Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 269-274 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadi M, Hosseini S H. The Acute Onset of Edema Caused by Risperidone in a Schizophrenic Patient: A Case Report. Pharm Biomed Res 2024; 10 (3) :269-274
URL: http://pbr.mazums.ac.ir/article-1-635-en.html
URL: http://pbr.mazums.ac.ir/article-1-635-en.html
1- Psychiatry and Behavioral Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 616 kb]
(1272 Downloads)
| Abstract (HTML) (1908 Views)
Full-Text: (1709 Views)
Introduction
Asecond-generation atypical antipsychotic called risperidone is frequently used to treat psychiatric disorders like schizophrenia and bipolar disorder [1-4]. A benzoxazole derivative called risperidone was first used in 1993 [4, 5]. It has a high affinity for serotonin type-2 (5-hydroxytryptamine, 5-HT2), dopamine (D2), and 1-adrenergic receptors, resulting in antagonism at these receptors [1, 6, 7].
Risperidone has a few side effects, including extrapyramidal symptoms, dizziness, sedation, insomnia, headache, anxiety, nausea, constipation, and weight gain. However, it is extremely effective in treating childhood and adolescent behavioral disorders, including hyperactivity, aggression, self-injurious states, irritability, and stereotypes [2, 5, 7].
Drug-induced peripheral edema is prevalent, particularly with beta-blockers, calcium channel blockers, nonsteroidal anti-inflammatory medications, and several hormonal medicines. Still, it is uncommon with new-generation antipsychotics [1, 8].
However, in other case reports, edema was noted as an uncommon but severe adverse effect of risperidone that happened in a dose-dependent way [9]. Risperidone seldom causes leg edema, particularly in low doses used for schizophrenia maintenance therapy [2, 9].
An innovative antipsychotic drug called aripiprazole is thought to stabilize the dopamine-serotonin system [10]. It is a partial agonist at serotonin (5-HT) 1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist at dopamine D2 receptors [11]. The fact that aripiprazole does not affect body weight, triglyceride levels, prolactin levels, or sedation [12] is also notable. It has typically been used to treat schizophrenia, bipolar disorder, major depressive disorder, and anxiety disorders [2, 5].
In this paper, a sub-acute onset of bilateral leg edema was reported after initiation of low-dose risperidone treatment in a patient. To our knowledge, this report was the only case study testifying such a high incidence of edema after administrating a low dosage of risperidone (1 mg) in the literature. A search was performed through relevance on PubMed, EMBASE, and Google Scholar, with keywords: “Edema,” “peripheral edema,” and “aripiprazole.” There are few case reports on aripiprazole resulting in edema in patients. In most reports, risperidone successfully was switched to aripiprazole, and edema was suppressed completely [1, 2, 13, 14]. Thus, the dose-dependent manner of our patient to aripiprazole could be considered a rare reaction. The patient’s response to aripiprazole, which appears to be dose-dependent, is an interesting and unusual observation worth further consideration.
Case Presentation
A 55-year-old single unemployed woman presented with auditory hallucination symptoms and delusion of persecution with an unremarkable medical and psychiatric history. Her symptoms had begun about two years before her visit. She was diagnosed as a case of schizophrenia. The patient started on risperidone 1 mg/d, and then the dose was increased to 2 mg/d. A few days after increasing risperidone dosage, she developed marked bilateral swelling over her hands, legs, and face, accompanied by difficulty in breathing. She did not have a history of edema, hepatic or renal dysfunction, thyroid disorder, cardiac dysfunction, or peripheral vascular disease. Symptoms of swelling were not accompanied by itching, pain, fever, lymphadenopathy, chest pain, and abdominal distension. She was not taking any other medication, so that a drug reaction could be ruled out. Complete laboratory examinations were done, including complete blood count, serum electrolytes, liver function test, renal function test, thyroid function test, rheumatological test, immunological examination, and urine analysis. All reports were within normal limits (Table 1).

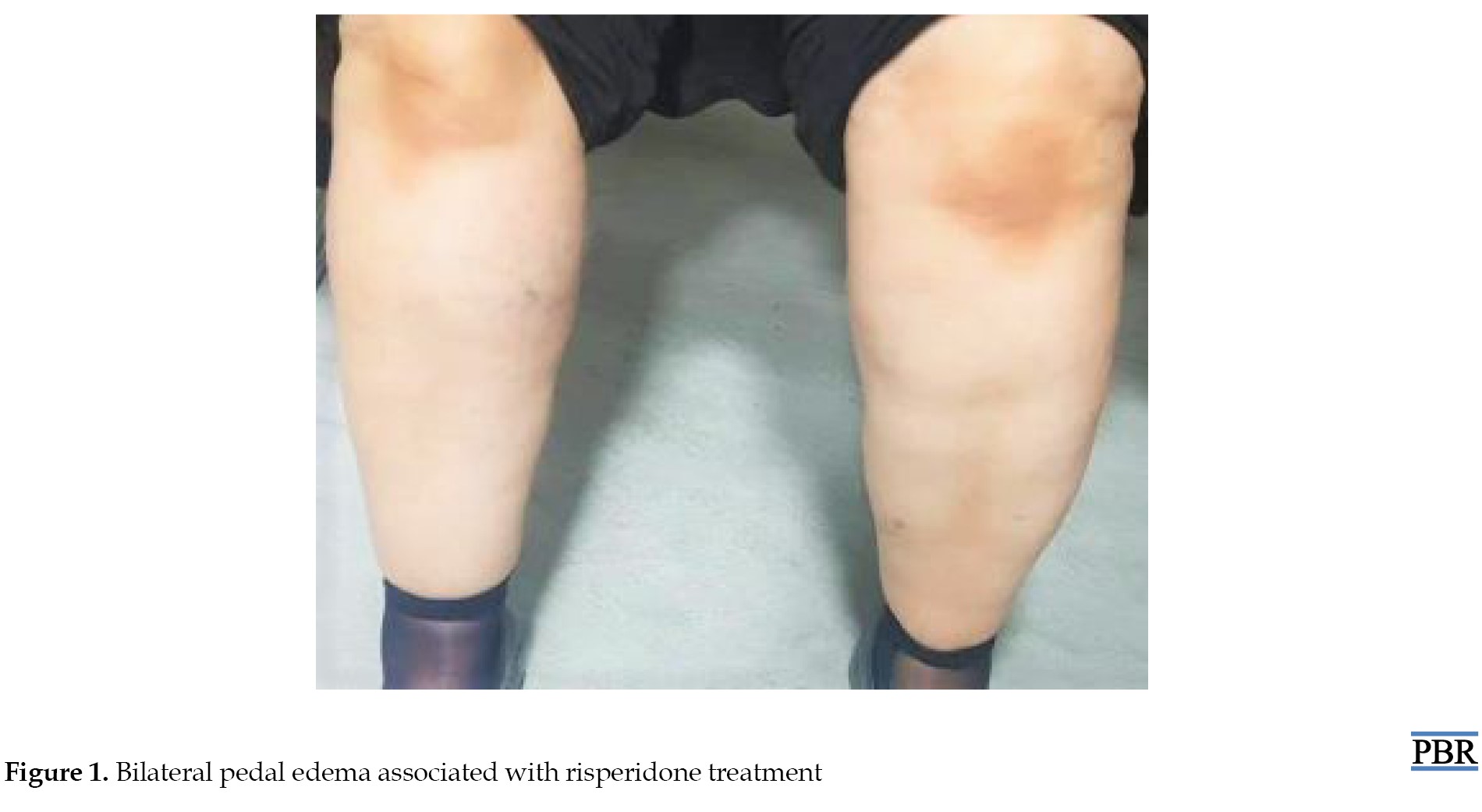
An ECG and chest radiography revealed no signs of pathology. Also, she did not use any other drugs, vitamins, and supplements for the last month. Risperidone treatment was discontinued for a week, and the edema resolved utterly 48-72 hours after risperidone discontinuation entirely without any medical intervention. However, our patient’s psychotic symptoms returned, and risperidone, 1 mg/d, was again prescribed. Remarkable edema was noted for both feet again 2-3 days after the onset of risperidone therapy. On physical examination, her feet, ankles, and pretibial region were grade 3 plus edematous. Subsequently, a diagnosis of risperidone-associated angioedema was made (Figure 1). Eventually, the patient was administered aripiprazole (10 mg/d). Two or three months after introducing aripiprazole (10 mg/d), she developed swelling of both feet. It has been decided that the medication dosage should be reduced to 5 mg/d. Within one week of reducing aripiprazole (5 mg/d), the edema resolved. Also, no edema-associated side-effect was observed over 6 months of follow-up.
Since the patient was on risperidone monotherapy, no drug interactions or additive effects from other medications were considered. As per Naranjo adverse drug reaction probability scale, the rating for the index case was 9, which corresponds to a definite adverse drug reaction associated with risperidone [15]. To our knowledge, the Naranjo scale was 6 or 7 as probable in most cases. This definite reaction shows the severe edema in inpatients, which might be considered an essential responsibility. We also performed the Naranjo adverse drug reaction scale, giving a score of 6, indicating a possible association between edema and aripiprazole treatment.
Discussion
Risperidone seldom causes the side effect of edema [4-6]. Some case reports link the edema after risperidone medication in a dose-dependent way [2, 7, 11].
Faruki noted a 55-year-old male with schizoaffective disorder and bipolar type I showed aggression and suicidal thoughts. He was prescribed low-dose risperidone; he experienced leg pain and edema. Stopping the medication helped the pain but not the swelling. Inadequate information on edema as a side effect led to noncompliance [16].
The other study reported a case of pedal edema linked to oral risperidone use in a 35-year-old male patient with aggressive behavior. The patient developed bilateral below-knee pedal edema within a week of starting risperidone, which improved after reducing the dose and completely resolved in one week [5].
Another report describes a seven-year-old child who develops face and lip edema one week after taking risperidone at a dosage increase from 0.25 mg to 0.5 mg. After stopping risperidone, the face edema disappeared completely after three days. Without any signs of edema, the patient was switched and learned how to take aripiprazole 5 mg/d [17].
A case study of an 80-year-old with significant depression and psychotic symptoms was provided by Hosseini and Ahmadi [7]. She was given prescriptions for citalopram (20 mg/d) and risperidone (2 mg/night). After 20 days, she experienced extreme swelling in her hands and feet, extending from the wrist to the tips of her fingers or toes. Eventually, quetiapine and citalopram were administered to the patient [7].
Orum et al. described two male patients who developed reciprocal pedal edema following treatment with risperidone (case A: 51-year-old with bipolar disorder type 2; Case B: 55-year-old with psychotic disorder + minor mental impairment). They concluded that risperidone treatment for the elderly should be carefully planned because even very low doses of the drug may increase the risk of edema [1].
Also, the other study reported a rare case of leg edema in a schizophrenia patient due to a low risperidone dose. A 37-year-old man developed leg edema after switching from aripiprazole to risperidone. The patient’s lower legs had minimal pitting edema, which improved after switching to amisulpride. No other cases of leg edema with risperidone were reported among 200 patients monitored [2].
In the other report [4], risperidone (4 mg) was recommended for a 37-year-old lady who had schizophrenia for three years. She complained the next day about her hands and feet growing. Pretibial and periorbital zones both expanded at the same time. Researchers stated that the onset of edema following the use of a prescription and its disappearance following the withdrawal of the drug suggests that edema was a side effect of risperidone [4].
Between 1/100 and 1/1000 people experience risperidone-related leg edema with an unclear mechanism [6]. No hematological or immunological abnormalities were found [5, 6, 17].
Some researchers believe that rapid increases in antipsychotic dosage may contribute to the development of peripheral edema [1, 18]. Additionally, it has been hypothesized that being older is a risk factor, especially for people with severe edema [18].
The likelihood of developing edema following the use of an atypical antipsychotic medication varies greatly, from a day to a few months [1, 18]. Physicians should be aware of this risperidone side effect and switch patients to an antipsychotic medication with a different pharmacodynamics profile, such as aripiprazole if the edematous response is suspected [4, 7, 17]. However, in our situation, the patient did not tolerate the 10 mg/day of aripiprazole well, and bilateral edema returned.
Even though there have been previously published instances of edema related to risperidone usage, our case was unique in its sensitivity to a low dose of risperidone (1 mg/night). In contrast, based on the clear reaction of the Naranjo scale of risperidone in our patient, the prescribed dosage in previous publications was at least 2 mg [2]. Edema returned after stopping risperidone and switching to aripiprazole (10 mg/d). There is limited information available on Aripiprazole side effects. According to most reports, aripiprazole is well tolerated by patients [1, 4, 13, 17]. Aripiprazole also appears to have anti-inflammatory properties in carrageenan-induced paw edema in male rats, according to studies [12].
Conclusion
In conclusion, this study reported a case of risperidone-induced edema. Therefore, more research is needed to determine the precise mechanism, risk factors, dose dependence, and features of edema generated by antipsychotic medications. However, this example demonstrates that even at low maintenance dosages, risperidone can cause edema.
Ethical Considerations
Compliance with ethical guidelines
The study was completed following the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research established by the Ministry of Health and Medical Education and the Ministry of Science, Research and Technology, Iran. The patient agreed to participate in the present study and signed written consent forms.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Supervision: Seyyed Hamzeh Hosseini; Investigation and writing: All authors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgments
The authors thank Mazandaran University of Medical Sciences.
References
Asecond-generation atypical antipsychotic called risperidone is frequently used to treat psychiatric disorders like schizophrenia and bipolar disorder [1-4]. A benzoxazole derivative called risperidone was first used in 1993 [4, 5]. It has a high affinity for serotonin type-2 (5-hydroxytryptamine, 5-HT2), dopamine (D2), and 1-adrenergic receptors, resulting in antagonism at these receptors [1, 6, 7].
Risperidone has a few side effects, including extrapyramidal symptoms, dizziness, sedation, insomnia, headache, anxiety, nausea, constipation, and weight gain. However, it is extremely effective in treating childhood and adolescent behavioral disorders, including hyperactivity, aggression, self-injurious states, irritability, and stereotypes [2, 5, 7].
Drug-induced peripheral edema is prevalent, particularly with beta-blockers, calcium channel blockers, nonsteroidal anti-inflammatory medications, and several hormonal medicines. Still, it is uncommon with new-generation antipsychotics [1, 8].
However, in other case reports, edema was noted as an uncommon but severe adverse effect of risperidone that happened in a dose-dependent way [9]. Risperidone seldom causes leg edema, particularly in low doses used for schizophrenia maintenance therapy [2, 9].
An innovative antipsychotic drug called aripiprazole is thought to stabilize the dopamine-serotonin system [10]. It is a partial agonist at serotonin (5-HT) 1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist at dopamine D2 receptors [11]. The fact that aripiprazole does not affect body weight, triglyceride levels, prolactin levels, or sedation [12] is also notable. It has typically been used to treat schizophrenia, bipolar disorder, major depressive disorder, and anxiety disorders [2, 5].
In this paper, a sub-acute onset of bilateral leg edema was reported after initiation of low-dose risperidone treatment in a patient. To our knowledge, this report was the only case study testifying such a high incidence of edema after administrating a low dosage of risperidone (1 mg) in the literature. A search was performed through relevance on PubMed, EMBASE, and Google Scholar, with keywords: “Edema,” “peripheral edema,” and “aripiprazole.” There are few case reports on aripiprazole resulting in edema in patients. In most reports, risperidone successfully was switched to aripiprazole, and edema was suppressed completely [1, 2, 13, 14]. Thus, the dose-dependent manner of our patient to aripiprazole could be considered a rare reaction. The patient’s response to aripiprazole, which appears to be dose-dependent, is an interesting and unusual observation worth further consideration.
Case Presentation
A 55-year-old single unemployed woman presented with auditory hallucination symptoms and delusion of persecution with an unremarkable medical and psychiatric history. Her symptoms had begun about two years before her visit. She was diagnosed as a case of schizophrenia. The patient started on risperidone 1 mg/d, and then the dose was increased to 2 mg/d. A few days after increasing risperidone dosage, she developed marked bilateral swelling over her hands, legs, and face, accompanied by difficulty in breathing. She did not have a history of edema, hepatic or renal dysfunction, thyroid disorder, cardiac dysfunction, or peripheral vascular disease. Symptoms of swelling were not accompanied by itching, pain, fever, lymphadenopathy, chest pain, and abdominal distension. She was not taking any other medication, so that a drug reaction could be ruled out. Complete laboratory examinations were done, including complete blood count, serum electrolytes, liver function test, renal function test, thyroid function test, rheumatological test, immunological examination, and urine analysis. All reports were within normal limits (Table 1).

An ECG and chest radiography revealed no signs of pathology. Also, she did not use any other drugs, vitamins, and supplements for the last month. Risperidone treatment was discontinued for a week, and the edema resolved utterly 48-72 hours after risperidone discontinuation entirely without any medical intervention. However, our patient’s psychotic symptoms returned, and risperidone, 1 mg/d, was again prescribed. Remarkable edema was noted for both feet again 2-3 days after the onset of risperidone therapy. On physical examination, her feet, ankles, and pretibial region were grade 3 plus edematous. Subsequently, a diagnosis of risperidone-associated angioedema was made (Figure 1). Eventually, the patient was administered aripiprazole (10 mg/d). Two or three months after introducing aripiprazole (10 mg/d), she developed swelling of both feet. It has been decided that the medication dosage should be reduced to 5 mg/d. Within one week of reducing aripiprazole (5 mg/d), the edema resolved. Also, no edema-associated side-effect was observed over 6 months of follow-up.
Since the patient was on risperidone monotherapy, no drug interactions or additive effects from other medications were considered. As per Naranjo adverse drug reaction probability scale, the rating for the index case was 9, which corresponds to a definite adverse drug reaction associated with risperidone [15]. To our knowledge, the Naranjo scale was 6 or 7 as probable in most cases. This definite reaction shows the severe edema in inpatients, which might be considered an essential responsibility. We also performed the Naranjo adverse drug reaction scale, giving a score of 6, indicating a possible association between edema and aripiprazole treatment.
Discussion
Risperidone seldom causes the side effect of edema [4-6]. Some case reports link the edema after risperidone medication in a dose-dependent way [2, 7, 11].
Faruki noted a 55-year-old male with schizoaffective disorder and bipolar type I showed aggression and suicidal thoughts. He was prescribed low-dose risperidone; he experienced leg pain and edema. Stopping the medication helped the pain but not the swelling. Inadequate information on edema as a side effect led to noncompliance [16].
The other study reported a case of pedal edema linked to oral risperidone use in a 35-year-old male patient with aggressive behavior. The patient developed bilateral below-knee pedal edema within a week of starting risperidone, which improved after reducing the dose and completely resolved in one week [5].
Another report describes a seven-year-old child who develops face and lip edema one week after taking risperidone at a dosage increase from 0.25 mg to 0.5 mg. After stopping risperidone, the face edema disappeared completely after three days. Without any signs of edema, the patient was switched and learned how to take aripiprazole 5 mg/d [17].
A case study of an 80-year-old with significant depression and psychotic symptoms was provided by Hosseini and Ahmadi [7]. She was given prescriptions for citalopram (20 mg/d) and risperidone (2 mg/night). After 20 days, she experienced extreme swelling in her hands and feet, extending from the wrist to the tips of her fingers or toes. Eventually, quetiapine and citalopram were administered to the patient [7].
Orum et al. described two male patients who developed reciprocal pedal edema following treatment with risperidone (case A: 51-year-old with bipolar disorder type 2; Case B: 55-year-old with psychotic disorder + minor mental impairment). They concluded that risperidone treatment for the elderly should be carefully planned because even very low doses of the drug may increase the risk of edema [1].
Also, the other study reported a rare case of leg edema in a schizophrenia patient due to a low risperidone dose. A 37-year-old man developed leg edema after switching from aripiprazole to risperidone. The patient’s lower legs had minimal pitting edema, which improved after switching to amisulpride. No other cases of leg edema with risperidone were reported among 200 patients monitored [2].
In the other report [4], risperidone (4 mg) was recommended for a 37-year-old lady who had schizophrenia for three years. She complained the next day about her hands and feet growing. Pretibial and periorbital zones both expanded at the same time. Researchers stated that the onset of edema following the use of a prescription and its disappearance following the withdrawal of the drug suggests that edema was a side effect of risperidone [4].
Between 1/100 and 1/1000 people experience risperidone-related leg edema with an unclear mechanism [6]. No hematological or immunological abnormalities were found [5, 6, 17].
Some researchers believe that rapid increases in antipsychotic dosage may contribute to the development of peripheral edema [1, 18]. Additionally, it has been hypothesized that being older is a risk factor, especially for people with severe edema [18].
The likelihood of developing edema following the use of an atypical antipsychotic medication varies greatly, from a day to a few months [1, 18]. Physicians should be aware of this risperidone side effect and switch patients to an antipsychotic medication with a different pharmacodynamics profile, such as aripiprazole if the edematous response is suspected [4, 7, 17]. However, in our situation, the patient did not tolerate the 10 mg/day of aripiprazole well, and bilateral edema returned.
Even though there have been previously published instances of edema related to risperidone usage, our case was unique in its sensitivity to a low dose of risperidone (1 mg/night). In contrast, based on the clear reaction of the Naranjo scale of risperidone in our patient, the prescribed dosage in previous publications was at least 2 mg [2]. Edema returned after stopping risperidone and switching to aripiprazole (10 mg/d). There is limited information available on Aripiprazole side effects. According to most reports, aripiprazole is well tolerated by patients [1, 4, 13, 17]. Aripiprazole also appears to have anti-inflammatory properties in carrageenan-induced paw edema in male rats, according to studies [12].
Conclusion
In conclusion, this study reported a case of risperidone-induced edema. Therefore, more research is needed to determine the precise mechanism, risk factors, dose dependence, and features of edema generated by antipsychotic medications. However, this example demonstrates that even at low maintenance dosages, risperidone can cause edema.
Ethical Considerations
Compliance with ethical guidelines
The study was completed following the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research established by the Ministry of Health and Medical Education and the Ministry of Science, Research and Technology, Iran. The patient agreed to participate in the present study and signed written consent forms.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Supervision: Seyyed Hamzeh Hosseini; Investigation and writing: All authors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgments
The authors thank Mazandaran University of Medical Sciences.
References
- Orum M, Almis BH, Karaca HT. Rapid onset of pedal edema associated with risperidone in two male patients: Simultaneous clinical cases. J Mood Disord. 2017; 7(4):237. [DOI:10.5455/jmood.20170710023745]
- Tunç S, Başbuğ HS. Leg oedema due to low-dose risperidone during maintenance monotherapy of schizophrenia. Psychiatry Clin Psychopharmacol. 2018; 28(1):104-6. [DOI:10.1080/24750573.2017.1379718]
- Aman MG, Arnold LE, McDougle CJ, Vitiello B, Scahill L, Davies M, et al. Acute and long-term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol. 2005; 15(6):869-84. [DOI:10.1089/cap.2005.15.869] [PMID]
- Asan O, Yaylaci ET, Göka E. A case of generalized edema associated with risperidone monotherapy. Turkish J Clinical Psychiatry. 2019; 22:501-3. [DOI:10.5505/kpd.2019.37029]
- Thakur A, Niranjan V, Rastogi P, Razdan R. Acute oedema associated with risperidone use: A report. Gen Psychiatr. 2020; 33(4):e100203. [DOI:10.1136/gpsych-2020-100203] [PMID]
- Tamam L, Ozpoyraz N, Unal M. Oedema associated with risperidone. Clin Drug Investig. 2002; 22(6):411-4. [DOI:10.2165/00044011-200222060-00011]
- Hosseini SH, Ahmadi A. Peripheral edema occurring during treatment with risperidone combined with citalopram. Case Rep Med. 2012; 2012:540732. [DOI:10.1155/2012/540732] [PMID]
- Almis BH, Çelik M. Facial edema after olanzapine addition to sertraline: A case report. Anadolu J Psychiatry. 2017; 18(Suppl.1):46-7. [Link]
- Ravasia S. Risperidone-induced edema. Can J Psychiatry. 2001; 46(5):453-4. [DOI:10.1177/070674370104600523] [PMID]
- Bandelow B, Meier A. Aripiprazole, a “dopamine-serotonin system stabilizer” in the treatment of psychosis. German J Psychiatry. 2003; 6(1):9-16. [Link]
- Sanders RD, Lehrer DS. Edema associated with addition of risperidone to valproate treatment. J Clin Psychiatry. 1998; 59(12):689-90. [DOI:10.4088/JCP.v59n1208e] [PMID]
- Adedayo L, Olawuyi D, Ojo A, Bamidele O, Onasanwo S, Ayoka A. The role of aripiprazole (an antipsychotic drug) in the resolution of acute peripheral inflammation in male wistar rats. J Pharm Res Int. 2017; 17(6):1-8. [DOI:10.9734/JPRI/2017/32601]
- Munshi S, Mukherjee S, Saha I, Sen S. Pedal edema associated with atypical antipsychotics. Indian J Pharmacol. 2016; 48(1):88-90. [DOI:10.4103/0253-7613.174571] [PMID]
- Gundogmus I, Unsal C, Akgun A, Bolu A, Celik C, Uzun O. Risperidone-induced pretibial edema: A case report. Ann Med Res. 2020; 27(1):429-30. [DOI:10.5455/annalsmedres.2019.10.648]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30(2):239-45.[DOI:10.1038/clpt.1981.154] [PMID]
- Faruki F, Gupta UD, Anwar A, Desai S. Case report: Risperidone induced peripheral oedema. Int J Med Pharm Case Rep. 2021; 14(3):25-8. [DOI:10.9734/ijmpcr/2021/v14i330136]
- Ayaydin H, Ulgar SB. Low-dose risperidone-induced facial edema in a child with conduct disorder. Psychiatry Behav Sci. 2018; 8(1):35-7. [DOI:10.5455/jmood.20180123081055]
- Umar MU, Abdullahi AT. Self-limiting atypical antipsychotics-induced edema: Clinical cases and systematic review. Indian J Psychol Med. 2016; 38(3):182-8. [DOI:10.4103/0253-7176.183089] [PMID]
Type of Study: case report |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |