Volume 7, Issue 3 (2021)
Pharm Biomed Res 2021, 7(3): 191-200 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Joshi B C, Panthri N, Prasad N, Virk J K. Pharmacognostic and Physico-chemical Investigation of Barleria cristata Linn. (Leaf) for Quality Control Assessment. Pharm Biomed Res 2021; 7 (3) :191-200
URL: http://pbr.mazums.ac.ir/article-1-358-en.html
URL: http://pbr.mazums.ac.ir/article-1-358-en.html
1- Department of Pharmacognosy, School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University, Balawala, Uttarakhand, India.
2- School of Pharmacy, Adarsh Vijendra Institute of Pharmaceutical Sciences, Shobhit University, Uttar Pradesh, India.
2- School of Pharmacy, Adarsh Vijendra Institute of Pharmaceutical Sciences, Shobhit University, Uttar Pradesh, India.
Full-Text [PDF 2289 kb]
(1336 Downloads)
| Abstract (HTML) (2489 Views)
Full-Text: (2121 Views)
Introduction
The genus Barleria (family: Acanthaceae) is native to the tropical regions of Asia and Africa; it expands its greatest species diversity that invades in open woodland. In the family Acanthaceae, Barleria is the third largest genus with 300 species [1]. In India, 32 species of Barleriahave were reported with 29 subspecies, and 6 varieties [2]. Barleria cristata Linn. is an erect evergreen heavily branched shrub, i.e., commonly known as Raktajhinti and VajraDanti. It is mainly cultivated in South China, South East Asia, subtropical, and the tropical regions of India [3]. It is also considered a potential enviro nmental weed in waste places and can easily grow along the roadside [4]. This plant is distributed throughout India in the form of hedges around fields and gardens (Figure 1).
.png)
The ethnomedicinal reports also classified B. cristata among the potential traditional medicines in India acclaimed for treating toothache, anemia, snakebite, diabetes, lungs disorders, blood diseases, and inflammatory conditions [5]. Mainly, its leaves are considerably used to reduce inflammations moreover also chewed for relief in toothache. In other words, the medicinal activities of a potent plant depend upon the matrix of secondary metabolites found in it. The phytochemical profile of the investigated plant presented the presence of triterpenes (oleanolic acid), flavonoid compounds (luteolin & 7-methoxy luteolin), phenolic compounds (p-coumaric acid & a-tocopherol), iridoid glycosides (barlerin & schanshiside methyl ester), and phenylethanoid glycosides (desrhamnosylacteoside, acteoside, & poliumoside) [6, 7].
Pharmacological studies reported that this plant possesses several biological activities, including anti-inflammatory, antibacterial, anti-diabetic, anti-oxidant, anti-fungal, hepato-protective, anti-plasmodial, anti-oxidant, and cytotoxic properties [8]. This plant is of significant value and lacking in standardization parameters based on the literature; therefore, detailed morphological estimation, physicochemical evaluation, and phytochemical group screening are required. Such measures can be helpful to avoid any ambiguity; accordingly, they enable us in establishing its standardization parameters as well as to authenticate its therapeutic value by pinpointing the exact plant species.
Materials and Methods
The analytical grade chemicals and solvents used in the study were obtained from the Loba Chemie Pvt Ltd., Central Drug House Pvt Ltd, Sigma-Aldrich Corporation, Ranbaxy Fine Chemicals Ltd., and HiMedia Laboratories Pvt. Ltd. companies. The instruments viz. Soxhlet’s extractor (Perfit), Water bath (Perfit), Muffle furnace (Jembo), and Microscope (OLYMPUS, India) were used for the experimental work.
Fresh leaves of the plant were collected from the herbal garden of the School of Pharmaceutical Sciences, SBS University, Balawala, Dehradun (India) in December. The plant was identified by referring to the published standard floras. The botanical name of B. crostata was verified from the plant list was recommended by the Global Strategy for Plant Conservation (www.theplantlist.org; accessed 02.01.2020).
Fresh leaves were first macroscopically examined for their color, odor, taste, shape, size, and texture characteristics concerning the correct identification. They, the investigated characters were compared with the available literature. The microscopic study of leaves was performed by preparing freehand sections; cleared with 5% KOH solution, then stained with concentrated hydrochloric acid-phloroglucinol (1:1), and mounted with 50% glycerin solution [9]. The same procedure was followed for the microscopic studies of the powdered material of B. cristata leaves.
The biochemical parameters were determined as per the guidelines of the World Health Organization (WHO) [10]. The parameters, such as moisture content (loss on drying method), ash values (total ash, acid insoluble ash, & water-soluble ash), and extractive values (water-soluble & alcohol-soluble extractives) were determined using the powdered drug [10, 11].
The fluorescence analysis was determined by treating the small quantities of dried powdered leaves with different chemicals; it was observed at different wavelengths of ultraviolet (254 nm & 366 nm) and visible light [12].
The leaves of B. cristata were shade dried and powdered. The powder (30 g) was defatted with petroleum ether (60-80°C); subsequently, marc was extracted by a continuous hot extraction process using 80% v/v ethanol in water. It was filtered and concentrated under reduced pressure and the percentage yield was calculated.
The preliminary phytochemical screening was conducted for various phytoconstituents, such as proteins, amino acids, carbohydrates, steroids, glycosides, alkaloids, terpenoids, flavonoids, saponins, tannins, and phenolic compounds to be present in the prepared extract of B. cristata, i.e., qualitatively described as per the standard procedures [13].
Thin Layer Chromatography (TLC) glass plates were prepared using silica gel G. The plates were activated at 110°C for 30min. TLC plates were developed in a TLC chamber using chloroform: methanol (9:1) as mobile phase. Thin layer chromatograms were visualized under 254 nm of UV light [14].
The obtained results were calculated in the triplicates manner. The Mean±SEM was applied in Microsoft Excel for statistical analysis.
Results
To ensure the evident reproducibility of quality standards of herbal products, the proper control of the starting material is critical; therefore, the first relevant step was the authentication of the plant material. The standardization of potential medicinal plants is rapidly increasing. For this purpose, despite the modern techniques, identifying plant drugs performed by pharmacognostic studies was more reliable [15]. The macroscopical examination of leaves of B. cristata was first conducted, as it is an initial requirement of the standardization. This examination is a mixture of qualitative evaluation based on the study of morphological features and the sensory profile of the drug.
The characteristic features identified after the morphology of B. cristata are listed in Table 1.
.png)
The leaves were found to be dark green on the ventral surface, while pale green on the dorsal surface with distinguished long petioles. Leaf-blades were found to be ovate elliptical in shape, margins particularly entire, acute-acuminate at apex, and cuneate at base. The leaves manifested reticulate venation and petiolate with the pubescent surface. Morphological parameters help explain an exclusive drug with a major focus on qualitative and quantitative microscopy [15].
The transverse section of B. cristata indicated that the first layer, as a single layer of the epidermis, is protected by a cuticle. The upper and lower epidermis presented a single layer of the polygonal parenchymatous cell, which contained covering trichomes on its surface. The epidermal layer was followed by a 5-7 layer thick collenchymatous cells network. Next to this layer was the ground tissue layer, i.e., composed of parenchymatous cells woven together with intercellular spaces in between. The vascular bundle was situated centrally; xylem vessels were arranged in a radial row surrounded by the phloem. The vascular bundle existing was of open and bicollateral type (xylem layer was sandwiched between phloem layers) is presented in Figure 2.
.png)
Powdered drug histology is a crucial variable for the initial identification of crude plant materials as fragments or powder; the detection of adulterants; reveal the cellular organization, and their distribution [16, 17]. The microscopic observation of B. cristata indicated the presence of parenchyma cells, collenchyma cells, fiber, xylem vessels, and calcium oxalate crystal (Figure 3).
.png)
The advanced quality control practices in herbal industries require the correct identification of the dried plants or powdered plants material; accordingly, it can facilitate the quick detection and prevention of the adulterants, if any. The challenge ahead of this investigation is to validate the therapeutic efficacy and safety of the plant.
The pharmacognostic evaluation of plant material, physicochemical parameters of the powdered drug, and phytochemical screening of the extract were also performed in this research. Extractive values, ash values, and loss on drying were also calculated.
The Mean±SD percentage of loss on drying of powder was measured as 5.03±0.31 w/w. The value appeared to be less than necessary for the growth of microorganisms, such as mould, bacteria, yeast, and fungi that brought changes in the chemical composition of the crude drugs [18].
The total Mean±SD percentage of ash, water-soluble ash, and acid insoluble ash were found to be 16.11±0.38, 11.10±0.23, and 1.50±0.18 w/w, respectively. The extractive values were compiled in Table 2.
.png)
The physicochemical constants can be used as a valuable source of information as well as assessing the purity and quality of crude drugs. The total ash values of the drug provided data on the inorganic composition, i.e., earthy matter and other impurities present along with the drug [17]. The extractive values presented an idea about the chemical constituents of crude drugs. These values are helpful in the estimation of the solubility of a specific constituent in a particular solvent [16]; the percentage of extractive values exceeded 30%, suggesting that a significant amount of polar compounds may be present in the polar extracts [22].
The fluorescence analysis of powder was performed after examining it with several solvents and chemicals. The fluorescence was observed at 254 nm and 366 nm comparing its change of color in visible light. The observations are presented in Table 3.
.png)
This analysis enables us to perform qualitative investigations which can be useful as reference data in recognizing the adulterants [15].
The plant drug was first defatted with petroleum ether, followed by ethanol for obtaining the ethanolic extract of defatted plant material. The percentage yield of leaves extracts was calculated and checked for its color and consistency (Table 4).
.png)
The qualitative preliminary phytochemical screening of the major plant extracts of B. cristata highlighted the presence of all the major phytoconstituents, such as steroids, triterpenoids, glycosides, flavonoids, phenolic compounds, tannins, carbohydrates, proteins, and amino acids (Table 5).
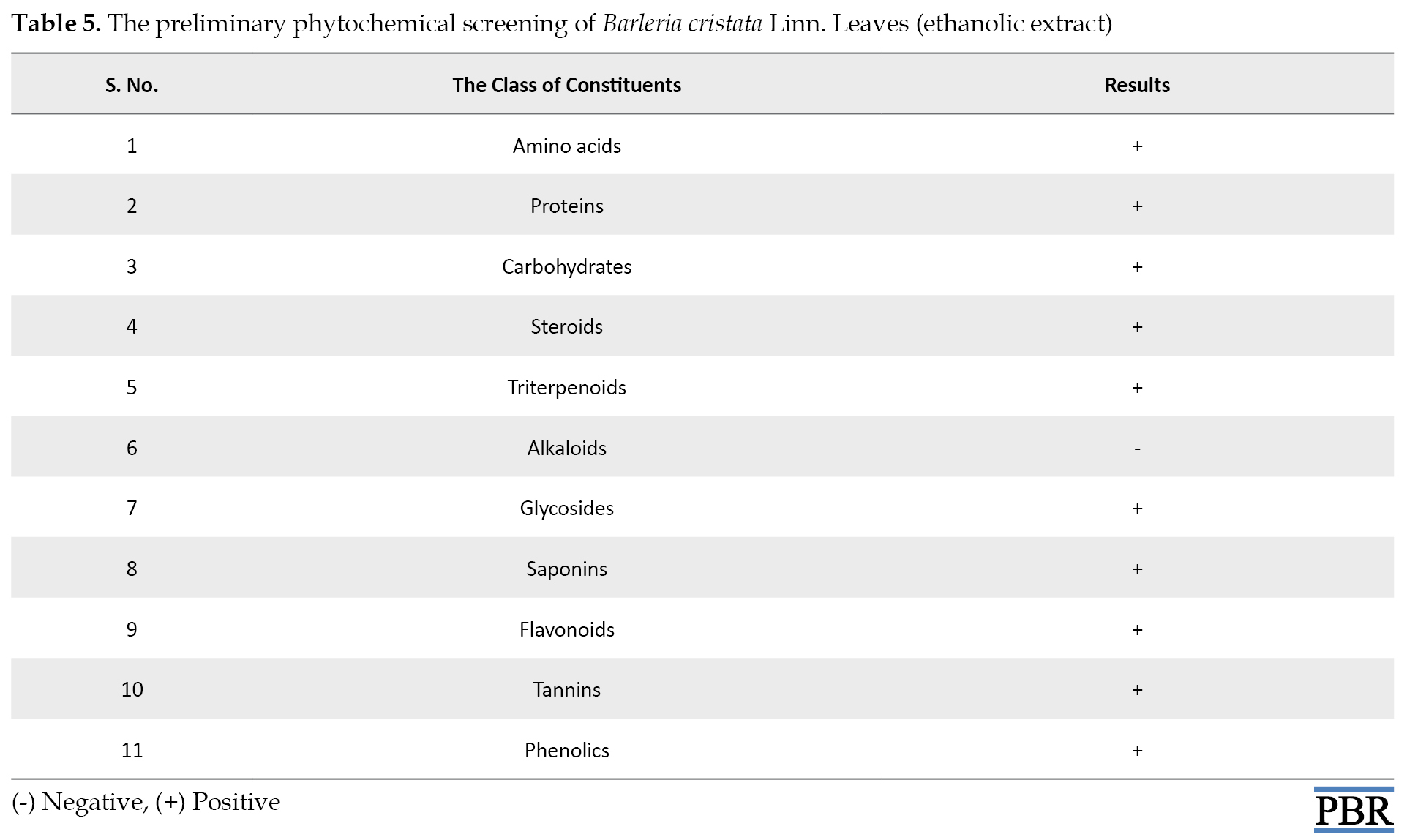
Baskar et al. [20] also revealed the presence of the same phytochemicals in B. cristata. leaves ethanolic extract. Therefore, our investigation was consistent with the above-mentioned results.
The spots were observed and Rf values were noted (Table 6).
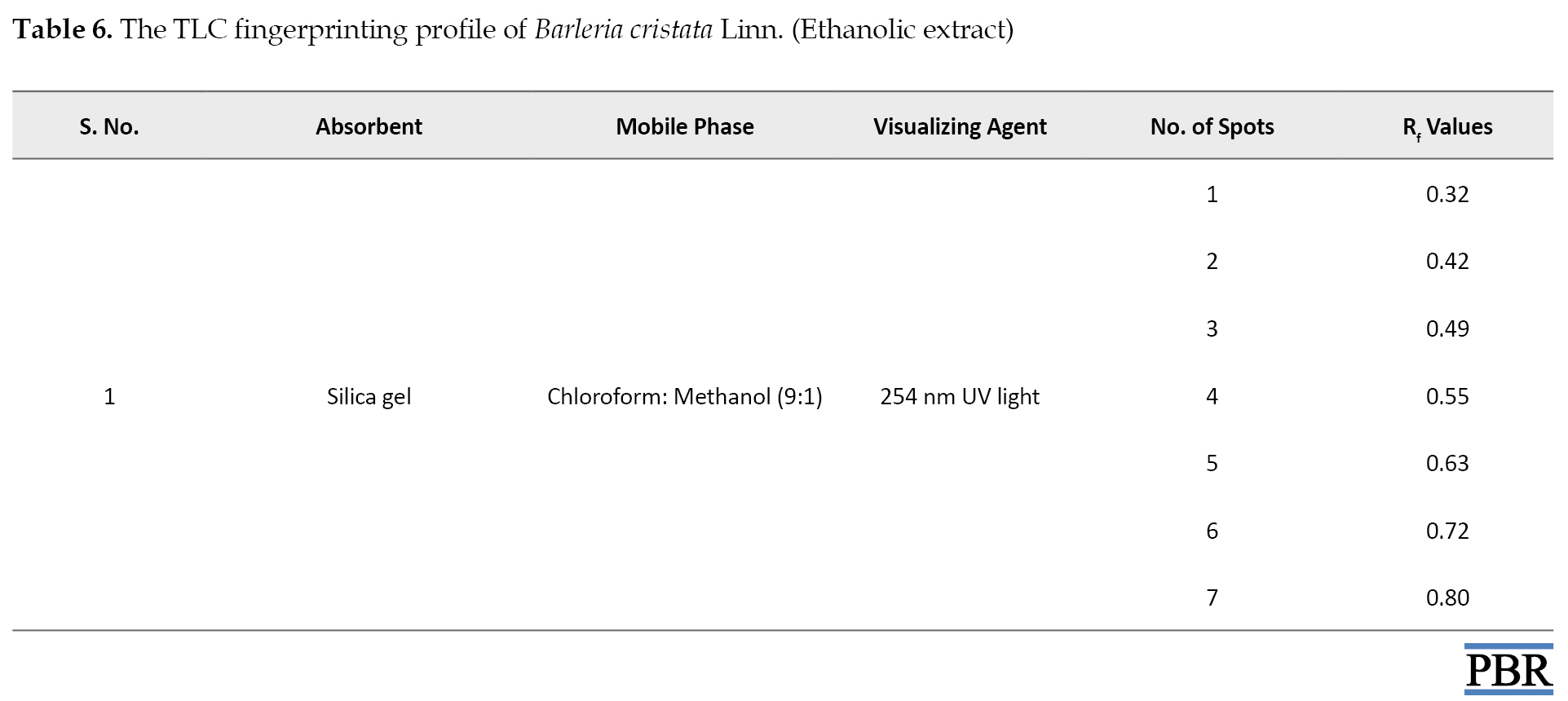
The TLC chromatogram is manifested in Figure 4.
.png)
The obtained chromatogram can be used as an additional information perk concerning the identification and standardization of Barleria cristata leaves. The present study result can serve as a key factor to identify the adulteration even in the powdered form of the drug; morphological and microscopical studies serve as a basis for physically differentiating the drug. These studies also suggested that the observed pharmacognostic and physiochemical parameters are of great value in quality control.
Discussion
Most of the population on the globe depend upon herbal medicines as their primary health care. The WHO emphasizes the need for the physicochemical and phytochemical evaluation of crude drug materials for developing standardized quality control monographs and their drug profiles. In the present study, pharmacognostic evaluation and physicochemical analysis of B. cristata were determined as per the WHO standard guidelines. Macroscopic studies help in determining the identity and degree of purity of herbal materials. Additionally, microscopic examinations are beneficial for identifying broken or powdered materials. The pharmacognostic investigations are required to set up some physical parameters; they play a key role in obtaining the standard monograph for a crude drug as the value of these parameters mostly remains constant for a particular plant species. Based on the WHO guidelines, standard procedures were followed to provide significant values for various physicochemical parameters [10]. These parameters are critical for detecting drug adulteration or the improper management of raw materials.
The ash value gives an idea of inorganic composition and other impurities present in plant drugs. The amount of moisture content present in a crude drug is directly related to its stability. This is because of the odds of microbial growth induced by it. The shelf life of the drug is inversely proportional to the moisture content present in it [14]. The extractive values inform about the nature of the chemical constituents present in a crude drug, i.e., determined in different solvents using cold and hot maceration methods. The extractive values also give an idea of the solubility of the specific constituent in a particular solvent [23]. Plants are recognized for their multi-utility and secondary metabolites as a unique gift of nature. The secondary metabolites of plants are considered major therapeutic agents to cure diverse classes of diseases [24]. The purpose of phytochemical screening of the plant material is to identify the class of chemical compounds present. The phytochemical screening of the leaves extract of B. cristata was performed; it would be of considerable use in identifying this drug and monograph preparation in the future.
The thin layer chromatograms were examined under short UV (254 nm) and long UV (366 nm); their values play a significant role in the preliminary separation and determination of plant constituents. Several leaf measurements were used to study microscopic features, i.e., not easily characterized by general microscopy. This finding is useful to supplement the existing information concerning the identification and standardization of B. cristata leaves even in the powdered form of the plant drug to distinguish it from the drug and its adulterant. These studies also suggested that the observed pharmacognostic and physiochemical parameters are of great value in the quality control and monograph development of this drug.
Conclusion
The quality control of a crude drug is a very delicate parameter for establishing its quality and purity values; extensive care is required in this field, as approximately 4 billion individuals (almost 80% of the world population) residing in developing regions depends upon the plant-based medicine as their primary source of healthcare. The significant parameters of a crude drug can be established by establishing its careful standardization; therefore, the WHO has emphasized quality control for herbal drugs. Barleria cristata is conventionally used for treating various disease conditions without its established standardization values and concerns related to its quality level.
The significant outcome of the present study could therefore be used in establishing a bridge between identification and the investigation of the plant as well as the development of quality control parameters of this plant species. Various quality control parameters are helpful to identify and authenticate Barleria cristata. The present work facilitates providing information regarding the standardization, identification, and performing further research on this plant. The collected data from its pharmacognostical, physicochemical, and phytochemical screening can serve as a source of information for preparing its monograph in the future; they can also be used as a tool for standardizing this medicinal plant.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
The study was supported by the Gaurav Bharti Shiksha Sansthan, Dehradun, India, which runs the School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We gratefully acknowledge the financial support of the Gaurav Bharti Shiksha Sansthan, Dehradun, India, which runs the School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University.
References
The genus Barleria (family: Acanthaceae) is native to the tropical regions of Asia and Africa; it expands its greatest species diversity that invades in open woodland. In the family Acanthaceae, Barleria is the third largest genus with 300 species [1]. In India, 32 species of Barleriahave were reported with 29 subspecies, and 6 varieties [2]. Barleria cristata Linn. is an erect evergreen heavily branched shrub, i.e., commonly known as Raktajhinti and VajraDanti. It is mainly cultivated in South China, South East Asia, subtropical, and the tropical regions of India [3]. It is also considered a potential enviro nmental weed in waste places and can easily grow along the roadside [4]. This plant is distributed throughout India in the form of hedges around fields and gardens (Figure 1).
.png)
The ethnomedicinal reports also classified B. cristata among the potential traditional medicines in India acclaimed for treating toothache, anemia, snakebite, diabetes, lungs disorders, blood diseases, and inflammatory conditions [5]. Mainly, its leaves are considerably used to reduce inflammations moreover also chewed for relief in toothache. In other words, the medicinal activities of a potent plant depend upon the matrix of secondary metabolites found in it. The phytochemical profile of the investigated plant presented the presence of triterpenes (oleanolic acid), flavonoid compounds (luteolin & 7-methoxy luteolin), phenolic compounds (p-coumaric acid & a-tocopherol), iridoid glycosides (barlerin & schanshiside methyl ester), and phenylethanoid glycosides (desrhamnosylacteoside, acteoside, & poliumoside) [6, 7].
Pharmacological studies reported that this plant possesses several biological activities, including anti-inflammatory, antibacterial, anti-diabetic, anti-oxidant, anti-fungal, hepato-protective, anti-plasmodial, anti-oxidant, and cytotoxic properties [8]. This plant is of significant value and lacking in standardization parameters based on the literature; therefore, detailed morphological estimation, physicochemical evaluation, and phytochemical group screening are required. Such measures can be helpful to avoid any ambiguity; accordingly, they enable us in establishing its standardization parameters as well as to authenticate its therapeutic value by pinpointing the exact plant species.
Materials and Methods
The analytical grade chemicals and solvents used in the study were obtained from the Loba Chemie Pvt Ltd., Central Drug House Pvt Ltd, Sigma-Aldrich Corporation, Ranbaxy Fine Chemicals Ltd., and HiMedia Laboratories Pvt. Ltd. companies. The instruments viz. Soxhlet’s extractor (Perfit), Water bath (Perfit), Muffle furnace (Jembo), and Microscope (OLYMPUS, India) were used for the experimental work.
Fresh leaves of the plant were collected from the herbal garden of the School of Pharmaceutical Sciences, SBS University, Balawala, Dehradun (India) in December. The plant was identified by referring to the published standard floras. The botanical name of B. crostata was verified from the plant list was recommended by the Global Strategy for Plant Conservation (www.theplantlist.org; accessed 02.01.2020).
Fresh leaves were first macroscopically examined for their color, odor, taste, shape, size, and texture characteristics concerning the correct identification. They, the investigated characters were compared with the available literature. The microscopic study of leaves was performed by preparing freehand sections; cleared with 5% KOH solution, then stained with concentrated hydrochloric acid-phloroglucinol (1:1), and mounted with 50% glycerin solution [9]. The same procedure was followed for the microscopic studies of the powdered material of B. cristata leaves.
The biochemical parameters were determined as per the guidelines of the World Health Organization (WHO) [10]. The parameters, such as moisture content (loss on drying method), ash values (total ash, acid insoluble ash, & water-soluble ash), and extractive values (water-soluble & alcohol-soluble extractives) were determined using the powdered drug [10, 11].
The fluorescence analysis was determined by treating the small quantities of dried powdered leaves with different chemicals; it was observed at different wavelengths of ultraviolet (254 nm & 366 nm) and visible light [12].
The leaves of B. cristata were shade dried and powdered. The powder (30 g) was defatted with petroleum ether (60-80°C); subsequently, marc was extracted by a continuous hot extraction process using 80% v/v ethanol in water. It was filtered and concentrated under reduced pressure and the percentage yield was calculated.
The preliminary phytochemical screening was conducted for various phytoconstituents, such as proteins, amino acids, carbohydrates, steroids, glycosides, alkaloids, terpenoids, flavonoids, saponins, tannins, and phenolic compounds to be present in the prepared extract of B. cristata, i.e., qualitatively described as per the standard procedures [13].
Thin Layer Chromatography (TLC) glass plates were prepared using silica gel G. The plates were activated at 110°C for 30min. TLC plates were developed in a TLC chamber using chloroform: methanol (9:1) as mobile phase. Thin layer chromatograms were visualized under 254 nm of UV light [14].
The obtained results were calculated in the triplicates manner. The Mean±SEM was applied in Microsoft Excel for statistical analysis.
Results
To ensure the evident reproducibility of quality standards of herbal products, the proper control of the starting material is critical; therefore, the first relevant step was the authentication of the plant material. The standardization of potential medicinal plants is rapidly increasing. For this purpose, despite the modern techniques, identifying plant drugs performed by pharmacognostic studies was more reliable [15]. The macroscopical examination of leaves of B. cristata was first conducted, as it is an initial requirement of the standardization. This examination is a mixture of qualitative evaluation based on the study of morphological features and the sensory profile of the drug.
The characteristic features identified after the morphology of B. cristata are listed in Table 1.
.png)
The leaves were found to be dark green on the ventral surface, while pale green on the dorsal surface with distinguished long petioles. Leaf-blades were found to be ovate elliptical in shape, margins particularly entire, acute-acuminate at apex, and cuneate at base. The leaves manifested reticulate venation and petiolate with the pubescent surface. Morphological parameters help explain an exclusive drug with a major focus on qualitative and quantitative microscopy [15].
The transverse section of B. cristata indicated that the first layer, as a single layer of the epidermis, is protected by a cuticle. The upper and lower epidermis presented a single layer of the polygonal parenchymatous cell, which contained covering trichomes on its surface. The epidermal layer was followed by a 5-7 layer thick collenchymatous cells network. Next to this layer was the ground tissue layer, i.e., composed of parenchymatous cells woven together with intercellular spaces in between. The vascular bundle was situated centrally; xylem vessels were arranged in a radial row surrounded by the phloem. The vascular bundle existing was of open and bicollateral type (xylem layer was sandwiched between phloem layers) is presented in Figure 2.
.png)
Powdered drug histology is a crucial variable for the initial identification of crude plant materials as fragments or powder; the detection of adulterants; reveal the cellular organization, and their distribution [16, 17]. The microscopic observation of B. cristata indicated the presence of parenchyma cells, collenchyma cells, fiber, xylem vessels, and calcium oxalate crystal (Figure 3).
.png)
The advanced quality control practices in herbal industries require the correct identification of the dried plants or powdered plants material; accordingly, it can facilitate the quick detection and prevention of the adulterants, if any. The challenge ahead of this investigation is to validate the therapeutic efficacy and safety of the plant.
The pharmacognostic evaluation of plant material, physicochemical parameters of the powdered drug, and phytochemical screening of the extract were also performed in this research. Extractive values, ash values, and loss on drying were also calculated.
The Mean±SD percentage of loss on drying of powder was measured as 5.03±0.31 w/w. The value appeared to be less than necessary for the growth of microorganisms, such as mould, bacteria, yeast, and fungi that brought changes in the chemical composition of the crude drugs [18].
The total Mean±SD percentage of ash, water-soluble ash, and acid insoluble ash were found to be 16.11±0.38, 11.10±0.23, and 1.50±0.18 w/w, respectively. The extractive values were compiled in Table 2.
.png)
The physicochemical constants can be used as a valuable source of information as well as assessing the purity and quality of crude drugs. The total ash values of the drug provided data on the inorganic composition, i.e., earthy matter and other impurities present along with the drug [17]. The extractive values presented an idea about the chemical constituents of crude drugs. These values are helpful in the estimation of the solubility of a specific constituent in a particular solvent [16]; the percentage of extractive values exceeded 30%, suggesting that a significant amount of polar compounds may be present in the polar extracts [22].
The fluorescence analysis of powder was performed after examining it with several solvents and chemicals. The fluorescence was observed at 254 nm and 366 nm comparing its change of color in visible light. The observations are presented in Table 3.
.png)
This analysis enables us to perform qualitative investigations which can be useful as reference data in recognizing the adulterants [15].
The plant drug was first defatted with petroleum ether, followed by ethanol for obtaining the ethanolic extract of defatted plant material. The percentage yield of leaves extracts was calculated and checked for its color and consistency (Table 4).
.png)
The qualitative preliminary phytochemical screening of the major plant extracts of B. cristata highlighted the presence of all the major phytoconstituents, such as steroids, triterpenoids, glycosides, flavonoids, phenolic compounds, tannins, carbohydrates, proteins, and amino acids (Table 5).

Baskar et al. [20] also revealed the presence of the same phytochemicals in B. cristata. leaves ethanolic extract. Therefore, our investigation was consistent with the above-mentioned results.
The spots were observed and Rf values were noted (Table 6).

The TLC chromatogram is manifested in Figure 4.
.png)
The obtained chromatogram can be used as an additional information perk concerning the identification and standardization of Barleria cristata leaves. The present study result can serve as a key factor to identify the adulteration even in the powdered form of the drug; morphological and microscopical studies serve as a basis for physically differentiating the drug. These studies also suggested that the observed pharmacognostic and physiochemical parameters are of great value in quality control.
Discussion
Most of the population on the globe depend upon herbal medicines as their primary health care. The WHO emphasizes the need for the physicochemical and phytochemical evaluation of crude drug materials for developing standardized quality control monographs and their drug profiles. In the present study, pharmacognostic evaluation and physicochemical analysis of B. cristata were determined as per the WHO standard guidelines. Macroscopic studies help in determining the identity and degree of purity of herbal materials. Additionally, microscopic examinations are beneficial for identifying broken or powdered materials. The pharmacognostic investigations are required to set up some physical parameters; they play a key role in obtaining the standard monograph for a crude drug as the value of these parameters mostly remains constant for a particular plant species. Based on the WHO guidelines, standard procedures were followed to provide significant values for various physicochemical parameters [10]. These parameters are critical for detecting drug adulteration or the improper management of raw materials.
The ash value gives an idea of inorganic composition and other impurities present in plant drugs. The amount of moisture content present in a crude drug is directly related to its stability. This is because of the odds of microbial growth induced by it. The shelf life of the drug is inversely proportional to the moisture content present in it [14]. The extractive values inform about the nature of the chemical constituents present in a crude drug, i.e., determined in different solvents using cold and hot maceration methods. The extractive values also give an idea of the solubility of the specific constituent in a particular solvent [23]. Plants are recognized for their multi-utility and secondary metabolites as a unique gift of nature. The secondary metabolites of plants are considered major therapeutic agents to cure diverse classes of diseases [24]. The purpose of phytochemical screening of the plant material is to identify the class of chemical compounds present. The phytochemical screening of the leaves extract of B. cristata was performed; it would be of considerable use in identifying this drug and monograph preparation in the future.
The thin layer chromatograms were examined under short UV (254 nm) and long UV (366 nm); their values play a significant role in the preliminary separation and determination of plant constituents. Several leaf measurements were used to study microscopic features, i.e., not easily characterized by general microscopy. This finding is useful to supplement the existing information concerning the identification and standardization of B. cristata leaves even in the powdered form of the plant drug to distinguish it from the drug and its adulterant. These studies also suggested that the observed pharmacognostic and physiochemical parameters are of great value in the quality control and monograph development of this drug.
Conclusion
The quality control of a crude drug is a very delicate parameter for establishing its quality and purity values; extensive care is required in this field, as approximately 4 billion individuals (almost 80% of the world population) residing in developing regions depends upon the plant-based medicine as their primary source of healthcare. The significant parameters of a crude drug can be established by establishing its careful standardization; therefore, the WHO has emphasized quality control for herbal drugs. Barleria cristata is conventionally used for treating various disease conditions without its established standardization values and concerns related to its quality level.
The significant outcome of the present study could therefore be used in establishing a bridge between identification and the investigation of the plant as well as the development of quality control parameters of this plant species. Various quality control parameters are helpful to identify and authenticate Barleria cristata. The present work facilitates providing information regarding the standardization, identification, and performing further research on this plant. The collected data from its pharmacognostical, physicochemical, and phytochemical screening can serve as a source of information for preparing its monograph in the future; they can also be used as a tool for standardizing this medicinal plant.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
The study was supported by the Gaurav Bharti Shiksha Sansthan, Dehradun, India, which runs the School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University.
Authors' contributions
All authors contributed equally in preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We gratefully acknowledge the financial support of the Gaurav Bharti Shiksha Sansthan, Dehradun, India, which runs the School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University.
References
- Balkwill MJ, Balkwill K. A preliminary analysis of distribution patterns in a large, pantropical genus, Barleria L. (Acanthaceae). J Biogeogr. 1998; 25(1):95-110. [DOI:10.1046/j.1365-2699.1998.251120.x]
- Ghosh T, Mukherjee SK, Debnath HS. Comparative taxonomic studies of four species of Barleria L. (Tribe justicieae seafft/benth. & Hook. F. - Acanthaceae) of N. E. India. Paper presented at: International Seminar on Multidisciplinary Approaches in Angiosperm Systematics. 2009:113-7. https://www.researchgate.net/publication/270279481_
- Chowdhury N, Al Hasan A, Tareq FS, Ahsan M, Azam AZ. 4-Hydroxy-trans-cinnamate Derivatives and Triterpene from Barleria cristata. Dhaka Univ J Pharm Sci. 2013; 12(2):143-5. [DOI:10.3329/dujps.v12i2.17623]
- Quattrocchi U. CRC world dictionary of palms: Common names, scientific names, eponyms, synonyms, and etymology. Boca Raton: CRC Press; 2017. [DOI:10.1201/9781315155449]
- Gambhire MN, Wankhede SS, Juvekar AR. Antiinflammatory activity of aqueous extract of Barleria cristata leaves. J Young Pharm. 2009; 1(3):220-4. [DOI:10.4103/0975-1483.57068]
- El-Mawla A, Ahmed AS, Ibraheim ZZ, Ernst L. Phenylethanoid glycosides from Barleria cristata L. Callus cultures. Bull Pharm Sci. 2005; 28(2):199-204. [DOI:10.21608/bfsa.2005.65382]
- Hemalatha K, Hareeka N, Sunitha D. Chemical constituents isolated from leaves of Barleria cristata Linn. Int J Pharm Bio Sci. 2012; 3(1):609-15. https://ijpbs.net/abstract.php?article=MTI2OA==
- Charoenchai P, Vajrodaya S, Somprasong W, Mahidol C, Ruchirawat S, Kittakoop P. Part 1: Antiplasmodial, cytotoxic, radical scavenging and antioxidant activities of Thai plants in the family Acanthaceae. Planta Med. 2010; 76(16):1940-3. [DOI:10.1055/s-0030-1250045] [PMID]
- Khandelwal KR. Practical pharmacognosy. Pune: Nirali Prakashan; 2008. https://www.google.com/books/edition/Practical_Pharmacognosy/SgYUFD_lkK4C?hl=en&gbpv=0
- World Health Organization. Quality control methods for herbal materials. Geneva: World Health Organization; 2011. https://apps.who.int/iris/bitstream/handle/10665/44479/9789241500739_eng.pdf
- Junejo JA, Ghoshal A, Mondal P, Nainwal L, Zaman K, Singh KD, et al. In-vivo toxicity evaluation and phytochemical, physicochemical analysis of diplaziumesculentum (Retz.) Sw. leaves a traditionally used North-Eastern Indian vegetable. Adv Biores. 2015; 6(5):175-81. https://www.researchgate.net/profile/linksf
- Kokoski CJ, Kokoski RJ, Slama FJ. Fluorescence of powdered vegetable drugs under ultraviolet radiation. J Am Pharm Assoc Am Pharm Assoc. 1958; 47(10):715-7. [DOI:10.1002/jps.3030471010] [PMID]
- Harborne JB, Harborne AJ . Phytochemical methods a guide to modern techniques of plant analysis. Dordrecht: Springer Netherlands; 1998. https://www.google.com/books/edition/Phytochemical_Methods_A_Guide_to_Modern/2yvqeRtE8CwC?hl=en&gbpv=0
- Bladt S. Plant drug analysis: A thin layer chromatography atlas. Heidelberg: Springer Berlin Heidelberg; 2009. https://www.google.com/books/edition/Plant_Drug_Analysis/CdVKAAAAQBAJ?hl=en&gbpv=0
- Upadhyay P, Joshi BC, Sundriyal A, Mukhija M. Pharmacognostic standardization and physicochemical evaluation of Caesalpinia crista L. Root for quality control assessment. J Nat Sci Med. 2019; 2(3):135-40. https://www.jnsmonline.org/article.asp?issn=2589-627X;year=2019;volume=2;issue=3;spage=135;epage=140;aulast=Upadhyay
- Kabra A, Sharma R, Singla S, Kabra R, Baghel US. Pharmacognostic characterization of Myrica esculenta leaves. J Ayurveda Integr Med. 2019; 10(1):18-24. [DOI:10.1016/j.jaim.2017.07.012] [PMID] [PMCID]
- Alam F, Najum us Saqib Q. Pharmacognostic standardization and preliminary phytochemical studies of Gaultheria trichophylla. Pharm Biol. 2015; 53(12):1711-8. [DOI:10.3109/13880209.2014.1003355] [PMID]
- Suresh V, Arunachalam G. Pharmacognostical and preliminary phytochemical studies of bark of Nyctanthes arbor-tristis Linn. Res J Pharmacogn Phytochem. 2012; 2(5):411-6. https://www.indianjournals.com/ijor.aspx?target=ijor:rjpp&volume=2&issue=5&article=016
- Kumar H, Agrawal R, Kumar V. Barleria cristata: Perspective towards phytopharmacological aspects. J Pharm Pharmacol. 2018; 70(4):475-87. [DOI:10.1111/jphp.12881] [PMID]
- Baskar S, Selvan G, Anbarasu R, Raja V. Phytochemical, trace metals assessment and antimicrobial efficacy of Barleria cristata. Int J Pharmacol Res. 2015; 5(10):257-63. https://ssjournals.com/index.php/ijpr/article/view/2743/2024
- Joshi BC, Chauhan N, Singh S, Uniyal S. Pharmacognostic and phytochemical evaluation of Nyctanthes arbortristis stem. Int J Pharmacognosy. 2018; 5(6):376-81. https://ijpjournal.com/bft-article/pharmacognostic-and-phytochemical-evaluation-of-nyctanthes-arbor-tristis-stem/
- Kumar V, Singh S, Kondalkar SA, Srivastava B, Sisodia BS, Barthi B, et al. High resolution GC/MS analysis of the Holoptelea integrifoli’s leaves and their medicinal qualities. BiocatalAgric Biotechnol. 2019; 22:101405. [DOI:10.1016/j.bcab.2019.101405]
- Shah G, Chawla A, Baghel US, Rahar S, Singh PS, Dhawan RK. Pharmacognostic standardization of leaves of Melaleuca leucadendron. Pharmacogn J. 2013; 5(4):143-8. [DOI:10.1016/j.phcgj.2013.07.008]
- Kumar V, Singh S, Singh A, Subhose V, Prakash O. Assessment of heavy metal ions, essential metal ions, and antioxidant properties of the most common herbal drugs in Indian Ayurvedic hospital: For ensuring quality assurance of certain Ayurvedic drugs. Biocatal Agric Biotechnol. 2019; 18:101018. [DOI:10.1016/j.bcab.2019.01.056]
Type of Study: Original Research |
Subject:
Pharmacognosy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









