Volume 6, Issue 2 (2020)
Pharm Biomed Res 2020, 6(2): 157-168 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khodabandeh Shahraki F, Nabiuni M, Farhadi M, Amini E. The Effect of Hydroalcoholic Rosmarinus Officinalis (Rosemary) on Memory Retention Deficit in Young Offspring Rat Induced by Maternal Sleep Deprivation. Pharm Biomed Res 2020; 6 (2) :157-168
URL: http://pbr.mazums.ac.ir/article-1-295-en.html
URL: http://pbr.mazums.ac.ir/article-1-295-en.html
1- Department of Animal Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
2- Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
3- Department of Microbiology, Karaj branch, Islamic Azad University, Karaj, Iran
2- Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
3- Department of Microbiology, Karaj branch, Islamic Azad University, Karaj, Iran
Keywords: Maternal sleep deprivation, Cognitive impairment, Memory deficit, Hippocampus, Neurogenesis, Oxidative stress, Rosemary
Full-Text [PDF 6742 kb]
(1807 Downloads)
| Abstract (HTML) (3600 Views)
Histopathological study of the hippocampus
Effect of sleep deprivation on neuronal morphology and density as depicted by histopathological sections of rat brains
Histopathological evaluation of hippocampal regions of the rat brain was conducted under light microscopy. Figure 2 displays the representative images of brain sections from different groups of animals. Brains of the control and the SD-treated group with rosemary (100 mg/kg) showed comparatively undamaged and optimum sized neuronal cells as compared with the sleep-deprived groups (SD and SD treated with rosemary extract dose of 50, or 200 mg/kg). However, the disorganization of various cell layers increased the level of cellular spongiosis, and the density of cells observed specifically in the Dentate Gyrus (DG) of the hippocampus and the brains of sleep-deprived animals.
Full-Text: (2146 Views)
Introduction
Sleep is a passive recovery process, characterized by cessation of the sensory, motor, and mental functions connecting the central nervous system to the external environment [1]. Sleep is necessary for the proper function of memory and learning. Thus, sleep deprivation has detrimental effects on learning, brain development, and alertness [2]. Studies have demonstrated that sleep deprivation is associated not only with high financial, social, and human costs but also with cognitive and motor functions. Cognitive indices such as working memory are particularly susceptible to sleep deprivation [3].
Sleep disturbance is a common phenomenon in many neurological diseases, including Alzheimer disease, Parkinson disease, and autism [4]. Patients with dementia are known for having symptoms such as insomnia, restlessness, decreased, or prolonged sleep. These sleep disturbances lead to cognitive and functional decline accompanied by abnormal behavior and finally depression in some patients. In this regard, it is pertinent to mention that sleep behavioral disorder with rapid eye movement is one of the major sleep disorders associated with neurological abnormalities [5].
According to previous investigations, sleep improves memory by enhancing the functional integration and cell survival of the new hippocampal neurons. Regarding the learning and memory space, the relationship between sleep and neurogenesis indicates that the hippocampus is an important structure for memory formation and one of the distinct brain regions, which is an essential part of adult neurogenesis. Hippocampus contributes to memory and learning by increasing neurogenesis and neuronal production, so that learning deficits are associated with the reduction of neurogenesis. On the other hand, both neurogenesis and hippocampus memory formation are susceptible to sleep deprivation. In other words, sleep deprivation alters the electrophysiological and molecular properties of the hippocampal neurons [6, 7]. According to the findings of the researchers, maternal sleep deprivation has a major impact on the baby’s brain during pregnancy; this impact includes memory, learning, and other cognitive functions.
The researchers concluded that neurogenesis in pregnant rats increased by 65% and reached its peak on the seventh day of pregnancy before decreasing and again reached its highest level at the end of pregnancy [8]. Studies have shown that sleep deprivation is a strong oxidative stress response. Free radicals like Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are the result of several cellular signaling pathways as a defense mechanism. An excessive amount of free radicals causes oxidative stress that damages cells, tissues, and organs, resulting in several diseases, like cancer, cardiovascular diseases, Alzheimer Disease (AD), and even accelerate the aging process. Hippocampus is a part of the brain which plays an important role in cognition, mood regulation, response to stress, learning, and memory. The hippocampus is one of the most vulnerable brain regions in oxidative stress.
The maintenance of a normal redox state in the hippocampus is essential for the prevention of cognitive functions decline in aging. Antioxidants can slow down or prevent the resulting damages caused by free radicals through delaying, intercepting, and inhibiting their activities or via breaking the chain reaction of oxidation. These compounds can prevent the progression of the disease by removing free radicals. Studies have also revealed that various neurological diseases are accompanied by increased oxidative stress and free radical production, which, in turn, can result in tissue damage and cell death. Application of antioxidant compounds can also play a pivotal role in preventing antioxidant damage induced by sleep deprivation [9-11].
Rosemarinus officinalis L. an evergreen perennial aromatic shrub belongs to the family Labiatae. It is a popular household plant, commonly called rosemary, which is native to the north and south coasts of the Mediterranean Sea. Rosemary has many effective compounds with antioxidant properties. Andrade et al. studied the antioxidant and pro-oxidant properties of rosemary. Its main constituents with antioxidant properties are carnosic acid and carnosol that are responsible for 90% of the properties. Both are inhibitors of lipid peroxidation in liposomal and microsomal systems; they are good scavengers of CCl3O2 (peroxyl radicals), reduce cytochrome c, and scavenge hydroxyl radicals. Particularly, carnosic acid scavenges H2O2, but could also act as a substrate for the peroxidase system. The antioxidant properties depend on the fruiting stages.
The increase in the concentration of polyphenols, which include carnosol, rosmarinic acid, and hesperidin, during the fruiting stage, is directly related to the enhancement of the extract antioxidant property. Rosmarinic acid and hesperidin have been cited in the literature as important free radical scavengers. This plant assumes significance in traditional medicine owing to its medicinal properties and treating cognitive function deficiencies. Rosemary contains essential oils of 0.6 to 2, including 1,8-cineoles, alpha pineol, camphor, porroneol, carvacrol with phenolic detergents. Flavones and caffeic acid are derivatives of rosemarynum acid. Rosmarinic acid acts as antioxidant compounds [12].
These compounds can treat memory deficit by removing free radicals. Neuronal deletion, particularly in the cortex region, is now known to lead to cognitive impairment in acquired learning skills and memory. Different in vitro and in vivo models evaluated rosemary and its efficacy in the management of patients with Alzheimer disease. The studies have demonstrated that extracts from plants of the Lamiaceae family are active in the inhibition of Acetylcholinesterase (AChE) and β-amyloid deposits. Besides, antioxidant, cytoprotective, and anti-inflammatory activities have been observed in Lamiaceae plant extracts. There are reports that compounds like 1,8-cineole and alpha-terpineol inhibit AChE, and notably, 1,8-cineole is the most potent inhibitor [13]. The purpose of this study was to investigate the protective effect of rosemary extract against side effects of maternal sleep deprivation in neonate rats such as memory deficit. Neurotransmitter interactions may be responsible for the cognitive enhancements observed in the present study.
Material and Methods
Study animals
Adult female Wistar rats were used in this study. The animals were maintained in the laboratory of Animal Breeding Laboratory of the Faculty of Biological Sciences of Kharazmi University. The animals were housed in cages under standard environmental (light/dark cycle 12/12 hours, the temperature of 22 ± 2°C with free access to food and water). The ethical considerations regarding the amount of sleep deprivation by the box were met following the protocols and guidelines for the care and use of laboratory animals in the Laboratory of Neuroscience and Laboratory Research at the American Institute of Laboratory Animals. To prevent the aggressive severity of sleep deprivation, the necessary measures were taken, as far as possible, following international guidelines. The dose of rosemary extract was adjusted according to the WHO and Food and Drug Administration.
Ethical approval
The authors declare that the experiments done on animals were conducted following the local Ethics Committee regulations on the care and use of laboratory animals (IR.IAU.K.REC.1395.34).
Preparing rosemary hydroalcoholic extract
The whole plant of Rosmarinus officinalis was collected freshly from a cultivation farm of the north of Iran in 2016. The plant was identified and authenticated by Dr Ghahremaninejad (the senior botanist of the Department of Botany, Kharazmi University of Tehran) and a voucher specimen was deposited accordingly in the herbarium of the same department. The medicinal parts of the plant, the fresh leaves, were separated, dried in shade, pulverized by a mechanical grinder, passed through a 40-mesh sieve, and stored in an airtight container for further use.
Preparation of the plant extract
About 500 g of powdered dry parts of the plant were extracted successively with 70% v/v ethanol at 68°C in the Soxhlet apparatus. The extracts were collected in 5-L conical flasks, filtered, and the solvent was evaporated to dryness under reduced pressure in an Eyela Rotary Evaporator (Japan) at 40°C-45°C and was stored in a vacuum desiccator. The dark green liquid extract so obtained was concentrated under vacuum and the resulting dried extract was lyophilized and preserved in a refrigerator at 4°C until further use. The extract was dissolved in 1% normal saline and was used for the experimental purpose [14].
Study Experiment
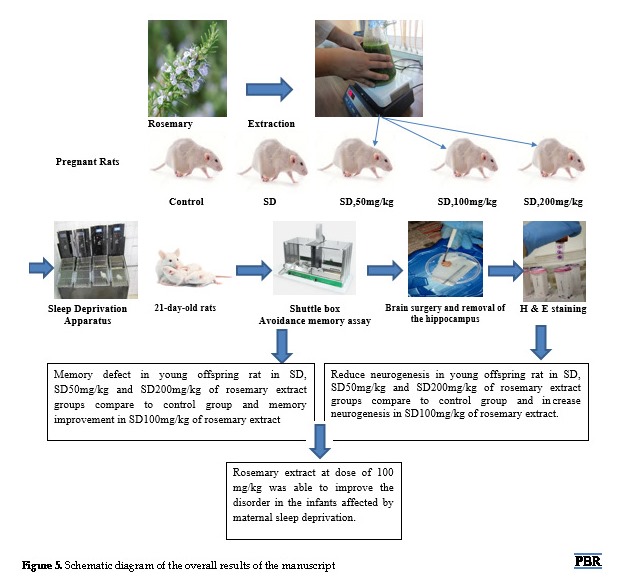
After confirmation of pregnancy based on vaginal plug formation, the rats were divided into 5 study groups of 4 weighing 170-220 g. The first group (control) consisted of pregnant rats without treatment. The second group included pregnant rats, which underwent Sleep Deprivation (SD) for 72 hours (24 hours on the 7th day, 24 hours on the 14th day, and 24 hours on the 21st day of pregnancy (SD group). The third, fourth, and fifth groups were pregnant rats that were deprived of sleep on the 7th, 14th, and 21st days of pregnancy but received rosemary extract at doses of 50 mg/kg, 100 mg/kg, and 200 mg/kg per day during pregnancy (SD 50 mg/kg, SD 100 mg/kg, SD 200 mg/kg).
Sleep deprivation apparatus
The sleep deprivation device was a machine with a calibrated tank measuring 120×30×50 cm divided into four parts of 30×30×50 cm. The device has two circular plates with a diameter of 15 cm and an edge of 3 mm. These plates are driven by a 1-W DC motor on a Plexiglas sheet at a speed of 1.5 cm/s. They move upward and downward in opposite directions and collide at the surface of the water. In this process, the animals have to turn around in the upright platform to prevent getting wet or drowning and since the cycle is repeated in less than 1 minute, the rats need to stay awake for 24 to 72 hours.
Behavioral procedure
Training: Shuttle box device
The shuttle box is used to compute an avoidance memory and includes two white and black boxes and a guillotine door between the two sections. A metal grid is embedded in the bottom of the black box that attaches to the electrical generator next to the device. Rats received 5 footshocks (1.5 mA, 50 Hz frequency, and 5 s long) at 30-s intervals and were removed from the apparatus 1 min after the last footshock [15].
Histological studies
For this purpose, all 21-day-old rats, in the control, SD, SD 50 mg/kg, SD 100 mg/kg, and SD 200 mg/kg were sacrificed by decapitation and their brains were removed using aseptic techniques. The hippocampus was quickly dissected out and placed in the sterile tubes. Two brain hemispheres were isolated after wiping into a fixative (formalin 10%) for storage and fixation of the brain tissue. After fixation, dehydration, clarification, paraffin penetration, blocking, cutting of block, staining with hematoxylin and eosin (H and E), and Nissl staining was performed.
Four rats in each group were selected for light microscopy studies. Under deep anesthesia, their brains were prefixed by a transracial perfusion 2500 mL Normal Saline (NS) followed by 250 mL of 4% paraformaldehyde (PFA, Sigma) in 0.1 M phosphate buffer pre-fixation. The brains were cut in the coronal plane into 3- to 5- mm sections. The fixation step was performed using 10% formalin at 4°C for 72 h. For histological studies, the brain samples were embedded in paraffin, and 5-µm coronal sections (one from every 5 sections) were prepared using a rotary microtome. For light microscopy observation, the tissue sections were stained with H & E staining according to the standard protocol [16].
For Nissle staining, the sections mounted on gelatin-coated slides were dehydrated with ascending series of ethanol, treated with xylene for 5 min, and rehydrated in descending series of ethanol and distilled water. Then, the sections were treated with a 1% Cresyl violet (Sigma) solution for 3 min followed by differentiation in acetic acid in 100% ethanol for 5 s. The sections were finally dehydrated in the ascending series of ethanol, treated with xylene and coverslipped using DPX mounting medium. Finally, the images were analyzed using a light field microscope (Sigma-Aldrich, Inc., USA) [17].
Data analysis
Statistical analyses were done by One-way ANOVA and repeated-measures t Tukey tests. Also, *P≤0.05; **P≤0.01; and ***P≤0.001 were considered significant.
Results
Behavioral tests results
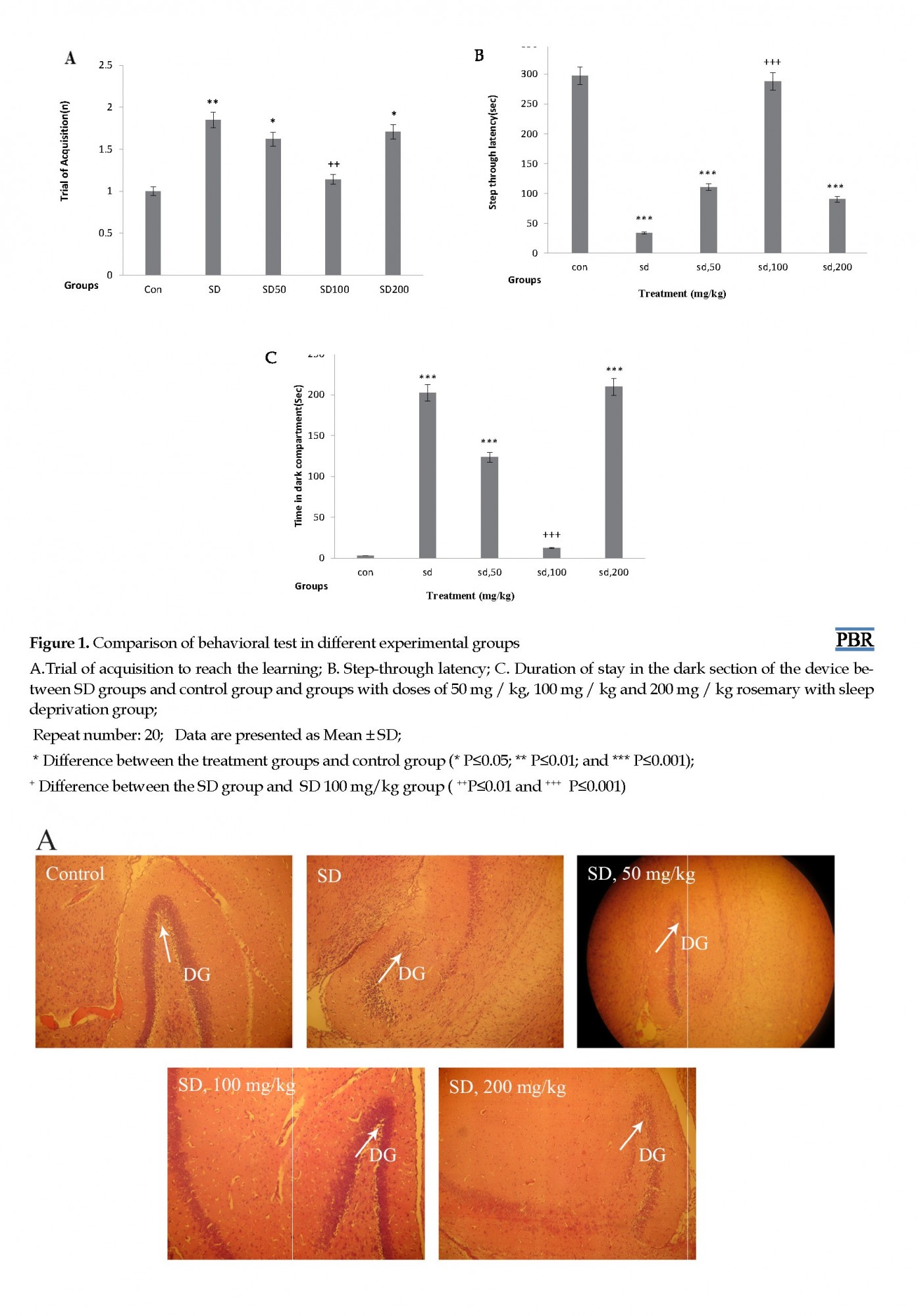
In this part, for the evaluation of learning and memory, three items of the trial of acquisition, step-through latency, and time spent in dark compartments were examined. In the number of trials to achieve learning, there was a significant difference between the SD group and the control group (P≤0.01) and also between SD 50 mg/kg and SD 200 mg/kg with the control group (P≤0.05). However, there was no significant difference between SD100 mg /kg and the control group.
To ensure that there is no sensory/motor dysfunction in animals, the initial latency was measured and compared. Comparison of groups showed that there was a significant difference in step-through latency
in the acquisition trial between SD, SD 50 mg/kg, and SD 200 mg/kg groups with the control group (P≤0.001).
The memory assessment test 24h following the training indicated that the number of acquisition trials for the animals between dark and light chambers was statistically significant among the groups. Extract groups (SD 50 mg/kg, SD 200 mg/kg) and the SD group compared to the control group spent more time in the dark area. Stop time in the device dark part of the SD and the extract groups (SD 50 mg/kg and SD 200 mg/kg) was significantly more than that of the control group (P≤0.001). While the difference between the control group and the extract group (SD 100 mg/kg) during the stay in the dark area was not significant. This means that rosemary extract at a dose of 100 mg/kg can prevent the effects of sleep deprivation and reduce the stop in the dark part of the device, but rosemary with the doses of 200 mg/kg and 50 mg/kg failed to affect maternal sleep deprivation (Figure 1).
Sleep is a passive recovery process, characterized by cessation of the sensory, motor, and mental functions connecting the central nervous system to the external environment [1]. Sleep is necessary for the proper function of memory and learning. Thus, sleep deprivation has detrimental effects on learning, brain development, and alertness [2]. Studies have demonstrated that sleep deprivation is associated not only with high financial, social, and human costs but also with cognitive and motor functions. Cognitive indices such as working memory are particularly susceptible to sleep deprivation [3].
Sleep disturbance is a common phenomenon in many neurological diseases, including Alzheimer disease, Parkinson disease, and autism [4]. Patients with dementia are known for having symptoms such as insomnia, restlessness, decreased, or prolonged sleep. These sleep disturbances lead to cognitive and functional decline accompanied by abnormal behavior and finally depression in some patients. In this regard, it is pertinent to mention that sleep behavioral disorder with rapid eye movement is one of the major sleep disorders associated with neurological abnormalities [5].
According to previous investigations, sleep improves memory by enhancing the functional integration and cell survival of the new hippocampal neurons. Regarding the learning and memory space, the relationship between sleep and neurogenesis indicates that the hippocampus is an important structure for memory formation and one of the distinct brain regions, which is an essential part of adult neurogenesis. Hippocampus contributes to memory and learning by increasing neurogenesis and neuronal production, so that learning deficits are associated with the reduction of neurogenesis. On the other hand, both neurogenesis and hippocampus memory formation are susceptible to sleep deprivation. In other words, sleep deprivation alters the electrophysiological and molecular properties of the hippocampal neurons [6, 7]. According to the findings of the researchers, maternal sleep deprivation has a major impact on the baby’s brain during pregnancy; this impact includes memory, learning, and other cognitive functions.
The researchers concluded that neurogenesis in pregnant rats increased by 65% and reached its peak on the seventh day of pregnancy before decreasing and again reached its highest level at the end of pregnancy [8]. Studies have shown that sleep deprivation is a strong oxidative stress response. Free radicals like Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are the result of several cellular signaling pathways as a defense mechanism. An excessive amount of free radicals causes oxidative stress that damages cells, tissues, and organs, resulting in several diseases, like cancer, cardiovascular diseases, Alzheimer Disease (AD), and even accelerate the aging process. Hippocampus is a part of the brain which plays an important role in cognition, mood regulation, response to stress, learning, and memory. The hippocampus is one of the most vulnerable brain regions in oxidative stress.
The maintenance of a normal redox state in the hippocampus is essential for the prevention of cognitive functions decline in aging. Antioxidants can slow down or prevent the resulting damages caused by free radicals through delaying, intercepting, and inhibiting their activities or via breaking the chain reaction of oxidation. These compounds can prevent the progression of the disease by removing free radicals. Studies have also revealed that various neurological diseases are accompanied by increased oxidative stress and free radical production, which, in turn, can result in tissue damage and cell death. Application of antioxidant compounds can also play a pivotal role in preventing antioxidant damage induced by sleep deprivation [9-11].
Rosemarinus officinalis L. an evergreen perennial aromatic shrub belongs to the family Labiatae. It is a popular household plant, commonly called rosemary, which is native to the north and south coasts of the Mediterranean Sea. Rosemary has many effective compounds with antioxidant properties. Andrade et al. studied the antioxidant and pro-oxidant properties of rosemary. Its main constituents with antioxidant properties are carnosic acid and carnosol that are responsible for 90% of the properties. Both are inhibitors of lipid peroxidation in liposomal and microsomal systems; they are good scavengers of CCl3O2 (peroxyl radicals), reduce cytochrome c, and scavenge hydroxyl radicals. Particularly, carnosic acid scavenges H2O2, but could also act as a substrate for the peroxidase system. The antioxidant properties depend on the fruiting stages.
The increase in the concentration of polyphenols, which include carnosol, rosmarinic acid, and hesperidin, during the fruiting stage, is directly related to the enhancement of the extract antioxidant property. Rosmarinic acid and hesperidin have been cited in the literature as important free radical scavengers. This plant assumes significance in traditional medicine owing to its medicinal properties and treating cognitive function deficiencies. Rosemary contains essential oils of 0.6 to 2, including 1,8-cineoles, alpha pineol, camphor, porroneol, carvacrol with phenolic detergents. Flavones and caffeic acid are derivatives of rosemarynum acid. Rosmarinic acid acts as antioxidant compounds [12].
These compounds can treat memory deficit by removing free radicals. Neuronal deletion, particularly in the cortex region, is now known to lead to cognitive impairment in acquired learning skills and memory. Different in vitro and in vivo models evaluated rosemary and its efficacy in the management of patients with Alzheimer disease. The studies have demonstrated that extracts from plants of the Lamiaceae family are active in the inhibition of Acetylcholinesterase (AChE) and β-amyloid deposits. Besides, antioxidant, cytoprotective, and anti-inflammatory activities have been observed in Lamiaceae plant extracts. There are reports that compounds like 1,8-cineole and alpha-terpineol inhibit AChE, and notably, 1,8-cineole is the most potent inhibitor [13]. The purpose of this study was to investigate the protective effect of rosemary extract against side effects of maternal sleep deprivation in neonate rats such as memory deficit. Neurotransmitter interactions may be responsible for the cognitive enhancements observed in the present study.
Material and Methods
Study animals
Adult female Wistar rats were used in this study. The animals were maintained in the laboratory of Animal Breeding Laboratory of the Faculty of Biological Sciences of Kharazmi University. The animals were housed in cages under standard environmental (light/dark cycle 12/12 hours, the temperature of 22 ± 2°C with free access to food and water). The ethical considerations regarding the amount of sleep deprivation by the box were met following the protocols and guidelines for the care and use of laboratory animals in the Laboratory of Neuroscience and Laboratory Research at the American Institute of Laboratory Animals. To prevent the aggressive severity of sleep deprivation, the necessary measures were taken, as far as possible, following international guidelines. The dose of rosemary extract was adjusted according to the WHO and Food and Drug Administration.
Ethical approval
The authors declare that the experiments done on animals were conducted following the local Ethics Committee regulations on the care and use of laboratory animals (IR.IAU.K.REC.1395.34).
Preparing rosemary hydroalcoholic extract
The whole plant of Rosmarinus officinalis was collected freshly from a cultivation farm of the north of Iran in 2016. The plant was identified and authenticated by Dr Ghahremaninejad (the senior botanist of the Department of Botany, Kharazmi University of Tehran) and a voucher specimen was deposited accordingly in the herbarium of the same department. The medicinal parts of the plant, the fresh leaves, were separated, dried in shade, pulverized by a mechanical grinder, passed through a 40-mesh sieve, and stored in an airtight container for further use.
Preparation of the plant extract
About 500 g of powdered dry parts of the plant were extracted successively with 70% v/v ethanol at 68°C in the Soxhlet apparatus. The extracts were collected in 5-L conical flasks, filtered, and the solvent was evaporated to dryness under reduced pressure in an Eyela Rotary Evaporator (Japan) at 40°C-45°C and was stored in a vacuum desiccator. The dark green liquid extract so obtained was concentrated under vacuum and the resulting dried extract was lyophilized and preserved in a refrigerator at 4°C until further use. The extract was dissolved in 1% normal saline and was used for the experimental purpose [14].
Study Experiment
After confirmation of pregnancy based on vaginal plug formation, the rats were divided into 5 study groups of 4 weighing 170-220 g. The first group (control) consisted of pregnant rats without treatment. The second group included pregnant rats, which underwent Sleep Deprivation (SD) for 72 hours (24 hours on the 7th day, 24 hours on the 14th day, and 24 hours on the 21st day of pregnancy (SD group). The third, fourth, and fifth groups were pregnant rats that were deprived of sleep on the 7th, 14th, and 21st days of pregnancy but received rosemary extract at doses of 50 mg/kg, 100 mg/kg, and 200 mg/kg per day during pregnancy (SD 50 mg/kg, SD 100 mg/kg, SD 200 mg/kg).
Sleep deprivation apparatus
The sleep deprivation device was a machine with a calibrated tank measuring 120×30×50 cm divided into four parts of 30×30×50 cm. The device has two circular plates with a diameter of 15 cm and an edge of 3 mm. These plates are driven by a 1-W DC motor on a Plexiglas sheet at a speed of 1.5 cm/s. They move upward and downward in opposite directions and collide at the surface of the water. In this process, the animals have to turn around in the upright platform to prevent getting wet or drowning and since the cycle is repeated in less than 1 minute, the rats need to stay awake for 24 to 72 hours.
Behavioral procedure
Training: Shuttle box device
The shuttle box is used to compute an avoidance memory and includes two white and black boxes and a guillotine door between the two sections. A metal grid is embedded in the bottom of the black box that attaches to the electrical generator next to the device. Rats received 5 footshocks (1.5 mA, 50 Hz frequency, and 5 s long) at 30-s intervals and were removed from the apparatus 1 min after the last footshock [15].
Histological studies
For this purpose, all 21-day-old rats, in the control, SD, SD 50 mg/kg, SD 100 mg/kg, and SD 200 mg/kg were sacrificed by decapitation and their brains were removed using aseptic techniques. The hippocampus was quickly dissected out and placed in the sterile tubes. Two brain hemispheres were isolated after wiping into a fixative (formalin 10%) for storage and fixation of the brain tissue. After fixation, dehydration, clarification, paraffin penetration, blocking, cutting of block, staining with hematoxylin and eosin (H and E), and Nissl staining was performed.
Four rats in each group were selected for light microscopy studies. Under deep anesthesia, their brains were prefixed by a transracial perfusion 2500 mL Normal Saline (NS) followed by 250 mL of 4% paraformaldehyde (PFA, Sigma) in 0.1 M phosphate buffer pre-fixation. The brains were cut in the coronal plane into 3- to 5- mm sections. The fixation step was performed using 10% formalin at 4°C for 72 h. For histological studies, the brain samples were embedded in paraffin, and 5-µm coronal sections (one from every 5 sections) were prepared using a rotary microtome. For light microscopy observation, the tissue sections were stained with H & E staining according to the standard protocol [16].
For Nissle staining, the sections mounted on gelatin-coated slides were dehydrated with ascending series of ethanol, treated with xylene for 5 min, and rehydrated in descending series of ethanol and distilled water. Then, the sections were treated with a 1% Cresyl violet (Sigma) solution for 3 min followed by differentiation in acetic acid in 100% ethanol for 5 s. The sections were finally dehydrated in the ascending series of ethanol, treated with xylene and coverslipped using DPX mounting medium. Finally, the images were analyzed using a light field microscope (Sigma-Aldrich, Inc., USA) [17].
Data analysis
Statistical analyses were done by One-way ANOVA and repeated-measures t Tukey tests. Also, *P≤0.05; **P≤0.01; and ***P≤0.001 were considered significant.
Results
Behavioral tests results
In this part, for the evaluation of learning and memory, three items of the trial of acquisition, step-through latency, and time spent in dark compartments were examined. In the number of trials to achieve learning, there was a significant difference between the SD group and the control group (P≤0.01) and also between SD 50 mg/kg and SD 200 mg/kg with the control group (P≤0.05). However, there was no significant difference between SD100 mg /kg and the control group.
To ensure that there is no sensory/motor dysfunction in animals, the initial latency was measured and compared. Comparison of groups showed that there was a significant difference in step-through latency
in the acquisition trial between SD, SD 50 mg/kg, and SD 200 mg/kg groups with the control group (P≤0.001).
The memory assessment test 24h following the training indicated that the number of acquisition trials for the animals between dark and light chambers was statistically significant among the groups. Extract groups (SD 50 mg/kg, SD 200 mg/kg) and the SD group compared to the control group spent more time in the dark area. Stop time in the device dark part of the SD and the extract groups (SD 50 mg/kg and SD 200 mg/kg) was significantly more than that of the control group (P≤0.001). While the difference between the control group and the extract group (SD 100 mg/kg) during the stay in the dark area was not significant. This means that rosemary extract at a dose of 100 mg/kg can prevent the effects of sleep deprivation and reduce the stop in the dark part of the device, but rosemary with the doses of 200 mg/kg and 50 mg/kg failed to affect maternal sleep deprivation (Figure 1).
Histopathological study of the hippocampus
Effect of sleep deprivation on neuronal morphology and density as depicted by histopathological sections of rat brains
Histopathological evaluation of hippocampal regions of the rat brain was conducted under light microscopy. Figure 2 displays the representative images of brain sections from different groups of animals. Brains of the control and the SD-treated group with rosemary (100 mg/kg) showed comparatively undamaged and optimum sized neuronal cells as compared with the sleep-deprived groups (SD and SD treated with rosemary extract dose of 50, or 200 mg/kg). However, the disorganization of various cell layers increased the level of cellular spongiosis, and the density of cells observed specifically in the Dentate Gyrus (DG) of the hippocampus and the brains of sleep-deprived animals.
Histopathological examination of the hippocampus indicated that in the control group all cells of the hippocampus were intact, their nuclei were completely distinct and darker than the cytoplasm and the DG region contained many cells. While in the SD group, the number of DG cells decreased significantly, and the shape of the cells was domed or triangular with a non-explicit core in the control group (Figure 2).
Effect of SD on neurons number in histopathological sections of rat brains
Histopathological assessment of rat hippocampal regions was conducted after Nissl staining under light microscopy. Representative images of brain sections from experimental groups are exhibited in Figure 3. It is shown that the larger shape of the DG region in the SD group and SD plus rosemary at doses of 50 and 200 mg/kg was changed from a rounded state with a distinct nucleus to a triangular state with an indistinct nucleus. An increase in the number of microglial cells and a lower number of neurons were observed in the SD group and SD treated with 50 and 200 mg/kg rosemary extract. It is also observed that most cells in the DG region in the rosemary group (at a dose of 100 mg/kg) have a clear corpuscular appearance and the ratio of pyramidal cells to neurons has decreased. This attenuation is not significantly different from that of the control group (400x magnification).
Effect of SD on neurons number in histopathological sections of rat brains
Histopathological assessment of rat hippocampal regions was conducted after Nissl staining under light microscopy. Representative images of brain sections from experimental groups are exhibited in Figure 3. It is shown that the larger shape of the DG region in the SD group and SD plus rosemary at doses of 50 and 200 mg/kg was changed from a rounded state with a distinct nucleus to a triangular state with an indistinct nucleus. An increase in the number of microglial cells and a lower number of neurons were observed in the SD group and SD treated with 50 and 200 mg/kg rosemary extract. It is also observed that most cells in the DG region in the rosemary group (at a dose of 100 mg/kg) have a clear corpuscular appearance and the ratio of pyramidal cells to neurons has decreased. This attenuation is not significantly different from that of the control group (400x magnification).
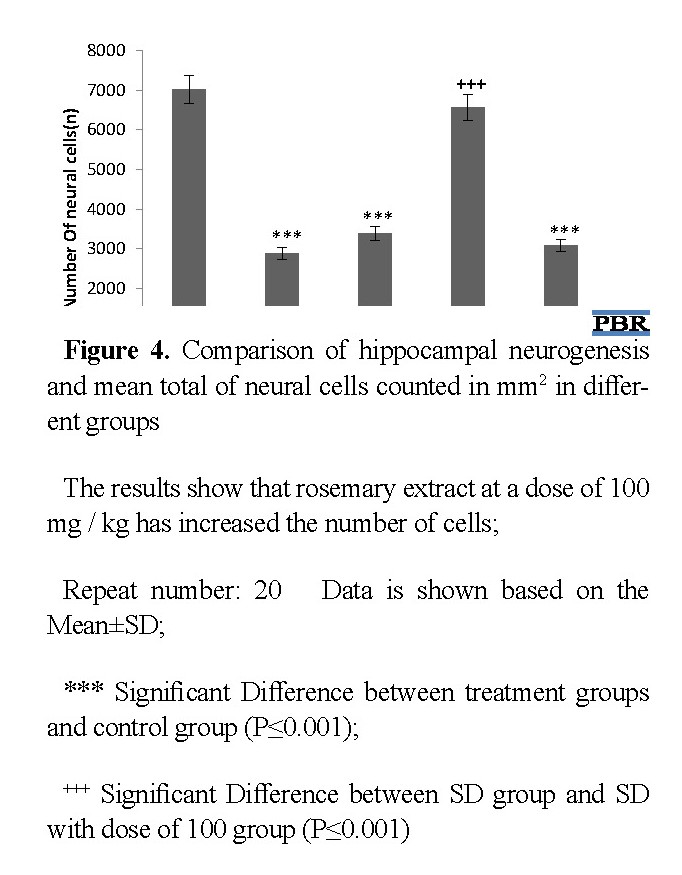
Total number of cells
After histological examination, the number of cells in the groups included control, SD, and SD groups with 50 mg/kg, 100 mg/kg, and 200 mg/kg rosemary extract were calculated (Figure 4). The average number of cells in the control, SD, and SD plus rosemary extract (50,100, and 200 mg/kg) groups were 7021, 2880, 3380, 6557, and 3090, respectively.
After histological examination, the number of cells in the groups included control, SD, and SD groups with 50 mg/kg, 100 mg/kg, and 200 mg/kg rosemary extract were calculated (Figure 4). The average number of cells in the control, SD, and SD plus rosemary extract (50,100, and 200 mg/kg) groups were 7021, 2880, 3380, 6557, and 3090, respectively.
Comparison of the cell numbers showed a significant difference between SD, SD 50 mg/kg, and SD 200 mg/kg with the control group (P≤0.001), which means that rosemary extract at 50 mg/kg and 200 mg kg could not stand the effect of sleep deprivation and did not improve neurogenesis in the hippocampus of newborns. However, there was no significant difference between the number of cells in the control group and the SD 100 mg/kg, which means that the rosemary extract at a dose of 100 mg/kg had a corrective effect on the sleep deprivation via increment of neurogenesis. On the other hand, there was no significant difference in the number of cells between the SD plus rosemary extract 50 mg /kg and 200 mg/kg groups with sleep deprivation group. However, there was a significant difference in the number of cells in the 100 mg/kg extract group compared with the SD group (P ≤0.001) (Figure 5).
Discussion
The present study showed that maternal sleep deprivation in the rats results in impaired neonatal avoidance memory consolidation and deficit in learning. In recent years, several research studies were conducted on the relationship between maternal sleep deprivation during pregnancy and increased short- and long-term childhood disorders. According to a study, maternal sleep deprivation could result in several harmful outcomes in the offspring and would damage the mother-child relationship. Sleep deprivation during pregnancy is also a serious public health problem that affects both mothers and infants [18]. Durmer et al. and Zhao et al. reported that maternal sleep deprivation would affect children’s 3D learning and memory through changes in the pre-adolescent brain [19, 20]. In 2017, Aswathy et al. proved that sleep deprivation in the last trimester of pregnancy causes symptoms similar to depression in infants. The brain develops rapidly during this period and continues after birth [5].
One is the probability of a relationship between sleep and neurogenesis of learning and memory space, which has been confirmed by various studies. The hippocampus is an important structure for the formation of memory and one of the distinct brain regions that constitute an essential part of adult neurogenesis. Neonatal brain tissue examination and observation of the brain tissue (especially the hippocampus and cell count of this area) under the optical microscope showed a lower number of cells in the hippocampus in the neonatal group affected by maternal sleep deprivation compared with the control group. Therefore, the volume of the hippocampus in the maternal newborns group is reduced by sleep deprivation compared with the control infants, which is visible in tissue samples. Maternal sleep deprivation, besides reduction in the production of neuronal cells, destroys functional and cognitive processes. Studies have shown that sleep deprivation impairs cognitive functions such as memory, learning, and so on [4].
In the present study, hippocampus analysis under a microscope showed that in the group of maternal neonates, the number of microglia cells or pyramidal cells is greater than the neurons or rounded cells in the dentate gyrus and CA1 region. However, in the control group, all cells in the DG and CA1 are rounded cells with distinct nuclei similar to the neurons. Thus, neurogenesis in the hippocampus of maternal sleep-deprived infants decreases compared with the control group infants with an increasing number of glial cells.
The effect of rosemary extract can be created using several useful compounds such as sugar and antioxidants. In adults, the production of new neurons in the hippocampus takes place in the Subgranular Zone (SGZ) of the dentate gyrus. Neural progenitor or stem cells go through processes of proliferation, differentiation, migration, and maturation. This process is exquisitely sensitive to oxidative stress, and perturbation in the redox balance in the neurogenic microenvironment can lead to reduced neurogenesis. However, even low doses of irradiation can lead to persistent elevation of oxidative stress and sustained suppression of hippocampal neurogenesis. The results of this study suggest that, regardless of the subcellular location, SOD deficiency leads to a significant reduction in the production of new neurons in the SGZ of the hippocampal dentate gyrus. In exchange, the generation of new glial cells significantly increases.
Several studies have reported that maternal sleep deprivation during pregnancy increases the number of microglia or pyramidal cells in the neonate’s brain and reduces the number of neurons, in other words, neurogenesis. Zhao et al. reported that sleep deprivation during pregnancy would impair the development of the brain and memory function by affecting neurogenesis in the hippocampus and glial cell growth in neonates. Also, these researchers demonstrated that offspring affected by maternal sleep deprivation showed a severe attenuation in hippocampal neurogenesis and an increase in the number of microglia. Three-dimensional spatial learning and memory are also affected in these infants [20]. Arathi et al. demonstrated that the activity of microglia and their number in the brains of infants affected by maternal sleep deprivation had increased in comparison to normal infants whose mothers were not deprived of sleep [4]. Heinwood et al. elucidated that sleep deprivation and stress induced by it in pregnant mice can impair neonatal brain development [21]. Wadhwa et al. argued that hippocampal neurogenesis would decline remarkably in neonatal rats affected by maternal sleep deprivation [22].
This study confirmed the beneficial effects of 100 mg/kg rosemary hydroalcoholic extract on learning and memory using a passive avoidance test to obtain new information, preserve and recall information stored in 21-day-old infants affected by maternal sleep deprivation. Significant differences were observed in the experimental groups treated with different doses of rosemary compared with the control group. In 2008, Kumar et al. as well as Kwon showed that rosmarinic acid suppresses oxidative stress, induces neuroprotective effect, and improves the deleterious effects of sleep deprivation [23, 24].
Similar to the present investigation, Zanella et al. study (2012) reported that hydroalcoholic rosemary extract in a dose-dependent manner improves learning and short-term memory. They found that treatment with 150 and 300 mg/kg of rosemary extract (hydroalcoholic) would improve new social short-term memory. Also, they suggested that treatment with 150 mg/kg of rosemary extract improves long-term memory when prescribed in the consolidation phase of learning [25]. Studies have shown that rosemary extract has antioxidant properties and carnosol, carnosic acid, and rosmarinic acid are known as antioxidant compounds [26, 27].
Several studies indicated that sleep deprivation induces oxidative stress in neurons. Melatonin and its metabolites are potential digestive radicals that play an important role in aging and nervous system degradation by acting as a neurotransmitter and antioxidant. Previous reports have indicated that treatment with antioxidant compounds such as melatonin receptor agonist has a sleep-promoting effect and are therefore useful in preventing damages of sleep deprivation. A reduction in the production and secretion of melatonin leads to systemic changes in metabolic processes, such as energy metabolism, especially in the brain. Sleep deprivation is one of the damages associated with changes in the secretion of melatonin. Melatonin copes with the involvement of a GABAergic system against oxidative stress caused by sleep deprivation and plays an important role in reducing the pathological effects [2].
The antioxidant substances accumulated in the neural tissue are effective factors in preventing and treating disorders caused by oxidative damage. Rosemary extract can also have a positive effect on memory. In this study, the beneficial effect of rosemary hydroalcoholic extract on learning and memory in terms of obtaining new information, maintaining, and remembering information stored in newborn infants under the influence of sleep deprivation at 21 days was confirmed by passive avoidance test and significant differences were observed in the experimental groups treated with different doses of rosemary compared with the control group. More specifically, passive avoidance learning and memory indicators increased in the extract group (100 mg/kg). Although treatment in the period of pregnancy with different doses of rosemary extract showed a reduction of neuron density in the rosemary extract groups (50 mg/kg and 200 mg/kg) compared with the control group.
The research reveals clear morphological evidence of
dose-dependent (250 mg and 500 mg) antifertility potential of rosemary in male albino rats [28]. Therefore, using rosemary extract in high doses may also have destructive effects on the brain.
Brain is especially sensitive to oxidative stress due to its high content of readily oxidizable fatty acids, high consumption of oxygen, and low levels of antioxidants. The increased ROS and also lowered levels of antioxidants are associated with the pathogenesis of neurodegenerative diseases such as depression and cognitive impairment. However, the enhancement of the antioxidant system may be effective to combat excessive ROS production. Evidence suggests that several natural antioxidants may be effective against stress-induced mental health complications [29].
This study aimed to investigate the effect of hydroalcoholic rosemary extract on learning and memory in the offspring of sleep-deprived rats using a passive avoidance memory test. In summary, the biochemical and histopathological data indicated that rosemary extract at a dose of 100 mg/kg can prevent the deleterious effects of maternal sleep deprivation on infants’ brains, including impaired learning and avoidant memory in rats. Rosemary is one of the most effective compounds with antioxidant properties that digest the active species of proxy nitrite nitrogen and is a free radical digester. Therefore, based on the findings of this study, rosemary and its antioxidant property can be used to reduce the complications of brain injuries.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Islamic Azad University of Karaj, (Ethical Code: IR.IAU.K.REC.1395.34). All Ethical Considerations were observed throughout the research.
Funding
The project was carried out in the Sleep and Cognition Laboratory under the supervision of the Faculty of Biological Sciences of Kharazmi University of Tehran.
Authors' contributions
All authors contributed to the studies performed and in the preparation of the manuscript.
Conflict of interest
Authors declare no conflict of interest in this study
References
Meerloa P, Mistlbergerb RE, Jacobs BL, Heller HC, McGintye D. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev. 2009; 13(3):187-94. [DOI:10.1016/j.smrv.2008.07.004] [PMID] [PMCID]
Lennington JB, Yang Z, Conover JC. Neural stem cells and the regulation of adult neurogenesis. Reprod Biol Endocrinol. 2003; 1:99. [DOI:10.1186/1477-7827-1-99] [PMID] [PMCID]
Pires GN, Andersen ML, Giovenardi M, Tufik S. Sleep impairment during pregnancy: Possible implications on mother-infant relationship. Med Hypotheses. 2010; 75(6):578-82. [DOI:10.1016/j.mehy.2010.07.036] [PMID]
Radhakrishnan A, Aswathy BS, Kumar VM, Gulia KK. Sleep deprivation during late pregnancy produces hyperactivity and increased risk-taking behavior in offspring. Brain Res. 2015; 1596:88-98. [DOI:10.1016/j.brainres.2014.11.021] [PMID]
Aswathy BS, Velayudhan MK, Kamalesh KG. The effects of rapid eye movement sleep deprivation during late pregnancy on newborns sleep. J Sleep Res. 2018: 27(2):197-205. [PMID]
Baddeley A. Working memory: Theories, models, and controversies. Annu Rev Psychol 2012; 63:1-29. [DOI:10.1146/annurev-psych-120710-100422] [PMID]
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013; 153(6):1219-27. [DOI:10.1016/j.cell.2013.05.002] [PMID] [PMCID]
Peng Y, Wang W, Tan T, He W, Dong Z, Wang YT, et al. Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Mol Brain. 2016; 9:17. [DOI:10.1186/s13041-016-0197-3] [PMID] [PMCID]
Azimzadeh K, Jafarpour H, Adldoost S. Sertraline alters level of adenosine deaminase activity, oxidative stress markers and cardiac biomarkers (homocysteine cardiac troponin I) in rats. Pharm Biomed Res. 2017; 3(3):17-22. [DOI:10.29252/pbr.3.3.17]
Heidari Z, Mohammadi Sh, Yousefi Taba M. Protective effect of curcumin on the density of hippo-campal dark neurons in mice model of aging induced by d-galactose: A histopathological study. Pharm Biomed Res. 2019; 5(4):63-8. [DOI:10.18502/pbr.v5i4.2398]
Linseman DA. Targeting oxidative stress for neuro protection. Antioxid Redox Signal. 2009; 11(3):421-4. [DOI:10.1089/ars.2008.2236] [PMID]
Andrade JM, Faustino C, Garcia C, Ladeiras D, Reis CP, Rijo P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci OA. 2018; 4(4):FSO283. [DOI:10.4155/fsoa-2017-0124] [PMID] [PMCID]
Ozarowski M, Mikolajczak PL, Bobklewlcz-Kozlowska T, Kujawski R, Mrozikiewicz PM. Neuroactive compounds from medicinal plants of the Lamiaceae family showing potentially beneficial activity in treatment of Alzheimer’s disease. Herba Pol. 2009; 55(4):148-63. https://www.cabdirect.org/cabdirect/abstract/20113007909
Cattaneo L, Cicconi R, Mignogna G, Giorgi A, Mattei M, Graziani G, et al. Anti-proliferative effect of Rosmarinus officinalis L. extract on human melanoma A375 cells. Plos One. 2015; 10(7):e0132439. [DOI:10.1371/journal.pone.0132439] [PMID] [PMCID]
Javad-Moosavi BBZ, Vaezi GH, Nasehi M, Haeri-Rouhani SA, Zarrindast MR. Critical role of CA1 muscarinic receptors on memory acquisition deficit induced by total (TSD) and REM sleep deprivation (RSD). Prog Neuro-Psychopharmacol Biol Psychiatry. 2017; 79(Pt B):128-35. [DOI:10.1016/j.pnpbp.2017.05.024] [PMID]
Miller DJ, Balaram O, Young NA, Kaas JH. Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat. 2014; 8:1-11. [DOI:10.3389/fnana.2014.00036]
Kadar A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissle staining. J Neurosci Methods. 2009; 184(1):115-8. [DOI:10.1016/j.jneumeth.2009.07.010] [PMID] [PMCID]
Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Med Rev. 2010; 14(2):107-14. [DOI:10.1016/j.smrv.2009.05.001] [PMID] [PMCID]
Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005; 25(1):117-29. [DOI:10.1055/s-2005-867080] [PMID]
Zhao Q, Xie X, Fan Y, Zhang J, Jiang W, Wu X, Yan S, et al. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation induced cognitive impairment. Sci Rep. 2015; 5:9513. [DOI:10.1038/srep09513] [PMID] [PMCID]
Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012; 22(6):1442-54. [DOI:10.1093/cercor/bhr229] [PMID]
Wadhwa M, Prabhakar A, Ray K, Roy K, Jha PK, Kishore K, et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017; 4(1):222. [DOI:10.1186/s12974-017-0998-z] [PMID] [PMCID]
Kumar A, Singh A. Possible nitric oxide modulation in protective effect of (Curcuma longa, Zingiberaceae) against sleep deprivation-induced behavioral alterations and oxidative damage in mice. Phytomedicine. 2008; 15(8):577-86. [DOI:10.1016/j.phymed.2008.02.003] [PMID]
Kwon KJ, Lee EJ, Kim MK, Jeon SJ, Choi YY, Shin CY, et al. The potential role of melatonin on sleep deprivation induced cognitive impairments: Implication of FMRP on cognitive function. Neuroscience, 2015; 301:403-14. [DOI:10.1016/j.neuroscience.2015.05.079] [PMID]
Zanella CA, Treichel H, Luiscan R, Roman S. The effect of acute administration of the hydro alcolcohlic extract of rosemary (Rosmarinus Officinolis L. Lamiaceae) in animal models of memory. Braz J Pharm Sci. 2012; 48(3):389-97. [DOI:10.1590/S1984-82502012000300005]
Seham MA, Mona A, Amira AB. Study on the effect of Rosemary extract on some neurotransmitters and their related lons in different brain areas of adult male albino rat. J Applied Sci Res. 2010; 1400-23. https://www.cabdirect.org/cabdirect/abstract/20103325218
Tamadoni M, Haji Ghasem Kashani M, Ghorbanian MT, Abrari K, Arashpour R. [Neuroprotective effects of carnosic acid on the hippocampus of 6- hydroxydopamine injured rats (Persian)]. Koomesh. 2014; 15(2):232-41. http://koomeshjournal.semums.ac.ir/article-1-1922-en.html
Salah El-Din RA, El-Shahat AER, Elmansy R. An electron microscopic study of the antifertility potential of Rosemary (Rosmarinus Officinalis L.) in male albino rats. Int J Morphol. 2012; 30(2):666-72. [DOI:10.4067/S0717-95022012000200051]
Heidari-Vala H, Ebrahimi Hariry R, Sadeghi MR, Akhondi MM, Ghaffari Novin M, Heidari M. Evaluation of an aqueous-ethanolic extract from Rosmarinus officinalis (Rosemary) for its activity on the hormonal and cellular function of testes in adult male rat. Iran J Pharm Res. 2013; 12(2):445-51. [PMID] [PMCID]
The present study showed that maternal sleep deprivation in the rats results in impaired neonatal avoidance memory consolidation and deficit in learning. In recent years, several research studies were conducted on the relationship between maternal sleep deprivation during pregnancy and increased short- and long-term childhood disorders. According to a study, maternal sleep deprivation could result in several harmful outcomes in the offspring and would damage the mother-child relationship. Sleep deprivation during pregnancy is also a serious public health problem that affects both mothers and infants [18]. Durmer et al. and Zhao et al. reported that maternal sleep deprivation would affect children’s 3D learning and memory through changes in the pre-adolescent brain [19, 20]. In 2017, Aswathy et al. proved that sleep deprivation in the last trimester of pregnancy causes symptoms similar to depression in infants. The brain develops rapidly during this period and continues after birth [5].
One is the probability of a relationship between sleep and neurogenesis of learning and memory space, which has been confirmed by various studies. The hippocampus is an important structure for the formation of memory and one of the distinct brain regions that constitute an essential part of adult neurogenesis. Neonatal brain tissue examination and observation of the brain tissue (especially the hippocampus and cell count of this area) under the optical microscope showed a lower number of cells in the hippocampus in the neonatal group affected by maternal sleep deprivation compared with the control group. Therefore, the volume of the hippocampus in the maternal newborns group is reduced by sleep deprivation compared with the control infants, which is visible in tissue samples. Maternal sleep deprivation, besides reduction in the production of neuronal cells, destroys functional and cognitive processes. Studies have shown that sleep deprivation impairs cognitive functions such as memory, learning, and so on [4].
In the present study, hippocampus analysis under a microscope showed that in the group of maternal neonates, the number of microglia cells or pyramidal cells is greater than the neurons or rounded cells in the dentate gyrus and CA1 region. However, in the control group, all cells in the DG and CA1 are rounded cells with distinct nuclei similar to the neurons. Thus, neurogenesis in the hippocampus of maternal sleep-deprived infants decreases compared with the control group infants with an increasing number of glial cells.
The effect of rosemary extract can be created using several useful compounds such as sugar and antioxidants. In adults, the production of new neurons in the hippocampus takes place in the Subgranular Zone (SGZ) of the dentate gyrus. Neural progenitor or stem cells go through processes of proliferation, differentiation, migration, and maturation. This process is exquisitely sensitive to oxidative stress, and perturbation in the redox balance in the neurogenic microenvironment can lead to reduced neurogenesis. However, even low doses of irradiation can lead to persistent elevation of oxidative stress and sustained suppression of hippocampal neurogenesis. The results of this study suggest that, regardless of the subcellular location, SOD deficiency leads to a significant reduction in the production of new neurons in the SGZ of the hippocampal dentate gyrus. In exchange, the generation of new glial cells significantly increases.
Several studies have reported that maternal sleep deprivation during pregnancy increases the number of microglia or pyramidal cells in the neonate’s brain and reduces the number of neurons, in other words, neurogenesis. Zhao et al. reported that sleep deprivation during pregnancy would impair the development of the brain and memory function by affecting neurogenesis in the hippocampus and glial cell growth in neonates. Also, these researchers demonstrated that offspring affected by maternal sleep deprivation showed a severe attenuation in hippocampal neurogenesis and an increase in the number of microglia. Three-dimensional spatial learning and memory are also affected in these infants [20]. Arathi et al. demonstrated that the activity of microglia and their number in the brains of infants affected by maternal sleep deprivation had increased in comparison to normal infants whose mothers were not deprived of sleep [4]. Heinwood et al. elucidated that sleep deprivation and stress induced by it in pregnant mice can impair neonatal brain development [21]. Wadhwa et al. argued that hippocampal neurogenesis would decline remarkably in neonatal rats affected by maternal sleep deprivation [22].
This study confirmed the beneficial effects of 100 mg/kg rosemary hydroalcoholic extract on learning and memory using a passive avoidance test to obtain new information, preserve and recall information stored in 21-day-old infants affected by maternal sleep deprivation. Significant differences were observed in the experimental groups treated with different doses of rosemary compared with the control group. In 2008, Kumar et al. as well as Kwon showed that rosmarinic acid suppresses oxidative stress, induces neuroprotective effect, and improves the deleterious effects of sleep deprivation [23, 24].
Similar to the present investigation, Zanella et al. study (2012) reported that hydroalcoholic rosemary extract in a dose-dependent manner improves learning and short-term memory. They found that treatment with 150 and 300 mg/kg of rosemary extract (hydroalcoholic) would improve new social short-term memory. Also, they suggested that treatment with 150 mg/kg of rosemary extract improves long-term memory when prescribed in the consolidation phase of learning [25]. Studies have shown that rosemary extract has antioxidant properties and carnosol, carnosic acid, and rosmarinic acid are known as antioxidant compounds [26, 27].
Several studies indicated that sleep deprivation induces oxidative stress in neurons. Melatonin and its metabolites are potential digestive radicals that play an important role in aging and nervous system degradation by acting as a neurotransmitter and antioxidant. Previous reports have indicated that treatment with antioxidant compounds such as melatonin receptor agonist has a sleep-promoting effect and are therefore useful in preventing damages of sleep deprivation. A reduction in the production and secretion of melatonin leads to systemic changes in metabolic processes, such as energy metabolism, especially in the brain. Sleep deprivation is one of the damages associated with changes in the secretion of melatonin. Melatonin copes with the involvement of a GABAergic system against oxidative stress caused by sleep deprivation and plays an important role in reducing the pathological effects [2].
The antioxidant substances accumulated in the neural tissue are effective factors in preventing and treating disorders caused by oxidative damage. Rosemary extract can also have a positive effect on memory. In this study, the beneficial effect of rosemary hydroalcoholic extract on learning and memory in terms of obtaining new information, maintaining, and remembering information stored in newborn infants under the influence of sleep deprivation at 21 days was confirmed by passive avoidance test and significant differences were observed in the experimental groups treated with different doses of rosemary compared with the control group. More specifically, passive avoidance learning and memory indicators increased in the extract group (100 mg/kg). Although treatment in the period of pregnancy with different doses of rosemary extract showed a reduction of neuron density in the rosemary extract groups (50 mg/kg and 200 mg/kg) compared with the control group.
The research reveals clear morphological evidence of
dose-dependent (250 mg and 500 mg) antifertility potential of rosemary in male albino rats [28]. Therefore, using rosemary extract in high doses may also have destructive effects on the brain.
Brain is especially sensitive to oxidative stress due to its high content of readily oxidizable fatty acids, high consumption of oxygen, and low levels of antioxidants. The increased ROS and also lowered levels of antioxidants are associated with the pathogenesis of neurodegenerative diseases such as depression and cognitive impairment. However, the enhancement of the antioxidant system may be effective to combat excessive ROS production. Evidence suggests that several natural antioxidants may be effective against stress-induced mental health complications [29].
This study aimed to investigate the effect of hydroalcoholic rosemary extract on learning and memory in the offspring of sleep-deprived rats using a passive avoidance memory test. In summary, the biochemical and histopathological data indicated that rosemary extract at a dose of 100 mg/kg can prevent the deleterious effects of maternal sleep deprivation on infants’ brains, including impaired learning and avoidant memory in rats. Rosemary is one of the most effective compounds with antioxidant properties that digest the active species of proxy nitrite nitrogen and is a free radical digester. Therefore, based on the findings of this study, rosemary and its antioxidant property can be used to reduce the complications of brain injuries.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by Islamic Azad University of Karaj, (Ethical Code: IR.IAU.K.REC.1395.34). All Ethical Considerations were observed throughout the research.
Funding
The project was carried out in the Sleep and Cognition Laboratory under the supervision of the Faculty of Biological Sciences of Kharazmi University of Tehran.
Authors' contributions
All authors contributed to the studies performed and in the preparation of the manuscript.
Conflict of interest
Authors declare no conflict of interest in this study
References
Meerloa P, Mistlbergerb RE, Jacobs BL, Heller HC, McGintye D. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev. 2009; 13(3):187-94. [DOI:10.1016/j.smrv.2008.07.004] [PMID] [PMCID]
Lennington JB, Yang Z, Conover JC. Neural stem cells and the regulation of adult neurogenesis. Reprod Biol Endocrinol. 2003; 1:99. [DOI:10.1186/1477-7827-1-99] [PMID] [PMCID]
Pires GN, Andersen ML, Giovenardi M, Tufik S. Sleep impairment during pregnancy: Possible implications on mother-infant relationship. Med Hypotheses. 2010; 75(6):578-82. [DOI:10.1016/j.mehy.2010.07.036] [PMID]
Radhakrishnan A, Aswathy BS, Kumar VM, Gulia KK. Sleep deprivation during late pregnancy produces hyperactivity and increased risk-taking behavior in offspring. Brain Res. 2015; 1596:88-98. [DOI:10.1016/j.brainres.2014.11.021] [PMID]
Aswathy BS, Velayudhan MK, Kamalesh KG. The effects of rapid eye movement sleep deprivation during late pregnancy on newborns sleep. J Sleep Res. 2018: 27(2):197-205. [PMID]
Baddeley A. Working memory: Theories, models, and controversies. Annu Rev Psychol 2012; 63:1-29. [DOI:10.1146/annurev-psych-120710-100422] [PMID]
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013; 153(6):1219-27. [DOI:10.1016/j.cell.2013.05.002] [PMID] [PMCID]
Peng Y, Wang W, Tan T, He W, Dong Z, Wang YT, et al. Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Mol Brain. 2016; 9:17. [DOI:10.1186/s13041-016-0197-3] [PMID] [PMCID]
Azimzadeh K, Jafarpour H, Adldoost S. Sertraline alters level of adenosine deaminase activity, oxidative stress markers and cardiac biomarkers (homocysteine cardiac troponin I) in rats. Pharm Biomed Res. 2017; 3(3):17-22. [DOI:10.29252/pbr.3.3.17]
Heidari Z, Mohammadi Sh, Yousefi Taba M. Protective effect of curcumin on the density of hippo-campal dark neurons in mice model of aging induced by d-galactose: A histopathological study. Pharm Biomed Res. 2019; 5(4):63-8. [DOI:10.18502/pbr.v5i4.2398]
Linseman DA. Targeting oxidative stress for neuro protection. Antioxid Redox Signal. 2009; 11(3):421-4. [DOI:10.1089/ars.2008.2236] [PMID]
Andrade JM, Faustino C, Garcia C, Ladeiras D, Reis CP, Rijo P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci OA. 2018; 4(4):FSO283. [DOI:10.4155/fsoa-2017-0124] [PMID] [PMCID]
Ozarowski M, Mikolajczak PL, Bobklewlcz-Kozlowska T, Kujawski R, Mrozikiewicz PM. Neuroactive compounds from medicinal plants of the Lamiaceae family showing potentially beneficial activity in treatment of Alzheimer’s disease. Herba Pol. 2009; 55(4):148-63. https://www.cabdirect.org/cabdirect/abstract/20113007909
Cattaneo L, Cicconi R, Mignogna G, Giorgi A, Mattei M, Graziani G, et al. Anti-proliferative effect of Rosmarinus officinalis L. extract on human melanoma A375 cells. Plos One. 2015; 10(7):e0132439. [DOI:10.1371/journal.pone.0132439] [PMID] [PMCID]
Javad-Moosavi BBZ, Vaezi GH, Nasehi M, Haeri-Rouhani SA, Zarrindast MR. Critical role of CA1 muscarinic receptors on memory acquisition deficit induced by total (TSD) and REM sleep deprivation (RSD). Prog Neuro-Psychopharmacol Biol Psychiatry. 2017; 79(Pt B):128-35. [DOI:10.1016/j.pnpbp.2017.05.024] [PMID]
Miller DJ, Balaram O, Young NA, Kaas JH. Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat. 2014; 8:1-11. [DOI:10.3389/fnana.2014.00036]
Kadar A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissle staining. J Neurosci Methods. 2009; 184(1):115-8. [DOI:10.1016/j.jneumeth.2009.07.010] [PMID] [PMCID]
Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: Is there a relationship? Sleep Med Rev. 2010; 14(2):107-14. [DOI:10.1016/j.smrv.2009.05.001] [PMID] [PMCID]
Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005; 25(1):117-29. [DOI:10.1055/s-2005-867080] [PMID]
Zhao Q, Xie X, Fan Y, Zhang J, Jiang W, Wu X, Yan S, et al. Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation induced cognitive impairment. Sci Rep. 2015; 5:9513. [DOI:10.1038/srep09513] [PMID] [PMCID]
Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012; 22(6):1442-54. [DOI:10.1093/cercor/bhr229] [PMID]
Wadhwa M, Prabhakar A, Ray K, Roy K, Jha PK, Kishore K, et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017; 4(1):222. [DOI:10.1186/s12974-017-0998-z] [PMID] [PMCID]
Kumar A, Singh A. Possible nitric oxide modulation in protective effect of (Curcuma longa, Zingiberaceae) against sleep deprivation-induced behavioral alterations and oxidative damage in mice. Phytomedicine. 2008; 15(8):577-86. [DOI:10.1016/j.phymed.2008.02.003] [PMID]
Kwon KJ, Lee EJ, Kim MK, Jeon SJ, Choi YY, Shin CY, et al. The potential role of melatonin on sleep deprivation induced cognitive impairments: Implication of FMRP on cognitive function. Neuroscience, 2015; 301:403-14. [DOI:10.1016/j.neuroscience.2015.05.079] [PMID]
Zanella CA, Treichel H, Luiscan R, Roman S. The effect of acute administration of the hydro alcolcohlic extract of rosemary (Rosmarinus Officinolis L. Lamiaceae) in animal models of memory. Braz J Pharm Sci. 2012; 48(3):389-97. [DOI:10.1590/S1984-82502012000300005]
Seham MA, Mona A, Amira AB. Study on the effect of Rosemary extract on some neurotransmitters and their related lons in different brain areas of adult male albino rat. J Applied Sci Res. 2010; 1400-23. https://www.cabdirect.org/cabdirect/abstract/20103325218
Tamadoni M, Haji Ghasem Kashani M, Ghorbanian MT, Abrari K, Arashpour R. [Neuroprotective effects of carnosic acid on the hippocampus of 6- hydroxydopamine injured rats (Persian)]. Koomesh. 2014; 15(2):232-41. http://koomeshjournal.semums.ac.ir/article-1-1922-en.html
Salah El-Din RA, El-Shahat AER, Elmansy R. An electron microscopic study of the antifertility potential of Rosemary (Rosmarinus Officinalis L.) in male albino rats. Int J Morphol. 2012; 30(2):666-72. [DOI:10.4067/S0717-95022012000200051]
Heidari-Vala H, Ebrahimi Hariry R, Sadeghi MR, Akhondi MM, Ghaffari Novin M, Heidari M. Evaluation of an aqueous-ethanolic extract from Rosmarinus officinalis (Rosemary) for its activity on the hormonal and cellular function of testes in adult male rat. Iran J Pharm Res. 2013; 12(2):445-51. [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Cellular and Molecular Biology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |