Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 125-138 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Imam-Fulani A O, Shuaib A O, Olajide L O, Afolabi H O, Inyang J, Onoshi O O, et al . Caffeine Modulates Oxidative Stress and Neuroinflammation in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Model of Parkinson’s Disease in Female Mice. Pharm Biomed Res 2025; 11 (2) :125-138
URL: http://pbr.mazums.ac.ir/article-1-667-en.html
URL: http://pbr.mazums.ac.ir/article-1-667-en.html
Aminat Omolola Imam-Fulani *1 

 , Abdulrahman Olanrewaju Shuaib2
, Abdulrahman Olanrewaju Shuaib2 

 , Lateefah Omotoyosi Olajide3
, Lateefah Omotoyosi Olajide3 

 , Halimat Oluwakemi Afolabi3
, Halimat Oluwakemi Afolabi3 

 , Jerry Inyang3
, Jerry Inyang3 

 , Oluwadamilola Onoshi Onoshi3
, Oluwadamilola Onoshi Onoshi3 

 , Taofeekat Temitope Ogunsesan3
, Taofeekat Temitope Ogunsesan3 

 , Oyindamola Bisola Adekunle3
, Oyindamola Bisola Adekunle3 

 , Taiwo Elijah Eso3
, Taiwo Elijah Eso3 

 , Oluwaseyi Deborah Babatunde3
, Oluwaseyi Deborah Babatunde3 

 , Edwin Ayolola Makanjuola3
, Edwin Ayolola Makanjuola3 
 , Olamilekan Abdulfatai Alimi3
, Olamilekan Abdulfatai Alimi3 

 , Hafeez Ajibola Olorunoje3
, Hafeez Ajibola Olorunoje3 

 , Kamaldeen Olalekan Sanusi4
, Kamaldeen Olalekan Sanusi4 




 , Abdulrahman Olanrewaju Shuaib2
, Abdulrahman Olanrewaju Shuaib2 

 , Lateefah Omotoyosi Olajide3
, Lateefah Omotoyosi Olajide3 

 , Halimat Oluwakemi Afolabi3
, Halimat Oluwakemi Afolabi3 

 , Jerry Inyang3
, Jerry Inyang3 

 , Oluwadamilola Onoshi Onoshi3
, Oluwadamilola Onoshi Onoshi3 

 , Taofeekat Temitope Ogunsesan3
, Taofeekat Temitope Ogunsesan3 

 , Oyindamola Bisola Adekunle3
, Oyindamola Bisola Adekunle3 

 , Taiwo Elijah Eso3
, Taiwo Elijah Eso3 

 , Oluwaseyi Deborah Babatunde3
, Oluwaseyi Deborah Babatunde3 

 , Edwin Ayolola Makanjuola3
, Edwin Ayolola Makanjuola3 
 , Olamilekan Abdulfatai Alimi3
, Olamilekan Abdulfatai Alimi3 

 , Hafeez Ajibola Olorunoje3
, Hafeez Ajibola Olorunoje3 

 , Kamaldeen Olalekan Sanusi4
, Kamaldeen Olalekan Sanusi4 


1- Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria. & Department of Neuroscience, Medical School, University of Minnesota, Minneapolis, United States.
2- Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria. & Department of Human Physiology, Faculty of Basic Medical Sciences, Al-Hikmah University, Ilorin, Nigeria.
3- Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria.
4- Department of Human Physiology, Faculty of Basic Medical Sciences, Al-Hikmah University, Ilorin, Nigeria.
2- Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria. & Department of Human Physiology, Faculty of Basic Medical Sciences, Al-Hikmah University, Ilorin, Nigeria.
3- Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria.
4- Department of Human Physiology, Faculty of Basic Medical Sciences, Al-Hikmah University, Ilorin, Nigeria.
Full-Text [PDF 1153 kb]
(300 Downloads)
| Abstract (HTML) (861 Views)
Full-Text: (354 Views)
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to motor dysfunction and a variety of non-motor symptoms [1]. It is the second most common neurodegenerative disorder, affecting millions worldwide. While the precise etiology of PD remains elusive, oxidative stress, neuroinflammation, and the accumulation of misfolded proteins, such as α-synuclein are widely recognized as critical contributors to the pathophysiology of the disease [2-4] a neurodegenerative disorder characterized by distinct aging-independent loss of dopaminergic neurons in substantia nigra pars compacta (SNpc). These pathological processes not only compromise neuronal integrity but also activate glial cells, leading to a cycle of inflammation and neurodegeneration [5].
Caffeine, a widely consumed stimulant found in coffee and kola nut, has garnered attention for its potential neuroprotective properties [6]. Epidemiological studies have suggested an inverse relationship between caffeine consumption and the risk of developing PD, prompting research into its mechanistic effects on neuroprotection [7]. Caffeine is known to exert antioxidant effects, enhancing the activity of key enzymes, such as superoxide dismutase (SOD) and catalase (CAT), which mitigate oxidative stress by neutralizing reactive oxygen species [8, 9]. Additionally, caffeine’s ability to modulate neuroinflammation by inhibiting pro-inflammatory cytokines presents a promising avenue for therapeutic intervention [10].
In this study, we aimed to investigate the effects of caffeine on neurobehavioral performance, oxidative stress markers, and inflammatory mediators in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced model of PD in female Swiss mice. We hypothesized that caffeine would enhance antioxidant defense, reduce oxidative stress, and attenuate neuroinflammation, thereby preserving dopaminergic neurons and improving motor function. Through a comprehensive analysis of biochemical, immunohistochemical, and behavioral outcomes, this study elucidated the potential of caffeine as a therapeutic agent for mitigating the effects of PD. This study uniquely highlights the systemic and organ-specific effects of caffeine in mitigating MPTP-induced neurodegeneration, employing a comprehensive approach that integrates serum and tissue-specific biochemical analyses in female mice—a less frequently studied cohort—offering novel insights into sex-specific therapeutic implications for PD.
Materials and methods
Experimental animals
Twenty-five female Swiss mice weighing between 20–30 g were obtained from the Department of Biochemistry, University of Ilorin, Ilorin, Nigeria. The animals were housed at the College of Health Sciences, University of Ilorin. The animals were kept at a temperature of 22–25 °C, 55% relative humidity, and under a 12-hour light/dark cycle. The mice had free access to food and water and were acclimatized for two weeks before conducting any training, treatment, or experiment. After acclimatization, required neurobehavioral training was conducted for three consecutive days before the induction of PD and treatments.
Drug preparation and treatments
Preparation of caffeine
One gram of caffeine (Sigma-Aldrich; USA) was dissolved in 200 mL of distilled water. The mixture was prepared every two days and stored in the refrigerator.
Preparation of MPTP
PD induction using MPTP followed an established method [11] where 100 mg of MPTP powder (Sigma-Aldrich, Product number: M0896; USA) was dissolved in 10 mL of 0.9% normal saline. The solution was divided into ten 1.5 mL Eppendorf tubes and stored at –20 °C. Prior to use, the solution was diluted to a final concentration of 5 mg/mL.
PD induction using MPTP
Mice were given a daily intraperitoneal (ip) injection of 25 mg/kg MPTP for five days to establish the PD model. Mice that failed the motor tests (latency of more than 9 seconds on the pole test and falling off and/or walking longer than 60 seconds on the beam test) were included in the PD model experimental groups. Mice that passed the motor test were excluded from the experiment.
Experimental design
A healthy mice group, control (n=5), received normal saline (vehicle) while the PD model experimental groups were divided into four groups (n=5 each):
The MPTP induced-PD group administered 0.2 mL normal saline (vehicle) (PD).
The MPTP-induced PD group was treated with levodopa (10 mg/kg, daily, p.o.) (L-DOPA).
The MPTP-induced PD group administered high-dose caffeine (10 mg/kg, daily, [ip]) (H-Caf).
The MPTP-induced PD group administered low-dose caffeine (5 mg/kg, daily, [ip]) (L-Caf).
After the last dose administration, the animals were subjected to behavioral tests, including the pole test, beam-walk test, and open field test.
Behavioral tests
Pole test
The pole test is often used to assess motor coordination in PD mice. A metal rod with a diameter of 1 cm and a length of 50 cm was wrapped with black tape to prevent the mice from slipping, and the bottom of the metal rod was placed in a cushioned cage. Each mouse was placed head down on the top of the rod, and the time it took to return to the cage freely was recorded, ending when the hind limbs reached the bottom of the cage. Before the formal tests, the mice were trained for two days.
Beam walk test
The beam walk test is used to analyze mice’s gait in an environment that challenges their ability to balance themselves. A narrow beam (1-3 cm wide) was elevated between a pole and their home cage (to attract the mice to the finish point). The mice were placed at one end of the narrow beam and allowed to walk across the narrow beam from the start end to the cage with feed. The time it takes a mouse to move from start to end was recorded.
Open field test
The open-field test was conducted in a square arena with dimensions of 50 cm × 50 cm × 40 cm (length, width, and height). The arena was divided into 25 squares. During the experiment, mice were placed in the center of the open field maze and allowed to move freely for three minutes. Grooming frequency, rearing frequency, freezing frequency, and center line crossing were recorded over 5 minutes using a camera. The maze was cleaned with alcohol between trials.
Euthanasia and tissue collection
At the end of the experiment, mice (n=2 per group) were anesthetized using ketamine and xylazine cocktail (1 mL of ketamine (100 mg/mL), 8.5 mL of phosphate-buffered saline, and 0.5 mL of xylazine). A 0.2 mL dose of the cocktail was administered to 20 g mice, after which the thoracic region was opened to expose the heart. The animals were perfused using phosphate-buffered saline (PBS) and the brain tissues were collected and then transferred to PBS until they sank. The tissues were embedded in paraffin.
Immunohistochemical staining
The brain tissues were sectioned, dewaxed in water, subjected to antigen retrieval, and blocked with bovine serum albumin (BSA) to prevent nonspecific protein binding. The tissues were incubated overnight at 4 °C with the following primary antibodies: Rabbit anti-tyrosine hydroxylase TH (ThermoFisher, USA; #PA5-85167) at 1:1500, rabbit anti-ionized calcium-binding adapter molecule 1 IBA1 (Cell Signaling, USA; #17198) at 1:1250, and rabbit anti-α-synuclein antibody (Cell Signaling Technology). Subsequently, the tissues were washed on a decolorization shaker with PBS and then incubated with the corresponding secondary antibody at room temperature for 50 min. After mounting the sections, they were observed under a fluorescence microscope and images were captured.
Biochemical analyses
Approximately 0.1- 0.2 g of brain tissue was rinsed and weighed in ice-cold normal saline and put into a 5 mL homogenizing tube. With water bathing, the corresponding volume of the homogenization medium (0.86% normal saline) was added into the glass homogenizing tube according to the ratio of weight (g) to volume (mL)=1:9. The tissue was fully ground to render a 10% homogenate. The prepared homogenate was centrifuged at 2500 rpm for 15 min, and the supernatant was used for detection. In addition to the brain, biochemical analyses of serum, liver, and heart tissues were conducted to comprehensively evaluate the systemic and organ-specific effects. The following detection kits were used: SOD detection kit (WST-1, USA; E-BC-K020-M), CAT detection kit (WST-1, USA; E-BC-K031-S), GSH-Px detection kit (E-BC-K096-M), MDA detection kit (TBARS Microplate, PN: FR40); TNF-α detection kit (ELISA, USA; E-EL-R001996T), and IL-1α detection kit (ELISA, USA; E-EL-R001296T).
Statistical analysis
Results are expressed as Mean±SD and Mean±SEM. Data were analyzed using GraphPad Prism software, version 9.5.1 (GraphPad Software, San Diego, CA, USA). Statistical evaluations of the differences between the group mean values were tested by one-way analysis of variance (ANOVA), followed by the Tukey Post – hoc test. Differences were considered statistically significant at P<0.05.
Results
Neurobehavioral activity
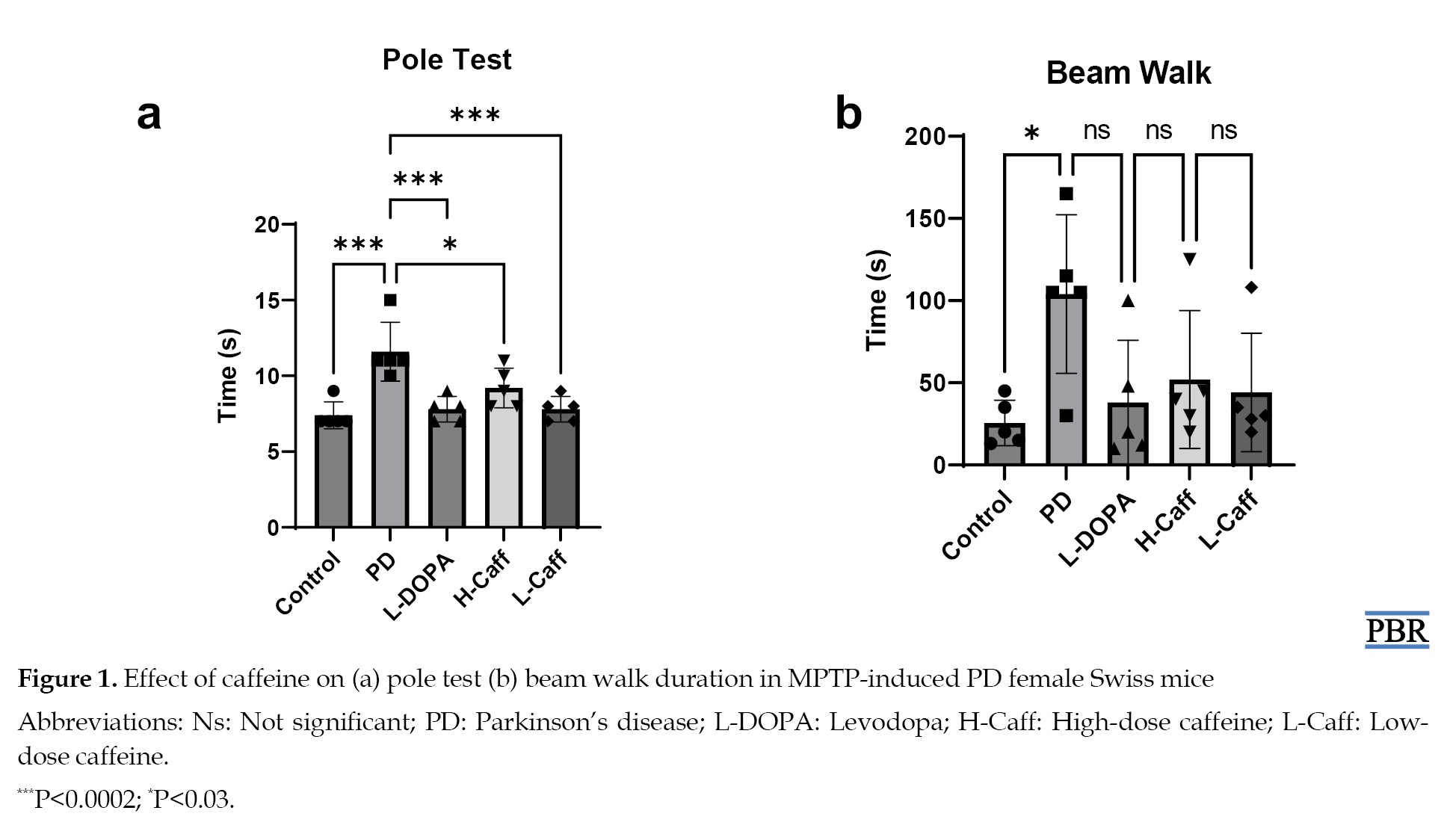
Caffeine enhanced motor performance
MPTP-treated mice exhibited significant motor dysfunction, characterized by an increased time on the pole test compared to the control group. Caffeine administration significantly (P<0.05) improved motor performance by reducing the time spent on the pole (Figure 1). Similarly, MPTP-induced mice showed a significantly reduced ability in the beam-walk test, with significantly (P<0.05) longer walking times compared to the control group. Treatment with caffeine alleviated this motor dysfunction by reducing the walking times (Figure 1).
Caffeine reduced anxiety
A significant reduction (P<0.05) in rearing frequency was observed in low caffeine-administered mice compared to control. However, both caffeine-treated groups demonstrated a significant increase (P<0.05) in rearing frequency compared to MPTP-induced mice (Table 1).
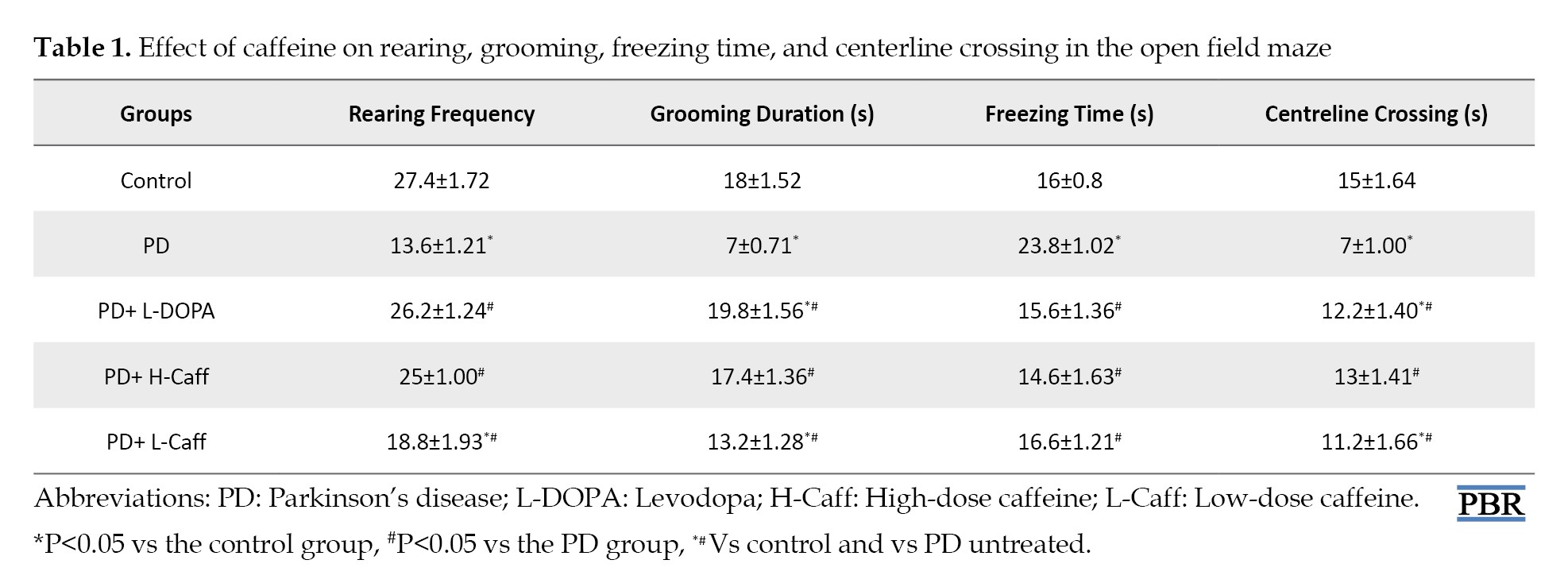
Mice treated with low-dose caffeine exhibited a significant reduction (P<0.05) in grooming time compared to the controls, while the high-dose caffeine group showed no significant difference compared to the controls. Both caffeine-treated groups displayed a significant increase (P<0.05) in grooming time compared to the MPTP-induced group (Table 1).
There were no significant differences in freezing frequency between the caffeine-treated groups and controls, but both caffeine-treated groups showed a significant reduction (P<0.05) in freezing frequency compared to MPTP-induced mice (Table 1).
Finally, there was a significant reduction (P<0.05) in centerline crossing in low caffeine-treated groups compared to controls. However, compared to MPTP-induced mice, both caffeine-treated groups showed a significant increase (P<0.05) in centerline crossing (Table 1).
Brain biochemical analysis
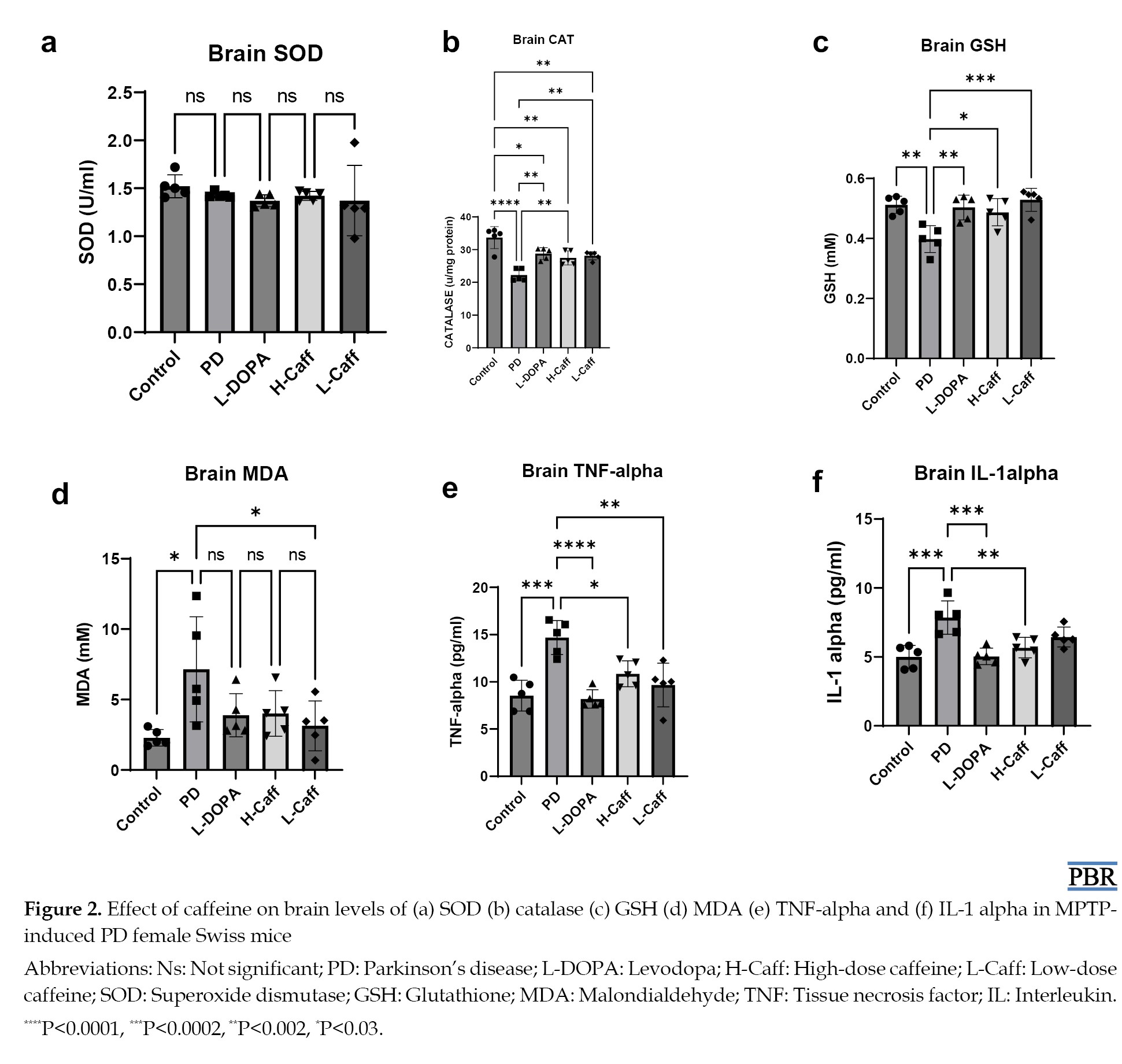
Caffeine increased antioxidant activity and reduced oxidative stress
No significant differences in SOD levels were observed across the groups (Figure 2a). However, the levels of CAT and GSH in the brain tissue were significantly elevated in both caffeine-treated groups compared to the MPTP-induced group (Figures 2b and 2c). In contrast, MDA levels were significantly reduced in the low caffeine-treated groups compared to the MPTP group (Figure 2d).
Caffeine reduced neuroinflammation
TNF-α and IL-1α levels in the brain tissue were significantly elevated in the MPTP-induced group compared to controls. Caffeine treatment significantly reduced TNF-α and IL-1α levels compared to the MPTP group (Figures 2e and 2f).
Serum biochemical analysis
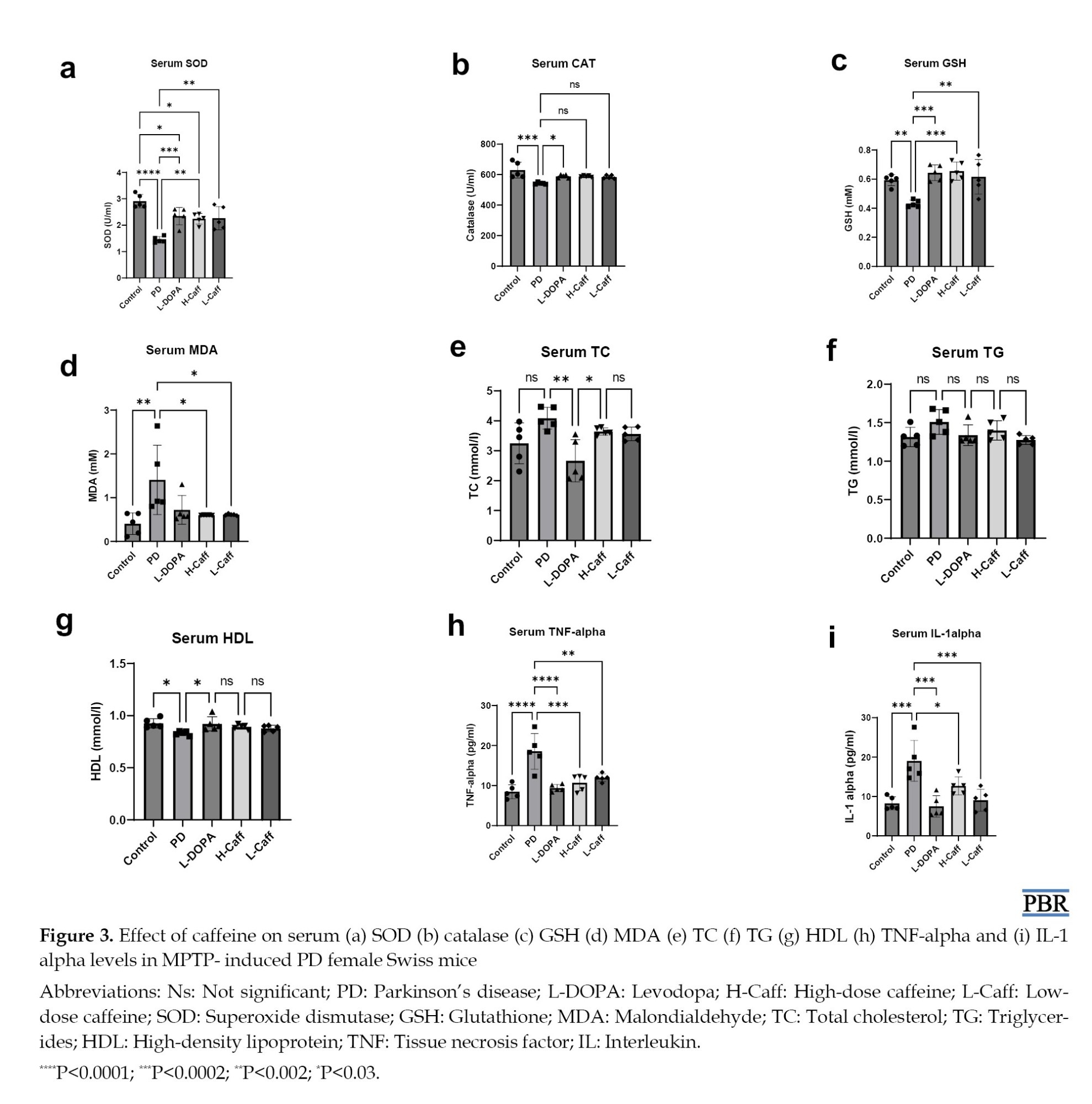
Caffeine increased antioxidant activity and reduced serum oxidative stress markers
Serum SOD levels were significantly (P<0.05) lower in the MPTP-induced group compared to the control group. Both caffeine-treated groups showed a marked increase in SOD levels relative to the MPTP group (Figure 3a). Moreover, serum CAT activity was significantly reduced in the MPTP-induced group compared to the control group. Although all treated groups showed a trend toward increased CAT activity, only the L-DOPA-treated group displayed a statistically significant (P<0.05) increase (Figure 3b).
Furthermore, a significant (P<0.05) decrease in serum GSH levels was observed in the MPTP-induced group compared to the control group. Treatment with caffeine and L-DOPA caused a significant increase in GSH levels relative to the MPTP group (Figure 3c).
Also, serum MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. Treatment with caffeine showed a significant reduction in MDA levels compared to the MPTP group, and no significant differences were observed between the treated groups and the control group (Figure 3d).
Caffeine caused no alteration in the serum lipid profile
There was no significant difference in serum levels of TC, TG, and HDL between the MPTP-induced, control, and treatment groups (Figures 3e and 3g). However, the L-DOPA group exhibited a significant (P<0.05) reduction in TC and HDL levels compared to the MPTP-induced group (Figure 3e).
Caffeine reduced serum inflammatory makers
Serum TNF-α levels were significantly (P<0.05) increased in the MPTP-induced group compared to the control group. Groups treated with caffeine exhibited a significant reduction in TNF-α levels compared to the MPTP group, with no significant differences between the treatment groups and the control group (Figure 3h).
Similarly, serum IL-1α levels were significantly higher in the MPTP-induced group compared to the control group. Groups treated with caffeine showed a significant (P<0.05) reduction in IL-1 alpha levels relative to the MPTP group (Figure 3i).
Heart biochemical parameters
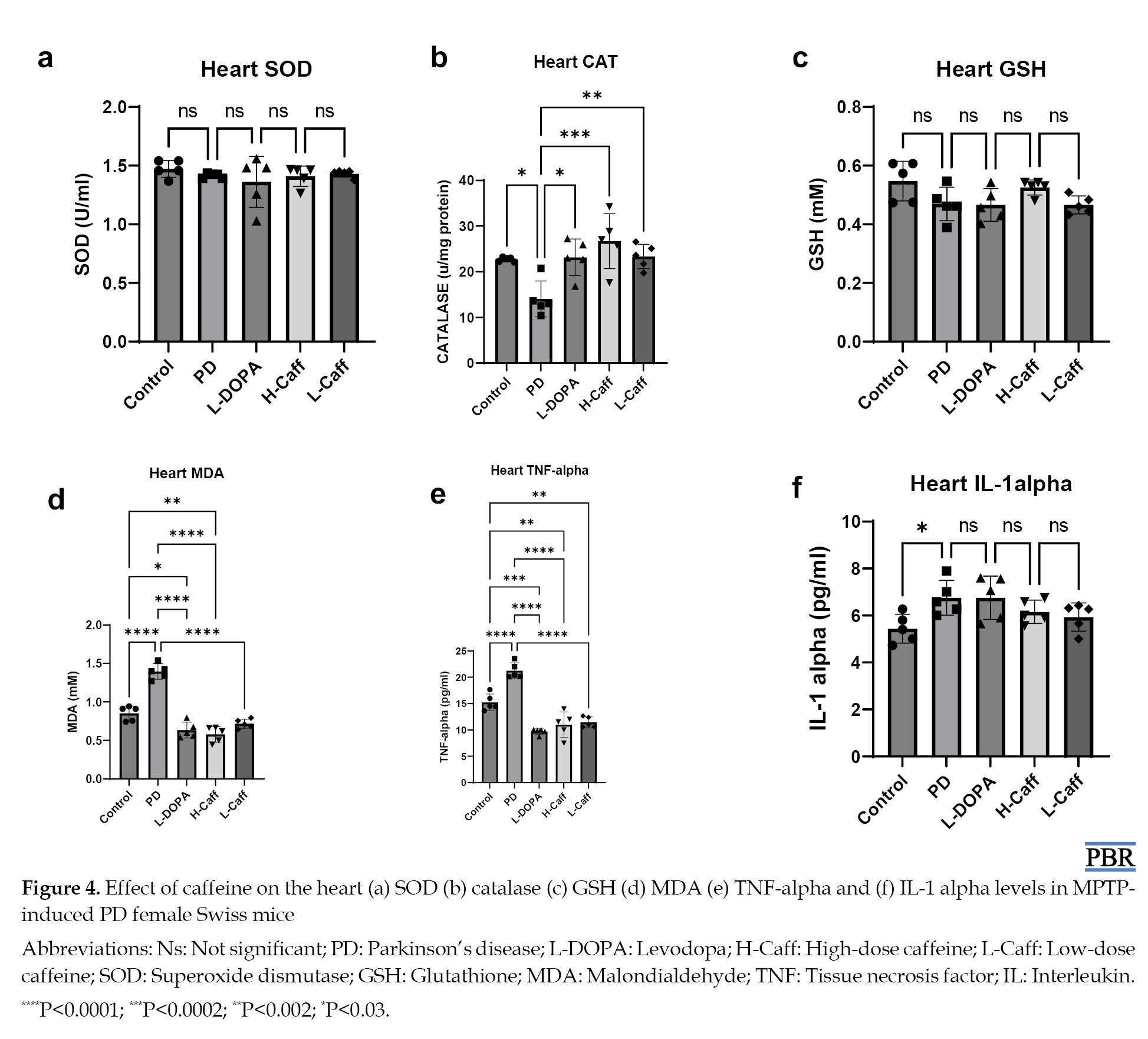
Caffeine increased heart antioxidant activity and reduced oxidative stress
SOD levels in the heart showed no significant (P>0.05) differences across all experimental groups, including the MPTP-induced and treatment groups (Figure 4a). Moreover, heart CAT activity was significantly (P<0.05) reduced in the MPTP-induced group compared to the control group. All treatment groups exhibited a significant increase in CAT activity compared to the MPTP group. However, there were no significant differences between the treatment groups and the control group (Figure 4b).
However, no significant (P>0.05) differences in GSH levels were observed in the heart across any of the groups, including the MPTP-induced and treated groups (Figure 4c).
Also, heart MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups showed a significant reduction in MDA levels relative to the MPTP group (Figure 4d).
Caffeine reduced heart inflammatory markers
TNF-α levels in the heart were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups exhibited a significant reduction in TNF-α levels compared to the MPTP group (Figure 4e).
Moreover, heart IL-1α levels were significantly (P<0.05) elevated in both the MPTP-induced compared to the control group. However, there were no significant differences in IL-1α levels across the treatment groups (Figure 4f).
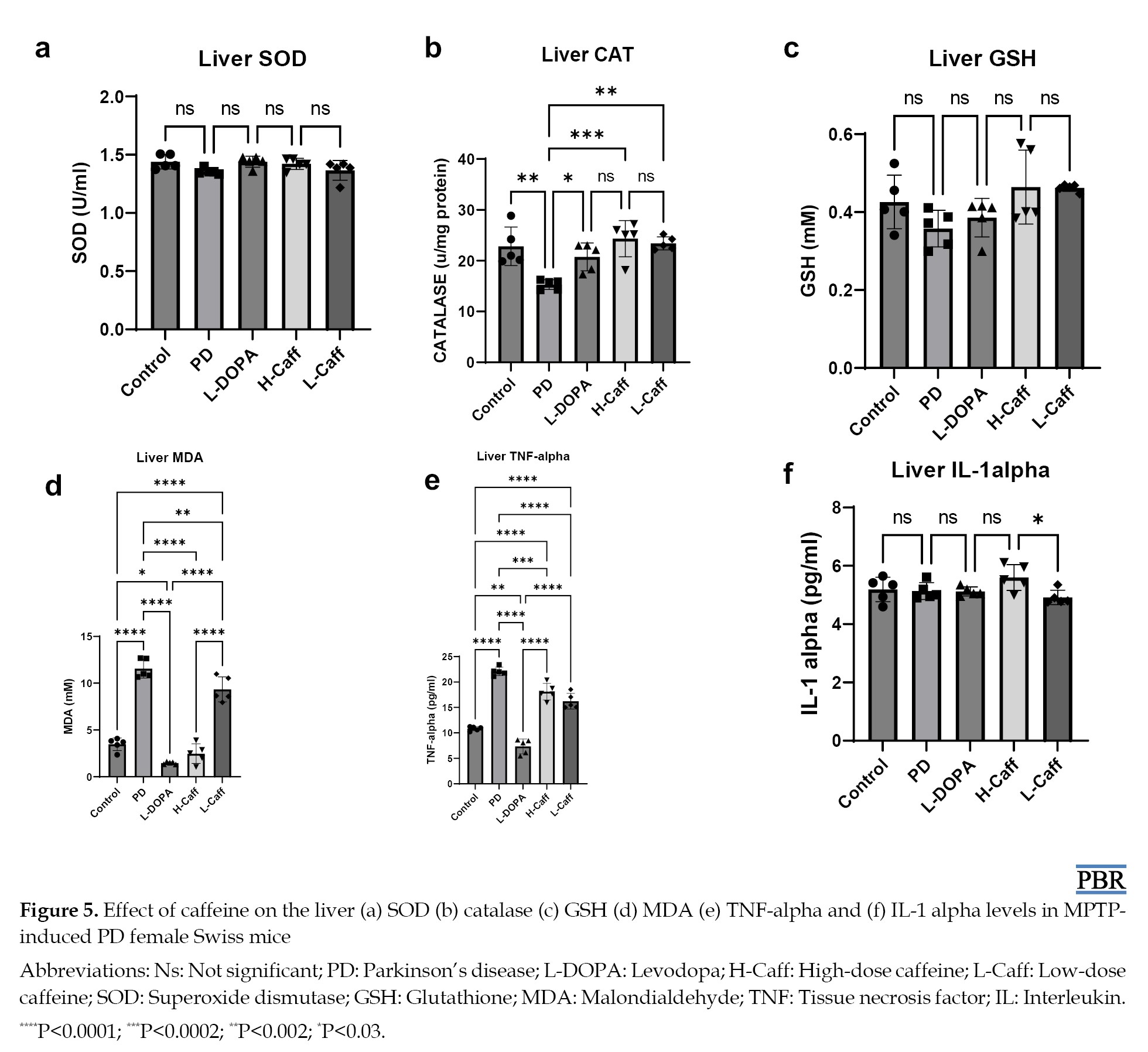
Liver biochemical parameters
Caffeine increased liver antioxidant activity and reduced oxidative stress
Liver SOD levels showed no significant differences across any of the groups (Figure 5a). Moreover, CAT activity in the liver was significantly (P<0.05) reduced in the MPTP-induced group compared to the control group. All treated groups exhibited a significant increase in CAT activity compared to the MPTP group, but no significant differences were observed between the treated groups and the control group (Figure 5b).
However, liver GSH levels did not show any significant differences between the MPTP-induced group and the control group (Figure 5c).
Also, liver MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups exhibited significant reductions in MDA levels compared to the MPTP group (Figure 5d).
Caffeine reduced liver inflammatory markers
Liver TNF-α levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treated groups showed significant reductions in TNF-α levels compared to the MPTP group (Figure 5e).
However, no significant differences in liver IL-1α levels were observed between any of the groups, including the MPTP-induced and control groups (Figure 5f).
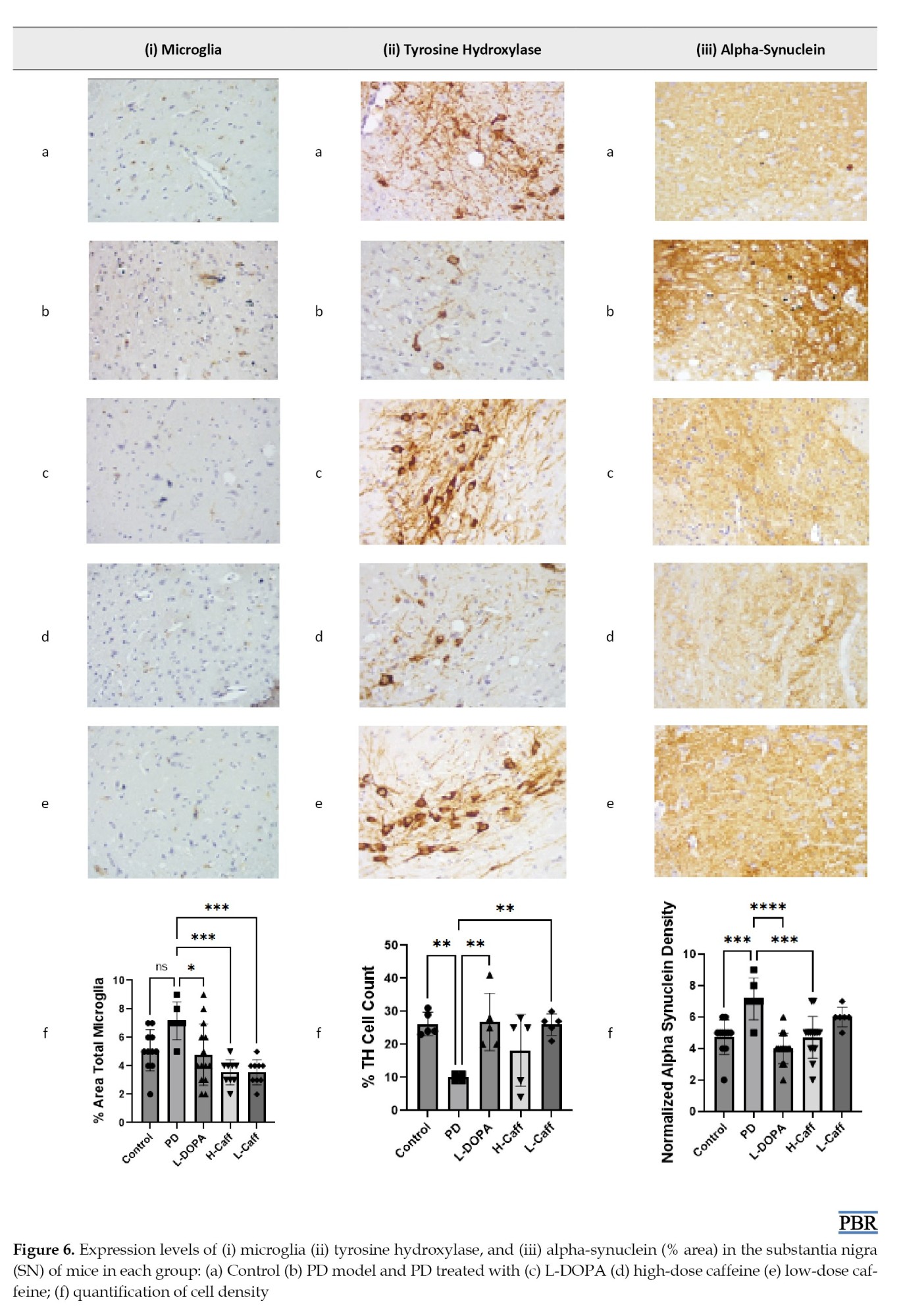
Immunohistochemical analysis
Total microglia count
A significant increase in total microglia (Iba-1 expression) was observed in the MPTP-induced group compared to the control group. All treatment groups showed a significant (P<0.05) reduction in Iba-1 expression in the brain compared to the MPTP-induced group (Figure 6).
Caffeine’s effect on neurodegeneration in the substantia nigra
TH-positive cells in the substantia nigra were significantly (P<0.05) reduced in the MPTP-induced group compared to both the control and treatment groups (Figure 6).
Reduction of alpha-synucleinopathy in the substantia nigra
The expression of α-synuclein in the substantia nigra was significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. The groups treated with low-dose caffeine and L-DOPA exhibited significantly (P<0.05) lower α-synuclein levels compared to the MPTP-induced group (Figure 6).
Discussion
PD is characterized by progressive neurodegeneration, particularly in the dopaminergic neurons of the substantia nigra, leading to motor dysfunction and other debilitating symptoms [1]. While various treatments aim to manage PD symptoms, there remains an ongoing search for neuroprotective agents that can slow disease progression. In this context, caffeine has emerged as a promising compound due to its antioxidant, anti-inflammatory, and neuroprotective properties [12, 13] such as PD, prompting an investigation into its potential therapeutic effects in PD models.
The observed improvement in motor performance, as evidenced by reduced times on the pole and beam-walk tests, is consistent with studies that have reported similar outcomes. For instance, caffeine administration improved motor coordination in 6-hydroxydopamine (6-OHDA)-induced PD models, correlating with enhanced dopaminergic activity [14]. Similarly, caffeine’s action on adenosine A2A receptors, which are known to influence motor control [15], may mediate these effects. The results of the current study reinforce the idea that caffeine may counteract the motor deficits associated with dopaminergic degeneration by enhancing neurotransmission pathways that support motor function. Moreover, the significant reduction in rearing frequency in caffeine-treated mice suggests an anxiolytic effect, which is further substantiated by the significant increase in rearing frequency compared to MPTP-induced mice. The anxiolytic effects of caffeine may be attributed to its role as an antagonist of adenosine receptors, which has been shown to influence anxiety-related behavior [16]. Additionally, the significant reduction in freezing frequency and center line duration in caffeine-treated groups compared to MPTP-induced mice indicates that caffeine may ameliorate anxiety-related behaviors commonly associated with stress and fear. This is consistent with studies indicating that caffeine’s effects can modulate anxiety-like behaviors [16].
The results from the brain biochemical analysis demonstrated that caffeine enhances antioxidant activity and mitigates neuroinflammation in MPTP-induced PD models. The observed elevation in brain CAT and GSH levels, the significant decrease in MDA, a marker of lipid peroxidation and oxidative stress, as well as an increase in serum SOD levels in the caffeine-treated groups indicates enhanced antioxidant capacity. This is consistent with studies indicating that caffeine can boost endogenous antioxidant defenses [12, 17] such as PD, thereby reducing oxidative stress. Similar outcomes from the heart and liver tissues show caffeine’s potential to enhance antioxidant defenses and mitigate oxidative damage in neurodegenerative models. As previously reported in other models [16], the protective effects of caffeine may be mediated through nervous and systemic antioxidants in this particular model.
Moreover, the significant reduction in TNF-α and IL-1α levels in the brain and serum of caffeine-treated mice compared to the MPTP-induced group highlights caffeine’s anti-inflammatory properties. Neuroinflammation is a critical component of PD pathology, and the ability of caffeine to modulate inflammatory cytokines is well-documented. A recent review indicated that caffeine could significantly reduce pro-inflammatory cytokines in experimental models of neurodegeneration, suggesting a neuroprotective mechanism against inflammation-related neuronal damage [18]. In this study, caffeine demonstrated significant anti-inflammatory effects by reducing pro-inflammatory cytokines, such as TNF-α and IL-1α in the brain, serum, heart, and liver tissues. These results highlight caffeine’s potential as a neuroprotective agent, particularly through its anti-inflammatory mechanisms in this PD model. Notably, we found that caffeine did not significantly alter serum lipid profile. These results suggest that caffeine’s primary effects in this model may lie in its antioxidant and anti-inflammatory properties rather than lipid modulation.
Furthermore, the immunohistochemical analysis revealed significant insights into the effects of caffeine on microglial activation, neurodegeneration, and protein aggregation in the MPTP-induced model of PD. The observed significant increase in total microglia (as indicated by Iba-1 expression) in the MPTP-induced group is consistent with numerous studies demonstrating that microglial activation is a hallmark of neuroinflammation in neurodegenerative diseases, including PD. Microglia, the resident immune cells of the brain, become activated in response to neuronal injury, and their increased count indicates a neuroinflammatory response [19, 20]. This heightened activation can contribute to neuronal degeneration and loss. In contrast, the significant reduction in Iba-1 expression in all treatment groups suggests that caffeine administration effectively mitigates microglial activation. This finding is supported by research indicating that caffeine possesses anti-inflammatory properties that can attenuate microglial activation and neuroinflammation [10]. The results imply that caffeine’s mechanism of action may involve the modulation of microglial activity, leading to decreased neuroinflammation and potential protection against neurodegeneration.
Moreover, the significant reduction in TH-positive cells in the substantia nigra of the MPTP-induced group underscores the neurodegenerative impact of MPTP. Furthermore, the preservation of TH-positive cells in the caffeine treatment groups indicates that caffeine may protect dopaminergic neurons from MPTP-induced degeneration. Previous research found that caffeine administration preserved dopaminergic neuronal integrity and function in Caenorhabditis elegans models [21], supporting the notion that caffeine has neuroprotective effects in the context of PD. The mechanism behind this protective effect may involve the inhibition of neuroinflammation and oxidative stress, both of which contribute to dopaminergic neuronal loss.
In addition, the elevated expression of α-synuclein in the MPTP-induced group highlights the pathological aggregation of this protein, which is a central feature of PD pathology. Increased α-synuclein levels can contribute to neuronal dysfunction and death, as aggregated forms of this protein are toxic to neurons [22]. Importantly, the significant reduction in α-synuclein levels in the treatment groups compared to the MPTP-induced group suggests that caffeine may attenuate the pathological accumulation of this protein.
Conclusion
This study provides evidence that caffeine exerts neuroprotective effects in an MPTP-induced model of PD in female Swiss mice. Our findings demonstrate that caffeine administration significantly improves motor performance and reduces anxiety-like behaviors, underscoring its potential role in enhancing the quality of life for individuals with PD. Biochemically, caffeine effectively increases antioxidant activity and decreases oxidative stress markers in both the brain and serum, suggesting a systemic and localized protective mechanism against neurodegeneration. Furthermore, the ability of caffeine to attenuate neuroinflammation, as evidenced by reduced levels of TNF-α and IL-1α, highlights its anti-inflammatory properties, which are crucial for mitigating the pathophysiological processes associated with PD. The molecular basis of these outcomes could be attributed to the reduced microglial activation and preservation of dopaminergic neurons in the substantia nigra, along with decreased α-synuclein expression. Collectively, these results advocate for the potential therapeutic application of caffeine in managing PD, warranting further investigation into its mechanisms and efficacy in clinical settings. Future research could elucidate the precise molecular pathways through which caffeine exerts its protective effects and explore optimal dosing strategies for therapeutic use.
Ethical Considerations
Compliance with ethical guidelines
The animals were treated humanely in accordance with the care and use of animals of the Ethical Committee of the Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria (UERC approval No.: UERC/ASN/2024/2686).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Supervision, and Validation: Aminat Omolola Imam-Fulani; Data curation: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, Halimat Oluwakemi Afolabi, Jerry Inyang, Oluwadamilola Onoshi Onoshi, Taofeekat Temitope Ogunsesan, Oyindamola Bisola Adekunle, Taiwo Elijah Eso, Oluwaseyi Deborah Babatunde, Edwin Ayolola Makanjuola, Olamilekan Abdulfatai Alimi, Halimat Oluwakemi Afolabi; Visualization, formal analysis and writing of the original draft: Aminat Omolola Imam-Fulani and Abdulrahman Olanrewaju Shuaib; Investigation: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, Halimat Oluwakemi Afolabi, Jerry Inyang, Oluwadamilola Onoshi Onoshi, Taofeekat Temitope Ogunsesan, Oyindamola Bisola Adekunle, Taiwo Elijah Eso, Oluwaseyi Deborah Babatunde, Edwin Ayolola Makanjuola, Olamilekan Abdulfatai Alimi, and Hafeez Ajibola Olorunoje; Methodology: Aminat Omolola Imam-Fulani, AOS; Project administration: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Halimat Oluwakemi Afolabi, Jerry Inyang; Resources; Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide; Software: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, and Kamaldeen Olalekan Sanusi; Review and editing: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, and Kamaldeen Olalekan Sanusi.
Conflict of interest
The authors reported no conflict of interest.
Acknowledgments
The authors would like to express their appreciation for the efforts of Garba Abdulrasheed, the laboratory technologist of the Department of Physiology at the University of Ilorin, Ilorin, Nigeria.
References
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to motor dysfunction and a variety of non-motor symptoms [1]. It is the second most common neurodegenerative disorder, affecting millions worldwide. While the precise etiology of PD remains elusive, oxidative stress, neuroinflammation, and the accumulation of misfolded proteins, such as α-synuclein are widely recognized as critical contributors to the pathophysiology of the disease [2-4] a neurodegenerative disorder characterized by distinct aging-independent loss of dopaminergic neurons in substantia nigra pars compacta (SNpc). These pathological processes not only compromise neuronal integrity but also activate glial cells, leading to a cycle of inflammation and neurodegeneration [5].
Caffeine, a widely consumed stimulant found in coffee and kola nut, has garnered attention for its potential neuroprotective properties [6]. Epidemiological studies have suggested an inverse relationship between caffeine consumption and the risk of developing PD, prompting research into its mechanistic effects on neuroprotection [7]. Caffeine is known to exert antioxidant effects, enhancing the activity of key enzymes, such as superoxide dismutase (SOD) and catalase (CAT), which mitigate oxidative stress by neutralizing reactive oxygen species [8, 9]. Additionally, caffeine’s ability to modulate neuroinflammation by inhibiting pro-inflammatory cytokines presents a promising avenue for therapeutic intervention [10].
In this study, we aimed to investigate the effects of caffeine on neurobehavioral performance, oxidative stress markers, and inflammatory mediators in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced model of PD in female Swiss mice. We hypothesized that caffeine would enhance antioxidant defense, reduce oxidative stress, and attenuate neuroinflammation, thereby preserving dopaminergic neurons and improving motor function. Through a comprehensive analysis of biochemical, immunohistochemical, and behavioral outcomes, this study elucidated the potential of caffeine as a therapeutic agent for mitigating the effects of PD. This study uniquely highlights the systemic and organ-specific effects of caffeine in mitigating MPTP-induced neurodegeneration, employing a comprehensive approach that integrates serum and tissue-specific biochemical analyses in female mice—a less frequently studied cohort—offering novel insights into sex-specific therapeutic implications for PD.
Materials and methods
Experimental animals
Twenty-five female Swiss mice weighing between 20–30 g were obtained from the Department of Biochemistry, University of Ilorin, Ilorin, Nigeria. The animals were housed at the College of Health Sciences, University of Ilorin. The animals were kept at a temperature of 22–25 °C, 55% relative humidity, and under a 12-hour light/dark cycle. The mice had free access to food and water and were acclimatized for two weeks before conducting any training, treatment, or experiment. After acclimatization, required neurobehavioral training was conducted for three consecutive days before the induction of PD and treatments.
Drug preparation and treatments
Preparation of caffeine
One gram of caffeine (Sigma-Aldrich; USA) was dissolved in 200 mL of distilled water. The mixture was prepared every two days and stored in the refrigerator.
Preparation of MPTP
PD induction using MPTP followed an established method [11] where 100 mg of MPTP powder (Sigma-Aldrich, Product number: M0896; USA) was dissolved in 10 mL of 0.9% normal saline. The solution was divided into ten 1.5 mL Eppendorf tubes and stored at –20 °C. Prior to use, the solution was diluted to a final concentration of 5 mg/mL.
PD induction using MPTP
Mice were given a daily intraperitoneal (ip) injection of 25 mg/kg MPTP for five days to establish the PD model. Mice that failed the motor tests (latency of more than 9 seconds on the pole test and falling off and/or walking longer than 60 seconds on the beam test) were included in the PD model experimental groups. Mice that passed the motor test were excluded from the experiment.
Experimental design
A healthy mice group, control (n=5), received normal saline (vehicle) while the PD model experimental groups were divided into four groups (n=5 each):
The MPTP induced-PD group administered 0.2 mL normal saline (vehicle) (PD).
The MPTP-induced PD group was treated with levodopa (10 mg/kg, daily, p.o.) (L-DOPA).
The MPTP-induced PD group administered high-dose caffeine (10 mg/kg, daily, [ip]) (H-Caf).
The MPTP-induced PD group administered low-dose caffeine (5 mg/kg, daily, [ip]) (L-Caf).
After the last dose administration, the animals were subjected to behavioral tests, including the pole test, beam-walk test, and open field test.
Behavioral tests
Pole test
The pole test is often used to assess motor coordination in PD mice. A metal rod with a diameter of 1 cm and a length of 50 cm was wrapped with black tape to prevent the mice from slipping, and the bottom of the metal rod was placed in a cushioned cage. Each mouse was placed head down on the top of the rod, and the time it took to return to the cage freely was recorded, ending when the hind limbs reached the bottom of the cage. Before the formal tests, the mice were trained for two days.
Beam walk test
The beam walk test is used to analyze mice’s gait in an environment that challenges their ability to balance themselves. A narrow beam (1-3 cm wide) was elevated between a pole and their home cage (to attract the mice to the finish point). The mice were placed at one end of the narrow beam and allowed to walk across the narrow beam from the start end to the cage with feed. The time it takes a mouse to move from start to end was recorded.
Open field test
The open-field test was conducted in a square arena with dimensions of 50 cm × 50 cm × 40 cm (length, width, and height). The arena was divided into 25 squares. During the experiment, mice were placed in the center of the open field maze and allowed to move freely for three minutes. Grooming frequency, rearing frequency, freezing frequency, and center line crossing were recorded over 5 minutes using a camera. The maze was cleaned with alcohol between trials.
Euthanasia and tissue collection
At the end of the experiment, mice (n=2 per group) were anesthetized using ketamine and xylazine cocktail (1 mL of ketamine (100 mg/mL), 8.5 mL of phosphate-buffered saline, and 0.5 mL of xylazine). A 0.2 mL dose of the cocktail was administered to 20 g mice, after which the thoracic region was opened to expose the heart. The animals were perfused using phosphate-buffered saline (PBS) and the brain tissues were collected and then transferred to PBS until they sank. The tissues were embedded in paraffin.
Immunohistochemical staining
The brain tissues were sectioned, dewaxed in water, subjected to antigen retrieval, and blocked with bovine serum albumin (BSA) to prevent nonspecific protein binding. The tissues were incubated overnight at 4 °C with the following primary antibodies: Rabbit anti-tyrosine hydroxylase TH (ThermoFisher, USA; #PA5-85167) at 1:1500, rabbit anti-ionized calcium-binding adapter molecule 1 IBA1 (Cell Signaling, USA; #17198) at 1:1250, and rabbit anti-α-synuclein antibody (Cell Signaling Technology). Subsequently, the tissues were washed on a decolorization shaker with PBS and then incubated with the corresponding secondary antibody at room temperature for 50 min. After mounting the sections, they were observed under a fluorescence microscope and images were captured.
Biochemical analyses
Approximately 0.1- 0.2 g of brain tissue was rinsed and weighed in ice-cold normal saline and put into a 5 mL homogenizing tube. With water bathing, the corresponding volume of the homogenization medium (0.86% normal saline) was added into the glass homogenizing tube according to the ratio of weight (g) to volume (mL)=1:9. The tissue was fully ground to render a 10% homogenate. The prepared homogenate was centrifuged at 2500 rpm for 15 min, and the supernatant was used for detection. In addition to the brain, biochemical analyses of serum, liver, and heart tissues were conducted to comprehensively evaluate the systemic and organ-specific effects. The following detection kits were used: SOD detection kit (WST-1, USA; E-BC-K020-M), CAT detection kit (WST-1, USA; E-BC-K031-S), GSH-Px detection kit (E-BC-K096-M), MDA detection kit (TBARS Microplate, PN: FR40); TNF-α detection kit (ELISA, USA; E-EL-R001996T), and IL-1α detection kit (ELISA, USA; E-EL-R001296T).
Statistical analysis
Results are expressed as Mean±SD and Mean±SEM. Data were analyzed using GraphPad Prism software, version 9.5.1 (GraphPad Software, San Diego, CA, USA). Statistical evaluations of the differences between the group mean values were tested by one-way analysis of variance (ANOVA), followed by the Tukey Post – hoc test. Differences were considered statistically significant at P<0.05.
Results
Neurobehavioral activity
Caffeine enhanced motor performance
MPTP-treated mice exhibited significant motor dysfunction, characterized by an increased time on the pole test compared to the control group. Caffeine administration significantly (P<0.05) improved motor performance by reducing the time spent on the pole (Figure 1). Similarly, MPTP-induced mice showed a significantly reduced ability in the beam-walk test, with significantly (P<0.05) longer walking times compared to the control group. Treatment with caffeine alleviated this motor dysfunction by reducing the walking times (Figure 1).
Caffeine reduced anxiety
A significant reduction (P<0.05) in rearing frequency was observed in low caffeine-administered mice compared to control. However, both caffeine-treated groups demonstrated a significant increase (P<0.05) in rearing frequency compared to MPTP-induced mice (Table 1).

Mice treated with low-dose caffeine exhibited a significant reduction (P<0.05) in grooming time compared to the controls, while the high-dose caffeine group showed no significant difference compared to the controls. Both caffeine-treated groups displayed a significant increase (P<0.05) in grooming time compared to the MPTP-induced group (Table 1).
There were no significant differences in freezing frequency between the caffeine-treated groups and controls, but both caffeine-treated groups showed a significant reduction (P<0.05) in freezing frequency compared to MPTP-induced mice (Table 1).
Finally, there was a significant reduction (P<0.05) in centerline crossing in low caffeine-treated groups compared to controls. However, compared to MPTP-induced mice, both caffeine-treated groups showed a significant increase (P<0.05) in centerline crossing (Table 1).
Brain biochemical analysis
Caffeine increased antioxidant activity and reduced oxidative stress
No significant differences in SOD levels were observed across the groups (Figure 2a). However, the levels of CAT and GSH in the brain tissue were significantly elevated in both caffeine-treated groups compared to the MPTP-induced group (Figures 2b and 2c). In contrast, MDA levels were significantly reduced in the low caffeine-treated groups compared to the MPTP group (Figure 2d).
Caffeine reduced neuroinflammation
TNF-α and IL-1α levels in the brain tissue were significantly elevated in the MPTP-induced group compared to controls. Caffeine treatment significantly reduced TNF-α and IL-1α levels compared to the MPTP group (Figures 2e and 2f).
Serum biochemical analysis
Caffeine increased antioxidant activity and reduced serum oxidative stress markers
Serum SOD levels were significantly (P<0.05) lower in the MPTP-induced group compared to the control group. Both caffeine-treated groups showed a marked increase in SOD levels relative to the MPTP group (Figure 3a). Moreover, serum CAT activity was significantly reduced in the MPTP-induced group compared to the control group. Although all treated groups showed a trend toward increased CAT activity, only the L-DOPA-treated group displayed a statistically significant (P<0.05) increase (Figure 3b).
Furthermore, a significant (P<0.05) decrease in serum GSH levels was observed in the MPTP-induced group compared to the control group. Treatment with caffeine and L-DOPA caused a significant increase in GSH levels relative to the MPTP group (Figure 3c).
Also, serum MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. Treatment with caffeine showed a significant reduction in MDA levels compared to the MPTP group, and no significant differences were observed between the treated groups and the control group (Figure 3d).
Caffeine caused no alteration in the serum lipid profile
There was no significant difference in serum levels of TC, TG, and HDL between the MPTP-induced, control, and treatment groups (Figures 3e and 3g). However, the L-DOPA group exhibited a significant (P<0.05) reduction in TC and HDL levels compared to the MPTP-induced group (Figure 3e).
Caffeine reduced serum inflammatory makers
Serum TNF-α levels were significantly (P<0.05) increased in the MPTP-induced group compared to the control group. Groups treated with caffeine exhibited a significant reduction in TNF-α levels compared to the MPTP group, with no significant differences between the treatment groups and the control group (Figure 3h).
Similarly, serum IL-1α levels were significantly higher in the MPTP-induced group compared to the control group. Groups treated with caffeine showed a significant (P<0.05) reduction in IL-1 alpha levels relative to the MPTP group (Figure 3i).
Heart biochemical parameters
Caffeine increased heart antioxidant activity and reduced oxidative stress
SOD levels in the heart showed no significant (P>0.05) differences across all experimental groups, including the MPTP-induced and treatment groups (Figure 4a). Moreover, heart CAT activity was significantly (P<0.05) reduced in the MPTP-induced group compared to the control group. All treatment groups exhibited a significant increase in CAT activity compared to the MPTP group. However, there were no significant differences between the treatment groups and the control group (Figure 4b).
However, no significant (P>0.05) differences in GSH levels were observed in the heart across any of the groups, including the MPTP-induced and treated groups (Figure 4c).
Also, heart MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups showed a significant reduction in MDA levels relative to the MPTP group (Figure 4d).
Caffeine reduced heart inflammatory markers
TNF-α levels in the heart were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups exhibited a significant reduction in TNF-α levels compared to the MPTP group (Figure 4e).
Moreover, heart IL-1α levels were significantly (P<0.05) elevated in both the MPTP-induced compared to the control group. However, there were no significant differences in IL-1α levels across the treatment groups (Figure 4f).
Liver biochemical parameters
Caffeine increased liver antioxidant activity and reduced oxidative stress
Liver SOD levels showed no significant differences across any of the groups (Figure 5a). Moreover, CAT activity in the liver was significantly (P<0.05) reduced in the MPTP-induced group compared to the control group. All treated groups exhibited a significant increase in CAT activity compared to the MPTP group, but no significant differences were observed between the treated groups and the control group (Figure 5b).
However, liver GSH levels did not show any significant differences between the MPTP-induced group and the control group (Figure 5c).
Also, liver MDA levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treatment groups exhibited significant reductions in MDA levels compared to the MPTP group (Figure 5d).
Caffeine reduced liver inflammatory markers
Liver TNF-α levels were significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. All treated groups showed significant reductions in TNF-α levels compared to the MPTP group (Figure 5e).
However, no significant differences in liver IL-1α levels were observed between any of the groups, including the MPTP-induced and control groups (Figure 5f).
Immunohistochemical analysis
Total microglia count
A significant increase in total microglia (Iba-1 expression) was observed in the MPTP-induced group compared to the control group. All treatment groups showed a significant (P<0.05) reduction in Iba-1 expression in the brain compared to the MPTP-induced group (Figure 6).
Caffeine’s effect on neurodegeneration in the substantia nigra
TH-positive cells in the substantia nigra were significantly (P<0.05) reduced in the MPTP-induced group compared to both the control and treatment groups (Figure 6).
Reduction of alpha-synucleinopathy in the substantia nigra
The expression of α-synuclein in the substantia nigra was significantly (P<0.05) elevated in the MPTP-induced group compared to the control group. The groups treated with low-dose caffeine and L-DOPA exhibited significantly (P<0.05) lower α-synuclein levels compared to the MPTP-induced group (Figure 6).
Discussion
PD is characterized by progressive neurodegeneration, particularly in the dopaminergic neurons of the substantia nigra, leading to motor dysfunction and other debilitating symptoms [1]. While various treatments aim to manage PD symptoms, there remains an ongoing search for neuroprotective agents that can slow disease progression. In this context, caffeine has emerged as a promising compound due to its antioxidant, anti-inflammatory, and neuroprotective properties [12, 13] such as PD, prompting an investigation into its potential therapeutic effects in PD models.
The observed improvement in motor performance, as evidenced by reduced times on the pole and beam-walk tests, is consistent with studies that have reported similar outcomes. For instance, caffeine administration improved motor coordination in 6-hydroxydopamine (6-OHDA)-induced PD models, correlating with enhanced dopaminergic activity [14]. Similarly, caffeine’s action on adenosine A2A receptors, which are known to influence motor control [15], may mediate these effects. The results of the current study reinforce the idea that caffeine may counteract the motor deficits associated with dopaminergic degeneration by enhancing neurotransmission pathways that support motor function. Moreover, the significant reduction in rearing frequency in caffeine-treated mice suggests an anxiolytic effect, which is further substantiated by the significant increase in rearing frequency compared to MPTP-induced mice. The anxiolytic effects of caffeine may be attributed to its role as an antagonist of adenosine receptors, which has been shown to influence anxiety-related behavior [16]. Additionally, the significant reduction in freezing frequency and center line duration in caffeine-treated groups compared to MPTP-induced mice indicates that caffeine may ameliorate anxiety-related behaviors commonly associated with stress and fear. This is consistent with studies indicating that caffeine’s effects can modulate anxiety-like behaviors [16].
The results from the brain biochemical analysis demonstrated that caffeine enhances antioxidant activity and mitigates neuroinflammation in MPTP-induced PD models. The observed elevation in brain CAT and GSH levels, the significant decrease in MDA, a marker of lipid peroxidation and oxidative stress, as well as an increase in serum SOD levels in the caffeine-treated groups indicates enhanced antioxidant capacity. This is consistent with studies indicating that caffeine can boost endogenous antioxidant defenses [12, 17] such as PD, thereby reducing oxidative stress. Similar outcomes from the heart and liver tissues show caffeine’s potential to enhance antioxidant defenses and mitigate oxidative damage in neurodegenerative models. As previously reported in other models [16], the protective effects of caffeine may be mediated through nervous and systemic antioxidants in this particular model.
Moreover, the significant reduction in TNF-α and IL-1α levels in the brain and serum of caffeine-treated mice compared to the MPTP-induced group highlights caffeine’s anti-inflammatory properties. Neuroinflammation is a critical component of PD pathology, and the ability of caffeine to modulate inflammatory cytokines is well-documented. A recent review indicated that caffeine could significantly reduce pro-inflammatory cytokines in experimental models of neurodegeneration, suggesting a neuroprotective mechanism against inflammation-related neuronal damage [18]. In this study, caffeine demonstrated significant anti-inflammatory effects by reducing pro-inflammatory cytokines, such as TNF-α and IL-1α in the brain, serum, heart, and liver tissues. These results highlight caffeine’s potential as a neuroprotective agent, particularly through its anti-inflammatory mechanisms in this PD model. Notably, we found that caffeine did not significantly alter serum lipid profile. These results suggest that caffeine’s primary effects in this model may lie in its antioxidant and anti-inflammatory properties rather than lipid modulation.
Furthermore, the immunohistochemical analysis revealed significant insights into the effects of caffeine on microglial activation, neurodegeneration, and protein aggregation in the MPTP-induced model of PD. The observed significant increase in total microglia (as indicated by Iba-1 expression) in the MPTP-induced group is consistent with numerous studies demonstrating that microglial activation is a hallmark of neuroinflammation in neurodegenerative diseases, including PD. Microglia, the resident immune cells of the brain, become activated in response to neuronal injury, and their increased count indicates a neuroinflammatory response [19, 20]. This heightened activation can contribute to neuronal degeneration and loss. In contrast, the significant reduction in Iba-1 expression in all treatment groups suggests that caffeine administration effectively mitigates microglial activation. This finding is supported by research indicating that caffeine possesses anti-inflammatory properties that can attenuate microglial activation and neuroinflammation [10]. The results imply that caffeine’s mechanism of action may involve the modulation of microglial activity, leading to decreased neuroinflammation and potential protection against neurodegeneration.
Moreover, the significant reduction in TH-positive cells in the substantia nigra of the MPTP-induced group underscores the neurodegenerative impact of MPTP. Furthermore, the preservation of TH-positive cells in the caffeine treatment groups indicates that caffeine may protect dopaminergic neurons from MPTP-induced degeneration. Previous research found that caffeine administration preserved dopaminergic neuronal integrity and function in Caenorhabditis elegans models [21], supporting the notion that caffeine has neuroprotective effects in the context of PD. The mechanism behind this protective effect may involve the inhibition of neuroinflammation and oxidative stress, both of which contribute to dopaminergic neuronal loss.
In addition, the elevated expression of α-synuclein in the MPTP-induced group highlights the pathological aggregation of this protein, which is a central feature of PD pathology. Increased α-synuclein levels can contribute to neuronal dysfunction and death, as aggregated forms of this protein are toxic to neurons [22]. Importantly, the significant reduction in α-synuclein levels in the treatment groups compared to the MPTP-induced group suggests that caffeine may attenuate the pathological accumulation of this protein.
Conclusion
This study provides evidence that caffeine exerts neuroprotective effects in an MPTP-induced model of PD in female Swiss mice. Our findings demonstrate that caffeine administration significantly improves motor performance and reduces anxiety-like behaviors, underscoring its potential role in enhancing the quality of life for individuals with PD. Biochemically, caffeine effectively increases antioxidant activity and decreases oxidative stress markers in both the brain and serum, suggesting a systemic and localized protective mechanism against neurodegeneration. Furthermore, the ability of caffeine to attenuate neuroinflammation, as evidenced by reduced levels of TNF-α and IL-1α, highlights its anti-inflammatory properties, which are crucial for mitigating the pathophysiological processes associated with PD. The molecular basis of these outcomes could be attributed to the reduced microglial activation and preservation of dopaminergic neurons in the substantia nigra, along with decreased α-synuclein expression. Collectively, these results advocate for the potential therapeutic application of caffeine in managing PD, warranting further investigation into its mechanisms and efficacy in clinical settings. Future research could elucidate the precise molecular pathways through which caffeine exerts its protective effects and explore optimal dosing strategies for therapeutic use.
Ethical Considerations
Compliance with ethical guidelines
The animals were treated humanely in accordance with the care and use of animals of the Ethical Committee of the Department of Physiology, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria (UERC approval No.: UERC/ASN/2024/2686).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Supervision, and Validation: Aminat Omolola Imam-Fulani; Data curation: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, Halimat Oluwakemi Afolabi, Jerry Inyang, Oluwadamilola Onoshi Onoshi, Taofeekat Temitope Ogunsesan, Oyindamola Bisola Adekunle, Taiwo Elijah Eso, Oluwaseyi Deborah Babatunde, Edwin Ayolola Makanjuola, Olamilekan Abdulfatai Alimi, Halimat Oluwakemi Afolabi; Visualization, formal analysis and writing of the original draft: Aminat Omolola Imam-Fulani and Abdulrahman Olanrewaju Shuaib; Investigation: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, Halimat Oluwakemi Afolabi, Jerry Inyang, Oluwadamilola Onoshi Onoshi, Taofeekat Temitope Ogunsesan, Oyindamola Bisola Adekunle, Taiwo Elijah Eso, Oluwaseyi Deborah Babatunde, Edwin Ayolola Makanjuola, Olamilekan Abdulfatai Alimi, and Hafeez Ajibola Olorunoje; Methodology: Aminat Omolola Imam-Fulani, AOS; Project administration: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Halimat Oluwakemi Afolabi, Jerry Inyang; Resources; Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide; Software: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, and Kamaldeen Olalekan Sanusi; Review and editing: Aminat Omolola Imam-Fulani, Abdulrahman Olanrewaju Shuaib, Lateefah Omotoyosi Olajide, and Kamaldeen Olalekan Sanusi.
Conflict of interest
The authors reported no conflict of interest.
Acknowledgments
The authors would like to express their appreciation for the efforts of Garba Abdulrasheed, the laboratory technologist of the Department of Physiology at the University of Ilorin, Ilorin, Nigeria.
References
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017; 3:17013. [DOI:10.1038/nrdp.2017.13] [PMID]
- Srinivasan E, Chandrasekhar G, Chandrasekar P, Anbarasu K, Vickram AS, Karunakaran R, et al. Alpha-synuclein aggregation in parkinson's disease. Front Med. 2021; 8:736978. [DOI:10.3389/fmed.2021.736978] [PMID]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013; 3(4):461-91. [DOI:10.3233/JPD-130230] [PMID]
- Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023; 8(1):267. [DOI:10.1038/s41392-023-01486-5] [PMID]
- Adamu A, Li S, Gao F, Xue G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front Aging Neurosci. 2024; 16:1347987. [DOI:10.3389/fnagi.2024.1347987] [PMID]
- Sanusi KO, Usman UZ, Usman D, Adeshina KA, Uthman YA, Jimoh L, et al. The therapeutic potential of cola nitida in health and disease: A review. Biol Med Nat Prod Chem. 2023; 12(2):637-43. [DOI:10.22541/au.169104269.99169022/v1]
- Zhao Y, Lai Y, Konijnenberg H, Huerta JM, Vinagre-Aragon A, Sabin JA, et al. Association of coffee consumption and prediagnostic caffeine metabolites with incident parkinson disease in a population-based cohort. Neurology. 2024; 102(8):e209201. [DOI:10.1212/WNL.0000000000209201] [PMID]
- Barcelos RP, Souza MA, Amaral GP, Stefanello ST, Bresciani G, Fighera MR, et al. Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci. 2014; 96(1-2):40-5. [DOI:10.1016/j.lfs.2013.12.002] [PMID]
- Hegde KR, Coleman M, Hauri R. Modulation of activity and expression of antioxidant enzymes in retina by ROS: Effect of caffeine. Invest Ophthalmol Visual Sci. 2023; 64(8):4473. [Link]
- Madeira MH, Boia R, Ambrósio AF, Santiago AR. Having a coffee break: The impact of caffeine consumption on microglia-mediated inflammation in neurodegenerative diseases. Mediators Inflamm. 2017; 2017:4761081. [DOI:10.1155/2017/4761081] [PMID]
- Gibrat C, Saint-Pierre M, Bousquet M, Lévesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J Neurochem. 2009;109(5):1469-1482. [DOI: 10.1111/j.1471-4159.2009.06072.x] [PMID]
- Ikram M, Park TJ, Ali T, Kim MO. Antioxidant and neuroprotective effects of caffeine against alzheimer's and parkinson's disease: Insight into the role of Nrf-2 and A2AR Signaling. Antioxidants. 2020; 9(9):902. [DOI:10.3390/antiox9090902] [PMID]
- Imam-Fulani AO, Sanusi KO, Owoyele BV. Effects of acetone extract of Cola nitida on brain sodium-potassium adenosine triphosphatase activity and spatial memory in healthy and streptozotocin-induced diabetic female Wistar rats. J Basic Clin Physiol Pharmacol. 2018; 29(4):411-6. [DOI:10.1515/jbcpp-2016-0019] [PMID]
- Aguiar LM, Nobre HV Jr, Macêdo DS, Oliveira AA, Freitas RM, Vasconcelos SM, et al. Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesion in rats. Pharmacol Biochem Behav. 2006; 84(3):415-9. [DOI:10.1016/j.pbb.2006.05.027] [PMID]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011; 31(27):10067-75. [DOI:10.1523/JNEUROSCI.6730-10.2011] [PMID]
- Liu C, Wang L, Zhang C, Hu Z, Tang J, Xue J, et al. Caffeine intake and anxiety: A meta-analysis. Front Psychol. 2024; 15:1270246. [DOI:10.3389/fpsyg.2024.1270246] [PMID]
- Reddy VS, Shiva S, Manikantan S, Ramakrishna S. Pharmacology of caffeine and its effects on the human body. Eur J Med Chem Reports. 2024; 10:100138. [DOI:10.1016/j.ejmcr.2024.100138]
- Ruggiero M, Calvello R, Porro C, Messina G, Cianciulli A, Panaro MA. Neurodegenerative Diseases: Can Caffeine Be a Powerful Ally to Weaken Neuroinflammation? Int J Mol Sci. 2022 Oct;23(21). [DOI:10.3390/ijms232112958] [PMID]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010; 7(4):354-65. [DOI:10.1016/j.nurt.2010.05.014] [PMID]
- Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021; 18(1):258. [DOI:10.1186/s12974-021-02309-6] [PMID]
- Manalo RVM, Medina PMB. Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic caenorhabditis elegans. Front Neurosci. 2018; 12:137. [DOI:10.3389/fnins.2018.00137] [PMID]
- Maor G, Dubreuil RR, Feany MB. α-synuclein promotes neuronal dysfunction and death by disrupting the binding of ankyrin to ß-spectrin. J Neurosci. 2023; 43(9):1614-26. [DOI:10.1101/2023.06.02.543481]
Type of Study: Original Research |
Subject:
Cellular and Molecular Biology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |