Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 81-94 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Singh S, Singh A, Singh R, Maurya A, Nishad U, Tyagi P et al . Lipid Nanocarrier Gel: Promising Novel Drug Delivery System. Pharm Biomed Res 2025; 11 (2) :81-94
URL: http://pbr.mazums.ac.ir/article-1-678-en.html
URL: http://pbr.mazums.ac.ir/article-1-678-en.html
Shivam Singh *1 

 , Arpita Singh1
, Arpita Singh1 

 , Ritunja Singh1
, Ritunja Singh1 

 , Anupama Maurya1
, Anupama Maurya1 

 , Urmila Nishad1
, Urmila Nishad1 

 , Priyanka Tyagi1
, Priyanka Tyagi1 

 , Harshit Yadav1
, Harshit Yadav1 




 , Arpita Singh1
, Arpita Singh1 

 , Ritunja Singh1
, Ritunja Singh1 

 , Anupama Maurya1
, Anupama Maurya1 

 , Urmila Nishad1
, Urmila Nishad1 

 , Priyanka Tyagi1
, Priyanka Tyagi1 

 , Harshit Yadav1
, Harshit Yadav1 


1- Department of Pharmaceutics, Seth Vishambhar Nath Institute of Pharmacy, Lucknow, India.
Keywords: Nanocarrier, Lipid, Bioavailability, Lipid Nanocarriers (LNCs), Solid lipid nanoparticles (SLNs)
Full-Text [PDF 1073 kb]
(306 Downloads)
| Abstract (HTML) (618 Views)
Full-Text: (416 Views)
Introduction
In today’s healthcare landscape, improving drug delivery requires innovative approaches that maximize therapeutic outcomes while minimizing side effects [1]. Traditional methods, such as oral tablets, injections, and topical creams deliver drugs without specialized carriers, which can lead to poor bioavailability, rapid clearance, and non-specific distribution [2-4]. For instance, oral drugs may degrade in the stomach or be cleared before reaching the target, reducing effectiveness. Lipids, commonly known as fats, are biologically important molecules involved in energy storage, membrane structure, and metabolic regulation [5, 6]. They include simple lipids (e.g. triglycerides, waxes), complex lipids (e.g. phospholipids), and related compounds, like sterols and fat-soluble vitamins. Their ability to dissolve in organic solvents while remaining insoluble in water has enabled their application in drug delivery systems [5]. Recently, lipids have been used to create nanoscale carriers that encapsulate active substances, offering protection and controlled release [7]. These lipid-based systems improve the delivery of drugs in medicine, cosmetics, and pharmacology [8, 9]. Lipid nanocarriers (LNCs) are an emerging class of drug delivery vehicles that combine lipid chemistry with nanotechnology to improve solubility, stability, and bioavailability of therapeutics [10]. Incorporating LNCs into gels results in lipid nanocarrier gels (LNC gels), which offer benefits, such as enhanced skin penetration, sustained drug release, and targeted delivery ideal for topical and transdermal use [11, 12].
Nanotechnology plays a major role across disciplines, particularly in enhancing drug delivery through precise targeting and efficient therapeutic action [13]. Nanocarriers, typically less than one micron in size, possess high surface-area-to-volume ratios and unique biodistribution profiles, making them suitable for diverse pharmaceutical applications [14, 15]. Their nanoscale properties allow for the modulation of drug kinetics, toxicity, and therapeutic index through surface and structural engineering [16]. However, despite progress, challenges, such as biological barriers, formulation complexity, and stability limit the widespread adoption of nanostructured lipid carriers (NLCs) [17-19]. NLCs are especially useful in chronic wound treatment, where sustained drug release is vital [20]. Their surface charge (zeta potential) and morphology (e.g. spheres, cones, and cylinders) significantly influence stability, targeting, and therapeutic performance [21, 22]. Nano-modification strategies are being explored to enhance drug action, especially in wound healing applications [23]. NLCs protect therapeutic agents, provide controlled release, and improve drug absorption, making them effective for both synthetic and natural compounds [24, 25]. Preclinical and clinical research has demonstrated their ability to enhance wound healing outcomes by delivering consistent drug levels and supporting tissue regeneration [26, 27].
Composition of LNCs
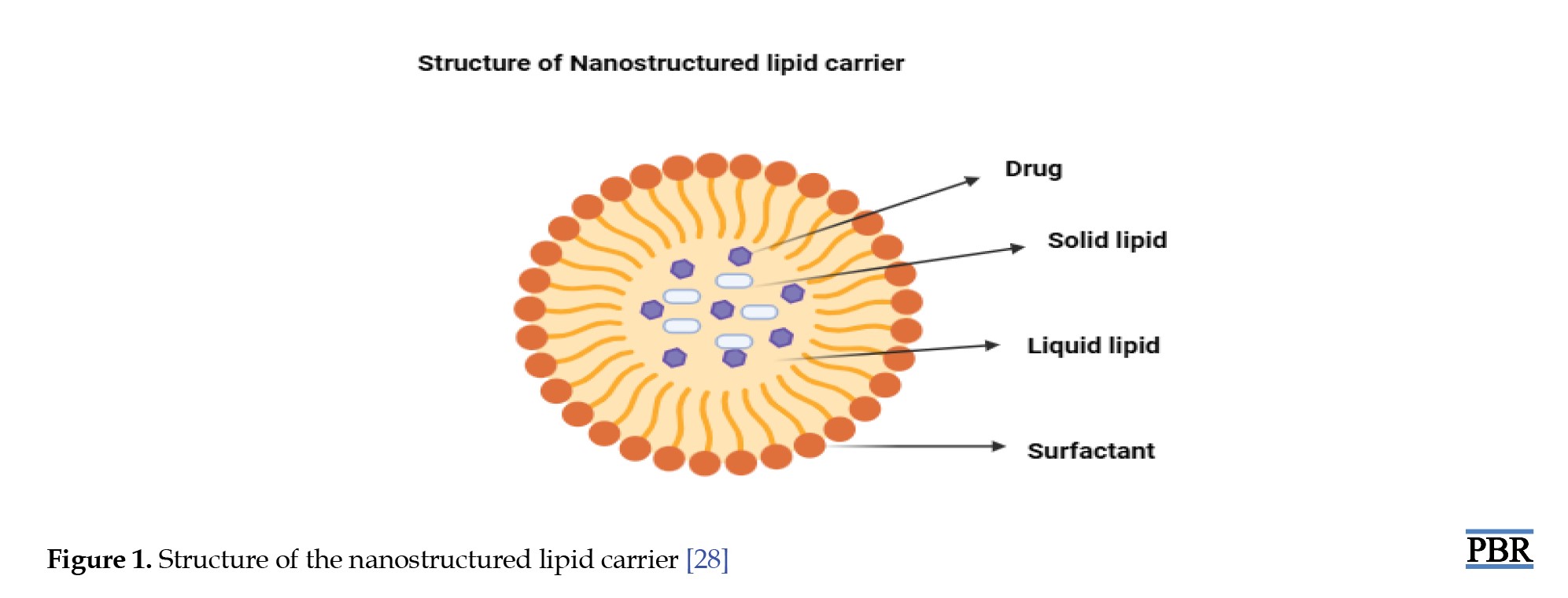
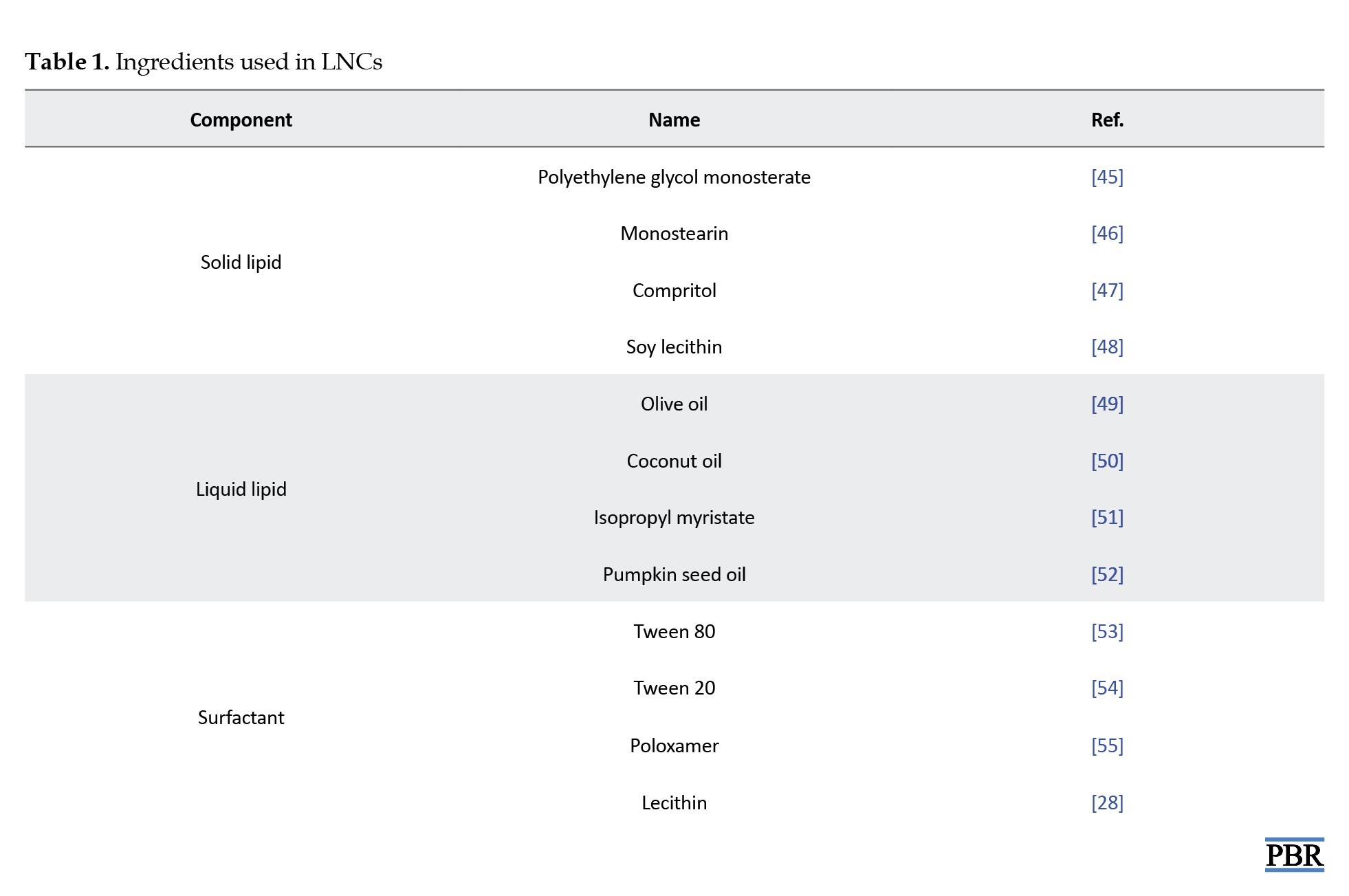
LNCs, particularly NLCs, are composed of carefully selected lipids, surfactants, and other functional ingredients that collectively determine their physicochemical properties and therapeutic efficacy (Figure 1). The selection of lipids is critical, as their type and structure significantly influence the characteristics of the resulting nanoparticles, including stability, particle size, drug loading capacity, and release behavior [29]. Two important factors to consider during lipid selection are the solubility of the drug within the lipid matrix and the drug’s partition coefficient, both of which affect encapsulation efficiency and the rate of drug release [30]. Additionally, the viscosity and melting point of the lipids play a crucial role in particle formation—lipids with higher viscosity and melting points tend to produce larger particles, impacting dispersion uniformity and system stability [31]. Surfactants are another key component in lipid nanocarrier systems. They serve multiple functions, most notably enhancing drug solubility, dissolution, and membrane permeability, which contributes to improved drug absorption and therapeutic outcomes [32]. The choice of surfactant is influenced by its hydrophilic-lipophilic balance (HLB), which affects not only emulsion formation but also particle size and the interaction between lipids and aqueous phases [33]. As emulsifiers, surfactants reduce the interfacial tension between lipid and water phases, promoting compatibility and preventing phase separation, thus enhancing the formulation’s overall stability [34].
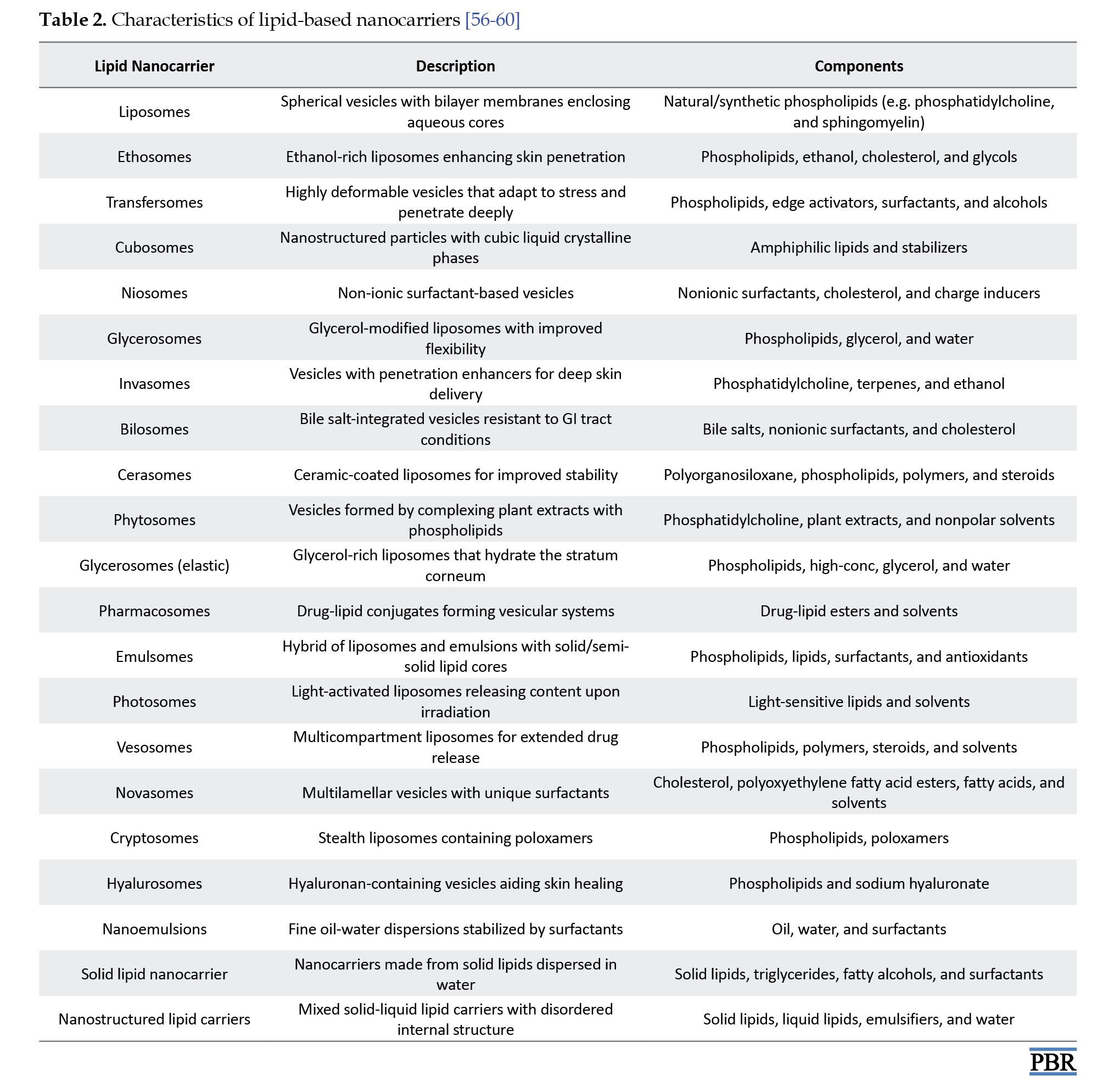
Furthermore, lipid crystallization behavior, modulated by surfactants and other factors, can influence the morphology, size, and surface characteristics of colloidal particles parameters that directly affect the nanocarrier’s drug delivery performance and physical stability under varying conditions [35]. Beyond lipids and surfactants, other ingredients are often incorporated to enhance the performance of NLCs. Surface modifiers improve the physical stability and biocompatibility of the formulation while facilitating targeted drug delivery across epithelial barriers [36]. These surface modifications also contribute to immune evasion by reducing the likelihood of phagocytic uptake by macrophages in the reticuloendothelial system (RES), thereby prolonging systemic circulation and enhancing therapeutic outcomes [37]. Because lipid-based drug delivery systems utilize biodegradable and biocompatible lipids, they are well-suited for applications requiring controlled drug release, targeted delivery, and protection of pharmaceuticals from degradation [38]. Moreover, these systems provide versatility in delivering a wide range of therapeutic agents, including growth factors, gene therapies, and cytokines offering more adaptability than traditional drug delivery methods [39]. They are also capable of targeting specific cells and tissues, enabling the delivery of complex therapeutic entities, such as nucleic acids, peptides, and proteins [40]. Lipid-based delivery systems can be broadly categorized into vesicular systems and lipid nanoparticles. Vesicular systems are formed when amphiphilic molecules self-assemble in aqueous environments into highly structured arrangements containing one or more concentric lipid bilayers [41]. Lipid nanoparticles, on the other hand, include several types, such as lipid-drug conjugates (LDCs), solid lipid nanoparticles (SLNs), and NLCs [42]. NLCs are further subclassified based on their internal structural organization into multiple oil/fat/water (O/F/W) systems, amorphous types, and highly imperfect crystalline structures. These variations influence the drug loading capacity and release behavior of the formulation, making NLCs a highly adaptable platform for advanced drug delivery applications [29] (Figure 2, Tables 1 and 2).
Preparation method of LNCs
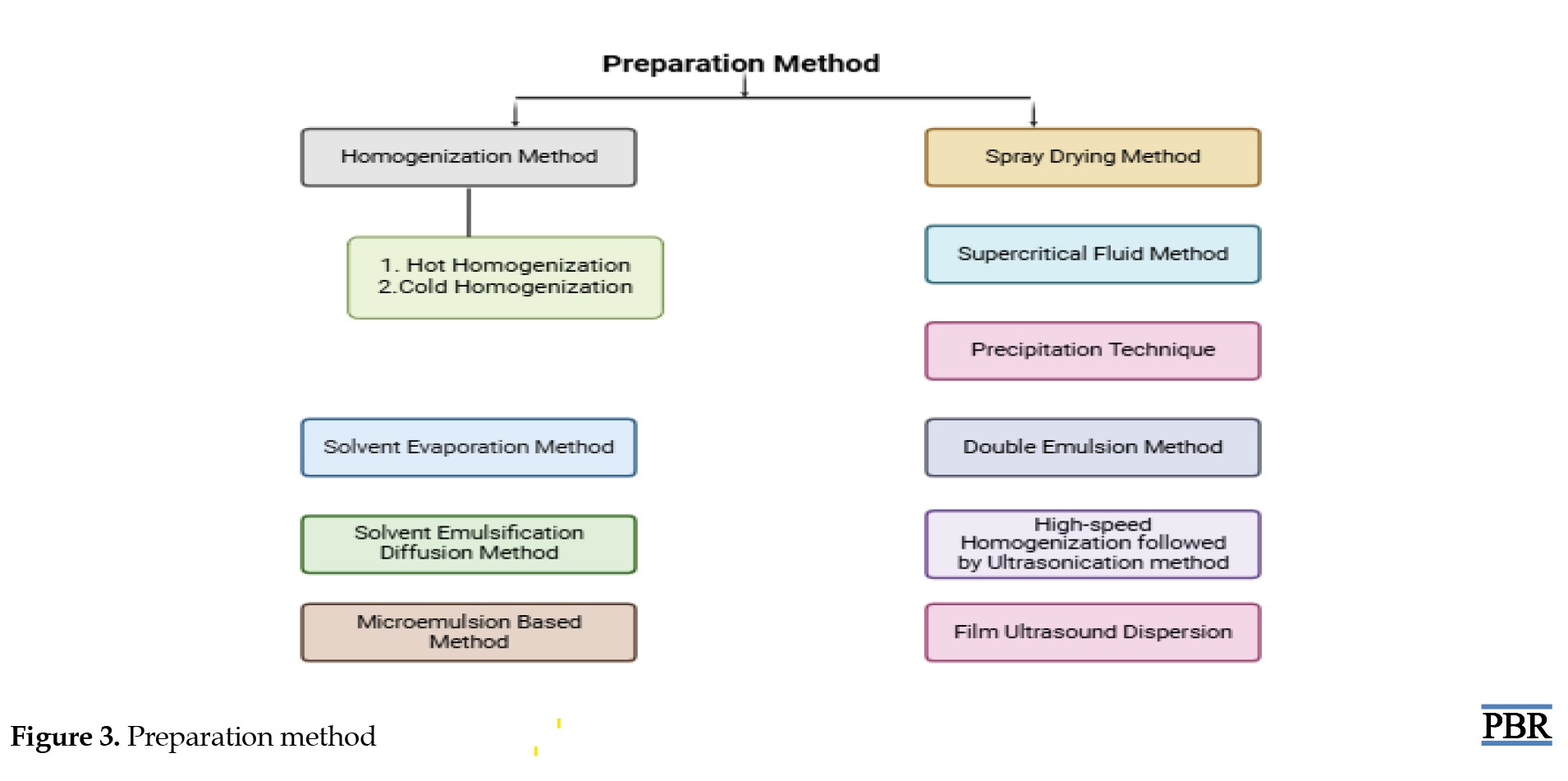
High-pressure homogenization techniques (hot and cold methods)
In the hot homogenization method, the drug is initially blended into a melted lipid mixture. At the same time, a heated surfactant solution is prepared. These two components are combined and processed using a high-pressure homogenizer, resulting in the formation of nanostructured lipid carriers. In the cold homogenization method, the drug is also first mixed with molten lipids, but this blend is rapidly cooled and then mechanically ground into fine particles. These particles are then mixed with a chilled surfactant solution and further processed under high pressure to yield the final nanocarriers.
Solvent-based techniques (evaporation and diffusion)
The solvent evaporation technique involves dissolving the drug and lipid in a water-insoluble organic solvent, which is then combined with a surfactant solution. This mixture is heated to remove the solvent through evaporation, leading to the production of lipid nanoparticles. On the other hand, in the solvent diffusion method, the drug-lipid mixture is dissolved in a water-soluble organic solvent, and then blended with a surfactant solution. This mixture is diluted with water to initiate solvent diffusion. The final nanocarrier formulation is obtained by drying the system through freeze-drying or filtration [61-64, 53] (Figure 3).
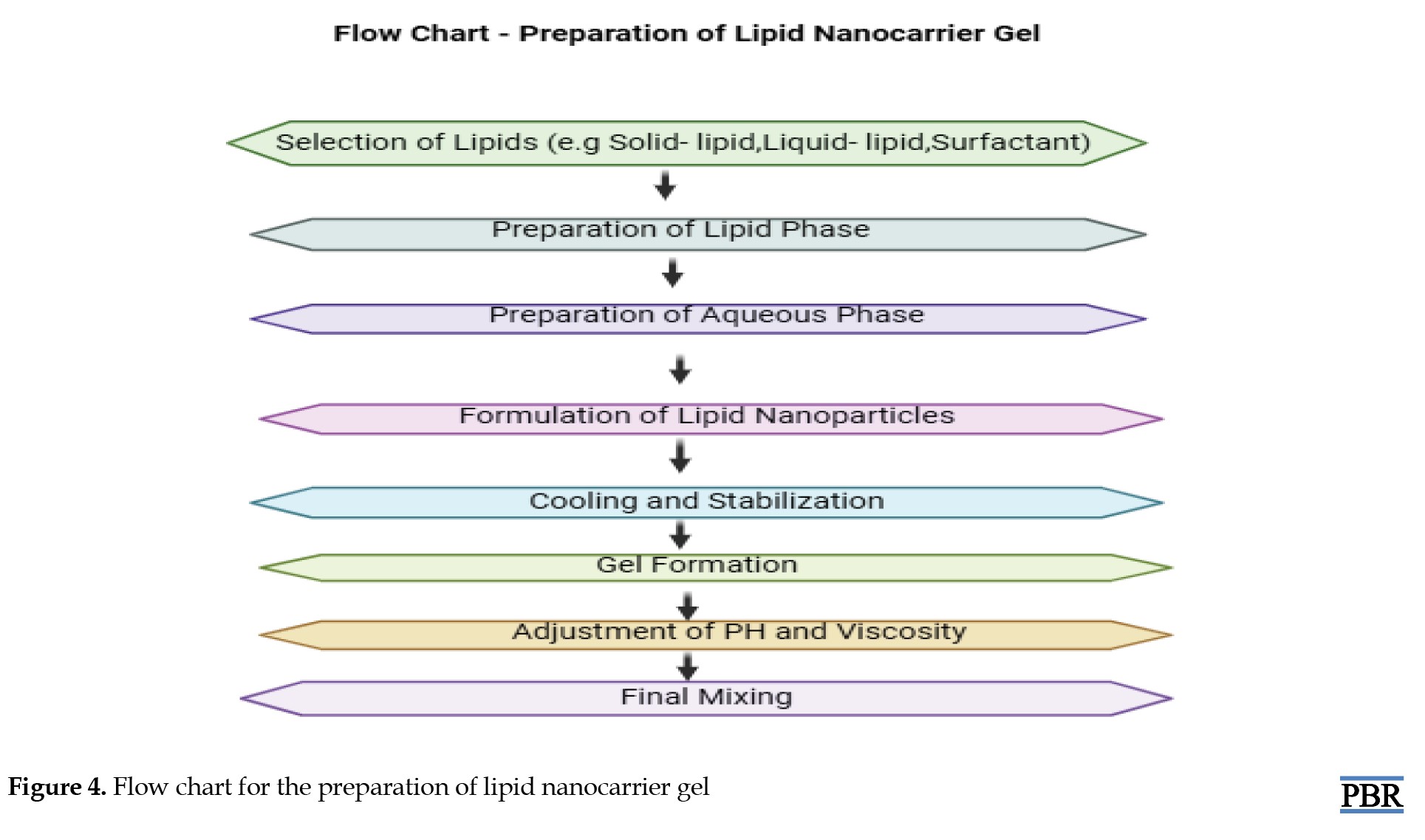
Lipid nano-carrier gel
Lipid core
Lipid molecules (micelles or liposomes) will be grouped in a spherical shape in the center. Phospholipids, which have hydrophilic heads and hydrophobic tails, would normally make up these lipids. A bilayer or monolayer structure is created with the hydrophilic heads oriented outward and the hydrophobic tails directed inward [28].
Encapsulated active ingredients
Inside the lipid core or in the surrounding gel matrix, there may be encapsulated substances, like drugs, vitamins, or other therapeutic agents. These molecules are usually hydrophobic and interact with the hydrophobic core of the lipid bilayer [65].
Gel matrix
Surrounding or embedding these lipid nanoparticles, the gel matrix could be a clear or slightly opaque network, composed of polymers such as hydroxyethyl cellulose or carbomers. This gel structure aids in controlling the release of the encapsulated materials and stabilizing the lipid carriers [66].
Nanoscale size
The lipids themselves are nanoscale in size, resembling small, compact spheres or vesicles dispersed throughout the gel matrix. This would appear as a cloudy or translucent gel when viewed under a microscope [67] (Figure 4).
Advantages of lipid nanocarrier gels
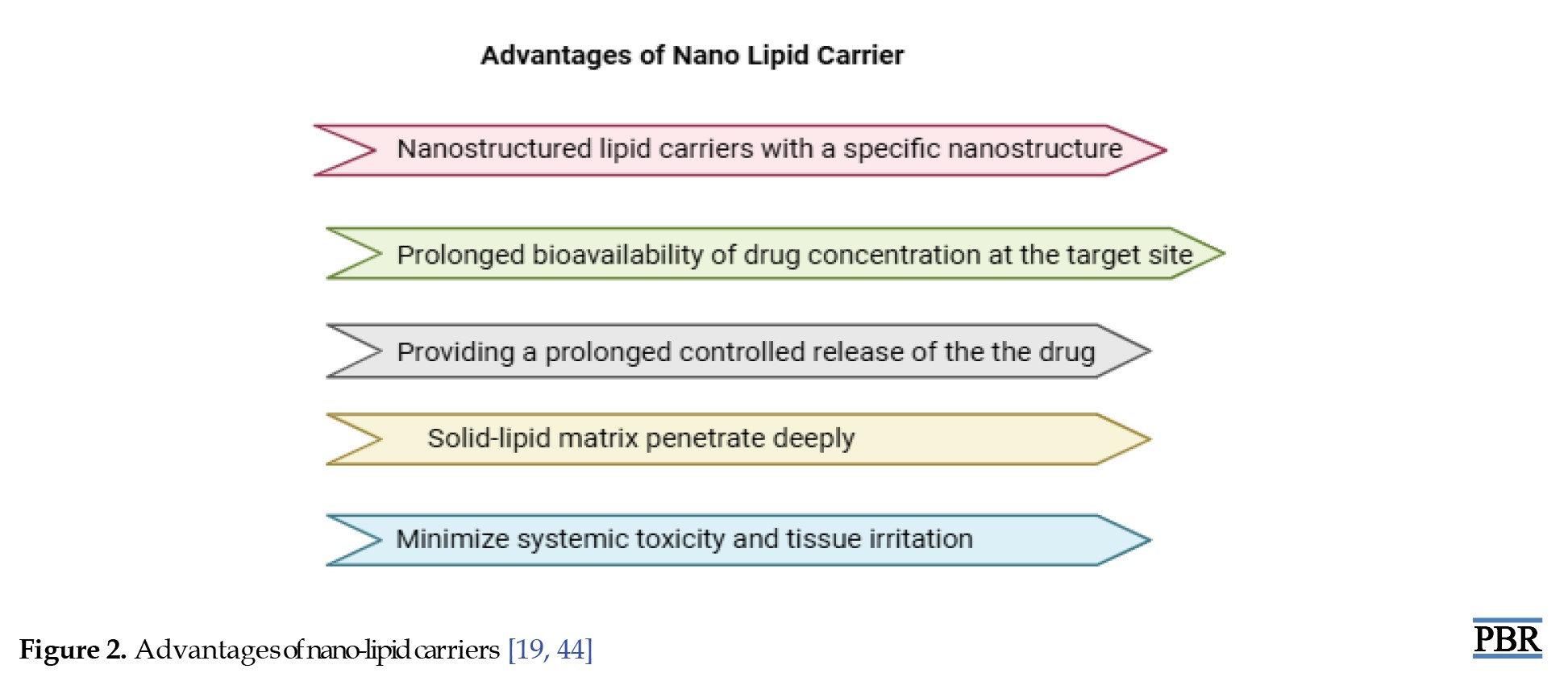
LNC gels offer several significant advantages in drug delivery systems. One of the primary benefits is enhanced bioavailability. Drugs that are poorly water-soluble can achieve improved solubility and stability when incorporated into LNC gels, which leads to better absorption and more effective therapeutic outcomes [69]. These gels also enable targeted and controlled drug delivery. The release of the active substance can be precisely modulated through diffusion or stimuli-responsive mechanisms, such as pH or temperature sensitivity, ensuring the medication reaches the intended site efficiently [70]. Additionally, by encapsulating active agents within lipid carriers, LNC gels help reduce the toxicity of drugs and minimize adverse side effects [71]. LNC gels are particularly effective in enhancing skin penetration. Due to their nanoscale size, lipid carriers can transport drugs deeper into the skin, increasing efficacy in treating conditions, like psoriasis, acne, and localized pain [72, 73]. Furthermore, the lipid matrix protects the drug from environmental degradation, such as oxidation or enzymatic breakdown, enhancing the overall stability of the formulation [74].
Applications of LNC gels
LNC gels have wide-ranging applications in modern therapeutics and cosmetics. In topical drug delivery, they are highly valued for delivering medications directly to the skin to treat conditions, such as psoriasis, eczema, fungal infections, and acne [75]. In transdermal drug delivery, these gels enable systemic absorption through the skin, offering a non-invasive alternative for managing conditions, like chronic pain and hormonal imbalances [76, 47]. The cosmetic industry also utilizes LNC gels due to their ability to protect and release active ingredients, such as vitamins and anti-aging compounds. These gels provide sustained delivery and better skin compatibility, making them suitable for skincare formulations [78, 79]. In oncology, LNC gels are being explored as targeted systems for localized delivery of anticancer drugs, reducing systemic side effects and improving drug accumulation at tumor sites [80].
Challenges and limitations
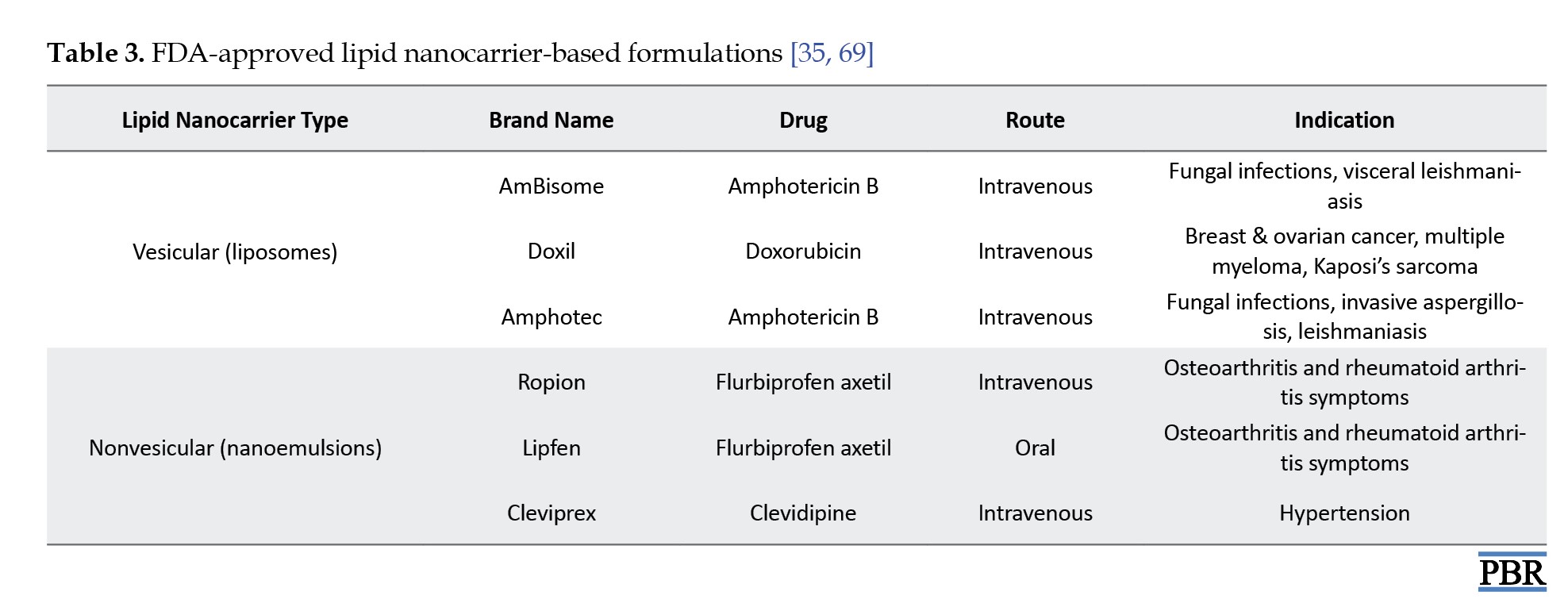
Despite their advantages, LNC gels present several challenges. One major limitation is the long-term stability of LNCs within gel formulations. Under certain storage conditions, issues, like phase separation and particle aggregation can compromise the system’s effectiveness [81]. Additionally, the gel base and surfactants used may cause skin irritation in sensitive individuals, particularly with long-term use or high concentrations, necessitating thorough safety testing [66]. Scaling up production for commercial manufacturing also poses difficulties. Maintaining consistent particle size, drug distribution, and reproducibility on a large scale requires complex formulation controls [82]. Furthermore, regulatory hurdles exist for nanotechnology-based products. Agencies, such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) demand extensive safety and efficacy data before approving new nano-formulations for clinical use [83] (Table 3).
Recent advancements
Indomethacin-loaded NLCs (IND-NLC) demonstrated exceptional encapsulation efficiency of over 99%, with a low polydispersity index (PDI=0.139), a particle size of 168.1 nm, and a surface charge of -30.1 mV. The formulation maintained its stability for over 60 days at both 4 °C and 25 °C. At a dose of 15.7 μM, IND-NLC exhibited potent anticancer activity by significantly inhibiting the proliferation of MDA-MB-468 breast cancer cells, confirming its potential as an effective anticancer drug delivery system [84].
Thymol-loaded NLCs were incorporated into various gel formulations to enable sustained release. Among these, carbomer-based gels showed superior skin retention, especially within the dermis and epidermis. While the NLC-thymol gels displayed prolonged efficacy against Cutibacterium acnes, their performance against Staphylococcus epidermidis was slightly lower than that of free thymol. Nonetheless, the thymol-loaded NLC gel formulation successfully maintained beneficial skin microbiota, establishing its potential as a promising topical therapy for acne vulgaris [76].
Magnolol-loaded NLCs (MA-NLC) were successfully synthesized with a particle size of 19.67 nm, a PDI of 0.21, and a zeta potential of -5.18 mV. These physicochemical properties contributed to enhanced safety and reduced pulmonary irritation. When administered via nebulization, MA-NLC showed improved lung targeting and prolonged retention, effectively overcoming issues related to low solubility and bioavailability. In animal models of chronic obstructive pulmonary disease (COPD), MA-NLC significantly improved oxidative stress markers and reduced inflammation, highlighting its therapeutic promise [85].
Bergenin-loaded NLCs (BNNLCo) were developed to enhance oral drug delivery efficiency. The formulation achieved an encapsulation efficiency of 80.6%, with a particle size of 174.3 nm, a PDI of 0.086, and a zeta potential of -35.6 mV. Compared to pure bergenin, BNNLCo demonstrated a 4.27-fold increase in relative bioavailability, a 3.2-fold enhancement in intestinal permeability, and a sustained drug release profile of 74.2% over 12 hours. Additionally, it achieved significant edema reduction (71.78% at 6 hours) and maintained prolonged therapeutic effects, suggesting its value in improving oral treatment outcomes [86].
Quercetin-loaded NLCs incorporated into chitosan hydrogels showed over 95% encapsulation efficiency and excellent long-term stability, retaining 88.63% of quercetin after ten months under natural daylight. Electrostatic interactions helped stabilize the NLCs within the chitosan matrix. In vitro studies demonstrated improved skin penetration and greater deposition of hydrophobic active compounds, establishing the NLC–chitosan hydrogel system as a promising vehicle for topical drug delivery [77].
Ketoconazole (KTZ)-loaded NLCs exhibited significantly improved retention in the skin, including a 2.58-fold increase in the stratum corneum, a 6.35-fold rise in the viable epidermis, and a 6.41-fold enhancement in transdermal permeation. The formulation displayed strong antifungal activity with effective inhibition of Candida albicans in planktonic, hyphal, and biofilm phases. NLC-based hydrogels also offered optimal spreadability, shear-thinning behavior, and minimal cytotoxicity on human skin fibroblasts, supporting their use in candidiasis treatment [87].
Luteolin-loaded NLCs (LUT-NLC) showed sustained drug release for up to 36 hours, with a particle size of 199.9 nm and encapsulation efficiency of 99.81%. Compared to conventional luteolin gel, LUT-NLC demonstrated superior skin penetration (78.89 μg/cm²). Fluorescent dye-tracking confirmed drug accumulation in the dermis, epidermis, and hair follicles. In an imiquimod-induced psoriasis model, LUT-NLC gels significantly reduced inflammatory cytokines (TNF-α, IL-6, IL-17, and IL-23) and alleviated both cutaneous and systemic symptoms, making it a promising therapeutic for psoriasis [88].
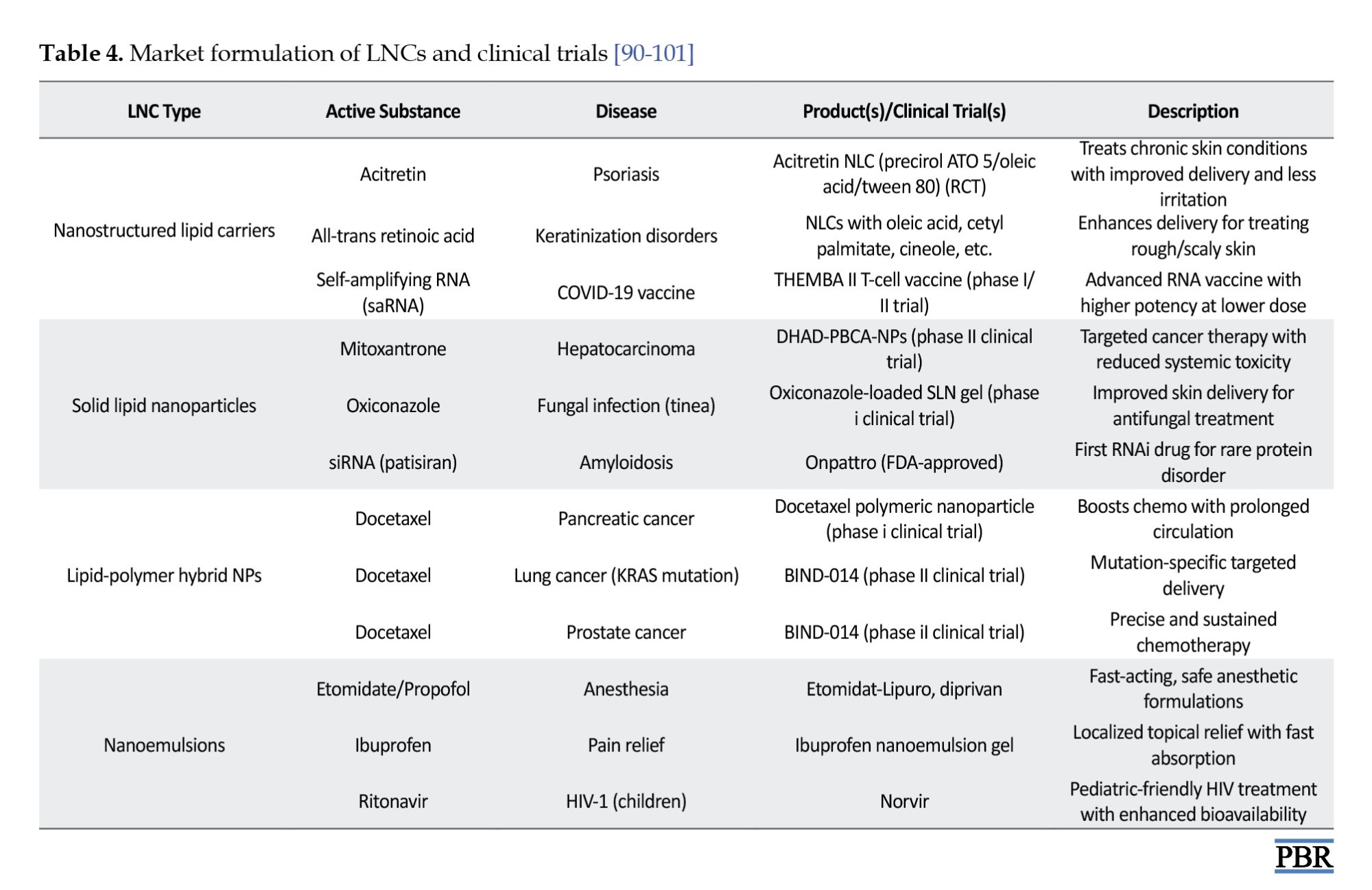
Zingiber officinale extract (ZOE)-loaded NLCs displayed excellent stability at 0.4% concentration, with particle sizes ranging from 302–344 nm and PDI values between 0.14 and 0.23 over 28 days. At 2% ZOE concentration, high encapsulation efficiency was achieved. Morphological assessments revealed well-formed, uniformly distributed particles. Compared to conventional topical formulations, ZOE-NLC gels exhibited enhanced rheological properties and improved stability, reinforcing their potential for effective topical drug delivery [89] (Table 4).
Conclusion
Featuring several uses in dermatology, cosmetics, and pharmaceuticals, LNC gels are a promising and adaptable drug delivery technology. LNC gels can enhance a range of pharmaceuticals’ solubility, stability, and effectiveness by combining the benefits of LNCs with the stability and controlled-release characteristics of gel matrices. Even while issues, like scalability, stability, and skin irritation still exist, more research and technical developments might eventually allow LNC gels to reach their full clinical and commercial potential. NLCs have been shown to greatly increase the effectiveness of drugs used to promote wound healing. Numerous studies demonstrate that NLCs are efficient at carrying a range of materials, such as hydrophobic chemicals, essential oils, plant extracts, and physiologically active molecules, like proteins and nucleic acids, which are essential for the repair of wounds. NLCs speed up wound healing and regeneration by improving these chemicals’ stability, absorption, and regulated release. NLC formulations are flexible enough to be customized to meet individual therapeutic demands since they may include a range of surfactants, lipids, and preparations, both liquid and solid techniques, such solvent evaporation, high-pressure homogenization, and microemulsion procedures. Because NLCs may administer medications at a steady, sustained pace, they are very useful for treating chronic wounds that need prolonged therapy periods. To investigate the usage of NLCs for a larger variety of medications, including more recent synthetic and natural substances, more study is necessary. Furthermore, thorough safety to ensure the long-term security and efficacy of NLCs, research is required, particularly in the management of long-term injuries, like as burns, diabetic ulcers, and other chronic wounds.
Ethical Considerations
Compliance with ethical guidelines
As this is a review article, there were no ethical issues or human/animal involvement requiring ethical approval.
Funding
This study did not receive financial support from any public, private, or non-profit funding organizations.
Authors' contributions
All authors equally participated in the study’s conception, design, data acquisition and analysis, interpretation of findings, and preparation of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
References
In today’s healthcare landscape, improving drug delivery requires innovative approaches that maximize therapeutic outcomes while minimizing side effects [1]. Traditional methods, such as oral tablets, injections, and topical creams deliver drugs without specialized carriers, which can lead to poor bioavailability, rapid clearance, and non-specific distribution [2-4]. For instance, oral drugs may degrade in the stomach or be cleared before reaching the target, reducing effectiveness. Lipids, commonly known as fats, are biologically important molecules involved in energy storage, membrane structure, and metabolic regulation [5, 6]. They include simple lipids (e.g. triglycerides, waxes), complex lipids (e.g. phospholipids), and related compounds, like sterols and fat-soluble vitamins. Their ability to dissolve in organic solvents while remaining insoluble in water has enabled their application in drug delivery systems [5]. Recently, lipids have been used to create nanoscale carriers that encapsulate active substances, offering protection and controlled release [7]. These lipid-based systems improve the delivery of drugs in medicine, cosmetics, and pharmacology [8, 9]. Lipid nanocarriers (LNCs) are an emerging class of drug delivery vehicles that combine lipid chemistry with nanotechnology to improve solubility, stability, and bioavailability of therapeutics [10]. Incorporating LNCs into gels results in lipid nanocarrier gels (LNC gels), which offer benefits, such as enhanced skin penetration, sustained drug release, and targeted delivery ideal for topical and transdermal use [11, 12].
Nanotechnology plays a major role across disciplines, particularly in enhancing drug delivery through precise targeting and efficient therapeutic action [13]. Nanocarriers, typically less than one micron in size, possess high surface-area-to-volume ratios and unique biodistribution profiles, making them suitable for diverse pharmaceutical applications [14, 15]. Their nanoscale properties allow for the modulation of drug kinetics, toxicity, and therapeutic index through surface and structural engineering [16]. However, despite progress, challenges, such as biological barriers, formulation complexity, and stability limit the widespread adoption of nanostructured lipid carriers (NLCs) [17-19]. NLCs are especially useful in chronic wound treatment, where sustained drug release is vital [20]. Their surface charge (zeta potential) and morphology (e.g. spheres, cones, and cylinders) significantly influence stability, targeting, and therapeutic performance [21, 22]. Nano-modification strategies are being explored to enhance drug action, especially in wound healing applications [23]. NLCs protect therapeutic agents, provide controlled release, and improve drug absorption, making them effective for both synthetic and natural compounds [24, 25]. Preclinical and clinical research has demonstrated their ability to enhance wound healing outcomes by delivering consistent drug levels and supporting tissue regeneration [26, 27].
Composition of LNCs
LNCs, particularly NLCs, are composed of carefully selected lipids, surfactants, and other functional ingredients that collectively determine their physicochemical properties and therapeutic efficacy (Figure 1). The selection of lipids is critical, as their type and structure significantly influence the characteristics of the resulting nanoparticles, including stability, particle size, drug loading capacity, and release behavior [29]. Two important factors to consider during lipid selection are the solubility of the drug within the lipid matrix and the drug’s partition coefficient, both of which affect encapsulation efficiency and the rate of drug release [30]. Additionally, the viscosity and melting point of the lipids play a crucial role in particle formation—lipids with higher viscosity and melting points tend to produce larger particles, impacting dispersion uniformity and system stability [31]. Surfactants are another key component in lipid nanocarrier systems. They serve multiple functions, most notably enhancing drug solubility, dissolution, and membrane permeability, which contributes to improved drug absorption and therapeutic outcomes [32]. The choice of surfactant is influenced by its hydrophilic-lipophilic balance (HLB), which affects not only emulsion formation but also particle size and the interaction between lipids and aqueous phases [33]. As emulsifiers, surfactants reduce the interfacial tension between lipid and water phases, promoting compatibility and preventing phase separation, thus enhancing the formulation’s overall stability [34].
Furthermore, lipid crystallization behavior, modulated by surfactants and other factors, can influence the morphology, size, and surface characteristics of colloidal particles parameters that directly affect the nanocarrier’s drug delivery performance and physical stability under varying conditions [35]. Beyond lipids and surfactants, other ingredients are often incorporated to enhance the performance of NLCs. Surface modifiers improve the physical stability and biocompatibility of the formulation while facilitating targeted drug delivery across epithelial barriers [36]. These surface modifications also contribute to immune evasion by reducing the likelihood of phagocytic uptake by macrophages in the reticuloendothelial system (RES), thereby prolonging systemic circulation and enhancing therapeutic outcomes [37]. Because lipid-based drug delivery systems utilize biodegradable and biocompatible lipids, they are well-suited for applications requiring controlled drug release, targeted delivery, and protection of pharmaceuticals from degradation [38]. Moreover, these systems provide versatility in delivering a wide range of therapeutic agents, including growth factors, gene therapies, and cytokines offering more adaptability than traditional drug delivery methods [39]. They are also capable of targeting specific cells and tissues, enabling the delivery of complex therapeutic entities, such as nucleic acids, peptides, and proteins [40]. Lipid-based delivery systems can be broadly categorized into vesicular systems and lipid nanoparticles. Vesicular systems are formed when amphiphilic molecules self-assemble in aqueous environments into highly structured arrangements containing one or more concentric lipid bilayers [41]. Lipid nanoparticles, on the other hand, include several types, such as lipid-drug conjugates (LDCs), solid lipid nanoparticles (SLNs), and NLCs [42]. NLCs are further subclassified based on their internal structural organization into multiple oil/fat/water (O/F/W) systems, amorphous types, and highly imperfect crystalline structures. These variations influence the drug loading capacity and release behavior of the formulation, making NLCs a highly adaptable platform for advanced drug delivery applications [29] (Figure 2, Tables 1 and 2).


Preparation method of LNCs
High-pressure homogenization techniques (hot and cold methods)
In the hot homogenization method, the drug is initially blended into a melted lipid mixture. At the same time, a heated surfactant solution is prepared. These two components are combined and processed using a high-pressure homogenizer, resulting in the formation of nanostructured lipid carriers. In the cold homogenization method, the drug is also first mixed with molten lipids, but this blend is rapidly cooled and then mechanically ground into fine particles. These particles are then mixed with a chilled surfactant solution and further processed under high pressure to yield the final nanocarriers.
Solvent-based techniques (evaporation and diffusion)
The solvent evaporation technique involves dissolving the drug and lipid in a water-insoluble organic solvent, which is then combined with a surfactant solution. This mixture is heated to remove the solvent through evaporation, leading to the production of lipid nanoparticles. On the other hand, in the solvent diffusion method, the drug-lipid mixture is dissolved in a water-soluble organic solvent, and then blended with a surfactant solution. This mixture is diluted with water to initiate solvent diffusion. The final nanocarrier formulation is obtained by drying the system through freeze-drying or filtration [61-64, 53] (Figure 3).
Lipid nano-carrier gel
Lipid core
Lipid molecules (micelles or liposomes) will be grouped in a spherical shape in the center. Phospholipids, which have hydrophilic heads and hydrophobic tails, would normally make up these lipids. A bilayer or monolayer structure is created with the hydrophilic heads oriented outward and the hydrophobic tails directed inward [28].
Encapsulated active ingredients
Inside the lipid core or in the surrounding gel matrix, there may be encapsulated substances, like drugs, vitamins, or other therapeutic agents. These molecules are usually hydrophobic and interact with the hydrophobic core of the lipid bilayer [65].
Gel matrix
Surrounding or embedding these lipid nanoparticles, the gel matrix could be a clear or slightly opaque network, composed of polymers such as hydroxyethyl cellulose or carbomers. This gel structure aids in controlling the release of the encapsulated materials and stabilizing the lipid carriers [66].
Nanoscale size
The lipids themselves are nanoscale in size, resembling small, compact spheres or vesicles dispersed throughout the gel matrix. This would appear as a cloudy or translucent gel when viewed under a microscope [67] (Figure 4).
Advantages of lipid nanocarrier gels
LNC gels offer several significant advantages in drug delivery systems. One of the primary benefits is enhanced bioavailability. Drugs that are poorly water-soluble can achieve improved solubility and stability when incorporated into LNC gels, which leads to better absorption and more effective therapeutic outcomes [69]. These gels also enable targeted and controlled drug delivery. The release of the active substance can be precisely modulated through diffusion or stimuli-responsive mechanisms, such as pH or temperature sensitivity, ensuring the medication reaches the intended site efficiently [70]. Additionally, by encapsulating active agents within lipid carriers, LNC gels help reduce the toxicity of drugs and minimize adverse side effects [71]. LNC gels are particularly effective in enhancing skin penetration. Due to their nanoscale size, lipid carriers can transport drugs deeper into the skin, increasing efficacy in treating conditions, like psoriasis, acne, and localized pain [72, 73]. Furthermore, the lipid matrix protects the drug from environmental degradation, such as oxidation or enzymatic breakdown, enhancing the overall stability of the formulation [74].
Applications of LNC gels
LNC gels have wide-ranging applications in modern therapeutics and cosmetics. In topical drug delivery, they are highly valued for delivering medications directly to the skin to treat conditions, such as psoriasis, eczema, fungal infections, and acne [75]. In transdermal drug delivery, these gels enable systemic absorption through the skin, offering a non-invasive alternative for managing conditions, like chronic pain and hormonal imbalances [76, 47]. The cosmetic industry also utilizes LNC gels due to their ability to protect and release active ingredients, such as vitamins and anti-aging compounds. These gels provide sustained delivery and better skin compatibility, making them suitable for skincare formulations [78, 79]. In oncology, LNC gels are being explored as targeted systems for localized delivery of anticancer drugs, reducing systemic side effects and improving drug accumulation at tumor sites [80].
Challenges and limitations
Despite their advantages, LNC gels present several challenges. One major limitation is the long-term stability of LNCs within gel formulations. Under certain storage conditions, issues, like phase separation and particle aggregation can compromise the system’s effectiveness [81]. Additionally, the gel base and surfactants used may cause skin irritation in sensitive individuals, particularly with long-term use or high concentrations, necessitating thorough safety testing [66]. Scaling up production for commercial manufacturing also poses difficulties. Maintaining consistent particle size, drug distribution, and reproducibility on a large scale requires complex formulation controls [82]. Furthermore, regulatory hurdles exist for nanotechnology-based products. Agencies, such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) demand extensive safety and efficacy data before approving new nano-formulations for clinical use [83] (Table 3).

Recent advancements
Indomethacin-loaded NLCs (IND-NLC) demonstrated exceptional encapsulation efficiency of over 99%, with a low polydispersity index (PDI=0.139), a particle size of 168.1 nm, and a surface charge of -30.1 mV. The formulation maintained its stability for over 60 days at both 4 °C and 25 °C. At a dose of 15.7 μM, IND-NLC exhibited potent anticancer activity by significantly inhibiting the proliferation of MDA-MB-468 breast cancer cells, confirming its potential as an effective anticancer drug delivery system [84].
Thymol-loaded NLCs were incorporated into various gel formulations to enable sustained release. Among these, carbomer-based gels showed superior skin retention, especially within the dermis and epidermis. While the NLC-thymol gels displayed prolonged efficacy against Cutibacterium acnes, their performance against Staphylococcus epidermidis was slightly lower than that of free thymol. Nonetheless, the thymol-loaded NLC gel formulation successfully maintained beneficial skin microbiota, establishing its potential as a promising topical therapy for acne vulgaris [76].
Magnolol-loaded NLCs (MA-NLC) were successfully synthesized with a particle size of 19.67 nm, a PDI of 0.21, and a zeta potential of -5.18 mV. These physicochemical properties contributed to enhanced safety and reduced pulmonary irritation. When administered via nebulization, MA-NLC showed improved lung targeting and prolonged retention, effectively overcoming issues related to low solubility and bioavailability. In animal models of chronic obstructive pulmonary disease (COPD), MA-NLC significantly improved oxidative stress markers and reduced inflammation, highlighting its therapeutic promise [85].
Bergenin-loaded NLCs (BNNLCo) were developed to enhance oral drug delivery efficiency. The formulation achieved an encapsulation efficiency of 80.6%, with a particle size of 174.3 nm, a PDI of 0.086, and a zeta potential of -35.6 mV. Compared to pure bergenin, BNNLCo demonstrated a 4.27-fold increase in relative bioavailability, a 3.2-fold enhancement in intestinal permeability, and a sustained drug release profile of 74.2% over 12 hours. Additionally, it achieved significant edema reduction (71.78% at 6 hours) and maintained prolonged therapeutic effects, suggesting its value in improving oral treatment outcomes [86].
Quercetin-loaded NLCs incorporated into chitosan hydrogels showed over 95% encapsulation efficiency and excellent long-term stability, retaining 88.63% of quercetin after ten months under natural daylight. Electrostatic interactions helped stabilize the NLCs within the chitosan matrix. In vitro studies demonstrated improved skin penetration and greater deposition of hydrophobic active compounds, establishing the NLC–chitosan hydrogel system as a promising vehicle for topical drug delivery [77].
Ketoconazole (KTZ)-loaded NLCs exhibited significantly improved retention in the skin, including a 2.58-fold increase in the stratum corneum, a 6.35-fold rise in the viable epidermis, and a 6.41-fold enhancement in transdermal permeation. The formulation displayed strong antifungal activity with effective inhibition of Candida albicans in planktonic, hyphal, and biofilm phases. NLC-based hydrogels also offered optimal spreadability, shear-thinning behavior, and minimal cytotoxicity on human skin fibroblasts, supporting their use in candidiasis treatment [87].
Luteolin-loaded NLCs (LUT-NLC) showed sustained drug release for up to 36 hours, with a particle size of 199.9 nm and encapsulation efficiency of 99.81%. Compared to conventional luteolin gel, LUT-NLC demonstrated superior skin penetration (78.89 μg/cm²). Fluorescent dye-tracking confirmed drug accumulation in the dermis, epidermis, and hair follicles. In an imiquimod-induced psoriasis model, LUT-NLC gels significantly reduced inflammatory cytokines (TNF-α, IL-6, IL-17, and IL-23) and alleviated both cutaneous and systemic symptoms, making it a promising therapeutic for psoriasis [88].
Zingiber officinale extract (ZOE)-loaded NLCs displayed excellent stability at 0.4% concentration, with particle sizes ranging from 302–344 nm and PDI values between 0.14 and 0.23 over 28 days. At 2% ZOE concentration, high encapsulation efficiency was achieved. Morphological assessments revealed well-formed, uniformly distributed particles. Compared to conventional topical formulations, ZOE-NLC gels exhibited enhanced rheological properties and improved stability, reinforcing their potential for effective topical drug delivery [89] (Table 4).

Conclusion
Featuring several uses in dermatology, cosmetics, and pharmaceuticals, LNC gels are a promising and adaptable drug delivery technology. LNC gels can enhance a range of pharmaceuticals’ solubility, stability, and effectiveness by combining the benefits of LNCs with the stability and controlled-release characteristics of gel matrices. Even while issues, like scalability, stability, and skin irritation still exist, more research and technical developments might eventually allow LNC gels to reach their full clinical and commercial potential. NLCs have been shown to greatly increase the effectiveness of drugs used to promote wound healing. Numerous studies demonstrate that NLCs are efficient at carrying a range of materials, such as hydrophobic chemicals, essential oils, plant extracts, and physiologically active molecules, like proteins and nucleic acids, which are essential for the repair of wounds. NLCs speed up wound healing and regeneration by improving these chemicals’ stability, absorption, and regulated release. NLC formulations are flexible enough to be customized to meet individual therapeutic demands since they may include a range of surfactants, lipids, and preparations, both liquid and solid techniques, such solvent evaporation, high-pressure homogenization, and microemulsion procedures. Because NLCs may administer medications at a steady, sustained pace, they are very useful for treating chronic wounds that need prolonged therapy periods. To investigate the usage of NLCs for a larger variety of medications, including more recent synthetic and natural substances, more study is necessary. Furthermore, thorough safety to ensure the long-term security and efficacy of NLCs, research is required, particularly in the management of long-term injuries, like as burns, diabetic ulcers, and other chronic wounds.
Ethical Considerations
Compliance with ethical guidelines
As this is a review article, there were no ethical issues or human/animal involvement requiring ethical approval.
Funding
This study did not receive financial support from any public, private, or non-profit funding organizations.
Authors' contributions
All authors equally participated in the study’s conception, design, data acquisition and analysis, interpretation of findings, and preparation of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
References
- Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023; 9(6):e17488. [DOI:10.1016/j.heliyon.2023.e17488] [PMID]
- Jain KK. Drug delivery systems - an overview. Methods Mol Biol. 2008; 437:1-50. [DOI:10.1007/978-1-59745-210-6_1] [PMID]
- Homayun B, Lin X, Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019; 11(3):129. [DOI:10.3390/pharmaceutics11030129] [PMID]
- Lee VH, Dodda-Kashi S, Grass GM, Rubas W. Oral route of peptide and protein drug delivery. In: Lee V, editor. Peptide and protein drug delivery. Boca Raton: CRC Press; 2024. [DOI:10.1201/9781003573715-18]
- Duché G, Sanderson JM. The chemical reactivity of membrane lipids. Chem Rev. 2024; 124(6):3284-30. [DOI:10.1021/acs.chemrev.3c00608] [PMID]
- Reszczyńska E, Hanaka A. Lipids composition in plant membranes. Cell Biochem Biophys. 2020; 78(4):401-14. [DOI:10.1007/s12013-020-00947-w] [PMID]
- Ren R, Lim C, Li S, Wang Y, Song J, Lin TW, et al. Recent advances in the development of lipid-, metal-, carbon-, and polymer-based nanomaterials for antibacterial applications. Nanomaterials. 2022; 12(21):3855. [DOI:10.3390/nano12213855] [PMID]
- Sonawane D, Pokharkar V. Quercetin-loaded nanostructured lipid carrier in situ gel for brain targeting through intranasal route: formulation, in vivo pharmacokinetic and pharmacodynamic studies. AAPS PharmSciTech. 2024; 25(2):30. [DOI:10.1208/s12249-024-02736-7] [PMID]
- Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: Recent advances in drug delivery. J Drug Target. 2012; 20(10):813-30. [DOI:10.3109/1061186X.2012.716845] [PMID]
- Khan S, Sharma A, Jain V. An overview of nanostructured lipid carriers and its application in drug delivery through different routes. Adv Pharm Bull. 2023; 13(3):446-60. [DOI:10.34172/apb.2023.056] [PMID]
- Gilani SJ, Jumah MNB, Zafar A, Imam SS, Yasir M, Khalid M, et al. Formulation and evaluation of nano lipid carrier-based ocular gel system: Optimization to antibacterial activity. Gels. 2022; 8(5):255. [DOI:10.3390/gels8050255] [PMID]
- Kumbhar PS, Manjappa AS, Shah RR, Nadaf SJ, Disouza JI. Nanostructured lipid carrier-based gel for repurposing simvastatin in localized treatment of breast cancer: formulation design, development, and in vitro and in vivo characterization. AAPS PharmSciTech. 2023; 24(5):106. [DOI:10.1208/s12249-023-02565-0] [PMID]
- Saxena SK, Nyodu R, Kumar S, Maurya VK. Current advances in nanotechnology and medicine. In: Saxena SK, Khurana SMP, editors. NanoBioMedicine. New York: Springer; 2020. [DOI:10.1007/978-981-32-9898-9_1]
- Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019; 144:57-77. [DOI:10.1016/j.addr.2019.07.010] [PMID]
- Wu H, Tang L, Dong H, Zhi M, Guo L, Hong X, et al. Shape and size dependence of pharmacokinetics, biodistribution, and toxicity of gold nanoparticles. Mol Pharm. 2025; 22(1):196-208. [DOI:10.1021/acs.molpharmaceut.4c00832] [PMID]
- Bourquin J, Milosevic A, Hauser D, Lehner R, Blank F, Petri-Fink A, et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv Mater. 2018; 30(19):e1704307. [DOI:10.1002/adma.201704307] [PMID]
- Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 2016; 44(1):27-40. [DOI:10.3109/21691401.2014.909822] [PMID]
- Singhvi G, Patil S, Girdhar V, Dubey SK. Nanocarriers for topical drug delivery: Approaches and advancements. Nanosci Nanotechnol Asia. 2019; 9(3):329-36. [DOI:10.2174/2210681208666180320122534]
- Mall J, Naseem N, Haider MF, Rahman MA, Khan S, Siddiqui SN. Nanostructured lipid carriers as a drug delivery system: A comprehensive review with therapeutic applications. Intelligent Pharm. 2024 [Unpublished]. [DOI:10.1016/j.ipha.2024.09.005]
- Ullah A, Ullah M, Lee GJ, Lim SI. A review of recent advances in nanotechnology for the delivery of therapeutics in wound healing. J Pharm Invest. 2024; 55:33-54. [DOI:10.1007/s40005-024-00692-9]
- Shrestha S, Wang B, Dutta P. Nanoparticle processing: Understanding and controlling aggregation. Adv Colloid Interface Sci. 2020; 279:102162. [DOI:10.1016/j.cis.2020.102162] [PMID]
- Stolarczyk JK, Deak A, Brougham DF. Nanoparticle assemblies: nanoparticle clusters: Assembly and control over internal order, current capabilities, and future potential (Adv. Mater. 27/2016). Adv Mater. 2016; 28(27):5764. [DOI:10.1002/adma.201505350] [PMID]
- Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019; 17(1):82. [DOI:10.1186/s12951-019-0514-y] [PMID]
- Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine. 2016; 12(1):143-61. [DOI:10.1016/j.nano.2015.09.004] [PMID]
- Goel R, Mishra R, Singh N, Rajora A, Singh R, Gaur PK. Nanostructured lipid carriers: Enhancing herbal medicine delivery. In: Baghel Chauhan S, Singh I, Singh Grewal A, Kaur R, editors. Lipid based nanocarriers for drug delivery. New York: Nova; 2024. [Link]
- Faheem S, Hameed H, Paiva-Santos AC, Zaman M, Sarwar HS, Majeed I. Niosome-based gels: A smart nano-carrier for effective and advanced transdermal drug delivery. Int J Polym Mater Polym Biomater. 2024; 74.4:1-9. [DOI:10.1080/00914037.2024.2335169]
- Abdel-Mageed HM, Abd El Aziz AE, Mohamed SA, AbuelEzz NZ. The tiny big world of solid lipid nanoparticles and nanostructured lipid carriers: an updated review. J Microencapsul. 2022; 39(1):72-94. [DOI:10.1080/02652048.2021.2021307] [PMID]
- Wathoni N, Suhandi C, Elamin KM, Lesmana R, Hasan N, Mohammed AFA, et al. Advancements and challenges of nanostructured lipid carriers for wound healing applications. Int J Nanomedicine. 2024; 19:8091-113. [DOI:10.2147/IJN.S478964] [PMID]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013; 19:29-43. [DOI:10.1016/j.ifset.2013.03.002]
- Pan Y, Tikekar RV, Nitin N. Distribution of a model bioactive within solid lipid nanoparticles and nanostructured lipid carriers influences its loading efficiency and oxidative stability. Int J Pharm. 2016; 511(1):322-30. [DOI:10.1016/j.ijpharm.2016.07.019] [PMID]
- Westesen K. Novel lipid-based colloidal dispersions as potential drug administration systems-expectations and reality. Colloid Polym Sci. 2000; 278:608-18. [DOI:10.1007/s003969900257]
- Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, et al. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. 2020; 17(3):357-77. [DOI:10.1080/17425247.2020.1727883] [PMID]
- Hogarth C, Arnold K, Wright S, Elkateb H, Rannard S, McDonald TO. Navigating the challenges of lipid nanoparticle formulation: The role of unpegylated lipid surfactants in enhancing drug loading and stability. Nanoscale Adv. 2023; 6(2):669-79. [DOI:10.1039/D3NA00484H] [PMID]
- Das R, Mallik N, Adhikari A, Bhattarai A. A comprehensive review on the creation, description, and utilization of surfactants containing multiple hydroxyl groups. Int J Polym Sci. 2024; 2024(1):6120535. [DOI:10.1155/2024/6120535]
- Mittal P, Singla M, Smriti, Kapoor R, Kumar D, Gupta S, et al. Paclitaxel loaded Capmul MCM and tristearin based nanostructured lipid carriers (NLCs) for glioblastoma treatment: Screening of formulation components by quality by design (QbD) approach. Discov Nano. 2024; 19(1):175. [DOI:10.1186/s11671-024-04132-3] [PMID]
- Xie Y, Li P, Fu D, Yang F, Sui X, Huang B, et al. CBD-loaded nanostructured lipid carriers: Optimization, characterization, and stability. ACS Omega. 2024; 9(39):40632-43. [DOI:10.1021/acsomega.4c04771] [PMID]
- Patel D, Solanki J, Kher MM, Azagury A. A review: Surface engineering of lipid-based drug delivery systems. Small. 2024; 20(43):e2401990. [DOI:10.1002/smll.202401990] [PMID]
- Satapathy BS, Mishra A, Mohanty K, Pattnaik S, Tripathy S, Biswal B. Lipid nanocarrier-based bigel of piper betel oil for analgesic and anti-inflammatory applications. J Microencapsul. 2025; 42(1):47-69. [DOI:10.1080/02652048.2024.2430651] [PMID]
- Li H, Lin Z, Ouyang L, Lin C, Zeng R, Liu G, et al. Lipid nanoparticle: Advanced drug delivery systems for promotion of angiogenesis in diabetic wounds. J Liposome Res. 2025; 35(1):76-85. [DOI:10.1080/08982104.2024.2378962] [PMID]
- Lin Y, Chen X, Wang K, Liang L, Zhang H. An overview of nanoparticle-based delivery platforms for mRNA vaccines for treating cancer. Vaccines. 2024; 12(7):727. [DOI:10.3390/vaccines12070727] [PMID]
- Kang Y, Zhang S, Wang G, Yan Z, Wu G, Tang L, et al. Nanocarrier-based transdermal drug delivery systems for dermatological therapy. Pharmaceutics. 2024; 16(11):1384. [DOI:10.3390/pharmaceutics16111384] [PMID]
- Arora R, Katiyar SS, Kushwah V, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin Drug Deliv. 2017; 14(2):165-77. [DOI:10.1080/17425247.2017.1264386] [PMID]
- Singhal GB, Patel RP, Prajapati BG, Patel NA. Solid lipid nanoparticles and nano lipid carriers: As novel solid lipid based drug carrier. Int Res J Pharm. 2011; 2(2):20-52. [Link]
- Schoenfelder H, Wiedemann Y, Lunter DJ. Development and characterization of topical formulation for maintenance therapy containing sorbitan monostearate with and without PEG-100-stearate. Int J Cosmet Sci. 2025; 47(2):223-33. [DOI:10.1111/ics.13023] [PMID]
- Wang Y, Deng Y, Mao S, Jin S, Wang J, Bi D. Characterization and body distribution of beta-elemene solid lipid nanoparticles (SLN). Drug Dev Ind Pharm. 2005; 31(8):769-78. [DOI:10.1080/03639040500216329] [PMID]
- D'Souza A, Shegokar R. Nanostructured lipid carriers (NLCs) for drug delivery: Role of liquid lipid (oil). Curr Drug Deliv. 2021; 18(3):249-70. [DOI:10.2174/1567201817666200423083807] [PMID]
- Arezoumand KS, Alizadeh E, Esmaeillou M, Ghasemi M, Alipour S, Pilehvar-Soltanahmadi Y, Zarghami N. The emu oil emulsified in egg lecithin and butylated hydroxytoluene enhanced the proliferation, stemness gene expression, and in vitro wound healing of adipose-derived stem cells. In Vitro Cell Dev Biol Anim. 2018; 54(3):205-16. [DOI:10.1007/s11626-018-0228-8] [PMID]
- Dobreva M, Stefanov S, Andonova V. Natural lipids as structural components of solid lipid nanoparticles and nanostructured lipid carriers for topical delivery. Curr Pharm Des. 2020; 26(36):4524-35. [DOI:10.2174/1381612826666200514221649] [PMID]
- Dalwadi S, Thakkar V, Prajapati B. Optimizing neuroprotective nano-structured lipid carriers for transdermal delivery through artificial neural network. Pharm Nanotechnol. 2025; 13(1):184-98. [DOI:10.2174/0122117385294969240326052312] [PMID]
- Fitriani EW, Avanti C, Rosana Y, Surini S. Nanostructured lipid carriers: A prospective dermal drug delivery system for natural active ingredients. Pharmacia. 2024; 71:e115849. [DOI:10.3897/pharmacia.71.e115849]
- Ahmed OAA, Fahmy UA, Bakhaidar R, El-Moselhy MA, Alfaleh MA, Ahmed AF, et al. Pumpkin oil-based nanostructured lipid carrier system for antiulcer effect in nsaid-induced gastric ulcer model in rats. Int J Nanomedicine. 2020; 15:2529-39. [DOI:10.2147/IJN.S247252] [PMID]
- Alatawi HM, Alhwiti SS, Alsharif KA, Albalawi SS, Abusaleh SM, Sror GK, et al. Nanostructured lipid carriers (NLCs) as effective drug delivery systems: Methods of preparation and their therapeutic applications. Recent Pat Nanotechnol. 2024; 18(2):179-89. [DOI:10.2174/1872210517666230120142439] [PMID]
- Kanojiya PS, Wadetwar RN, Atole PG, Thakrani KC, Gawande NP. Sustained delivery of statistically optimized transfersomal gel of miconazole nitrate for vaginal candidiasis. J Dispers Sci Technol. 2025; 46(2):333-50. [DOI:10.1080/01932691.2023.2289621]
- Elbardisy B, Boraie N, Galal S. Tadalafil nanoemulsion mists for treatment of pediatric pulmonary hypertension via nebulization. Pharmaceutics. 2022; 14(12):2717. [DOI:10.3390/pharmaceutics14122717] [PMID]
- Gupta P, Mazumder R, Padhi S. Glycerosomes: Advanced liposomal drug delivery system. Indian J Pharm Sci. 2020; 82(3):385-97. [DOI:10.36468/pharmaceutical-sciences.661]
- Karami Z, Saghatchi Zanjani MR, Hamidi M. Nanoemulsions in CNS drug delivery: Recent developments, impacts and challenges. Drug Discov Today. 2019; 24(5):1104-115. [DOI:10.1016/j.drudis.2019.03.021] [PMID]
- Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018; 103:598-613. [DOI:10.1016/j.biopha.2018.04.055] [PMID]
- Tan C, Hosseini SF, Jafari SM. Cubosomes and hexosomes as novel nanocarriers for bioactive compounds. J Agric Food Chem. 2022; 70(5):1423-37. [DOI:10.1021/acs.jafc.1c06747] [PMID]
- Lohumi A. A novel drug delivery system: Niosomes review. J Drug Deliv Ther. 2012; 2(5):129-35. [DOI:10.22270/jddt.v2i5.274]
- Chinthaginjala H, Bogavalli V, Hindustan AA, Pathakamuri J, Pullaganti SS, Gowni A, et al. Nanostructured lipid carriers: A potential era of drug delivery systems. Ind J Pharm Educ Res. 2024; 58(1):21-33. [DOI:10.5530/ijper.58.1.3]
- Qian L, Cook MT, Dreiss CA. In situ gels for nasal delivery: Formulation, characterization and applications. Macromolecular Materials and Engineering. 2025; 2400356. [DOI:10.1002/mame.202400356]
- Jahan S, Aqil M, Ahad A, Imam SS, Waheed A, Qadir A, et al. Nanostructured lipid carrier for transdermal gliclazide delivery: Development and optimization by box-behnken design. Inorganic and Nano-Metal Chemistry. 2024; 54(5):474-87. [DOI:10.1080/24701556.2021.2025097]
- Soni S, Maheshwari RK, Sah AK. Nanostructured lipid carrier: Beneficial role in oral drug delivery system. BioNanoScience. 2024; 14:3988–4005. [DOI:10.1007/s12668-024-01416-x]
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020; 25(20):4781. [DOI:10.3390/molecules25204781] [PMID]
- Munir M, Zaman M, Waqar MA, Khan MA, Alvi MN. Solid lipid nanoparticles: A versatile approach for controlled release and targeted drug delivery. J Liposome Res. 2024; 34(2):335-48. [DOI:10.1080/08982104.2023.2268711] [PMID]
- Swami AB, Nagoba SN. Design, development and optimization of nanostructured lipid carrier containing anticancer drug. Afr J Biomed Res. 2024; 27(s3):1406-15. [DOI:10.53555/AJBR.v27i3S.2312]
- Taher SS, Al-Kinani KK. In vivo brain pharmacokinetics of dolutegravir sodium-loaded nanostructured lipid carrier in situ gel: Comparative study with an intravenous drug solution. Al-Rafidain J Med Sci. 2025; 8(1):115-25. [DOI:10.54133/ajms.v8i1.1692]
- Singh A, Iqubal MK, Mittal S, Qizilbash FF, Sartaz A, Kumar S, et al. Designing and evaluation of dermal targeted combinatorial nanostructured lipid carrier gel loaded with curcumin and resveratrol for accelerating cutaneous wound healing. Particulate Sci Technol. 2024; 42(1):88-106. [DOI:10.1080/02726351.2023.2205348]
- Domingues Bianchin M, Borowicz SM, da Rosa Monte Machado G, Pippi B, Stanisçuaski Guterres S, et al. Lipid core nanoparticles as a broad strategy to reverse fluconazole resistance in multiple Candida species. Colloids Surf B Biointerfaces. 2019; 175:523-9. [DOI:10.1016/j.colsurfb.2018.12.011] [PMID]
- Pal P, Sambhakar S, Paliwal S. Revolutionizing ophthalmic care: A review of ocular hydrogels from pathologies to therapeutic applications. Curr Eye Res. 2025; 50(1):1-17. [DOI:10.1080/02713683.2024.2396385] [PMID]
- Yu G, Jie K, Huang F. Supramolecular amphiphiles based on host-guest molecular recognition motifs. Chem Rev. 2015; 115(15):7240-303. [DOI:10.1021/cr5005315] [PMID]
- Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014; 15(3):694-708. [DOI:10.1208/s12249-014-0088-9] [PMID]
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009; 26(6):523-80. [DOI:10.1615/CritRevTherDrugCarrierSyst.v26.i6.10] [PMID]
- Passos JS, Apolinario AC, Ishida K, Martins TS, Lopes LB. Nanostructured lipid carriers loaded into in situ gels for breast cancer local treatment. Eur J Pharm Sci. 2024; 192:106638. [DOI:10.1016/j.ejps.2023.106638] [PMID]
- Elsewedy HS, Shehata TM, Genedy SM, Siddiq KM, Asiri BY, Alshammari RA, et al. Enhancing the topical antibacterial activity of fusidic acid via embedding into cinnamon oil nano-lipid carrier. Gels. 2024; 10(4):268. [DOI:10.3390/gels10040268] [PMID]
- Folle C, Marqués AM, Díaz-Garrido N, Carvajal-Vidal P, Sánchez López E, Suñer-Carbó J, et al. Gel-dispersed nanostructured lipid carriers loading thymol designed for dermal pathologies. Int J Nanomedicine. 2024; 19:1225-48. [DOI:10.2147/IJN.S433686] [PMID]
- Sun R, Xia Q, Sun Y. A novel strategy for topical administration by combining chitosan hydrogel beads with nanostructured lipid carriers: Preparation, characterization, and evaluation. Gels. 2024; 10(3):160. [DOI:10.3390/gels10030160] [PMID]
- Jamshaid U, Anton N, Elhassan M, Conzatti G, Vandamme TF. Novel hydrogels based on the nano-emulsion formulation process: Development, rheological characterization, and study as a drug delivery system. Pharmaceutics. 2024; 16(6):812. [DOI:10.3390/pharmaceutics16060812] [PMID]
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res Pharm Sci. 2018; 13(4):288-303. [DOI:10.4103/1735-5362.235156] [PMID]
- Zhang F, Zhang J, Zhang W. Recent advances in nanotechnology for the treatment of fungal keratitis. Eur J Ophthalmol. 2024; 34(1):18-29. [DOI:10.1177/11206721231174653] [PMID]
- Mirza R, Shah KU, Khan AU, Fawad M, Rehman AU, Ahmed N, et al. Statistical design and optimization of nano-transfersomes based chitosan gel for transdermal delivery of cefepime. Drug Dev Ind Pharm. 2024; 50(6):511-23. [DOI:10.1080/03639045.2024.2353098] [PMID]
- Moazeni M, Kelidari H, Kazeminejad A, Gholizadeh N, Haghani I, Saravani A, Parsay S, Nasirzadeh Y, Mofarrah R, Amini A. Addressing filamentous fungi-related onychomycosis in the era of antifungal resistance: Assessment of Zataria multiflora nanostructured lipid carrier topical gel in a double-blinded clinical trial. Current Med Mycol. 2025; 11. [Link]
- Montenegro L, Lai F, Offerta A, Sarpietro MG, Micicche L, Maccioni AM, Valenti D, Fadda AM. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J Drug Deliv Sci Technol. 2016; 32:100-12. [DOI:10.1016/j.jddst.2015.10.003]
- Thiruchenthooran V, Espina M, Świtalska M, Bonilla-Vidal L, Wietrzyk J, Garcia ML, et al. Combination of indomethacin with nanostructured lipid carriers for effective anticancer therapy. Int J Nanomedicine. 2024; 19:7033-48. [DOI:10.2147/IJN.S464239] [PMID]
- Jia B, He J, Zhang Y, Dang W, Xing B, Yang M, et al. Pulmonary delivery of magnolol-loaded nanostructured lipid carriers for COPD treatment. Int J Pharm. 2024; 662:124495. [DOI:10.1016/j.ijpharm.2024.124495] [PMID]
- Zafar A, Yasir M, Panda DS, Singh L. Bergenin nano-lipid carrier to improve the oral delivery: development, optimization, in vitro and in vivo evaluation. J Drug Deliv Sci Technol. 2024; 96:105655. [DOI:10.1016/j.jddst.2024.105655]
- Chilamakuri SN, Kumar A, Nath AG, Gupta A, Selvaraju S, Basrani S, et al. Development and in-vitro evaluation of eugenol-based nanostructured lipid carriers for effectual topical treatment against C. albicans. J Pharm Sci. 2024; 113(3):772-84. [DOI:10.1016/j.xphs.2023.11.031] [PMID]
- Xu H, Hu H, Zhao M, Shi C, Zhang X. Preparation of luteolin loaded nanostructured lipid carrier based gel and effect on psoriasis of mice. Drug Deliv Transl Res. 2024; 14(3):637-54. [DOI:10.1007/s13346-023-01418-4] [PMID]
- Shazwani SS, Marlina A, Misran M. Development of nanostructured lipid carrier-loaded flavonoid-enriched zingiber officinale. ACS Omega. 2024; 9(15):17379-88. [DOI:10.1021/acsomega.4c00091] [PMID]
- Agrawal Y, Petkar KC, Sawant KK. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 2010; 401(1-2):93-102. [DOI:10.1016/j.ijpharm.2010.09.007] [PMID]
- Charoenputtakun P, Pamornpathomkul B, Opanasopit P, Rojanarata T, Ngawhirunpat T. Terpene composited lipid nanoparticles for enhanced dermal delivery of all-trans-retinoic acids. Biol Pharm Bull. 2014; 37(7):1139-48. [DOI:10.1248/bpb.b14-00015] [PMID]
- Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur J Pharm Biopharm. 2013; 85(3 Pt A):427-43. [DOI:10.1016/j.ejpb.2013.07.002] [PMID]
- Zhou Q, Sun X, Zeng L, Liu J, Zhang Z. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine. 2009; 5(4):419-23. [DOI:10.1016/j.nano.2009.01.009] [PMID]
- Holtze C. Large-scale droplet production in microfluidic devices an industrial perspective. J Phys D. 2013; 46(11):114008. [DOI:10.1088/0022-3727/46/11/114008]
- Maeki M, Okada Y, Uno S, Sugiura K, Suzuki Y, Okuda K, et al. Mass production system for RNA-loaded lipid nanoparticles using piling up microfluidic devices. Appl Mater Today. 2023; 31:101754. [DOI:10.1016/j.apmt.2023.101754]
- Carvajal-Vidal P, González-Pizarro R, Araya C, Espina M, Halbaut L, Gómez de Aranda I, et al. Nanostructured lipid carriers loaded with Halobetasol propionate for topical treatment of inflammation: Development, characterization, biopharmaceutical behavior and therapeutic efficacy of gel dosage forms. Int J Pharm. 2020; 585:119480. [DOI:10.1016/j.ijpharm.2020.119480] [PMID]
- Luzzati V, Tardieu A, Gulik-Krzywicki T. Polymorphism of lipids. Nature. 1968; 217(5133):1028-30. [DOI:10.1038/2171028a0] [PMID]
- Vetten MA, Yah CS, Singh T, Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomedicine. 2014; 10(7):1391-9. [DOI:10.1016/j.nano.2014.03.017] [PMID]
- Packer M, Gyawali D, Yerabolu R, Schariter J, White P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat Commun. 2021; 12(1):6777. [DOI:10.1038/s41467-021-26926-0] [PMID]
- Mayer M, Doenicke A, Nebauer AE, Hepting L. [Propofol and etomidate-Lipuro for induction of general anesthesia. Hemodynamics, vascular compatibility, subjective findings and postoperative nausea (German)]. Anaesthesist. 1996 Nov;45(11):1082-4. [DOI:10.1007/s001010050343] [PMID]
- Patel HH, Pearn ML, Patel PM, Roth DM. General anesthetics and therapeutic gases. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman's: The pharmacological basis of therapeutics, 13e. New York: McGraw Hill; 2025. [Link]
Type of Study: Review article |
Subject:
Nanotechnology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |