Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 105-114 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bunu S J, Baba H, Alfred-Ugbenbo D. In-silico Analysis and Anti-inflammatory Evaluation of Synthesized Amide Derivatives of Long-chain Fatty Acids. Pharm Biomed Res 2025; 11 (2) :105-114
URL: http://pbr.mazums.ac.ir/article-1-666-en.html
URL: http://pbr.mazums.ac.ir/article-1-666-en.html
1- Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Nigeria. & Drug Analysis and Research Center, Ebisamdex Global Ventures Ltd, Yenagoa, Nigeria.
2- Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, University of Calabar, University of Calabar, Nigeria.
3- Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Bayelsa Medical University, Yenagoa, Nigeria.
2- Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, University of Calabar, University of Calabar, Nigeria.
3- Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Bayelsa Medical University, Yenagoa, Nigeria.
Keywords: Nociception, Anti-inflammatory Agents, Nociceptive, Carrageenan-induced edema, Molecular mechanisms
Full-Text [PDF 1394 kb]
(228 Downloads)
| Abstract (HTML) (665 Views)
Full-Text: (191 Views)
Introduction
Fatty acids (FAs) are essential nutrients that regulate an organism’s health and growth in mammals [1]. Long-chain FAs (LCFAs) are FAs formed by the hydrolysis of neutral lipids and have aliphatic tails containing 13-21 carbon atoms. Three of the most prevalent LCFAs in anaerobic digesters are palmitate, oleate, and stearate. LCFAs have significant functions in cellular energy metabolism, acting as both a vital energy source and signaling molecules [2, 3]. LCFAs can be classified as saturated or unsaturated based on the presence of at least one double bond in their carbon chains. LCFAs enter cells via passive diffusion and protein-mediated translocation across the plasma membrane, in which FA translocase (FAT/CD36), plasma membrane FA-binding protein (FABPpm), FA transport protein (FATP), and caveolin-1 are thought to play critical roles. Cells absorb LCFAs, which bind to FA-binding proteins (FABPs) and transport them to certain organelles, where they are activated into acyl-CoA to target specific metabolic pathways [4]. LCFA-CoA esters boost their oxidation by acting as allosteric inhibitors of acetyl-CoA carboxylase. This decreases malonyl-CoA synthesis and relieves the inhibition of carnitine palmitoyl-transferase-1, promoting LCFA-CoA transport into mitochondria for β-oxidation [2, 5]. LCFAs are not only important metabolites but also contribute to various physiological processes, including the activation of protein kinase C (PKC) isoforms and nuclear transcription factors, such as peroxisome proliferator-activated receptors (PPARs) [6].
FAs are digested and produced as energy sources throughout biological processes. The metabolic network’s primary sources of free FAs (FFAs) include long- and medium-chain FAs generated mostly from dietary triglycerides, as well as short-chain FAs (SCFAs) produced by gut microbial fermentation of otherwise indigestible food fiber [7]. FFAs not only serve as energy sources but also as natural ligands for a group of orphan G protein-coupled receptors (GPCRs) known as free FAs receptors (FFARs), essentially intertwining metabolism and immunity in multiple ways, such as through inflammation regulation and secretion of peptide hormones [7]. Several factors can induce inflammation, including a blood clot, an immune system issue, cancer, an infection, chemical exposure, a physical injury, or a neurological condition, like Alzheimer’s disease or depression. Many infections, particularly those caused by viral, bacterial, fungal, or protozoan pathogens, can produce inflammation. Inflammations can have far-reaching medical repercussions since persistent inflammation can aid cancer and trigger autoimmune illnesses [8].
Amide derivatives of FAs are a type of lipid that consists of two chemical moieties, a FAs, and a biogenic amine, joined together by an amide bond, structurally related to the endocannabinoid anandamide (N-arachidonoylethanolamine) [9]. Palmitoyl-ethanolamide belongs to the anandamide class as a possible anti-inflammatory and anti-nociceptive agent. Anandamide is a known endogenous ligand for cannabinoid receptors [10]. Research and analysis of anti-inflammatory agents are common because these drug compounds are employed frequently in current medical practice globally, as all known human disease pathophysiology passes through inflammatory stages [11-15]. The lipid signal is becoming more congested as more FAs amide derivatives are discovered and considered potential therapeutic targets. The identification of N-arachidonoyl-ethanolamine as an endogenous ligand of cannabinoid type-1 and type-2 receptors, as well as the development of various-omics technologies, can lead to the discovery of a large number of naturally occurring N-acyl-amines [16]. Aside from being used as anti-inflammatory agents, reprogramming of lipid metabolism is emerging as a hallmark of cancer, although the role of specific FA species and associated enzymes in carcinogenesis is unclear. While prior research has focused on the participation of LCFAs, such as palmitate in cancer, less attention has been paid to the impact of very LCFAs (VLCFAs) [16].
Studies on palmitoyl ethanolamide (palmitoylamino acids) have shown good anti-inflammatory and anti-nociceptive effects in vitro, but the exact mechanism of action is yet to be elucidated and confirmed, and it was suspected to be related to the inhibition of FAs amide hydrolase (FAAH), the enzyme that metabolizes anandamide and palmitoyl ethanolamide in vivo [10]. Hence, we conducted an in-silico molecular docking analysis using the Arabidopsis thaliana FAAH proteins (PDB ID: 6DII) on the synthesized amide derivatives of LCFAs and tested their biological activities to correlate and possibly validate the biological mechanism behind their anti-inflammatory and anti-nociceptive properties. FAAH is an amidase signature (AS) enzyme in most multicellular organisms. FAAH hydrolyzes lipid signaling molecules, specifically N-acyl ethanolamines (NAEs), terminating their activity. The crystal structure of A. thaliana FAAH was determined, and critical residues were discovered for substrate-specific interactions [17]. In vertebrates, the endocannabinoid signaling pathway is a key lipid regulatory route that influences various physiological and behavioral functions. NAEs are a class of FAs derivatives that function in this pathway. Their signaling activity is ended by FAAH, which hydrolyzes NAEs to ethanolamine and their corresponding FFAs [18].
Materials and Methods
Synthesis and characterization
Several medicines have been developed through the synthetic route, with some in clinical use and others in the pre-clinical phase [19, 20]. The synthesis of the amide derivatives was accomplished using commercially available starting materials (glycine, β-alanine, γ-aminobutyric acid (GABA), and palmitoyl chloride) that met analytical standards and required no further purification. To obtain the amide derivatives of the LCFAs, for N-palmitoyl glycine, glycine (0.546 g, 7.28 mmol) was dissolved in 10 mL of dichloromethane (DCM) and added to 1 mL of triethylamine and palmitoyl chloride (2.2 mL, 7.28 mmol) with constant stirring for 4 hours. The mixture was filtered and washed with HCl (10 mL), and the combined organic filtrate was dried over anhydrous Na2SO4, evaporated in a vacuum, and recrystallized from a 1:1 methanol and water solution. For N-palmitoyl GABA, (0.34 g, 3.3 mmol) was dissolved in 15 mL 1,4-dioxane and stirred in a solution of 1N NaOH (3.3 mL, 3.3 mmol) added under ice and stirred continuously for 15 minutes. The addition of palmitoyl chloride (3.3 mmol) was followed by 1N NaOH (3.3 mL, 3.3 mmol), and the mixture was stirred at room temperature for 4 hours. The mixture was acidified with 1N HCl to a pH of 3, the solid was collected, and purification was performed (pH 5-6). The final product was recrystallized following the previous procedure. Finally, 1 mL of triethylamine (TEA) was added to N-palmitoyl alanine, β-alanine (0.590 g, 6.6 mmol) in dry dichloromethane (10 mL) while stirring in an ice-cold chamber (0-5 °C), followed by the dropwise addition of palmitoyl chloride and a final wash with DCM (10 mL). The ice was removed, and the resulting syrupy mixture was stirred for 2 hours at room temperature, diluted with DCM (20 mL), and washed with 10 mL of 1N HCl and water. The combined organic portion was dried over anhydrous Na2SO4, and the solvent was removed under vacuum, resulting in a whitish crude solid that was recrystallized as described above.
CH3(CH2)14COCl + H2N(CH2)nCOOH CH3(CH2)14CONH(CH2)nCOOH
Scheme 1. Synthetic route (n=1–3).
The synthesized compounds were characterized using an IR spectrum obtained from a Buck Scientific IR M500 instrument (Buck Scientific Inc., USA), and 1H and 13C NMR spectra were recorded on a Varian Gemini 200 (250 MHz) (Varian Inc., USA).
Anti-inflammatory and antinociceptive studies
The raw paw edema model was used to assess the anti-inflammatory and antinociceptive properties of the synthesized amide derivatives of LCFAs. Three doses were tested, including 20, 50, and 100 mg/kg. One of the test compounds was injected into the subplantar area of the right hind paw after an hour of carrageenan suspension (0.1 mL, 1%) in 0.9% NaCl. Paw thickness was determined for 5 hours using a veneer caliper. The anti-inflammatory activity was evaluated using the following formula [21]: Activity =100-[100×(average drug treated/average for control)]. The analgesic activity was evaluated using the following formula [22, 23]: Percentage inhibition (%) =100-[100×(average drug response/average control response)]. The analgesic properties were assessed in a group of five mice administered oral doses of 20, 50, and 100 mg/kg of the test compounds. After 1 hour, the mice were injected intraperitoneally (i.p.) with 0.2 mL of a 0.6% acetic acid solution. Acetylsalicylic acid, also known as aspirin (100 mg/kg), was administered orally as a reference drug, while Tween 80(10%), used to solubilize the synthesized drugs, was used as a negative control. The mean abdominal constrictions per group were used as an indicator of analgesic activity. The percentage inhibitions of abdominal constrictions by the test compounds were compared to the control group. All results were statistically analyzed by one-way analysis of variance (ANOVA), with a P of <0.005 considered significant.
Molecular docking
Arabidopsis FAAH in complex with methyl linolenyl fluorophosphonate (PDB ID: 6DII), obtained from the Protein Data Bank (PDB) was used for the molecular docking studies from the determined FAAH crystal structure of A. thaliana, and critical substrate-specific residues for protein-ligand interactions [17]. Hence, inhibiting these proteins may provide insight into the ability of these compounds to act as anti-inflammatory or anti-nociceptive agents. Acetylsalicylic acid and linolenyl fluorophosphonate were used as standard controls in the in-silico molecular docking studies. The test compounds included N-palmitoyl glycine, N-palmitoyl alanine, and N-palmitoyl GABA, respectively. Molecular modeling and docking of the binding protein with ketamine and its analogs (ligands) were conducted using the Maestro software with the OPLS3 2018 force field [24, 25].
Results
The results are presented in tables and charts following analysis on Microsoft Excel spreadsheet, and Microsoft word version 2019. Figures were prepare using PowerPoint and Python software.
Discussion
The amide derivatives of LCFAs were obtained in high yield and purity, as shown by their elemental characterization and physicochemical parameter evaluation [10]. We subjected the compounds to in silico molecular docking, which showed promising results compared with the standards, with comparable affinity levels to a known inhibitor of the A. thaliana FAs amide hydrolase enzyme, linolenyl fluorophosphonate (-6.85 kcal/mol), and higher affinity than the well-known anti-inflammatory and analgesic agent, acetylsalicylic acid (-5.61 kcal/mol). Among the amide derivatives, N-palmitoyl alanine exhibited the highest protein binding affinity (-6.67 kcal/mol), followed by N-palmitoyl GABA (-6.48 kcal/mol) and N-palmitoyl glycine (-6.38 kcal/mol), respectively (Table 1). This depicts that the synthesized amide derivatives of LCFAs are promising lead compounds in the inhibition of biological inflammatory and nociceptive pathways.
The in vivo anti-inflammatory activity of the synthesized amide derivatives of LCFAs was evaluated using a carrageenan-induced paw edema assay [26, 27]. The anti-inflammatory assessment revealed a dose-dependent bioactivity from 20 mg/kg to 50 mg/kg; further increments in the dose led to decreased activity. The compounds also demonstrated significant anti-nociceptive activity compared with the ASA control. N-palmitoyl glycine exhibited the highest dose-dependent edema inhibition (among the three compounds) ranging from 47 to 49% as the dose increased from 20 to 50 mg/kg, while aspirin, the standard drug (100 mg/kg), produced a 51% inhibition of edema. For the analgesic activity, at 100 mg/kg, N-palmitoyl glycine exhibited an 83.2 % inhibition of writhing compared to a 74.3% inhibition by the standard drug, aspirin (100 mg/kg). N-palmitoyl glycine produced the most pronounced anti-nociceptive effect (Table 2). This is similar to the in silico studies, where N-palmitoyl glycine showed a better protein-binding affinity compared to the aspirin standard (-6.38 kcal/mol vs. -5.61 kcal/mol).
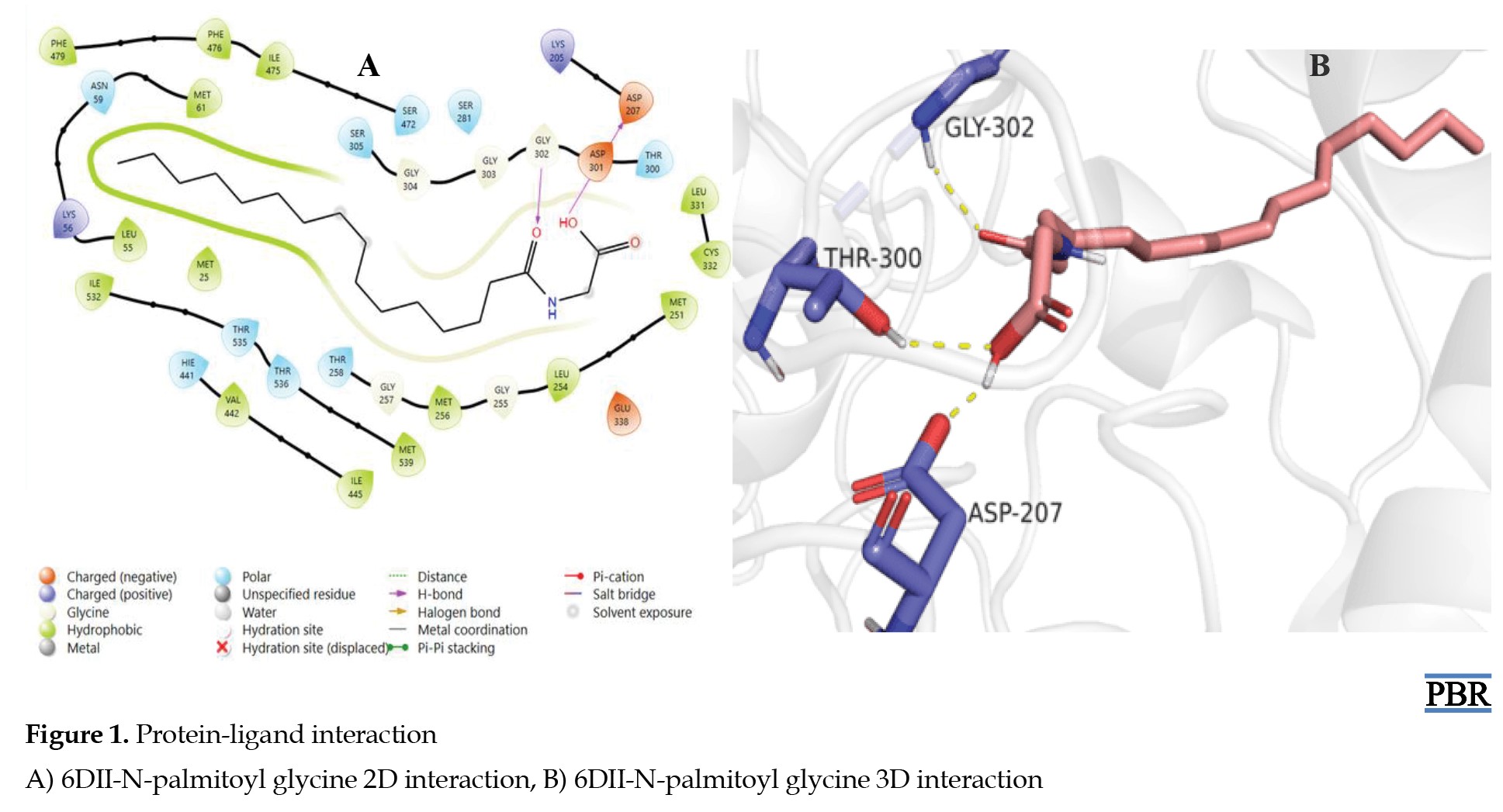
The protein-ligand binding of all the synthesized amide derivatives of the LCFAs was conducted via in silico molecular docking studies using the A. thaliana FAAH protein (PDB ID: 6DII). The interaction between 6DII and N-palmitoyl glycine showed that N-palmitoyl glycine had hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 1A).
Fatty acids (FAs) are essential nutrients that regulate an organism’s health and growth in mammals [1]. Long-chain FAs (LCFAs) are FAs formed by the hydrolysis of neutral lipids and have aliphatic tails containing 13-21 carbon atoms. Three of the most prevalent LCFAs in anaerobic digesters are palmitate, oleate, and stearate. LCFAs have significant functions in cellular energy metabolism, acting as both a vital energy source and signaling molecules [2, 3]. LCFAs can be classified as saturated or unsaturated based on the presence of at least one double bond in their carbon chains. LCFAs enter cells via passive diffusion and protein-mediated translocation across the plasma membrane, in which FA translocase (FAT/CD36), plasma membrane FA-binding protein (FABPpm), FA transport protein (FATP), and caveolin-1 are thought to play critical roles. Cells absorb LCFAs, which bind to FA-binding proteins (FABPs) and transport them to certain organelles, where they are activated into acyl-CoA to target specific metabolic pathways [4]. LCFA-CoA esters boost their oxidation by acting as allosteric inhibitors of acetyl-CoA carboxylase. This decreases malonyl-CoA synthesis and relieves the inhibition of carnitine palmitoyl-transferase-1, promoting LCFA-CoA transport into mitochondria for β-oxidation [2, 5]. LCFAs are not only important metabolites but also contribute to various physiological processes, including the activation of protein kinase C (PKC) isoforms and nuclear transcription factors, such as peroxisome proliferator-activated receptors (PPARs) [6].
FAs are digested and produced as energy sources throughout biological processes. The metabolic network’s primary sources of free FAs (FFAs) include long- and medium-chain FAs generated mostly from dietary triglycerides, as well as short-chain FAs (SCFAs) produced by gut microbial fermentation of otherwise indigestible food fiber [7]. FFAs not only serve as energy sources but also as natural ligands for a group of orphan G protein-coupled receptors (GPCRs) known as free FAs receptors (FFARs), essentially intertwining metabolism and immunity in multiple ways, such as through inflammation regulation and secretion of peptide hormones [7]. Several factors can induce inflammation, including a blood clot, an immune system issue, cancer, an infection, chemical exposure, a physical injury, or a neurological condition, like Alzheimer’s disease or depression. Many infections, particularly those caused by viral, bacterial, fungal, or protozoan pathogens, can produce inflammation. Inflammations can have far-reaching medical repercussions since persistent inflammation can aid cancer and trigger autoimmune illnesses [8].
Amide derivatives of FAs are a type of lipid that consists of two chemical moieties, a FAs, and a biogenic amine, joined together by an amide bond, structurally related to the endocannabinoid anandamide (N-arachidonoylethanolamine) [9]. Palmitoyl-ethanolamide belongs to the anandamide class as a possible anti-inflammatory and anti-nociceptive agent. Anandamide is a known endogenous ligand for cannabinoid receptors [10]. Research and analysis of anti-inflammatory agents are common because these drug compounds are employed frequently in current medical practice globally, as all known human disease pathophysiology passes through inflammatory stages [11-15]. The lipid signal is becoming more congested as more FAs amide derivatives are discovered and considered potential therapeutic targets. The identification of N-arachidonoyl-ethanolamine as an endogenous ligand of cannabinoid type-1 and type-2 receptors, as well as the development of various-omics technologies, can lead to the discovery of a large number of naturally occurring N-acyl-amines [16]. Aside from being used as anti-inflammatory agents, reprogramming of lipid metabolism is emerging as a hallmark of cancer, although the role of specific FA species and associated enzymes in carcinogenesis is unclear. While prior research has focused on the participation of LCFAs, such as palmitate in cancer, less attention has been paid to the impact of very LCFAs (VLCFAs) [16].
Studies on palmitoyl ethanolamide (palmitoylamino acids) have shown good anti-inflammatory and anti-nociceptive effects in vitro, but the exact mechanism of action is yet to be elucidated and confirmed, and it was suspected to be related to the inhibition of FAs amide hydrolase (FAAH), the enzyme that metabolizes anandamide and palmitoyl ethanolamide in vivo [10]. Hence, we conducted an in-silico molecular docking analysis using the Arabidopsis thaliana FAAH proteins (PDB ID: 6DII) on the synthesized amide derivatives of LCFAs and tested their biological activities to correlate and possibly validate the biological mechanism behind their anti-inflammatory and anti-nociceptive properties. FAAH is an amidase signature (AS) enzyme in most multicellular organisms. FAAH hydrolyzes lipid signaling molecules, specifically N-acyl ethanolamines (NAEs), terminating their activity. The crystal structure of A. thaliana FAAH was determined, and critical residues were discovered for substrate-specific interactions [17]. In vertebrates, the endocannabinoid signaling pathway is a key lipid regulatory route that influences various physiological and behavioral functions. NAEs are a class of FAs derivatives that function in this pathway. Their signaling activity is ended by FAAH, which hydrolyzes NAEs to ethanolamine and their corresponding FFAs [18].
Materials and Methods
Synthesis and characterization
Several medicines have been developed through the synthetic route, with some in clinical use and others in the pre-clinical phase [19, 20]. The synthesis of the amide derivatives was accomplished using commercially available starting materials (glycine, β-alanine, γ-aminobutyric acid (GABA), and palmitoyl chloride) that met analytical standards and required no further purification. To obtain the amide derivatives of the LCFAs, for N-palmitoyl glycine, glycine (0.546 g, 7.28 mmol) was dissolved in 10 mL of dichloromethane (DCM) and added to 1 mL of triethylamine and palmitoyl chloride (2.2 mL, 7.28 mmol) with constant stirring for 4 hours. The mixture was filtered and washed with HCl (10 mL), and the combined organic filtrate was dried over anhydrous Na2SO4, evaporated in a vacuum, and recrystallized from a 1:1 methanol and water solution. For N-palmitoyl GABA, (0.34 g, 3.3 mmol) was dissolved in 15 mL 1,4-dioxane and stirred in a solution of 1N NaOH (3.3 mL, 3.3 mmol) added under ice and stirred continuously for 15 minutes. The addition of palmitoyl chloride (3.3 mmol) was followed by 1N NaOH (3.3 mL, 3.3 mmol), and the mixture was stirred at room temperature for 4 hours. The mixture was acidified with 1N HCl to a pH of 3, the solid was collected, and purification was performed (pH 5-6). The final product was recrystallized following the previous procedure. Finally, 1 mL of triethylamine (TEA) was added to N-palmitoyl alanine, β-alanine (0.590 g, 6.6 mmol) in dry dichloromethane (10 mL) while stirring in an ice-cold chamber (0-5 °C), followed by the dropwise addition of palmitoyl chloride and a final wash with DCM (10 mL). The ice was removed, and the resulting syrupy mixture was stirred for 2 hours at room temperature, diluted with DCM (20 mL), and washed with 10 mL of 1N HCl and water. The combined organic portion was dried over anhydrous Na2SO4, and the solvent was removed under vacuum, resulting in a whitish crude solid that was recrystallized as described above.
CH3(CH2)14COCl + H2N(CH2)nCOOH CH3(CH2)14CONH(CH2)nCOOH
Scheme 1. Synthetic route (n=1–3).
The synthesized compounds were characterized using an IR spectrum obtained from a Buck Scientific IR M500 instrument (Buck Scientific Inc., USA), and 1H and 13C NMR spectra were recorded on a Varian Gemini 200 (250 MHz) (Varian Inc., USA).
Anti-inflammatory and antinociceptive studies
The raw paw edema model was used to assess the anti-inflammatory and antinociceptive properties of the synthesized amide derivatives of LCFAs. Three doses were tested, including 20, 50, and 100 mg/kg. One of the test compounds was injected into the subplantar area of the right hind paw after an hour of carrageenan suspension (0.1 mL, 1%) in 0.9% NaCl. Paw thickness was determined for 5 hours using a veneer caliper. The anti-inflammatory activity was evaluated using the following formula [21]: Activity =100-[100×(average drug treated/average for control)]. The analgesic activity was evaluated using the following formula [22, 23]: Percentage inhibition (%) =100-[100×(average drug response/average control response)]. The analgesic properties were assessed in a group of five mice administered oral doses of 20, 50, and 100 mg/kg of the test compounds. After 1 hour, the mice were injected intraperitoneally (i.p.) with 0.2 mL of a 0.6% acetic acid solution. Acetylsalicylic acid, also known as aspirin (100 mg/kg), was administered orally as a reference drug, while Tween 80(10%), used to solubilize the synthesized drugs, was used as a negative control. The mean abdominal constrictions per group were used as an indicator of analgesic activity. The percentage inhibitions of abdominal constrictions by the test compounds were compared to the control group. All results were statistically analyzed by one-way analysis of variance (ANOVA), with a P of <0.005 considered significant.
Molecular docking
Arabidopsis FAAH in complex with methyl linolenyl fluorophosphonate (PDB ID: 6DII), obtained from the Protein Data Bank (PDB) was used for the molecular docking studies from the determined FAAH crystal structure of A. thaliana, and critical substrate-specific residues for protein-ligand interactions [17]. Hence, inhibiting these proteins may provide insight into the ability of these compounds to act as anti-inflammatory or anti-nociceptive agents. Acetylsalicylic acid and linolenyl fluorophosphonate were used as standard controls in the in-silico molecular docking studies. The test compounds included N-palmitoyl glycine, N-palmitoyl alanine, and N-palmitoyl GABA, respectively. Molecular modeling and docking of the binding protein with ketamine and its analogs (ligands) were conducted using the Maestro software with the OPLS3 2018 force field [24, 25].
Results
The results are presented in tables and charts following analysis on Microsoft Excel spreadsheet, and Microsoft word version 2019. Figures were prepare using PowerPoint and Python software.
Discussion
The amide derivatives of LCFAs were obtained in high yield and purity, as shown by their elemental characterization and physicochemical parameter evaluation [10]. We subjected the compounds to in silico molecular docking, which showed promising results compared with the standards, with comparable affinity levels to a known inhibitor of the A. thaliana FAs amide hydrolase enzyme, linolenyl fluorophosphonate (-6.85 kcal/mol), and higher affinity than the well-known anti-inflammatory and analgesic agent, acetylsalicylic acid (-5.61 kcal/mol). Among the amide derivatives, N-palmitoyl alanine exhibited the highest protein binding affinity (-6.67 kcal/mol), followed by N-palmitoyl GABA (-6.48 kcal/mol) and N-palmitoyl glycine (-6.38 kcal/mol), respectively (Table 1). This depicts that the synthesized amide derivatives of LCFAs are promising lead compounds in the inhibition of biological inflammatory and nociceptive pathways.
The in vivo anti-inflammatory activity of the synthesized amide derivatives of LCFAs was evaluated using a carrageenan-induced paw edema assay [26, 27]. The anti-inflammatory assessment revealed a dose-dependent bioactivity from 20 mg/kg to 50 mg/kg; further increments in the dose led to decreased activity. The compounds also demonstrated significant anti-nociceptive activity compared with the ASA control. N-palmitoyl glycine exhibited the highest dose-dependent edema inhibition (among the three compounds) ranging from 47 to 49% as the dose increased from 20 to 50 mg/kg, while aspirin, the standard drug (100 mg/kg), produced a 51% inhibition of edema. For the analgesic activity, at 100 mg/kg, N-palmitoyl glycine exhibited an 83.2 % inhibition of writhing compared to a 74.3% inhibition by the standard drug, aspirin (100 mg/kg). N-palmitoyl glycine produced the most pronounced anti-nociceptive effect (Table 2). This is similar to the in silico studies, where N-palmitoyl glycine showed a better protein-binding affinity compared to the aspirin standard (-6.38 kcal/mol vs. -5.61 kcal/mol).
The protein-ligand binding of all the synthesized amide derivatives of the LCFAs was conducted via in silico molecular docking studies using the A. thaliana FAAH protein (PDB ID: 6DII). The interaction between 6DII and N-palmitoyl glycine showed that N-palmitoyl glycine had hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 1A).
Additionally, N-palmitoyl glycine formed hydrogen pi-pi bonding interactions with the carboxylic acid (OH), the amide carbonyl functional group (C=O), and the residues ASP-207, GLY-302, and THR-300 of the protein (Figure 1B). This depicts that the amide C=O and OH groups might be required for the bioactivity of N-palmitoyl glycine.
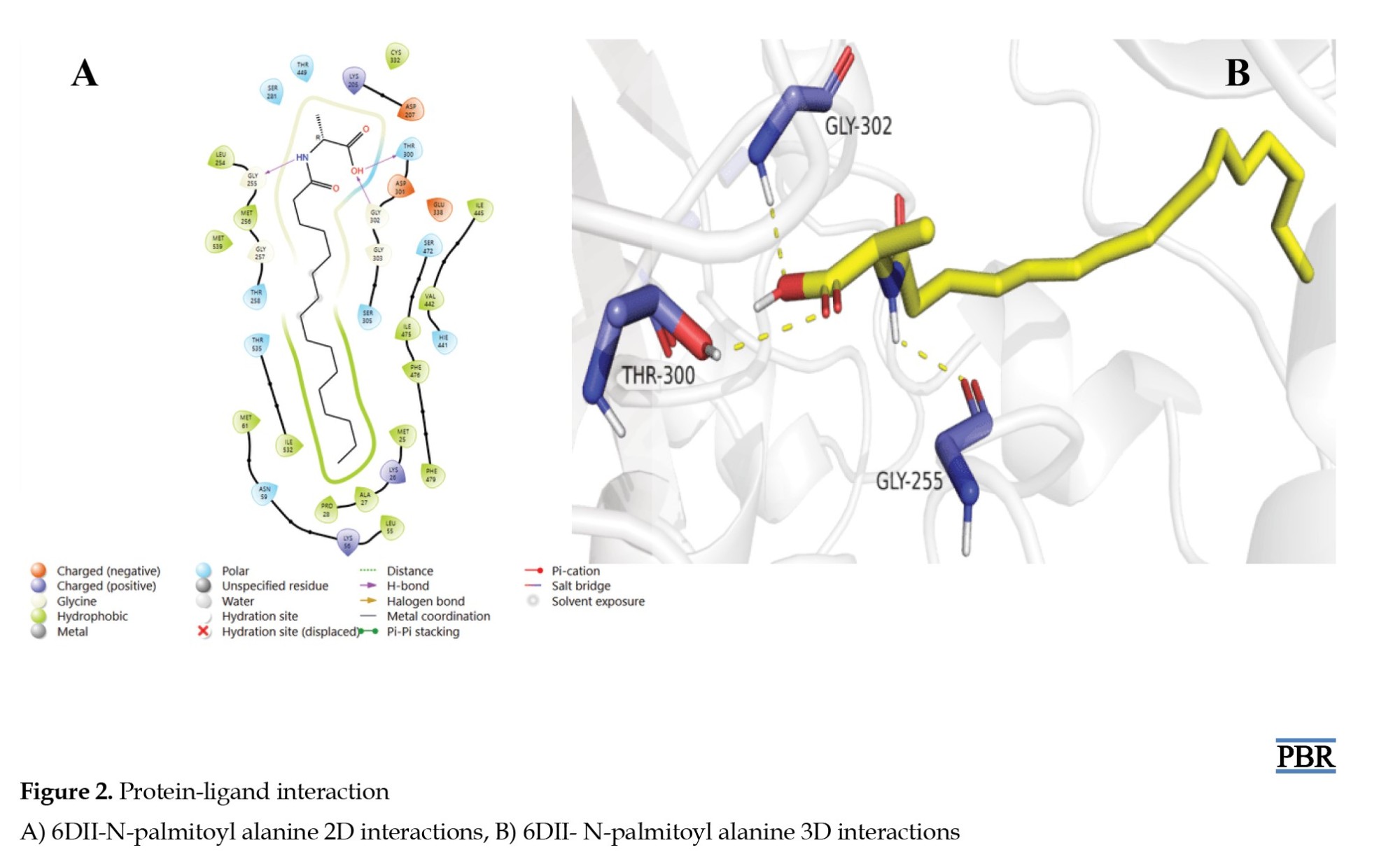
The interaction of 6DII with N-palmitoyl alanine showed hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 2A).
The interaction of 6DII with N-palmitoyl alanine showed hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 2A).
N-palmitoyl alanine also formed hydrogen pi-pi bonding interactions between the amide nitrogen functional group (N-H), the carboxylic acid (OH), and the residues GLY-255, THR-300, and GLY-302 of the protein, respectively (Figure 2B). This depicts that the N-H and OH groups of N-palmitoyl alanine may be required for its bioactivity.
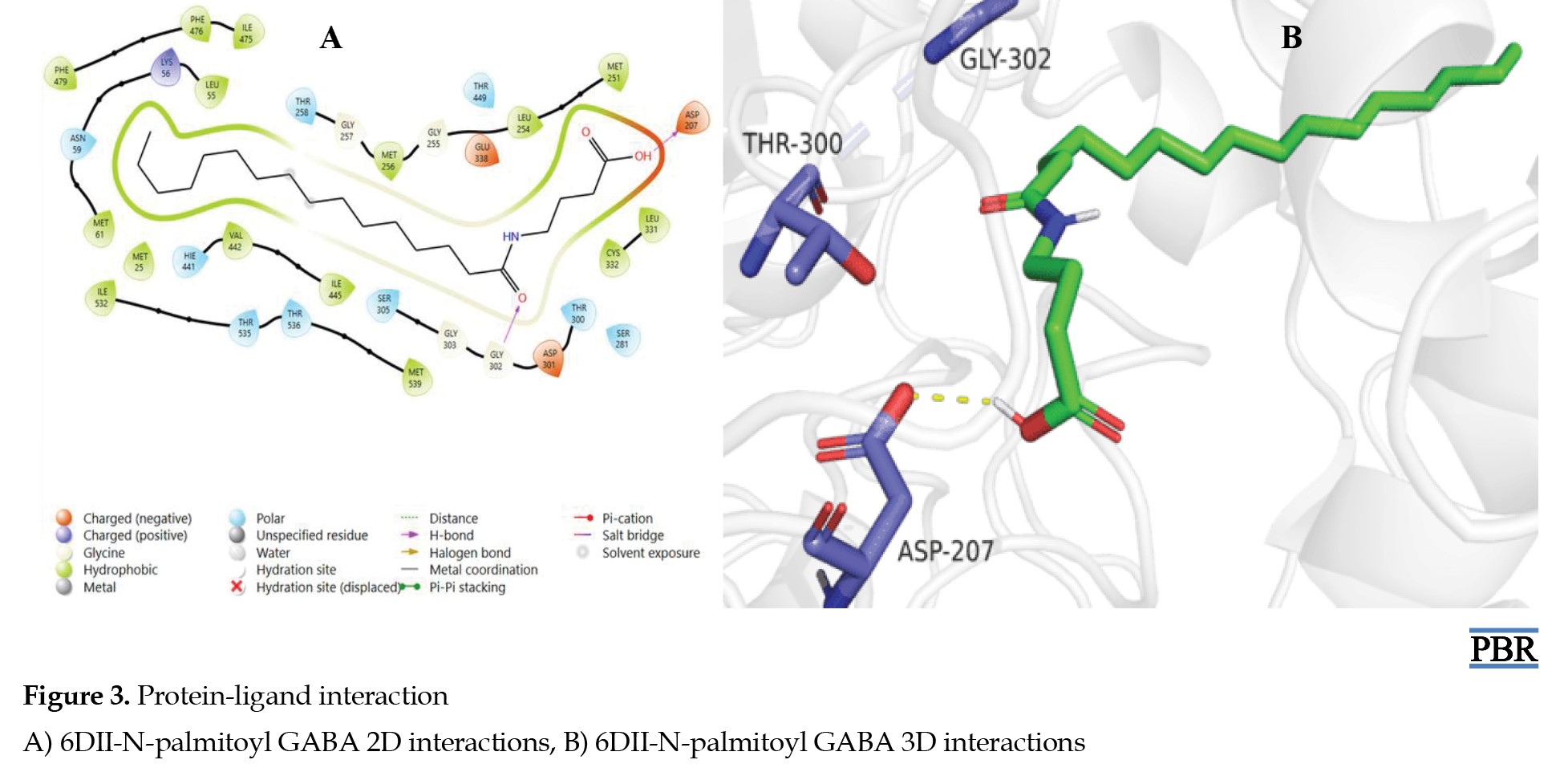
The interaction of N-palmitoyl GABA with the protein (6DII) showed hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 3A).

The interaction of N-palmitoyl GABA with the protein (6DII) showed hydrophobic, polar, positive, and negatively charged interactions with different residues of the protein (Figure 3A).
N-palmitoyl alanine also formed hydrogen pi-pi bonding interactions between the amide N-H and OH with ASP-207 and GLY-302, respectively, of the protein (Figure 3B). This suggests that the C=O and OH groups of N-palmitoyl GABA might be required for its bioactivity.
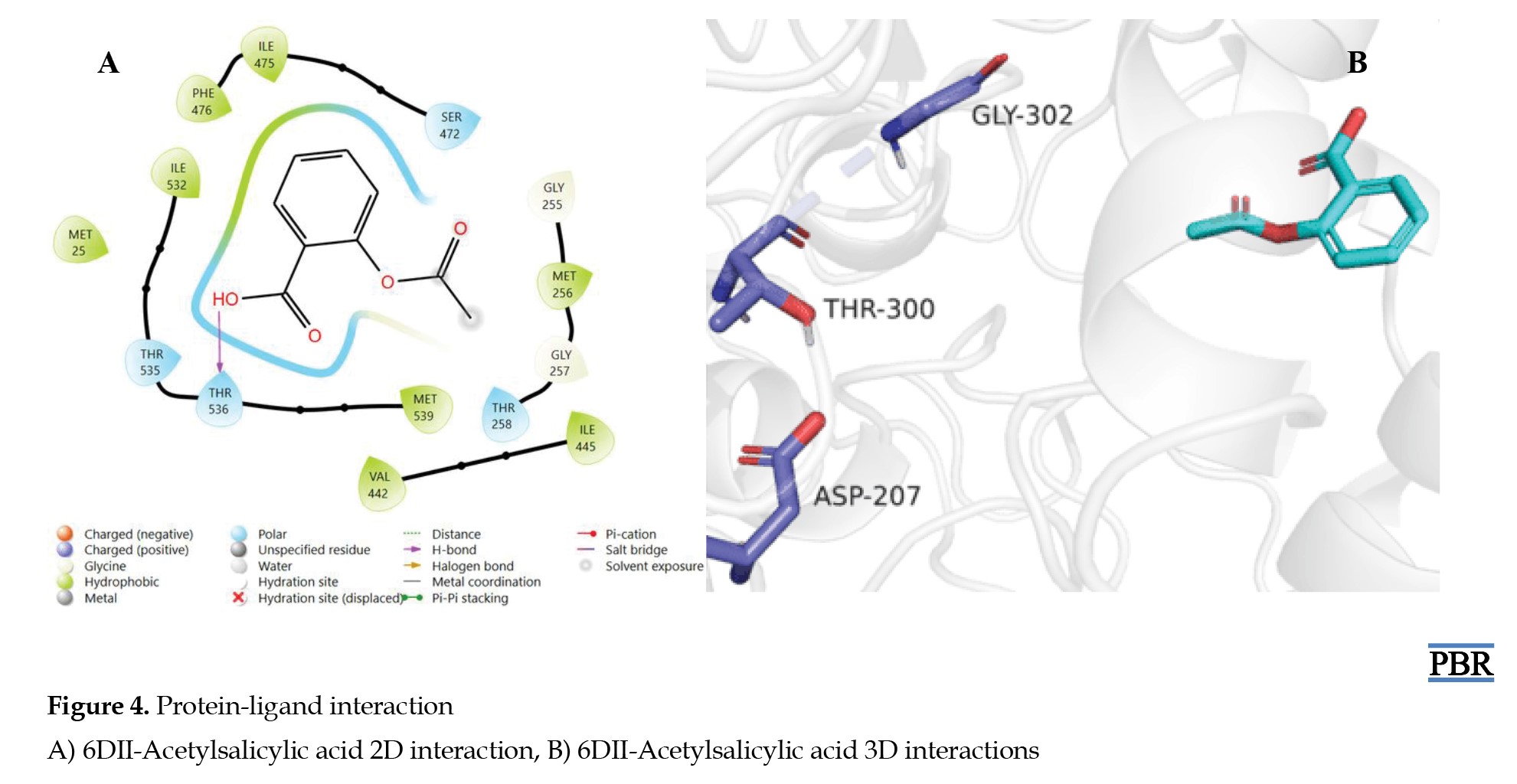
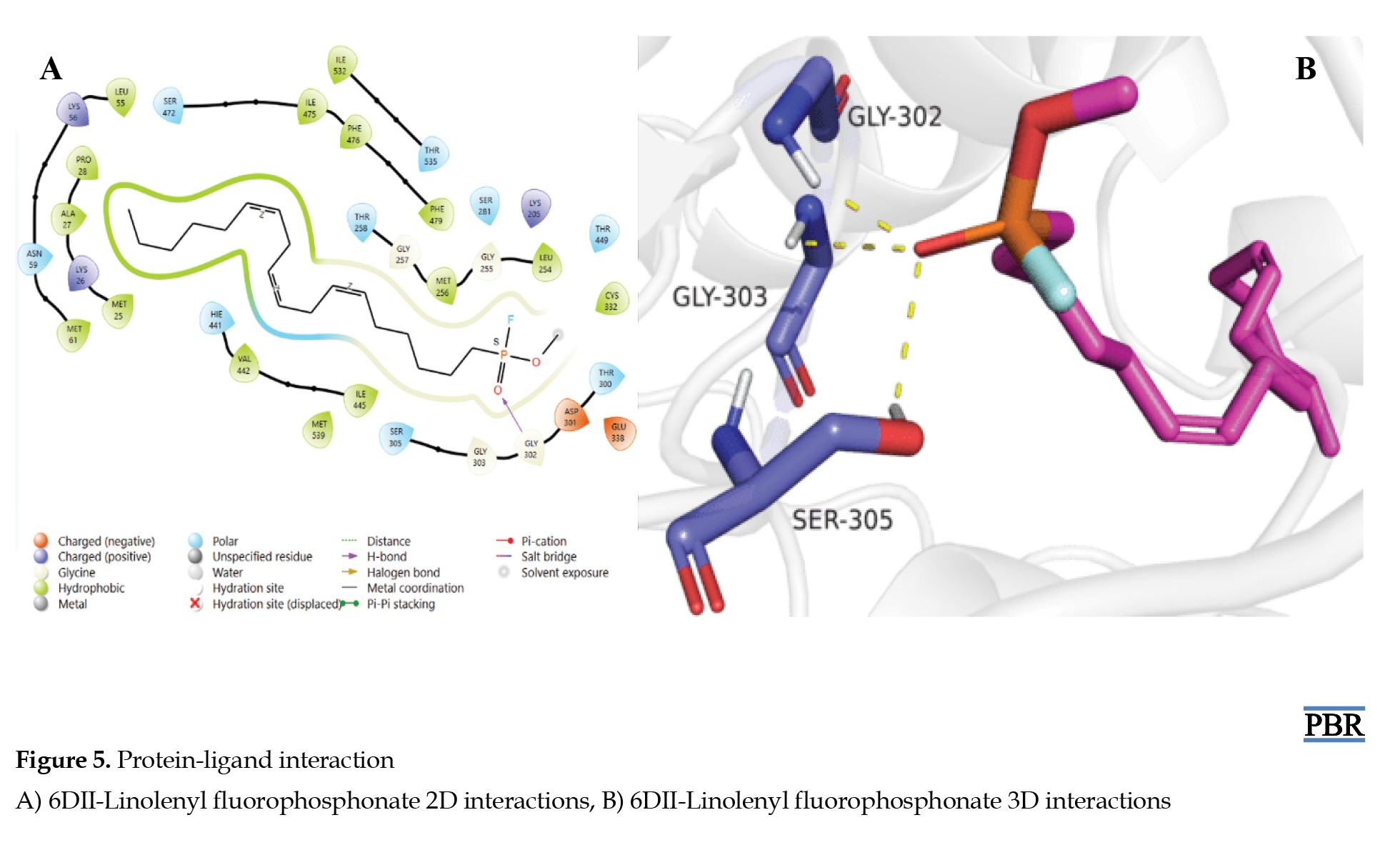
For the standard drugs, acetylsalicylic acid formed hydrogen interactions between the carboxylic acid hydrogen (OH) and the THR-536 residue of the protein (Figures 4A and 4B), while linolenyl fluorophosphonate showed hydrogen interactions between the oxygen of the phosphonate group (P=O) and GLY-302, GLY-303, and SER-305 residues of the protein (Figures 5A and 5B).
For the standard drugs, acetylsalicylic acid formed hydrogen interactions between the carboxylic acid hydrogen (OH) and the THR-536 residue of the protein (Figures 4A and 4B), while linolenyl fluorophosphonate showed hydrogen interactions between the oxygen of the phosphonate group (P=O) and GLY-302, GLY-303, and SER-305 residues of the protein (Figures 5A and 5B).
FAs and vitamins have been reported to protect chemotherapy-induced intestinal mucositis in cancer [28-30]. Hence, the role of FAs is very significant in the current medical practice. The current study was an attempt to suggest a possible biological mechanism for the synthesized amide derivatives of LCFAs. Based on the obtained results, the protein binding mode of the compounds was similar to that of the identified inhibitor (linolenyl fluorophosphonate) of A. thaliana FAAH proteins (PDB ID: 6DII), which bound to the GLY-302, GLY-303, SER-305 residues of the protein via the oxygen atom of the phosphonate group. This is very similar to the protein binding mechanism of our novel amide derivatives of long-chain FAs, as revealed by our molecular docking studies. N-palmitoyl alanine showed the highest protein binding (inhibitory) affinity, binding to GLY-255, THR-300, and GLY-302 through the amide N-H and OH functional groups. Additionally, N-palmitoyl glycine, which demonstrated the highest bioactivity, also showed significant protein binding affinity (inhibition), binding to ASP-207, GLY-302, and THR-300 via the carboxylic acid (OH) and amide carbonyl functional group (C=O). This is also comparable to N-palmitoyl GABA, which binds to ASP-207 and GLY-302 through the N-H and OH functional group.
The stronger effect at 50 mg/kg compared to 100 mg/kg in Table 2 may result from a biphasic or hormetic response, where higher doses saturate target pathways or trigger counteracting mechanisms.
Also, pharmacokinetic factors at increased dosage could reduce efficacy [31]. While this finding requires further validation, it aligns with reports of non-linear dose responses for some bioactive compounds.
Conclusion
There was a common protein binding mode for the synthesized compounds and the identified inhibitor of A. thaliana FAs amide hydrolase (PDB ID: 6DII), which involved binding to specific residues of the protein via unique functional groups. Therefore, it can be suggested that the ability of these amide derivatives of LCFAs to biochemically inhibit the inflammatory and nociceptive pathways may be due to the presence of the amide nitrogen and carbonyl oxygen (N-H, C=O) and carboxylic acid (OH) functional groups that bind to the GLY-255, THR-300, GLY-302, and ASP-207 residues of the protein. Thus, these compounds could serve as novel lead molecules in the discovery and design of effective anti-inflammatory and analgesic drugs.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research and Ethics Committee of the Faculty of Pharmacy, Niger Delta University, Amassoma, Nigeria (Code: BH-0014).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to this work.
Conflict of interest
The authors declared no conflict of interest.
References
The stronger effect at 50 mg/kg compared to 100 mg/kg in Table 2 may result from a biphasic or hormetic response, where higher doses saturate target pathways or trigger counteracting mechanisms.

Also, pharmacokinetic factors at increased dosage could reduce efficacy [31]. While this finding requires further validation, it aligns with reports of non-linear dose responses for some bioactive compounds.
Conclusion
There was a common protein binding mode for the synthesized compounds and the identified inhibitor of A. thaliana FAs amide hydrolase (PDB ID: 6DII), which involved binding to specific residues of the protein via unique functional groups. Therefore, it can be suggested that the ability of these amide derivatives of LCFAs to biochemically inhibit the inflammatory and nociceptive pathways may be due to the presence of the amide nitrogen and carbonyl oxygen (N-H, C=O) and carboxylic acid (OH) functional groups that bind to the GLY-255, THR-300, GLY-302, and ASP-207 residues of the protein. Thus, these compounds could serve as novel lead molecules in the discovery and design of effective anti-inflammatory and analgesic drugs.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Research and Ethics Committee of the Faculty of Pharmacy, Niger Delta University, Amassoma, Nigeria (Code: BH-0014).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to this work.
Conflict of interest
The authors declared no conflict of interest.
References
- He Q, Chen Y, Wang Z, He H, Yu P. Cellular uptake, metabolism and sensing of long-chain fatty acids. Front Biosci. 2023; 28(1):10. [DOI:10.31083/j.fbl2801010] [PMID]
- Pinkosky SL, Scott JW, Desjardins EM, Smith BK, Day EA, Ford RJ, et al. Long-chain fatty acyl-CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nat Metab. 2020; 2(9):873-81. [DOI:10.1038/s42255-020-0245-2] [PMID]
- Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997; 323(Pt 1):1-12. [DOI:10.1042/bj3230001] [PMID]
- Sassa T, Kihara A. Metabolism of very long-chain fatty acids: Genes and pathophysiology. Biomol Ther (Seoul). 2014; 22(2):83-92. [DOI: 10.4062/biomolther.2014.017] [PMID]
- Bortz WM, Lynen F. The inhibition of acetyl coa carboxylase by long chain acyl coa derivatives. Biochem Z. 1963; 337:505-9. [PMID]
- Stahl A. A current review of fatty acid transport proteins (SLC27). Pflugers Arch. 2004; 447(5):722-7. [DOI:10.1007/s00424-003-1106-z] [PMID]
- Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020; 100(1):171-210. [DOI:10.1152/physrev.00041.2018] [PMID]
- Roe K. An inflammation classification system using cytokine parameters. Scand J Immunol. 2021; 93(2):e12970. [DOI:10.1111/sji.12970] [PMID]
- Bhandari S, Bisht KS, Merkler DJ. The biosynthesis and metabolism of the N-acylated aromatic amino acids: N-acylphenylalanine, N-acyltyrosine, N-acyltryptophan, and N-acylhistidine. Front Mol Biosci. 2022; 8:801749. [DOI:10.3389/fmolb.2021.801749] [PMID]
- Baba H, Usifoh CO, Nwid LL. Synthesis of some long chain fatty acid amide derivatives with possible anti-inflammatory and anti-nociceptive effect. West Afr J Pharm. 2014; 25(1):1-8. [Link]
- Jacob BB, Baba H, Oluwadiya JO. Synthesis, characterization and evaluation of anti-inflammatory and antimicrobial properties of some cinnamic acid derivatives. Niger J Pharm Res. 2020; 16(1):1-8.1 [DOI:10.4314/njpr.v16i1.1]
- Bunu SJ, Alfred-Ugbenbo DE, Miediegha OY, Baba HA. Characterization and molecular docking of cinnamic acid derivatives: Potential inhibitors of cyclo-oxygenase enzymes. Innovare J Life Sci. 2023; 11:41-6. [DOI:10.22159/ijls.2023.v11i1.49501]
- Bunu SJ, Miediegha O, Awala EV, Alfred Ugbenbo D, Baba H, Usifoh CO. Synthesis and in silico analysis of chalcone derivatives as potential prostaglandin synthetase inhibitors. Biomed J Sci Tech Res. 2024; 55(2):46709-20. [DOI:10.26717/BJSTR.2024.55.008662]
- Awala EV, Bunu SJ, Haruna B, Oluwadiya JO. Synthesis, antimicrobial, and anti-inflammatory evaluation of epoxide and 4-methoxy-and 4, 6-diphenyl-2-thiopyrimidine derivatives of chalcones. Scholars Acad J Pharm. 2019; 8(8):436-2. [DOI:10.21276/sajp.2019.8.8.9]
- Dode E, Bunu SJ, Garando OR. Assessment of different brands of diclofenac tablets: An evaluation utilizing uv spectroscopy and disintegration test methods. World J Bio Pharm Health Sci. 2023; 14(2):1-6. [DOI:10.30574/wjbphs.2023.14.2.0201]
- Battista N, Bari M, Bisogno T. N-acyl amino acids: Metabolism, molecular targets, and role in biological processes. Biomolecules. 2019; 9(12):822. [DOI:10.3390/biom9120822] [PMID]
- Aziz M, Chapman KD. Fatty acid amide hydrolases: an expanded capacity for chemical communication? Trends Plant Sci. 2020; 25(3):236-49. [DOI:10.1016/j.tplants.2019.11.002] [PMID]
- Wang YS, Shrestha R, Kilaru A, Wiant W, Venables BJ, Chapman KD, et al. Manipulation of arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines. Proc Natl Acad Sci U S A. 2006; 103(32):12197-202. [DOI:10.1073/pnas.0603571103] [PMID]
- Vaikosen EN, Bunu JS, Miediegha O, Chilaka UP, Echendu CE, Usifoh CO. Synthesis and antimicrobial evaluation of some schiff base derivatives. Pharm Drug Dev. 2024; 3(2):1-4. [DOI:10.58489/2836-2322/031]
- Li CQ, Lei HM, Hu QY, Li GH, Zhao PJ. Recent advances in the synthetic biology of natural drugs. Front Bioeng Biotechnol. 2021; 9:691152. [DOI:10.3389/fbioe.2021.691152] [PMID]
- Michaelis J, Vissers MC, Winterbourn CC. Human neutrophil collagenase cleaves alpha 1-antitrypsin. Biochem J. 1990; 270(3):809-14. [DOI:10.1042/bj2700809] [PMID]
- Di Chiacchio A, Rimoli MG, Avallone L, Arena F, Abignente E, Filippelli W, et al. 2-Phenylimidazo [1, 2-a] pyridine-3-carboxylic acid derivatives: Synthesis and antiinflammatory activity. Archiv der Pharmazie. 1998; 331(9):273-8. [DOI:10.1002/(SICI)1521-4184(19989)331:93.0.CO;2-R]
- Valencia E, Feria M, Díaz JG, González A, Bermejo J. Antinociceptive, anti-inflammatory and antipyretic effects of lapidin, a bicyclic sesquiterpene. Planta Med. 1994; 60(5):395-9. [DOI:10.1055/s-2006-959517] [PMID]
- Schrödinger. LLC, New York: Schrödinger; 2020. [Link]
- Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010; 24(5):417-22. [DOI:10.1007/s10822-010-9352-6] [PMID]
- Vinegar R, Truax JF, Selph JL, Johnston PR. Antagonism of pain and hyperalgesia. In: Vane JR, Ferreira SH, editors. Anti inflammatory drugs. Berlin: Springer; 1979. [DOI:10.1007/978-3-642-66891-3_8]
- Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969; 166(1):96-103. [DOI:10.1016/S0022-3565(25)28209-X] [PMID]
- Bunu JS, Miediegha O, Agbolo T, Adugo M, Usifoh OC. Review of vitamin a structural analogues and their pharmacokinetic parameters. Open Access J Biomed Sci. 2022; 4(4):1923-33. [DOI:10.38125/OAJBS.000466]
- Ebeshi UB, Bunu JS, Vaikosen NE, Kashimawo JA, Enoch MU, Chukwuemerie LO. Quality assessment of ascorbic acid tablet formulations and comparative analysis with edible fruits. J Basic Soc Pharm Res. 2022; 2(4):57-66. [DOI:10.52968/27455330]
- Alcorta A, López-Gómez L, Capasso R, Abalo R. Vitamins and fatty acids against chemotherapy-induced intestinal mucositis. Pharmacol Ther. 2024; 261:108689. [DOI:10.1016/j.pharmthera.2024.108689] [PMID]
- Bauer S, Demetri GD, Halilovic E, Dummer R, Meille C, Tan DSW, et al. Pharmacokinetic-pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies. Br J Cancer. 2021; 125(5):687-98. [DOI:10.1038/s41416-021-01444-4] [PMID]
Type of Study: Original Research |
Subject:
Medicine and traditional medicine
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |