Volume 10, Issue 4 (2024)
Pharm Biomed Res 2024, 10(4): 331-344 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Islam M M, Mahmud I, Rahaman M H, Asaduzzaman S M. Investigating Computational Insights Into Plant-derived Terpenoids of Bangladesh: Prospects for Anti-rheumatoid Arthritis Medication. Pharm Biomed Res 2024; 10 (4) :331-344
URL: http://pbr.mazums.ac.ir/article-1-633-en.html
URL: http://pbr.mazums.ac.ir/article-1-633-en.html
1- Department of Pharmacy, Noakhali Science and Technology University, Noakhali, Bangladesh.
2- Department of Biochemistry and Molecular Biology, National University, Dhaka, Bangladesh.
3- Department of Pharmacy, East West University, Dhaka, Bangladesh.
2- Department of Biochemistry and Molecular Biology, National University, Dhaka, Bangladesh.
3- Department of Pharmacy, East West University, Dhaka, Bangladesh.
Full-Text [PDF 3348 kb]
(700 Downloads)
| Abstract (HTML) (2047 Views)
Full-Text: (417 Views)
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that is inflammatory and persistent, and linked to early mortality, systemic complications, and progressive disability [1]. Numerous illnesses, including lung conditions, kidney problems, and cardiovascular and cerebrovascular disorders, can coexist with RA. The development of RA is influenced by several risk factors, including environmental and genetic variables [2]. Medications for RA therapy are being developed. These medications include glucocorticoids, methotrexate, Janus kinase inhibitors, and interleukin (IL)-6 inhibitors, as well as nonsteroidal anti-inflammatory medicines (e.g. aspirin, ibuprofen, and naproxen) [3]. However, each class of these medications has its side effects [4, 5]. Plant-based natural remedies are usually considered safe and effective medications with reduced adverse effects [6, 7].
Numerous preclinical studies have shown that natural plant extracts and compounds reduce RA symptoms considerably [8, 9, 10]. Plant secondary metabolites are known as bioactive substances. Because of the enormous variation in their structures, they have a wide range of pharmacological activities and are useful in the treatment and prevention of many different diseases [11]. Several plants are used traditionally for the treatment of RA. These plants contain a variety of secondary metabolites. Terpenoids are the largest plant compound group, having numerous pharmacological activities, including antitumor, anti-inflammatory, antibacterial, antiviral, and antimalarial activities [12]. Nevertheless, in-depth research has not been done to determine whether these compounds can certainly be used to treat RA. For rational drug design, computational drug design forecasts molecular-level interactions between therapeutic drugs and targets. This meticulous methodology increases the likelihood of discovering medicinal compounds with minimal adverse effects. Molecular docking can be used to virtually screen large compound libraries, which can help guide optimization [13]. Therefore, we focus on the evaluation of anti-RA properties of terpenoids of some Bangladeshi plants by molecular docking analysis and perform the pharmacokinetic evaluation. This study can be helpful in synthesizing novel and safe medications for RA.
Materials and Methods
Ligand selection and preparation
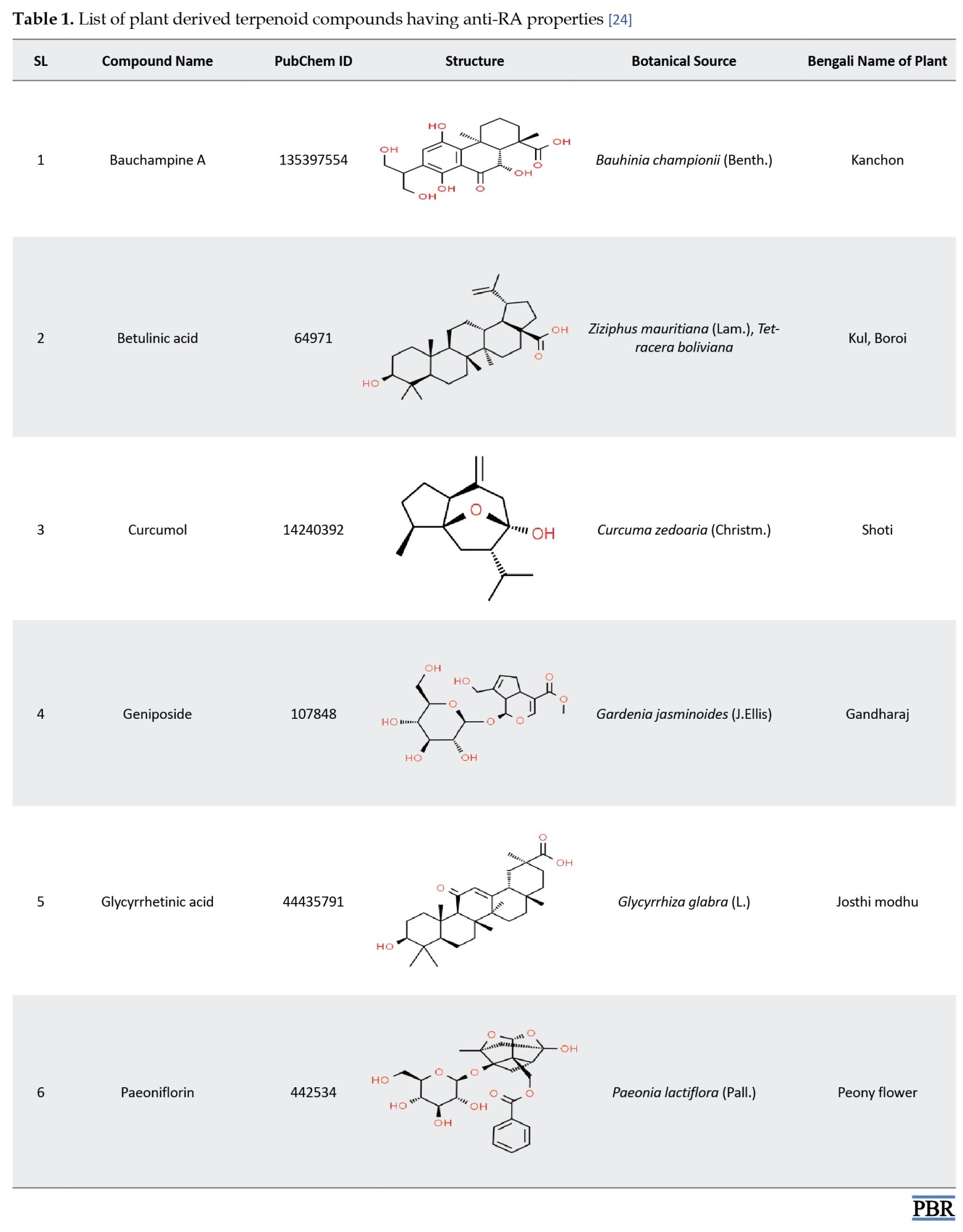
An extensive literature survey was performed to find out the plant sources used for the treatment of RA. Then, the responsible plant compounds were also listed in the databases. Among the different classes of compounds, we selected only the terpenoids. Due to the anti-inflammatory activity of terpenoids, these compounds can be used to suppress RA-related symptoms [14-17]. The plants that are available in Bangladesh were finally selected. These compounds show their anti-RA activity by various mechanisms. For example, betulinic acid showed chondroprotective activity [18], curcumol had an immunoregulatory effect [19], geniposide revealed both anti-inflammatory [20] and immunosuppressive activities [21], paeoniflorin possessed antioxidant and anti-inflammatory activities [22]. The list of the selected compounds is provided in Table 1.
The six compounds were used as ligands for the subsequent in silico studies. After that, the 3D conformer of the ligands was obtained in structure data file format from the PubChem database [23, 24].
Absorption, distribution, metabolism, excretion and toxicity analysis
Pharmacokinetics studies the drug molecules’ absorption, distribution, metabolism, and excretion (ADME) characteristics, which is essential in drug design procedures. Computationally, all these attributes form the basis of computer-based drug design. Inappropriate compounds are also eliminated based on these characteristics. The Swiss-ADME website is used to evaluate preliminary pharmacokinetics results during the drug discovery process. The Swiss-ADME online website is a public web server for predicting small compounds’ pharmacokinetics and drug-likeness characteristics [25]. When developing new medications, toxicity evaluation may be done to evaluate a chemical’s adverse or harmful effects [26]. In this regard, the pkCSM internet server was implemented for quickly analyzing toxicological properties, which is accessible to the public [27].
Receptor protein selection and preparation
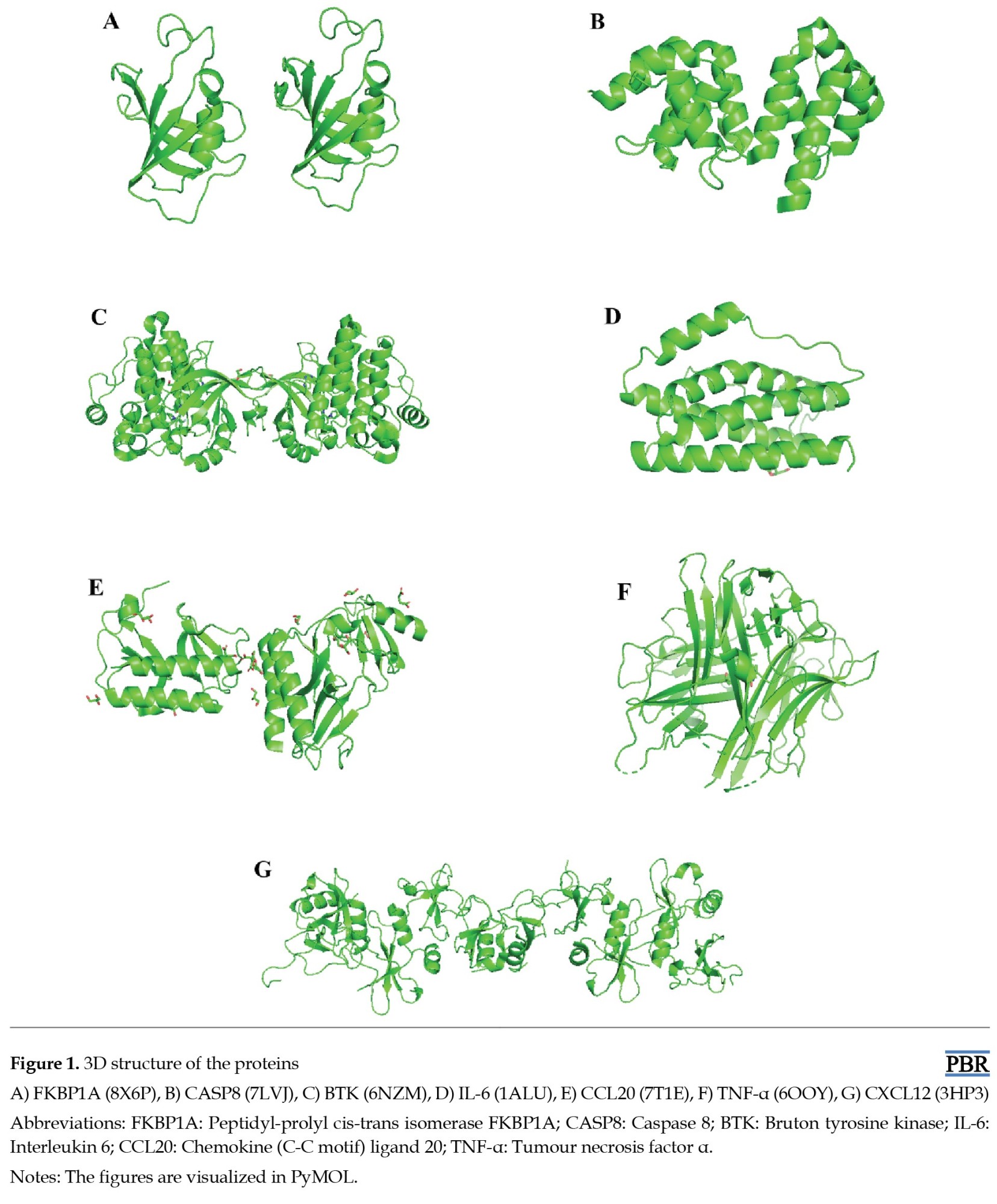
RA is an autoimmune disorder that mainly affects the bone joint and causes severe pain and swelling at the affected places. There are several pathways involved in the progression of the disease. Since cytokines are intimately implicated in the RA process, they have long been investigated and evaluated as possible targets of RA. Patients with RA may have high levels of inflammatory cytokines (including tumor necrosis factor-α [TNF-α], IL-6) in their peripheral blood or serum or synovium [28, 29]. According to research, chemokines influence angiogenesis and draw leukocytes, which contribute to the underlying pathophysiology of RA. The CXC and CC chemokines play vital roles in this process [30, 31]. Peptidyl-prolyl cis-trans isomerase FKBP1A, Bruton tyrosine kinase (BTK), and caspase 8 (CASP8) are also involved in the pathogenesis of RA [32-34]. Based on the published articles, we selected six proteins as receptor proteins for molecular docking: FKBP1A (8X6P), CASP8 (7LVJ), BTK (6NZM), IL-6 (1ALU), CCL20 (7T1E), CXCL12 (3HP3), and TNF-α (6OOY) (Figure 1). The protein structures were obtained from the Protein Data Bank. Then, the protein was imported into the PyMOL 2.5.7.0 tool [35]. PyMOL tool was used to eliminate heteroatoms, ligands, and water molecules from the protein. Lastly, the modified protein was saved in protein data bank format. Then, protein minimization was done by Swiss-PDBViewer 4.10 [36]. By this process, the proteins are prepared for molecular docking.
Molecular docking and visualization of the receptor-ligand interaction
The modified proteins were input into the PyRx 0.8 docking tool [37]. Then, the modified proteins were transformed into macromolecules. After that, the ligand molecules were imported into the PyRx 0.8. Then, the ligands were converted into PDBQT format. Consequently, they were turned into Autodock ligands. After that, proteins and ligands were selected, and the software was run. After that, the file containing the binding affinity of the ligands with the target molecule and the ligands was saved. The prepared combined structures were loaded into the Biovia Discovery Studio 21.1.0 tool, and the ligands and the receptors were defined individually [38]. Finally, the molecular interactions between the targeted protein and selected ligands were viewed and analyzed. In addition, 2D and 3D visualizations were performed.
Results
Absorption, distribution, metabolism, excretion and toxicity analysis
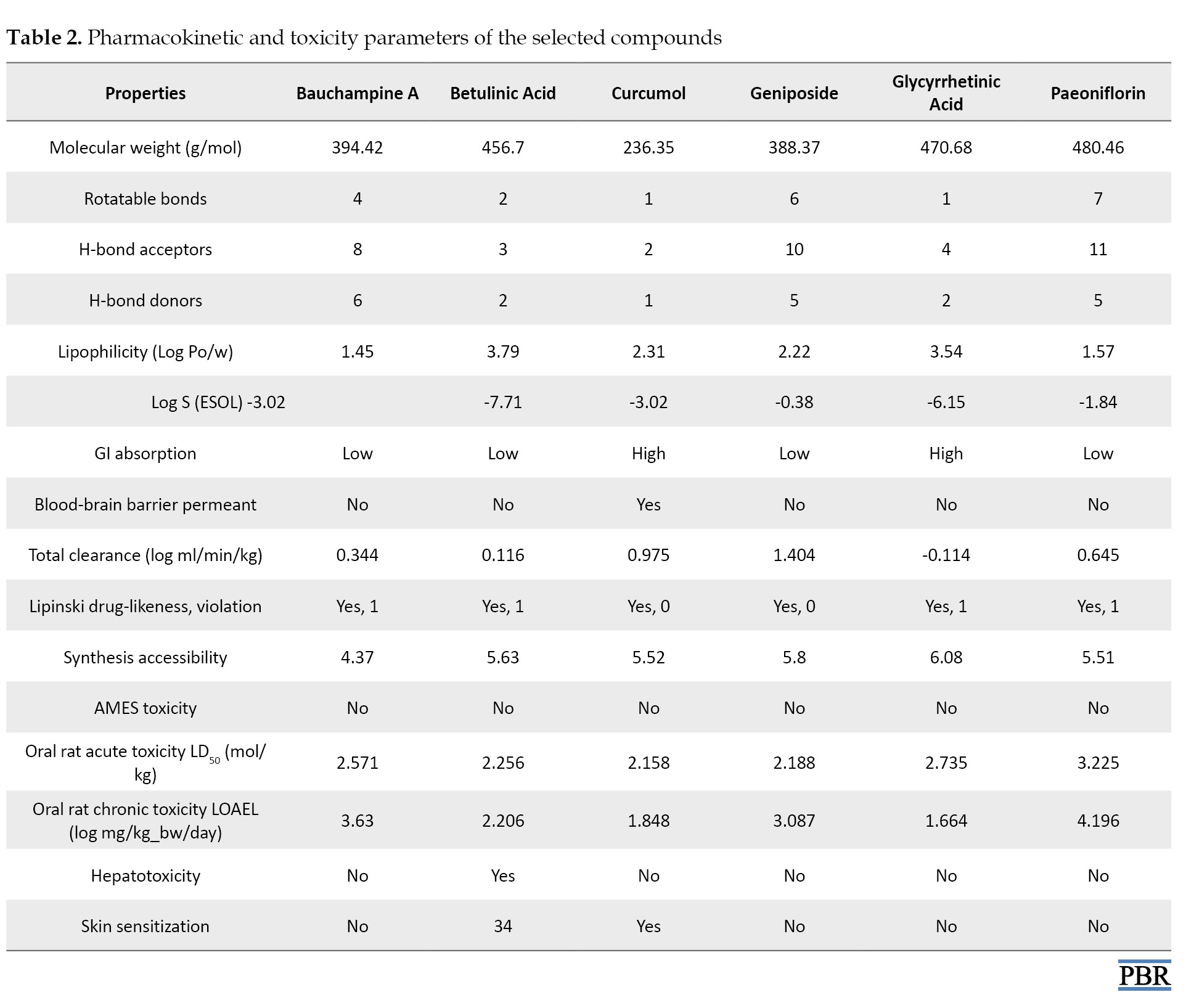
With the help of the Swiss-ADME server, we analyzed the ADME features of the selected compounds for anti-RA activity. Studied properties include molecular weight, number of rotatable bonds, number of H-bond acceptors, number of H-bond donors, lipophilicity (Log Po/w), water solubility (Log S -ESOL), gastrointestinal absorption, The blood-brain barrier permeant, Lipinski drug-likeliness properties, and synthesis feasibility. Analyzing a chemical substance’s toxicity endpoints, including mutagenicity, carcinogenicity, and other characteristics, is one technique to quantify and assess a chemical substance’s toxicity. The pkCSM server was used for the toxicity identification of a molecule, an essential stage in in-silico drug development. We studied properties like AMES toxicity, oral rat acute toxicity (LD50), oral rat chronic toxicity (LOAEL), hepatotoxicity, and skin sensitization of the compounds. We also performed the total clearance of the compounds using the pkCSM server. The findings are shown in Table 2.
Molecular docking
The docking results of the selected compounds are shown in Table 3.
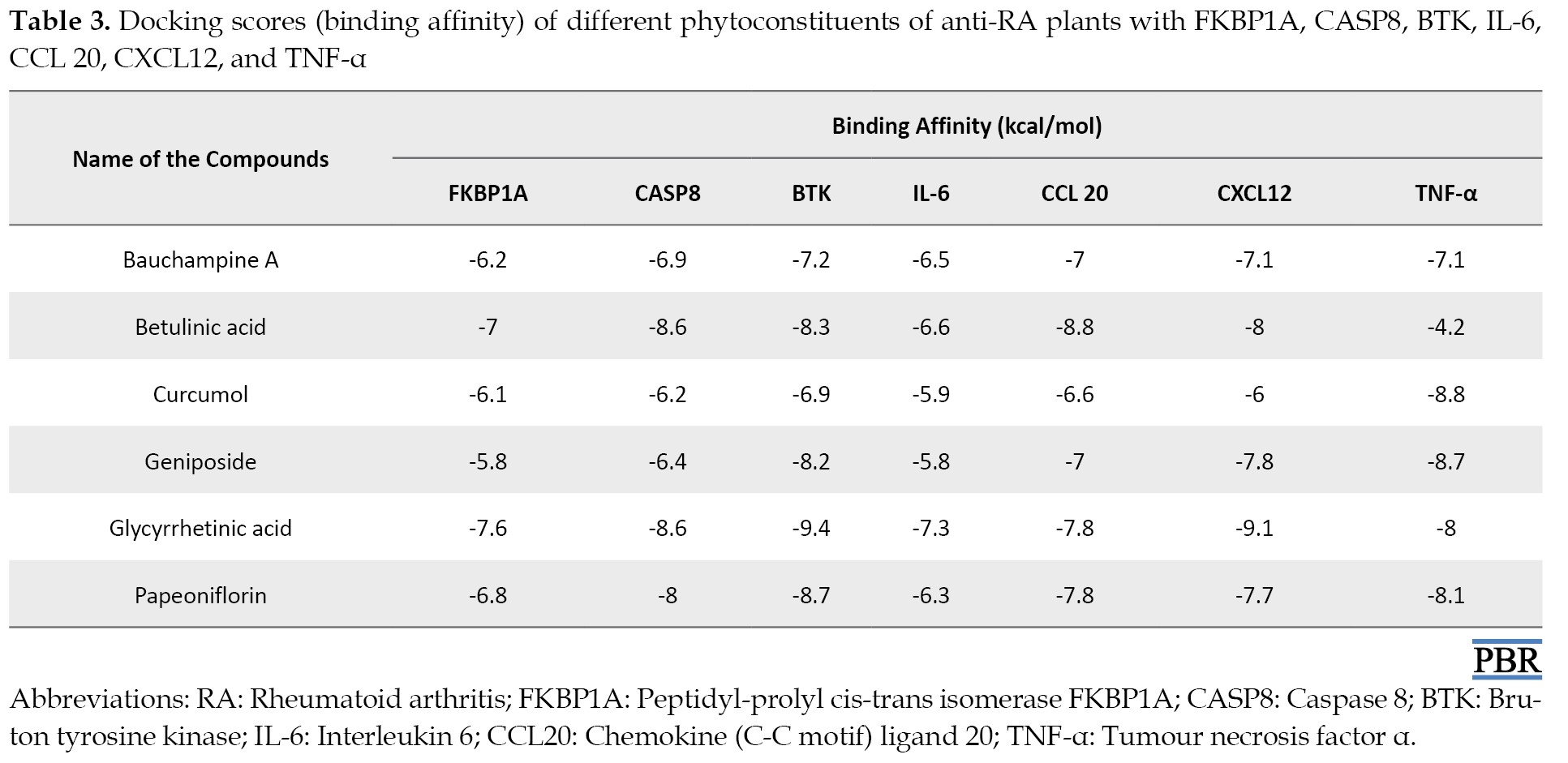
The overall binding energy for all compounds was satisfactory. The binding energies range from -4.2 to -9.4. However, the highest docking score for bauchampine A was with BTK (-7.2 kcal/mol), betulinic acid with CCL20 (-8.8 kcal/mol), curcumol with TNF-α (-8.8 kcal/mol), geniposide with TNF-α (-8.7 kcal/mol), glycyrrhetinic acid with BTK (-9.4 kcal/mol), and papeoniflorin with BTK (-8.7 kcal/mol).
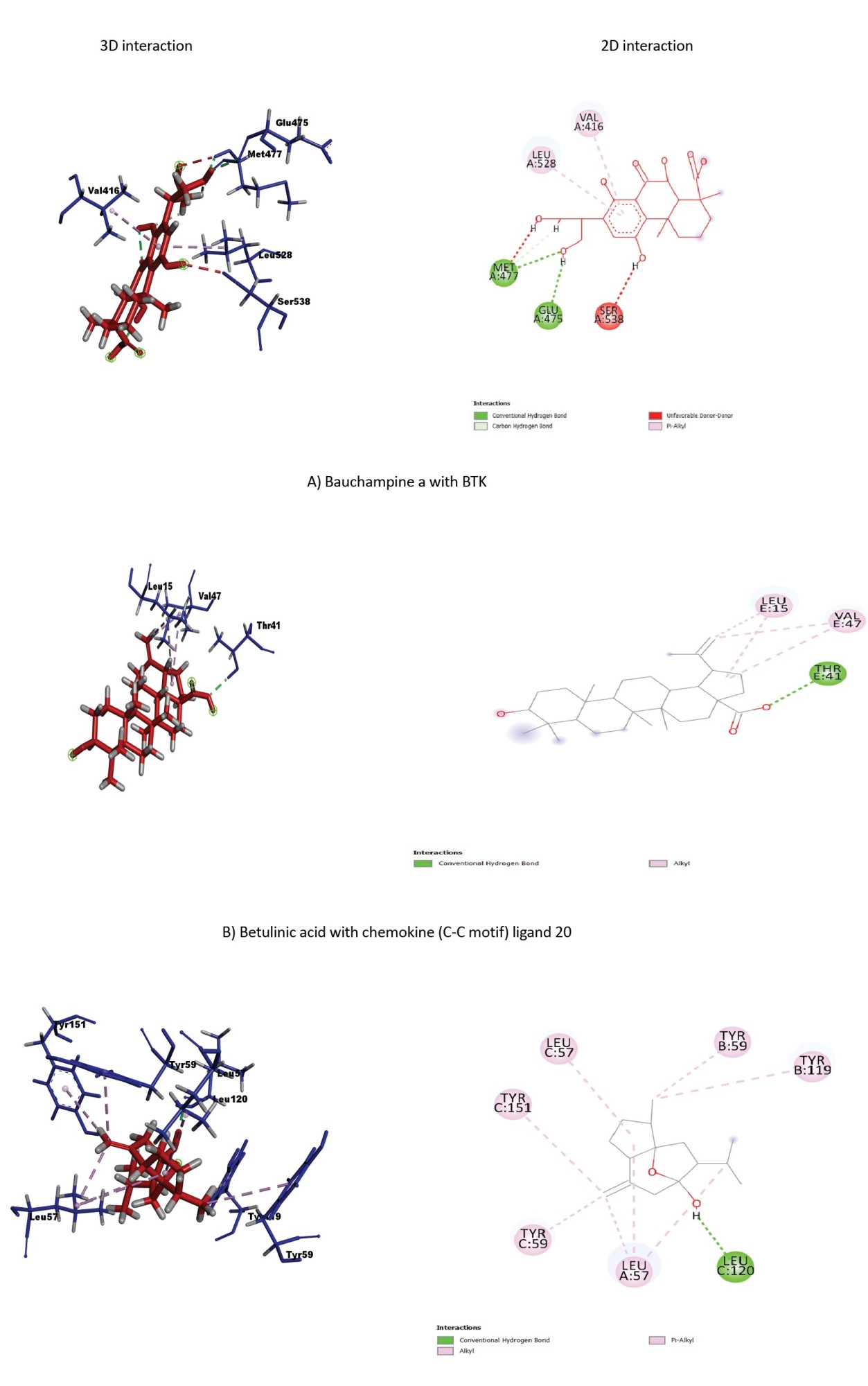
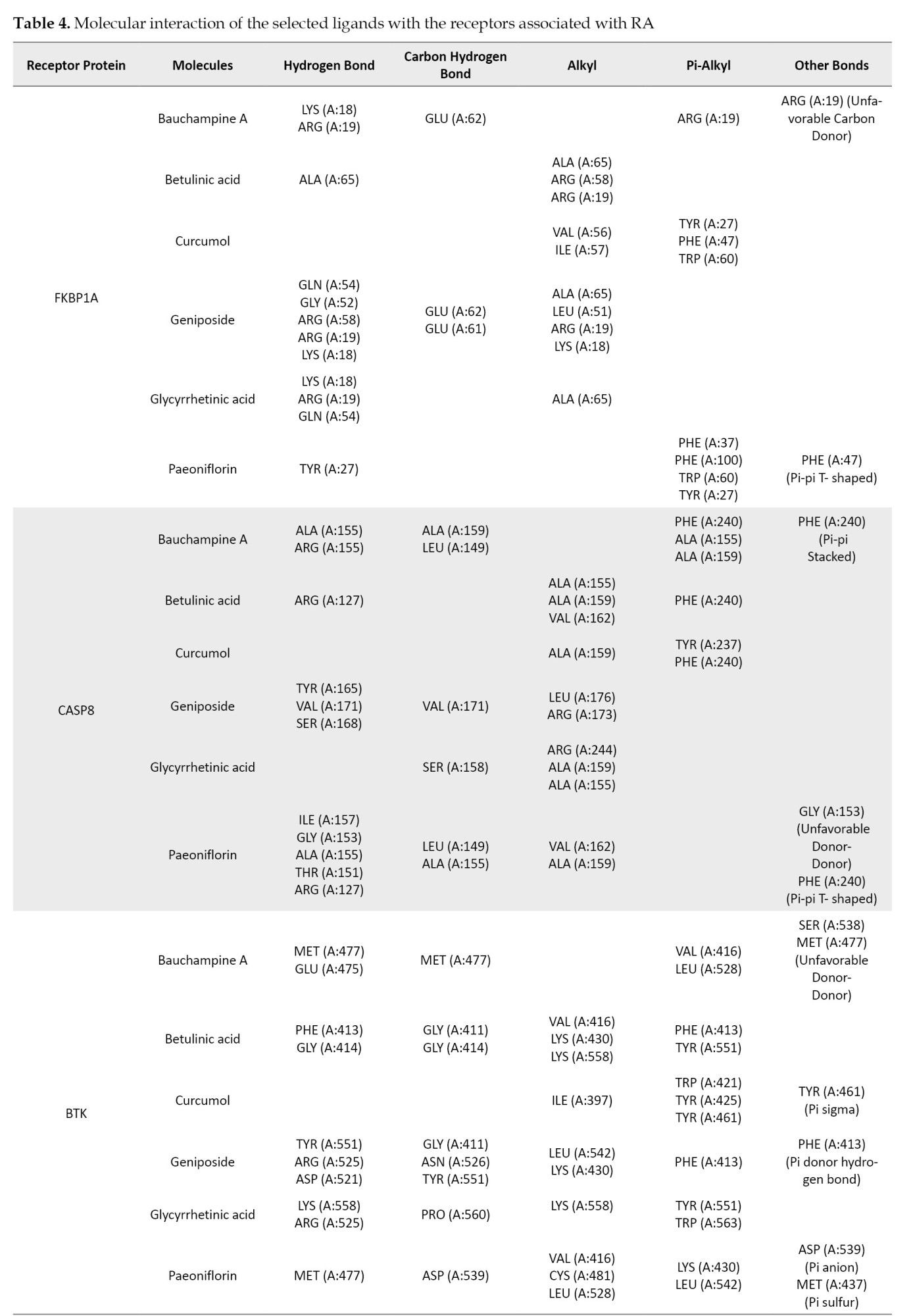
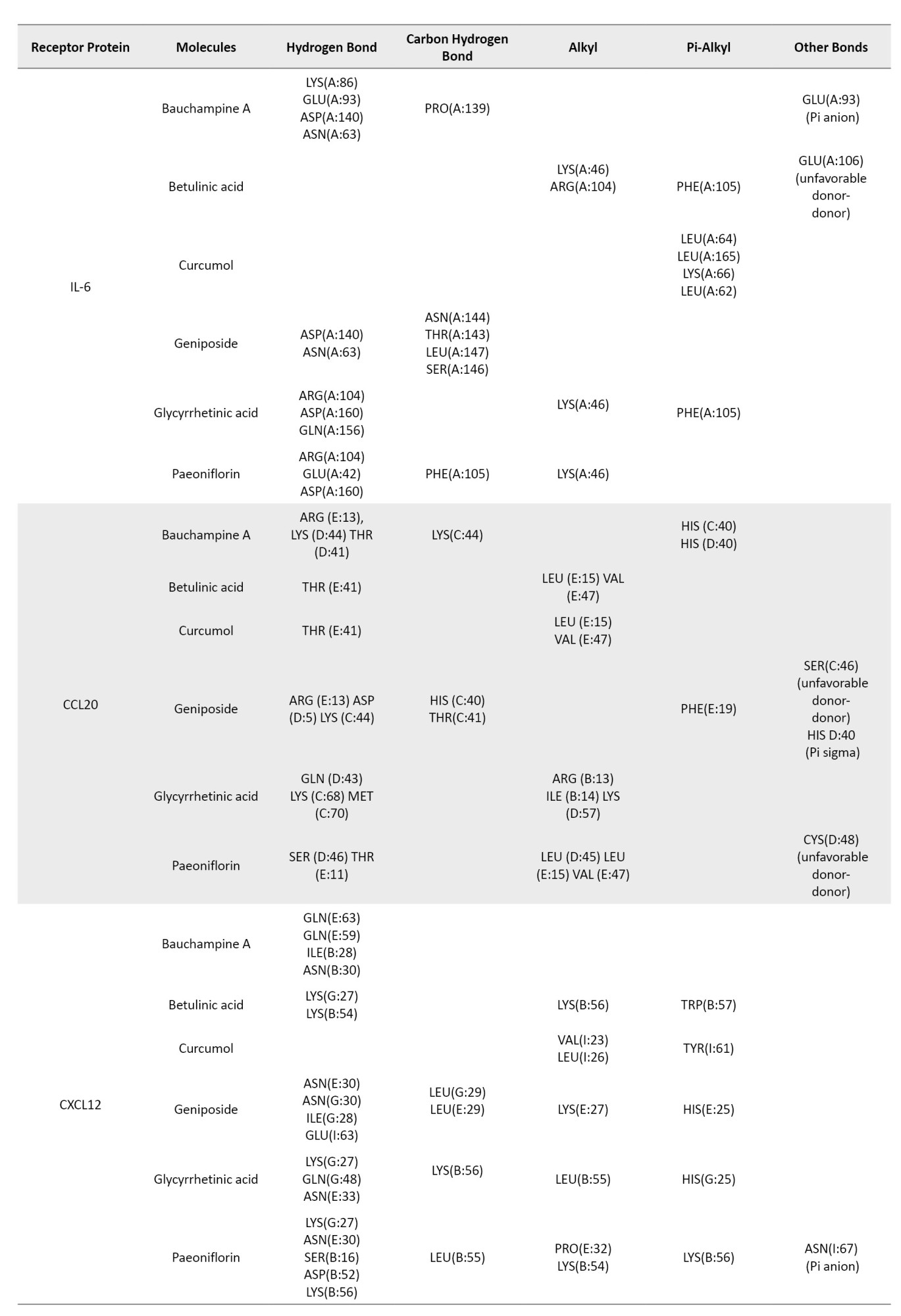
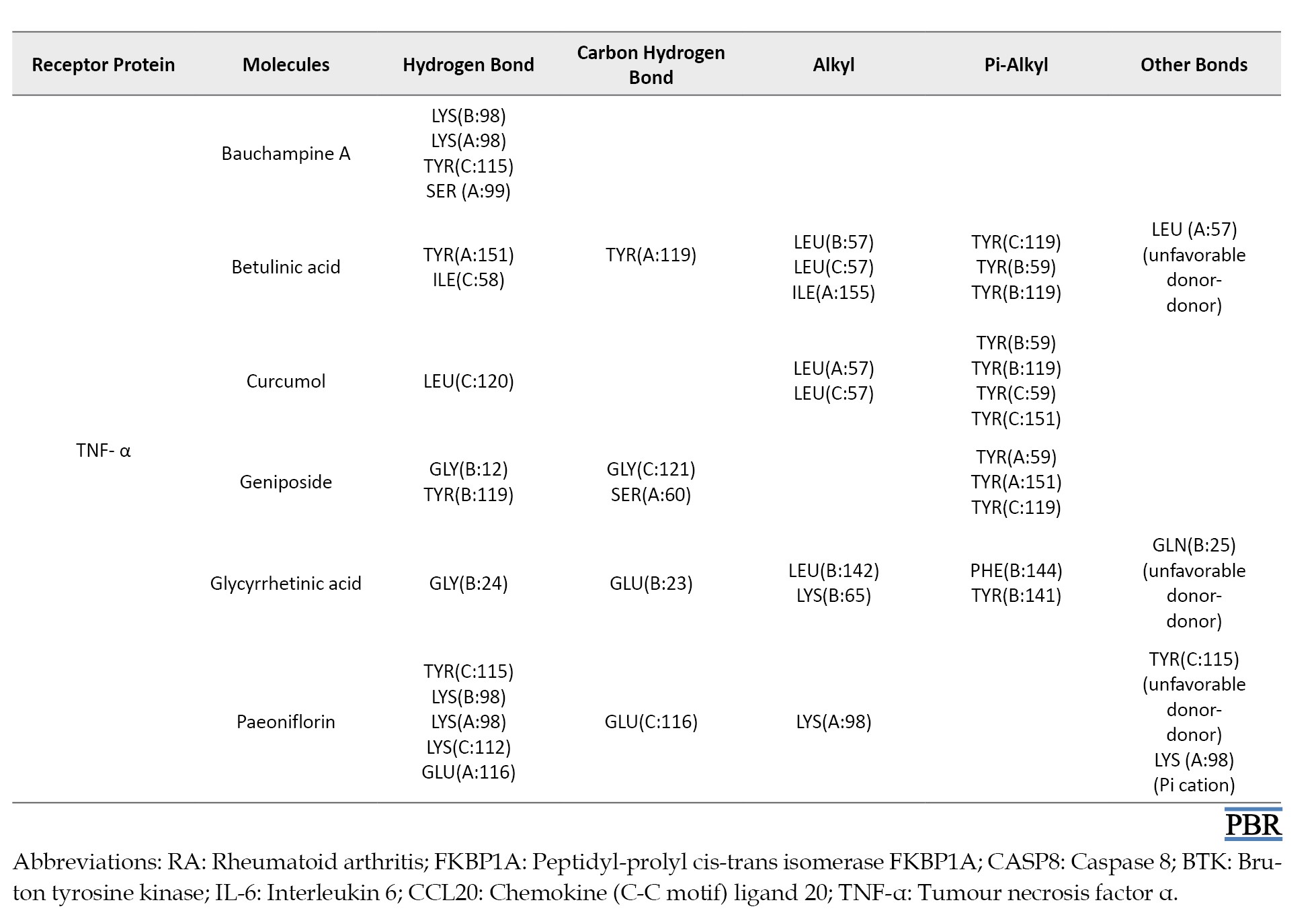
The ligand-receptor interactions of the highest scores are shown in Figure 2. Bauchampine A is bound to FKBP1A, CASP8, BTK, IL-6, CCL20, CXCL12, and TNF-α by forming a conventional hydrogen bond with LYS(A:18) and ARG(A:19); ALA(A:155) and ARG(A:155); MET(A:477) and GLU(A:475); LYS(A:86), GLU(A:93), ASP(A:140) and ASN(A:63); ARG(E:13), LYS(D:44) and THR(D:41); GLN(E:63), GLN(E:59), ILE(B:28), and ASN(B:30); LYS(B:98), LYS(A:98), TYR(C:115) and SER(A:99), respectively. In addition, the carbonyl group formed a hydrogen bond with GLU(A:62), ALA(A:159) and LEU(A:149), MET(A:477), PRO(A:139), LYS(C:44) of FKBP1A, CASP8, BTK, IL-6, and CCL20, respectively. The other bonds between bauchampine A and the receptors include unfavorable donor, pi-alkyl, pi donor hydrogen bond, pi-pi stacked interaction, and pi anion interaction. The detailed interaction of the other ligands and receptors is compiled in Table 4.
Discussion
The hands and feet are the main areas affected by RA, a systemic poly-articular chronic inflammatory joint disease. Pathological manifestations of RA include pannus development, inflammatory cell infiltration, hyperplasia of the synovial membrane, and degeneration of the bone and cartilage in the joints. We studied six terpenoid compounds from Bangladeshi plants as potential candidates for anti-RA agents. The seven targets were selected as they have a profound role in RA’s pathogenesis. Lipinski’s criteria were supported by an analysis of the docked drugs’ pharmacokinetics, which included examining their ADME characteristics. Toxicity tests were conducted on the substance to evaluate the chemical’s possible harm to humans and animals.
ADME screening is necessary for a compound to meet the quality standards required to be considered a prospective drug candidate. A medicine candidate with a large molecular weight may have less permeability through a biological barrier. The lipophilicity of the target molecule is shown by the logarithm of its distribution coefficient among the hydrophilic and lipophilic phases. The log P indicates the degree to which the body captivates the drug. The degree to which the body absorbs a medicine is inversely correlated with its log P. Betulinic acid showed the highest log P (3.79) and bauchampine A possessed the lowest value (1.54). The log S value indicates the pharmaceutical candidate’s solubility. The lowest log S value was found for betulinic acid (-7.71) and the highest value was shown by geniposide (-0.38). A drug’s ability to function as both an acceptor and a donor of hydrogen bonds determines how easily it can pass through a cell membrane. Rotatable bond numbers enable bioavailability; the optimal range for these values lies within 10. All the compounds showed rotatable bond numbers within this limit.
A substance’s toxicity is its capacity to cause harm to people or animals. Typically, animal models are employed to evaluate toxicity. This model is a lengthy and costly process. Because the in-silico method is inexpensive, short-lived, and does not need any animal object, it helps establish a compound’s toxicity profile for drug development. The toxicological test servers (pkCSM) ascertain whether a medicine is carcinogenic. The studied compounds showed no toxicity in ‘AMES toxicity’ profiling; all were safe in hepatotoxicity testing except betulinic acid, and only curcumol showed a skin sensitization effect.
To help execute rational drug design, molecular docking techniques can be utilized to determine the binding affinities of different ligands for the target protein structure. This method can help comprehend the dynamics of interactions and possible binding mechanisms, which can be used to apply more stringent inhibition [39]. The docking scores of these compounds revealed excellent binding properties with the targets. Individual compounds have distinct binding affinity with different targets. The BTK has the highest scores for the compounds. The bonds among the compounds and the receptors are also diverse.
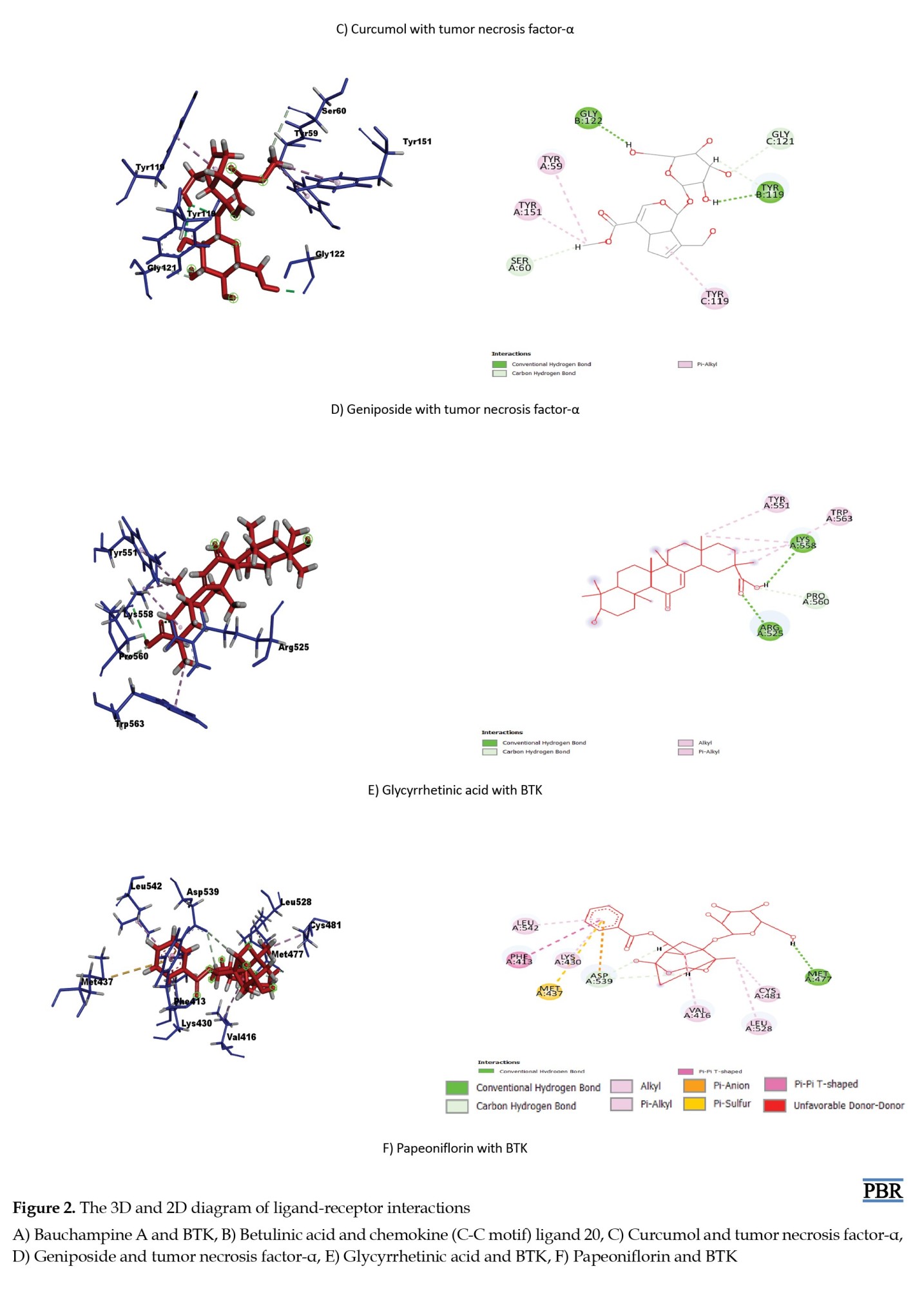
BTK plays a vital role in mononuclear cells of the innate immune system, especially in dendritic cells and macrophages. Moreover, it is a direct regulator of the NLRP3 inflammasome, a key innate inflammatory machinery [40]. Studies revealed that inhibition of BTK resulted in the downregulation of B cells and thus reduced RA-mediated inflammation [41, 42]. In our study, bauchampine A, glycyrrhetinic acid, and papeoniflorin showed the most significant binding affinity with BTK. These compounds have a selective affinity to BTK, which can be used to inhibit the protein. There is growing interest in inhibiting BTK for anti-RA medication development [43], so these compounds may serve as potential candidates.
Another crucial inflammatory cytokine in this study is TNF-α. Excessive upregulation of TNF-α is associated with chronic inflammation that can result in autoimmune diseases [44, 45]. Blocking the destructive activity of TNF has been used in the treatment of RA for several years [46, 47]. Curcumol and geniposide showed the highest binding energy score with TNF-α, which is also promising for blocking this pathway [48, 49].
The chemokines are actively involved in inflammatory disorders, and CCL20 is a vital chemokine [50]. The binding energy of betulinic acid with CCL20 was the highest. The role of CCL20 in the pathogenesis of RA is unclear, though the patients contain higher levels of the chemokine than healthy individuals [51, 52]. The compound has the most affinity to the chemokine than other proteins.
Conclusion
Plant-derived terpenoid compounds can serve as effective leads for developing anti-RA medications. In this study, terpenoid molecules were tested against several proteins engaged in RA pathogenesis. This study revealed the tested compounds’ binding site and affinity for their targets. The compounds showed significant binding scores with different receptor proteins. Glycyrrhetinic acid showed the highest binding scores with BTK. The pharmacokinetic properties were also evaluated to confirm their drug-likeness. Almost every compound showed potential drug-like pharmacokinetic parameters. The toxicity profiling of the terpenoids also showed auspicious results. The present study may aid the development of effective drugs for rheumatic arthritis.
Limitations
The computational approaches used in this study have several limitations that impact their effectiveness. In this study, the accuracy of predictions is often constrained by simplified models that may not fully capture complex molecular interactions or the dynamic nature of biological systems. Additionally, predicting critical drug properties like absorption, distribution, metabolism, excretion, and toxicity remains challenging and prone to errors. Moreover, the results from in silico studies require experimental validation, which can reveal discrepancies and unforeseen issues, such as drug-drug interactions or off-target effects, that were not predicted. Further in-depth research, as well as in vitro and in vivo investigations, should be conducted to get above the study’s constraints and enhance the computational techniques employed.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Md Monirul Islam; Methodology: Iqbal Mahmud, and Md Habibur Rahaman; Data analysis: Md Monirul Islam, and Iqbal Mahmud; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to all related to this work.
References
Rheumatoid arthritis (RA) is a systemic autoimmune disease that is inflammatory and persistent, and linked to early mortality, systemic complications, and progressive disability [1]. Numerous illnesses, including lung conditions, kidney problems, and cardiovascular and cerebrovascular disorders, can coexist with RA. The development of RA is influenced by several risk factors, including environmental and genetic variables [2]. Medications for RA therapy are being developed. These medications include glucocorticoids, methotrexate, Janus kinase inhibitors, and interleukin (IL)-6 inhibitors, as well as nonsteroidal anti-inflammatory medicines (e.g. aspirin, ibuprofen, and naproxen) [3]. However, each class of these medications has its side effects [4, 5]. Plant-based natural remedies are usually considered safe and effective medications with reduced adverse effects [6, 7].
Numerous preclinical studies have shown that natural plant extracts and compounds reduce RA symptoms considerably [8, 9, 10]. Plant secondary metabolites are known as bioactive substances. Because of the enormous variation in their structures, they have a wide range of pharmacological activities and are useful in the treatment and prevention of many different diseases [11]. Several plants are used traditionally for the treatment of RA. These plants contain a variety of secondary metabolites. Terpenoids are the largest plant compound group, having numerous pharmacological activities, including antitumor, anti-inflammatory, antibacterial, antiviral, and antimalarial activities [12]. Nevertheless, in-depth research has not been done to determine whether these compounds can certainly be used to treat RA. For rational drug design, computational drug design forecasts molecular-level interactions between therapeutic drugs and targets. This meticulous methodology increases the likelihood of discovering medicinal compounds with minimal adverse effects. Molecular docking can be used to virtually screen large compound libraries, which can help guide optimization [13]. Therefore, we focus on the evaluation of anti-RA properties of terpenoids of some Bangladeshi plants by molecular docking analysis and perform the pharmacokinetic evaluation. This study can be helpful in synthesizing novel and safe medications for RA.
Materials and Methods
Ligand selection and preparation
An extensive literature survey was performed to find out the plant sources used for the treatment of RA. Then, the responsible plant compounds were also listed in the databases. Among the different classes of compounds, we selected only the terpenoids. Due to the anti-inflammatory activity of terpenoids, these compounds can be used to suppress RA-related symptoms [14-17]. The plants that are available in Bangladesh were finally selected. These compounds show their anti-RA activity by various mechanisms. For example, betulinic acid showed chondroprotective activity [18], curcumol had an immunoregulatory effect [19], geniposide revealed both anti-inflammatory [20] and immunosuppressive activities [21], paeoniflorin possessed antioxidant and anti-inflammatory activities [22]. The list of the selected compounds is provided in Table 1.

The six compounds were used as ligands for the subsequent in silico studies. After that, the 3D conformer of the ligands was obtained in structure data file format from the PubChem database [23, 24].
Absorption, distribution, metabolism, excretion and toxicity analysis
Pharmacokinetics studies the drug molecules’ absorption, distribution, metabolism, and excretion (ADME) characteristics, which is essential in drug design procedures. Computationally, all these attributes form the basis of computer-based drug design. Inappropriate compounds are also eliminated based on these characteristics. The Swiss-ADME website is used to evaluate preliminary pharmacokinetics results during the drug discovery process. The Swiss-ADME online website is a public web server for predicting small compounds’ pharmacokinetics and drug-likeness characteristics [25]. When developing new medications, toxicity evaluation may be done to evaluate a chemical’s adverse or harmful effects [26]. In this regard, the pkCSM internet server was implemented for quickly analyzing toxicological properties, which is accessible to the public [27].
Receptor protein selection and preparation
RA is an autoimmune disorder that mainly affects the bone joint and causes severe pain and swelling at the affected places. There are several pathways involved in the progression of the disease. Since cytokines are intimately implicated in the RA process, they have long been investigated and evaluated as possible targets of RA. Patients with RA may have high levels of inflammatory cytokines (including tumor necrosis factor-α [TNF-α], IL-6) in their peripheral blood or serum or synovium [28, 29]. According to research, chemokines influence angiogenesis and draw leukocytes, which contribute to the underlying pathophysiology of RA. The CXC and CC chemokines play vital roles in this process [30, 31]. Peptidyl-prolyl cis-trans isomerase FKBP1A, Bruton tyrosine kinase (BTK), and caspase 8 (CASP8) are also involved in the pathogenesis of RA [32-34]. Based on the published articles, we selected six proteins as receptor proteins for molecular docking: FKBP1A (8X6P), CASP8 (7LVJ), BTK (6NZM), IL-6 (1ALU), CCL20 (7T1E), CXCL12 (3HP3), and TNF-α (6OOY) (Figure 1). The protein structures were obtained from the Protein Data Bank. Then, the protein was imported into the PyMOL 2.5.7.0 tool [35]. PyMOL tool was used to eliminate heteroatoms, ligands, and water molecules from the protein. Lastly, the modified protein was saved in protein data bank format. Then, protein minimization was done by Swiss-PDBViewer 4.10 [36]. By this process, the proteins are prepared for molecular docking.
Molecular docking and visualization of the receptor-ligand interaction
The modified proteins were input into the PyRx 0.8 docking tool [37]. Then, the modified proteins were transformed into macromolecules. After that, the ligand molecules were imported into the PyRx 0.8. Then, the ligands were converted into PDBQT format. Consequently, they were turned into Autodock ligands. After that, proteins and ligands were selected, and the software was run. After that, the file containing the binding affinity of the ligands with the target molecule and the ligands was saved. The prepared combined structures were loaded into the Biovia Discovery Studio 21.1.0 tool, and the ligands and the receptors were defined individually [38]. Finally, the molecular interactions between the targeted protein and selected ligands were viewed and analyzed. In addition, 2D and 3D visualizations were performed.
Results
Absorption, distribution, metabolism, excretion and toxicity analysis
With the help of the Swiss-ADME server, we analyzed the ADME features of the selected compounds for anti-RA activity. Studied properties include molecular weight, number of rotatable bonds, number of H-bond acceptors, number of H-bond donors, lipophilicity (Log Po/w), water solubility (Log S -ESOL), gastrointestinal absorption, The blood-brain barrier permeant, Lipinski drug-likeliness properties, and synthesis feasibility. Analyzing a chemical substance’s toxicity endpoints, including mutagenicity, carcinogenicity, and other characteristics, is one technique to quantify and assess a chemical substance’s toxicity. The pkCSM server was used for the toxicity identification of a molecule, an essential stage in in-silico drug development. We studied properties like AMES toxicity, oral rat acute toxicity (LD50), oral rat chronic toxicity (LOAEL), hepatotoxicity, and skin sensitization of the compounds. We also performed the total clearance of the compounds using the pkCSM server. The findings are shown in Table 2.

Molecular docking
The docking results of the selected compounds are shown in Table 3.

The overall binding energy for all compounds was satisfactory. The binding energies range from -4.2 to -9.4. However, the highest docking score for bauchampine A was with BTK (-7.2 kcal/mol), betulinic acid with CCL20 (-8.8 kcal/mol), curcumol with TNF-α (-8.8 kcal/mol), geniposide with TNF-α (-8.7 kcal/mol), glycyrrhetinic acid with BTK (-9.4 kcal/mol), and papeoniflorin with BTK (-8.7 kcal/mol).
The ligand-receptor interactions of the highest scores are shown in Figure 2. Bauchampine A is bound to FKBP1A, CASP8, BTK, IL-6, CCL20, CXCL12, and TNF-α by forming a conventional hydrogen bond with LYS(A:18) and ARG(A:19); ALA(A:155) and ARG(A:155); MET(A:477) and GLU(A:475); LYS(A:86), GLU(A:93), ASP(A:140) and ASN(A:63); ARG(E:13), LYS(D:44) and THR(D:41); GLN(E:63), GLN(E:59), ILE(B:28), and ASN(B:30); LYS(B:98), LYS(A:98), TYR(C:115) and SER(A:99), respectively. In addition, the carbonyl group formed a hydrogen bond with GLU(A:62), ALA(A:159) and LEU(A:149), MET(A:477), PRO(A:139), LYS(C:44) of FKBP1A, CASP8, BTK, IL-6, and CCL20, respectively. The other bonds between bauchampine A and the receptors include unfavorable donor, pi-alkyl, pi donor hydrogen bond, pi-pi stacked interaction, and pi anion interaction. The detailed interaction of the other ligands and receptors is compiled in Table 4.



Discussion
The hands and feet are the main areas affected by RA, a systemic poly-articular chronic inflammatory joint disease. Pathological manifestations of RA include pannus development, inflammatory cell infiltration, hyperplasia of the synovial membrane, and degeneration of the bone and cartilage in the joints. We studied six terpenoid compounds from Bangladeshi plants as potential candidates for anti-RA agents. The seven targets were selected as they have a profound role in RA’s pathogenesis. Lipinski’s criteria were supported by an analysis of the docked drugs’ pharmacokinetics, which included examining their ADME characteristics. Toxicity tests were conducted on the substance to evaluate the chemical’s possible harm to humans and animals.
ADME screening is necessary for a compound to meet the quality standards required to be considered a prospective drug candidate. A medicine candidate with a large molecular weight may have less permeability through a biological barrier. The lipophilicity of the target molecule is shown by the logarithm of its distribution coefficient among the hydrophilic and lipophilic phases. The log P indicates the degree to which the body captivates the drug. The degree to which the body absorbs a medicine is inversely correlated with its log P. Betulinic acid showed the highest log P (3.79) and bauchampine A possessed the lowest value (1.54). The log S value indicates the pharmaceutical candidate’s solubility. The lowest log S value was found for betulinic acid (-7.71) and the highest value was shown by geniposide (-0.38). A drug’s ability to function as both an acceptor and a donor of hydrogen bonds determines how easily it can pass through a cell membrane. Rotatable bond numbers enable bioavailability; the optimal range for these values lies within 10. All the compounds showed rotatable bond numbers within this limit.
A substance’s toxicity is its capacity to cause harm to people or animals. Typically, animal models are employed to evaluate toxicity. This model is a lengthy and costly process. Because the in-silico method is inexpensive, short-lived, and does not need any animal object, it helps establish a compound’s toxicity profile for drug development. The toxicological test servers (pkCSM) ascertain whether a medicine is carcinogenic. The studied compounds showed no toxicity in ‘AMES toxicity’ profiling; all were safe in hepatotoxicity testing except betulinic acid, and only curcumol showed a skin sensitization effect.
To help execute rational drug design, molecular docking techniques can be utilized to determine the binding affinities of different ligands for the target protein structure. This method can help comprehend the dynamics of interactions and possible binding mechanisms, which can be used to apply more stringent inhibition [39]. The docking scores of these compounds revealed excellent binding properties with the targets. Individual compounds have distinct binding affinity with different targets. The BTK has the highest scores for the compounds. The bonds among the compounds and the receptors are also diverse.
BTK plays a vital role in mononuclear cells of the innate immune system, especially in dendritic cells and macrophages. Moreover, it is a direct regulator of the NLRP3 inflammasome, a key innate inflammatory machinery [40]. Studies revealed that inhibition of BTK resulted in the downregulation of B cells and thus reduced RA-mediated inflammation [41, 42]. In our study, bauchampine A, glycyrrhetinic acid, and papeoniflorin showed the most significant binding affinity with BTK. These compounds have a selective affinity to BTK, which can be used to inhibit the protein. There is growing interest in inhibiting BTK for anti-RA medication development [43], so these compounds may serve as potential candidates.
Another crucial inflammatory cytokine in this study is TNF-α. Excessive upregulation of TNF-α is associated with chronic inflammation that can result in autoimmune diseases [44, 45]. Blocking the destructive activity of TNF has been used in the treatment of RA for several years [46, 47]. Curcumol and geniposide showed the highest binding energy score with TNF-α, which is also promising for blocking this pathway [48, 49].
The chemokines are actively involved in inflammatory disorders, and CCL20 is a vital chemokine [50]. The binding energy of betulinic acid with CCL20 was the highest. The role of CCL20 in the pathogenesis of RA is unclear, though the patients contain higher levels of the chemokine than healthy individuals [51, 52]. The compound has the most affinity to the chemokine than other proteins.
Conclusion
Plant-derived terpenoid compounds can serve as effective leads for developing anti-RA medications. In this study, terpenoid molecules were tested against several proteins engaged in RA pathogenesis. This study revealed the tested compounds’ binding site and affinity for their targets. The compounds showed significant binding scores with different receptor proteins. Glycyrrhetinic acid showed the highest binding scores with BTK. The pharmacokinetic properties were also evaluated to confirm their drug-likeness. Almost every compound showed potential drug-like pharmacokinetic parameters. The toxicity profiling of the terpenoids also showed auspicious results. The present study may aid the development of effective drugs for rheumatic arthritis.
Limitations
The computational approaches used in this study have several limitations that impact their effectiveness. In this study, the accuracy of predictions is often constrained by simplified models that may not fully capture complex molecular interactions or the dynamic nature of biological systems. Additionally, predicting critical drug properties like absorption, distribution, metabolism, excretion, and toxicity remains challenging and prone to errors. Moreover, the results from in silico studies require experimental validation, which can reveal discrepancies and unforeseen issues, such as drug-drug interactions or off-target effects, that were not predicted. Further in-depth research, as well as in vitro and in vivo investigations, should be conducted to get above the study’s constraints and enhance the computational techniques employed.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Md Monirul Islam; Methodology: Iqbal Mahmud, and Md Habibur Rahaman; Data analysis: Md Monirul Islam, and Iqbal Mahmud; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to all related to this work.
References
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365(23):2205-19. [DOI:10.1056/nejmra1004965] [PMID]
- van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018; 32(2):174-187. [DOI:10.1016/j.berh.2018.10.005] [PMID]
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017; 389(10086):2338-48. [DOI:10.1016/s0140-6736(17)31491-5] [PMID]
- Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur J Med Chem. 2018; 158:502-16. [DOI:10.1016/j.ejmech.2018.09.027] [PMID]
- Min HK, Kim SH, Kim HR, Lee SH. Therapeutic utility and adverse effects of biologic disease-modifying anti-rheumatic drugs in inflammatory arthritis. Int J Mol Sci. 2022; 23(22):13913. [DOI:10.3390/ijms232213913] [PMID] [PMCID]
- Luo C, Xu X, Wei X, Feng W, Huang H, Liu H, et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol Res. 2019; 148:104409. [DOI:10.1016/j.phrs.2019.104409] [PMID]
- Voon FL, Sulaiman MR, Akhtar MN, Idris MF, Akira A, Perimal EK, et al. Cardamonin (2',4'-dihydroxy-6'-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol. 2017; 794:127-34. [DOI:10.1016/j.ejphar.2016.11.009] [PMID]
- Pu J, Fang FF, Li XQ, Shu ZH, Jiang YP, Han T, et al. Matrine exerts a strong anti-arthritic effect on type ii collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci. 2016; 17(9):1410. [DOI:10.3390/ijms17091410] [PMID] [PMCID]
- Doss HM, Ganesan R, Rasool M. Trikatu, an herbal compound ameliorates rheumatoid arthritis by the suppression of inflammatory immune responses in rats with adjuvant-induced arthritis and on cultured fibroblast like synoviocytes via the inhibition of the NFκB signaling pathway. Chem Biol Interact. 2016; 258:175-86. [DOI:10.1016/j.cbi.2016.09.003] [PMID]
- Xu T, Liu S, Zhao J, Feng G, Pi Z, Song F, et al. A study on the effective substance of the Wu-tou formula based on the metabonomic method using UPLC-Q-TOF-HDMS. Mol Biosyst. 2015; 11(11):3081-91. [DOI:10.1039/c5mb00454c] [PMID]
- Azmir J, Zaidul IS, Rahman MM, Sharif KM, Mohamed A, Sahena F, et al. Techniques for extraction of bioactive compounds from plant materials: A review. J Food Eng. 2013; 117(4):426-36. [DOI:10.1016/j.jfoodeng.2013.01.014]
- Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in pharmacological activities of terpenoids. Nat Prod Commun. 2020; 15(3):1934578X20903555. [DOI:10.1177/1934578X20903555]
- Morris GM, Lim-Wilby M. Molecular docking. Methods Mol Biol. 2008; 443:365-82. [DOI:10.1007/978-1-59745-177-2_19] [PMID]
- Carvalho AMS, Heimfarth L, Santos KA, Guimarães AG, Picot L, Almeida JRGS, et al. Terpenes as possible drugs for the mitigation of arthritic symptoms-A systematic review. Phytomedicine. 2019; 57:137-47. [DOI:10.1016/j.phymed.2018.10.028] [PMID]
- Del Prado-Audelo ML, Cortés H, Caballero-Florán IH, González-Torres M, Escutia-Guadarrama L, Bernal-Chávez SA, et al. Therapeutic applications of terpenes on inflammatory diseases. Front Pharmacol. 2021; 12:704197. [DOI:10.3389/fphar.2021.704197] [PMID] [PMCID]
- Ge J, Liu Z, Zhong Z, Wang L, Zhuo X, Li J,et al. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg Chem. 2022; 124:105817. [DOI:10.1016/j.bioorg.2022.105817] [PMID]
- Faustino C, Pinheiro L, Duarte N. Triterpenes as potential drug candidates for rheumatoid arthritis treatment. Life. 2023; 13(7):1514. [DOI:10.3390/life13071514] [PMID] [PMCID]
- Li N, Gong Z, Li X, Ma Q, Wu M, Liu D, et al. Betulinic acid inhibits the migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Int Immunopharmacol. 2019; 67:186-93. [DOI:10.1016/j.intimp.2018.11.042] [PMID]
- Wang H, Wang Y, Jiang X, Wang Z, Zhong B, Fang Y. The molecular mechanism of curcumol on inducing cell growth arrest and apoptosis in Jurkat cells, a model of CD4+T cells. Int Immunopharmacol. 2014; 21(2):375-82. [DOI:10.1016/j.intimp.2014.05.021] [PMID]
- Wang R, Wu H, Chen J, Li SP, Dai L, Zhang ZR, et al. Antiinflammation effects and mechanisms study of geniposide on rats with collagen-induced arthritis. Phytother Res. 2017; 31(4):631-7. [DOI:10.1002/ptr.5775] [PMID]
- Li F, Dai M, Wu H, Deng R, Fu J, Zhang Z, et al. Immunosuppressive effect of geniposide on mitogen-activated protein kinase signalling pathway and their cross-talk in fibroblast-like synoviocytes of adjuvant arthritis rats. Molecules. 2018; 23(1):91. [DOI:10.3390/molecules23010091] [PMID] [PMCID]
- Jia Z, He J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp Ther Med. 2016; 11(2):655-9. [DOI:10.3892/etm.2015.2908] [PMID] [PMCID]
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res. 2023; 51(D1):D1373-80. [PMID]
- Li XZ, Zhang SN. Herbal compounds for rheumatoid arthritis: Literatures review and cheminformatics prediction. Phytother Res. 2020; 34(1):51-66. [DOI:10.1002/ptr.6509] [PMID]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7:42717. [DOI:10.1038/srep42717] [PMID] [PMCID]
- Krewski D, Acosta D Jr, Andersen M, Anderson H, Bailar JC 3rd, Boekelheide K, et al. Toxicity testing in the 21st century: a vision and a strategy. J Toxicol Environ Health B Crit Rev. 2010; 13(2-4):51-138. [DOI:10.1080/10937404.2010.483176] [PMID] [PMCID]
- Pires DE, Blundell TL, Ascher DB. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015; 58(9):4066-72. [DOI:10.1021/acs.jmedchem.5b00104] [PMID] [PMCID]
- Radner H, Aletaha D. Anti-TNF in rheumatoid arthritis: An overview. Wien Med Wochenschr. 2015; 165(1-2):3-9. [DOI:10.1007/s10354-015-0344-y] [PMID]
- Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007; 37(Suppl 1):S34-45. [DOI:10.1002/eji.200737772] [PMID] [PMCID]
- Szekanecz Z, Vegvari A, Szabo Z, Koch AE. Chemokines and chemokine receptors in arthritis. Front Biosci. 2010; 2(1):153-67. [DOI:10.2741/s53] [PMID] [PMCID]
- Yang MH, Wu FX, Xie CM, Qing YF, Wang GR, Guo XL, et al. Expression of CC chemokine ligand 5 in patients with rheumatoid arthritis and its correlation with disease activity and medication. Chin Med Sci J. 2009; 24(1):50-4. [DOI:10.1016/s1001-9294(09)60059-6] [PMID]
- Ansalone C, Ainsworth RI, Nygaard G, Ai R, Prideaux EB, Hammaker D, et al. Caspase-8 variant g regulates rheumatoid arthritis fibroblast-like synoviocyte aggressive behavior. ACR Open Rheumatol. 2022; 4(4):288-99. [DOI:10.1002/acr2.11384] [PMID] [PMCID]
- Schafer PH, Kivitz AJ, Ma J, Korish S, Sutherland D, Li L, et al. Spebrutinib (CC-292) affects markers of B cell activation, chemotaxis, and osteoclasts in patients with rheumatoid arthritis: Results from a mechanistic study. Rheumatol Ther. 2020; 7(1):101-19. [DOI:10.1007/s40744-019-00182-7] [PMID] [PMCID]
- Huang QL, Zhou FJ, Wu CB, Xu C, Qian WY, Fan DP, et al. Circulating biomarkers for predicting infliximab response in rheumatoid arthritis: A systematic bioinformatics analysis. Med Sci Monit. 2017; 23:1849-55. [DOI:10.12659/msm.900897] [PMID] [PMCID]
- PyMOL. The PyMOL Molecular Graphics System, Version 3.0 Schrödinger, LLC [Internet]. 2024[Updated 2024 January 10]. Available from: [Link]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis. 1997; 18(15):2714-23. [PMID]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015; 1263:243-50. [DOI:10.1007/978-1-4939-2269-7_19] [PMID]
- Dassault Syst’emes. BIOVIA Discovery Studio Visualizer, version 21.1.0. San Diego: Dassault Syst’emes; 2021. [Link]
- Liu Z, Liu Y, Zeng G, Shao B, Chen M, Li Z, et al. Application of molecular docking for the degradation of organic pollutants in the environmental remediation: A review. Chemosphere. 2018; 203:139-50. [DOI:10.1016/j.chemosphere.2018.03.179] [PMID]
- Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. Bruton's tyrosine kinase: an emerging key player in innate immunity. Front Immunol. 2017; 8:1454. [DOI:10.3389/fimmu.2017.01454] [PMID] [PMCID]
- Lv J, Wu J, He F, Qu Y, Zhang Q, Yu C. Development of bruton's tyrosine kinase inhibitors for rheumatoid arthritis. Curr Med Chem. 2018; 25(42):5847-59. [DOI:10.2174/0929867325666180316121951] [PMID]
- Arneson LC, Carroll KJ, Ruderman EM. Bruton's tyrosine kinase inhibition for the treatment of rheumatoid arthritis. Immunotargets Ther. 2021; 10:333-42. [DOI:10.2147%2FITT.S288550] [PMID] [PMCID]
- Akasaka D, Iguchi S, Kaneko R, Yoshiga Y, Kajiwara D, Nakachi Y, et al. Novel Bruton's tyrosine kinase inhibitor TAS5315 suppresses the progression of inflammation and joint destruction in rodent collagen-induced arthritis. Plos One. 2023; 18(2):e0282117. [DOI:10.1371/journal.pone.0282117] [PMID] [PMCID]
- Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021; 22(5):2719. [DOI:10.3390/ijms22052719] [PMID] [PMCID]
- Hassany AE, Tharwat S, Mansour M, Enein AF. TNF-α in the serum and synovial fluid of patients with rheumatoid arthritis: Correlation with sonographic parameters: A cross-sectional study. Egypt Rheumatol Rehabil. 2024; 51(1):22. [DOI:10.1186/s43166-024-00256-7]
- Zamri F, de Vries TJ. Use of TNF inhibitors in rheumatoid arthritis and implications for the periodontal status: For the benefit of both? Front Immunol. 2020; 11:591365. [DOI:10.3389/fimmu.2020.591365] [PMID] [PMCID]
- Ceban F, Xu J. The evolution of TNF-α blockade for the treatment of rheumatoid arthritis. J Undergrad Life Sci. 2022; 16(1):1-12. [DOI:10.33137/juls.v16i1.39048]
- Tarachand SP, Thirumoorthy G, Lakshmaiah VV, Nagella P. In silico molecular docking study of Andrographis paniculata phytochemicals against TNF-α as a potent anti-rheumatoid drug. J Biomol Struct Dyn. 2023; 41(7):2687-97. [DOI:10.1080/07391102.2022.2037463] [PMID]
- Agnihotri P, Deka H, Chakraborty D, Monu, Saquib M, Kumar U, Biswas S. Anti-inflammatory potential of selective small compounds by targeting TNF-α & NF-kB signaling: A comprehensive molecular docking and simulation study. J Biomol Struct Dyn. 2023; 41(23):13815-28. [DOI:10.1080/07391102.2023.2196692] [PMID]
- Murayama MA, Shimizu J, Miyabe C, Yudo K, Miyabe Y. Chemokines and chemokine receptors as promising targets in rheumatoid arthritis. Front Immunol. 2023; 14:1100869. [DOI:10.3389/fimmu.2023.1100869] [PMID] [PMCID]
- Wang L, Hong X, Du H. Association between serum chemokine ligand 20 levels and disease activity and Th1/Th2/Th17-related cytokine levels in rheumatoid Arthritis. J Interferon Cytokine Res. 2023; 43(11):512-7. [DOI:10.1089/jir.2023.0057] [PMID]
- Pournazari M, Feizollahi P, Roghani SA, Assar S, Soufivand P, Soleymani B, et al. Increased plasma levels of CCL20 in peripheral blood of rheumatoid arthritis patients and its association with clinical and laboratory parameters. Clin Rheumatol. 2022; 41(1):265-270. [DOI:10.1007/s10067-021-05899-x] [PMID]
Type of Study: Original Research |
Subject:
Ehtnopharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |