Volume 9, Issue 3 (2023)
Pharm Biomed Res 2023, 9(3): 201-210 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Muhammad Fannami I, Hyedima Garba S, Oliver Hamman W, Chiroma S M. Acute Toxicity Study of Methanol Extract of Baobab (Adansonia digitata Linn) Fruit Shell Extract in Mice. Pharm Biomed Res 2023; 9 (3) :201-210
URL: http://pbr.mazums.ac.ir/article-1-475-en.html
URL: http://pbr.mazums.ac.ir/article-1-475-en.html
1- Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Maiduguri Borno-Nigeria, Maiduguri, Nigeria.
2- Department of Human Anatomy, Faculty of Basic Medical Sciences, Ahmadu Bello University, Zaria, Nigeria.
2- Department of Human Anatomy, Faculty of Basic Medical Sciences, Ahmadu Bello University, Zaria, Nigeria.
Full-Text [PDF 3021 kb]
(663 Downloads)
| Abstract (HTML) (2390 Views)
Full-Text: (723 Views)
Introduction
Medicinal plants and plant extracts and products form an essential part of our daily life and are considered to be the mainstay of traditional medicine [1, 2]. They are used in the treatment and prevention of several diseases, particularly chronic and non-communicable ones, in nature [2]. The postulation that medicinal plants are natural and their preparations are harmless for the treatment of many diseases may have influenced their wide use by 70% of Asians and Africans [3, 4]. However, their origins should not assure their safety, since there are no sufficient studies on the efficacy, safety, and toxicity to support their beneficial claims [2]. Against this backdrop, the scientific community has developed three concepts. Firstly, a study must be conducted to show the safety profiles of any product/compound that are claimed to be beneficial. Secondly, the assessment of its chemical constituents, and thirdly to set guidelines to examine the proposed folkloric applications. Therefore, every medicinal plant requires verification for public acceptance and subsequently the necessity of toxicological reports [2].
Baobab (Adansonia digitata L.) is a part of Africa’s indigenous iconic tree, which belongs to the Bombacaceae family and is widely distributed across sub-Saharan Africa and Western Madagascar [5]. The tree is fondly called “the small pharmacy tree” as all its parts, such as bark, leaves, and fruits have been reported to be an important source of traditional medicine, food, shelter, and livelihood to the communities producing them in Africa [6-8]. Recently, a dramatic increase has been seen in the global demand for baobab raw materials, especially its fruit pulp by food industries, which resulted in its increased importance and commercial value [5]. The baobab fruit pulp has been approved in Europe as a food ingredient since 2008, while in the United States, it received a Generally Recognized Safe (GRAS) status in 2009 [8].
Due to its high demand, baobab fruits are gathered in small or large quantities and sold to commercial companies or vendors for further processing into different products, such as baobab pulp powder, seed oil, fruit juice, and other valued products. During these operational processes, a huge quantity of baobab fruit shells (BFS) is generated as a waste product, which has less or no economic value. It is usually thrown away around the harvesting or processing areas; thus, littering the environment [6], affecting farmlands, and harboring dangerous reptiles. Despite the fact that baobab is a valuable plant with several applications, the BFS has not yet been the subject of serious investigations. In the last two decades, only a handful of studies have been reported on the usefulness of BFS [9]. Extracts of BSF have been reported to exhibit high antioxidant capacity, probably due to the presence of bioactive compounds. Some studies reported the usefulness of BFS in the production of activated carbon for the treatment of environmental pollutants [10, 11]. More recently, Ismail et al. [6] for the first time characterized the major phenolic constituents of BSF and also demonstrated their antioxidants and inhibitory effects on α-glucosidase and α-amylase in vitro. These may serve as a novel approach to the Figureht against type 2 diabetes.
However, after an extensive literature search by the authors, there is a dearth of information on in vivo studies on the oral acute toxicity of BFS extract. Consequently, this study presented the first attempt to evaluate the single-dose toxicity of methanolic extract of BFS in mice. The mice were monitored for the physical sign of toxicity and mortality. Their serum biochemical parameters for kidney and liver functions were evaluated, besides the histopathology of kidneys, liver, and brain, which are vital organs in toxicology studies.
Materials and Methods
Material
Plant material
A. digitata L fruit shell was purchased from Gamboru market Maiduguri, Borno State. The taxonomic identification of the fruit shell was established by a Botanist in the Department of Biological Sciences, University of Maiduguri. The specimen is deposited at the herbarium Department of Human Anatomy, the University of Maiduguri with voucher number UM/HAH/2021/001.
Chemical substances
Ketamine injection (Swiss Parental, PVT LTD, India), methanol (Sigma Chemical Company, Saint Louis, MO, USA), and all other chemicals used in this study were of analytical grade.
Preparation of methanolic extract of A. digitata fruit shell
BFS were rinsed with tap water for the removal of dust and other impurities, dried under shade, pounded into powder, and sieved. Then, 1 kg of the powdered shell was soaked in 80% methanol for three days with agitations at some intervals. The mixture was filtered using filter paper (Watchman No. 3) and concentrated to dryness using a rotary vacuum evaporator to obtain the extract. Afterward, 44.5 g of methanolic extract from BFS was obtained and kept airtight for later use. It was dissolved in distilled water before oral administration to the rats.
Phytochemical screening (qualitative and quantitative)
Qualitative screening of methanol extract of BFS
Test for alkaloids, saponin, carbohydrates, proteins and amino acids, flavonoids, tannins, cardenolide, saponin glycosides, terpenoids, cardiac glycoside, free anthraquinones, phenols, and combined anthraquinones was carried out using a standard method [12, 13].
Quantitative measurement of methanol extract of BFS
The quantities of alkaloids, phenols, saponin, tannin, and flavonoids were all measured [14-17].
Animals
Experimentations were conducted on female mice weighing between 18 to 25 g, approximately 5-6 weeks old. They were kept in the Animal House, Department of Biochemistry University Maiduguri, in accordance with the principles for the care and use of laboratory animals. The laboratory is well ventilated and the mice were exposed to 12 hours light/dark cycle and administered with standard grower mash (Vital Feed, Grand Cereal Jos, Nigeria) (proteins and amino acids, carbohydrates, fats, minerals, and vitamins) and water ad libitum.
Acute toxicity study
The study was conducted in two phases as earlier described [18], all mice were weighed before the dose administration. In phase I, four groups of mice (n=3) were orally administered with distilled water or BFS at doses of 10, 100, and 1000 mg/kg, respectively. The mice were observed for the first 4 hours and subsequently for 14 days for signs of toxicity; body weakness, hyperactivity, lack of appetite, sleep, erection of hair, brushing of the nose on the floor, salivation, stretching of the body, coma, or death. In phase II, four groups of one mouse each were orally administered with distilled water or BFS at doses of 1600, 2900, and 5000 mg/kg, respectively. They were also observed for the first 4 hours and the subsequent 14 days for any signs of toxicity and mortality. All mice were fasted overnight and euthanized on day 15 of the experiment where blood samples were taken and organs harvested.
Collection of blood samples
Blood samples were collected through cardiac puncture and kept in plain bottles. They were centrifuged at 5000 rpm for 5 minutes. The serum was transferred into another plain bottle and used for biochemical analyses.
Relative organ weight
After euthanasia on day 15, organs, like the liver, kidneys, brain, heart, lungs, and spleen were carefully harvested and weighed in grams. The relative organ weight of each mouse was then calculated as:
Relative organ weight=Absolute organ weight (g)×100/Body weight of mouse on sacrifice day (g).
Biochemical analysis
Biochemical analyses carried out include the measurement of the activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) as well as serum total protein (TP), and albumin (ALB) to assess the liver function. While creatinine (CREA), urea, sodium (Na+), potassium (K+), chlorine (Cl-), and bicarbonate (HCO3) concentrations were evaluated for kidney function. All the biomarkers were processed using the available commercial kits according to the manufacturer’s manual.
Histology
The liver and kidneys were harvested and fixed in 10% formalin, while the brain was fixed in 10% Bouin’s fluid. After seven days, the tissues underwent routine histological preparations and were stained with Harris hematoxylin for 15 minutes and counter-stained in 1% alcoholic eosin for 30 seconds, and then mounted in DPX [19]. The prepared slides were qualitatively evaluated using a light microscope. Micrographs were snapped using a microscope (MBJX-ISCOPE, Los Angeles, USA) equipped with a digital camera (M500, X64, v. 3.7) at different magnifications.
Statistical analysis
All values obtained were expressed as Mean±SE. The one-way analysis of variance (ANOVA) was used to determine the difference between groups, followed by Tukey’s post hoc test, and P<0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software, version 9 (San Diego, CA, USA).
Results
Types of phytochemicals present in methanol extract of BFS
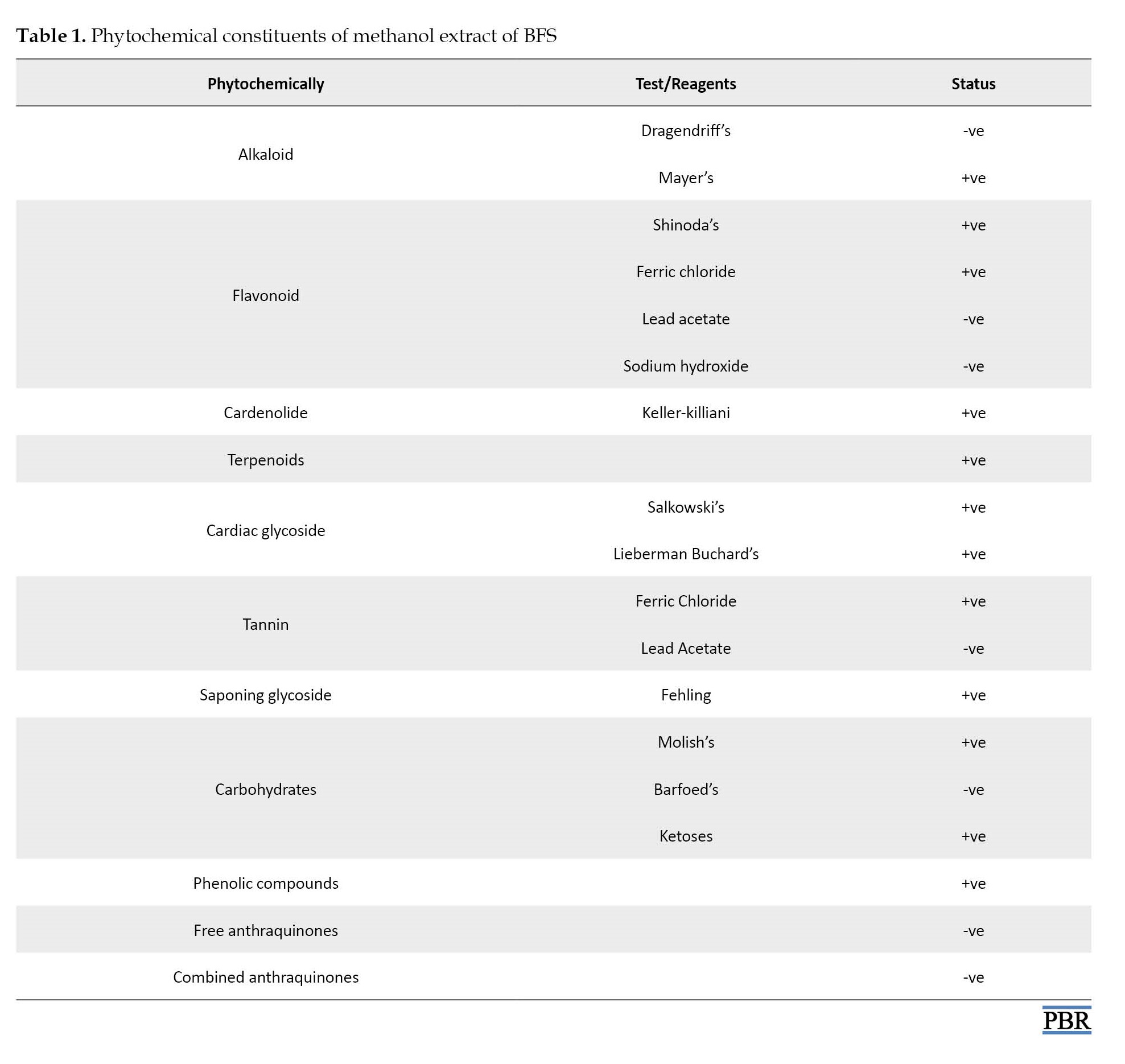
Qualitative preliminary phytochemical screening of BFS revealed the existence of different phyto-constituents (Table 1).
Quantities of phytochemicals present in methanol extract of BFS
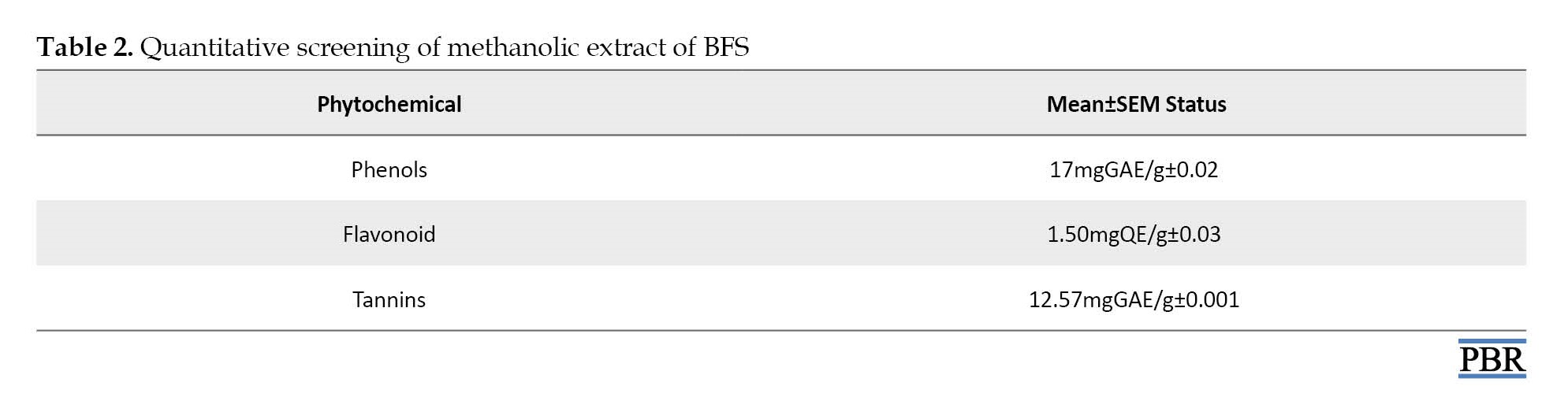
The phytochemicals found in the methanol extract of BFS were further subjected to quantitative measurements to find out the quantity of each compound therein (Table 2).
Clinical signs
No mortality was recorded and no serious sign of toxicity was seen in the mice within the 14 days of observation after a single dose of BFS ranging from 10 to 5000 mg/kg. Therefore, the extract seemed to be safe at 5000 mg/kg body weight and the LD50 was considered to be >5000 mg/kg.
Effect of methanol extract of BFS on anthropometric parameters in mice
Body weight
A steady increase in body weight was observed among all the groups of mice administered with single but different doses of BFS ranging from 10 mg/kg to 1000 mg/kg after 14 days (Table 3). However, the one-way ANOVA revealed no observable differences in the initial body weight [F(3, 8)=1.674, P=0.2488], final body weight [F(3, 8)=1.097, P=0.4048], and weight gain [F(3, 8)=0.8018, P=0.5270] among all the experimental groups of mice.

Mice relative organ weight
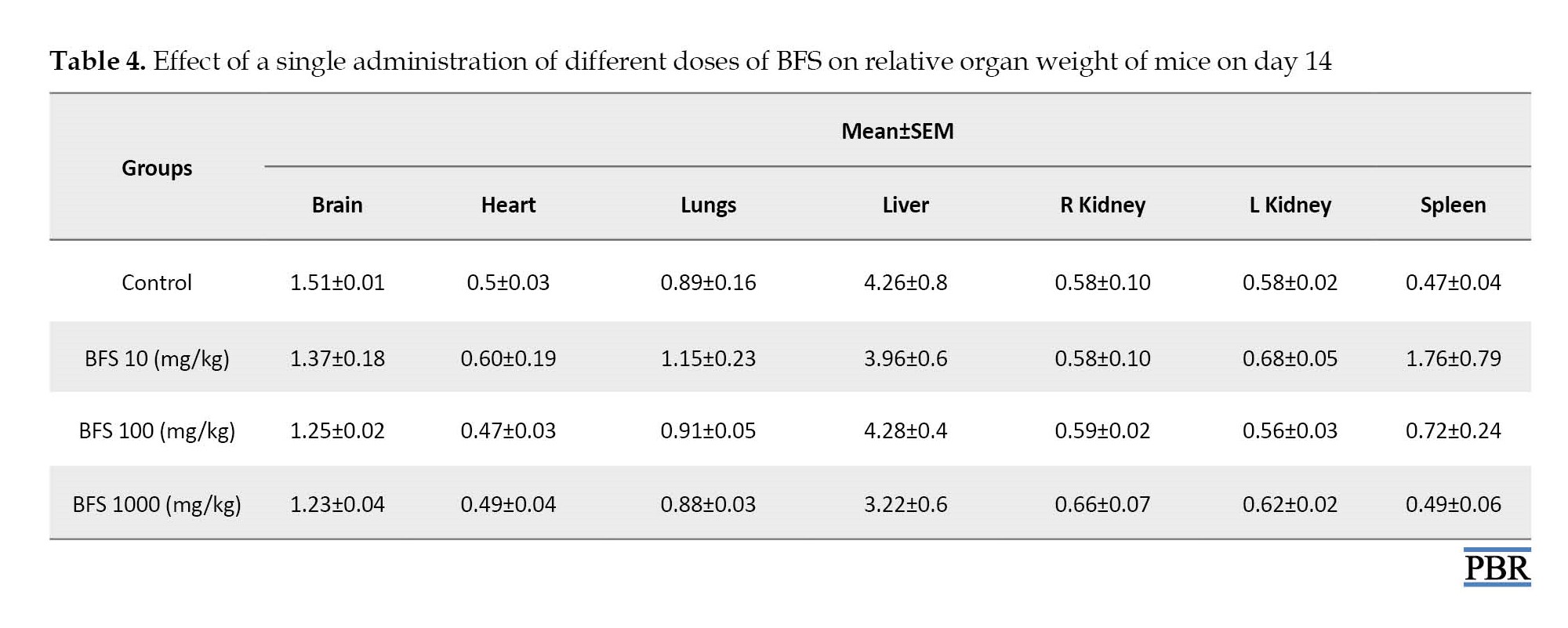
The values for relative organ weights were indicated in Table 4. One-way ANOVA results showed no statistically significant differences (P>0.05) in the relative organ weights evaluated, which included brain [F(3, 8)=1.427, P=0.3050], heart [F(3, 8)=0.3575, P=0.7854], lungs [F(3, 8)=0.8167, P=0.5199], liver [F(3, 8)=1.115, P=0.3985], left kidney [F(3, 8)=2.503, P=0.1332], right kidney [F(3, 8)=0.2554, P=0.8555], and spleen [F(3, 8)=2.115, P=0.1766] among all the treatment groups.
Effect of methanol extract of BFS on biochemical parameters in mice
Serum levels of liver function biomarkers
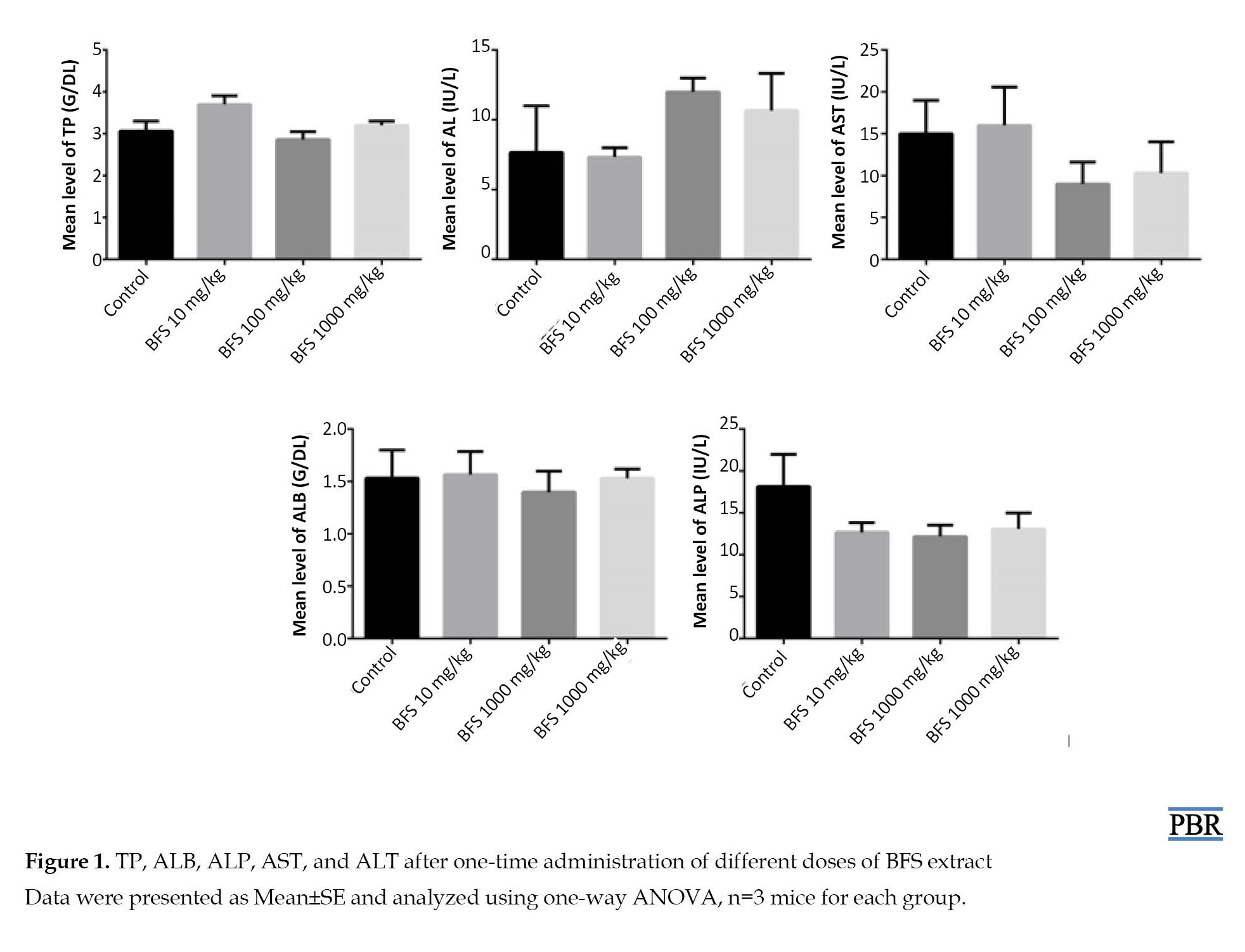
The toxicological effect of methanol extract of BFS was evaluated on liver function biomarkers. One-way ANOVA results showed that there was no significant change (P>0.05) in the serum levels of ALT [F[F(3, 8)=1.060, P=0.4182], ALP [F(3, 8)=1.436, P=0.3025], AST [F(3, 8)=0.8171, P=0.5197], TP [F(3, 8)=3.549, P=0.0675], and ALB [F(3, 8)=0.1561, P=0.9228] at all treatment doses of BFS in comparison to the control group of mice (Figure 1).
Serum levels of kidneys function biomarkers
The toxicological effect of methanol extract of BFS was ascertained on the kidney function parameters. One-way ANOVA showed no statistical significant differences (P>0.05) in the serum levels of urea [F(3, 8)=1.431, P=0.3040], creatinine [F(3, 8)=0.7520, P=0.5523], sodium [F(3, 8)=1.347, P=0.3262], potassium [F(3, 8)=1.209, P=0.3672], and chloride [F(3, 8)=4.608, P=0.0373] compared to the control group (Figure 2). However, one-way ANOVA revealed a statistically significant difference in the level of bicarbonate [F(3, 8)=5.602, P=0.0229]. Tukey’s post hoc test confirmed the marked increase (P<0.05) in bicarbonate levels in mice receiving BSF 10 mg/kg compared to the control group.

Effect of methanol extract of BFS on the histology of kidneys, liver, and cerebellum in mice
Histology of liver
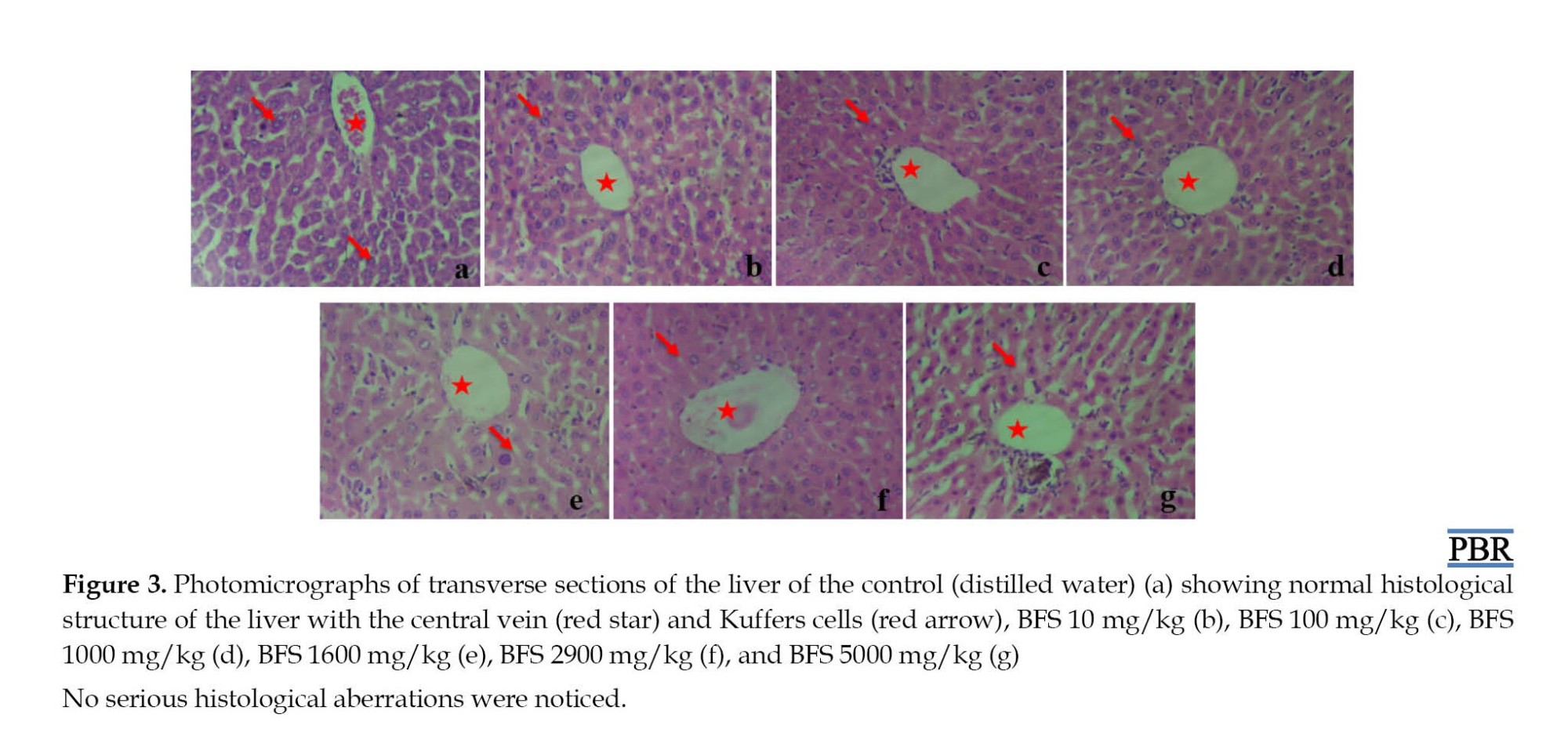
A qualitative analysis of the liver histology was conducted to ascertain the effects of methanol extract of BFS. The photomicrograph of the control mice livers showed normal sinusoids, hepatocytes, and central vein (Figure 3a). The photomicrographs of the livers of mice that received BFS ranging from 10 mg/kg to 5000 mg/kg body weight also showed no significant difference than the control group (Figure 3b-g).
Histology of kidneys
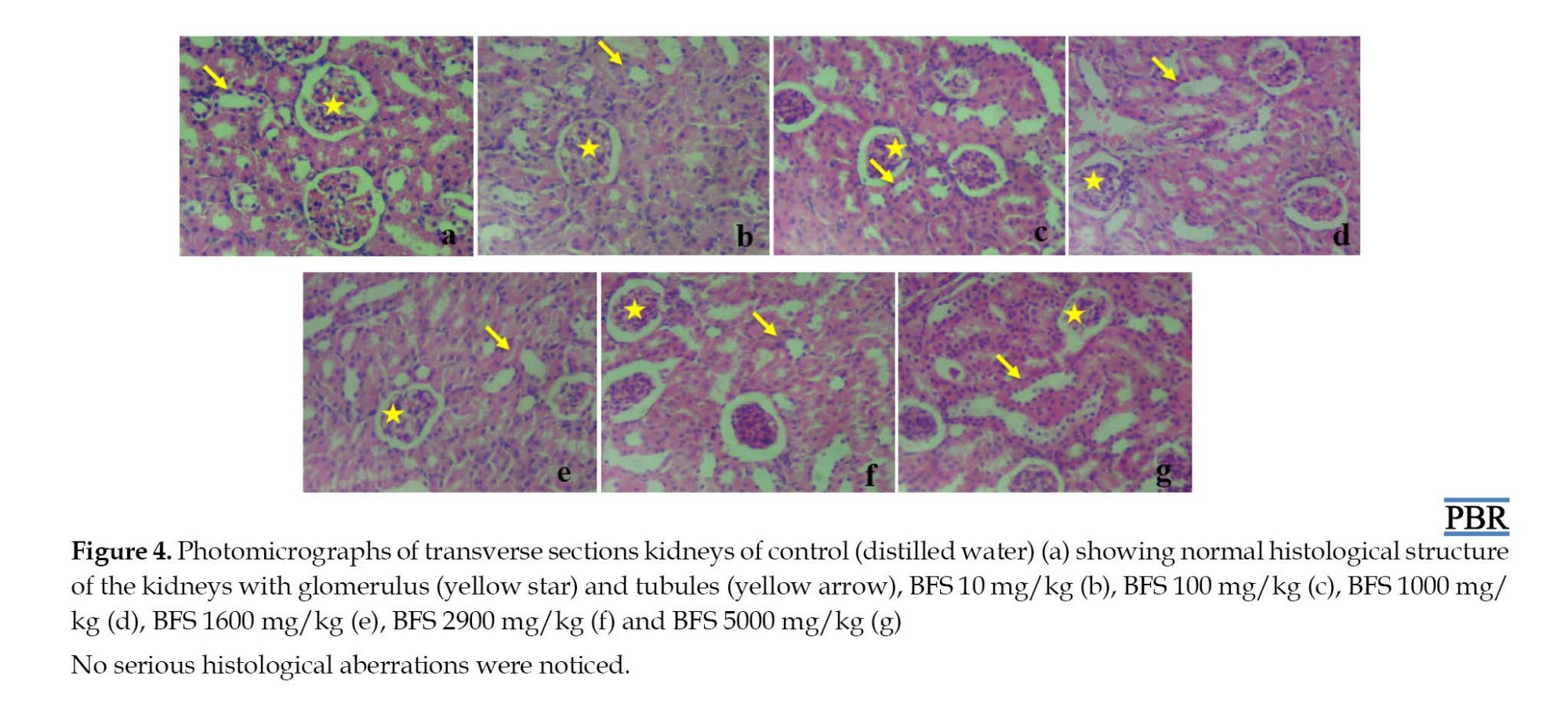
Qualitative analysis of the mice’s kidney histology was carried out to ascertain the effects of methanol extract of BFS. Photomicrographs of the control mice kidneys showed normal convoluted tubules and glomeruli (Figure 4a). The photomicrographs of the kidneys of mice that received a single dose of BFS ranging from 10 mg/kg to 5000 mg/kg body weight also showed a normal appearance of convoluted tubules and glomeruli (Figure 4b-g).
Histology of the cerebellum
Qualitative analysis of the histology of the mice cerebellar cortex was performed to check the effects of single-dose exposure to methanol extract of BFS. Photomicrograph of the cerebellar cortex of the control group of mice showed normal cell layers, which included the Molecular Layer (ML), middle Purkinje cells layer (PCL), and inner granular layer (GL) with aggregation of granular cells (Figure 5a). The photomicrographs of the cerebellum of mice that received a single dose of BFS ranging from 10 mg/kg to 5000 mg/kg (Figure 5b-g) also showed a similar pattern as there were no obvious histological aberrations.

Discussions
In this study, qualitative analysis of a methanolic extract of A. digitata fruit shell indicated the presence of cardenolide, terpenoids, cardiac glycoside, saponin glycoside alkaloid, and phenolic compounds. Similar results were reported after the phytochemical screening of the aqueous extract of A. digitata fruit pulp [20], which revealed the presence of tannins, saponin, phenol, glycosides, flavonoids, and alkaloids. In the present study, quantitative estimation of phytochemicals showed total flavonoid content to be 1.50 mgQE/g, which showed the presence of Quercetin. Quercetin is among the most commonly studied flavonoids, which possesses many health-promoting potentials, including anti-inflammatory, anti-diabetic, and anti-cancer properties, and also reduces the risk of cardiovascular diseases [21, 22]. These potentials are associated with its potent antioxidant capacity by strengthening endogenous defense against free radicals, while the anti-cancer activity of quercetin is due to its ability to bind cellular receptors and proteins, which controls mechanisms of cell signaling and also inhibits enzymes responsible for the carcinogens activation [23]. Alkaloids are chemical compounds mostly containing basic nitrogen atoms and are used as a remedy for gout with analgesic, antihypertensive, vasodilator, muscle relaxant, antitumor, and anti-malarial activity [20, 24]. The presence of these classes of compounds, especially flavonoids and alkaloids in the aqueous extract of the fruit pulp of A. digitata could be responsible for its various medicinal and anti-oxidative uses [25]. Flavonoid is considered to have good antioxidant, antiviral, anti-inflammatory, anti-allergic, and anticancer properties and are appropriate therapeutic agents [26].
The present acute toxicity study showed that oral administration of methanol extract of BSF up to the dose of 5000 mg/kg body weight did not cause any persistent clinical signs nor mortality in mice. Consequently, this study proved that BSF was non-toxic up to 5000 mg/kg, and relatively safe to use. Substances that are higher than 5000 mg/kg via the oral route of administration are considered non-toxic [27]. Weight modulation is a very important characteristic of numerous medicinal agents [28, 29]. The present study explored body weight as well as relative organ weights in order to ascertain the toxicity of BFS extract. However, no changes in the body weights nor the relative organ weight of their liver, kidneys, brain, heart, lungs, and spleen were observed. This is suggestive of the safe nature of the extract at the doses tested. Further, no biochemical derangement was observed in both liver and kidney function biomarkers analyzed, while the histology of vital organs of metabolism, including the liver and kidney was intact besides the normal appearance of the mice brain’s cerebellum.
Conclusion
In conclusion, the acute oral dose of BFS methanolic extract up to 5000 mg/kg showed no evidence of toxicity or treatment-related mortality in mice. No changes were observed in the body and relative organs weights, liver and kidney function biomarkers, and also in the histology of their liver, kidneys, and cerebellum. Hence, the LD50 of BFS is more than 5000 mg/kg in mice and can be said to have a high margin of safety. However, a longer study duration is recommended to evaluate its toxic effects on fertility, teratogenicity, and carcinogenic potentials.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Postgraduate Board of Studies, Faculty of Basic Medical Sciences, the University of Maiduguri, which is in accordance with the ARRIVE guidelines for the Care and Use of Animals with code PGA/18/01/01/08807.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
All authors appreciated the valuable contributions made by NI Dibal, Ephraim Ayuba, and Sunday of the Human Anatomy Department University of Maiduguri.
References
Medicinal plants and plant extracts and products form an essential part of our daily life and are considered to be the mainstay of traditional medicine [1, 2]. They are used in the treatment and prevention of several diseases, particularly chronic and non-communicable ones, in nature [2]. The postulation that medicinal plants are natural and their preparations are harmless for the treatment of many diseases may have influenced their wide use by 70% of Asians and Africans [3, 4]. However, their origins should not assure their safety, since there are no sufficient studies on the efficacy, safety, and toxicity to support their beneficial claims [2]. Against this backdrop, the scientific community has developed three concepts. Firstly, a study must be conducted to show the safety profiles of any product/compound that are claimed to be beneficial. Secondly, the assessment of its chemical constituents, and thirdly to set guidelines to examine the proposed folkloric applications. Therefore, every medicinal plant requires verification for public acceptance and subsequently the necessity of toxicological reports [2].
Baobab (Adansonia digitata L.) is a part of Africa’s indigenous iconic tree, which belongs to the Bombacaceae family and is widely distributed across sub-Saharan Africa and Western Madagascar [5]. The tree is fondly called “the small pharmacy tree” as all its parts, such as bark, leaves, and fruits have been reported to be an important source of traditional medicine, food, shelter, and livelihood to the communities producing them in Africa [6-8]. Recently, a dramatic increase has been seen in the global demand for baobab raw materials, especially its fruit pulp by food industries, which resulted in its increased importance and commercial value [5]. The baobab fruit pulp has been approved in Europe as a food ingredient since 2008, while in the United States, it received a Generally Recognized Safe (GRAS) status in 2009 [8].
Due to its high demand, baobab fruits are gathered in small or large quantities and sold to commercial companies or vendors for further processing into different products, such as baobab pulp powder, seed oil, fruit juice, and other valued products. During these operational processes, a huge quantity of baobab fruit shells (BFS) is generated as a waste product, which has less or no economic value. It is usually thrown away around the harvesting or processing areas; thus, littering the environment [6], affecting farmlands, and harboring dangerous reptiles. Despite the fact that baobab is a valuable plant with several applications, the BFS has not yet been the subject of serious investigations. In the last two decades, only a handful of studies have been reported on the usefulness of BFS [9]. Extracts of BSF have been reported to exhibit high antioxidant capacity, probably due to the presence of bioactive compounds. Some studies reported the usefulness of BFS in the production of activated carbon for the treatment of environmental pollutants [10, 11]. More recently, Ismail et al. [6] for the first time characterized the major phenolic constituents of BSF and also demonstrated their antioxidants and inhibitory effects on α-glucosidase and α-amylase in vitro. These may serve as a novel approach to the Figureht against type 2 diabetes.
However, after an extensive literature search by the authors, there is a dearth of information on in vivo studies on the oral acute toxicity of BFS extract. Consequently, this study presented the first attempt to evaluate the single-dose toxicity of methanolic extract of BFS in mice. The mice were monitored for the physical sign of toxicity and mortality. Their serum biochemical parameters for kidney and liver functions were evaluated, besides the histopathology of kidneys, liver, and brain, which are vital organs in toxicology studies.
Materials and Methods
Material
Plant material
A. digitata L fruit shell was purchased from Gamboru market Maiduguri, Borno State. The taxonomic identification of the fruit shell was established by a Botanist in the Department of Biological Sciences, University of Maiduguri. The specimen is deposited at the herbarium Department of Human Anatomy, the University of Maiduguri with voucher number UM/HAH/2021/001.
Chemical substances
Ketamine injection (Swiss Parental, PVT LTD, India), methanol (Sigma Chemical Company, Saint Louis, MO, USA), and all other chemicals used in this study were of analytical grade.
Preparation of methanolic extract of A. digitata fruit shell
BFS were rinsed with tap water for the removal of dust and other impurities, dried under shade, pounded into powder, and sieved. Then, 1 kg of the powdered shell was soaked in 80% methanol for three days with agitations at some intervals. The mixture was filtered using filter paper (Watchman No. 3) and concentrated to dryness using a rotary vacuum evaporator to obtain the extract. Afterward, 44.5 g of methanolic extract from BFS was obtained and kept airtight for later use. It was dissolved in distilled water before oral administration to the rats.
Phytochemical screening (qualitative and quantitative)
Qualitative screening of methanol extract of BFS
Test for alkaloids, saponin, carbohydrates, proteins and amino acids, flavonoids, tannins, cardenolide, saponin glycosides, terpenoids, cardiac glycoside, free anthraquinones, phenols, and combined anthraquinones was carried out using a standard method [12, 13].
Quantitative measurement of methanol extract of BFS
The quantities of alkaloids, phenols, saponin, tannin, and flavonoids were all measured [14-17].
Animals
Experimentations were conducted on female mice weighing between 18 to 25 g, approximately 5-6 weeks old. They were kept in the Animal House, Department of Biochemistry University Maiduguri, in accordance with the principles for the care and use of laboratory animals. The laboratory is well ventilated and the mice were exposed to 12 hours light/dark cycle and administered with standard grower mash (Vital Feed, Grand Cereal Jos, Nigeria) (proteins and amino acids, carbohydrates, fats, minerals, and vitamins) and water ad libitum.
Acute toxicity study
The study was conducted in two phases as earlier described [18], all mice were weighed before the dose administration. In phase I, four groups of mice (n=3) were orally administered with distilled water or BFS at doses of 10, 100, and 1000 mg/kg, respectively. The mice were observed for the first 4 hours and subsequently for 14 days for signs of toxicity; body weakness, hyperactivity, lack of appetite, sleep, erection of hair, brushing of the nose on the floor, salivation, stretching of the body, coma, or death. In phase II, four groups of one mouse each were orally administered with distilled water or BFS at doses of 1600, 2900, and 5000 mg/kg, respectively. They were also observed for the first 4 hours and the subsequent 14 days for any signs of toxicity and mortality. All mice were fasted overnight and euthanized on day 15 of the experiment where blood samples were taken and organs harvested.
Collection of blood samples
Blood samples were collected through cardiac puncture and kept in plain bottles. They were centrifuged at 5000 rpm for 5 minutes. The serum was transferred into another plain bottle and used for biochemical analyses.
Relative organ weight
After euthanasia on day 15, organs, like the liver, kidneys, brain, heart, lungs, and spleen were carefully harvested and weighed in grams. The relative organ weight of each mouse was then calculated as:
Relative organ weight=Absolute organ weight (g)×100/Body weight of mouse on sacrifice day (g).
Biochemical analysis
Biochemical analyses carried out include the measurement of the activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) as well as serum total protein (TP), and albumin (ALB) to assess the liver function. While creatinine (CREA), urea, sodium (Na+), potassium (K+), chlorine (Cl-), and bicarbonate (HCO3) concentrations were evaluated for kidney function. All the biomarkers were processed using the available commercial kits according to the manufacturer’s manual.
Histology
The liver and kidneys were harvested and fixed in 10% formalin, while the brain was fixed in 10% Bouin’s fluid. After seven days, the tissues underwent routine histological preparations and were stained with Harris hematoxylin for 15 minutes and counter-stained in 1% alcoholic eosin for 30 seconds, and then mounted in DPX [19]. The prepared slides were qualitatively evaluated using a light microscope. Micrographs were snapped using a microscope (MBJX-ISCOPE, Los Angeles, USA) equipped with a digital camera (M500, X64, v. 3.7) at different magnifications.
Statistical analysis
All values obtained were expressed as Mean±SE. The one-way analysis of variance (ANOVA) was used to determine the difference between groups, followed by Tukey’s post hoc test, and P<0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism software, version 9 (San Diego, CA, USA).
Results
Types of phytochemicals present in methanol extract of BFS
Qualitative preliminary phytochemical screening of BFS revealed the existence of different phyto-constituents (Table 1).

Quantities of phytochemicals present in methanol extract of BFS
The phytochemicals found in the methanol extract of BFS were further subjected to quantitative measurements to find out the quantity of each compound therein (Table 2).

Clinical signs
No mortality was recorded and no serious sign of toxicity was seen in the mice within the 14 days of observation after a single dose of BFS ranging from 10 to 5000 mg/kg. Therefore, the extract seemed to be safe at 5000 mg/kg body weight and the LD50 was considered to be >5000 mg/kg.
Effect of methanol extract of BFS on anthropometric parameters in mice
Body weight
A steady increase in body weight was observed among all the groups of mice administered with single but different doses of BFS ranging from 10 mg/kg to 1000 mg/kg after 14 days (Table 3). However, the one-way ANOVA revealed no observable differences in the initial body weight [F(3, 8)=1.674, P=0.2488], final body weight [F(3, 8)=1.097, P=0.4048], and weight gain [F(3, 8)=0.8018, P=0.5270] among all the experimental groups of mice.

Mice relative organ weight
The values for relative organ weights were indicated in Table 4. One-way ANOVA results showed no statistically significant differences (P>0.05) in the relative organ weights evaluated, which included brain [F(3, 8)=1.427, P=0.3050], heart [F(3, 8)=0.3575, P=0.7854], lungs [F(3, 8)=0.8167, P=0.5199], liver [F(3, 8)=1.115, P=0.3985], left kidney [F(3, 8)=2.503, P=0.1332], right kidney [F(3, 8)=0.2554, P=0.8555], and spleen [F(3, 8)=2.115, P=0.1766] among all the treatment groups.

Effect of methanol extract of BFS on biochemical parameters in mice
Serum levels of liver function biomarkers
The toxicological effect of methanol extract of BFS was evaluated on liver function biomarkers. One-way ANOVA results showed that there was no significant change (P>0.05) in the serum levels of ALT [F[F(3, 8)=1.060, P=0.4182], ALP [F(3, 8)=1.436, P=0.3025], AST [F(3, 8)=0.8171, P=0.5197], TP [F(3, 8)=3.549, P=0.0675], and ALB [F(3, 8)=0.1561, P=0.9228] at all treatment doses of BFS in comparison to the control group of mice (Figure 1).

Serum levels of kidneys function biomarkers
The toxicological effect of methanol extract of BFS was ascertained on the kidney function parameters. One-way ANOVA showed no statistical significant differences (P>0.05) in the serum levels of urea [F(3, 8)=1.431, P=0.3040], creatinine [F(3, 8)=0.7520, P=0.5523], sodium [F(3, 8)=1.347, P=0.3262], potassium [F(3, 8)=1.209, P=0.3672], and chloride [F(3, 8)=4.608, P=0.0373] compared to the control group (Figure 2). However, one-way ANOVA revealed a statistically significant difference in the level of bicarbonate [F(3, 8)=5.602, P=0.0229]. Tukey’s post hoc test confirmed the marked increase (P<0.05) in bicarbonate levels in mice receiving BSF 10 mg/kg compared to the control group.

Effect of methanol extract of BFS on the histology of kidneys, liver, and cerebellum in mice
Histology of liver
A qualitative analysis of the liver histology was conducted to ascertain the effects of methanol extract of BFS. The photomicrograph of the control mice livers showed normal sinusoids, hepatocytes, and central vein (Figure 3a). The photomicrographs of the livers of mice that received BFS ranging from 10 mg/kg to 5000 mg/kg body weight also showed no significant difference than the control group (Figure 3b-g).

Histology of kidneys
Qualitative analysis of the mice’s kidney histology was carried out to ascertain the effects of methanol extract of BFS. Photomicrographs of the control mice kidneys showed normal convoluted tubules and glomeruli (Figure 4a). The photomicrographs of the kidneys of mice that received a single dose of BFS ranging from 10 mg/kg to 5000 mg/kg body weight also showed a normal appearance of convoluted tubules and glomeruli (Figure 4b-g).

Histology of the cerebellum
Qualitative analysis of the histology of the mice cerebellar cortex was performed to check the effects of single-dose exposure to methanol extract of BFS. Photomicrograph of the cerebellar cortex of the control group of mice showed normal cell layers, which included the Molecular Layer (ML), middle Purkinje cells layer (PCL), and inner granular layer (GL) with aggregation of granular cells (Figure 5a). The photomicrographs of the cerebellum of mice that received a single dose of BFS ranging from 10 mg/kg to 5000 mg/kg (Figure 5b-g) also showed a similar pattern as there were no obvious histological aberrations.

Discussions
In this study, qualitative analysis of a methanolic extract of A. digitata fruit shell indicated the presence of cardenolide, terpenoids, cardiac glycoside, saponin glycoside alkaloid, and phenolic compounds. Similar results were reported after the phytochemical screening of the aqueous extract of A. digitata fruit pulp [20], which revealed the presence of tannins, saponin, phenol, glycosides, flavonoids, and alkaloids. In the present study, quantitative estimation of phytochemicals showed total flavonoid content to be 1.50 mgQE/g, which showed the presence of Quercetin. Quercetin is among the most commonly studied flavonoids, which possesses many health-promoting potentials, including anti-inflammatory, anti-diabetic, and anti-cancer properties, and also reduces the risk of cardiovascular diseases [21, 22]. These potentials are associated with its potent antioxidant capacity by strengthening endogenous defense against free radicals, while the anti-cancer activity of quercetin is due to its ability to bind cellular receptors and proteins, which controls mechanisms of cell signaling and also inhibits enzymes responsible for the carcinogens activation [23]. Alkaloids are chemical compounds mostly containing basic nitrogen atoms and are used as a remedy for gout with analgesic, antihypertensive, vasodilator, muscle relaxant, antitumor, and anti-malarial activity [20, 24]. The presence of these classes of compounds, especially flavonoids and alkaloids in the aqueous extract of the fruit pulp of A. digitata could be responsible for its various medicinal and anti-oxidative uses [25]. Flavonoid is considered to have good antioxidant, antiviral, anti-inflammatory, anti-allergic, and anticancer properties and are appropriate therapeutic agents [26].
The present acute toxicity study showed that oral administration of methanol extract of BSF up to the dose of 5000 mg/kg body weight did not cause any persistent clinical signs nor mortality in mice. Consequently, this study proved that BSF was non-toxic up to 5000 mg/kg, and relatively safe to use. Substances that are higher than 5000 mg/kg via the oral route of administration are considered non-toxic [27]. Weight modulation is a very important characteristic of numerous medicinal agents [28, 29]. The present study explored body weight as well as relative organ weights in order to ascertain the toxicity of BFS extract. However, no changes in the body weights nor the relative organ weight of their liver, kidneys, brain, heart, lungs, and spleen were observed. This is suggestive of the safe nature of the extract at the doses tested. Further, no biochemical derangement was observed in both liver and kidney function biomarkers analyzed, while the histology of vital organs of metabolism, including the liver and kidney was intact besides the normal appearance of the mice brain’s cerebellum.
Conclusion
In conclusion, the acute oral dose of BFS methanolic extract up to 5000 mg/kg showed no evidence of toxicity or treatment-related mortality in mice. No changes were observed in the body and relative organs weights, liver and kidney function biomarkers, and also in the histology of their liver, kidneys, and cerebellum. Hence, the LD50 of BFS is more than 5000 mg/kg in mice and can be said to have a high margin of safety. However, a longer study duration is recommended to evaluate its toxic effects on fertility, teratogenicity, and carcinogenic potentials.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Postgraduate Board of Studies, Faculty of Basic Medical Sciences, the University of Maiduguri, which is in accordance with the ARRIVE guidelines for the Care and Use of Animals with code PGA/18/01/01/08807.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
All authors appreciated the valuable contributions made by NI Dibal, Ephraim Ayuba, and Sunday of the Human Anatomy Department University of Maiduguri.
References
- Adewale OB, Onasanya A, Anadozie SO, Abu MF, Akintan IA, Ogbole CJ, et al. Evaluation of acute and subacute toxicity of aqueous extract of crassocephalum rubens leaves in rats. J Ethnopharmacol. 2016; 188:153-8. [DOI:10.1016/j.jep.2016.05.003] [PMID]
- Kale OE, Awodele O, Akindele AJ. Subacute and subchronic oral toxicity assessments of acridocarpus smeathmannii (dc.) guill. & perr. root in wistar rats. Toxicol Rep. 2019; 6:161-75. [DOI:10.1016/j.toxrep.2019.01.005] [PMID] [PMCID]
- World Health Organization (WHO). The selection and use of essential medicines: Report of the WHO expert committee, 2015 (including the 19th WHO model list of essential medicines and the 5th WHO model list of essential medicines for children). Gevena: World Health Organization; 2015. [Link]
- Osagie-Eweka SE, Orhue NEJ, Omogbai EKI, Amaechina FC. Oral acute and sub-chronic toxicity assessment of aqueous leaf extract of simarouba glauca dc (paradise tree). Toxicol Rep. 2021; 8:239-47. [DOI:10.1016/j.toxrep.2021.01.008] [PMID] [PMCID]
- Kamatou GP, Vermaak I, Viljoen AM. An updated review of adansonia digitata: A commercially important African tree. S Afr JBot. 2011; 77(4):908-19. [DOI:10.1016/j.sajb.2011.08.010]
- Ismail BB, Pu Y, Guo M, Ma X, Liu D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (adansonia digitata) fruit pulp. Food Chem. 2019; 277:279-88. [DOI:10.1016/j.foodchem.2018.10.056] [PMID]
- Tsetegho Sokeng AJ, Sobolev AP, Di Lorenzo A, Xiao J, Mannina L, Capitani D, et al. Metabolite characterization of powdered fruits and leaves from adansonia digitata l.(baobab): A multi-methodological approach. Food Chem. 2019; 272:93-108. [DOI:10.1016/j.foodchem.2018.08.030] [PMID]
- Ismail BB, Yusuf HL, Pu Y, Zhao H, Guo M, Liu D. Ultrasound-assisted adsorption/desorption for the enrichment and purification of flavonoids from baobab (adansonia digitata) fruit pulp. Ultrason Sonochem. 2020; 65:104980. [DOI:10.1016/j.ultsonch.2020.104980] [PMID]
- Kempe A, Neinhuis C, Lautenschläger T. Adansonia digitata and adansonia gregorii fruit shells serve as a protection against high temperatures experienced during wildfires. Bot Stud. 2018; 59(1):7. [DOI:10.1186/s40529-018-0223-0] [PMID] [PMCID]
- Kabbashi NA, Mirghani ME, Alam MZ, Qudsieh SY, Bello IA. Characterization of the Baobab fruit shells as adsorption material. Int Food Res J. 2017; 24(Suppl):S472-4. [Link]
- Vunain E, Kenneth D, Biswick T. Synthesis and characterization of low-cost activated carbon prepared from Malawian baobab fruit shells by H3PO4 activation for removal of Cu (II) ions: Equilibrium and kinetics studies. Appl Water Sci. 2017; 7(8):4301-19. [DOI:10.1007/s13201-017-0573-x]
- No author. EU Reference laboratory for alternatives to animal testing (EURL ECVAM) [Internet]. 2013 [Updated 2022 November]. Available from: [Link]
- Evans WC. Trease and Evans’ Pharmacognosy. London: Elsevier Health Sciences; 2009. [Link]
- Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob J Pure Appl Sci. 2002; 8(2):203-08. [DOI:10.4314/gjpas.v8i2.16033]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (moringa oleifera Lam.) leaves. J Agric Food Chem. 2003; 51(8):2144-55. [DOI:10.1021/jf020444+] [PMID]
- Siddhuraju P, Manian S. The antioxidant activity and free radical-scavenging capacity of dietary phenolic extracts from horse gram (macrotyloma uniflorum (lam.) verdc.) seeds. Food Chem. 2007; 105(3):950-8. [DOI:10.1016/j.foodchem.2007.04.040]
- Makkar HP, Siddhuraju P, Becker K. Plant secondary metabolites. Totowa: Humana Press; 2007. [Link]
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54(4):275-87. [DOI:10.1007/BF01234480] [PMID]
- Benard SA, Afolabi OO, Olutunde OA. Hibiscus extact-iron-van gieson: A new morphological staining technique in neuro-histology. Afr J Cell Pathol. 2016; 7(5):30-4. [Link]
- Zagga AI, Abduljabbar IA, Garko MB, Tsoho B, Gbande S. Phytochemical composition of adansonia digitata l. leaf extracts. Paper presented at: The 6th NSCB Biodiversity Conferences. 6-12 May 2018: Uyo, Nigeria.
- Talari S, Ramaswamy N. Determination of total phenolics and flavonoids in different parts of globally Endangered tree species adansonia digitata L. Int J Ayu Alt Med. 2016; 4(1):24-9. [Link]
- Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Mohammed H. African natural products with potential antioxidants and hepatoprotectives properties: A review. Clin Phytoscience. 2017; 2:23. [DOI:10.1186/s40816-016-0037-0]
- Olatunde A, Nigam M, Singh RK, Panwar AS, Lasisi A, Alhumaydhi FA, et al. Cancer and diabetes: The interlinking metabolic pathways and repurposing actions of antidiabetic drugs. Cancer Cell Int. 2021; 21(1):499. [DOI:10.1186/s12935-021-02202-5] [PMID] [PMCID]
- Kamanula M, Munthali CR, Dziwapo A, Kamanula JF. Mineral and phytochemical composition of baobab (adansonia digitata l.) root tubers from selected natural populations of Malawi. Malawi Med J. 2018; 30(4):250-5. [DOI:10.4314/mmj.v30i4.7] [PMID] [PMCID]
- Althwab SA, Alsattame M, Al-mundarij TI, Hamad EM, Mousa HM. protective effect of baobab fruit pulp (adansonia digitata l.) from oxidative stress induced in rats by high-fat diet. Life Sci J. 2019; 16(1):63-71. [Link]
- Tedeschi E, Menegazzi M, Yao Y, Suzuki H, Förstermann U, Kleinert H. Green tea inhibits human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1α activation. Mol Pharmacol. 2004; 65(1):111-20. [DOI:10.1124/mol.65.1.111] [PMID]
- Kennedy GL Jr, Ferenz RL, Burgess BA. Estimation of acute oral toxicity in rates by determination of the approximate lethal dose rather than the ld50. J Appl Toxicol. 1986; 6(3):145-8. [DOI:10.1002/jat.2550060302] [PMID]
- Akindele AJ, Unachukwu EG, Osiagwu DD. 90 Days toxicological assessment of hydroethanolic leaf extract of ipomoea asarifolia (desr.) roem. and schult. (convolvulaceae) in rats. J Ethnopharmacol. 2015; 174:582-94. [DOI:10.1016/j.jep.2015.03.044] [PMID]
- Awodele O, Badru WA, Busari AA, Kale OE, Ajayi TB, Udeh RO, et al. Toxicological evaluation of therapeutic and supra-therapeutic doses of cellgevity® on reproductive function and biochemical indices in wistar rats. BMC Pharmacol Toxicol. 2018; 19(1):68. [DOI:10.1186/s40360-018-0253-y] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Ehtnopharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









