Volume 6, Issue 2 (2020)
Pharm Biomed Res 2020, 6(2): 123-132 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Doudi M, Hooshmandi Z, Saedi S, Setorki M. Effects of Kombucha Tea on Side Effects of High Cholesterol Diet in Rabbits. Pharm Biomed Res 2020; 6 (2) :123-132
URL: http://pbr.mazums.ac.ir/article-1-294-en.html
URL: http://pbr.mazums.ac.ir/article-1-294-en.html
1- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran.
2- Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran.
3- Department of Biology, Izeh Branch, Islamic Azad University, Izeh, Iran.
2- Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran.
3- Department of Biology, Izeh Branch, Islamic Azad University, Izeh, Iran.
Full-Text [PDF 2833 kb]
(1865 Downloads)
| Abstract (HTML) (3749 Views)
Full-Text: (3371 Views)
Introduction
Kombucha is a traditional beverage consumed since 220 BC. It is prepared by fermenting sweetened black tea [1, 2]. The beverage is consumed worldwide, andits popularity derives from its therapeutic benefits on relieving symptoms of metabolic diseases, hemorrhoids, rheumatism. It also reduces blood pressure, boosts immune response, relieves arthritis, and even cures cancer and AIDS [1-3].
The fermentation is done using a symbiotic culture of yeasts and bacteria (e.g. acetic acid bacteria) at room temperature for 10-12 days [3]. The yeasts ferment the sugar (i.e. sucrose) to ethanol in the sweetened tea medium. Then the bacteria oxidize the alcohol to produce acetic acid, thereby decreasing pH and producing antimicrobial metabolites which in turn reduce the competition of other bacteria and fungi [4]. Previous studies have revealed a broad spectrum of yeast species associated with kombucha products, including Candida, Kloeckera, Pichia, Saccharomyces, Saccharomycoides, Schizosaccharomyces, Torulospora, and Zygosaccharomyces [2]. However, only a few bacteria, especially Acetobacter xylinum strains which produce cellulose, have been reported to be involved in kombucha processing [5, 6].
Therapeutic properties of the fermented liquid have been mainly ascribed to the presence of acetic, lactic, and gluconic acids as major chemical compounds. The last compound is considered the key therapeutic agent and functions as a detoxification agent in the liver [2, 6]. Besides, the antimicrobial activity of kombucha beverage is related to the presence of usinic and acetic acids as well as other ingredients, such as bacteriocins and tea-derived phenolic compounds [2]. In other studies, flavor compounds such as alcohols, aldehydes, ketones, esters, and amino acids have also been reported at different levels, suggesting the consistency in the metabolisms during kombucha fermentation [7].
Despite its traditional and long-term consumption, there are controversial claims on kombucha’s therapeutic benefits which are scientifically unsubstantiated. For example, Murphy et al. stated the pharmacological effectiveness of kombucha beverage as a therapeutic agent and therefore recommended its consumption in the food [1].
A growing body of research has investigated the microbiological, compositional [8-11], health beneficial, fermentation characteristics [3, 6, 12], and toxicological effects of kombucha tea [13]. Previous investigations suggested and even claimed that fermented kombucha tea could reduce blood cholesterol levels, treat obesity, and protect against diabetes [6, 12]. Given that lipid disorder is one of the most common causes of diabetes, this research aimed to evaluate the effectiveness of kombucha tea in reducing cholesterol uptake, as well as treating pathological and immunological side effects (i.e. oxidative stress) caused by high cholesterol diets in New Zealand white rabbit (Oryctolagus cuniculus) model.
Materials and Methods
Kombucha preparation
Iranian black tea was obtained from Lahijan City (north of Iran). The tea leaves (3 g/L) were infused into freshly boiled water for 30 min and sweetened with 200 g/L sucrose. After removing the leaves, the tea was allowed to cool at room temperature and then 30 g kombucha mat from a previous batch was added to the sweetened tea. After fermentation at room temperature for 2 months, the fungi mat was removed. The preparation of kombucha beverage was done in sterile containers and utensils to prevent contamination with external microorganisms [14].
Animal maintenance and experimental diets
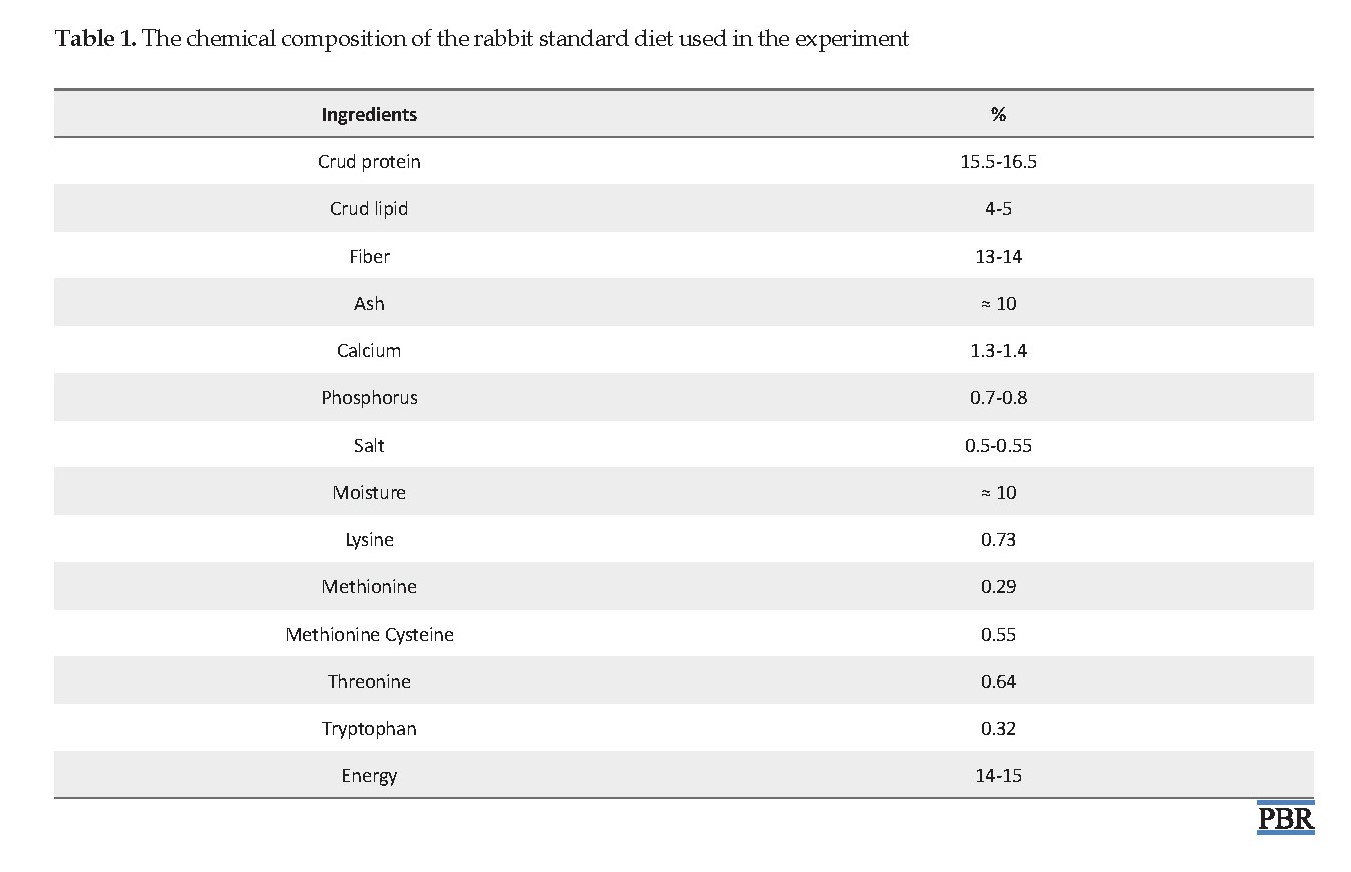
A total of 32 adult male New Zealand rabbits (average weight of 2000±129 g) were obtained from the Pasteur Institute of Tehran (Iran). Before starting the procedure, the rabbits were acclimated to the experimental condition for seven days at 25-30°C with a 12:12 h light/dark cycle and fed by a standard diet (Table 1). The tests on animals were done based on the ethical guidelines of working with laboratory animals approved by the Ethics Committee at the Deputy of Research and Technology of Islamic Azad University (Iran).
The animals were randomly assigned into four groups each containing 8 animals. First, all rabbits were fasted for 12-15 hours. Then, their blood samples were collected via a coronary perfusion catheter to determine baseline values for biochemical and experimental indices. Next, the experimental groups received the following treatments: 1. Control group, fed with standard pellets without cholesterol; 2. HCh+St group, fed with high cholesterol diet in which cholesterol (1 g in 2 mL olive oil) was gavaged once each day along with the standard diet; 3. HCh+Kom group, fed with high cholesterol diet as HCh+St group along with 5 mL of kombucha beverage once each day; 4. Kom+St group, fed with standard diet along with 5 mL of kombucha beverage once each day. The animals were fed each daily with 100 g of the desired diet with food and water ad libitum. Also, the same volume of olive oil as the second and third groups was given to the animals of the first and third groups [15]. The treatments were followed for 40 days. In the end, the blood samples were collected again for the assessment of biochemical and experimental indices. Also, the rabbits’ liver and pancreas tissues were dissected for histopathological analysis.
Blood biochemical indices
Blood samples were centrifuged at 3500 rpm for 20 minutes to obtain serum and plasma. Atherosclerosis-associated lipids, including cholesterol (CHO), triglycerides (TG), Low-Density Lipoprotein (LDL), High-Density Lipoprotein (HDL), Creatine Phosphokinase (CPK), ferritin, interleukin-6 (IL-6), Interleukin-1 (IL-1), Malondialdehyde (MDA) were measured. Also, Alanine Transaminase (ALT) and serum Aspartate Transaminase (AST) were analyzed with a UV/VIS spectrophotometer (WTW SpectroFlex 6600, Germany) using a commercial kit (Pars Azmoon, Iran). Creatine Phosphokinase (CPK) activity was determined using (CK) kit according to the manufacturer’s instructions. MDA levels were estimated using the Thiobarbituric Acid (TBA) method by the assessment of the absorbance at the wavelength of 532 nm [16]. The measurements were carried out according to the manufacturer’s instructions.
Histopathological analysis
The liver and pancreas tissues of the rabbits were dissected out and fixed in 10% formalin, then dehydrated in gradual ethanol (50%–100%), cleared in xylene, and embedded in paraffin. Finally, the sections were prepared and stained by hematoxylin and eosin (H&E) for light microscope observation (Nikon-YF100, Japan).
Statistical analysis
Results are presented as Mean±SD. Differences between the baseline values (first day) and the final values were calculated and then introduced to one-way ANOVA for comparing between groups. Next, the pair-wise multiple comparisons were performed using LSD (least significance difference) posttest. In all steps, P-values less than 0.05 were considered significant.
Results
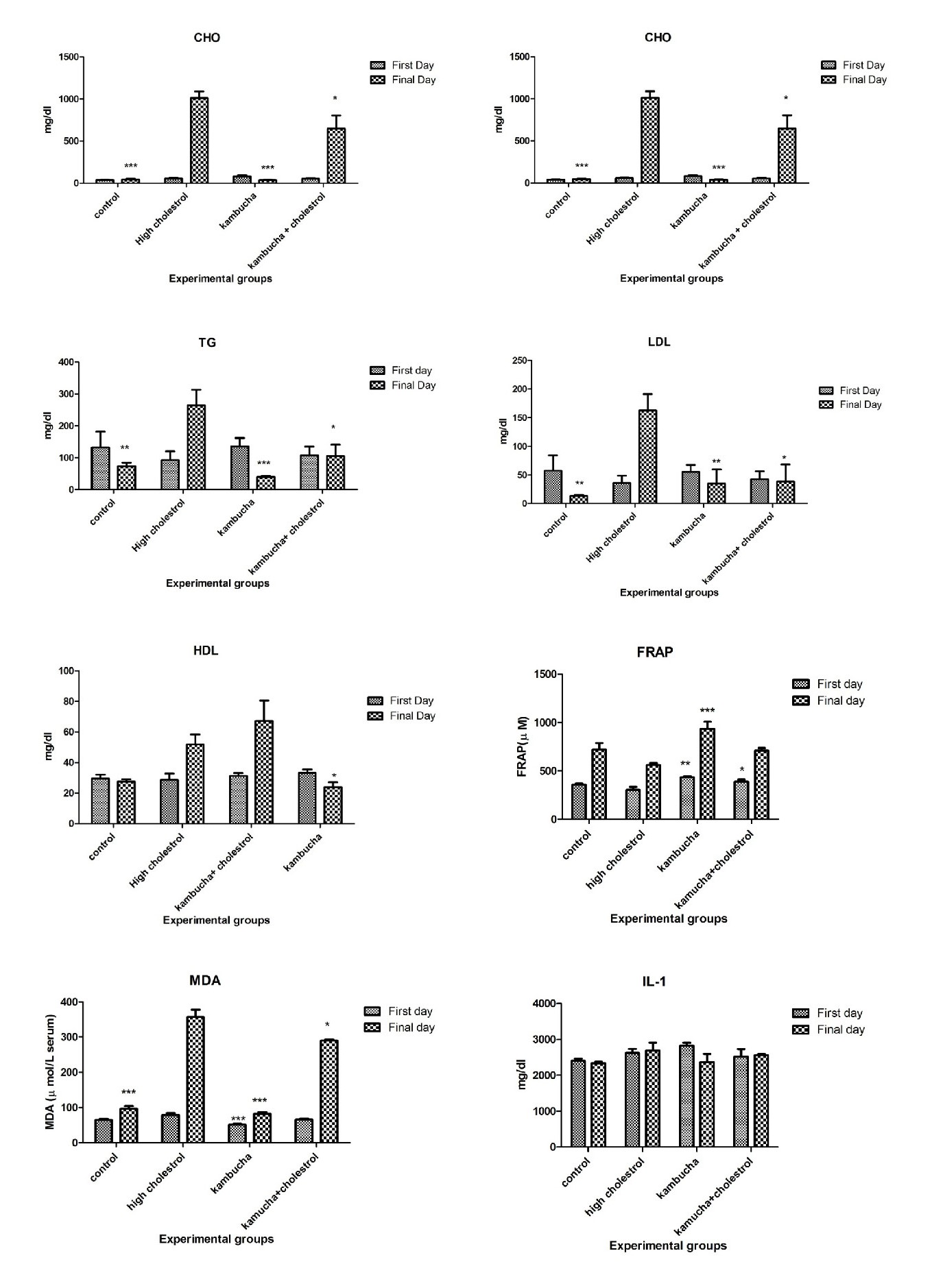
The concentration of CHO, TG, LDL, HDL, MDA, IL-1, IL-6, ferritin, and CPK were different in the experimental groups. Plasma CHO exhibited the highest level in the group who only received the high cholesterol diet (HCh+St) compared with the other groups (P<0.05), mostly with the HCh+Kom group. That is, the kombucha beverage used in the HCh+Kom regime significantly reduced the amount of CHO when compared with the HCh+St group (Figure 1A). Treatment with high cholesterol diet (HCh+St) significantly increased plasma TG, whereas the rabbits gavaged with kombucha beverage (HCh+Kom) displayed no significant changes when compared with the control group (P<0.05) (Figure 1 B). Likewise, plasma LDL exhibited almost the same response to the treatments (P<0.05) (Figure 1 C). On the other hand, HCh+Kom treatment significantly increased HDL level compared with the HCh+St and Kom+St treatments, especially the second one (P<0.05) (Figure 1 D).
Kombucha is a traditional beverage consumed since 220 BC. It is prepared by fermenting sweetened black tea [1, 2]. The beverage is consumed worldwide, andits popularity derives from its therapeutic benefits on relieving symptoms of metabolic diseases, hemorrhoids, rheumatism. It also reduces blood pressure, boosts immune response, relieves arthritis, and even cures cancer and AIDS [1-3].
The fermentation is done using a symbiotic culture of yeasts and bacteria (e.g. acetic acid bacteria) at room temperature for 10-12 days [3]. The yeasts ferment the sugar (i.e. sucrose) to ethanol in the sweetened tea medium. Then the bacteria oxidize the alcohol to produce acetic acid, thereby decreasing pH and producing antimicrobial metabolites which in turn reduce the competition of other bacteria and fungi [4]. Previous studies have revealed a broad spectrum of yeast species associated with kombucha products, including Candida, Kloeckera, Pichia, Saccharomyces, Saccharomycoides, Schizosaccharomyces, Torulospora, and Zygosaccharomyces [2]. However, only a few bacteria, especially Acetobacter xylinum strains which produce cellulose, have been reported to be involved in kombucha processing [5, 6].
Therapeutic properties of the fermented liquid have been mainly ascribed to the presence of acetic, lactic, and gluconic acids as major chemical compounds. The last compound is considered the key therapeutic agent and functions as a detoxification agent in the liver [2, 6]. Besides, the antimicrobial activity of kombucha beverage is related to the presence of usinic and acetic acids as well as other ingredients, such as bacteriocins and tea-derived phenolic compounds [2]. In other studies, flavor compounds such as alcohols, aldehydes, ketones, esters, and amino acids have also been reported at different levels, suggesting the consistency in the metabolisms during kombucha fermentation [7].
Despite its traditional and long-term consumption, there are controversial claims on kombucha’s therapeutic benefits which are scientifically unsubstantiated. For example, Murphy et al. stated the pharmacological effectiveness of kombucha beverage as a therapeutic agent and therefore recommended its consumption in the food [1].
A growing body of research has investigated the microbiological, compositional [8-11], health beneficial, fermentation characteristics [3, 6, 12], and toxicological effects of kombucha tea [13]. Previous investigations suggested and even claimed that fermented kombucha tea could reduce blood cholesterol levels, treat obesity, and protect against diabetes [6, 12]. Given that lipid disorder is one of the most common causes of diabetes, this research aimed to evaluate the effectiveness of kombucha tea in reducing cholesterol uptake, as well as treating pathological and immunological side effects (i.e. oxidative stress) caused by high cholesterol diets in New Zealand white rabbit (Oryctolagus cuniculus) model.
Materials and Methods
Kombucha preparation
Iranian black tea was obtained from Lahijan City (north of Iran). The tea leaves (3 g/L) were infused into freshly boiled water for 30 min and sweetened with 200 g/L sucrose. After removing the leaves, the tea was allowed to cool at room temperature and then 30 g kombucha mat from a previous batch was added to the sweetened tea. After fermentation at room temperature for 2 months, the fungi mat was removed. The preparation of kombucha beverage was done in sterile containers and utensils to prevent contamination with external microorganisms [14].
Animal maintenance and experimental diets
A total of 32 adult male New Zealand rabbits (average weight of 2000±129 g) were obtained from the Pasteur Institute of Tehran (Iran). Before starting the procedure, the rabbits were acclimated to the experimental condition for seven days at 25-30°C with a 12:12 h light/dark cycle and fed by a standard diet (Table 1). The tests on animals were done based on the ethical guidelines of working with laboratory animals approved by the Ethics Committee at the Deputy of Research and Technology of Islamic Azad University (Iran).
The animals were randomly assigned into four groups each containing 8 animals. First, all rabbits were fasted for 12-15 hours. Then, their blood samples were collected via a coronary perfusion catheter to determine baseline values for biochemical and experimental indices. Next, the experimental groups received the following treatments: 1. Control group, fed with standard pellets without cholesterol; 2. HCh+St group, fed with high cholesterol diet in which cholesterol (1 g in 2 mL olive oil) was gavaged once each day along with the standard diet; 3. HCh+Kom group, fed with high cholesterol diet as HCh+St group along with 5 mL of kombucha beverage once each day; 4. Kom+St group, fed with standard diet along with 5 mL of kombucha beverage once each day. The animals were fed each daily with 100 g of the desired diet with food and water ad libitum. Also, the same volume of olive oil as the second and third groups was given to the animals of the first and third groups [15]. The treatments were followed for 40 days. In the end, the blood samples were collected again for the assessment of biochemical and experimental indices. Also, the rabbits’ liver and pancreas tissues were dissected for histopathological analysis.
Blood biochemical indices
Blood samples were centrifuged at 3500 rpm for 20 minutes to obtain serum and plasma. Atherosclerosis-associated lipids, including cholesterol (CHO), triglycerides (TG), Low-Density Lipoprotein (LDL), High-Density Lipoprotein (HDL), Creatine Phosphokinase (CPK), ferritin, interleukin-6 (IL-6), Interleukin-1 (IL-1), Malondialdehyde (MDA) were measured. Also, Alanine Transaminase (ALT) and serum Aspartate Transaminase (AST) were analyzed with a UV/VIS spectrophotometer (WTW SpectroFlex 6600, Germany) using a commercial kit (Pars Azmoon, Iran). Creatine Phosphokinase (CPK) activity was determined using (CK) kit according to the manufacturer’s instructions. MDA levels were estimated using the Thiobarbituric Acid (TBA) method by the assessment of the absorbance at the wavelength of 532 nm [16]. The measurements were carried out according to the manufacturer’s instructions.
Histopathological analysis
The liver and pancreas tissues of the rabbits were dissected out and fixed in 10% formalin, then dehydrated in gradual ethanol (50%–100%), cleared in xylene, and embedded in paraffin. Finally, the sections were prepared and stained by hematoxylin and eosin (H&E) for light microscope observation (Nikon-YF100, Japan).
Statistical analysis
Results are presented as Mean±SD. Differences between the baseline values (first day) and the final values were calculated and then introduced to one-way ANOVA for comparing between groups. Next, the pair-wise multiple comparisons were performed using LSD (least significance difference) posttest. In all steps, P-values less than 0.05 were considered significant.
Results
The concentration of CHO, TG, LDL, HDL, MDA, IL-1, IL-6, ferritin, and CPK were different in the experimental groups. Plasma CHO exhibited the highest level in the group who only received the high cholesterol diet (HCh+St) compared with the other groups (P<0.05), mostly with the HCh+Kom group. That is, the kombucha beverage used in the HCh+Kom regime significantly reduced the amount of CHO when compared with the HCh+St group (Figure 1A). Treatment with high cholesterol diet (HCh+St) significantly increased plasma TG, whereas the rabbits gavaged with kombucha beverage (HCh+Kom) displayed no significant changes when compared with the control group (P<0.05) (Figure 1 B). Likewise, plasma LDL exhibited almost the same response to the treatments (P<0.05) (Figure 1 C). On the other hand, HCh+Kom treatment significantly increased HDL level compared with the HCh+St and Kom+St treatments, especially the second one (P<0.05) (Figure 1 D).
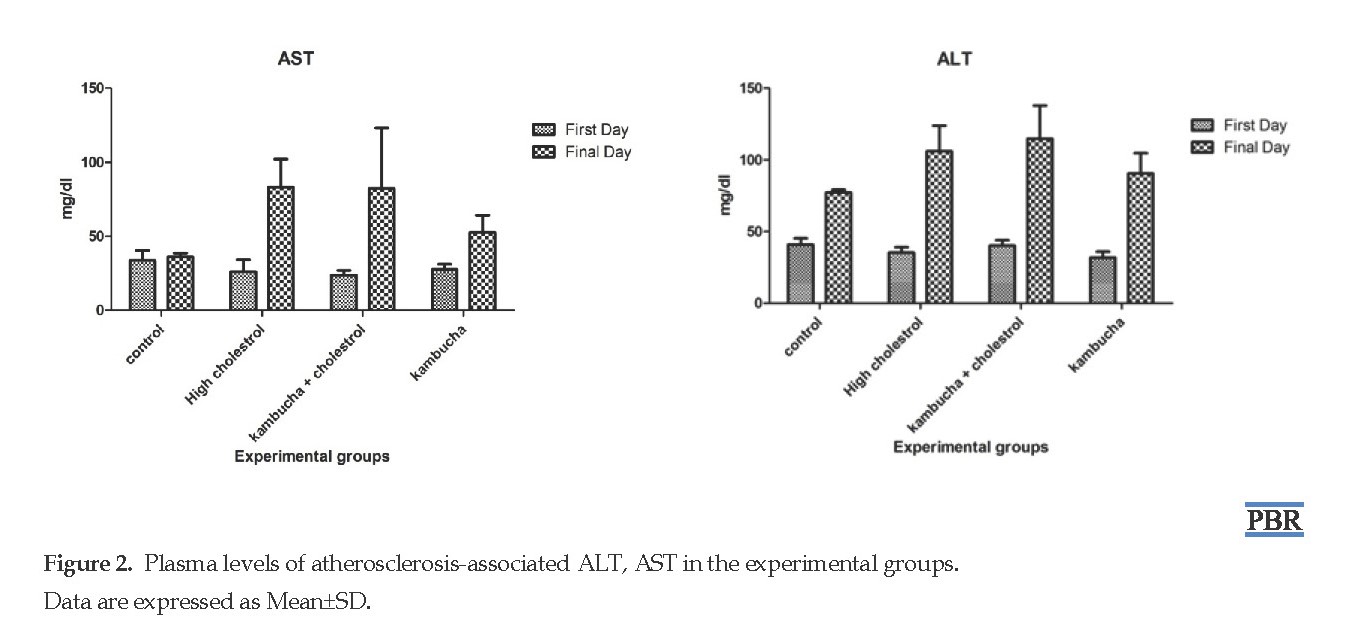
The values of MDA (Figure 1 F), ferritin (Figure 1 I), and CPK (Figure 1 J) in the Kom+St treatment were significantly higher than those in the other groups (P< 0.05), followed by HCh+Kom. No significant difference was observed in the values of IL-1 and IL-6 (Figure 1 G and H, respectively) as well as ALT and AST activity (Figure 2) between the experimental groups and the control group.
Analysis of the green kombucha tea with cell spectrophotometer
The acetic acid in kombucha green tea was detected by cell spectrophotometer (model SpectroFlex 6600) in Poyan Engineering Group of Isfahan University of Technology, Isfahan Scientific and Research Town. First, we prepared various percentages of acetic acid. Next, 2 mL of the sample and 2 mL of the rhodamine reagent were added to the cell spectrophotometer in that order, and the absorption of each sample was read using the cell spectrophotometer.
Table 2 presents the wavelength and absorption levels of different concentrations of 3-month fermented kombucha tea compared with different concentrations of acetic acid using the cell spectrophotometer. By comparing the optical absorption of different concentrations of chemical acetic acid at 510 nm wavelength with the same concentrations of acetic acid obtained from green kombucha tea (Figure 1), one can conclude that the acetic acid obtained by 3-month fermented green kombucha tea has a higher acidic power because of its higher absorption of light (Figure 3).
The acetic acid in kombucha green tea was detected by cell spectrophotometer (model SpectroFlex 6600) in Poyan Engineering Group of Isfahan University of Technology, Isfahan Scientific and Research Town. First, we prepared various percentages of acetic acid. Next, 2 mL of the sample and 2 mL of the rhodamine reagent were added to the cell spectrophotometer in that order, and the absorption of each sample was read using the cell spectrophotometer.
Table 2 presents the wavelength and absorption levels of different concentrations of 3-month fermented kombucha tea compared with different concentrations of acetic acid using the cell spectrophotometer. By comparing the optical absorption of different concentrations of chemical acetic acid at 510 nm wavelength with the same concentrations of acetic acid obtained from green kombucha tea (Figure 1), one can conclude that the acetic acid obtained by 3-month fermented green kombucha tea has a higher acidic power because of its higher absorption of light (Figure 3).
Histopathological observations
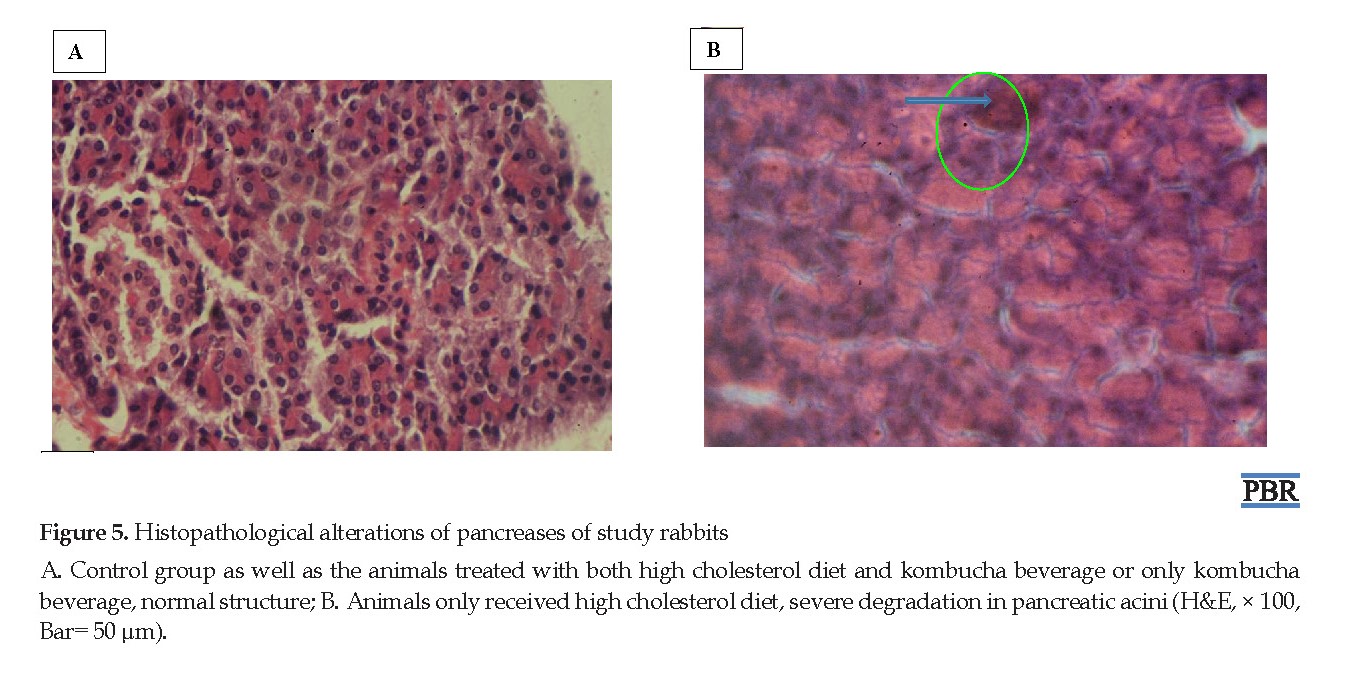
Histopathological analysis of the liver tissues in the control group revealed normal hepatic cells with well-preserved cytoplasm, prominent nucleus, nucleolus, and visible central veins, although with a little hyperemia in the sinusoid (Figure 4A). However, foam cells with acidophilic appearance were observed in the animals received HCh+St diet (Figure 4B) and hyperemia in the sinusoid was shown in those treated with HCh+Kom and Kom+St (Figure 4C and D, respectively). Pancreas sections exhibited a normal structure in the control group and the animals received HCh+Kom and Kom+St (Figure 5A). Still, severe degradation in pancreatic acini was observed in the animals received HCO+St diet (Figure 5B).
Histopathological analysis of the liver tissues in the control group revealed normal hepatic cells with well-preserved cytoplasm, prominent nucleus, nucleolus, and visible central veins, although with a little hyperemia in the sinusoid (Figure 4A). However, foam cells with acidophilic appearance were observed in the animals received HCh+St diet (Figure 4B) and hyperemia in the sinusoid was shown in those treated with HCh+Kom and Kom+St (Figure 4C and D, respectively). Pancreas sections exhibited a normal structure in the control group and the animals received HCh+Kom and Kom+St (Figure 5A). Still, severe degradation in pancreatic acini was observed in the animals received HCO+St diet (Figure 5B).
Discussion
Atherosclerosis-associated lipids (CHO, TG, LDL, and HDL) level, the activity of liver functional enzymes (ALT and AST), MDA, IL-1, IL-6, ferritin, and CPK levels, as well as the histological structure of liver and pancreas tissues, were evaluated in white rabbits following exposure to high cholesterol dietary along with oral administration of kombucha beverage. Rabbits are highly sensitive to cholesterol-rich diets and usually exhibit the response as hypercholesterolemia [17-19]. Elevated cholesterol levels and in some cases triglycerides are considered as risk factors for the development of atherosclerosis and cardiovascular diseases [20].
Atherosclerosis-associated lipids (CHO, TG, LDL, and HDL) level, the activity of liver functional enzymes (ALT and AST), MDA, IL-1, IL-6, ferritin, and CPK levels, as well as the histological structure of liver and pancreas tissues, were evaluated in white rabbits following exposure to high cholesterol dietary along with oral administration of kombucha beverage. Rabbits are highly sensitive to cholesterol-rich diets and usually exhibit the response as hypercholesterolemia [17-19]. Elevated cholesterol levels and in some cases triglycerides are considered as risk factors for the development of atherosclerosis and cardiovascular diseases [20].
The current study demonstrated the highest plasma CHO level in the rabbits treated only with a cholesterol-rich diet (i.e. HCh+St) but the effect was significantly less in those received HCh+Kom regime, suggesting that kombucha beverage effectively disallows the absorption of CHO in the intestinal tissue. The observed lower levels of blood cholesterol in the HCh+Kom group has been documented in a previous study on duck [21], alloxan-induced diabetic rats [22], and Wistar rats [23], which were treated with kombucha.
The present study demonstrated that the administration of kombucha significantly reduced plasma TG levels in HCh+Kom treated group when compared with the animals only received HCh+St. Similar results have been reported by Aloulou et al. (2012) who administrated kombucha to diabetic rats and observed a considerable decrease in plasma TG and cholesterol; the reduction in plasma TG and cholesterol was attributed to the reduction in pancreatic lipase activity which, in turn, is responsible for the hydrolysis of non-absorbable dietary triglycerides into absorbable monoglycerides and free fatty acids [20]. Also, kombucha induced lowered serum levels of TC, TG, VLDL-C, and LDL-C by 26%, 27%, 28%, and 36%, respectively, and increased the serum level of high-density lipoprotein cholesterol [23]. Increasing the concentration of cholesterol level prevents the synthesis of LDL receptor proteins at the cellular level, and thus, cells are unable to absorb LDL through endothetic receptors, thereby increasing plasma LDL level [17]. Besides, insufficient function of the liver in very-low-density lipoprotein (VLDL) degradation occurs following a high intake of rich-cholesterol diets and consequently plasma VLDLs accumulation [24]. Hence, the observed elevation of plasma LDL in the HCh+St group could be attributed to the high detected cholesterol level and insufficient performance of the liver in VLDL degradation. Also, the present finding for HDL seems to be consistent with other research indicating that kombucha tea induces a significant increase in HDL-cholesterol [21-23]. The beneficial effects of kombucha tea are attributed to the presence of tea polyphenols, gluconic acid, glucuronic acid, lactic acid, vitamins, amino acids, antibiotics, and a variety of micronutrients produced during fermentation [23].
Ferritin maintains the intracellular iron balance and serves as a cytoprotective protein, which lowers oxygen free radical formation by sequestering intracellular iron [25]. Our finding demonstrated that the concentration of serum ferritin was significantly lower in the rabbits treated with Kom+St or HCh-Kom when compared with those received HCh+St. Given that ferritin is a lipid oxidation catalyst, the higher concentration of ferritin in HCh+St could be attributed to higher stress in the animals administrated with high cholesterol regime. The same holds for MDA which is frequently used as an indicator of lipid peroxidation.
Serum CK is an enzyme whose high concentration reflects injury, inflammation, or necrosis of muscles. Therefore, high levels of CPK usually indicate some degrees of stress or injury to the heart or other muscles. The animals administered with kombucha displayed lower serum CK concentrations when compared with those received high cholesterol diet (i.e. HCh+St), suggesting that kombucha tea possesses a protective effect against muscle injury or heart attack as an antihypercholesterolemic agent.
ALP and AST are biomarkers of liver health, and clinically critical for the diagnosis of hepatopancreas dysfunction. Being high in the cytoplasm of the liver cells, these plasmatic enzymes pass through the cell membrane and enter the bloodstream during injuries [26, 27]. In the present study, no significant change was observed in the activity of ALT and AST between all of the animal groups, and the findings do not support the previous research for hypercholesterolemic Wistar rats done by Bellassoued et al. (2015). They measured AST, ALT, and GGT indices and concluded that kombucha beverage could not protect the liver and kidney [23].
Histopathological analysis demonstrated that the HCh+St diet feeding would lead to the emergence of foam cells with an acidophilic appearance in the liver and severe degradation in pancreas acini. But both experimental groups who received kombucha tea (i.e. HCh+Kom and Kom+St) showed no irreversible abnormal structures, suggesting a potential protective effect of the beverage. These findings agreed with the findings of other studies [22, 23].
Ethical Considerations
Compliance with ethical guidelines
This is a research conducted in the Department of Biology at Islamic Azad University, Falavarjan Branch with Ethical Code IR.IAU.NAJAFABAD.REC.1396.67.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Made all of the tests: Zahra Hooshmandi; Designed the project: Monir Doudi; Statistically analyzed the data and prepared the article: Mahbubeh Setorki; and Edited the paper: Somayeh Saedi.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy of Research and Technology of Islamic Azad University, Izeh Branch for providing the proper laboratory facilities to perform this research project.
References
Murphy TE, Walia K, Farber JM. Safety aspects and guidance for consumerson the safe preparation, handling and storage of kombucha-A fermented tea beverage. Int J Food Protec Trend. 2018; 38(5):329-37. https://bit.ly/3i15d8a
Coton M, Pawtowski A, Taminiau B, Burgaud G, Deniel F, Coulloumme-Labarthe L, et al. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol Ecol. 2017; 93(5). [DOI:10.1093/femsec/fix048] [PMID]
De Filippis F, Troise AD, Vitaglione P, Ercolini D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018; 73:11-6. [DOI:10.1016/j.fm.2018.01.008] [PMID]
Kaufmann K. Kombucha rediscovered: Revised edition The medicinal benefits of an ancient healing tea. Summertown: Book Publishing Company; 2013. https://books.google.com/books/about/Kombucha_Rediscovered.html?id
Hyun J, Lee Y, Wang S, Kim J, Kim J, Cha J, et al. Kombucha tea prevents obese mice from developing hepatic steatosis and liver damage. Food Sci Biotechnol. 2016; 25(3):861-6. [DOI:10.1007/s10068-016-0142-3] [PMID] [PMCID]
Jayabalan R, Malbaša RV, Lončar ES, Vitas JS Sathishkumar M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Safety. 2014; 13(4):538-50. [DOI:10.1111/1541-4337.12073]
Vohra BM, Fazry S, Sairi F, Babul-Airianah O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of Kombucha. Mal J Fund Appl Sci. 2019; 15(2-1):298-302. [DOI:10.11113/mjfas.v15n2-1.1536]
Manikandan VS, Adhikari B, Chen A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst. 2018; 143(19):4537-54. [DOI:10.1039/C8AN00497H] [PMID]
Malbasa RV, Lončar ES, Kolarov LJA. L-lactic, L-ascorbic, total and volatile acids contents in dietetic Kombucha beverage. Rom Biotech Lett. 2002; 7:891-6. https://e-repository.org/rbl/vol.7/iss.5/2.pdf
Yang Z, Zhou F, Ji B, Li B, Luo Y, Yang L, et al. Symbiosis between microorganisms from kombucha and kefir: potential significance to the enhancement of kombucha function. Appl Biochem Biotechnol. 2010; 160(2):446-55. [DOI:10.1007/s12010-008-8361-6] [PMID]
Vitas JS, Malbaša RV, Grahovac JA, Lončar ES. The antioxidant activity of kombucha fermented milk products with stinging nettle and winter savory. Chem Ind Chem Eng. 2013; 19(1):129-39. [DOI:10.2298/CICEQ120205048V]
Liamkaew R, Chattrawanit J, Danvirutai P. Kombucha production by combinations of black tea and apple juice. Sci Techy RMUTT J. 2016; 6(2):139-46. http://www.sci.rmutt.ac.th/stj/index.php/stj/article/view/176
Prasad A, Clopton P, Ayers C, Khera A, De Lemos JA, Witztum JL, et al. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017; 37(6):1213-21. [DOI:10.1161/ATVBAHA.117.309101] [PMID] [PMCID]
Murugesan GS, Sathishkumar M, Jayabalan R, Binupriya AR, Swaminathan K, Yun SE. Hepatoprotective and curative properties of Kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotechnol. 2009; 19(4):397-402. [DOI:10.4014/jmb.0806.374] [PMID]
Asgary S, Jafari Dinani N, Madani HP. Mahzoni P. Effect of glycyrrhizaglabra extracts on aorta wall atherosclerotic lesion in hypercholesterolemic rabbits. Pakistan J Nutr. 2007; 6(4):313-7. [DOI:10.3923/pjn.2007.313.317]
Gupta A, Kant S, Gupta SK, Prakash S, Kalaivani M, Pandav CS, et al. Serum FRAP levels and pre-eclampsia among pregnant women in a rural community of northern India. J Clin Diagn Res. 2016; 10(10):LC12-LC15. [DOI:10.7860/JCDR/2016/18763.8745] [PMID] [PMCID]
Chekanov VS. Low frequency electrical impulses reduce atherosclerosis in cholesterol fed rabbits. Med Sci Monit, 2003. 9(8):BR302-9. [PMID]
Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation. 1999; 99(10):1355-62. [DOI:10.1161/01.CIR.99.10.1355] [PMID]
Asgary S, Rafieian-Kopaei M, Najafi S, Heidarian E, Sahebkar AH. Antihyperlipidemic effects of sesamum indicum L. in rabbits fed a high-fat diet. Sci World J. 2013; 2013:365892. [DOI:10.1155/2013/365892] [PMID] [PMCID]
Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, et al. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 2006; 103(2):252-60. [DOI:10.1016/j.jep.2005.08.033] [PMID]
Adriani L, Mayasari N, Kartasudjana R. The effect of feeding fermented kombucha tea on HLD, LDL and total cholesterol levels in the duck bloods. Biotechnol Anim Husbandry. 2011; 27(4):1749-55. [DOI:10.2298/BAH1104749A]
Aloulou A, Hamden K, Elloumi D, Bou Ali M, Hargafi K, Jaouadi B, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012; 12:63. [DOI:10.1186/1472-6882-12-63] [PMID] [PMCID]
Bellassoued K, Ghrab F, Makni-Ayadi F, Pelt JV, Elfeki A, Ammar E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm Biol. 2015; 53(11):1699-709. [DOI:10.3109/13880209.2014.1001408] [PMID]
Gillard BK, Rosales C, Xu B, Gotto Jr AM, Pownall HJ. Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins. J Clin Lipidol. 2018; 12(4):849-56. [DOI:10.1016/j.jacl.2018.04.001] [PMID] [PMCID]
Cheng HT, Yen CJ, Chang CC, Huang KT, Chen KH, Zhang RY, et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta. 2015; 1850(12):2506-17. [DOI:10.1016/j.bbagen.2015.09.018] [PMID]
Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol. 2013; 3(2):280-4. http://voitedsaja.tk/index/?Bkm9gr
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33(6):550-8. [DOI:10.1200/JCO.2014.57.9151] [PMID] [PMCID]
Serum CK is an enzyme whose high concentration reflects injury, inflammation, or necrosis of muscles. Therefore, high levels of CPK usually indicate some degrees of stress or injury to the heart or other muscles. The animals administered with kombucha displayed lower serum CK concentrations when compared with those received high cholesterol diet (i.e. HCh+St), suggesting that kombucha tea possesses a protective effect against muscle injury or heart attack as an antihypercholesterolemic agent.
ALP and AST are biomarkers of liver health, and clinically critical for the diagnosis of hepatopancreas dysfunction. Being high in the cytoplasm of the liver cells, these plasmatic enzymes pass through the cell membrane and enter the bloodstream during injuries [26, 27]. In the present study, no significant change was observed in the activity of ALT and AST between all of the animal groups, and the findings do not support the previous research for hypercholesterolemic Wistar rats done by Bellassoued et al. (2015). They measured AST, ALT, and GGT indices and concluded that kombucha beverage could not protect the liver and kidney [23].
Histopathological analysis demonstrated that the HCh+St diet feeding would lead to the emergence of foam cells with an acidophilic appearance in the liver and severe degradation in pancreas acini. But both experimental groups who received kombucha tea (i.e. HCh+Kom and Kom+St) showed no irreversible abnormal structures, suggesting a potential protective effect of the beverage. These findings agreed with the findings of other studies [22, 23].
Ethical Considerations
Compliance with ethical guidelines
This is a research conducted in the Department of Biology at Islamic Azad University, Falavarjan Branch with Ethical Code IR.IAU.NAJAFABAD.REC.1396.67.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Made all of the tests: Zahra Hooshmandi; Designed the project: Monir Doudi; Statistically analyzed the data and prepared the article: Mahbubeh Setorki; and Edited the paper: Somayeh Saedi.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy of Research and Technology of Islamic Azad University, Izeh Branch for providing the proper laboratory facilities to perform this research project.
References
Murphy TE, Walia K, Farber JM. Safety aspects and guidance for consumerson the safe preparation, handling and storage of kombucha-A fermented tea beverage. Int J Food Protec Trend. 2018; 38(5):329-37. https://bit.ly/3i15d8a
Coton M, Pawtowski A, Taminiau B, Burgaud G, Deniel F, Coulloumme-Labarthe L, et al. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol Ecol. 2017; 93(5). [DOI:10.1093/femsec/fix048] [PMID]
De Filippis F, Troise AD, Vitaglione P, Ercolini D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018; 73:11-6. [DOI:10.1016/j.fm.2018.01.008] [PMID]
Kaufmann K. Kombucha rediscovered: Revised edition The medicinal benefits of an ancient healing tea. Summertown: Book Publishing Company; 2013. https://books.google.com/books/about/Kombucha_Rediscovered.html?id
Hyun J, Lee Y, Wang S, Kim J, Kim J, Cha J, et al. Kombucha tea prevents obese mice from developing hepatic steatosis and liver damage. Food Sci Biotechnol. 2016; 25(3):861-6. [DOI:10.1007/s10068-016-0142-3] [PMID] [PMCID]
Jayabalan R, Malbaša RV, Lončar ES, Vitas JS Sathishkumar M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Safety. 2014; 13(4):538-50. [DOI:10.1111/1541-4337.12073]
Vohra BM, Fazry S, Sairi F, Babul-Airianah O. Effects of medium variation and fermentation time on the antioxidant and antimicrobial properties of Kombucha. Mal J Fund Appl Sci. 2019; 15(2-1):298-302. [DOI:10.11113/mjfas.v15n2-1.1536]
Manikandan VS, Adhikari B, Chen A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst. 2018; 143(19):4537-54. [DOI:10.1039/C8AN00497H] [PMID]
Malbasa RV, Lončar ES, Kolarov LJA. L-lactic, L-ascorbic, total and volatile acids contents in dietetic Kombucha beverage. Rom Biotech Lett. 2002; 7:891-6. https://e-repository.org/rbl/vol.7/iss.5/2.pdf
Yang Z, Zhou F, Ji B, Li B, Luo Y, Yang L, et al. Symbiosis between microorganisms from kombucha and kefir: potential significance to the enhancement of kombucha function. Appl Biochem Biotechnol. 2010; 160(2):446-55. [DOI:10.1007/s12010-008-8361-6] [PMID]
Vitas JS, Malbaša RV, Grahovac JA, Lončar ES. The antioxidant activity of kombucha fermented milk products with stinging nettle and winter savory. Chem Ind Chem Eng. 2013; 19(1):129-39. [DOI:10.2298/CICEQ120205048V]
Liamkaew R, Chattrawanit J, Danvirutai P. Kombucha production by combinations of black tea and apple juice. Sci Techy RMUTT J. 2016; 6(2):139-46. http://www.sci.rmutt.ac.th/stj/index.php/stj/article/view/176
Prasad A, Clopton P, Ayers C, Khera A, De Lemos JA, Witztum JL, et al. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017; 37(6):1213-21. [DOI:10.1161/ATVBAHA.117.309101] [PMID] [PMCID]
Murugesan GS, Sathishkumar M, Jayabalan R, Binupriya AR, Swaminathan K, Yun SE. Hepatoprotective and curative properties of Kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotechnol. 2009; 19(4):397-402. [DOI:10.4014/jmb.0806.374] [PMID]
Asgary S, Jafari Dinani N, Madani HP. Mahzoni P. Effect of glycyrrhizaglabra extracts on aorta wall atherosclerotic lesion in hypercholesterolemic rabbits. Pakistan J Nutr. 2007; 6(4):313-7. [DOI:10.3923/pjn.2007.313.317]
Gupta A, Kant S, Gupta SK, Prakash S, Kalaivani M, Pandav CS, et al. Serum FRAP levels and pre-eclampsia among pregnant women in a rural community of northern India. J Clin Diagn Res. 2016; 10(10):LC12-LC15. [DOI:10.7860/JCDR/2016/18763.8745] [PMID] [PMCID]
Chekanov VS. Low frequency electrical impulses reduce atherosclerosis in cholesterol fed rabbits. Med Sci Monit, 2003. 9(8):BR302-9. [PMID]
Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation. 1999; 99(10):1355-62. [DOI:10.1161/01.CIR.99.10.1355] [PMID]
Asgary S, Rafieian-Kopaei M, Najafi S, Heidarian E, Sahebkar AH. Antihyperlipidemic effects of sesamum indicum L. in rabbits fed a high-fat diet. Sci World J. 2013; 2013:365892. [DOI:10.1155/2013/365892] [PMID] [PMCID]
Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, et al. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 2006; 103(2):252-60. [DOI:10.1016/j.jep.2005.08.033] [PMID]
Adriani L, Mayasari N, Kartasudjana R. The effect of feeding fermented kombucha tea on HLD, LDL and total cholesterol levels in the duck bloods. Biotechnol Anim Husbandry. 2011; 27(4):1749-55. [DOI:10.2298/BAH1104749A]
Aloulou A, Hamden K, Elloumi D, Bou Ali M, Hargafi K, Jaouadi B, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012; 12:63. [DOI:10.1186/1472-6882-12-63] [PMID] [PMCID]
Bellassoued K, Ghrab F, Makni-Ayadi F, Pelt JV, Elfeki A, Ammar E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm Biol. 2015; 53(11):1699-709. [DOI:10.3109/13880209.2014.1001408] [PMID]
Gillard BK, Rosales C, Xu B, Gotto Jr AM, Pownall HJ. Rethinking reverse cholesterol transport and dysfunctional high-density lipoproteins. J Clin Lipidol. 2018; 12(4):849-56. [DOI:10.1016/j.jacl.2018.04.001] [PMID] [PMCID]
Cheng HT, Yen CJ, Chang CC, Huang KT, Chen KH, Zhang RY, et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta. 2015; 1850(12):2506-17. [DOI:10.1016/j.bbagen.2015.09.018] [PMID]
Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol. 2013; 3(2):280-4. http://voitedsaja.tk/index/?Bkm9gr
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33(6):550-8. [DOI:10.1200/JCO.2014.57.9151] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Pharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |