Volume 8, Issue 4 (2022)
Pharm Biomed Res 2022, 8(4): 279-290 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khalifa M A, Mohammad A E H, Ahmed B M. Acute and Subacute Toxicity Studies of Cyperus Papyrus Ash on Wistar Albino Rats. Pharm Biomed Res 2022; 8 (4) :279-290
URL: http://pbr.mazums.ac.ir/article-1-433-en.html
URL: http://pbr.mazums.ac.ir/article-1-433-en.html
1- Department of Pharmacology, School of Pharmacy, Omdurman Islamic University, Khartoum, Sudan.
2- Department of Pharmacology, Medicinal and Aromatic Plants and Traditional medicine Research Institute, National Centre for Research, Khartoum, Sudan.
2- Department of Pharmacology, Medicinal and Aromatic Plants and Traditional medicine Research Institute, National Centre for Research, Khartoum, Sudan.
Full-Text [PDF 4259 kb]
(515 Downloads)
| Abstract (HTML) (1111 Views)
Full-Text: (555 Views)
Introduction
The usage of medicinal plants as a key component of complementary and alternative medicine has acquired a renewed interest in developed countries for managing many health problems [1]. The increasing demand for herbal medicines for human use is causing a growing demand for the cultivation of medicinal plants. This demand has developed because of cost-effective and safe plant-derived products compared to commercially available synthetic drugs [2]. In the screening of medicinal plants, determining lethal dose (LD) 50% (the dose which has proved to be lethal [causing death] to 50% of the tested group of animals) is usually the initial step in assessing and evaluating the toxic characteristics of plants [3].
Papyrus (Cyperus papyrus, Cyperaceae) covers large areas in the tropical African wetlands [4]. Cyperus papyrus (CP) ash contains several minerals served as micronutrients, such as Na, K, Fe, and Mg in different percentages, and has a pH range of 10.23-10.55 which is classified as an alkaline substance [5].
The CP reeds ash has been traditionally used as a substitute for salt in western Kenya and earlier studies have indicated that potassium salt substitution derived from local papyrus reed has a favorable potassium/sodium (K/Na) ratio that is suitable for use to regulate high blood pressure in hypertensive patients. This salt has been traditionally used in painful spasms, eye diseases, ulcers, fever, diarrhea, and inflammations [6].
The ash content of elements was measured by using a variety of analytical methods, while the important method was the flame atomic absorption spectrophotometer (FAAS).
In the screening of medicinal plants, determination of LD50% (the dose which has proved to be lethal [causing death] to 50% of the tested group of animals) is usually an initial step in assessing and evaluating the toxic characteristics of this plant. It is an initial assessment of the toxic manifestations (as it provides information on health hazards likely to arise from short-term exposure to plants) and is one of the initial screening experiments performed with all compounds [3].
This study aims to evaluate the acute and subacute toxicity of CP ash on Wistar albino rats according to the Organization for Economic Cooperation and Development (OECD) guidelines on mortality, behavior, and some hematological, biochemical, and histopathological parameters.
Materials and Methods
Plant Material
Fresh aerial parts were harvested from their natural habitat in Khartoum, Sudan, in August 2019. Plant identification and voucher specimen no. (Y-2010-54-MAPTRI-H) referencing was done at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTRI) of the National Center for Research, Khartoum, Sudan.
Preparation and analysis of plant dry ash
One gram of dried, ground plant tissue was placed in a porcelain crucible, and then in a cool muffle furnace (small scale) and it turned to ash at 500°C overnight. The resulting ash was cooled and dissolved in 5 mL of hydrochloric acid 20%. Then, the solution was filtered through an acid-washed filter paper into a 50-mL volumetric flask, and the filter paper was washed. The solution was diluted with deionized water and mixed well. Subsequently, the elements were measured using 210/211VGP Atomic Absorption Spectrophotometer VER 3.94 C through different lamps.
Experimental animals
Both male and female Wistar albino rats (65-120 g) obtained from the animal house of MAPTRI were used for the acute and subacute toxicity studies. They were housed in polypropylene cages (485×350×200 mm) up to a maximum of 6 rats per cage, in a well-ventilated room with 12 h light/dark cycle and free access to clean drinking water and food (standard rat feed). They were allowed to acclimatize for one week before the experiment. The plant ash was administered orally and all animals had a regular supply of clean drinking water and food.
Acute toxicity testing
The acute oral toxicity of CP ash was evaluated in Wistar albino rats according to the procedures outlined by the OECD using the Aot425 software. Following the adaptation period, the rats were weighed and the dose was calculated in reference to the body weight. The plant ash was suspended in distilled water. For the limit test, a single high dose of 2000 mg/kg (for 5 animals) of plant ash was administered. Whereas the control group received distilled water by the oral route (10 mL/kg). Food was provided to the rats approximately 1 h after the treatment. The animals were observed for 30 min after dosing, and then 1, 2, 4, 24, and 48 h, respectively, for short-term outcomes followed by observation once a day for the next 12 days. All observations were recorded for each animal during the study period. Surviving animals were weighed, and food consumption was measured. Meanwhile, the visual observations for mortality, changes in physical appearance, behavioral patterns, or any other signs of illness were conducted daily during the study period.
Subacute toxicity testing
Subacute toxicity of CP ash was carried out in Wistar albino rats according to the OECD 407 guidelines, while the rats were divided into 4 groups (A, B, C, and D) of 6 rats in each group, and received an oral dose (through oral gavage) of 1750, 550, 175 mg/kg respectively, whereas groups E served as control and received the distilled water 10 ml/kg BW for consecutive 28 days. At the end of the experiment (day 29), all rats in each group were weighed and sacrificed after an overnight fast, under diethyl ether anesthesia, whereas the outstanding rats of group D were sacrificed similarly after extra free 14 days without the administration of testing compounds (recovery group). Blood samples were collected for hematological analysis and plasma was prepared for biochemical analysis through the orbital veins. The liver, kidney, lung, spleen, and heart were harvested immediately clean of blood using physiological saline and then fixed in 10% formalin for histopathological examination.
Histopathology examination
The fixed organs were dehydrated with 100% ethanol solution and embedded in paraffin. They were then processed into 6 to 8 microns by using microtone, stained using hematoxylin-eosin, and observed under a light microscope by a histopathologist as earlier described [7].
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software, version 5. The data were expressed as Mean±SEM. The 1-way analysis of variance (Dunnett’s multiple comparison test) was conducted to determine significant differences and P<0.05 were considered significant.
Results
Analysis of the Ash
Table 1 demonstrates the FAAS results for the analysis of CP ash elements with standard conditions.
.jpg)
No mortality was recorded in both male and female CP-administered rats at a dose of 2000 mg/kg body weight, as shown in Figure 1, Figure 2 and Table 2.
.jpg)
.jpg)
.jpg)
Subacute toxicity
Changes in body weight during subacute toxicity of CP ash are provided in the following Figure 3.
.jpg)
Hematological parameters
As a response: Many hematological parameters of the experimental animals were changed significantly with sub-acute exposure to CP ash when compared to control group, but all these abnormalities were reversed to the normal range with recovery group and having non-significant differences with control group.
Biochemical parameters
Many biochemical parameters of the experimental animals were changed significantly with sub-acute exposure to CP ash when compared to control group, but all these abnormalities were reversed to the normal range with recovery group and having non-significant differences with control group.
Histopathological examinations
The effects of CP ash on heart histopathology during subacute exposure are shown in the following Figure 4.
Regarding the effects of CP ash on the vital organs (heart, lung, kidney, liver, spleen, and small intestine) of the experimental rats administered for 28 successive days there was no any abnormalities observed.
.jpg)
The effects of CP ash on lung histopathology during subacute exposure are provided in the following Figure 5.
.jpg)
The effects of CP ash on kidney histopathology during subacute exposure are represented in the following Figure 6.
.jpg)
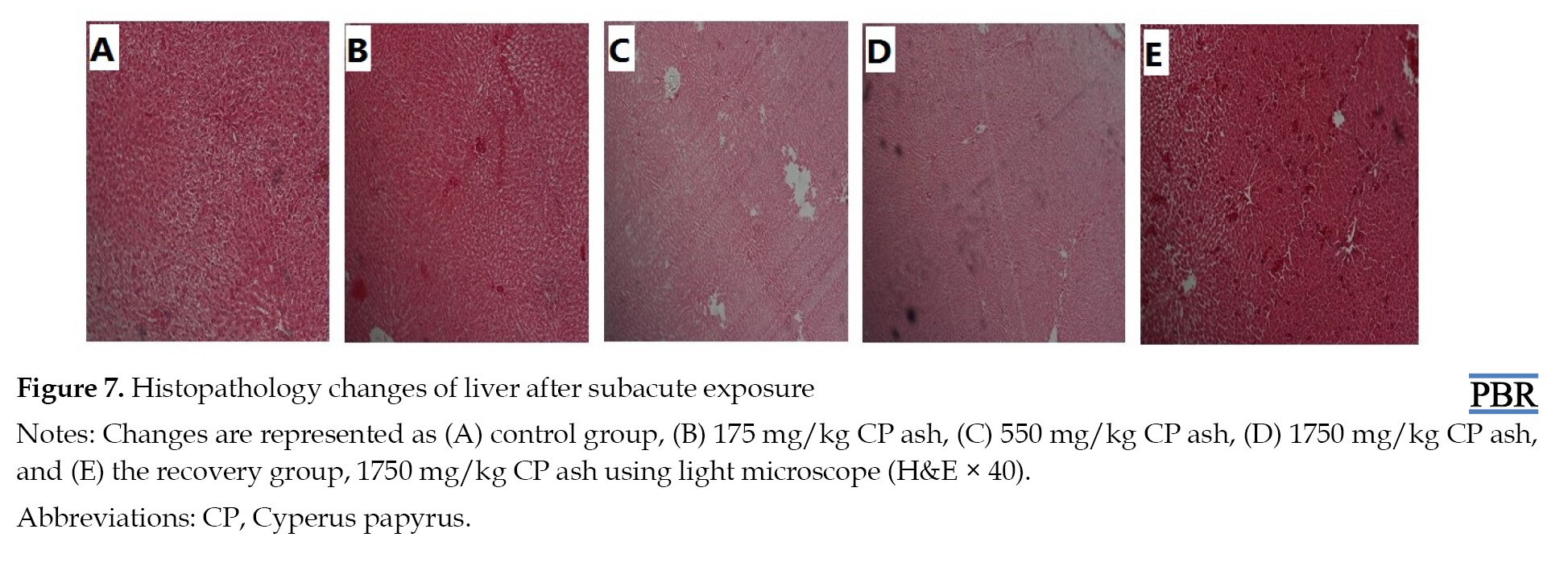
The effects of CP ash on liver histopathology during subacute exposure are presented in the following Figure 7.
The effects of CP ash on spleen histopathology during subacute exposure are represented in the following Figure 8.

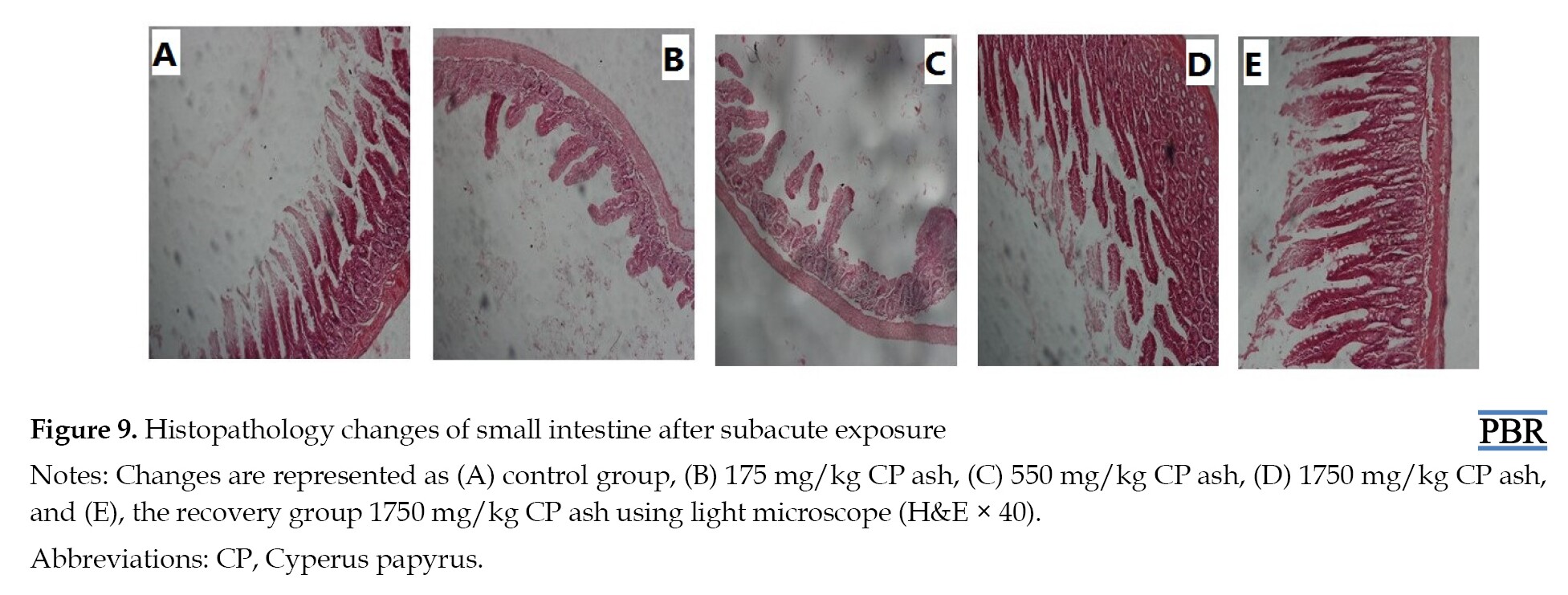
The effects of CP ash on small intestine histopathology during subacute exposure are represented in the following Figure 9.
Discussion
No mortality was recorded in female rats that were administered the CP ash at a dose of 2 g/kg and 5 g/kg–1 body weight as shown in Figure 1 and Figure 2. These results were compared to previous relevant studies, including Nazaré et al. 2019 which revealed that the LD50% value for CP was greater than 2000 mg/kg. Another study reported that the administration of different Cyperus species had LD50% greater than 5000 mg/kg, without exhibiting any signs of acute toxicity.
The study animals did not display death or any significant changes in behavioral patterns, such as body weight (Tables 1 and 3), trembling, diarrhea, salivation, breathing, impairment in food intake, water consumption, postural abnormalities, hair loss, sleep, lethargy, restlessness, or in physical appearances, such as eye color, mucous membrane, salivation, skin/fur effects, coma, injury, change in heart rate or blood pressure, when compared to the control group at the end of 14 days of general observation.
.jpg)
Meanwhile, no histopathology changes (necropsy) in the heart, lung, liver, spleen, kidney, or small intestine of study animals were observed when compared to the control group at the end of 14 days of general observation during acute toxicity testing as shown in Table 4.
.jpg)
According to the OECD guidance document for acute oral toxicity testing, compounds with LD50% values lower than 2g/kg–1 body weight are considered to be relatively safe, since values above this threshold are non-classified. Thus, the CP ash can be considered to be nontoxic at acute administration as the extract was well tolerated and there were no observed adverse effects.
After 28 days of administration of the plant ash, there was a significant decrease in the mean corpuscular volume (P<0.05), the lymphocyte count (P<0.05), and red cell distribution width (P<0.05). Meanwhile, a highly significant decrease was observed in lymphocyte percentage (P<0.01), hematocrit (P<0.01), and a significant increase in mean corpuscular hemoglobin concentration (P<0.05) in groups treated with the CP ash compared to the control group. All these abnormalities returned to the normal range in the recovery group. The rest of the parameters did not change significantly as shown in Table 5.
.jpg)
As demonstrated in Table 6, on plasma biochemical parameters in the experimental rats, there was a significant increase in the serum creatinine (P<0.01), total bilirubin (P<0.01), and alanine transaminase (P<0.01). A highly significant increase was observed in aspartate transaminase (P<0.01) when compared to the control group. All these abnormalities returned to the normal range in the recovery group, whereas the direct bilirubin was significantly decreased in the recovery group only (P<0.05). The rest of the parameters did not change significantly.

When comparing the hematological and biochemical results with previous studies of different Cyperus species, a significant decrease (P<0.05) was observed only in the activities of aspartate transaminase and alanine transaminase of animals treated Similarly. The decrease in renal function was only significant (P<0.05) in the concentration of urea in animals [8]. Another study revealed that the administration of different Cyperus species produces a significant increase in the red blood cell count, hemoglobin level, and hematocrit percentage [9]. The research findings contradicted with another study on different Cyperus species, which revealed no hematological or biochemical changes after the subacute exposure [10].
The effects of cyperus papyrus extract on some histopathological parameters in rat’s subacute toxicity
There were no tubular necrosis, changes in glomeruli, and cellular infiltrate in the interstitial kidney observed in all rat groups that received the CP extract when compared to the control group.
In the liver, there were no necrosis, inflammatory cellular infiltrate, and stasis in the bile cuniculi of the kidney in all rat groups that received the CP extract when compared to the control group.
For the histopathology of the spleen, no congestion in the red pulp, paucity of the white pulp, and increase in the hemosiderin macrophage level was observed in all rat groups that received the CP extract when compared to the control group.
There were no necrosis, inflammatory cells in alveoli, mucus or edema in alveoli, and mucus in bronchioles observed in the lung of all rat groups that received the CP extract when compared to the control group.
In the heart, no necrosis and inflammatory cellular infiltrate were seen in all rat groups that received CP extract when compared to the control group.
In the small intestine, normal villi were observed, and there were no inflammatory cells in the lumen of propria in all rat groups that received the CP extract when compared to the control group.
Conclusion
The administration of CP ash did not show any signs of acute toxicity on mortality or other illness in Wistar albino rats and the LD50% value was found to be greater than 2000 mg/kg body weight. In subacute toxicity, there were some hematological and biochemical changes in the treated animals compared to the control group; however, in the recovery group, these abnormalities were not significantly different from the control group. These findings were confirmed with histopathology, which revealed the absence of any abnormalities in the selected organ.
Ethical Considerations
Compliance with ethical guidelines
All experiments involving animals were performed with permission and under the strict guidance of the Institutional Animal Ethical Committee for Research on small animals, Faculty of Pharmacy, Omdurman Islamic University, with the permission letter (No.:OIU/IAEC/Exp.Ph.2019/6).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to MAPTRI (Sudan) for financial support, and express their gratitude toward Professor Abdoalwahab Hassan Ahmed and Leena Abdulaziz for their constant support.
References
The usage of medicinal plants as a key component of complementary and alternative medicine has acquired a renewed interest in developed countries for managing many health problems [1]. The increasing demand for herbal medicines for human use is causing a growing demand for the cultivation of medicinal plants. This demand has developed because of cost-effective and safe plant-derived products compared to commercially available synthetic drugs [2]. In the screening of medicinal plants, determining lethal dose (LD) 50% (the dose which has proved to be lethal [causing death] to 50% of the tested group of animals) is usually the initial step in assessing and evaluating the toxic characteristics of plants [3].
Papyrus (Cyperus papyrus, Cyperaceae) covers large areas in the tropical African wetlands [4]. Cyperus papyrus (CP) ash contains several minerals served as micronutrients, such as Na, K, Fe, and Mg in different percentages, and has a pH range of 10.23-10.55 which is classified as an alkaline substance [5].
The CP reeds ash has been traditionally used as a substitute for salt in western Kenya and earlier studies have indicated that potassium salt substitution derived from local papyrus reed has a favorable potassium/sodium (K/Na) ratio that is suitable for use to regulate high blood pressure in hypertensive patients. This salt has been traditionally used in painful spasms, eye diseases, ulcers, fever, diarrhea, and inflammations [6].
The ash content of elements was measured by using a variety of analytical methods, while the important method was the flame atomic absorption spectrophotometer (FAAS).
In the screening of medicinal plants, determination of LD50% (the dose which has proved to be lethal [causing death] to 50% of the tested group of animals) is usually an initial step in assessing and evaluating the toxic characteristics of this plant. It is an initial assessment of the toxic manifestations (as it provides information on health hazards likely to arise from short-term exposure to plants) and is one of the initial screening experiments performed with all compounds [3].
This study aims to evaluate the acute and subacute toxicity of CP ash on Wistar albino rats according to the Organization for Economic Cooperation and Development (OECD) guidelines on mortality, behavior, and some hematological, biochemical, and histopathological parameters.
Materials and Methods
Plant Material
Fresh aerial parts were harvested from their natural habitat in Khartoum, Sudan, in August 2019. Plant identification and voucher specimen no. (Y-2010-54-MAPTRI-H) referencing was done at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTRI) of the National Center for Research, Khartoum, Sudan.
Preparation and analysis of plant dry ash
One gram of dried, ground plant tissue was placed in a porcelain crucible, and then in a cool muffle furnace (small scale) and it turned to ash at 500°C overnight. The resulting ash was cooled and dissolved in 5 mL of hydrochloric acid 20%. Then, the solution was filtered through an acid-washed filter paper into a 50-mL volumetric flask, and the filter paper was washed. The solution was diluted with deionized water and mixed well. Subsequently, the elements were measured using 210/211VGP Atomic Absorption Spectrophotometer VER 3.94 C through different lamps.
Experimental animals
Both male and female Wistar albino rats (65-120 g) obtained from the animal house of MAPTRI were used for the acute and subacute toxicity studies. They were housed in polypropylene cages (485×350×200 mm) up to a maximum of 6 rats per cage, in a well-ventilated room with 12 h light/dark cycle and free access to clean drinking water and food (standard rat feed). They were allowed to acclimatize for one week before the experiment. The plant ash was administered orally and all animals had a regular supply of clean drinking water and food.
Acute toxicity testing
The acute oral toxicity of CP ash was evaluated in Wistar albino rats according to the procedures outlined by the OECD using the Aot425 software. Following the adaptation period, the rats were weighed and the dose was calculated in reference to the body weight. The plant ash was suspended in distilled water. For the limit test, a single high dose of 2000 mg/kg (for 5 animals) of plant ash was administered. Whereas the control group received distilled water by the oral route (10 mL/kg). Food was provided to the rats approximately 1 h after the treatment. The animals were observed for 30 min after dosing, and then 1, 2, 4, 24, and 48 h, respectively, for short-term outcomes followed by observation once a day for the next 12 days. All observations were recorded for each animal during the study period. Surviving animals were weighed, and food consumption was measured. Meanwhile, the visual observations for mortality, changes in physical appearance, behavioral patterns, or any other signs of illness were conducted daily during the study period.
Subacute toxicity testing
Subacute toxicity of CP ash was carried out in Wistar albino rats according to the OECD 407 guidelines, while the rats were divided into 4 groups (A, B, C, and D) of 6 rats in each group, and received an oral dose (through oral gavage) of 1750, 550, 175 mg/kg respectively, whereas groups E served as control and received the distilled water 10 ml/kg BW for consecutive 28 days. At the end of the experiment (day 29), all rats in each group were weighed and sacrificed after an overnight fast, under diethyl ether anesthesia, whereas the outstanding rats of group D were sacrificed similarly after extra free 14 days without the administration of testing compounds (recovery group). Blood samples were collected for hematological analysis and plasma was prepared for biochemical analysis through the orbital veins. The liver, kidney, lung, spleen, and heart were harvested immediately clean of blood using physiological saline and then fixed in 10% formalin for histopathological examination.
Histopathology examination
The fixed organs were dehydrated with 100% ethanol solution and embedded in paraffin. They were then processed into 6 to 8 microns by using microtone, stained using hematoxylin-eosin, and observed under a light microscope by a histopathologist as earlier described [7].
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software, version 5. The data were expressed as Mean±SEM. The 1-way analysis of variance (Dunnett’s multiple comparison test) was conducted to determine significant differences and P<0.05 were considered significant.
Results
Analysis of the Ash
Table 1 demonstrates the FAAS results for the analysis of CP ash elements with standard conditions.
.jpg)
No mortality was recorded in both male and female CP-administered rats at a dose of 2000 mg/kg body weight, as shown in Figure 1, Figure 2 and Table 2.
.jpg)
.jpg)
.jpg)
Subacute toxicity
Changes in body weight during subacute toxicity of CP ash are provided in the following Figure 3.
.jpg)
Hematological parameters
As a response: Many hematological parameters of the experimental animals were changed significantly with sub-acute exposure to CP ash when compared to control group, but all these abnormalities were reversed to the normal range with recovery group and having non-significant differences with control group.
Biochemical parameters
Many biochemical parameters of the experimental animals were changed significantly with sub-acute exposure to CP ash when compared to control group, but all these abnormalities were reversed to the normal range with recovery group and having non-significant differences with control group.
Histopathological examinations
The effects of CP ash on heart histopathology during subacute exposure are shown in the following Figure 4.
Regarding the effects of CP ash on the vital organs (heart, lung, kidney, liver, spleen, and small intestine) of the experimental rats administered for 28 successive days there was no any abnormalities observed.
.jpg)
The effects of CP ash on lung histopathology during subacute exposure are provided in the following Figure 5.
.jpg)
The effects of CP ash on kidney histopathology during subacute exposure are represented in the following Figure 6.
.jpg)
The effects of CP ash on liver histopathology during subacute exposure are presented in the following Figure 7.

The effects of CP ash on spleen histopathology during subacute exposure are represented in the following Figure 8.

The effects of CP ash on small intestine histopathology during subacute exposure are represented in the following Figure 9.

Discussion
No mortality was recorded in female rats that were administered the CP ash at a dose of 2 g/kg and 5 g/kg–1 body weight as shown in Figure 1 and Figure 2. These results were compared to previous relevant studies, including Nazaré et al. 2019 which revealed that the LD50% value for CP was greater than 2000 mg/kg. Another study reported that the administration of different Cyperus species had LD50% greater than 5000 mg/kg, without exhibiting any signs of acute toxicity.
The study animals did not display death or any significant changes in behavioral patterns, such as body weight (Tables 1 and 3), trembling, diarrhea, salivation, breathing, impairment in food intake, water consumption, postural abnormalities, hair loss, sleep, lethargy, restlessness, or in physical appearances, such as eye color, mucous membrane, salivation, skin/fur effects, coma, injury, change in heart rate or blood pressure, when compared to the control group at the end of 14 days of general observation.
.jpg)
Meanwhile, no histopathology changes (necropsy) in the heart, lung, liver, spleen, kidney, or small intestine of study animals were observed when compared to the control group at the end of 14 days of general observation during acute toxicity testing as shown in Table 4.
.jpg)
According to the OECD guidance document for acute oral toxicity testing, compounds with LD50% values lower than 2g/kg–1 body weight are considered to be relatively safe, since values above this threshold are non-classified. Thus, the CP ash can be considered to be nontoxic at acute administration as the extract was well tolerated and there were no observed adverse effects.
After 28 days of administration of the plant ash, there was a significant decrease in the mean corpuscular volume (P<0.05), the lymphocyte count (P<0.05), and red cell distribution width (P<0.05). Meanwhile, a highly significant decrease was observed in lymphocyte percentage (P<0.01), hematocrit (P<0.01), and a significant increase in mean corpuscular hemoglobin concentration (P<0.05) in groups treated with the CP ash compared to the control group. All these abnormalities returned to the normal range in the recovery group. The rest of the parameters did not change significantly as shown in Table 5.
.jpg)
As demonstrated in Table 6, on plasma biochemical parameters in the experimental rats, there was a significant increase in the serum creatinine (P<0.01), total bilirubin (P<0.01), and alanine transaminase (P<0.01). A highly significant increase was observed in aspartate transaminase (P<0.01) when compared to the control group. All these abnormalities returned to the normal range in the recovery group, whereas the direct bilirubin was significantly decreased in the recovery group only (P<0.05). The rest of the parameters did not change significantly.

When comparing the hematological and biochemical results with previous studies of different Cyperus species, a significant decrease (P<0.05) was observed only in the activities of aspartate transaminase and alanine transaminase of animals treated Similarly. The decrease in renal function was only significant (P<0.05) in the concentration of urea in animals [8]. Another study revealed that the administration of different Cyperus species produces a significant increase in the red blood cell count, hemoglobin level, and hematocrit percentage [9]. The research findings contradicted with another study on different Cyperus species, which revealed no hematological or biochemical changes after the subacute exposure [10].
The effects of cyperus papyrus extract on some histopathological parameters in rat’s subacute toxicity
There were no tubular necrosis, changes in glomeruli, and cellular infiltrate in the interstitial kidney observed in all rat groups that received the CP extract when compared to the control group.
In the liver, there were no necrosis, inflammatory cellular infiltrate, and stasis in the bile cuniculi of the kidney in all rat groups that received the CP extract when compared to the control group.
For the histopathology of the spleen, no congestion in the red pulp, paucity of the white pulp, and increase in the hemosiderin macrophage level was observed in all rat groups that received the CP extract when compared to the control group.
There were no necrosis, inflammatory cells in alveoli, mucus or edema in alveoli, and mucus in bronchioles observed in the lung of all rat groups that received the CP extract when compared to the control group.
In the heart, no necrosis and inflammatory cellular infiltrate were seen in all rat groups that received CP extract when compared to the control group.
In the small intestine, normal villi were observed, and there were no inflammatory cells in the lumen of propria in all rat groups that received the CP extract when compared to the control group.
Conclusion
The administration of CP ash did not show any signs of acute toxicity on mortality or other illness in Wistar albino rats and the LD50% value was found to be greater than 2000 mg/kg body weight. In subacute toxicity, there were some hematological and biochemical changes in the treated animals compared to the control group; however, in the recovery group, these abnormalities were not significantly different from the control group. These findings were confirmed with histopathology, which revealed the absence of any abnormalities in the selected organ.
Ethical Considerations
Compliance with ethical guidelines
All experiments involving animals were performed with permission and under the strict guidance of the Institutional Animal Ethical Committee for Research on small animals, Faculty of Pharmacy, Omdurman Islamic University, with the permission letter (No.:OIU/IAEC/Exp.Ph.2019/6).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to MAPTRI (Sudan) for financial support, and express their gratitude toward Professor Abdoalwahab Hassan Ahmed and Leena Abdulaziz for their constant support.
References
- Sanchez M, Gonzalez-Burgos E, Iglesias I, Lozano R, Gomez-Serranillos MP. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement Med Ther. 2020; 20(1):306. [PMID] [PMCID]
- Khan A, Mishra A, Hasan SM, Usmani A, Ubaid M, Khan N, et al. Biological and medicinal application of Cucumis sativus Linn. - review of current status with future possibilities. J Complement Integr Med. 2021. [DOI:10.1515/jcim-2020-0240] [PMID]
- J. Akhila JS, Shyamjith D, Alwar MC. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007; 93(7):917-20. [Link]
- Muthuri FM, Kinyamario JI. Nutritive value of papyrus (Cyperus papyrus, Cyperaceae), a tropical emergent macrophyte. Econ Bot. 1989; 43:23-30. [DOI:10.1007/BF02859321]
- Wangila TP, Tsimbiri P, Nyongesa P, Mule S, Wati N L, Kinyanjui T. Micronutrients I2, Fe2+, Mg, K and naconcentration in typhalatifoliaand cyperus papyrus reedSaltsof Busia and Lugari regions of western Kenya. Int J Sci Res. 2015; 4(1):175-80. [Link]
- Keter LK, Wekesa I, Tolo F, Mwaghadi Z, Mwitari P, Murunga S, et al. Development of a nutraceutical from natural products: A case study of a herbal-based low sodium table salt. Afr J Pharmacol Ther. 2013; 2(1):9-16. [Link]
- Gabe M. Techniques Histologiques. Paris: Mason. [Link]
- Silva NC, Goncalves SF, Araújo LS, Kasper AA, Fonseca AL, Sartoratto A, Castro KC, Moraes TM, Baratto LC, Varotti FD, Barata LE. In vitro and in vivo antimalarial activity of the volatile oil of Cyperus articulatus (Cyperaceae). Acta Amazonica. 2019; 49:334-42. [Link]
- Airaodion AI, Ogbuagu EO. Effect of Cyperus esculentus L.(tiger nut) milk on hepatic and renal indices of Wistar rat. Asian Journal of Research in Nephrology. 2020; 3(2):10-6. [Link]
- Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A. Acute and subacute toxicities of the ethanol extract from the rhizomes of Cyperus rotundus Linn. Mahidol University J Pharm Sci. 2005; 32:15-22. [Link]
Type of Study: Original Research |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








