Volume 7, Issue 2 (2021)
Pharm Biomed Res 2021, 7(2): 79-86 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shaki F, Teymoori M, Motafeghi F S, Hemmati N, Arab-Nozari M. L-arginine Ameliorated Mitochondrial Oxidative Damage Induced by Sub-chronic Exposure to Cadmium in Mice Kidney. Pharm Biomed Res 2021; 7 (2) :79-86
URL: http://pbr.mazums.ac.ir/article-1-376-en.html
URL: http://pbr.mazums.ac.ir/article-1-376-en.html
Fatemeh Shaki1 

 , Melika Teymoori2
, Melika Teymoori2 
 , Farzaneh Sadat Motafeghi2
, Farzaneh Sadat Motafeghi2 
 , Nasibeh Hemmati1
, Nasibeh Hemmati1 
 , Milad Arab-Nozari *3
, Milad Arab-Nozari *3 




 , Melika Teymoori2
, Melika Teymoori2 
 , Farzaneh Sadat Motafeghi2
, Farzaneh Sadat Motafeghi2 
 , Nasibeh Hemmati1
, Nasibeh Hemmati1 
 , Milad Arab-Nozari *3
, Milad Arab-Nozari *3 


1- Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Toxicology and Pharmacology, Faculty of Pharmacy, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran.
2- Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Toxicology and Pharmacology, Faculty of Pharmacy, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran.
Full-Text [PDF 831 kb]
(1380 Downloads)
| Abstract (HTML) (2630 Views)
Full-Text: (2039 Views)
Introduction
Heavy metals are among the most critical environmental hazards. Most metals accumulate in the food chain and the body to cause chronic problems. The toxicity of metals depends on the amount of absorption and also the duration of exposure [1]. These metals can cause several health problems, and their toxicity is significant for ecological, evolutionary, nutritional, and environmental reasons [2]. Cadmium is the seventh most toxic heavy metal according to the Agency for Toxic Substances and Disease Registry (ATSDR) classification and the main component of cigarette smoke and air pollution [3]. Cadmium poisoning occurs in both acute and chronic forms, mainly through respiratory and oral routes. This metal accumulates in plants, eventually reaches the human food chain, and is commonly found in fruits and vegetables [4].
In the United States, more than 500000 workers have been exposed to cadmium toxicity. Chinese researchers estimated the annual amount of cadmium industrial waste discharged into the environment is more than 680 tons [5]. Bone toxicity, testis toxicity, neurotoxicity, hepatotoxicity, and nephrotoxicity are the most significant adverse effects of cadmium accumulation in the human body [6]. Oxidative stress is considered the main mechanism of cadmium-induced tissue damages. It results from the overproduction of Reactive Oxygen Species (ROS) and the disability of endogenous antioxidants to neutralize them [6]. Previous studies also reported the role of oxidative stress in tissue damages induced by cadmium [7, 8]. For example, Fouad et al. reported that Intraperitoneal (IP) administration of cadmium chloride (2 mg/kg) resulted in testicular oxidative injury in rats via increasing Malondialdehyde (MDA) level, depleting Glutathione (GSH), and decreasing Superoxide Dismutase (SOD) and Catalase (CAT) activity [7].
Cadmium can also have a detrimental effect on mitochondria. Mitochondria are the main source of ROS generation in the cells [9]. Shi et al. reported the mitochondrial toxicity, dysfunction, and swelling in the kidney tissue of duck after exposure to cadmium chloride [10].
On the other hand, it was shown that antioxidant agents such as anthocyanin, zinc, and selenium could protect tissues from cadmium-induced damage by reducing apoptosis and blocking oxidative stress pathways [11, 12]. So, it is suggested that antioxidant compounds can prevent cadmium tissue toxicity.
L-Arginine is an essential amino acid for nutrition in young mammals and acts as an essential precursor to the synthesis of proline, polyamines, glutamate, and Nitric Oxide (NO). Arginine has multiple functions in the cardiovascular, immunological, endocrine, and cell growth system [13, 14]. Several studies reported that L-arginine could prevent oxidative stress via its antioxidant properties [15, 16]. Abd-Elrazek et al. reported that L-arginine ameliorated busulfan-induced testicular toxicity by increasing GSH storage and decreasing lipid peroxidation and DNA damage [16].
Because of the role of oxidative stress in the pathogenesis of cadmium toxicity and the beneficial antioxidant properties of L-arginine, we investigated the possible inhibitory effects of L-arginine against oxidative stress and mitochondrial damage caused by subchronic exposure to cadmium chloride in the kidney tissue of mice.
Materials and Methods
Chemicals and reagents
L-arginine was a product of Hakim Pharmaceutical Company, Tehran, Iran. Cadmium chloride, 5, 5’-dithiobis-[2-nitrobenzoic acid] (DTNB), and Thiobarbituric Acid (TBA) were purchased from Merk company (Germany). All other chemicals and reagents used in this study were obtained from the Sigma chemical company (Germany), with the highest available commercial grade.
Study animals
In this study, 42 male mice weighing between 25-26 g and four weeks old were obtained from the Institute for Laboratory Animals Research, Mazandaran University of Medical Sciences, Sari, Iran. They were housed under standard conditions of temperature 23±2ᴼC with regular 12/12 h light/dark cycle, with free access to food and water. All experimental procedures were conducted according to the ethical standard and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran (Ethical Number: IR.MAZUMZ.REC.1398.4844).
Animal grouping and drug administration
The rats were randomly divided into six groups (6 animals in each). Group 1 served as control (normal saline). Group 2 received cadmium chloride (2 mg/kg). Groups 3 to 5 received cadmium chloride (2 mg/kg) plus three doses of L-arginine (50, 100, and 200 mg/kg). The last group received cadmium chloride (2 mg/kg) plus vitamin C (500 mg/kg) as the positive control group. All the treatments were administered intraperitoneally for six consecutive weeks.
Preparation of samples
Twenty-four hours after receiving the last dose, the animals were anesthetized with ether, and a blood sample was taken directly from the heart (centrifuged at 3000 rpm for 15 minutes). The serum was then stored in a freezer to assess Blood Urea Nitrogen (BUN) and Creatinine (Cr). Then, kidney tissues were isolated from the animal for the experiments. Kidney tissues were homogenized and then centrifuged at 2000×g for 10 min at 4°C. Then, the supernatant was subjected to further centrifugation at 10000×g for 10 min. In this step, the supernatant was stored at -70°C for the evaluation of oxidative stress, and the mitochondrial sediment was suspending in Tris-HCl buffer (0.05 M Tris–HCl, 0.25M sucrose, 20 mM KCl, 2mM MgCl2, and 1mM Na2HPO4, pH of 7.4) for the evaluation of mitochondrial toxicity.
Measurement of protein concentration
Protein content was determined in kidney tissue with the Bradford method [17]. Bovine Serum Albumin (BSA) was used as standard. Homogenate samples were mixed with Coomassie blue, and after 10 min, the absorbance was determined at 595 nm by spectrophotometer (UV- 1601 PC, Shimadzu, Japan).
Measurement of lipid peroxidation
The content of MDA was determined using the Zhang et al. method [18]. Briefly, 0.1 mL Thiobarbituric Acid (TBA) was added to 0.2 mL of kidney tissue homogenate. All the samples were placed in a boiling water bath for 30 min. In the end, the tubes were shifted to an ice-bath, and 0.2 mL n-butanol was added to each tube. Then, they were centrifuged at 3500 rpm for 10 min. The amount of MDA formed in each sample was assessed by measuring the absorbance of the supernatant at 532 nm with an ELISA reader (Tecan, Rainbow Thermo, Austria). Tetramethoxypropane was used as standard, and MDA content was expressed as nmol/mg.
Measurement of glutathione content
GSH content was determined by DTNB as the indicator and spectrophotometer. Briefly, 0.1 mL of the kidney tissue homogenate was added into 0.1 mol/L of phosphate buffer and 0.04% DTNB in a total volume of 0.3 mL (PH 7.4). Then yellow color of samples was red at 412 nm on a spectrophotometer (UV- 1601 PC, Shimadzu, Japan). GSH content was expressed as nM [19].
Measurement of protein carbonyl
Determination of protein carbonyl was done by spectrophotometric method. Briefly, 200 µL of kidney tissues were homogenized. The samples were extracted in 500 µL of Trichloroacetic Acid (TCA) 20% (w/v). Then, the samples were placed at 4ᴼC for 15 min. The sediments were treated with 500 µL of 0.2% 2,4-dinitrophenylhydrazine (DNPH) and 500 µL of 2 N HCL for the control group, and samples were incubated at room temperature for 1 h with vortexing at 5 min intervals. Then, the proteins were precipitated by adding 55 µL of 20% TCA. The microtubes were centrifuged and washed three times with 1000 µL of the ethanol-ethyl acetate mixture. And the microtubes were dissolved in 200 µL of 6 M guanidine hydrochloride. The carbonyl content was determined by recording the absorbance at 365 nm wavelength [20].
Assessment of mitochondrial function:
The amount of 1 mL samples were mixed with 25 μL of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) solution and placed at 37°C for 30 min. Then, it was centrifuged at 1000 rpm for 10 minutes. The supernatant was discarded, and 1000 μL of Dimethyl Sulfoxide (DMSO) was added to the precipitate and dispersed. The resulting mixture was centrifuged at 1000 rpm for 10 minutes. Finally, 150-200 µL of the supernatant of each sample was put into a 96-well plate in each cell. Then, the absorbance was read using an ELISA reader [21].
Assessment of the mitochondrial membrane potential
About 1 mL sample was placed into a tube, and 2 mL of Mitochondrial Membrane Potential (MMP) buffer was added. Then, 25 µL of rhodamine dye was added to the tube mixture and placed at 37°C for 20 minutes. Then, the tube was centrifuged to separate. The fluorescence intensity of rhodamine was monitored using Shimadzu RF-5000U fluorescence spectrophotometer at the excitation and emission wavelengths of 490 and 535 nm [9].
Assessment of mitochondrial swelling
About 250 μL of the sample containing mitochondria was mixed with 750 μL of the swelling buffer (70 mM sucrose, 230 mM mannitol, 3 mM HEPES, 2 mM tris-phosphate, 5 mM succinate, and 1 μM of rotenone). Then, the absorbance was read using an ELISA reader (Tecan, Rainbow Thermo, Austria) [22].
Measurement of blood urea nitrogen and creatinine
Blood samples obtained from the heart of mice were sent to a laboratory for measuring BUN and Cr levels.
Statistical analysis
Results were reported as Mean±Standard Deviation (Mean±SD) from three repetitions of the experiment. The data were analyzed by GraphPad Prism 5 statistical software using ANOVA analysis followed by the post hoc Tukey test. The significance level was set at P<0.05. The Kolmogorov-Simonov normality test was used for variables in this study.
Results
Effect of L-arginine on lipid peroxidation in the mice kidney
The amount of GSH in the cadmium-treated group decreased compared to the control group (P<0.05). In L-arginine groups, the amount of GSH increased significantly (P<0.05) (Figure 2). Effect of L-arginine on protein carbonyl levels in the mice kidney
The concentration of protein carbonyl in the cadmium-treated group increased compared to the control group (P<0.05). In L-arginine treated groups, the amount of protein carbonyl significantly decreased compared to the cadmium group (P<0.05). This effect was dose-dependent, with the highest effect seen at 200 mg/kg and the lowest at 50 mg/kg. The effect of the maximum dose of L-arginine on protein carbonyl levels was more significant than the effect of vitamin C (Figure 3). Effect of L-arginine on mitochondrial function in the mice kidney
Mitochondrial function significantly decreased in the cadmium-treated group compared to the control group (P<0.05). In groups treated with L-arginine, mitochondrial function was significantly improved. This effect was dose-dependent, with the highest effect at 200 mg/kg and the lowest at 50 mg/kg. The effect of the maximum dose of L-arginine on mitochondrial function was greater than the effect of vitamin C (Figure 4). Effect of L-arginine on mitochondrial membrane potential in the mice kidney
The mitochondrial membrane potential collapsed (increase in absorbance) in the cadmium-treated group compared to the control group (P<0.05). On the other hand, in the collapse of L-arginine-treated mitochondrial membrane potential was significantly inhibited (decrease in absorbance) (Figure 5). Effect of L-arginine on mitochondrial swelling in the mice kidney
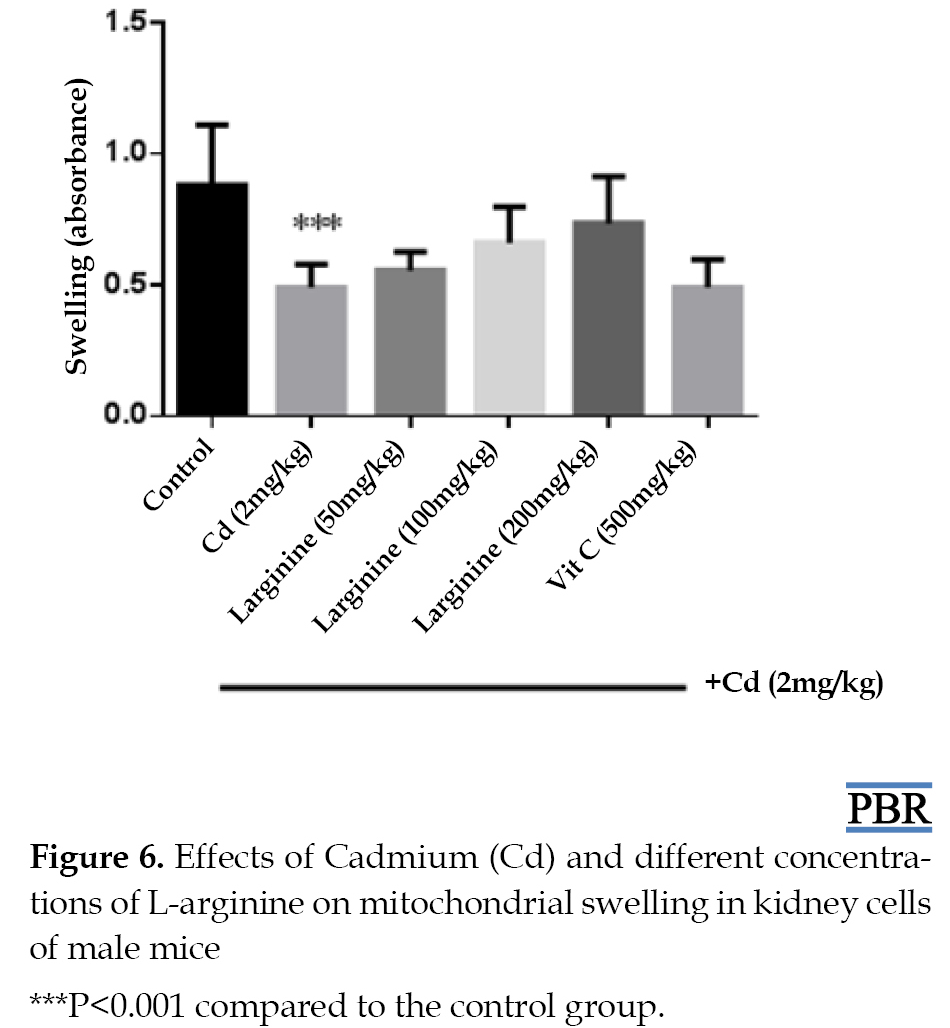
Cadmium treatment caused significant mitochondrial swelling (decrease in absorbance) compared to the control group (P<0.05). On the other hand, L-arginine reduced mitochondrial swelling induced by cadmium in mice, but this change was not statistically significant (Figure 6). Effect of L-arginine on blood urea nitrogen and creatinine levels in the mice kidney
The serum concentrations of BUN and Cr in the group that received cadmium increased significantly compared to the control group (P<0.05). In the groups receiving L-arginine, the concentration of urea and Cr significantly decreased compared to the cadmium group (P<0.05). This effect was dose-dependent, with the highest effect at 200 mg/kg and the lowest at 50 mg/kg (Table 1).
.png)
Discussion
In this study, we found that L-arginine inhibited oxidative stress and mitochondrial toxicity induced by subchronic exposure to cadmium chloride in male mice. The kidney is one of the main target organs in cadmium poisoning. Cadmium can cause glomerular dysfunction and nephropathy, leading to proteinuria, glycosuria, enzyme urea, and phosphaturia [23]. Cadmium toxicity occurs through indirect induction of ROS production in mitochondria and affects the expression of mitochondrial genes and impaired cellular respiratory activity [9, 24, 25].
We found that cadmium administration increased oxidative stress in mice kidneys via increasing MDA and protein carbonyl levels and decreasing GSH depletion. Other researchers also reported the role of oxidative stress in cadmium tissue toxicity [7, 8]. For example, Lamtai et al. found that IP administration of cadmium chloride (1 mg/kg) for eight weeks promoted oxidative stress in hippocampus tissue via increasing the levels of Nitric Oxide (NO) and Lipid Peroxidation (LPO), while the activities of CAT and SOD were significantly decreased in the hippocampus [8]. These studies confirmed our results about cadmium-induced oxidative damage. Interestingly, administration of L-arginine improved in kidney oxidative markers. Various studies have shown that L-arginine can reduce oxidative stress markers such as protein carbonyl, lipid peroxidation in various conditions in which oxidative stress is involved [15, 16]. Akinrinde et al. showed that L-arginine prevented fluoride-induced hepatotoxicity in rats via decreasing ROS, protein carbonyl, and MDA levels as well as increasing SOD and glutathione peroxidase activity [15].
In addition, it has been reported that L-arginine can trap free radicals [26]. MDA and protein carbonyl are the main end products of the oxidation of membrane lipids and proteins created by the action of free radicals [27, 28]. GSH is an important natural and non-enzymatic antioxidant in the body [29]. L-arginine stimulates the production of GSH by stimulating the endogenous synthesis of GSH, stimulating the expression of arginase (which itself stimulates the production of GSH by converting arginine to glutamate) [30]. So, L-arginine can potentiate antioxidant defense by preventing GSH depletion.
In this study, cadmium administration increased mitochondrial dysfunction, MMP collapse, and mitochondrial swelling in the kidney tissue. These results were in accordance with the results of previous studies, which reported mitochondrial toxicity of cadmium [31, 32]. For example, Bhattacharjee et al. declared that cadmium exposure led to increased oxidative stress markers (ROS and MDA production), mitochondrial oxidative toxicity, reduction of MMP, and mitochondrial dysfunction in rat liver and heart [32].
On the other hand, co-administration of L-arginine with cadmium reduced mitochondrial dysfunction, swelling, and MMP collapse. Previous studies also showed the mitochondrial protective effects of L-arginine [33, 34]. L-arginine administration improved mitochondrial function and inhibition of mitochondrial hydroxyl and superoxide radicals in brain cortex isolated mitochondria of streptomycin-treated rats [34]. L-arginine appears to prevent mitochondrial toxicity by preventing mitochondrial ROS generation.
In this study, cadmium administration raised serum levels of BUN and Cr. These findings in the cadmium-treated group may indicate renal impairment. However, co-administration of L-arginine decreased serum levels of BUN and Cr, indicating a protective effect of L-arginine against cadmium-induced kidney damage.
According to the study results, it is suggested that the renal protective effects of L-arginine might be due to its ability to inhibiting oxidative stress markers (including ROS, glutathione and protein oxidation, and lipid peroxidation). Furthermore, it could also protect the mitochondria from damage by preventing membrane potential collapse, swelling, and mitochondrial dysfunction. It was the first study that evaluated the protective effects of L-arginine against cadmium-induced nephrotoxicity. However, there were several limitations in this study, such as financial problems in providing materials and also gene expression analyses.
Conclusion
Overall, we showed that L-arginine has beneficial effects on nephrotoxicity and oxidative injury caused by cadmium in mice. Therefore, L-arginine would be evaluated for treatment or protection against cadmium-induced kidney injuries.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, (Code: IR.MAZUMZ.REC.1398.4844). All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This study was extracted from the PhD. dissertation of the second author at the Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences. Also, this study was supported by the Research Council of Mazandaran University of Medical Sciences (Grant number: 4844).
Authors' contributions
Methodology, data analysis, interpretation, and writing – review & editing: Fatemeh Shaki and Milad Arab-Nozari; Data collection: Farzaneh Sadat Motafeghi, Melika Teymoori, and Nasibeh Hemmati; Manuscript preparation: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Heavy metals are among the most critical environmental hazards. Most metals accumulate in the food chain and the body to cause chronic problems. The toxicity of metals depends on the amount of absorption and also the duration of exposure [1]. These metals can cause several health problems, and their toxicity is significant for ecological, evolutionary, nutritional, and environmental reasons [2]. Cadmium is the seventh most toxic heavy metal according to the Agency for Toxic Substances and Disease Registry (ATSDR) classification and the main component of cigarette smoke and air pollution [3]. Cadmium poisoning occurs in both acute and chronic forms, mainly through respiratory and oral routes. This metal accumulates in plants, eventually reaches the human food chain, and is commonly found in fruits and vegetables [4].
In the United States, more than 500000 workers have been exposed to cadmium toxicity. Chinese researchers estimated the annual amount of cadmium industrial waste discharged into the environment is more than 680 tons [5]. Bone toxicity, testis toxicity, neurotoxicity, hepatotoxicity, and nephrotoxicity are the most significant adverse effects of cadmium accumulation in the human body [6]. Oxidative stress is considered the main mechanism of cadmium-induced tissue damages. It results from the overproduction of Reactive Oxygen Species (ROS) and the disability of endogenous antioxidants to neutralize them [6]. Previous studies also reported the role of oxidative stress in tissue damages induced by cadmium [7, 8]. For example, Fouad et al. reported that Intraperitoneal (IP) administration of cadmium chloride (2 mg/kg) resulted in testicular oxidative injury in rats via increasing Malondialdehyde (MDA) level, depleting Glutathione (GSH), and decreasing Superoxide Dismutase (SOD) and Catalase (CAT) activity [7].
Cadmium can also have a detrimental effect on mitochondria. Mitochondria are the main source of ROS generation in the cells [9]. Shi et al. reported the mitochondrial toxicity, dysfunction, and swelling in the kidney tissue of duck after exposure to cadmium chloride [10].
On the other hand, it was shown that antioxidant agents such as anthocyanin, zinc, and selenium could protect tissues from cadmium-induced damage by reducing apoptosis and blocking oxidative stress pathways [11, 12]. So, it is suggested that antioxidant compounds can prevent cadmium tissue toxicity.
L-Arginine is an essential amino acid for nutrition in young mammals and acts as an essential precursor to the synthesis of proline, polyamines, glutamate, and Nitric Oxide (NO). Arginine has multiple functions in the cardiovascular, immunological, endocrine, and cell growth system [13, 14]. Several studies reported that L-arginine could prevent oxidative stress via its antioxidant properties [15, 16]. Abd-Elrazek et al. reported that L-arginine ameliorated busulfan-induced testicular toxicity by increasing GSH storage and decreasing lipid peroxidation and DNA damage [16].
Because of the role of oxidative stress in the pathogenesis of cadmium toxicity and the beneficial antioxidant properties of L-arginine, we investigated the possible inhibitory effects of L-arginine against oxidative stress and mitochondrial damage caused by subchronic exposure to cadmium chloride in the kidney tissue of mice.
Materials and Methods
Chemicals and reagents
L-arginine was a product of Hakim Pharmaceutical Company, Tehran, Iran. Cadmium chloride, 5, 5’-dithiobis-[2-nitrobenzoic acid] (DTNB), and Thiobarbituric Acid (TBA) were purchased from Merk company (Germany). All other chemicals and reagents used in this study were obtained from the Sigma chemical company (Germany), with the highest available commercial grade.
Study animals
In this study, 42 male mice weighing between 25-26 g and four weeks old were obtained from the Institute for Laboratory Animals Research, Mazandaran University of Medical Sciences, Sari, Iran. They were housed under standard conditions of temperature 23±2ᴼC with regular 12/12 h light/dark cycle, with free access to food and water. All experimental procedures were conducted according to the ethical standard and protocols approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, Sari, Iran (Ethical Number: IR.MAZUMZ.REC.1398.4844).
Animal grouping and drug administration
The rats were randomly divided into six groups (6 animals in each). Group 1 served as control (normal saline). Group 2 received cadmium chloride (2 mg/kg). Groups 3 to 5 received cadmium chloride (2 mg/kg) plus three doses of L-arginine (50, 100, and 200 mg/kg). The last group received cadmium chloride (2 mg/kg) plus vitamin C (500 mg/kg) as the positive control group. All the treatments were administered intraperitoneally for six consecutive weeks.
Preparation of samples
Twenty-four hours after receiving the last dose, the animals were anesthetized with ether, and a blood sample was taken directly from the heart (centrifuged at 3000 rpm for 15 minutes). The serum was then stored in a freezer to assess Blood Urea Nitrogen (BUN) and Creatinine (Cr). Then, kidney tissues were isolated from the animal for the experiments. Kidney tissues were homogenized and then centrifuged at 2000×g for 10 min at 4°C. Then, the supernatant was subjected to further centrifugation at 10000×g for 10 min. In this step, the supernatant was stored at -70°C for the evaluation of oxidative stress, and the mitochondrial sediment was suspending in Tris-HCl buffer (0.05 M Tris–HCl, 0.25M sucrose, 20 mM KCl, 2mM MgCl2, and 1mM Na2HPO4, pH of 7.4) for the evaluation of mitochondrial toxicity.
Measurement of protein concentration
Protein content was determined in kidney tissue with the Bradford method [17]. Bovine Serum Albumin (BSA) was used as standard. Homogenate samples were mixed with Coomassie blue, and after 10 min, the absorbance was determined at 595 nm by spectrophotometer (UV- 1601 PC, Shimadzu, Japan).
Measurement of lipid peroxidation
The content of MDA was determined using the Zhang et al. method [18]. Briefly, 0.1 mL Thiobarbituric Acid (TBA) was added to 0.2 mL of kidney tissue homogenate. All the samples were placed in a boiling water bath for 30 min. In the end, the tubes were shifted to an ice-bath, and 0.2 mL n-butanol was added to each tube. Then, they were centrifuged at 3500 rpm for 10 min. The amount of MDA formed in each sample was assessed by measuring the absorbance of the supernatant at 532 nm with an ELISA reader (Tecan, Rainbow Thermo, Austria). Tetramethoxypropane was used as standard, and MDA content was expressed as nmol/mg.
Measurement of glutathione content
GSH content was determined by DTNB as the indicator and spectrophotometer. Briefly, 0.1 mL of the kidney tissue homogenate was added into 0.1 mol/L of phosphate buffer and 0.04% DTNB in a total volume of 0.3 mL (PH 7.4). Then yellow color of samples was red at 412 nm on a spectrophotometer (UV- 1601 PC, Shimadzu, Japan). GSH content was expressed as nM [19].
Measurement of protein carbonyl
Determination of protein carbonyl was done by spectrophotometric method. Briefly, 200 µL of kidney tissues were homogenized. The samples were extracted in 500 µL of Trichloroacetic Acid (TCA) 20% (w/v). Then, the samples were placed at 4ᴼC for 15 min. The sediments were treated with 500 µL of 0.2% 2,4-dinitrophenylhydrazine (DNPH) and 500 µL of 2 N HCL for the control group, and samples were incubated at room temperature for 1 h with vortexing at 5 min intervals. Then, the proteins were precipitated by adding 55 µL of 20% TCA. The microtubes were centrifuged and washed three times with 1000 µL of the ethanol-ethyl acetate mixture. And the microtubes were dissolved in 200 µL of 6 M guanidine hydrochloride. The carbonyl content was determined by recording the absorbance at 365 nm wavelength [20].
Assessment of mitochondrial function:
The amount of 1 mL samples were mixed with 25 μL of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) solution and placed at 37°C for 30 min. Then, it was centrifuged at 1000 rpm for 10 minutes. The supernatant was discarded, and 1000 μL of Dimethyl Sulfoxide (DMSO) was added to the precipitate and dispersed. The resulting mixture was centrifuged at 1000 rpm for 10 minutes. Finally, 150-200 µL of the supernatant of each sample was put into a 96-well plate in each cell. Then, the absorbance was read using an ELISA reader [21].
Assessment of the mitochondrial membrane potential
About 1 mL sample was placed into a tube, and 2 mL of Mitochondrial Membrane Potential (MMP) buffer was added. Then, 25 µL of rhodamine dye was added to the tube mixture and placed at 37°C for 20 minutes. Then, the tube was centrifuged to separate. The fluorescence intensity of rhodamine was monitored using Shimadzu RF-5000U fluorescence spectrophotometer at the excitation and emission wavelengths of 490 and 535 nm [9].
Assessment of mitochondrial swelling
About 250 μL of the sample containing mitochondria was mixed with 750 μL of the swelling buffer (70 mM sucrose, 230 mM mannitol, 3 mM HEPES, 2 mM tris-phosphate, 5 mM succinate, and 1 μM of rotenone). Then, the absorbance was read using an ELISA reader (Tecan, Rainbow Thermo, Austria) [22].
Measurement of blood urea nitrogen and creatinine
Blood samples obtained from the heart of mice were sent to a laboratory for measuring BUN and Cr levels.
Statistical analysis
Results were reported as Mean±Standard Deviation (Mean±SD) from three repetitions of the experiment. The data were analyzed by GraphPad Prism 5 statistical software using ANOVA analysis followed by the post hoc Tukey test. The significance level was set at P<0.05. The Kolmogorov-Simonov normality test was used for variables in this study.
Results
Effect of L-arginine on lipid peroxidation in the mice kidney
Cadmium administration significantly increased kidney MDA levels compared to the control group (P<0.05). Administration of L-arginine significantly decreased MDA production and reduced lipid peroxidation (P<0.05). The effect of the highest dose of L-arginine was more than the effect of vitamin C on MDA levels (figure 1).
Effect of L-arginine on glutathione levels in the mice kidneyThe amount of GSH in the cadmium-treated group decreased compared to the control group (P<0.05). In L-arginine groups, the amount of GSH increased significantly (P<0.05) (Figure 2). Effect of L-arginine on protein carbonyl levels in the mice kidney
The concentration of protein carbonyl in the cadmium-treated group increased compared to the control group (P<0.05). In L-arginine treated groups, the amount of protein carbonyl significantly decreased compared to the cadmium group (P<0.05). This effect was dose-dependent, with the highest effect seen at 200 mg/kg and the lowest at 50 mg/kg. The effect of the maximum dose of L-arginine on protein carbonyl levels was more significant than the effect of vitamin C (Figure 3). Effect of L-arginine on mitochondrial function in the mice kidney
Mitochondrial function significantly decreased in the cadmium-treated group compared to the control group (P<0.05). In groups treated with L-arginine, mitochondrial function was significantly improved. This effect was dose-dependent, with the highest effect at 200 mg/kg and the lowest at 50 mg/kg. The effect of the maximum dose of L-arginine on mitochondrial function was greater than the effect of vitamin C (Figure 4). Effect of L-arginine on mitochondrial membrane potential in the mice kidney
The mitochondrial membrane potential collapsed (increase in absorbance) in the cadmium-treated group compared to the control group (P<0.05). On the other hand, in the collapse of L-arginine-treated mitochondrial membrane potential was significantly inhibited (decrease in absorbance) (Figure 5). Effect of L-arginine on mitochondrial swelling in the mice kidney
Cadmium treatment caused significant mitochondrial swelling (decrease in absorbance) compared to the control group (P<0.05). On the other hand, L-arginine reduced mitochondrial swelling induced by cadmium in mice, but this change was not statistically significant (Figure 6). Effect of L-arginine on blood urea nitrogen and creatinine levels in the mice kidney
The serum concentrations of BUN and Cr in the group that received cadmium increased significantly compared to the control group (P<0.05). In the groups receiving L-arginine, the concentration of urea and Cr significantly decreased compared to the cadmium group (P<0.05). This effect was dose-dependent, with the highest effect at 200 mg/kg and the lowest at 50 mg/kg (Table 1).
.png)
Discussion
In this study, we found that L-arginine inhibited oxidative stress and mitochondrial toxicity induced by subchronic exposure to cadmium chloride in male mice. The kidney is one of the main target organs in cadmium poisoning. Cadmium can cause glomerular dysfunction and nephropathy, leading to proteinuria, glycosuria, enzyme urea, and phosphaturia [23]. Cadmium toxicity occurs through indirect induction of ROS production in mitochondria and affects the expression of mitochondrial genes and impaired cellular respiratory activity [9, 24, 25].
We found that cadmium administration increased oxidative stress in mice kidneys via increasing MDA and protein carbonyl levels and decreasing GSH depletion. Other researchers also reported the role of oxidative stress in cadmium tissue toxicity [7, 8]. For example, Lamtai et al. found that IP administration of cadmium chloride (1 mg/kg) for eight weeks promoted oxidative stress in hippocampus tissue via increasing the levels of Nitric Oxide (NO) and Lipid Peroxidation (LPO), while the activities of CAT and SOD were significantly decreased in the hippocampus [8]. These studies confirmed our results about cadmium-induced oxidative damage. Interestingly, administration of L-arginine improved in kidney oxidative markers. Various studies have shown that L-arginine can reduce oxidative stress markers such as protein carbonyl, lipid peroxidation in various conditions in which oxidative stress is involved [15, 16]. Akinrinde et al. showed that L-arginine prevented fluoride-induced hepatotoxicity in rats via decreasing ROS, protein carbonyl, and MDA levels as well as increasing SOD and glutathione peroxidase activity [15].
In addition, it has been reported that L-arginine can trap free radicals [26]. MDA and protein carbonyl are the main end products of the oxidation of membrane lipids and proteins created by the action of free radicals [27, 28]. GSH is an important natural and non-enzymatic antioxidant in the body [29]. L-arginine stimulates the production of GSH by stimulating the endogenous synthesis of GSH, stimulating the expression of arginase (which itself stimulates the production of GSH by converting arginine to glutamate) [30]. So, L-arginine can potentiate antioxidant defense by preventing GSH depletion.
In this study, cadmium administration increased mitochondrial dysfunction, MMP collapse, and mitochondrial swelling in the kidney tissue. These results were in accordance with the results of previous studies, which reported mitochondrial toxicity of cadmium [31, 32]. For example, Bhattacharjee et al. declared that cadmium exposure led to increased oxidative stress markers (ROS and MDA production), mitochondrial oxidative toxicity, reduction of MMP, and mitochondrial dysfunction in rat liver and heart [32].
On the other hand, co-administration of L-arginine with cadmium reduced mitochondrial dysfunction, swelling, and MMP collapse. Previous studies also showed the mitochondrial protective effects of L-arginine [33, 34]. L-arginine administration improved mitochondrial function and inhibition of mitochondrial hydroxyl and superoxide radicals in brain cortex isolated mitochondria of streptomycin-treated rats [34]. L-arginine appears to prevent mitochondrial toxicity by preventing mitochondrial ROS generation.
In this study, cadmium administration raised serum levels of BUN and Cr. These findings in the cadmium-treated group may indicate renal impairment. However, co-administration of L-arginine decreased serum levels of BUN and Cr, indicating a protective effect of L-arginine against cadmium-induced kidney damage.
According to the study results, it is suggested that the renal protective effects of L-arginine might be due to its ability to inhibiting oxidative stress markers (including ROS, glutathione and protein oxidation, and lipid peroxidation). Furthermore, it could also protect the mitochondria from damage by preventing membrane potential collapse, swelling, and mitochondrial dysfunction. It was the first study that evaluated the protective effects of L-arginine against cadmium-induced nephrotoxicity. However, there were several limitations in this study, such as financial problems in providing materials and also gene expression analyses.
Conclusion
Overall, we showed that L-arginine has beneficial effects on nephrotoxicity and oxidative injury caused by cadmium in mice. Therefore, L-arginine would be evaluated for treatment or protection against cadmium-induced kidney injuries.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Committee of Animal Experimentation of Mazandaran University of Medical Sciences, (Code: IR.MAZUMZ.REC.1398.4844). All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This study was extracted from the PhD. dissertation of the second author at the Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical Sciences. Also, this study was supported by the Research Council of Mazandaran University of Medical Sciences (Grant number: 4844).
Authors' contributions
Methodology, data analysis, interpretation, and writing – review & editing: Fatemeh Shaki and Milad Arab-Nozari; Data collection: Farzaneh Sadat Motafeghi, Melika Teymoori, and Nasibeh Hemmati; Manuscript preparation: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014; 7(2):60-72. [DOI:10.2478/intox-2014-0009] [PMID] [PMCID]
- Jaishankar M, Mathew BB, Shah MS, Murthy KTP, Gowda SKR. Biosorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health. 2014; 2(1):1-6. [DOI:10.12691/jephh-2-1-1]
- Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA. Reduced graphene oxide mitigates cadmium-induced cytotoxicity and oxidative stress in HepG2 cells. Food Chem Toxicol. 2020; 143:111515. [DOI:10.1016/j.fct.2020.111515] [PMID]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010; 118(2):182-90. [DOI:10.1289/ehp.0901234] [PMID] [PMCID]
- Han JX, Shang Q, Du Y. Effect of environmental cadmium pollution on human health. Health. 2009; 1(3):159-66. [DOI:10.4236/health.2009.13026]
- Arab-Nozari M, Mohammadi E, Shokrzadeh M, Ahangar N, Talebpour Amiri F, Shaki F. Co-exposure to non-toxic levels of cadmium and fluoride induces hepatotoxicity in rats via triggering mitochondrial oxidative damage, apoptosis, and NF-kB pathways. Environ Sci Pollut Res Int. 2020; 27(19):24048-58. [DOI:10.1007/s11356-020-08791-4] [PMID]
- Fouad AA, Albuali WH, Jresat I. Simvastatin treatment ameliorates injury of rat testes induced by cadmium toxicity. Biol Trace Elem Res. 2013; 153(1-3):269-78. [DOI:10.1007/s12011-013-9667-y] [PMID]
- Lamtai M, Azirar S, Zghari O, Ouakki S, El Hessni A, Mesfioui A, et al. Melatonin ameliorates cadmium-induced affective and cognitive impairments and hippocampal oxidative stress in rat. Biol Trace Elem Res. 2021; 199(4):1445-55. [DOI:10.1080/01480545.2020.1858853] [PMID]
- Arab-Nozari M, Ahangar N, Mohammadi E, Lorigooini Z, Shokrzadeh M, Talebpour Amiri F, et al. Ginkgo biloba attenuated hepatotoxicity induced by combined exposure to cadmium and fluoride via modulating the redox imbalance, Bax/Bcl-2 and NF-kB signaling pathways in male rats. Mol Biol Rep. 2020; 47(9):6961-72. [DOI:10.1007/s11033-020-05755-2] [PMID]
- Shi L, Cao H, Luo J, Liu P, Wang T, Hu G, et al. Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in Duck. Ecotoxicol Environ Saf. 2017; 145:24-31. [DOI:10.1016/j.ecoenv.2017.07.006] [PMID]
- Kowalczyk E, Kopff A, Fijałkowski P, Kopff M, Niedworok J, Błaszczyk J, et al. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim Pol. 2003; 50(2):543-8. [DOI:10.18388/abp.2003_3707] [PMID]
- Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I. Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals. 2011; 24(6):981-92. [DOI:10.1007/s10534-011-9456-z] [PMID]
- Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008; 138(5):867-72. [DOI:10.1093/jn/138.5.867] [PMID]
- Kong X, Tan B, Yin Y, Gao H, Li X, Jaeger LA, et al. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. 2012; 23(9):1178-83. [DOI:10.1016/j.jnutbio.2011.06.012] [PMID]
- Akinrinde AS, Tijani M, Awodele OA, Oyagbemi AA. Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppression of oxidative stress and stimulation of nitric oxide production in rats. Toxicol Environ Health Sci. 2021; 13:57-64. [DOI:10.1007/s13530-020-00070-6]
- Abd‐Elrazek AM, Ahmed‐Farid OAH. Protective effect of L‐carnitine and L‐arginine against busulfan‐induced oligospermia in adult rat. Andrologia. 2018; 50(1):e12806. [DOI:10.1111/and.12806] [PMID]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72(1-2):248-54. [DOI:10.1016/0003-2697(76)90527-3] [PMID]
- Zhang F, Xu Z, Gao J, Xu B, Deng Y. In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol. 2008; 26(2):232-6. [DOI:10.1016/j.etap.2008.04.003] [PMID]
- Sadegh C, Schreck RP. The spectroscopic determination of aqueous sulfite using Ellman’s reagent. MURJ. 2003; 8:39-43. https://www.researchgate.net/publication/267683433_The_Spectroscopic_Determination_of_Aqueous_Sulfite_Using_Ellman's_Reagent
- Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121-6. [DOI:10.1016/S0076-6879(84)05016-3] [PMID]
- Shokrzadeh M, Zamani E, Mehrzad M, Norian Y, Shaki F. Protective effects of propofol against methamphetamine-induced neurotoxicity. Toxicol Int. 2015; 22(1):92-9. [DOI:10.4103/0971-6580.172250] [PMID] [PMCID]
- Arab-Nozari M, Zamani E, Latifi A, Shaki F. Mitochondrial toxicity of aluminium nanoparticles in comparison to its ionic form on isolated rat brain mitochondria. Bratisl Lek Listy. 2019; 120(7):516-22. [DOI:10.4149/BLL_2019_083] [PMID]
- Klaassen CD. Casarett and Doull’s toxicology: The basic science of poisons. New York: McGraw-Hill Education; 2018. https://books.google.com/books?id=_7CSvgAACAAJ&dq
- Casalino E, Sblano C, Landriscina C. Enzyme activity alteration by cadmium administration to rats: The possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys. 1997; 346(2):171-9. [DOI:10.1006/abbi.1997.0197] [PMID]
- Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Effects of cadmium chloride on some mitochondria-related activity and gene expression of human MDA-MB231 breast tumor cells. J Inorg Biochem. 2008; 102(8):1668-76. [DOI:10.1016/j.jinorgbio.2008.04.002] [PMID]
- McMurray CT, Tainer JA. Cancer, cadmium and genome integrity. Nat Genet. 2003; 34(3):239-41. [DOI:10.1038/ng0703-239] [PMID]
- Bogdański P, Suliburska J, Szulińska M, Sikora M, Walkowiak J, Jakubowski H. L-Arginine and vitamin C attenuate pro-atherogenic effects of high-fat diet on biomarkers of endothelial dysfunction in rats. Biomed Pharmacother. 2015; 76:100-6. [DOI:10.1016/j.biopha.2015.10.001] [PMID]
- Shaki F, Arab-Nozari M, Elahi P, Ghasemi M, Habibi E. [Ameliorative effect of Viola odorata. L. ethyl acetate extract against nephrotoxicity induced by chronic ethanol exposure in rats (Persian)]. J Mazandaran Univ Med Sci. 2019; 29(175):1-13. http://jmums.mazums.ac.ir/article-1-12642-en.html
- Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013; 1830(5):3143-53. [DOI:10.1016/j.bbagen.2012.09.008] [PMID] [PMCID]
- Liang M, Wang Z, Li H, Cai L, Pan J, He H, et al. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018; 115:315-28. [DOI:10.1016/j.fct.2018.03.029] [PMID]
- Pan YX, Luo Z, Zhuo MQ, Wei CC, Chen GH, Song YF. Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol. 2018; 199:12-20. [DOI:10.1016/j.aquatox.2018.03.017] [PMID]
- Bhattacharjee B, Pal PK, Chattopadhyay A, Bandyopadhyay D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020; 244:117324. [DOI:10.1016/j.lfs.2020.117324] [PMID]
- Mabalirajan U, Ahmad T, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B. L-arginine reduces mitochondrial dysfunction and airway injury in murine allergic airway inflammation. Int Immunopharmacol. 2010; 10(12):1514-9. [DOI:10.1016/j.intimp.2010.08.025] [PMID]
- Ortiz MDC, Lores-Arnaiz S, Borghese MFA, Balonga S, Lavagna A, Filipuzzi AL, et al. Mitochondrial dysfunction in brain cortex mitochondria of STZ-diabetic rats: Effect of l-Arginine. Neurochem Res. 2013; 38(12):2570-80. [DOI:10.1007/s11064-013-1172-3] [PMID]
Type of Study: Original Research |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





.png)
.png)
.png)