Volume 11, Issue 1 (2025)
Pharm Biomed Res 2025, 11(1): 23-30 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shokrzadeh M, Parash K, Rahmati M, Gharehkhani E. The Renal Protective Function of Chrysin in Cisplatin-induced Kidney Toxicity: Insights From Bee-derived Natural Compounds. Pharm Biomed Res 2025; 11 (1) :23-30
URL: http://pbr.mazums.ac.ir/article-1-642-en.html
URL: http://pbr.mazums.ac.ir/article-1-642-en.html
1- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. & Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Pharmacy, Ramsar Campus, Mazandaran University of Medical Sciences, Ramsar, Iran.
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Pharmacy, Ramsar Campus, Mazandaran University of Medical Sciences, Ramsar, Iran.
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 585 kb]
(433 Downloads)
| Abstract (HTML) (1191 Views)
Full-Text: (561 Views)
Introduction
Maintaining the health of the kidney, as a vital organ, has essential effects on the quality of an individual’s life [1]. Many components and materials could protect nephrons from injury and cure renal failure. At the same time, plenty of things damage it, such as drugs and free radicals that are produced due to metabolism, lack of activity, and diseases [2]. Cis-diaminedichloroplatinum, cisplatin (CDDP), is one of the chemotherapeutic medications most frequently used to treat various human cancers [3]. Still, renal toxicity is the dominant dose-limiting side effect and is noticed in almost 30% of sufferers [4]. The most severe form of acute kidney injury happens in 20%–30% of sufferers and results in CDDP-cutoff chemotherapy [5]. However, over an extended period of clinical medications, therapeutic failure is brought on by side effects like nephrotoxicity, neurotoxicity, and myelosuppression that impact the entire hematopoietic population and lead to the development of chemoresistance.
Preclinical research has shed light on the cellular and molecular mechanisms underlying cisplatin nephrotoxicity. These mechanisms impact intracellular stressors, such as endoplasmic reticulum stress, oxidative stress, mitochondrial pathology, and DNA damage [6]. For many years, one important factor that has been investigated in cisplatin-induced nephrotoxicity is oxidative stress. Research indicates that the primary mechanism by which cisplatin damages renal tubular and glomerular cells is by inducing oxidative stress, which results in cell necrosis and apoptosis, vascular defects, and robust immune response [7].
Accordingly, variant mixtures have been assessed in combination with cisplatin [8].
The organic and physiologically active flavone chrysin (5,7-dihydroxyflavone) is extracted from chamomile, Pleurotus ostreatus, and honeycomb [9]. Anticancer, neuroprotective, antiviral, antibacterial, anti-asthmatic, anti-inflammatory, hepatoprotective, nephroprotective, cardioprotective, antidepressant, and antiarthritic activities are the most candid pharmacological characteristics of chrysin [10]. It is thought to possess the ability to destroy free radicals because of the hydroxyl groups in CH at positions five and seven. Research has indicated that the CH group encompasses all effects, including those that are anti-inflammatory, anti-diabetic, anti-allergic, anti-apoptotic, and antioxidant [11]. The constant absorption of chrysin in humans is estimated to range from 0.5 to 3 grams per dose [12]. In this work, we investigated chrysin’s effects against cisplatin-induced nephrotoxicity using Vero cells as a renal model. Chrysin is known for its role in reducing oxidative stress.
Materials and Methods
Preparation of medicine and materials
Cisplatin was prepared in a vial under the brand Sigma-Aldrich (CAS:15663-27-1), and chrysin in powder under the brand Herbst (CAS: 480-40-0MF). The cell line was purchased from Pasteur Institute cell bank.
Cell lines
The Vero cell lines from the Pasteur Institute in Tehran, Iran, were cultivated in RPMI-1640 (Gibco, Berlin, Germany) supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 IU/mL penicillin (all from Gibco-BRL, Germany). In an atmosphere consisting of 5% CO2 and 95% air mixture, cell cultures were incubated at 37 °C after being adjusted for exponential growth [13].
MTT assay
Chrysin was applied in varying concentrations to renal cells (derived from the kidney cell line) based on the assessment of cell toxicity. This metabolic test is competitive and measures mitochondrial function. In the mitochondria of living cells, the yellow-colored MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), a tetrazole, is reduced to the unresolved purple-colored formazan.
Only in the presence of active mitochondrial reductase enzymes does the reduction occur.
As a result, the number of living cells can be used to determine this response precisely. Chrysin was prepared in 40, 80, 120, 160, and 200 µg/mL concentrations. While the positive control group was exposed to a half-maximal inhibitory concentration (IC50) dose of cisplatin, and the negative control group was left untreated [14].
Calculating reduced glutathione (GSH) levels
The reaction mixture, which included 0.4 mL of phosphate buffer, 0.1 mL of sodium azide, 0.1 mL of H2O2, 0.2 mL of glutathione (GSH), 0.2 mL of ethylenediaminetetraacetic acid (EDTA), and 0.2 mL of homogenate was incubated for 10 minutes at 37 °C. After adding 0.5 mL of trichloroacetic acid to stop the reaction, the tubes were centrifuged at 2000 rpm. Three milliliters of disodium hydrogen phosphate and 1 mL of DTNB, Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid), were added to the supernatant, and the color developed was read at 420 nm right away. The GSH oxidized activity of glutathione peroxidase (GPx) was expressed as μ mole/minute/mg of protein [15].
Measurement of oxidative stress
Approximately 4×104 cells per well were cultured in 96-well plates (black wall/clear bottom) for 24 hours. The medium was then aspirated, and the cells were washed twice with Hanks’ balanced salt solution (HBSS). Cells were then treated with the studied chrysin concentrations (40, 80, 120, 160, and 200 μg/mL) for 1 hour at 37 °C 24 h before cisplatin treatment (120 μg/mL). After treatment, the cells were washed twice with HBSS and incubated in 2 mL of fresh culture medium without FBS. 2’, 7’Dichlorodihydrofluorescein diacetate was added at a final concentration of 10 µM and incubated for 20 minutes. The cells were washed twice with phosphate-buffered saline and maintained in 1 mL of culture medium. We assessed reactive oxygen species (ROS) by immediately analyzing the cells with a fluorescent plate reader using 488 nm for excitation and detection at 535 nm. We selected untreated cells as a negative control and cells treated with 0.1 mM H2O2 as a positive control [16].
Statistical analysis
One-way analysis of variance and Tukey honestly significance differences (HSD) test were used for multiple data comparisons. P<0.05 was considered significant. IC50 values were calculated by PRISM software, version 8 using nonlinear regression. Standard deviations represent the average results of duplicate experiments.
IC50 values were compared using the student t-test, which measures the effectiveness of a substance in causing cell death or inhibiting cell growth. Therefore, the lower amount of IC50 represents higher toxicity of a compound leading to death or inhibition of cell growth. Data are presented as Mean±SD. Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test.
Results
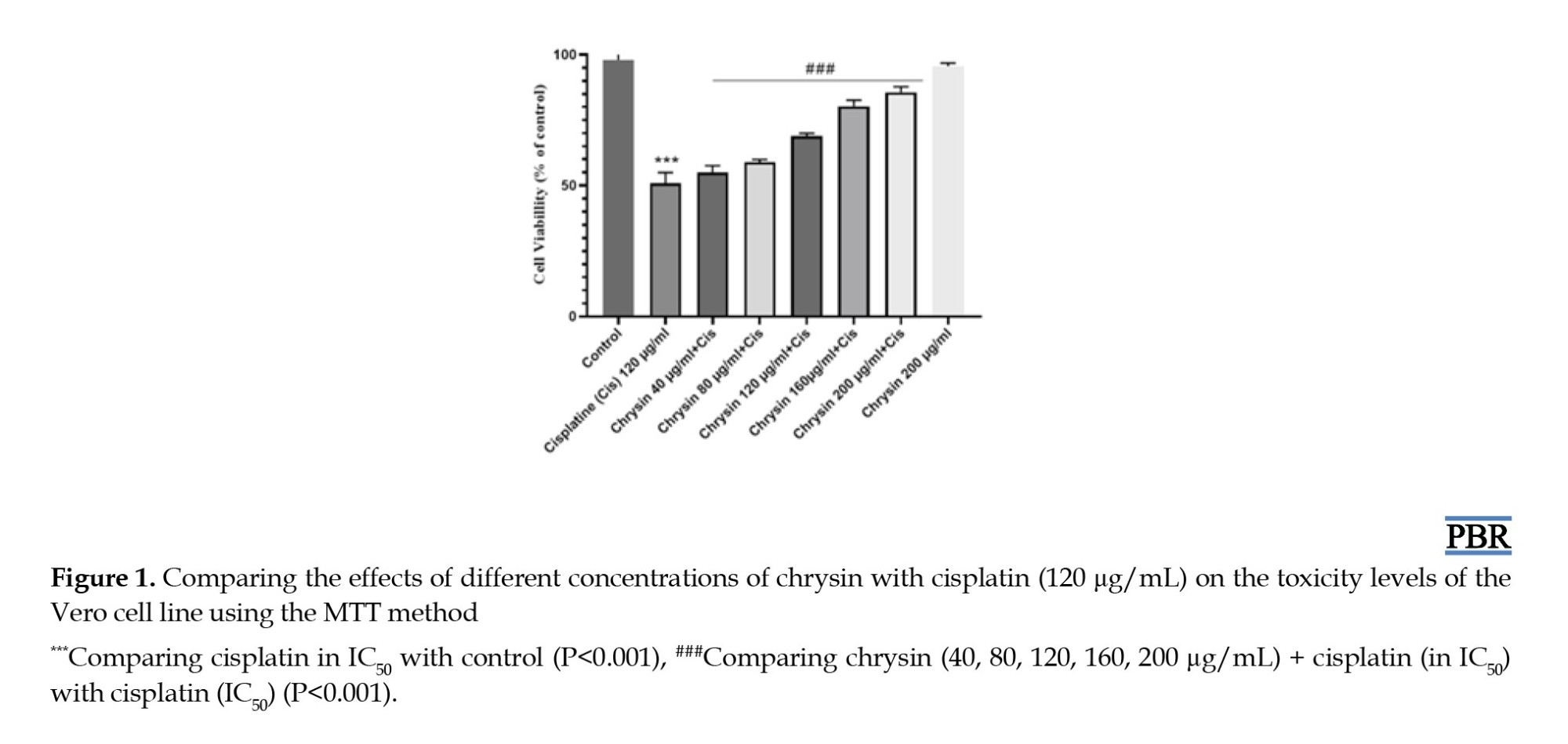
To study the effect of chrysin on cell viability upon exposure to cisplatin, after the MTT assay, Vero cells pretreated with chrysin exhibited different behavior than the control group. The results show that the maximum concentration has a significant effect in contrast to the control (Figure 1).
Influence of chrysin on glutathione content
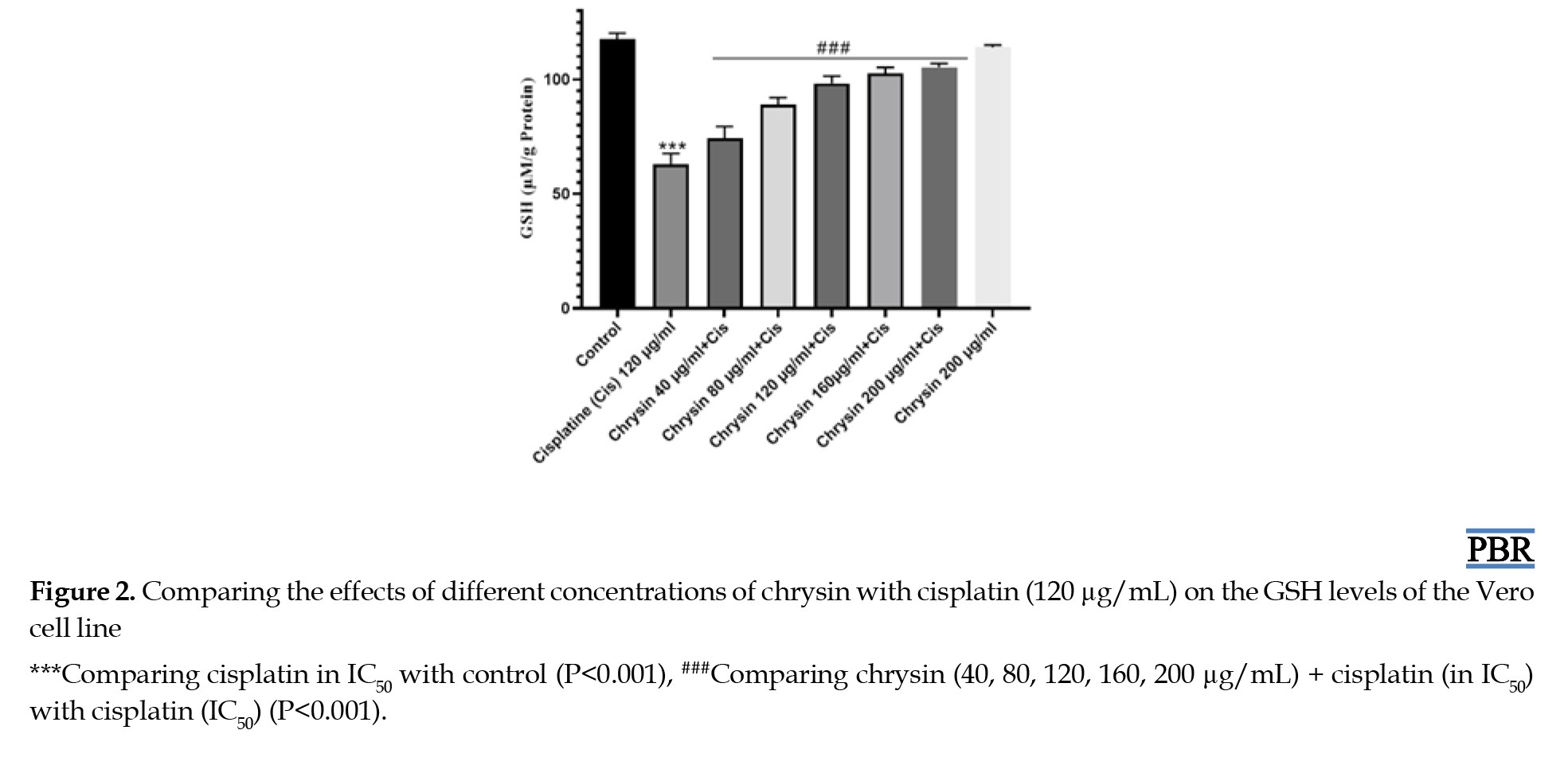
According to the results of the glutathione measurement method, treating the cells with chrysin changes the amount of glutathione compared to the negative control group (Figure 2).
Protective effect of chrysin on cisplatin-induced ROS generation
According to the results, 120 µg/mL cisplatin resulted in an increase in ROS levels compared to the control group (P<0.05) (Figure 3).
Discussion
Flavonoids are the main group of plant alternative metabolites with tremendous vital activities that are useful for human health. There is growing interest in studying flavonoids because they act through physiological mechanisms and many signaling pathways involved in many medical diseases [17].
Chrysin is a flavonoid found in fruits, vegetables, plants, and mainly in honey. Chrysin has antioxidant effects by enhancing the antioxidant system, defeating pro-oxidant enzymes, scavenging free radicals, and chelating redox-active transition metal ions [18].
Oxidative damage occurs when the production of ROS impairs the antioxidant system’s ability to destroy ROS, ultimately leading to cell damage [19]. One of the sources of ROS generation is chemical agents such as chemotherapy drugs [20].
Cisplatin is frequently utilized in the treatment of leukemia, lymphomas, breast cancer, testicular cancer, ovarian cancer, head and neck cancer, cervical cancer, and sarcomas [21]. Due to the high effectiveness of this drug in cancer, despite its side effects, its use is very common. Side effects such as DNA damage, cytotoxicity, ROS generation, mutagenicity, tumorigenesis, and genotoxicity have been mentioned in the research [22]. To protect patients from the side effects of cisplatin, physicians prescribe some diets and protective compounds along with the drug [23]. Chrysin can scavenge free radicals, and it belongs to its hydroxyl groups. Because of that, we designed this study based on the protective effect of chrysin against cisplatin-induced cytotoxicity on Vero cell lines.
Many studies have proved that chrysin could raise cell viability and protect cells. Talebi et al., in their studies about cardiometabolic diseases, found that chrysin could protect the cardiovascular system by enhancing the inherent antioxidative defense system, which helps cells to live [24].
In our investigation using the MTT method, we observed that chrysin elevated cell viability compared to the positive control (cisplatin at IC50). This correlation was evident as higher concentrations of chrysin led to increased cell viability. Our findings indicate that chrysin mitigates cisplatin-induced lipid peroxidation, xanthine oxidase activity, and reduction in glutathione levels, as well as a decline in the activities of antioxidant enzymes (catalase, glutathione reductase, superoxide dismutase (SOD), glutathione peroxidase, and glucose-6 phosphate dehydrogenase) and phase-II detoxifying enzymes (glutathione-S-transferase and quinone reductase) [25]. In another study on the effects of chrysin on cell protection in cadmium toxicity by Simsek et al., it was found that chrysin has cell protection properties and can prevent kidney toxicity and heal damaged cells [26]. The investigation into mechanisms showed that chrysin could decrease Dox-induced apoptosis by lowering the expression of p53, Bax, cytochrome c, and caspase-3. Also, suppressing pathways activated by doxorubicin, such as Akt and VEGF pathways, causes the survival of kidney cells [27].
GSH depletion is another problem caused by cisplatin toxicity and cell harm [28]. Based on our results in the group treated with cisplatin (in IC50), GSH depletion is obvious but decreased in groups pretreated with chrysin. Chrysin could increase the GSH level of cells by its potential antioxidant power [11]. Chrysin could decrease the genotoxicity damage of mitomycin c via inducing activation of the antioxidant system in the level of SOD, GPx, and GSH [29]. However, chrysin can initiate cellular apoptosis by GSH depletion in cancer cells and sensitize neoplasm to chemotherapy drugs [30]. Chrysin could act as a double-edged sword, depending on the situation; because of that, it could be used for prophylaxis or therapy [31]. Chrysin stimulates the transcription factor known as nuclear factor erythroid-derived 2-related factor (Nrf2), leading to upregulation of genes encoding antioxidant and phase II detoxification enzymes, including GSH reductase and GSH S-transferase (GST) [32].
As we mentioned, cisplatin could raise the generation of ROS, and chrysin, as a free radical scavenger, could reduce it [33, 34]. According to our results, groups treated with chrysin and cisplatin show ROS at high levels. The highest level belongs to a positive control (cisplatin in IC50 concentration) in contrast with the negative control. Still, based on the chrysin concentration, we could see decreasing in ROS level, and in the group with a single dose of chrysin (200 µg/mL), we saw the minimum level of ROS in treated groups. This means that cisplatin increases ROS, but in the groups exposed to chrysin at the same time, ROS production decreased and as the concentration of chrysin increased, the reduction in ROS production was more significant.
Conclusion
In the end, according to the data obtained from the test, chrysin can protect cells, and this feature is visible in the two parameters of glutathione and ROS production. Also, this substance helps cell survival, which was visible in the MTT test results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Vice-Chancellor of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.RIB.REC.1402.124).
Funding
The study was supported by Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 20911).
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank their peers for their valuable input and knowledge that significantly contributed to the study, even if they may not align with every interpretation or conclusion presented in this paper.
References
Maintaining the health of the kidney, as a vital organ, has essential effects on the quality of an individual’s life [1]. Many components and materials could protect nephrons from injury and cure renal failure. At the same time, plenty of things damage it, such as drugs and free radicals that are produced due to metabolism, lack of activity, and diseases [2]. Cis-diaminedichloroplatinum, cisplatin (CDDP), is one of the chemotherapeutic medications most frequently used to treat various human cancers [3]. Still, renal toxicity is the dominant dose-limiting side effect and is noticed in almost 30% of sufferers [4]. The most severe form of acute kidney injury happens in 20%–30% of sufferers and results in CDDP-cutoff chemotherapy [5]. However, over an extended period of clinical medications, therapeutic failure is brought on by side effects like nephrotoxicity, neurotoxicity, and myelosuppression that impact the entire hematopoietic population and lead to the development of chemoresistance.
Preclinical research has shed light on the cellular and molecular mechanisms underlying cisplatin nephrotoxicity. These mechanisms impact intracellular stressors, such as endoplasmic reticulum stress, oxidative stress, mitochondrial pathology, and DNA damage [6]. For many years, one important factor that has been investigated in cisplatin-induced nephrotoxicity is oxidative stress. Research indicates that the primary mechanism by which cisplatin damages renal tubular and glomerular cells is by inducing oxidative stress, which results in cell necrosis and apoptosis, vascular defects, and robust immune response [7].
Accordingly, variant mixtures have been assessed in combination with cisplatin [8].
The organic and physiologically active flavone chrysin (5,7-dihydroxyflavone) is extracted from chamomile, Pleurotus ostreatus, and honeycomb [9]. Anticancer, neuroprotective, antiviral, antibacterial, anti-asthmatic, anti-inflammatory, hepatoprotective, nephroprotective, cardioprotective, antidepressant, and antiarthritic activities are the most candid pharmacological characteristics of chrysin [10]. It is thought to possess the ability to destroy free radicals because of the hydroxyl groups in CH at positions five and seven. Research has indicated that the CH group encompasses all effects, including those that are anti-inflammatory, anti-diabetic, anti-allergic, anti-apoptotic, and antioxidant [11]. The constant absorption of chrysin in humans is estimated to range from 0.5 to 3 grams per dose [12]. In this work, we investigated chrysin’s effects against cisplatin-induced nephrotoxicity using Vero cells as a renal model. Chrysin is known for its role in reducing oxidative stress.
Materials and Methods
Preparation of medicine and materials
Cisplatin was prepared in a vial under the brand Sigma-Aldrich (CAS:15663-27-1), and chrysin in powder under the brand Herbst (CAS: 480-40-0MF). The cell line was purchased from Pasteur Institute cell bank.
Cell lines
The Vero cell lines from the Pasteur Institute in Tehran, Iran, were cultivated in RPMI-1640 (Gibco, Berlin, Germany) supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 IU/mL penicillin (all from Gibco-BRL, Germany). In an atmosphere consisting of 5% CO2 and 95% air mixture, cell cultures were incubated at 37 °C after being adjusted for exponential growth [13].
MTT assay
Chrysin was applied in varying concentrations to renal cells (derived from the kidney cell line) based on the assessment of cell toxicity. This metabolic test is competitive and measures mitochondrial function. In the mitochondria of living cells, the yellow-colored MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), a tetrazole, is reduced to the unresolved purple-colored formazan.
Only in the presence of active mitochondrial reductase enzymes does the reduction occur.
As a result, the number of living cells can be used to determine this response precisely. Chrysin was prepared in 40, 80, 120, 160, and 200 µg/mL concentrations. While the positive control group was exposed to a half-maximal inhibitory concentration (IC50) dose of cisplatin, and the negative control group was left untreated [14].
Calculating reduced glutathione (GSH) levels
The reaction mixture, which included 0.4 mL of phosphate buffer, 0.1 mL of sodium azide, 0.1 mL of H2O2, 0.2 mL of glutathione (GSH), 0.2 mL of ethylenediaminetetraacetic acid (EDTA), and 0.2 mL of homogenate was incubated for 10 minutes at 37 °C. After adding 0.5 mL of trichloroacetic acid to stop the reaction, the tubes were centrifuged at 2000 rpm. Three milliliters of disodium hydrogen phosphate and 1 mL of DTNB, Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid), were added to the supernatant, and the color developed was read at 420 nm right away. The GSH oxidized activity of glutathione peroxidase (GPx) was expressed as μ mole/minute/mg of protein [15].
Measurement of oxidative stress
Approximately 4×104 cells per well were cultured in 96-well plates (black wall/clear bottom) for 24 hours. The medium was then aspirated, and the cells were washed twice with Hanks’ balanced salt solution (HBSS). Cells were then treated with the studied chrysin concentrations (40, 80, 120, 160, and 200 μg/mL) for 1 hour at 37 °C 24 h before cisplatin treatment (120 μg/mL). After treatment, the cells were washed twice with HBSS and incubated in 2 mL of fresh culture medium without FBS. 2’, 7’Dichlorodihydrofluorescein diacetate was added at a final concentration of 10 µM and incubated for 20 minutes. The cells were washed twice with phosphate-buffered saline and maintained in 1 mL of culture medium. We assessed reactive oxygen species (ROS) by immediately analyzing the cells with a fluorescent plate reader using 488 nm for excitation and detection at 535 nm. We selected untreated cells as a negative control and cells treated with 0.1 mM H2O2 as a positive control [16].
Statistical analysis
One-way analysis of variance and Tukey honestly significance differences (HSD) test were used for multiple data comparisons. P<0.05 was considered significant. IC50 values were calculated by PRISM software, version 8 using nonlinear regression. Standard deviations represent the average results of duplicate experiments.
IC50 values were compared using the student t-test, which measures the effectiveness of a substance in causing cell death or inhibiting cell growth. Therefore, the lower amount of IC50 represents higher toxicity of a compound leading to death or inhibition of cell growth. Data are presented as Mean±SD. Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey’s post hoc test.
Results
To study the effect of chrysin on cell viability upon exposure to cisplatin, after the MTT assay, Vero cells pretreated with chrysin exhibited different behavior than the control group. The results show that the maximum concentration has a significant effect in contrast to the control (Figure 1).
Influence of chrysin on glutathione content
According to the results of the glutathione measurement method, treating the cells with chrysin changes the amount of glutathione compared to the negative control group (Figure 2).
Protective effect of chrysin on cisplatin-induced ROS generation
According to the results, 120 µg/mL cisplatin resulted in an increase in ROS levels compared to the control group (P<0.05) (Figure 3).
Discussion
Flavonoids are the main group of plant alternative metabolites with tremendous vital activities that are useful for human health. There is growing interest in studying flavonoids because they act through physiological mechanisms and many signaling pathways involved in many medical diseases [17].
Chrysin is a flavonoid found in fruits, vegetables, plants, and mainly in honey. Chrysin has antioxidant effects by enhancing the antioxidant system, defeating pro-oxidant enzymes, scavenging free radicals, and chelating redox-active transition metal ions [18].
Oxidative damage occurs when the production of ROS impairs the antioxidant system’s ability to destroy ROS, ultimately leading to cell damage [19]. One of the sources of ROS generation is chemical agents such as chemotherapy drugs [20].
Cisplatin is frequently utilized in the treatment of leukemia, lymphomas, breast cancer, testicular cancer, ovarian cancer, head and neck cancer, cervical cancer, and sarcomas [21]. Due to the high effectiveness of this drug in cancer, despite its side effects, its use is very common. Side effects such as DNA damage, cytotoxicity, ROS generation, mutagenicity, tumorigenesis, and genotoxicity have been mentioned in the research [22]. To protect patients from the side effects of cisplatin, physicians prescribe some diets and protective compounds along with the drug [23]. Chrysin can scavenge free radicals, and it belongs to its hydroxyl groups. Because of that, we designed this study based on the protective effect of chrysin against cisplatin-induced cytotoxicity on Vero cell lines.
Many studies have proved that chrysin could raise cell viability and protect cells. Talebi et al., in their studies about cardiometabolic diseases, found that chrysin could protect the cardiovascular system by enhancing the inherent antioxidative defense system, which helps cells to live [24].
In our investigation using the MTT method, we observed that chrysin elevated cell viability compared to the positive control (cisplatin at IC50). This correlation was evident as higher concentrations of chrysin led to increased cell viability. Our findings indicate that chrysin mitigates cisplatin-induced lipid peroxidation, xanthine oxidase activity, and reduction in glutathione levels, as well as a decline in the activities of antioxidant enzymes (catalase, glutathione reductase, superoxide dismutase (SOD), glutathione peroxidase, and glucose-6 phosphate dehydrogenase) and phase-II detoxifying enzymes (glutathione-S-transferase and quinone reductase) [25]. In another study on the effects of chrysin on cell protection in cadmium toxicity by Simsek et al., it was found that chrysin has cell protection properties and can prevent kidney toxicity and heal damaged cells [26]. The investigation into mechanisms showed that chrysin could decrease Dox-induced apoptosis by lowering the expression of p53, Bax, cytochrome c, and caspase-3. Also, suppressing pathways activated by doxorubicin, such as Akt and VEGF pathways, causes the survival of kidney cells [27].
GSH depletion is another problem caused by cisplatin toxicity and cell harm [28]. Based on our results in the group treated with cisplatin (in IC50), GSH depletion is obvious but decreased in groups pretreated with chrysin. Chrysin could increase the GSH level of cells by its potential antioxidant power [11]. Chrysin could decrease the genotoxicity damage of mitomycin c via inducing activation of the antioxidant system in the level of SOD, GPx, and GSH [29]. However, chrysin can initiate cellular apoptosis by GSH depletion in cancer cells and sensitize neoplasm to chemotherapy drugs [30]. Chrysin could act as a double-edged sword, depending on the situation; because of that, it could be used for prophylaxis or therapy [31]. Chrysin stimulates the transcription factor known as nuclear factor erythroid-derived 2-related factor (Nrf2), leading to upregulation of genes encoding antioxidant and phase II detoxification enzymes, including GSH reductase and GSH S-transferase (GST) [32].
As we mentioned, cisplatin could raise the generation of ROS, and chrysin, as a free radical scavenger, could reduce it [33, 34]. According to our results, groups treated with chrysin and cisplatin show ROS at high levels. The highest level belongs to a positive control (cisplatin in IC50 concentration) in contrast with the negative control. Still, based on the chrysin concentration, we could see decreasing in ROS level, and in the group with a single dose of chrysin (200 µg/mL), we saw the minimum level of ROS in treated groups. This means that cisplatin increases ROS, but in the groups exposed to chrysin at the same time, ROS production decreased and as the concentration of chrysin increased, the reduction in ROS production was more significant.
Conclusion
In the end, according to the data obtained from the test, chrysin can protect cells, and this feature is visible in the two parameters of glutathione and ROS production. Also, this substance helps cell survival, which was visible in the MTT test results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Vice-Chancellor of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.RIB.REC.1402.124).
Funding
The study was supported by Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 20911).
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results, and manuscript drafting. Each author approved the submission of the final version of the manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank their peers for their valuable input and knowledge that significantly contributed to the study, even if they may not align with every interpretation or conclusion presented in this paper.
References
- Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021; 7(1):52. [DOI:10.1038/s41572-021-00284-z] [PMID]
- Bridoux F, Leung N, Belmouaz M, Royal V, Ronco P, Nasr SH, et al. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. 2021; 99(3):570-80. [DOI:10.1016/j.kint.2020.11.010] [PMID]
- Movaliya VR, Zaveri M. In vitro nephroprotective activity of selected herbal plants on vero cell line. Int J Pharmacogn Chinese Med. 2020; 5(3):207-12. [Link]
- Wang XL, Wang L, Lin FL, Li SS, Lin TX, Jiang RW. Protective Effect of Penetratin Analogue-Tagged SOD1 on Cisplatin-Induced Nephrotoxicity through Inhibiting Oxidative Stress and JNK/p38 MAPK Signaling Pathway. Oxid Med Cell Longev. 2021; 2021:5526053. [DOI:10.1155/2021/5526053] [PMID] [PMCID]
- Miyoshi T, Uoi M, Omura F, Tsumagari K, Maesaki S, Yokota C. Risk factors for cisplatin-induced nephrotoxicity: A multicenter retrospective study. Oncology. 2021; 99(2):105-13. [DOI:10.1159/000510384] [PMID]
- Tang C, Livingston MJ, Safirstein R, Dong Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol. 2023; 19(1):53-72. [DOI:10.1038/s41581-022-00631-7] [PMID]
- Zhou J, Nie RC, Yin YX, Cai XX, Xie D, Cai MY. Protective effect of natural antioxidants on reducing Cisplatin-Induced nephrotoxicity. Dis Markers. 2022; 2022:1612348. [DOI:10.1155/2022/1612348] [PMID] [PMCID]
- Zugasti A, Rivera AL, Silva SY, Alfaro MA, Sierra CA. Effect of sodium dichloroacetate as single agent or in combination with Cisplatin in normal and human cervical cancer cell lines. Trop J Pharm Res. 2020; 19(3):467-74. [DOI:10.4314/tjpr.v19i3.2]
- Zhong X, Liu D, Jiang Z, Li C, Chen L, Xia Y, et al. Chrysin induced cell apoptosis and inhibited invasion through regulation of TET1 expression in gastric cancer cells. Onco Targets Ther. 2020; 13:3277-87. [DOI:10.2147/OTT.S246031] [PMID] [PMCID]
- Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J, et al. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021; 21(1):214. [DOI:10.1186/s12935-021-01906-y] [PMID] [PMCID]
- Temel Y, Kucukler S, Yıldırım S, Caglayan C, Kandemir FM. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2020; 393(3):325-37. [DOI:10.1007/s00210-019-01741-z] [PMID]
- Kucukler S, Benzer F, Yildirim S, Gur C, Kandemir FM, Bengu AS, et al. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: a biochemical and histopathological approach. Biol Trace Elem Res. 2021; 199(4):1501-14. [DOI:10.1007/s12011-020-02268-8] [PMID]
- Shokrzadeh M, Mohammadpour A, Modanloo M, Hassani M, Barghi NG, Niroomand P. Cytotoxic effects of Aripiprazole on MKN45 and NIH3T3 cell lines and Genotoxic effects on human peripheral blood Lymphocytes. Arq Gastroenterol. 2019; 56(2):155-9. [DOI:10.1590/s0004-2803.201900000-31] [PMID]
- Motafeghi F, Gerami M, Mortazavi P, Khayambashi B, Ghassemi-Barghi N, Shokrzadeh M. Green synthesis of silver nanoparticles, graphene, and silver-graphene nanocomposite using Melissa officinalis ethanolic extract: Anticancer effect on MCF-7 cell line. Iran J Basic Med Sci. 2023; 26(1):57-68. [PMID]
- Kumar A, Selvakumar S. Antiproliferative efficacy of Tabernaemontana divaricata against HEP2 cell line and Vero cell line. Pharmacogn Mag. 2015; 11(S 1):S46-52.[DOI:10.4103/0973-1296.157682] [PMID] [PMCID]
- Shokrzadeh M, Ghassemi-Barghi N. Antioxidant and genoprotective effects of amifostine against irinotecan toxicity in human hepatoma cells. Int J Cancer Res Ther. 2018; 3(1):1-5. [DOI:10.33140/IJCRT/03/01/00004]
- Samarghandian S, Farkhondeh T, Azimi-Nezhad M. Protective effects of chrysin against drugs and toxic agents. Dose Response. 2017; 15(2):1559325817711782.[DOI:10.1177/1559325817711782] [PMID] [PMCID]
- Farkhondeh T, Samarghandian S, Bafandeh F. The cardiovascular protective effects of chrysin: a narrative review on experimental researches. Cardiovasc Hematol Agents Med Chem. 2019; 17(1):17-27. [DOI:10.2174/1871525717666190114145137] [PMID] [PMCID]
- Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl4)-induced tissue injury in male Wistar rats. Toxicol Ind Health. 2011; 27(10):923-33. [DOI:10.1177/0748233711399324] [PMID]
- Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, et al. Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One. 2015; 10(8):e0135083. [DOI:10.1371/journal.pone.0135083] [PMID] [PMCID]
- Brown A, Kumar S, Tchounwou PB. Cisplatin-based chemotherapy of human cancers. J Cancer Sci Ther. 2019; 11(4):97. [PMID]
- Gonçalves EM, Ventura CA, Yano T, Rodrigues Macedo ML, Genari SC. Morphological and growth alterations in Vero cells transformed by cisplatin. Cell Biol Int. 2006; 30(6):485-94. [DOI:10.1016/j.cellbi.2005.12.007] [PMID]
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol. 2000; 21(4):513-20. [PMID]
- Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J, et al. Promising protective effects of chrysin in cardiometabolic diseases. Curr Drug Targets. 2022; 23(5):458-70. [DOI:10.2174/1389450122666211005113234] [PMID]
- Pingili RB, Pawar AK, Challa SR, Kodali T, Koppula S, Toleti V. A comprehensive review on hepatoprotective and nephroprotective activities of chrysin against various drugs and toxic agents. Chem Biol Interact. 2019; 308:51-60. [DOI:10.1016/j.cbi.2019.05.010] [PMID]
- Şimşek H, Akaras N, Gür C, Küçükler S, Kandemir FM. Beneficial effects of Chrysin on Cadmium-induced nephrotoxicity in rats: Modulating the levels of Nrf2/HO-1, RAGE/NLRP3, and Caspase-3/Bax/Bcl-2 signaling pathways. Gene. 2023; 875:147502. [DOI:10.1016/j.gene.2023.147502] [PMID]
- Mantawy EM, Esmat A, El-Bakly WM, Salah ElDin RA, El-Demerdash E. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci Rep. 2017; 7(1):4795. [DOI:10.1038/s41598-017-05005-9] [PMID] [PMCID]
- Yu F, Megyesi J, Price PM. Cytoplasmic initiation of cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2008; 295(1):F44-52. [DOI:10.1152/ajprenal.00593.2007] [PMID] [PMCID]
- Sassi A, Boubaker J, Loussaief A, Jomaa K, Ghedira K, Chekir-Ghedira L. Protective effect of chrysin, a dietary flavone against genotoxic and oxidative damage induced by Mitomycin C in Balb/C mice. Nutr Cancer. 2021; 73(2):329-38. [DOI:10.1080/01635581.2020.1749289] [PMID]
- Khan R, Khan AQ, Qamar W, Lateef A, Tahir M, Rehman MU, et al. Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: probable role of p38MAPK and p53. Toxicol Appl Pharmacol. 2012; 258(3):315-29. [DOI:10.1016/j.taap.2011.11.013] [PMID]
- Ciftci O, Ozdemir I, Aydin M, Beytur A. Beneficial effects of chrysin on the reproductive system of adult male rats. Andrologia. 2012; 44(3):181-6. [DOI:10.1111/j.1439-0272.2010.01127.x] [PMID]
- Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, et al. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 2013; 87(1):167-78. [DOI:10.1007/s00204-012-0913-4] [PMID]
- Sulaiman GM, Jabir MS, Hameed AH. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol. 2018; 46(S 1):708-20. [DOI:10.1080/21691401.2018.1434661] [PMID]
- Bragado P, Armesilla A, Silva A, Porras A. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007; 12(9):1733-42. [DOI:10.1007/s10495-007-0082-8] [PMID]
Type of Study: Original Research |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |