Volume 11, Issue 2 (2025)
Pharm Biomed Res 2025, 11(2): 115-124 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmed M A K, Mohammad A E H, Ahmed B M, Yousef B A. Wound Healing Properties of Cyperus Papyrus Ethanolic Extract in Wister Albino Rats. Pharm Biomed Res 2025; 11 (2) :115-124
URL: http://pbr.mazums.ac.ir/article-1-639-en.html
URL: http://pbr.mazums.ac.ir/article-1-639-en.html
Mohammed Ali Khalifa Ahmed *1 

 , Amna Elhassan Hamad Mohammad2
, Amna Elhassan Hamad Mohammad2 

 , Basher Mohamed Ahmed2
, Basher Mohamed Ahmed2 

 , Bashir A. Yousef3
, Bashir A. Yousef3 



 , Amna Elhassan Hamad Mohammad2
, Amna Elhassan Hamad Mohammad2 

 , Basher Mohamed Ahmed2
, Basher Mohamed Ahmed2 

 , Bashir A. Yousef3
, Bashir A. Yousef3 

1- Department of Pharmacology, Faculty of Pharmacy, Omdurman Islamic University, Khartoum, Sudan.
2- Department of Pharmacology, Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, Khartoum, Sudan.
3- Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan.
2- Department of Pharmacology, Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, Khartoum, Sudan.
3- Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan.
Full-Text [PDF 1880 kb]
(231 Downloads)
| Abstract (HTML) (677 Views)
Full-Text: (214 Views)
Introduction
Wounds are physical injuries that result in an opening or break of the skin, causing a disturbance in normal skin anatomy and physiology, and resulting in the loss of continuity of the epithelium, with or without loss of underlying connective tissues, according to the Wound Healing Society (WHS) [1]. Wounds affect a large number of people and seriously reduce the quality of life [2]. Generally, there are three phases of wound healing: Inflammatory, proliferative, and remodeling [1]. Several agents have been used in the management of wounds and a wide variety of treatment modalities are available for wound repair. Among these medicines, those of herbal origin have a significant impact on the treatment and healing of wounds [3]. Medicinal plants offer significant benefits for wound treatment, not only because they are inexpensive and affordable but also because they are generally safe and do not typically cause hypersensitivity reactions [4].
The increasing demand and availability of medicinal products have created a need to isolate and identify the principles responsible for their therapeutic activities and effectiveness. For instance, the stimulation of fibroblasts by plant extracts has been observed as one of the mechanisms, by which medicinal plants enhance the wound healing process [5].
Cyperus papyrus belongs to the family Cyperaceae, which comprises monocotyledonous graminoid flowering plants known as sedges, that superficially resemble grasses or rushes [6]. It contains small amounts of sesquiterpenes relative to monoterpenes obtained by gas chromatography; it also contains phenolic compounds, as well as Na, K, Mg, Fe, I, and proteins, which are considered micronutrients [7]. C. papyrus has traditionally been used in the treatment of painful spasms, eye diseases, ulcers, fever, diarrhea, and various inflammatory conditions.
Any pathophysiologic disturbances in the healing process result in delayed or halted healing and present problems that result in frustrating and expensive care, ultimately failing to meet patient and provider goals [8]. Wounds (both normal and diabetic wounds) can become significant issues and may respond poorly to medications [9].
This study was conducted to evaluate the wound-healing activity of C. papyrus ethanolic extract in experimentally induced excision wounds in rats.
Materials and Methods
Materials
Plant material
Fresh aerial parts were harvested from the Botanical Garden at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTRI), Khartoum, Sudan, in August 2019. Plant identification (specimen No. Y-2010-54-MAPTRI-H) was referenced at MAPTRI, Khartoum, Sudan. The freshly harvested leaves were then air-dried. A total of 100 g of the plant sample was coarsely powdered using a mortar and pestle, and a sample was extracted with 80% ethanol by soaking extraction, according to the method described by Sukhdev et al. [10]. The extract was air-dried in an evaporating dish until completely dry. The yield percentage was calculated, and the dried extract was weighed and stored in sealed plastic containers at 4 °C for subsequent experiments.
Chemicals and drugs
Carbopol 941 and triethanolamine were obtained from Spectrum Chemicals, Ltd, China. Povidone iodine 10% ointment was obtained from the Sudan market, Mathely Trading & Drugs.
Experimental animals
Healthy Wister albino rats of both sexes weighed between 100 and 120 g, were obtained from the animal house of MAPTRI, NCR. Each rat was housed alone in polypropylene (485×350×200 mm) cages at 25 °C and subjected to a 12:12h light–dark cycle, with free access to food and water ad libitum. The animals were allowed to acclimatize for one week before use.
Methods
Formulation of semi-solid preparations
As shown in Table 1, C.
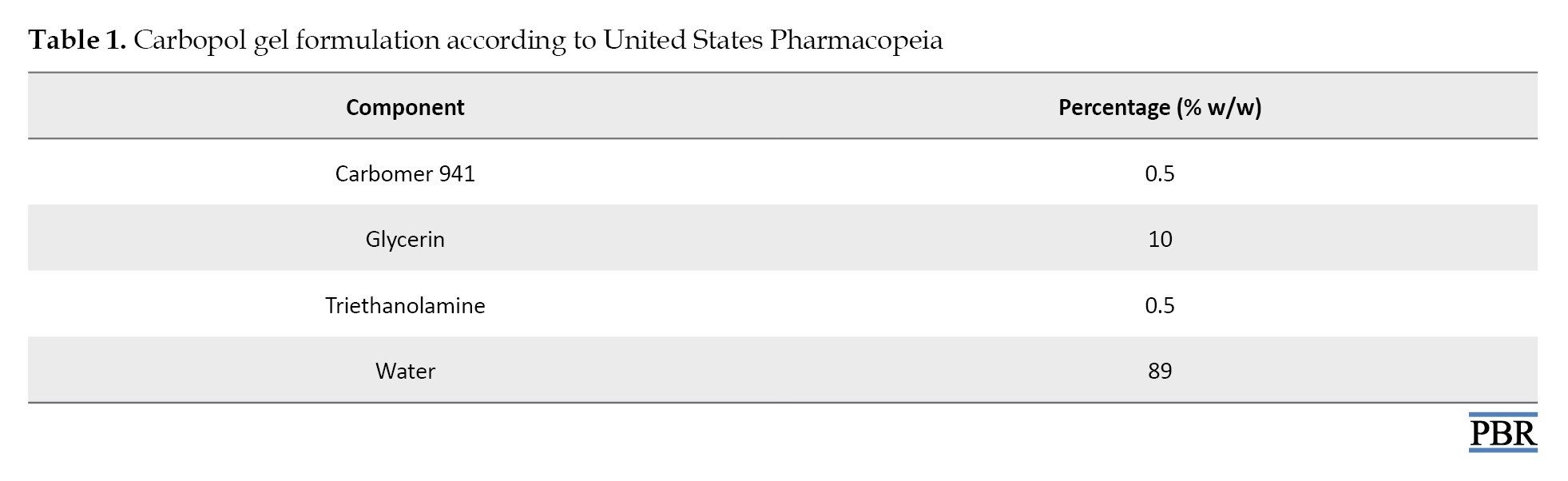
papyrus extract was dissolved in water, then mixed with glycerin, and the carbomer was added by sprinkling on the surface while constantly mixing at high speed. Triethanolamine was added with slow agitation until a clear viscous gel was formed.
Two concentrations were formulated from C. papyrus extract: 5% and 10%, whereas the gel base was prepared without the addition of the extract. The concentrations of 5% and 10% C. papyrus ethanolic extract were selected based on preliminary phytochemical screening, literature on topical herbal formulations, and pilot toxicity tests [11].
Wound-healing activity
The excision wound model was used to evaluate the wound-healing activity of ethanolic extract of C. papyrus [12]. The dorsal fur of the animals was shaved with an electric clipper. The anticipated area of the wound to be created was outlined on the back of the animals. A full-thickness excision wound with a circular area and a depth of 0.2 cm was created under sterile conditions. The entire wound was left open. The animals were closely observed for any signs of infection, and those that showed signs of infection were excluded from the study and replaced. The day of surgery was considered day zero. Wounded animals were randomly divided into five groups, with six animals in each group. Group I (control) did not receive any treatment; Group II received a simple Carbopol gel base; group III received povidone-iodine ointment 10% (no silver sulfadiazine or other advanced comparators were used due to availability at that time); group IV received 5% CP extract; and group V received 10% CP extract. Treatment was applied topically to all groups twice daily (every 12 hours), and the wound areas were measured each morning until complete closure was achieved.
The percentage of wound contraction was calculated, and the onset of healing was determined. Skin tissue was then collected from the wound area and placed in 10% formalin for histopathological examination.
Histopathology examination
The fixed tissues were dehydrated with 100% ethanol solution and embedded in paraffin. They were then processed into 6-8 microns using a microtone and then stained with hematoxylin-eosin. The samples were observed under a light microscope (40X) by a histopathologist, as previously described by [13]. The presence of the epithelial layer, fibrous tissues, inflammatory cells, and granulation tissues was assessed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 5. Data were expressed as mean and contraction percentages. The two-way ANOVA (followed by Bonferroni’s post-hoc test) was conducted to determine significant differences and P<0.05 were considered significant.
Results
Phytochemical screening
As shown in Table 2, the ethanolic extract of C.
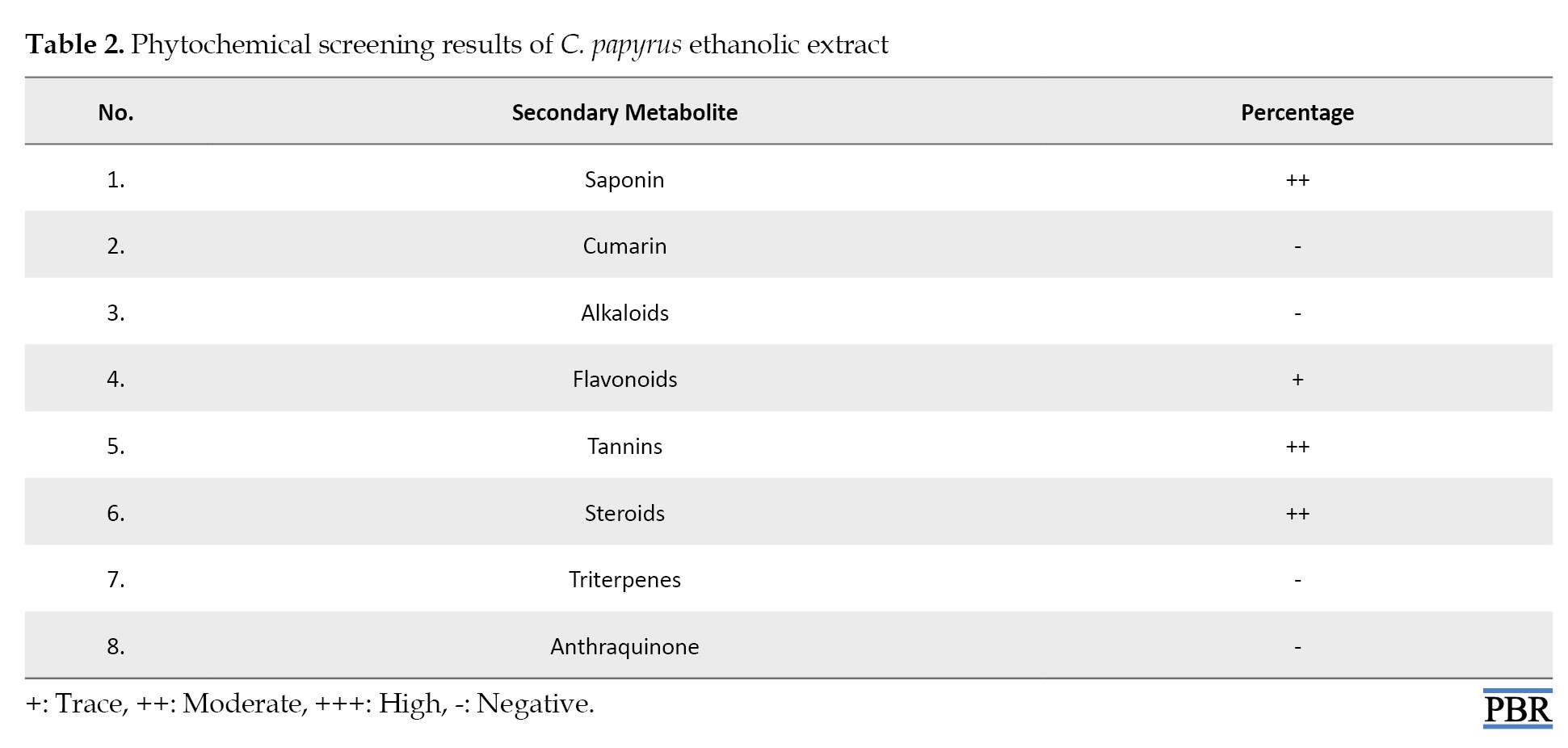
papyrus contains various secondary metabolites, including saponins, flavonoids, tannins, steroids, and other compounds, with different percentages.
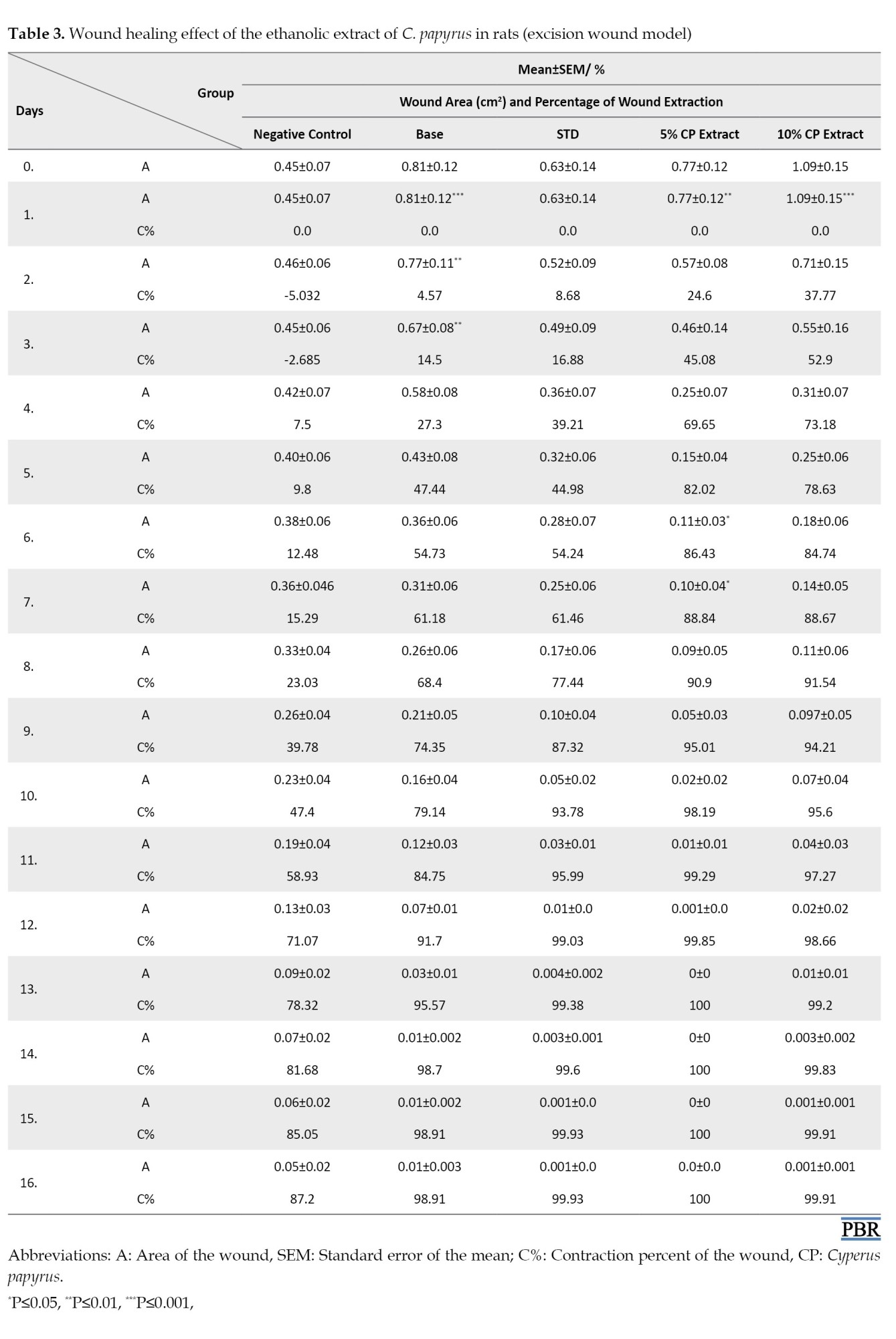
Wound area and contraction percentage
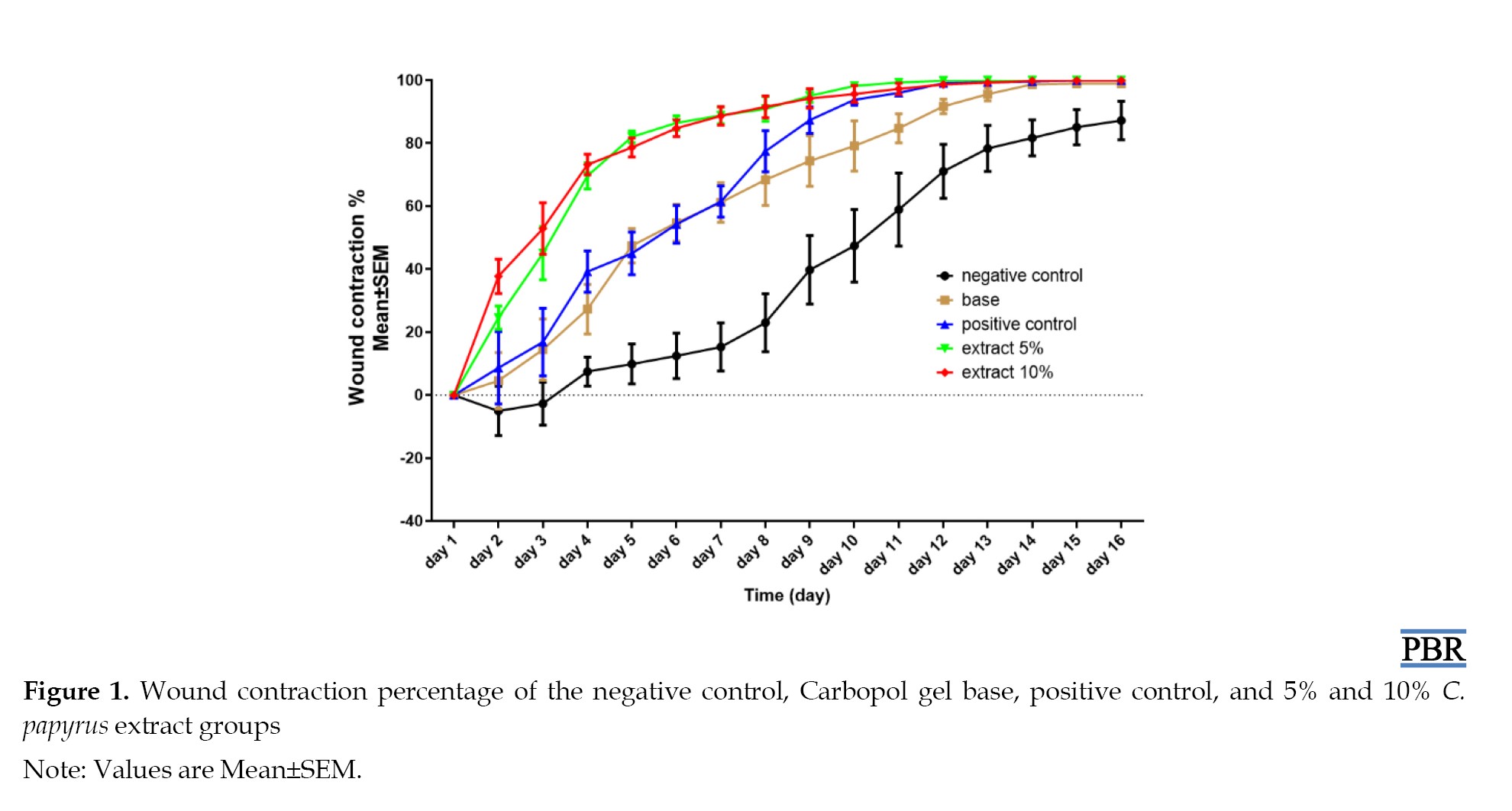
Mean contraction percentage and wound area increased more rapidly in animals that received C. papyrus extract than in animals that received the standard drug, the base group, or the control group as demonstrated in Table 3 and Figure 1.
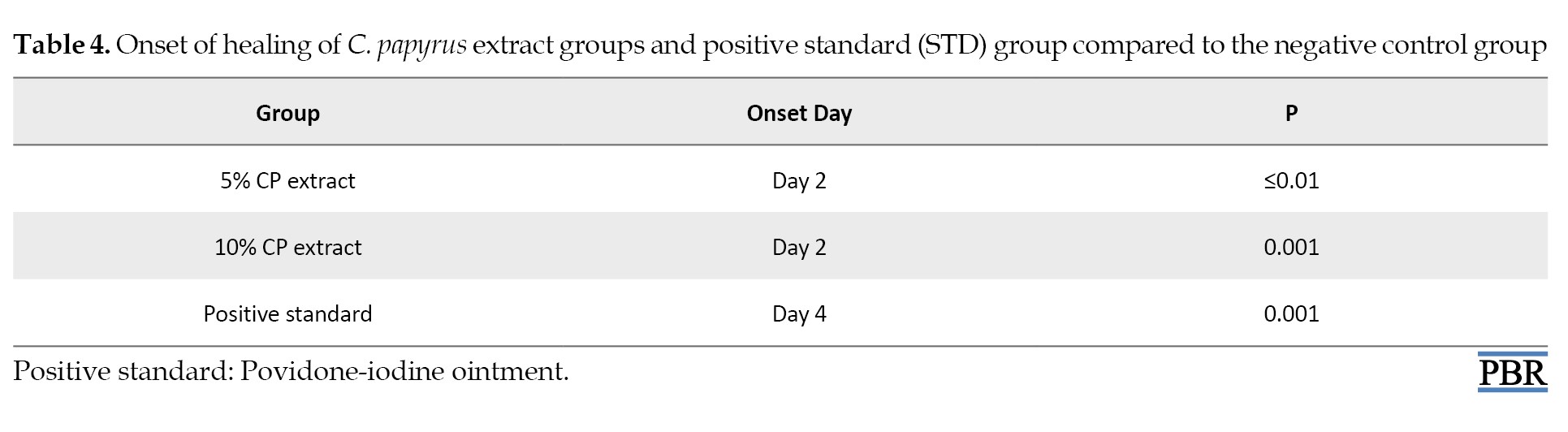
Onset of healing
Only two days were required to induce healing in animals that received C. papyrus extract formula, and four days for the standard drug when compared to the negative control group. Whereas the Carbopol gel base does not differ significantly in inducing healing compared to the negative control group as demonstrated in Table 4.
Histopathology findings
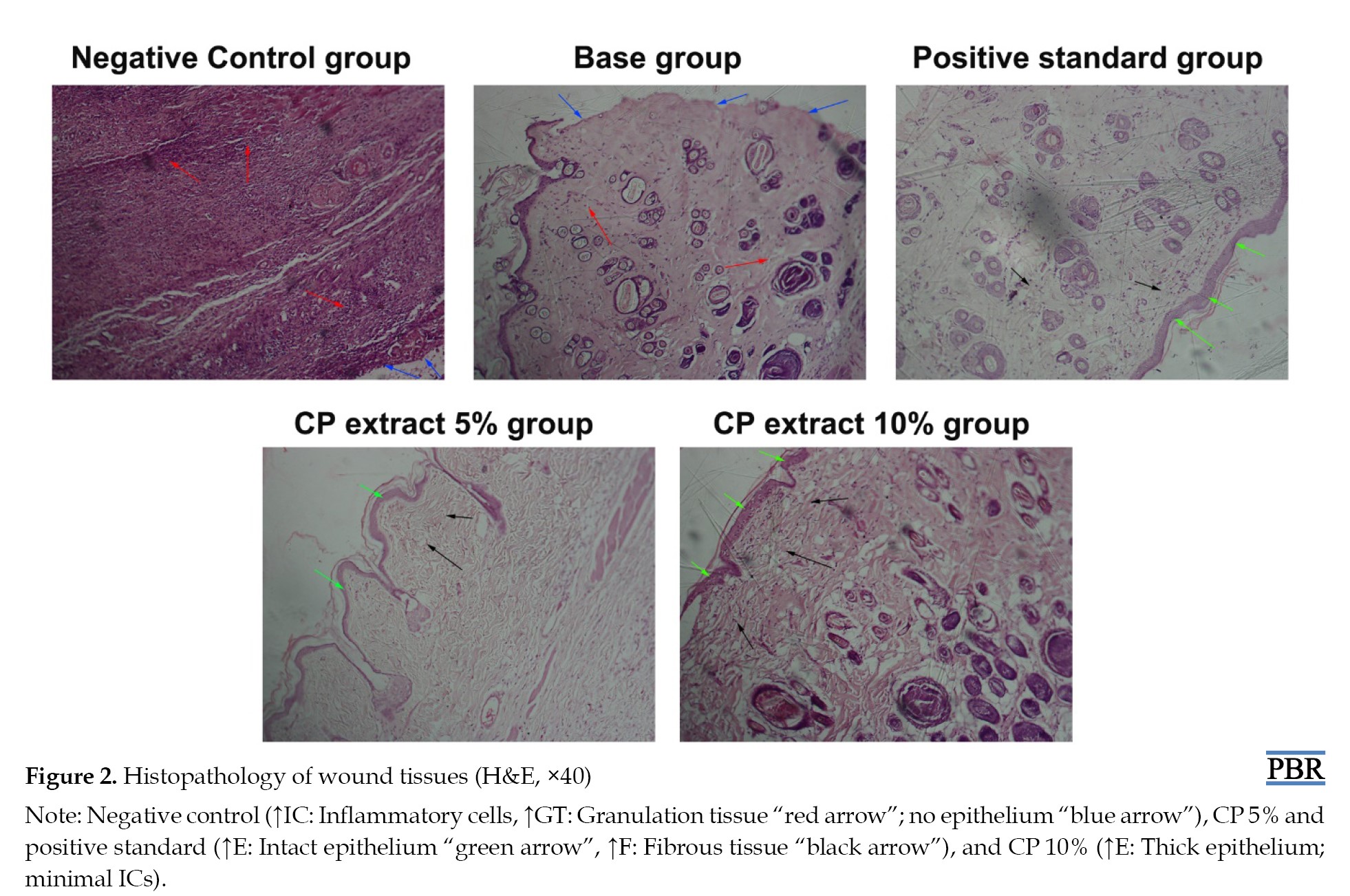
As shown in Figure 2, the histopathology changes in the wound area further demonstrated the wound-healing effect of the C. papyrus extract. The control group showed the presence of inflammatory cells (ICs) and granulation tissues, with an absence of the epithelial layer (EC) when compared to the CP extract-treated groups, which showed an absence of ICs and the presence of an intact epithelial layer and fibrous tissues.
Discussion
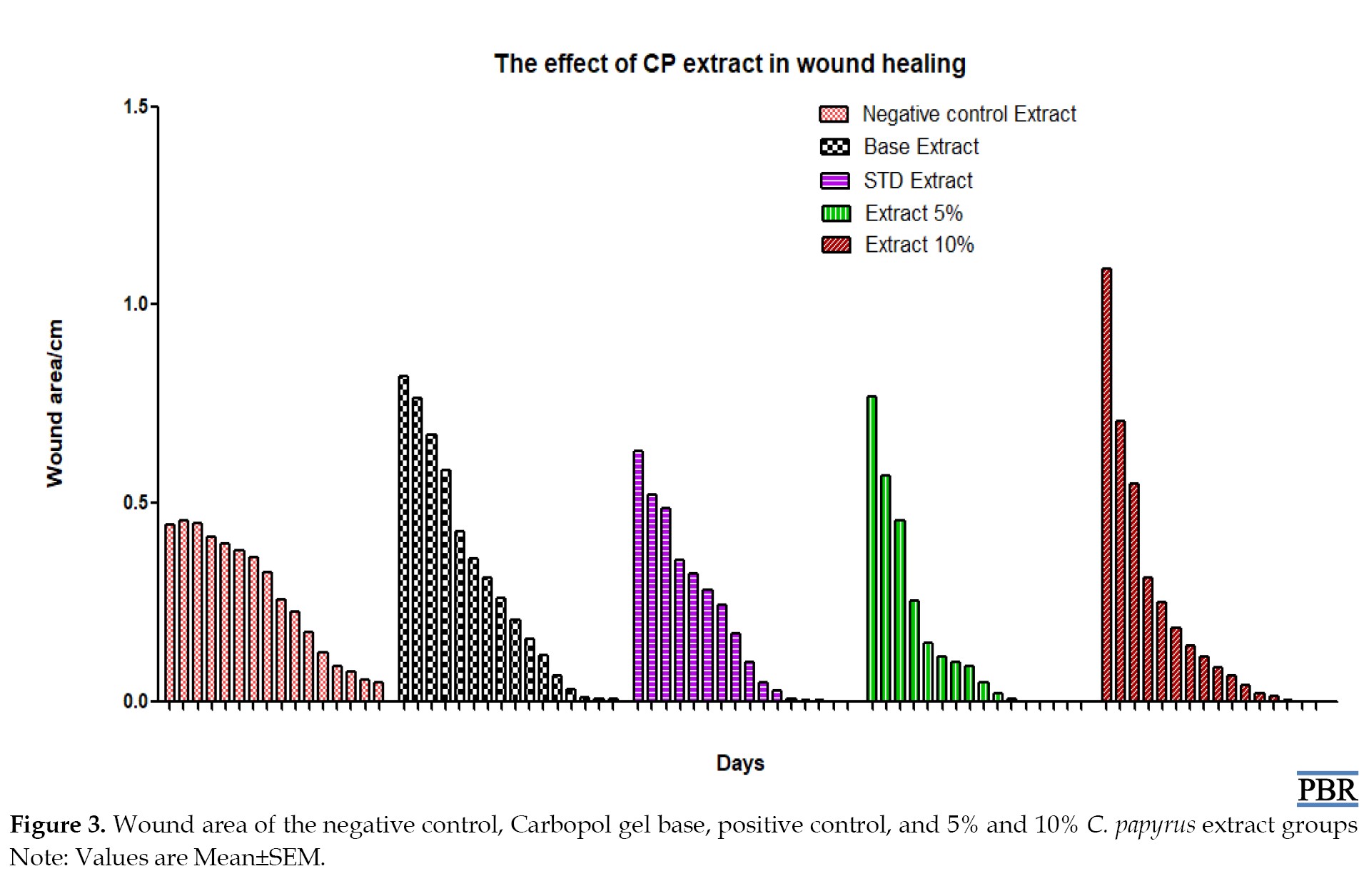
Wound contraction has a very crucial role in the closure of full-thickness skin wounds [14]. In the present study, the wound-healing potential activity of a gel containing C. papyrus ethanolic extract was investigated. The obtained results revealed a reduction in wound size in all rats; however, the animals treated with 5% and 10% C. papyrus ethanolic extract exhibited a significant (P≤0.001) reduction in wound area and consequently increased the contraction percentage more rapidly during the epithelization period compared to the control group. Results were expressed as Mean±SEM, with significant differences from the control group indicated. The onset of healing was found to be more rapid in the C. papyrus group compared to the other groups (P<0.01). Collagen, a main component of the extracellular matrix, significantly contributes to the wound strength. The wound-healing properties of C. papyrus extract are mainly due to its ability to enhance the formation of the epithelial layer (3 cross) and fibrous tissues (scant), as shown in Figure 3. In contrast, the negative control and base groups exhibited no healing due to the absence of epithelial and fibrous tissues, as well as the presence of inflammatory cells and granulation tissues at the end of the experiment (day 16), as demonstrated in Figure 3. The positive control (standard drug) showed partial healing, attributed to incomplete cross-linking of the epithelial layer and moderate fibrous tissue formation, as shown in Figure 3.
Although povidone-iodine was selected as the positive control in this study due to its well-established antiseptic and wound-healing properties, it is acknowledged that more advanced wound care agents, such as silver sulfadiazine, are commonly used in modern clinical practice for wound healing [15]. The absence of such a comparator limits the direct applicability of our findings to current clinical standards. Future investigations should include silver-based or other advanced wound-healing agents to comprehensively evaluate the relative efficacy of C. papyrus extract within a broader therapeutic context.
Flavonoids have important anti-inflammatory properties, as they reduce the levels of many inflammatory mediators, including PGE2, LTB-4, IL-1β, TNF-α, IL-6, IFN-γ, and COX while increasing anti-inflammatory mediators, particularly IL-10 [16]. They also produce an anti-oxidant effect and play a potential role in reepithelization in the wound area. Since the C. papyrus ethanolic extract contains flavonoids, it may contribute to its wound-healing properties.
The use of topical steroids, in conjunction with antibiotics and antifungals, can improve wound healing rates due to their anti-inflammatory action and ability to relieve pain associated with chronic wounds [17]. This can also be considered a major mechanism of wound healing properties observed in the C. papyrus ethanolic extract.
Medicinal plants that contain tannin (such as C. papyrus) indicate significant wound healing properties due to its function in the promotion of fibroblast proliferation and migration into wounds, as well as antibacterial activities [18].
Saponins have multiple mechanisms in wound healing. They effectively suppress inflammatory reactions during the early phase, promote re-epithelialization of the wound, and promote matrix synthesis throughout the wound healing process [19]. This is another additional wound-healing mechanism of the ethanolic extract of C. papyrus.
The wound healing effects observed in this study are due to the anti-inflammatory, antioxidant, and fibroblast-stimulating properties of phytoconstituents, such as flavonoids, tannins, and saponins present in the C. papyrus extract. However, it should be noted that these mechanistic insights are extrapolated from established literature, and our study did not include direct biochemical assays (e.g. cytokine profiling, oxidative stress markers) or molecular investigations (e.g. gene expression studies) to confirm these pathways. Future research should incorporate such analyses to elucidate the precise molecular and cellular mechanisms underpinning the wound-healing activity of this plant extract.
Conclusion
The C. papyrus ethanolic extract has wound healing activity, as the onset of healing was faster than in all other groups, and the closure percentage was higher in the C. papyrus extract group compared to the positive control, base, and negative control groups. The histopathology examinations revealed that in the C. papyrus ethanolic extract and positive control groups, a sufficient amount of epithelial layer and fibrous tissues were formed without the presence of inflammatory cells or granulation tissues. These results suggest that sericin has wound-healing effects without causing allergic reactions.
Limitations
Lack of modern comparators: Only povidone-iodine was used as a positive control, and the inclusion of advanced treatments (e.g. silver sulfadiazine) would strengthen clinical relevance. Mechanistic gaps: while phytochemicals (flavonoids and tannins) were identified, biochemical/molecular data (e.g. cytokine levels, antioxidant assays) are lacking to confirm their role. Animal model constraints: Results from healthy Wistar rats may not fully translate to chronic or diabetic wounds in humans. Short-term evaluation: The long-term effects of CP extract (e.g. scar quality, recurrence) were not assessed. Dose optimization: Only two concentrations (5% and 10%) were tested; a broader dose range might reveal optimal efficacy.
Sample size: Small group sizes (n=6) may limit statistical power for detecting subtle effects. It should be noted that the assumptions of normality and homogeneity of variances, which are prerequisites for ANOVA, were not formally tested in this study. Future analyses should incorporate these checks to strengthen the robustness of statistical inferences.
Ethical Considerations
Compliance with ethical guidelines
All experiments involving animals were performed with the permission and under the strict guidance of the Institutional Animal Ethical Committee for research on small animals at the Faculty of Pharmacy, Omdurman Islamic University, Omdurman, Sudan (Permission letter number: OIU/IAEC/Exp.Ph.2019/6). Although no anesthesia / analgesia was used due to the superficial nature of the wound model, every effort was made to reduce distress and ensure animal welfare.
Funding
Financial support was provided by the Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, Khartoum, Sudan.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to MAPTRI (Sudan) for their financial support and extend special thanks to Abdoalwahab Hassan Ahmed and Fakhreldien Yahia Osman for their continuous support, as well as to Leena Abdulaziz for her assistance with statistical analysis and encouragement.
References
Wounds are physical injuries that result in an opening or break of the skin, causing a disturbance in normal skin anatomy and physiology, and resulting in the loss of continuity of the epithelium, with or without loss of underlying connective tissues, according to the Wound Healing Society (WHS) [1]. Wounds affect a large number of people and seriously reduce the quality of life [2]. Generally, there are three phases of wound healing: Inflammatory, proliferative, and remodeling [1]. Several agents have been used in the management of wounds and a wide variety of treatment modalities are available for wound repair. Among these medicines, those of herbal origin have a significant impact on the treatment and healing of wounds [3]. Medicinal plants offer significant benefits for wound treatment, not only because they are inexpensive and affordable but also because they are generally safe and do not typically cause hypersensitivity reactions [4].
The increasing demand and availability of medicinal products have created a need to isolate and identify the principles responsible for their therapeutic activities and effectiveness. For instance, the stimulation of fibroblasts by plant extracts has been observed as one of the mechanisms, by which medicinal plants enhance the wound healing process [5].
Cyperus papyrus belongs to the family Cyperaceae, which comprises monocotyledonous graminoid flowering plants known as sedges, that superficially resemble grasses or rushes [6]. It contains small amounts of sesquiterpenes relative to monoterpenes obtained by gas chromatography; it also contains phenolic compounds, as well as Na, K, Mg, Fe, I, and proteins, which are considered micronutrients [7]. C. papyrus has traditionally been used in the treatment of painful spasms, eye diseases, ulcers, fever, diarrhea, and various inflammatory conditions.
Any pathophysiologic disturbances in the healing process result in delayed or halted healing and present problems that result in frustrating and expensive care, ultimately failing to meet patient and provider goals [8]. Wounds (both normal and diabetic wounds) can become significant issues and may respond poorly to medications [9].
This study was conducted to evaluate the wound-healing activity of C. papyrus ethanolic extract in experimentally induced excision wounds in rats.
Materials and Methods
Materials
Plant material
Fresh aerial parts were harvested from the Botanical Garden at the Medicinal and Aromatic Plants and Traditional Medicine Research Institute (MAPTRI), Khartoum, Sudan, in August 2019. Plant identification (specimen No. Y-2010-54-MAPTRI-H) was referenced at MAPTRI, Khartoum, Sudan. The freshly harvested leaves were then air-dried. A total of 100 g of the plant sample was coarsely powdered using a mortar and pestle, and a sample was extracted with 80% ethanol by soaking extraction, according to the method described by Sukhdev et al. [10]. The extract was air-dried in an evaporating dish until completely dry. The yield percentage was calculated, and the dried extract was weighed and stored in sealed plastic containers at 4 °C for subsequent experiments.
Chemicals and drugs
Carbopol 941 and triethanolamine were obtained from Spectrum Chemicals, Ltd, China. Povidone iodine 10% ointment was obtained from the Sudan market, Mathely Trading & Drugs.
Experimental animals
Healthy Wister albino rats of both sexes weighed between 100 and 120 g, were obtained from the animal house of MAPTRI, NCR. Each rat was housed alone in polypropylene (485×350×200 mm) cages at 25 °C and subjected to a 12:12h light–dark cycle, with free access to food and water ad libitum. The animals were allowed to acclimatize for one week before use.
Methods
Formulation of semi-solid preparations
As shown in Table 1, C.

papyrus extract was dissolved in water, then mixed with glycerin, and the carbomer was added by sprinkling on the surface while constantly mixing at high speed. Triethanolamine was added with slow agitation until a clear viscous gel was formed.
Two concentrations were formulated from C. papyrus extract: 5% and 10%, whereas the gel base was prepared without the addition of the extract. The concentrations of 5% and 10% C. papyrus ethanolic extract were selected based on preliminary phytochemical screening, literature on topical herbal formulations, and pilot toxicity tests [11].
Wound-healing activity
The excision wound model was used to evaluate the wound-healing activity of ethanolic extract of C. papyrus [12]. The dorsal fur of the animals was shaved with an electric clipper. The anticipated area of the wound to be created was outlined on the back of the animals. A full-thickness excision wound with a circular area and a depth of 0.2 cm was created under sterile conditions. The entire wound was left open. The animals were closely observed for any signs of infection, and those that showed signs of infection were excluded from the study and replaced. The day of surgery was considered day zero. Wounded animals were randomly divided into five groups, with six animals in each group. Group I (control) did not receive any treatment; Group II received a simple Carbopol gel base; group III received povidone-iodine ointment 10% (no silver sulfadiazine or other advanced comparators were used due to availability at that time); group IV received 5% CP extract; and group V received 10% CP extract. Treatment was applied topically to all groups twice daily (every 12 hours), and the wound areas were measured each morning until complete closure was achieved.
The percentage of wound contraction was calculated, and the onset of healing was determined. Skin tissue was then collected from the wound area and placed in 10% formalin for histopathological examination.
Histopathology examination
The fixed tissues were dehydrated with 100% ethanol solution and embedded in paraffin. They were then processed into 6-8 microns using a microtone and then stained with hematoxylin-eosin. The samples were observed under a light microscope (40X) by a histopathologist, as previously described by [13]. The presence of the epithelial layer, fibrous tissues, inflammatory cells, and granulation tissues was assessed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 5. Data were expressed as mean and contraction percentages. The two-way ANOVA (followed by Bonferroni’s post-hoc test) was conducted to determine significant differences and P<0.05 were considered significant.
Results
Phytochemical screening
As shown in Table 2, the ethanolic extract of C.

papyrus contains various secondary metabolites, including saponins, flavonoids, tannins, steroids, and other compounds, with different percentages.
Wound area and contraction percentage
Mean contraction percentage and wound area increased more rapidly in animals that received C. papyrus extract than in animals that received the standard drug, the base group, or the control group as demonstrated in Table 3 and Figure 1.

Onset of healing
Only two days were required to induce healing in animals that received C. papyrus extract formula, and four days for the standard drug when compared to the negative control group. Whereas the Carbopol gel base does not differ significantly in inducing healing compared to the negative control group as demonstrated in Table 4.

Histopathology findings
As shown in Figure 2, the histopathology changes in the wound area further demonstrated the wound-healing effect of the C. papyrus extract. The control group showed the presence of inflammatory cells (ICs) and granulation tissues, with an absence of the epithelial layer (EC) when compared to the CP extract-treated groups, which showed an absence of ICs and the presence of an intact epithelial layer and fibrous tissues.
Discussion
Wound contraction has a very crucial role in the closure of full-thickness skin wounds [14]. In the present study, the wound-healing potential activity of a gel containing C. papyrus ethanolic extract was investigated. The obtained results revealed a reduction in wound size in all rats; however, the animals treated with 5% and 10% C. papyrus ethanolic extract exhibited a significant (P≤0.001) reduction in wound area and consequently increased the contraction percentage more rapidly during the epithelization period compared to the control group. Results were expressed as Mean±SEM, with significant differences from the control group indicated. The onset of healing was found to be more rapid in the C. papyrus group compared to the other groups (P<0.01). Collagen, a main component of the extracellular matrix, significantly contributes to the wound strength. The wound-healing properties of C. papyrus extract are mainly due to its ability to enhance the formation of the epithelial layer (3 cross) and fibrous tissues (scant), as shown in Figure 3. In contrast, the negative control and base groups exhibited no healing due to the absence of epithelial and fibrous tissues, as well as the presence of inflammatory cells and granulation tissues at the end of the experiment (day 16), as demonstrated in Figure 3. The positive control (standard drug) showed partial healing, attributed to incomplete cross-linking of the epithelial layer and moderate fibrous tissue formation, as shown in Figure 3.
Although povidone-iodine was selected as the positive control in this study due to its well-established antiseptic and wound-healing properties, it is acknowledged that more advanced wound care agents, such as silver sulfadiazine, are commonly used in modern clinical practice for wound healing [15]. The absence of such a comparator limits the direct applicability of our findings to current clinical standards. Future investigations should include silver-based or other advanced wound-healing agents to comprehensively evaluate the relative efficacy of C. papyrus extract within a broader therapeutic context.
Flavonoids have important anti-inflammatory properties, as they reduce the levels of many inflammatory mediators, including PGE2, LTB-4, IL-1β, TNF-α, IL-6, IFN-γ, and COX while increasing anti-inflammatory mediators, particularly IL-10 [16]. They also produce an anti-oxidant effect and play a potential role in reepithelization in the wound area. Since the C. papyrus ethanolic extract contains flavonoids, it may contribute to its wound-healing properties.
The use of topical steroids, in conjunction with antibiotics and antifungals, can improve wound healing rates due to their anti-inflammatory action and ability to relieve pain associated with chronic wounds [17]. This can also be considered a major mechanism of wound healing properties observed in the C. papyrus ethanolic extract.
Medicinal plants that contain tannin (such as C. papyrus) indicate significant wound healing properties due to its function in the promotion of fibroblast proliferation and migration into wounds, as well as antibacterial activities [18].
Saponins have multiple mechanisms in wound healing. They effectively suppress inflammatory reactions during the early phase, promote re-epithelialization of the wound, and promote matrix synthesis throughout the wound healing process [19]. This is another additional wound-healing mechanism of the ethanolic extract of C. papyrus.
The wound healing effects observed in this study are due to the anti-inflammatory, antioxidant, and fibroblast-stimulating properties of phytoconstituents, such as flavonoids, tannins, and saponins present in the C. papyrus extract. However, it should be noted that these mechanistic insights are extrapolated from established literature, and our study did not include direct biochemical assays (e.g. cytokine profiling, oxidative stress markers) or molecular investigations (e.g. gene expression studies) to confirm these pathways. Future research should incorporate such analyses to elucidate the precise molecular and cellular mechanisms underpinning the wound-healing activity of this plant extract.
Conclusion
The C. papyrus ethanolic extract has wound healing activity, as the onset of healing was faster than in all other groups, and the closure percentage was higher in the C. papyrus extract group compared to the positive control, base, and negative control groups. The histopathology examinations revealed that in the C. papyrus ethanolic extract and positive control groups, a sufficient amount of epithelial layer and fibrous tissues were formed without the presence of inflammatory cells or granulation tissues. These results suggest that sericin has wound-healing effects without causing allergic reactions.
Limitations
Lack of modern comparators: Only povidone-iodine was used as a positive control, and the inclusion of advanced treatments (e.g. silver sulfadiazine) would strengthen clinical relevance. Mechanistic gaps: while phytochemicals (flavonoids and tannins) were identified, biochemical/molecular data (e.g. cytokine levels, antioxidant assays) are lacking to confirm their role. Animal model constraints: Results from healthy Wistar rats may not fully translate to chronic or diabetic wounds in humans. Short-term evaluation: The long-term effects of CP extract (e.g. scar quality, recurrence) were not assessed. Dose optimization: Only two concentrations (5% and 10%) were tested; a broader dose range might reveal optimal efficacy.
Sample size: Small group sizes (n=6) may limit statistical power for detecting subtle effects. It should be noted that the assumptions of normality and homogeneity of variances, which are prerequisites for ANOVA, were not formally tested in this study. Future analyses should incorporate these checks to strengthen the robustness of statistical inferences.
Ethical Considerations
Compliance with ethical guidelines
All experiments involving animals were performed with the permission and under the strict guidance of the Institutional Animal Ethical Committee for research on small animals at the Faculty of Pharmacy, Omdurman Islamic University, Omdurman, Sudan (Permission letter number: OIU/IAEC/Exp.Ph.2019/6). Although no anesthesia / analgesia was used due to the superficial nature of the wound model, every effort was made to reduce distress and ensure animal welfare.
Funding
Financial support was provided by the Medicinal and Aromatic Plants and Traditional Medicine Research Institute, National Centre for Research, Khartoum, Sudan.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to MAPTRI (Sudan) for their financial support and extend special thanks to Abdoalwahab Hassan Ahmed and Fakhreldien Yahia Osman for their continuous support, as well as to Leena Abdulaziz for her assistance with statistical analysis and encouragement.
References
- Nagori BP, Solanki R. Role of medicinal plants in wound healing. Res J Med Plant. 2011; 5(4):392-405. [DOI:10.3923/rjmp.2011.392.405]
- Ghildiyal S, Gautam MK, Joshi VK, Goel RK. Wound healing and antimicrobial activity of two classical formulations of Laghupanchamula in rats. J Ayurveda Integr Med. 2015; 6(4):241-7. [DOI:10.4103/0975-9476.157952] [PMID]
- Sathyanarayanan S, Muniyandi K, George E, Sivaraj D, Sasidharan SP, Thangaraj P. Chemical profiling of Pterolobium hexapetalum leaves by HPLC analysis and its productive wound healing activities in rats. Biomed Pharmacother. 2017; 95:287-97. [DOI:10.1016/j.biopha.2017.08.062] [PMID]
- Raina R, Parwez S, Verma PK, Pankaj NK. Medicinal plants and their role in wound healing. Online Veterinary J. 2008; 3(1):21. [Link]
- Oguntibeju OO. Medicinal plants and their effects on diabetic wound healing. Vet World. 2019; 12(5):653-63. [DOI:10.14202/vetworld.2019.653-663] [PMID]
- Kakarla L, Mathi P, Allu PR, Rama C, Botlagunta M. Identification of human cyclooxegenase-2 inhibitors from Cyperus scariosus (R.Br) rhizomes. Bioinformation. 2014; 10(10):637-46. [DOI:10.6026/97320630010637] [PMID]
- Ahmat NB, Zain WZ, Abdullah NA, Ramli NW, Hamid NA. Insecticidal, antimicrobial, antioxidant and phytochemistry of Cyperus species–a review. Int J Agr For Plant. 2021; 11. [Link]
- Swoboda L, Held J. Impaired wound healing in diabetes. J Wound Care. 2022; 31(10):882-5. [DOI:10.12968/jowc.2022.31.10.882] [PMID]
- Salazar JJ, Ennis WJ, Koh TJ. Diabetes medications: Impact on inflammation and wound healing. J Diabetes Complications. 2016; 30(4):746-52. [DOI:10.1016/j.jdiacomp.2015.12.017] [PMID]
- Sukhdev SH. An overview of extraction techniques for medicinal and aromatic plants. In: Sukhdev SH, Suman PSK, Gennaro L, Dev DR, editors. Extraction technologies for medicinal and aromatic plants. Vienna: United Nations Industrial Development Organization and the International centre for Science and High Technology; 2008.
- Toppo FA, Pawar RS. Development, optimization and evaluation of different herbal formulations for wound healing. Int J Pharm Pharm Sci. 2015; 7:447-52. [Link]
- Gul Satar NY, Cangul IT, Topal A, Kurt H, Ipek V, Onel GI. The effects of Tarantula cubensis venom on open wound healing in rats. J Wound Care. 2017; 26(2):66-71. [DOI:10.12968/jowc.2017.26.2.66] [PMID]
- Khalifa MA, Mohammad AE, Ahmed BM. Acute and subacute toxicity studies of cyperus papyrus ash on wistar albino rats. Pharm Biomed Res. 2022; 8(4):279-90. [DOI:10.32598/PBR.8.4.1021.1]
- Mori HM, Kawanami H, Kawahata H, Aoki M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016; 16:144. [DOI:10.1186/s12906-016-1128-7] [PMID]
- Abul Barkat H, Abul Barkat M, Ali R, Hadi H, Kasmuri AR. Old Wine in new Bottles: Silver Sulfadiazine Nanotherapeutics for Burn Wound Management. Int J Low Extrem Wounds. 2023; 2023:15347346231166980. [DOI:10.1177/15347346231166980] [PMID]
- Zulkefli N, Che Zahari CNM, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS, et al. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int J Mol Sci. 2023; 24(5):4607. [DOI:10.3390/ijms24054607] [PMID]
- Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016; 82(4):371-8. [DOI:10.4103/0378-6323.178903] [PMID]
- Su X, Liu X, Wang S, Li B, Pan T, Liu D, et al. Wound-healing promoting effect of total tannins from Entada phaseoloides (L.) Merr. in rats. Burns. 2017; 43(4):830-8. [DOI:10.1016/j.burns.2016.10.010] [PMID]
- Men SY, Huo QL, Shi L, Yan Y, Yang CC, Yu W, et al. Panax notoginseng saponins promotes cutaneous wound healing and suppresses scar formation in mice. J Cosmet Dermatol. 2020; 19(2):529-34. [DOI:10.1111/jocd.13042] [PMID]
Type of Study: Original Research |
Subject:
Pharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |