlorpromazine were recorded.
The aim of this study was to evaluate the role of chlorpromazine as an add-on therapy to the atazanavir/ritonavir regimen in the treatment of moderate COVID-19 patients. This hypothesis is mainly based on the activity of chlorpromazine against RNA viruses, like COVID-19 with established efficacy for in vitro studies [26-28].
Materials and Methods
Study design
This study was a pilot double-blind placebo-controlled randomized clinical trial. This study recruited patients from October 22, 2020, to January 7, 2021, at two educational hospitals of Mazandaran University of Medical Science, in the north of Iran. Written informed consent was obtained from all patients, or their legal representatives if they were unable to provide consent.
Sixty patients who had inclusion criteria entered the study after the goals of the study were explained. Patients were randomly assigned to one of the two parallel treatment groups using a block randomization method. To keep the blindness, a computer-based 5-digit random code was assigned to each patient.
In this study, 169 patients were studied, of whom 60 patients met the inclusion criteria and were randomized with a 1:1 ratio to receive chlorpromazine (Tehran-Shimi Pharmaceutical Company, Iran, 25 mg) three times a day or the placebo. Both groups received atazanavir /ritonavir 300 mg/100 mg (Mylan Pharmaceutical Pvt, India) once a day for up to 14 days in the hospital.
The placebo was made in the Department of Pharmaceutics of the School of Pharmacy using Avicel (microcrystalline cellulose).
Inclusion criteria
Patients who had all of these criteria were included: 1) The age of 18-70 years, 2) Being diagnosed with COVID-19 according to clinical symptoms, confirmed by reverse transcription polymerase chain reaction (RT-PCR) or CT scan of the lungs, 3) Intend to receive atazanavir/ritonavir as antiviral regimen, 4) The SpO2 of less than 94 mmHg (without oxygen), oral temperature >37.2 °C, respiratory rate (RR) >24/min, or having dyspnea.
Exclusion criteria
Patients who had each of these criteria were excluded: 1) Those who did not give informed consent; 2) Receiving antipsychotic medicines at present or during the last 14 days; 3) History of seizure, Parkinson’s disease, or dementia; 4) Liver dysfunction; 5) History of pheochromocytoma; 6) Allergy to phenothiazine drugs; 7) Existence of any major drug interactions between the patient’s routine medications and chlorpromazine; 8) Pregnancy or breastfeeding; 9) Previously treated for COVID-19 or enrolment in other trials.
Outcome measures
The primary outcome of the study was the improvement of SpO2.
Secondary outcomes were the day of hospitalization, the need for ICU admission, lymphocyte count, and the rate of conversion of RT-PCR positive test to negative result. Initial PCR was performed for all patients. The next PCR was performed on the fifth or discharge day, whichever was earlier, and if positive, it was repeated a week later.
The complete blood count (CBC) and biochemical tests, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK), lactate dehydrogenase (LDH), C - reactive protein (CRP) as well as erythrocyte sedimentation rate (ESR) were conducted on days 1, 3, 5, 7, and 10. Oxygen saturation and respiratory rate (RR) were evaluated for all days of hospitalization. On all days of hospitalization, patients were evaluated for any potential side effects caused by chlorpromazine. If the side effects were bothersome or serious, patients were excluded from the study.
Statistical analysis
The normality of data was checked by Shapiro-Wilk Test. Independent sample t-test or Mann-Whitney U test was used for the comparison of continuous variables between the two groups. Wilcoxon matched-pair signed-rank test and chi-square test were used for the comparison of continuous variables and qualitative data before and after treatment, respectively. Repeated measures ANOVA was used to determine the changes of variables (e.g. laboratory data) on different days in each group.
The trend of changes in laboratory data and O2 saturation was compared by the generalized estimating equation (GEE) model. SPSS software, version 26.0 was applied for statistical analysis.
Result
Patients
A total of sixty subjects were enrolled in this study, including the chlorpromazine group (n=30) and placebo group (n=30). Thirteen patients (ten patients in the CLPZ group and three patients in the placebo group) did not continue the study (Figure 1).

Table 1 shows the baseline demographic and clinical characteristics of the 47 patients who completed the study. The mean age of patients was 52±12.74 years, and 28% of the patients were men. The most common presenting symptoms were fever (defined as temperature ≥37.8) (61.7%), dyspnea (53.2%), elevated CRP (50.23%), and cough (38.3%). All patients had lung injury on the first day and the mean percentage of their lung involvements was 48.9±14.65.

The WBC counts were 5702±2437 cells/µL. On admission, lymphopenia (lymphocyte count <1500 cells/µL) was found in 33(70.2%) patients. On admission, patients had mild to moderate pneumonia as documented by clinical manifestations and CT findings. There were no between-group differences in baseline laboratory test results (e.g. CRP, WBC, and lymphocyte counts) and CT scores for lung lesions (Table 2).

Regarding the incidence of adverse effects, xerostomia was seen in five cases (16.6%) in the chlorpromazine group and one case (6.7%) in the placebo group. Somnolence occurred in ten cases (33.3%) of the chlorpromazine group and two cases of the placebo group. Dizziness was seen in four cases (13.3%) in the chlorpromazine group. Six patients from the drug group were excluded from the study due to intolerance to side effects, one case was due to xerostomia, three cases were due to somnolence, and the other two cases were due to somnolence and dizziness. The mortality rate in the chlorpromazine group was one case (3.3%) and in the placebo group, was two cases (6.7%) (Table 3).

Primary outcomes
There was no significant difference in oxygen saturation in the chlorpromazine group compared to the placebo group during the hospital stay.
Secondary outcome
The duration of hospitalization in the chlorpromazine group (7.4±2.7) was almost 0.8 days less than the placebo group (8.2±3), but the difference was not significant (P=0.2). In the placebo group, an RT-PCR test was performed for all individuals; out of 22 people whose RT-RT-PCR test were positive (81.5%) on the day one of hospitalization, five (18.5%) patients had a positive PCR test on the seventh day, which represents a decrease of 77.3%. On the other hand, in the chlorpromazine group, an RT-PCR test was performed for all individuals; out of 14 people who had an RT-PCR positive test (70%) on the first day of hospitalization, two (10%) patients on the seventh day were RT-PCR-positive indicating an 85.7% decrease (Table 4).

On the 14th day of hospitalization, none of the remaining 16 patients in the chlorpromazine group had a positive test (100% reduction) and in the placebo group, out of 20 patients, only one test was positive (95% reduction). The mean lymphocytes counts were 1190±657 cells/µL and 1353±518 cells/µL in the chlorpromazine and placebo groups on the first days of admission whereas they decreased to 1069±580 cells/µL and 1034±634 cells/µL after chlorpromazine and placebo treatments, respectively. The mean CRP levels on the first day for the placebo and chlorpromazine groups were 53.1±27.1 mg/dL and 46.8±24.8 mg/dL, which decreased to 44±30.2 mg/dL and 40.3±32.5 mg/dL after the treatment period, respectively. The median time from the onset of symptoms to enrolling in the trial was 6.43±1.9 days. The mean severity of lung involvement according to CT scan was 44.9±14.65% and the mean oxygen saturation on admission to the hospitalization was 93.62±2.8. The mean of ESR, CRP, CPK, and LDH levels in patients showed no significant difference between the two groups (Table 5).


Discussion
This randomized clinical trial found that adding chlorpromazine to the antiviral regimen of patients with moderate COVID-19 was not significantly effective on the SpO2 or days of ICU or non-ICU stay.
Lymphocyte count is one of the most significant and consistent trends, suggesting that this indicator might reflect the disease progression, and lymphopenia is a predictor of prognosis in COVID-19 patients [26]. Patients with severe disease had more prominent laboratory abnormalities (including lymphopenia and elevated CRP) than those with non-severe disease [27]. Lymphopenia (lymphocytes <1500 per mm3) is the most common lab finding in COVID-19 and is found in as many as 70% of the patients on admission. All patients had high CRP levels with an average of 50.23±29.35 on the first day of hospitalization.
This is the second clinical trial using chlorpromazine in patients with COVID-19, which was performed as a double-blind randomized study in two hospitals, while another study in France on chlorpromazine was a single-blind study [28].
Multiple drugs with in vitro antiviral activity against SARS-CoV-2 and/or immunomodulatory effects have been suggested to be clinically beneficial. In our study, atazanavir-ritonavir was prescribed as an antiviral regimen based on the national guideline regarding the treatment of COVID-19. Lymphopenia was observed in 70% of patients on admission, while lymphocyte counts showed no significant increase after 3, 5, 7, and 10 days of treatments to the normal range (P=0.67).
Elevated CRP levels (≥6 mg/dL) as positive CRP was observed in all patients on admission, while chlorpromazine could not significantly reduce CRP levels after 3, 5, 7, 10 days of treatment to the negative range and there was no statistically significant difference between the two groups according to changes in CRP during the treatment.
In a study conducted in 2014 to investigate the inhibitory effect of chlorpromazine on the Huh 7 cell line, the drug completely inhibited the proliferation of all seven cell lines at a dose of 12 μM. On the other hand, this study showed that the addition of chlorpromazine 1 hour prior to cell line infection led to a 2-log reduction of virus progeny titers, and the addition of chlorpromazine 1 hour after cell line infection reduced the virus by half to one log [29].
In a group of patients receiving the chlorpromazine, no life-threatening side effects were observed, and the annoying side effect in the patients was dry mouth.
In the treatment of HIV patients, atazanavir is a potent, well-tolerated, and effective once-daily protease inhibitor with a low pill burden [30]. A 96-week analysis suggested that the long-term efficacy of atazanavir/ritonavir monotherapy was inferior as compared to atazanavir/ritonavir plus two nucleosides reverse transcriptase inhibitors (NRTIs). Monotherapy was also associated with a lower incidence of adverse events [31].
Remdesivir demonstrated clinical benefit in a placebo-controlled trial in patients with severe COVID-19. However, its effect in patients with moderate disease who were randomized to a 10-day course of remdesivir, showed no statistically significant difference in clinical status (P=0.18 by Wilcoxon rank sum test) compared to standard care 11 days after treatment, but in a 5-day course, it showed a statistically significant difference (odds ratio, 1.65; 95% CI, 1.09-2.48; P=0.02) [32]. In this trial, atazanavir was considered the standard antiviral treatment for patients with moderate involvement, and in the case of disease progression or inadequate response, it was changed to another drug, including remedesivir, at the discretion of the clinician. According to the results, in the chlorpromazine group, fewer people (two vs. ten in the placebo group) needed to change their antiviral regimen to remdesivir (P=0.05).
Our study was designed according to our country’s guidelines and hydroxychloroquine was ordered. Remdesivir was ordered for severe COVID-19 patients and availability of this drug was limited; thus, we used atazanavir as the primary antiviral.
Conclusion
Based on this study, adding chlorpromazine is not effective in the alleviation of clinical symptoms and modifying laboratory tests of moderate to severe COVID-19 in hospitalized patients that received atazanavir/ritunavir.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1399.617) and registered at the Iranian Clinical Trial Registration Database (IRCT) (No.: IRCT20200913048708N1).
Funding
This study was supported by the Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: David Darvishnia, Roya Ghasemian, Ahmad Alikhani and Jafar Akbari; Methodology: Nematollah Ahangar, Hamideh Abbaspour and Ebrahim Salehifar; Investigation, writing the paper, review & editing: All authors; Data analysis: Sima Ramezaninejad, Hamid Reza Namvar and Zahra Akbari.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
We thank all patients who participated in this trial and their families. We appreciate the healthcare personnel at Emam Komeini Hospital and Razi Hospital of the Mazandaran University of Medical Sciences who have given their lives in the care of patients with COVID-19.
References
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271-80.e8. [DOI:10.1016/j.cell.2020.02.052] [PMID] [PMCID]
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020; 9(1):221-36. [DOI:10.1080/22221751.2020.1719902] [PMID] [PMCID]
- World Health Organization (WHO). Novel coronavirus - Thailand (ex-China). Geneva: World Health Organization 2020. [Link]
- Rothan H, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (covid-19) outbreak. J Autoimmun. 2020; 109:102433. [DOI:10.1016/j.jaut.2020.102433] [PMID] [PMCID]
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of covid-19. J Pharm Anal. 2020; 10(2):102-8. [DOI:10.1016/j.jpha.2020.03.001] [PMID] [PMCID]
- Kirchdoerfer RN, Wang N, Pallesen J, Wrapp D, Turner HL, Cottrell CA, Et al. Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Sci Rep. 2018; 8(1):15701. [DOI:10.1038/s41598-018-34171-7]
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in covid-19 patients with severe respiratory failure. Cell Host Microbe. 2020; 27(6):992.e3-1000. [DOI:10.1016/j.chom.2020.04.009] [PMID] [PMCID]
- Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, et al. An update on covid-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021; 19(7):877-88. [DOI:10.1080/14787210.2021.1863146] [PMID]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579: 270-3. [DOI:10.1038/s41586-020-2012-7] [PMID] [PMCID]
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020; 180(7):934-43. [DOI:10.1001/jamainternmed.2020.0994] [PMID] [PMCID]
- Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C,et al. Inhaled budesonide in the treatment of early covid-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021; 9(7):763-72. [DOI:10.1016/S2213-2600(21)00160-0] [PMID]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11):1061-9. [DOI:10.1001/jama.2020.1585] [PMID] [PMCID]
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020; 368:m1091. [DOI:10.1136/bmj.m1091] [PMID] [PMCID]
- Ohashi H, Watashi K, Saso W, Shionoya K, Iwanami S, Hirokawa T, et al. Potential anti-covid-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021; 24(4):102367. [DOI:10.1016/j.isci.2021.102367] [PMID] [PMCID]
- Contini A. Virtual screening of an FDA approved drugs database on two covid-19 coronavirus proteins. 2020. [Unpublished article] [DOI:10.26434/chemrxiv.11847381]
- Raja A, Lebbos J, Kirkpatrick P. Atazanavir sulphate. Nat Rev Drug Discov. 2003; 2(11):857-8. [DOI:10.1038/nrd1232] [PMID]
- Fintelman-Rodrigues N, Sacramento CQ, Lima CR, da Silva FS, Ferreira AC, Mattos M, et al. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. bioRxiv. 2020. [Unpublished article] [DOI:10.1101/2020.04.04.020925]
- Rahmani H, Davoudi-Monfared E, Nourian A, Nabiee M, Sadeghi S, Khalili H, et al. Comparing outcomes of hospitalized patients with moderate and severe covid-19 following treatment with hydroxychloroquine plus atazanavir/ritonavir. DARU. 2020; 28(2):625-34. [DOI:10.1007/s40199-020-00369-2] [PMID] [PMCID]
- Stip E, Rizvi TA, Mustafa F, Javaid S, Aburuz S, Ahmed NN, et al. The large action of chlorpromazine: Translational and transdisciplinary considerations in the face of covid-19. Front Pharmacol. 2020; 11:577678. [DOI:10.3389/fphar.2020.577678] [PMID] [PMCID]
- Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007; 81(16):8722-9. [DOI:10.1128/JVI.00253-07] [PMID] [PMCID]
- Dyall J, Coleman CM, Hart BJ, Venkataraman T, Holbrook MR, Kindrachuk J, et al. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014; 58(8):4885-93. [DOI:10.1128/AAC.03036-14] [PMID] [PMCID]
- Dyall J, Gross R, Kindrachuk J, Johnson RF, Olinger GG Jr, Hensley LE, et al. Middle East respiratory syndrome and severe acute respiratory syndrome: Current therapeutic options and potential targets for novel therapies. Drugs. 2017; 77(18):1935-66. [DOI:10.1007/s40265-017-0830-1] [PMID] [PMCID]
- Mengozzi M, Fantuzzi G, Faggioni R, Marchant A, Goldman M, Orencole S, et al. Chlorpromazine specifically inhibits peripheral and brain TNF production, and up-regulates IL-10 production, in mice. Immunology. 1994; 82(2):207-10. [PMID] [PMCID]
- Delay J, Deniker P, Harl JM. [Therapeutic use in psychiatry of phenothiazine of central elective action (4560 RP) (Hungarian)]. Ann Med Psychol. 1952; 110:112-7. [PMID]
- Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, et al. Safety, tolerability, and risks associated with first-and secondgeneration antipsychotics: A state-of-the-art clinical review. Ther Clin Risk Manag. 2017; 13:757-77. [DOI:10.2147/TCRM.S117321] [PMID] [PMCID]
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of covid-19: A descriptive and predictive study. Signal Transduct Target Ther. 2020; 5(1):33. [DOI:10.1038/s41392-020-0148-4] [PMID] [PMCID]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708-20. [DOI:10.1056/NEJMoa2002032] [PMID] [PMCID]
- National Library of Medicine. Repurposing of chlorpromazine in covid-19 treatment (reCoVery). Bethesda: National Library of Medicine; 2020. [Link]
- de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014; 58(8):4875-84. [DOI:10.1128/AAC.03011-14] [PMID] [PMCID]
- Murphy RL, Sanne I, Cahn P, Phanuphak P, Percival L, Kelleher T, et al. Dose-ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral-naive subjects: 48-week results. AIDS. 2003; 17(18):2603-14. [DOI:10.1097/00002030-200312050-00007] [PMID]
- Galli L, Spagnuolo V, Bigoloni A, D'Arminio Monforte A, Montella F, Antinori A, et al. Atazanavir/ritonavir monotherapy: 96 week efficacy, safety and bone mineral density from the MODAt randomized trial. J Antimicrob Chemother. 2016; 71(6):1637-42. [DOI:10.1093/jac/dkw031] [PMID]
- Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate covid-19: A randomized clinical trial. JAMA. 2020; 324(11):1048-57. DOI:10.1001/jama.2020.16349] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






