Volume 6, Issue 1 (2020)
Pharm Biomed Res 2020, 6(1): 91-104 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehmood R, Amaldoss M J N. Fabrication and Characterisation of Lavender Oil and Plant Phospholipid Based Sumatriptan Succinate Hybrid Nano Lipid Carriers. Pharm Biomed Res 2020; 6 (1) :91-104
URL: http://pbr.mazums.ac.ir/article-1-278-en.html
URL: http://pbr.mazums.ac.ir/article-1-278-en.html
1- School of Materials Science and Engineering, UNSW Sydney, NSW, Australia
Keywords: Nanostructured lipid carriers (NLCs), Zetasizer, Zeta potential, Transmission electron microscopy (TEM)
Full-Text [PDF 5310 kb]
(1882 Downloads)
| Abstract (HTML) (4091 Views)
Full-Text: (2373 Views)
Introduction
Nanostructured lipid carriers (NLCs) are the new generation of drug delivery systems. The matrix of NLCs is composed of lipid and oil. NLCs offer high drug loading and delivery with various drugs [1, 2]. NLCs are a versatile drug delivery system, which can be fabricated by using solid and liquid lipids, surfactant, co-surfactant, and stabilizer. NLCs offer formulation and therapeutic advantages such as improved stability, solubility, higher permeability, bioavailability, and targeting.
Besides these ingredients, cryoprotectant and charge modifiers may contribute to the synthesis of NLCs to improve the formulation characteristics. The preparation method of NLCs involves the combination of liquid and solid lipids in different ratios, along with the surfactant between the range of 1.5%-5% [3]. NLCs can be prepared by various methods such as high shear homogenization, ultrasonication, solvent diffusion, microemulsion, and double emulsification [4]. In this research, we have adopted a double emulsification method for the synthesis of NLCs. These carriers demonstrate various advantages over other lipid nanoparticles regarding the drug loading because their physical stability increases surface area and cellular uptake [5].
Migraine is a chronic disorder characterized by the paroxysmal episodes of a headache lasting for 4-72 h. Migraine attacks are 2.5 times more prevalent in women than men and usually strike the 25-44 years age group [6]. Intranasal therapy was practiced in the Indian system of medicines of Ayurveda for a long time. Currently, the nasal route is emerging as a second suitable route after the oral route [7]. The nasal drug delivery system is under intensive research due to the various advantages such as non-invasive approach, rapid absorption, quick onset of action, self-administration, convenient for long-term therapy, and prevention of drug degradation by first-pass effect [8]. However, the nasal route has few disadvantages, such as immunological reaction, nasal irritation, small absorption surface area and rapid mucociliary clearance [9]. The developed NLCs were evaluated in vitro in the Simulated Nasal Fluid (SNF) as the formulation has the scope of transforming to a nasal delivery after appropriate clinical trials.
LECIVA-S70 is a pure polyunsaturated phospholipid of a specialized grade for liposomal, dermatological preparation and specially designed for emulsion, fat infusions, nutraceutical formulations and parenteral use. It contains validated 70% phosphatidylcholine. The regular soy lecithin which is used in the manufacturing of chocolates, confectionaries, dietary supplements and pharmaceuticals have 10%-35% of phosphatidylcholine which is lower than LECIVA-S70. The phospholipids are classified as GRAS (Generally recognized as safe) by the United States FDA and demonstrating advantageous in drug delivery, facilitating absorption, increasing bioavailability, and reducing toxicity [10]. Lavender oil is reported with the properties of soothing and calming effect on the nerves. It is also used in relieving tension, depression, panic, hysteria and nervous exhaustion.
Lavender oil has been known for its medicinal properties in soothing headache and other types of pain [11, 12]. Lavender oil inclusion in this formula is an added advantage, which offers the natural approach in relieving migraines; moreover, synthesized NLCs retained the smell of lavender oil in the formulation. A tryptamine derivative, namely 3-[2-(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide or sumatriptan succinate, is a selective vascular serotonin agonist of 5-HT1 type 1. The agonist activities of 5-HT1B/1D receptors provide the relief of acute headache [13]. Sumatriptan succinate is an agonist against this particular receptor subtypes and no effect in other receptors [14]. Sumatriptan succinate is used via oral, subcutaneous, or intranasal route for the management of acute migraine with aura or without aura described as a classical and common migraine, respectively [15]. Sumatriptan succinate is available in the oral doses of 25 mg, 50 mg, and 100 mg. The maximum dose is 200 mg per 24 h. The subcutaneous injections are available at a dose of 6 mg/d. Also, another 12 mg dose would be required if migraine was reoccurred. The nasal spray of SS is available in the market, which is gaining popularity among the patients.
In this research work, the central composite design was used to fabricate SS-NLCs by using natural phospholipid of plant origin (LECIVA-S70) and migraine natural medicine lavender oil with the aid of surfactant and co-surfactant. The central composite design was chosen to study the effect of key ingredients in different concentrations in the formulation development. The central composite design approach minimizes the number of experimental trials to optimize the proportion of ingredients to develop the best formulation. The Fourier Transform Infrared Spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC) confirmed the physicochemical compatibility of drug and excipients. The X-ray Diffraction (XRD) and Transmission Electron Microscopy (TEM) analysis were done for the characterization. The hydrodynamic diameter and zeta potential were analyzed with Zetasizer. The in vitro drug release study was done with membrane diffusion technique.
Materials and Methods
Study materials
Natco Pharmaceuticals Pvt. Ltd. Hyderabad kindly presented the pure drug Sumatriptan Succinate (SS). LECIVA-S70 was received from VAV Life Sciences, Mumbai, India. Lavender oil and chloroform were obtained from the CDH chemicals, India.
Experimental design
A central composite design was developed to study the impact of lavender oil and LECIVA-S70 in sumatriptan succinate NLCs. The concentrations of two independent variables of lavender oil wt% (X1) and LECIVA-S70 wt% (X2) varied in 3 levels as low (-1), medium (0), and high (+1). The concentrations of lavender oil were 2.2, 4.4, and 6.6 wt.%, respectively, for a low, medium, and high level. Similarly, the concentrations of LECIVA-S70 were 0.1, 0.5, and 1 wt.%, respectively, for the low, medium and high level. The dependent variables were represented in terms of R1, R2, and R3, respectively for Z-average, Polydispersity Index (PDI), and % entrapment efficiency (%EE). The central composite design and composition of different ingredients in the formulation were presented in Table 1 and Table 2, respectively.
.jpg)
.jpg)
X-ray Diffraction (XRD) analysis
X-ray Diffraction (XRD) analysis was carried out to detect the crystallinity of the pure drug and its possible changes in the formulation. The XRD patterns of the pure drug (SS), dummy NLCs without the drug, and optimized NLCs (SNE9) were recorded with Bruker AXS D8 Advance and Cu monochromatic radiation (λ=1.5406˚A), at 40 kV and 40 mA, as the X-ray source. The light-spot position-sensitive detector recorded the scattering intensity. The diffractograms were recorded for 2Ѳ between 20° and 75°.
Fourier-transform infrared spectroscopy
The compatibility study plays a significant role in the development of a stable dosage form. The FTIR spectrum of the pure drug (SS) alone and drug with the other excipients was analyzed by FTIR (Bruker Alpha 2000, Germany). The samples were analyzed at a range from 4000-400 cm-1 between wavenumber and % transmittance. KBr pellet technique is used in the sample preparation with a 2 mg sample in 200 mg KBr. The significant peaks of the drug were identified in the pure and mixture sample to study their interaction and, experiments were repeated for reproducibility.
Differential scanning calorimetry
Differential Scanning Calorimetry (DSC) thermograms of pure drug, drug-loaded optimized formulation (SNE9), and optimized formulation without the drug were recorded on a DSC equipment (TA Instruments DSC SDT Q600) to analyze the physical nature of the drug and excipients in the formulation. A sample (4–5 mg) was weighed in an aluminum pan and then crimped. The samples were scanned at a temperature range of 25°C –400°C at a heat flow rate of 10°C/min and continuously purged with nitrogen at a flow rate of 100 mL/min. The data were analyzed by using the Universal Analysis 2000 software package (SDT Instruments).
Preparation of sumatriptan succinate NLCs
The first oil phase was prepared by adding molten LECIVA-S70 (brownish waxy phosphatidylcholine phosphorous lipid enriched fraction of Non-GMO Soya bean lecithin) into lavender oil and stirred at 1000 rpm for 45 min by using overhead stirrer at 50˚C while intermittently drops of chloroform were added. LECIVA-S70 and lavender oil mixture formed a thick slurry of oil phase 1. On the other side, 0.6% w/v Pluronic F-68 solution was prepared by using the Milli-Q water (10 mL). Then SS (35 mg) was added slowly and the mixture was stirred at 500 rpm for 30 min at 50˚C (aqueous phase 1). The aqueous phase 1 was added dropwise into the oil phase 1, at 1000 rpm for 1 h, followed by sonication for 15 min. The entire process was maintained at 50˚C until completion, which forms the primary emulsion (W/O). In another container, 0.6% w/v Pluronic F-68 solution was stirred at 50˚C (aqueous phase 2).
The primary emulsion was added dropwise into an aqueous phase 2 containing 0.6% w/v Pluronic F-68 in Milli-Q water (10 mL) and stirred for 1 h at 1000 rpm and homogenized at 8000 rpm for 15 min. The whole product was then added to 30 mL of ice-cold water (2˚C-4˚C) and stirred for another 30 min to stabilize the emulsion (W/O/W). The final solution was centrifuged at 8000 rpm for 15 min. Then, the pellet was collected and dispersed into a few milliliters of Milli-Q water and refrigerated at -80°C and freeze-dried to form NLCs. This is the recommended approach to increase the physical stability of the NLCs and to avoid phase inversion upon storage [5, 16] (Table 3).
.jpg)
Particle size analysis and zeta potential
The mean droplet size, PDI, and zeta potential of NLCs were analyzed by Dynamic Light Scattering (DLS) technique at 25°C using a scattering angle of 90° (Zetasizer, model ZS, Malvern Instruments, UK). The mean diameter of particles was analyzed with photon correlation spectroscopy (PCS) (DelsaTM Nano Common; Beckman Coulter) at a fixed angle of 90º. The particle size analysis data were analyzed by volume distribution ratio. The zeta potential of prepared NLCs was also investigated under the same conditions. The samples were previously diluted appropriately and sonicated for 3 min, then kept at a low conductivity zeta cell to meet the instrumental requirements before the measurement.
Entrapment efficiency
The NLCs were subjected to % drug entrapment efficiency (%EE) by centrifuging it at 4696 RCF for 45 min at 4ºC. If required, the centrifugation was repeated one or two times more to separate the supernatant properly. The free drug content was analyzed by UV spectrophotometer at 228 nm. The blank for the study was obtained from NLCs prepared without a drug (dummy NLCs). The supernatant of dummy NLCs was separated by applying 4696 RCF for 45 min. The following equation calculated the percentage of drug entrapped (Formoul 1):

Transmission Electron Microscopy (TEM)
The Transmission Electron Microscopy (TEM) (Jeol/JEM-2100) studies were carried out to analyze the physical features, shape, and surface morphology of nanoparticles. TEM is contrast-based, and by which there is a possibility of detecting the entrapped drug molecules. The samples were placed in a carbon-coated copper grid. The samples were already diluted appropriately with HPLC (high-performance liquid chromatography) grade distilled water and subjected to sonication for 3 min.
In vitro drug release by membrane diffusion technique
Sumatriptan succinate release from the NLCs was studied with membrane diffusion technique [17, 18]. The dialysis membrane has a pore size of 2.4 nm. Approximately the molecular weight cut-off of 12000-14000 was used [19]. The membrane was already soaked in HPLC grade distilled water for 12 h before enabling the diffusion of molecules through the membrane, which limits the nanoparticles and allows the free drug to move to the receptor compartment. This membrane was then tied at one end of the open-ended test tube, and another side remained open through which NLCs filled the tube. It is serving as a donor compartment of the diffusion studies. The amount of NLCs was equivalent to 3 mg of drug content calculated based on %EE. This set up was then kept vertical into a beaker containing 100 mL of Simulated Nasal Fluid (SNF).
The SNF was selected as a dissolution medium since the formulation can be used as a nasal formulation after appropriate clinical studies, and SS is also available in the market in the form of a nasal spray. The SNF was prepared by dissolving accurately weighed sodium chloride (NaCl) 8.77 mg/mL, potassium chloride (KCl) 2.98 mg/mL and calcium chloride (CaCl2) 0.59 mg/mL per litre in aqueous solution. The beaker containing 100 mL SNF was served as a receptor compartment. The temperature was maintained at 37±5ºC and 50 rpm was maintained throughout the study.
An aliquot of 5 mL of samples was withdrawn at various time intervals from the receiver compartment and replenished with the fresh volume of SNF to maintain the sink condition. Based on the amount of drug released at each time interval, the cumulative amount of drug release was calculated for a complete diffusion period. The system was replenished with the new media (5 mL) every time and samples were analyzed spectrophotometrically (UV SYSTRONICS; 2201, India) at 228 nm.
Various pharmacokinetic models were used to describe the in vitro drug release kinetics of the drug release profile [20]. The cumulative amount of SS released from the SLN formulation at different time intervals were fitted with the following models: cumulative drug release vs. time (zero-order kinetic model), log cumulative of % drug remaining vs. time (first-order kinetic model), and cumulative % drug release vs. square root of time (Higuchi model). The zero-order rate describes the system in which the drug release rate is independent of its concentration. The first order describes the release from a system, where the release rate is concentration-dependent. Higuchi explained the release of drug from NLCs as a square root of time-dependent process based on Fickian diffusion, log cumulative % drug release vs. log time (Korsmeyer-Peppas model), and cube root of drug % remaining in matrix vs. time (Hixson- The Crowell cube root law).
Stability studies
The stability of the NLCs is a critical parameter in the study. In the present study, the formulations were packed in a suitable container and subjected to stability study for one month at 4ºC and 27ºC±75% RH. The samples from the container were withdrawn at 1 day, 1 week, and 1 month for the analyses of their physical features, color change, % EE, and drug release.
Data analysis
The central composite design was analyzed by response surface method with a subtype of randomized design mode of quadratic process order in Design Expert 10.0.3. The results were analyzed appropriately by ANOVA and other statistical tests.
Results
Central composite design analysis
Figure 1A-C displays the results of different factors and responses. The significant impact of the factors on responses were detailed in the respective discussion section.
.jpg)
X-ray Diffraction (XRD) Analysis
The results of the XRD pattern of pure SS and optimized best formula of SLN without the drug were reported. The diffraction pattern of SS demonstrated the significant sharp characteristic peaks at 2Ѳ value of 13.27º, 16.30º, 20.56º, 22.06º, 22.72º, 26.92º, and 31.39º (Figure 2A-B).
.jpg)
Fourier-transform infrared spectroscopy
The Fourier Transform Infrared Spectroscopy (FTIR) analysis was done on all the ingredients as well as on the developed formulations. The drug SS demonstrated S=O, -C-N stretch at 1081.55 cm-1 and 1298.42 cm-1, respectively. The –C-S stretch and the N-H stretch were also observed at the 637.52 cm-1 and 3372.64 cm-1, respectively. The 3ºNH (tertiary amine) and O-H stretch are also present and observed at 3102.61 cm-1 and 1437.59 cm-1, respectively. The various spectrums were recorded by mixing the excipients with the drug. The spectrum of drug-excipients mixture was also demonstrated the same stretch as the pure drug spectrum itself. The SS with the lavender oil showed the S=O, C-N stretch at 1081.18 cm-1, and 1298.09 cm-1, respectively. The C-S and N-H stretch were also observed at 637.61 cm-1 and 3372.35 cm-1, respectively. Slightly different 3ºNH and O-H stretches were observed at the 3102.30 cm-1 and 1428.40 cm-1, respectively. Similarly, SS in combination with LECIVA-S70 demonstrated S=O stretch at 1081.55 cm-1, C-N stretch at 1299.09 cm-1, and C-S stretch at 637.30 cm-1 the same as those of SS. A slightly different N-H stretch was found at 3377.09 cm-1 which is negligible. The 3ºNH stretch was observed at 3101.97 cm-1 intact as that of SS. Similarly, O-H stretch was also seen at 1438.19 cm-1 (Figure 3).
.jpg)
Differential scanning calorimetry analysis
The DSC thermogram of SS pure drug demonstrated a sharp exothermic peak at 172.2ºC, which corresponds to the melting point of the drug. The physical mixture of SS and LECIVA-S70 was analyzed by DSC in which SS peak was slightly reduced to 168.3ºC due to the presence of LECIVA-S70 in the physical mixture. LECIVA-S70 did not show any peak due to its amorphous nature. The physical mixture of SS with all the ingredients (Lavender oil, LECIVA-S70, and Pluronic F-68) showed two peaks, one at 168.1ºC corresponding to SS and another at 57.1ºC corresponding to the melting point of Pluronic F-68. No peaks appeared for the lavender oil and LECIVA-S70 as those are not present in crystalline form (Figure 4).
.jpg)
Particle size analysis
The particle size analysis of all formulations was done with Zetasizer. The Mean±SD diameter of SNE1 and SNE2 was found to be 411.4±311.1 and 299.0±165.6, with the PDI of 0.298 and 0.207, respectively. The SNE3 and SNE6 formulations demonstrated that the Mean±SD diameter of 298.5±106.0 and 177.0 ±86.0 with the PDI of 0.227 and 0.183, respectively. The Mean±SD diameter of formulations SNE8 and SNE9 were found to be 204.9±100.6 and 398.8±242.6 with the PDI of 0.187 and 0.216, respectively. The PDI of the mentioned formulations ranged between 0.183 and 0.298 which was found to be satisfactory. The PDI of all formulations ranged between 0.245 and 0.560. The formulation SNE9 demonstrated the Z-average, PDI, %EE of 19.67, 0.216, and 82.8±0.7, respectively. The other formulations with better Z-average were SNE2 and SNE3, which recorded the values of 0.245 and 0.275, respectively (Table 4, Figure 5 A-C).
.jpg)
.jpg)
Zeta potential
The NLCs of SS were subjected to the study by using the instrument Delsa Nano CTM Beckman Coulter. All the formulations were reported with a negative charge. The values of the zeta potential were -9.01,-8.23,-17.18, respectively for SNE2, SNE3, and SNE9 (Table 5, Figure 6 A-C).
.jpg)
.jpg)
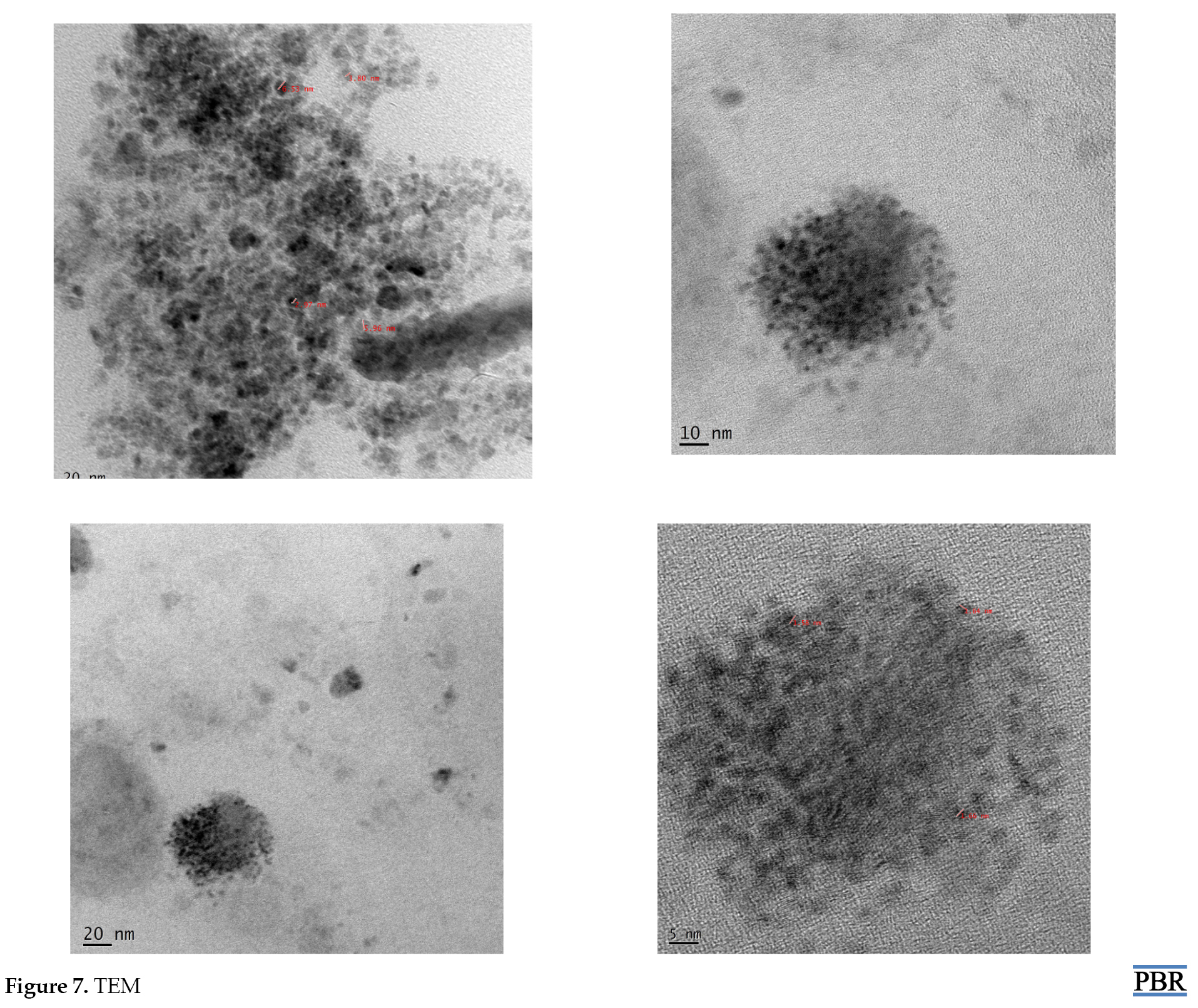
Transmission Electron Microscopy (TEM)
The TEM images of optimized SLN demonstrated that the particle was nearly spherical. The particle size values reported by the TEM imaging technique were about 1.5, 1.65, 2.97, 3.80, 5.96, and 6.53 nm (Figure 7 A-D).
In vitro study
The in vitro release study of NLC was performed using membrane diffusion technique. The SNF was used as diffusion media as the preparations can be used as a nasal formulation. The best formulation (SNE9) released only 51.4% of the drug 8 h after the diffusion study, which confirmed the controlled release pattern. It took 18 h to release 90% of the entrapped drug. Almost all the formulations released 25% of the drug in the first 6 h and less than 50% of the drug until 8 h of the diffusion study (Figure 8A-B).

Discussion
SS is widely used in the treatment of migraine and available in the form of tablet and spray. The aroma and medicinal property of lavender oil may offer a beneficial effect in the treatment of migraine, especially when it is combined with an antimigraine drug. The formulations were prepared according to the central composite design generated by Design Expert 10.0.3. The impact of two formulation factors, i.e. the amount of lavender oil (X1) and the amount of LECIVA-S70 (X2) on the 3 response variables of Z-average (R1), PDI (R2), and %EE (R3) were analyzed in the study. The significance of the designed model was determined by F value (Supplementary file 4, Table 1A-C).
The study results demonstrate that the Z-average and %EE were not significant in the design at the significance level of 0.05; however, PDI results were significant. The formulations characteristics were influenced by the selected concentration level of the lavender oil and LECIVA-S70 ingredients. Danaei et al. described that PDI and size play an important role in deciding the systemic use of nanoparticles in clinical settings [21]. Therefore, the method developed for the synthesis of nanoparticles should produce the nanoparticles with reproducible PDI. Thus the developed design is significant with the PDI and will help develop the lipid carrier of reproducible PDI for the therapeutic applications.
All the prepared NLCs were subjected to particle size analysis by Zetasizer. The size distribution was analyzed by taking size (d.nm) in the X-axis and % intensity in Y-axis. The mean particle size of all formulations was between 19.67 nm to 179.7 nm. The entrapment efficiency of all formulations varied between 60.1% and 65.5%. The best formulation has been selected based on the three critical parameters of smaller size, lower PDI, and higher %EE of the drug.
The formulations which demonstrated extreme deviations from these critical parameters were already eliminated from further studies such as TEM and in vitro drug release profile. Nine formulations were selected from the various trials, and the elimination criteria were set with the following parameters: formulations with %EE less than 60%, high Z-average, and high PDI. The formulation which falls in these categories has been eliminated from further studies. The formulation which fails to entrap more than 60% drug was not considered as the best formulation, and upon storage, they may deteriorate further due to the diffusion of the drug from the ruptured emulsion layers of NLCs. Therefore, they were eliminated from further studies.
The formulations with high Z-average tend to settle quicker than the smaller droplet, and this is reinforced by higher PDI. The formulations with high PDI demonstrate a high variation in the droplet size, which encourages an Ostwald ripening of smaller particles that further coalesces with the already available larger particles and weakens stability and phase separation. So, the formulations with higher PDI in the group were eliminated. The formulations which fall closer to these three parameters have also been eliminated. Amongst the 9 remaining formulations, the formulations (CNP4, CNP5, CNP7) which demonstrated the wide distribution range based on the size distribution pattern seen in the Malvern Zetasizer graph plotted for % intensity vs. size (nm.) were eliminated from this group (Supplementary file 1).
The formulations SNE1, SNE2, SNE3, SNE 6, SNE 8, and SNE 9 were subjected to further evaluation in Zetasizer Delsa NanoTM Common (Supplementary file 2) for the determination of distribution width (D10, D50, D90) and distribution diameter. Based on this report, three formulations of SNE2, SNE3, and SNE9 were picked for zeta potential determination (Table 5). Of these three formulations, SNE9 demonstrated the highest zeta potential; thus, it was declared as the best formulation of the group. However, SNE9 was selected as the best formulation based on the overall comparison.
The particle size was very narrow in all formulations. The best formula, SNE9, demonstrated the Z-average of 19.67±0.43, which is the lowest among the groups, and the PDI of SNE9 was 0.281±0.03. As shown in the surface response graph, the higher level of lavender oil and LECIVA-S70 concentration influences the lower range of globules in the emulsion, whereas PDI significantly increased by a higher concentration of LECIVA-S70 and lavender oil. Thus, the concentration of these two ingredients should be in the range between lower to medium level (-1,1) that corresponds to 2.2-4.4 for lavender oil and 0.1-0.5 for LEVICA-S70. This range helps fabricate the NLCs with smaller particle size and increase their stability. The higher concentration of LECIVA-S70 and lavender oil significantly influenced the higher encapsulation capacity as the layers of the globules were strong enough to accommodate more medication. On the other hand, the lower concentration of both ingredients makes %EE to fall below 60%.
The x-ray diffraction results exhibited the crystalline nature of the drug. The best formulation, SNE9, confirmed the presence of similar significant peaks in the diffractogram as the pure drug (SS) with less intensity. The intensity of the significant peaks was less in the formulation due to the conversion of the drug into the amorphous form which is confirmed by the diffused pattern of the diffractogram. The drug excipient compatibility was analyzed with FTIR. The spectrum of pure drug and its mixture with used excipients were recorded. The spectrum was analyzed for the significant peaks of pure drug and then compared with the spectrum of drug mixture with excipients. The overlay spectrum of pure medicine (SS) and the mixture was developed to confirm the absence of interaction between the drug and excipients in terms of chemical change.
The results demonstrated that the key ingredients, such as lavender oil and LECIVA-S70, have no significant interaction with the SS. The overall drug excipients compatibility study showed no interaction of drug and used excipients in terms of chemical change. Based on the results of the FTIR, the excipients were proceeded by the formulation process. The final best formulation of NLCs, i.e. SNE9, was analyzed by DSC and came out with a single exothermic peak at 116.4ºC, in which the onset of melting starts below 85ºC. The melting point reduction of SS from 172.2ºC (pure form) to 116.4ºC revealed that the entrapped crystalline SS had been partially converted into amorphous form within the NLCs.
All the NLCs of SS were subjected to particle size analysis by Malvern Zetasizer. Table 2 presents the results of Z-average, PDI, and % EE. The particle size distribution was analyzed by taking size (d.nm) in X-axis and % intensity in Y-axis. The results demonstrated that all the particles were within the nanorange with the low to moderate PDI. The SNE1, SNE2, SNE3, SNE6, SNE8, and SNE9 formulations were found to be the better and further subjected to Delsa Nano CTM Zetasizer analysis to obtain more detailed information.
In Delsa Nano CTM, the intensity distribution was plotted by taking diameter (nm) in X-axis, differential intensity % in Y-axis, and cumulative intensity (%) in Z-axis. The best formulation was SNE9 which demonstrated a bell-shaped curve. This formulation contained the highest concentration of lavender oil (6.6 wt.%) and LECIVA-S70 (1 wt.%). The remaining formulations have also demonstrated the nanorange size but, SNE9 demonstrated the highest zeta potential amongst the group; thus, it was selected as the best formulation.
The zeta potential of SNE9 was -17.18 mV, and the other formulations were reported with less zeta potential as compared to SNE9. The whole system favors the negative charge, and this supports the high stability due to electrostatic repulsion of the same charge.
The highest %EE of 82.8% was reported for SNE9, which revealed that smaller particles possess higher %EE. The formulation of SNE2 and SNE3 demonstrated 82.2% and 79.4% as the second and third highest %EE in the group. The remaining formulations %EE ranged between 60.1% and 65.5%.The TEM results revealed that the particle size obtained by the TEM imaging technique was less than the hydrodynamic diameter obtained by the dynamic light scattering technique with Zetasizer. This is probably due to the dehydration of nanoparticles during the sample preparation of the NLCs for TEM analysis and can be considered as dried particle size.
The drug entrapment or loading is visible from the TEM analysis by the presence of contrast part, which is covered by thin uniform, the evenly distributed translucent film; thus, the drug loading is confirmed in the NLCs. The particle size shown in the Zetasizer was slightly higher than what was reported with TEM because the Zetasizer measures the apparent diameter of the particle, which covers the surrounding hydrodynamic layer of the particles.
The larger size of nanoparticles in the Zetasizer results was not due to the aggregation of particles because the preparation was subjected to probe sonication for 5 min, which nullifies the chances of aggregation. However, the TEM and Zetasizer analyses confirm the presence of nanoparticles in the prepared emulsion and the entire system is within the nanorange.The in vitro study results revealed that despite the less viscous nature of formulations, the drug release was due to the drug entrapment within the multi-layers of the NLCs (Figure 8A-B). Furthermore, the matrix of the nanoparticles was in a uniform spherical shape, which controlled the drug release from the core. Since the drug release pattern demonstrated the controlled release profile, the data were fitted with various pharmacokinetic models. The results of the pharmacokinetic model analysis showed the best fit with the Korsmeyer-Peppas equation (Table 6, Supplementary file 3)
.jpg)
Conclusion
SS-lavender oil hybrid NLCs were prepared successfully. The results of FTIR and DSC revealed no incompatibility between the drug and excipients. The XRD pattern of the best formulation confirms the conversion of the crystalline form of the medicine to amorphous form. The particle size and zeta potential results confirmed the size in nanorange, and the best formulation, SNE9, demonstrated the highest zeta potential of -17.18 mV, which confirms the strong stability of the NLCs. The entrapment efficiency of the formulations ranged between 60% and 82%. The in vitro drug release study revealed that the drug release was found to be consistent, controlled, and reproducible. The drug release profile of the formulation fitted with various pharmacokinetic models. The Korsmeyer-Peppas equation demonstrated the best fit.
The TEM results confirmed the particle size within the nanorange and the presence of entrapped drug within the nanoparticles. The results of central composite design demonstrated that the concentration of LECIVA-S70 and lavender oil plays an essential role in the synthesis of NLCs with reproducible PDI which is crucial for systemic and translation into clinical uses. The developed NLCs were considered as the hybrid nanoparticles which possess the soothing aroma of lavender oil and therapeutic potential of the antimigraine drug (SS) in a single formulation. The formulation demonstrates the significant potential to be used as a nasal formulation after an appropriate clinical study.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This project is partially funded by Rayat Bahra Institute of Pharmacy, Hoshiarpur, Punjab, India.
Authors' contributions
Amaldoss Maria John Newton: Developed a scientific concept and methodology, reviewed all the drafts of manuscript and supervised the project. Reeta: Carried-out all the experimentation and data analysis, wrote the first draft of the manuscript. Rashid Mehmood: Provided technical support and helped in data interpretation.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to Natco Pharmaceuticals Pvt. Ltd. Hyderabad for providing sumatriptan succinate and VAV Life Sciences, Mumbai, India, for providing LECIVA-S70 and lavender oil.
References
Nanostructured lipid carriers (NLCs) are the new generation of drug delivery systems. The matrix of NLCs is composed of lipid and oil. NLCs offer high drug loading and delivery with various drugs [1, 2]. NLCs are a versatile drug delivery system, which can be fabricated by using solid and liquid lipids, surfactant, co-surfactant, and stabilizer. NLCs offer formulation and therapeutic advantages such as improved stability, solubility, higher permeability, bioavailability, and targeting.
Besides these ingredients, cryoprotectant and charge modifiers may contribute to the synthesis of NLCs to improve the formulation characteristics. The preparation method of NLCs involves the combination of liquid and solid lipids in different ratios, along with the surfactant between the range of 1.5%-5% [3]. NLCs can be prepared by various methods such as high shear homogenization, ultrasonication, solvent diffusion, microemulsion, and double emulsification [4]. In this research, we have adopted a double emulsification method for the synthesis of NLCs. These carriers demonstrate various advantages over other lipid nanoparticles regarding the drug loading because their physical stability increases surface area and cellular uptake [5].
Migraine is a chronic disorder characterized by the paroxysmal episodes of a headache lasting for 4-72 h. Migraine attacks are 2.5 times more prevalent in women than men and usually strike the 25-44 years age group [6]. Intranasal therapy was practiced in the Indian system of medicines of Ayurveda for a long time. Currently, the nasal route is emerging as a second suitable route after the oral route [7]. The nasal drug delivery system is under intensive research due to the various advantages such as non-invasive approach, rapid absorption, quick onset of action, self-administration, convenient for long-term therapy, and prevention of drug degradation by first-pass effect [8]. However, the nasal route has few disadvantages, such as immunological reaction, nasal irritation, small absorption surface area and rapid mucociliary clearance [9]. The developed NLCs were evaluated in vitro in the Simulated Nasal Fluid (SNF) as the formulation has the scope of transforming to a nasal delivery after appropriate clinical trials.
LECIVA-S70 is a pure polyunsaturated phospholipid of a specialized grade for liposomal, dermatological preparation and specially designed for emulsion, fat infusions, nutraceutical formulations and parenteral use. It contains validated 70% phosphatidylcholine. The regular soy lecithin which is used in the manufacturing of chocolates, confectionaries, dietary supplements and pharmaceuticals have 10%-35% of phosphatidylcholine which is lower than LECIVA-S70. The phospholipids are classified as GRAS (Generally recognized as safe) by the United States FDA and demonstrating advantageous in drug delivery, facilitating absorption, increasing bioavailability, and reducing toxicity [10]. Lavender oil is reported with the properties of soothing and calming effect on the nerves. It is also used in relieving tension, depression, panic, hysteria and nervous exhaustion.
Lavender oil has been known for its medicinal properties in soothing headache and other types of pain [11, 12]. Lavender oil inclusion in this formula is an added advantage, which offers the natural approach in relieving migraines; moreover, synthesized NLCs retained the smell of lavender oil in the formulation. A tryptamine derivative, namely 3-[2-(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide or sumatriptan succinate, is a selective vascular serotonin agonist of 5-HT1 type 1. The agonist activities of 5-HT1B/1D receptors provide the relief of acute headache [13]. Sumatriptan succinate is an agonist against this particular receptor subtypes and no effect in other receptors [14]. Sumatriptan succinate is used via oral, subcutaneous, or intranasal route for the management of acute migraine with aura or without aura described as a classical and common migraine, respectively [15]. Sumatriptan succinate is available in the oral doses of 25 mg, 50 mg, and 100 mg. The maximum dose is 200 mg per 24 h. The subcutaneous injections are available at a dose of 6 mg/d. Also, another 12 mg dose would be required if migraine was reoccurred. The nasal spray of SS is available in the market, which is gaining popularity among the patients.
In this research work, the central composite design was used to fabricate SS-NLCs by using natural phospholipid of plant origin (LECIVA-S70) and migraine natural medicine lavender oil with the aid of surfactant and co-surfactant. The central composite design was chosen to study the effect of key ingredients in different concentrations in the formulation development. The central composite design approach minimizes the number of experimental trials to optimize the proportion of ingredients to develop the best formulation. The Fourier Transform Infrared Spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC) confirmed the physicochemical compatibility of drug and excipients. The X-ray Diffraction (XRD) and Transmission Electron Microscopy (TEM) analysis were done for the characterization. The hydrodynamic diameter and zeta potential were analyzed with Zetasizer. The in vitro drug release study was done with membrane diffusion technique.
Materials and Methods
Study materials
Natco Pharmaceuticals Pvt. Ltd. Hyderabad kindly presented the pure drug Sumatriptan Succinate (SS). LECIVA-S70 was received from VAV Life Sciences, Mumbai, India. Lavender oil and chloroform were obtained from the CDH chemicals, India.
Experimental design
A central composite design was developed to study the impact of lavender oil and LECIVA-S70 in sumatriptan succinate NLCs. The concentrations of two independent variables of lavender oil wt% (X1) and LECIVA-S70 wt% (X2) varied in 3 levels as low (-1), medium (0), and high (+1). The concentrations of lavender oil were 2.2, 4.4, and 6.6 wt.%, respectively, for a low, medium, and high level. Similarly, the concentrations of LECIVA-S70 were 0.1, 0.5, and 1 wt.%, respectively, for the low, medium and high level. The dependent variables were represented in terms of R1, R2, and R3, respectively for Z-average, Polydispersity Index (PDI), and % entrapment efficiency (%EE). The central composite design and composition of different ingredients in the formulation were presented in Table 1 and Table 2, respectively.
.jpg)
.jpg)
X-ray Diffraction (XRD) analysis
X-ray Diffraction (XRD) analysis was carried out to detect the crystallinity of the pure drug and its possible changes in the formulation. The XRD patterns of the pure drug (SS), dummy NLCs without the drug, and optimized NLCs (SNE9) were recorded with Bruker AXS D8 Advance and Cu monochromatic radiation (λ=1.5406˚A), at 40 kV and 40 mA, as the X-ray source. The light-spot position-sensitive detector recorded the scattering intensity. The diffractograms were recorded for 2Ѳ between 20° and 75°.
Fourier-transform infrared spectroscopy
The compatibility study plays a significant role in the development of a stable dosage form. The FTIR spectrum of the pure drug (SS) alone and drug with the other excipients was analyzed by FTIR (Bruker Alpha 2000, Germany). The samples were analyzed at a range from 4000-400 cm-1 between wavenumber and % transmittance. KBr pellet technique is used in the sample preparation with a 2 mg sample in 200 mg KBr. The significant peaks of the drug were identified in the pure and mixture sample to study their interaction and, experiments were repeated for reproducibility.
Differential scanning calorimetry
Differential Scanning Calorimetry (DSC) thermograms of pure drug, drug-loaded optimized formulation (SNE9), and optimized formulation without the drug were recorded on a DSC equipment (TA Instruments DSC SDT Q600) to analyze the physical nature of the drug and excipients in the formulation. A sample (4–5 mg) was weighed in an aluminum pan and then crimped. The samples were scanned at a temperature range of 25°C –400°C at a heat flow rate of 10°C/min and continuously purged with nitrogen at a flow rate of 100 mL/min. The data were analyzed by using the Universal Analysis 2000 software package (SDT Instruments).
Preparation of sumatriptan succinate NLCs
The first oil phase was prepared by adding molten LECIVA-S70 (brownish waxy phosphatidylcholine phosphorous lipid enriched fraction of Non-GMO Soya bean lecithin) into lavender oil and stirred at 1000 rpm for 45 min by using overhead stirrer at 50˚C while intermittently drops of chloroform were added. LECIVA-S70 and lavender oil mixture formed a thick slurry of oil phase 1. On the other side, 0.6% w/v Pluronic F-68 solution was prepared by using the Milli-Q water (10 mL). Then SS (35 mg) was added slowly and the mixture was stirred at 500 rpm for 30 min at 50˚C (aqueous phase 1). The aqueous phase 1 was added dropwise into the oil phase 1, at 1000 rpm for 1 h, followed by sonication for 15 min. The entire process was maintained at 50˚C until completion, which forms the primary emulsion (W/O). In another container, 0.6% w/v Pluronic F-68 solution was stirred at 50˚C (aqueous phase 2).
The primary emulsion was added dropwise into an aqueous phase 2 containing 0.6% w/v Pluronic F-68 in Milli-Q water (10 mL) and stirred for 1 h at 1000 rpm and homogenized at 8000 rpm for 15 min. The whole product was then added to 30 mL of ice-cold water (2˚C-4˚C) and stirred for another 30 min to stabilize the emulsion (W/O/W). The final solution was centrifuged at 8000 rpm for 15 min. Then, the pellet was collected and dispersed into a few milliliters of Milli-Q water and refrigerated at -80°C and freeze-dried to form NLCs. This is the recommended approach to increase the physical stability of the NLCs and to avoid phase inversion upon storage [5, 16] (Table 3).
.jpg)
Particle size analysis and zeta potential
The mean droplet size, PDI, and zeta potential of NLCs were analyzed by Dynamic Light Scattering (DLS) technique at 25°C using a scattering angle of 90° (Zetasizer, model ZS, Malvern Instruments, UK). The mean diameter of particles was analyzed with photon correlation spectroscopy (PCS) (DelsaTM Nano Common; Beckman Coulter) at a fixed angle of 90º. The particle size analysis data were analyzed by volume distribution ratio. The zeta potential of prepared NLCs was also investigated under the same conditions. The samples were previously diluted appropriately and sonicated for 3 min, then kept at a low conductivity zeta cell to meet the instrumental requirements before the measurement.
Entrapment efficiency
The NLCs were subjected to % drug entrapment efficiency (%EE) by centrifuging it at 4696 RCF for 45 min at 4ºC. If required, the centrifugation was repeated one or two times more to separate the supernatant properly. The free drug content was analyzed by UV spectrophotometer at 228 nm. The blank for the study was obtained from NLCs prepared without a drug (dummy NLCs). The supernatant of dummy NLCs was separated by applying 4696 RCF for 45 min. The following equation calculated the percentage of drug entrapped (Formoul 1):

Transmission Electron Microscopy (TEM)
The Transmission Electron Microscopy (TEM) (Jeol/JEM-2100) studies were carried out to analyze the physical features, shape, and surface morphology of nanoparticles. TEM is contrast-based, and by which there is a possibility of detecting the entrapped drug molecules. The samples were placed in a carbon-coated copper grid. The samples were already diluted appropriately with HPLC (high-performance liquid chromatography) grade distilled water and subjected to sonication for 3 min.
In vitro drug release by membrane diffusion technique
Sumatriptan succinate release from the NLCs was studied with membrane diffusion technique [17, 18]. The dialysis membrane has a pore size of 2.4 nm. Approximately the molecular weight cut-off of 12000-14000 was used [19]. The membrane was already soaked in HPLC grade distilled water for 12 h before enabling the diffusion of molecules through the membrane, which limits the nanoparticles and allows the free drug to move to the receptor compartment. This membrane was then tied at one end of the open-ended test tube, and another side remained open through which NLCs filled the tube. It is serving as a donor compartment of the diffusion studies. The amount of NLCs was equivalent to 3 mg of drug content calculated based on %EE. This set up was then kept vertical into a beaker containing 100 mL of Simulated Nasal Fluid (SNF).
The SNF was selected as a dissolution medium since the formulation can be used as a nasal formulation after appropriate clinical studies, and SS is also available in the market in the form of a nasal spray. The SNF was prepared by dissolving accurately weighed sodium chloride (NaCl) 8.77 mg/mL, potassium chloride (KCl) 2.98 mg/mL and calcium chloride (CaCl2) 0.59 mg/mL per litre in aqueous solution. The beaker containing 100 mL SNF was served as a receptor compartment. The temperature was maintained at 37±5ºC and 50 rpm was maintained throughout the study.
An aliquot of 5 mL of samples was withdrawn at various time intervals from the receiver compartment and replenished with the fresh volume of SNF to maintain the sink condition. Based on the amount of drug released at each time interval, the cumulative amount of drug release was calculated for a complete diffusion period. The system was replenished with the new media (5 mL) every time and samples were analyzed spectrophotometrically (UV SYSTRONICS; 2201, India) at 228 nm.
Various pharmacokinetic models were used to describe the in vitro drug release kinetics of the drug release profile [20]. The cumulative amount of SS released from the SLN formulation at different time intervals were fitted with the following models: cumulative drug release vs. time (zero-order kinetic model), log cumulative of % drug remaining vs. time (first-order kinetic model), and cumulative % drug release vs. square root of time (Higuchi model). The zero-order rate describes the system in which the drug release rate is independent of its concentration. The first order describes the release from a system, where the release rate is concentration-dependent. Higuchi explained the release of drug from NLCs as a square root of time-dependent process based on Fickian diffusion, log cumulative % drug release vs. log time (Korsmeyer-Peppas model), and cube root of drug % remaining in matrix vs. time (Hixson- The Crowell cube root law).
Stability studies
The stability of the NLCs is a critical parameter in the study. In the present study, the formulations were packed in a suitable container and subjected to stability study for one month at 4ºC and 27ºC±75% RH. The samples from the container were withdrawn at 1 day, 1 week, and 1 month for the analyses of their physical features, color change, % EE, and drug release.
Data analysis
The central composite design was analyzed by response surface method with a subtype of randomized design mode of quadratic process order in Design Expert 10.0.3. The results were analyzed appropriately by ANOVA and other statistical tests.
Results
Central composite design analysis
Figure 1A-C displays the results of different factors and responses. The significant impact of the factors on responses were detailed in the respective discussion section.
.jpg)
X-ray Diffraction (XRD) Analysis
The results of the XRD pattern of pure SS and optimized best formula of SLN without the drug were reported. The diffraction pattern of SS demonstrated the significant sharp characteristic peaks at 2Ѳ value of 13.27º, 16.30º, 20.56º, 22.06º, 22.72º, 26.92º, and 31.39º (Figure 2A-B).
.jpg)
Fourier-transform infrared spectroscopy
The Fourier Transform Infrared Spectroscopy (FTIR) analysis was done on all the ingredients as well as on the developed formulations. The drug SS demonstrated S=O, -C-N stretch at 1081.55 cm-1 and 1298.42 cm-1, respectively. The –C-S stretch and the N-H stretch were also observed at the 637.52 cm-1 and 3372.64 cm-1, respectively. The 3ºNH (tertiary amine) and O-H stretch are also present and observed at 3102.61 cm-1 and 1437.59 cm-1, respectively. The various spectrums were recorded by mixing the excipients with the drug. The spectrum of drug-excipients mixture was also demonstrated the same stretch as the pure drug spectrum itself. The SS with the lavender oil showed the S=O, C-N stretch at 1081.18 cm-1, and 1298.09 cm-1, respectively. The C-S and N-H stretch were also observed at 637.61 cm-1 and 3372.35 cm-1, respectively. Slightly different 3ºNH and O-H stretches were observed at the 3102.30 cm-1 and 1428.40 cm-1, respectively. Similarly, SS in combination with LECIVA-S70 demonstrated S=O stretch at 1081.55 cm-1, C-N stretch at 1299.09 cm-1, and C-S stretch at 637.30 cm-1 the same as those of SS. A slightly different N-H stretch was found at 3377.09 cm-1 which is negligible. The 3ºNH stretch was observed at 3101.97 cm-1 intact as that of SS. Similarly, O-H stretch was also seen at 1438.19 cm-1 (Figure 3).
.jpg)
Differential scanning calorimetry analysis
The DSC thermogram of SS pure drug demonstrated a sharp exothermic peak at 172.2ºC, which corresponds to the melting point of the drug. The physical mixture of SS and LECIVA-S70 was analyzed by DSC in which SS peak was slightly reduced to 168.3ºC due to the presence of LECIVA-S70 in the physical mixture. LECIVA-S70 did not show any peak due to its amorphous nature. The physical mixture of SS with all the ingredients (Lavender oil, LECIVA-S70, and Pluronic F-68) showed two peaks, one at 168.1ºC corresponding to SS and another at 57.1ºC corresponding to the melting point of Pluronic F-68. No peaks appeared for the lavender oil and LECIVA-S70 as those are not present in crystalline form (Figure 4).
.jpg)
Particle size analysis
The particle size analysis of all formulations was done with Zetasizer. The Mean±SD diameter of SNE1 and SNE2 was found to be 411.4±311.1 and 299.0±165.6, with the PDI of 0.298 and 0.207, respectively. The SNE3 and SNE6 formulations demonstrated that the Mean±SD diameter of 298.5±106.0 and 177.0 ±86.0 with the PDI of 0.227 and 0.183, respectively. The Mean±SD diameter of formulations SNE8 and SNE9 were found to be 204.9±100.6 and 398.8±242.6 with the PDI of 0.187 and 0.216, respectively. The PDI of the mentioned formulations ranged between 0.183 and 0.298 which was found to be satisfactory. The PDI of all formulations ranged between 0.245 and 0.560. The formulation SNE9 demonstrated the Z-average, PDI, %EE of 19.67, 0.216, and 82.8±0.7, respectively. The other formulations with better Z-average were SNE2 and SNE3, which recorded the values of 0.245 and 0.275, respectively (Table 4, Figure 5 A-C).
.jpg)
.jpg)
Zeta potential
The NLCs of SS were subjected to the study by using the instrument Delsa Nano CTM Beckman Coulter. All the formulations were reported with a negative charge. The values of the zeta potential were -9.01,-8.23,-17.18, respectively for SNE2, SNE3, and SNE9 (Table 5, Figure 6 A-C).
.jpg)
.jpg)
Transmission Electron Microscopy (TEM)
The TEM images of optimized SLN demonstrated that the particle was nearly spherical. The particle size values reported by the TEM imaging technique were about 1.5, 1.65, 2.97, 3.80, 5.96, and 6.53 nm (Figure 7 A-D).

In vitro study
The in vitro release study of NLC was performed using membrane diffusion technique. The SNF was used as diffusion media as the preparations can be used as a nasal formulation. The best formulation (SNE9) released only 51.4% of the drug 8 h after the diffusion study, which confirmed the controlled release pattern. It took 18 h to release 90% of the entrapped drug. Almost all the formulations released 25% of the drug in the first 6 h and less than 50% of the drug until 8 h of the diffusion study (Figure 8A-B).

Discussion
SS is widely used in the treatment of migraine and available in the form of tablet and spray. The aroma and medicinal property of lavender oil may offer a beneficial effect in the treatment of migraine, especially when it is combined with an antimigraine drug. The formulations were prepared according to the central composite design generated by Design Expert 10.0.3. The impact of two formulation factors, i.e. the amount of lavender oil (X1) and the amount of LECIVA-S70 (X2) on the 3 response variables of Z-average (R1), PDI (R2), and %EE (R3) were analyzed in the study. The significance of the designed model was determined by F value (Supplementary file 4, Table 1A-C).
The study results demonstrate that the Z-average and %EE were not significant in the design at the significance level of 0.05; however, PDI results were significant. The formulations characteristics were influenced by the selected concentration level of the lavender oil and LECIVA-S70 ingredients. Danaei et al. described that PDI and size play an important role in deciding the systemic use of nanoparticles in clinical settings [21]. Therefore, the method developed for the synthesis of nanoparticles should produce the nanoparticles with reproducible PDI. Thus the developed design is significant with the PDI and will help develop the lipid carrier of reproducible PDI for the therapeutic applications.
All the prepared NLCs were subjected to particle size analysis by Zetasizer. The size distribution was analyzed by taking size (d.nm) in the X-axis and % intensity in Y-axis. The mean particle size of all formulations was between 19.67 nm to 179.7 nm. The entrapment efficiency of all formulations varied between 60.1% and 65.5%. The best formulation has been selected based on the three critical parameters of smaller size, lower PDI, and higher %EE of the drug.
The formulations which demonstrated extreme deviations from these critical parameters were already eliminated from further studies such as TEM and in vitro drug release profile. Nine formulations were selected from the various trials, and the elimination criteria were set with the following parameters: formulations with %EE less than 60%, high Z-average, and high PDI. The formulation which falls in these categories has been eliminated from further studies. The formulation which fails to entrap more than 60% drug was not considered as the best formulation, and upon storage, they may deteriorate further due to the diffusion of the drug from the ruptured emulsion layers of NLCs. Therefore, they were eliminated from further studies.
The formulations with high Z-average tend to settle quicker than the smaller droplet, and this is reinforced by higher PDI. The formulations with high PDI demonstrate a high variation in the droplet size, which encourages an Ostwald ripening of smaller particles that further coalesces with the already available larger particles and weakens stability and phase separation. So, the formulations with higher PDI in the group were eliminated. The formulations which fall closer to these three parameters have also been eliminated. Amongst the 9 remaining formulations, the formulations (CNP4, CNP5, CNP7) which demonstrated the wide distribution range based on the size distribution pattern seen in the Malvern Zetasizer graph plotted for % intensity vs. size (nm.) were eliminated from this group (Supplementary file 1).
The formulations SNE1, SNE2, SNE3, SNE 6, SNE 8, and SNE 9 were subjected to further evaluation in Zetasizer Delsa NanoTM Common (Supplementary file 2) for the determination of distribution width (D10, D50, D90) and distribution diameter. Based on this report, three formulations of SNE2, SNE3, and SNE9 were picked for zeta potential determination (Table 5). Of these three formulations, SNE9 demonstrated the highest zeta potential; thus, it was declared as the best formulation of the group. However, SNE9 was selected as the best formulation based on the overall comparison.
The particle size was very narrow in all formulations. The best formula, SNE9, demonstrated the Z-average of 19.67±0.43, which is the lowest among the groups, and the PDI of SNE9 was 0.281±0.03. As shown in the surface response graph, the higher level of lavender oil and LECIVA-S70 concentration influences the lower range of globules in the emulsion, whereas PDI significantly increased by a higher concentration of LECIVA-S70 and lavender oil. Thus, the concentration of these two ingredients should be in the range between lower to medium level (-1,1) that corresponds to 2.2-4.4 for lavender oil and 0.1-0.5 for LEVICA-S70. This range helps fabricate the NLCs with smaller particle size and increase their stability. The higher concentration of LECIVA-S70 and lavender oil significantly influenced the higher encapsulation capacity as the layers of the globules were strong enough to accommodate more medication. On the other hand, the lower concentration of both ingredients makes %EE to fall below 60%.
The x-ray diffraction results exhibited the crystalline nature of the drug. The best formulation, SNE9, confirmed the presence of similar significant peaks in the diffractogram as the pure drug (SS) with less intensity. The intensity of the significant peaks was less in the formulation due to the conversion of the drug into the amorphous form which is confirmed by the diffused pattern of the diffractogram. The drug excipient compatibility was analyzed with FTIR. The spectrum of pure drug and its mixture with used excipients were recorded. The spectrum was analyzed for the significant peaks of pure drug and then compared with the spectrum of drug mixture with excipients. The overlay spectrum of pure medicine (SS) and the mixture was developed to confirm the absence of interaction between the drug and excipients in terms of chemical change.
The results demonstrated that the key ingredients, such as lavender oil and LECIVA-S70, have no significant interaction with the SS. The overall drug excipients compatibility study showed no interaction of drug and used excipients in terms of chemical change. Based on the results of the FTIR, the excipients were proceeded by the formulation process. The final best formulation of NLCs, i.e. SNE9, was analyzed by DSC and came out with a single exothermic peak at 116.4ºC, in which the onset of melting starts below 85ºC. The melting point reduction of SS from 172.2ºC (pure form) to 116.4ºC revealed that the entrapped crystalline SS had been partially converted into amorphous form within the NLCs.
All the NLCs of SS were subjected to particle size analysis by Malvern Zetasizer. Table 2 presents the results of Z-average, PDI, and % EE. The particle size distribution was analyzed by taking size (d.nm) in X-axis and % intensity in Y-axis. The results demonstrated that all the particles were within the nanorange with the low to moderate PDI. The SNE1, SNE2, SNE3, SNE6, SNE8, and SNE9 formulations were found to be the better and further subjected to Delsa Nano CTM Zetasizer analysis to obtain more detailed information.
In Delsa Nano CTM, the intensity distribution was plotted by taking diameter (nm) in X-axis, differential intensity % in Y-axis, and cumulative intensity (%) in Z-axis. The best formulation was SNE9 which demonstrated a bell-shaped curve. This formulation contained the highest concentration of lavender oil (6.6 wt.%) and LECIVA-S70 (1 wt.%). The remaining formulations have also demonstrated the nanorange size but, SNE9 demonstrated the highest zeta potential amongst the group; thus, it was selected as the best formulation.
The zeta potential of SNE9 was -17.18 mV, and the other formulations were reported with less zeta potential as compared to SNE9. The whole system favors the negative charge, and this supports the high stability due to electrostatic repulsion of the same charge.
The highest %EE of 82.8% was reported for SNE9, which revealed that smaller particles possess higher %EE. The formulation of SNE2 and SNE3 demonstrated 82.2% and 79.4% as the second and third highest %EE in the group. The remaining formulations %EE ranged between 60.1% and 65.5%.The TEM results revealed that the particle size obtained by the TEM imaging technique was less than the hydrodynamic diameter obtained by the dynamic light scattering technique with Zetasizer. This is probably due to the dehydration of nanoparticles during the sample preparation of the NLCs for TEM analysis and can be considered as dried particle size.
The drug entrapment or loading is visible from the TEM analysis by the presence of contrast part, which is covered by thin uniform, the evenly distributed translucent film; thus, the drug loading is confirmed in the NLCs. The particle size shown in the Zetasizer was slightly higher than what was reported with TEM because the Zetasizer measures the apparent diameter of the particle, which covers the surrounding hydrodynamic layer of the particles.
The larger size of nanoparticles in the Zetasizer results was not due to the aggregation of particles because the preparation was subjected to probe sonication for 5 min, which nullifies the chances of aggregation. However, the TEM and Zetasizer analyses confirm the presence of nanoparticles in the prepared emulsion and the entire system is within the nanorange.The in vitro study results revealed that despite the less viscous nature of formulations, the drug release was due to the drug entrapment within the multi-layers of the NLCs (Figure 8A-B). Furthermore, the matrix of the nanoparticles was in a uniform spherical shape, which controlled the drug release from the core. Since the drug release pattern demonstrated the controlled release profile, the data were fitted with various pharmacokinetic models. The results of the pharmacokinetic model analysis showed the best fit with the Korsmeyer-Peppas equation (Table 6, Supplementary file 3)
.jpg)
Conclusion
SS-lavender oil hybrid NLCs were prepared successfully. The results of FTIR and DSC revealed no incompatibility between the drug and excipients. The XRD pattern of the best formulation confirms the conversion of the crystalline form of the medicine to amorphous form. The particle size and zeta potential results confirmed the size in nanorange, and the best formulation, SNE9, demonstrated the highest zeta potential of -17.18 mV, which confirms the strong stability of the NLCs. The entrapment efficiency of the formulations ranged between 60% and 82%. The in vitro drug release study revealed that the drug release was found to be consistent, controlled, and reproducible. The drug release profile of the formulation fitted with various pharmacokinetic models. The Korsmeyer-Peppas equation demonstrated the best fit.
The TEM results confirmed the particle size within the nanorange and the presence of entrapped drug within the nanoparticles. The results of central composite design demonstrated that the concentration of LECIVA-S70 and lavender oil plays an essential role in the synthesis of NLCs with reproducible PDI which is crucial for systemic and translation into clinical uses. The developed NLCs were considered as the hybrid nanoparticles which possess the soothing aroma of lavender oil and therapeutic potential of the antimigraine drug (SS) in a single formulation. The formulation demonstrates the significant potential to be used as a nasal formulation after an appropriate clinical study.
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This project is partially funded by Rayat Bahra Institute of Pharmacy, Hoshiarpur, Punjab, India.
Authors' contributions
Amaldoss Maria John Newton: Developed a scientific concept and methodology, reviewed all the drafts of manuscript and supervised the project. Reeta: Carried-out all the experimentation and data analysis, wrote the first draft of the manuscript. Rashid Mehmood: Provided technical support and helped in data interpretation.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to Natco Pharmaceuticals Pvt. Ltd. Hyderabad for providing sumatriptan succinate and VAV Life Sciences, Mumbai, India, for providing LECIVA-S70 and lavender oil.
References
- Fang G, Tang B, Chao Y, Xu H, Gou J, Zhang Y, et al. Cysteine-functionalized nanostructured lipid carriers for oral delivery of docetaxel: A permeability and pharmacokinetic study. Mol Pharm. 2015; 12(7):2384-95. [DOI:10.1021/acs.molpharmaceut.5b00081] [PMID]
- Mohamad NE, Abu N, Rahman HS, Ky H, Ho WY, Lim KL, et al. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of Zerumbone in 4T1 challenged mice. RSC Adv. 2015; 5(28):22066-74. [DOI:10.1039/C5RA00144G]
- Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009; 366(1):170-84. [DOI:10.1016/j.ijpharm.2008.10.003] [PMID]
- Li Q, Cai T, Huang Y, Xia X, Cole SPC, Cai Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials (Basel). 2017; 7(6):E122. [DOI:10.3390/nano7060122] [PMID] [PMCID]
- Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine. 2016; 12(1):143-61. [DOI:10.1016/j.nano.2015.09.004] [PMID]
- Saunders EFH, Nazir R, Kamali M, Ryan KA, Evans S, Langenecker S, et al. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry. 2014; 75(5):512-9. [DOI:10.4088/JCP.13m08623] [PMID] [PMCID]
- Djupesland PG. Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013; 3(1):42-62. [DOI:10.1007/s13346-012-0108-9] [PMID] [PMCID]
- Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013; 4(11):1443-67. [DOI:10.4155/tde.13.104] [PMID] [PMCID]
- Touitou E, Illum L. Nasal drug delivery. Drug Deliv Transl Res. 2013; 3(1):1-3. [DOI:10.1007/s13346-012-0111-1] [PMID]
- van Hoogevest P, Wendel A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol. 2014; 116(9):1088-107. [DOI:10.1002/ejlt.201400219] [PMID] [PMCID]
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013; 2013:681304. [DOI:10.1155/2013/681304] [PMID] [PMCID]
- Sasannejad P, Saeedi M, Shoeibi A, Gorji A, Abbasi M, Foroughipour M. Lavender essential oil in the treatment of migraine headache: A placebo-controlled clinical trial. Euro Neurol. 2012; 67(5):288-91. [DOI:10.1159/000335249] [PMID]
- Paczkowska M, Mizera M, Sałat K, Furgała A, Popik P, Knapik-Kowalczuk J, et al. Enhanced pharmacological efficacy of sumatriptan due to modification of its physicochemical properties by inclusion in selected cyclodextrins. Sci Rep. 2018; 8(1):16184. [DOI:10.1038/s41598-018-34554-w] [PMID] [PMCID]
- Fullerton T, Gengo FM. Sumatriptan: A selective 5-Hydroxytryptamine receptor agonist for the acute treatment of migraine. Ann Pharmacother. 1992; 26(6):800-8. [DOI:10.1177/106002809202600611] [PMID]
- Ishkanian G, Blumenthal H, Webster CJ, Richardson MS, Ames M. Efficacy of sumatriptan tablets in migraineurs self-described or physician-diagnosed as having sinus headache: A randomized, double-blind, placebo-controlled study. Clin Ther. 2007; 29(1):99-109. [DOI:10.1016/j.clinthera.2007.01.012] [PMID]
- Varshosaz J, Eskandari S, Tabbakhian M. Freeze-drying of nanostructure lipid carriers by different carbohydrate polymers used as cryoprotectants. Carbohydr Polym. 2012; 88(4):1157-63. [DOI:10.1016/j.carbpol.2012.01.051]
- Hansraj GP, Singh SK, Kumar P. Sumatriptan succinate loaded chitosan solid lipid nanoparticles for enhanced anti-migraine potential. Int J Biol Macromol. 2015; 81:467-76. [DOI:10.1016/j.ijbiomac.2015.08.035] [PMID]
- Kamel AO, Awad GAS, Geneidi AS, Mortada ND. Preparation of intravenous stealthy acyclovir nanoparticles with increased mean residence time. AAPS Pharm Sci Tech. 2009; 10(4):1427-36. [DOI:10.1208/s12249-009-9342-y] [PMID] [PMCID]
- Thatipamula R, Palem C, Gannu R, Mudragada S, Yamsani M. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru. 2011; 19(1):23-32.
- Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J Control Release. 2004; 95(3):627-38. [DOI:10.1016/j.jconrel.2004.01.005] [PMID]
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018; 10(2):E57. [DOI:10.3390/pharmaceutics10020057] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
Nanotechnology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








