Volume 10, Issue 4 (2024)
Pharm Biomed Res 2024, 10(4): 345-354 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Begum K, Ripon M A R, Akter T, Islam M M, Begum R, Hossain M S. Investigating the Effects of Olanzapine in Changes in Metabolic Syndrome in High-fat diet-induced Obese Mice. Pharm Biomed Res 2024; 10 (4) :345-354
URL: http://pbr.mazums.ac.ir/article-1-629-en.html
URL: http://pbr.mazums.ac.ir/article-1-629-en.html
Khadiza Begum1 

 , Md Abdur Rahman Ripon1
, Md Abdur Rahman Ripon1 
 , Tabassum Akter1
, Tabassum Akter1 
 , Md Monirul Islam1
, Md Monirul Islam1 
 , Rahima Begum1
, Rahima Begum1 
 , Mohammad Salim Hossain *1
, Mohammad Salim Hossain *1 



 , Md Abdur Rahman Ripon1
, Md Abdur Rahman Ripon1 
 , Tabassum Akter1
, Tabassum Akter1 
 , Md Monirul Islam1
, Md Monirul Islam1 
 , Rahima Begum1
, Rahima Begum1 
 , Mohammad Salim Hossain *1
, Mohammad Salim Hossain *1 

1- Department of Pharmacy, Noakhali Science and Technology University, Noakhali, Bangladesh.
Full-Text [PDF 1185 kb]
(411 Downloads)
| Abstract (HTML) (920 Views)
Full-Text: (262 Views)
Introduction
The increased prevalence of obesity has become a major health problem worldwide for all ages. Obesity rates have risen significantly as a result of recent lifestyle changes. World Obesity Federation forecasts that if current trends continue, it will cost about US$ 1.2 trillion per year to treat the consequences of obesity by 2025 [1]. Obesity is linked to a higher risk of cardiovascular and all-cause mortality [2]. Increased adiposity increases the risk of insulin resistance, hypertension, hyperglycemia, and dyslipidemia [3]. As an endocrine organ, adipose tissue secretes a variety of hormones and messengers including adiponectin, resistin, angiotensin, leptin, tumor necrosis factor-alpha (TNF-α), and fatty acids that act at distant sites [4]. Leptin is released by adipose tissue in direct proportion to its bulk, and it binds to specific cell surface receptors in the hypothalamus, acting as a powerful inhibitor of food intake [4].
Antipsychotic medicines are widely used to treat mental diseases, such as bipolar disorder, delusional disorder, and schizophrenia. Second-generation antipsychotics (SGAs), also known as atypical antipsychotics, carry a reduced prevalence of extrapyramidal syndromes like dystonia, hypokinesia, tremors, and akathisia than do first-generation or typical antipsychotics [5]. Out of all SGAs, olanzapine (OLA) has the greatest impact on weight gain and metabolic alterations that may raise the risk of metabolic syndrome and associated diseases such as cardiovascular disease, prediabetes, and type 2 diabetes [6, 7]. It antagonizes dopamine receptor D2 and 5-HT receptor (5-HTR) 2A/C [8]. OLA is a regularly given medicine for the short-term treatment of acute psychosis, psychotic and manic-depressive disorders, and agitated states in delirium and dementia, as well as the long-term therapy of chronic psychotic disorders, such as schizophrenia [9]. However, some research reveals that OLA has severe metabolic side effects associated with obesity, including weight gain, increased adiposity, hepatosteatosis, hyperphagia, dyslipidemia, hyperglycemia, insulin resistance, increased fat oxidation and impairments in glucose homeostasis [10-12]. Thus, the prevalence of obesity is higher among people with schizophrenia than among healthy people due to the side effects of SGAs. However, if OLA and fat combine to disrupt metabolism, it may be very undesirable leading to further complications which are also supported by Townsend et al., [10]. The mechanisms behind metabolic disruption caused by SGAs are likely complex and include both the central and peripheral neural systems [13, 14].
Except for central nervous system study, little is known about the effects of OLA on peripheral organs, such as the liver and adipose tissue which are crucial for lipid metabolic homeostasis. Besides, how the status of metabolic disturbance shapes the metabolic reaction to prolonged OLA administration that changes liver function, lipid profiles, and obesity-mediated inflammation is an open question. Considering these clinically significant facts, this investigation evaluates the effect of OLA treatment on pre-existing metabolic disturbance in high-fat diet (HFD)-induced obese mice and observes the findings for future adaptation with metabolic disturbances.
Materials and Methods
Experimental animals and diets
A total number of 15 mature Swiss albino mice (female, weight=22-25 g) were used in this study. Mice were obtained from the animal house of the International Center for Diarrheal Disease Research, Bangladesh (ICDDR, B). The mice were housed in separate metal cages while maintaining constant environment and nutritional conditions and under controlled conditions of 12-h dark/light cycles. Through the particular nipple, unlimited supplies of clean, fresh drinking water were provided. Water and food were given to the mice twice a day and their cages were cleaned regularly. Seven days were dedicated to acclimatization before the start of the study. Every animal experiment was carried out at the Department of Pharmacy, Noakhali Science and Technology University, Bangladesh.
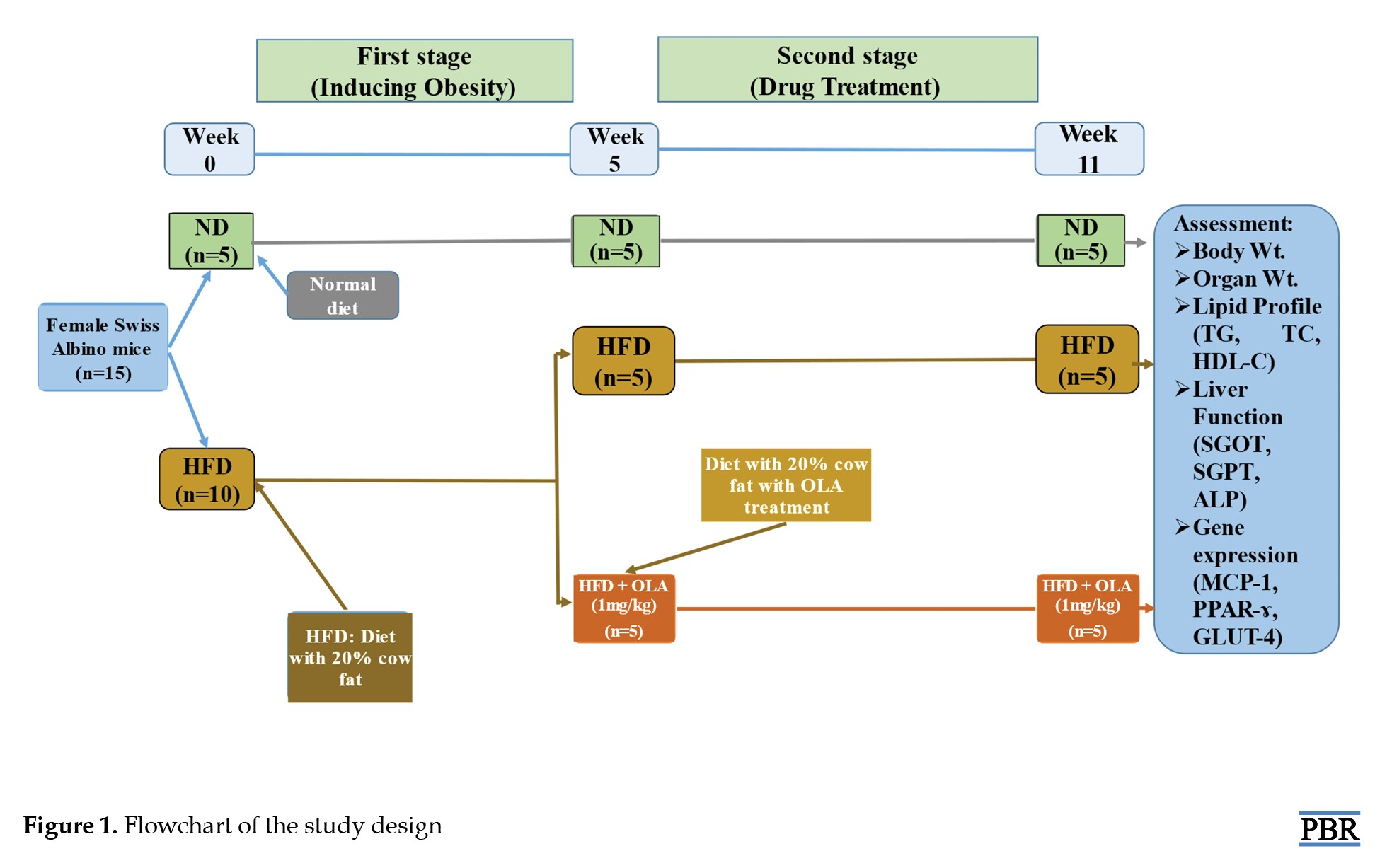
Study design
At first, a total of 15 mice (5 weeks old) were randomly categorized into two groups, namely the HFD group which consisted of 10 mice with HFD for five weeks in a row, and the normal diet (ND) group, which consisted of 5 mice given normal diets for five weeks in a row. The study design has been summarized and represented in Figure 1. Throughout the duration, the body weight of all mice was determined by a weight balance machine daily and the average weight of each group of mice was determined. After 5 weeks, the HFD group was further divided into two groups with 5 mice. One remained with the previous name HFD group. Another was renamed with HFD+OLA which was given 1 mg/kg dose of OLA via oral gavage for 6 weeks. All of these two groups were continued with regular HFD and the previous ND group was continued with normal diets. Accordingly, the overall mice groups after 5 weeks in this study design are as follows: Group 1 (n=5), including a control group, fed with only ND and water: Group 2 (n=5), including the HFD group, was provided with HFD (normal diet along with 20% cow fat) and water; and group 3 (n=5), including HFD with OLA (HFD + OLA) group, was given HFD along with OLA (1 mg/kg of body weight) and water.
Method of serum collection for biochemical tests and liver function tests
The mice’s heart was used to extract blood, which was then left to stand at room temperature for 30 min to allow it to clot. After that, blood samples were centrifuged in a centrifuge machine for 10 min at 2600 rounds per min (rpm) to extract serum for tests of liver function (aspartate transferase [AST]/serum glutamic-oxaloacetic transaminase [SGOT], alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase [SGPT], and alkaline phosphatase [ALP] level) and biochemical parameters (triglycerides [TG], total cholesterol [TC], and high-density lipoprotein cholesterol [HDL-C] level) according to the procedure applied in our previous study [15]. Before analysis, the serum was finally transferred into an Eppendorf tube and kept in a freezer at -80 ºC. Using Human Diagnostics kits (Germany) in a semi-automated chemistry analyzer (Mindray BA-88A), the test was conducted following the guidelines provided in the relevant manufacturing protocol.
Determination of organ weight
After 6 weeks of drug treatment, the mice were anesthetized by using chloroform and dissected by using a dissection box, and their abdominal fat was collected and weighed separately.
Extraction of messenger ribonucleic acid from adipose tissue, quantification and analysis
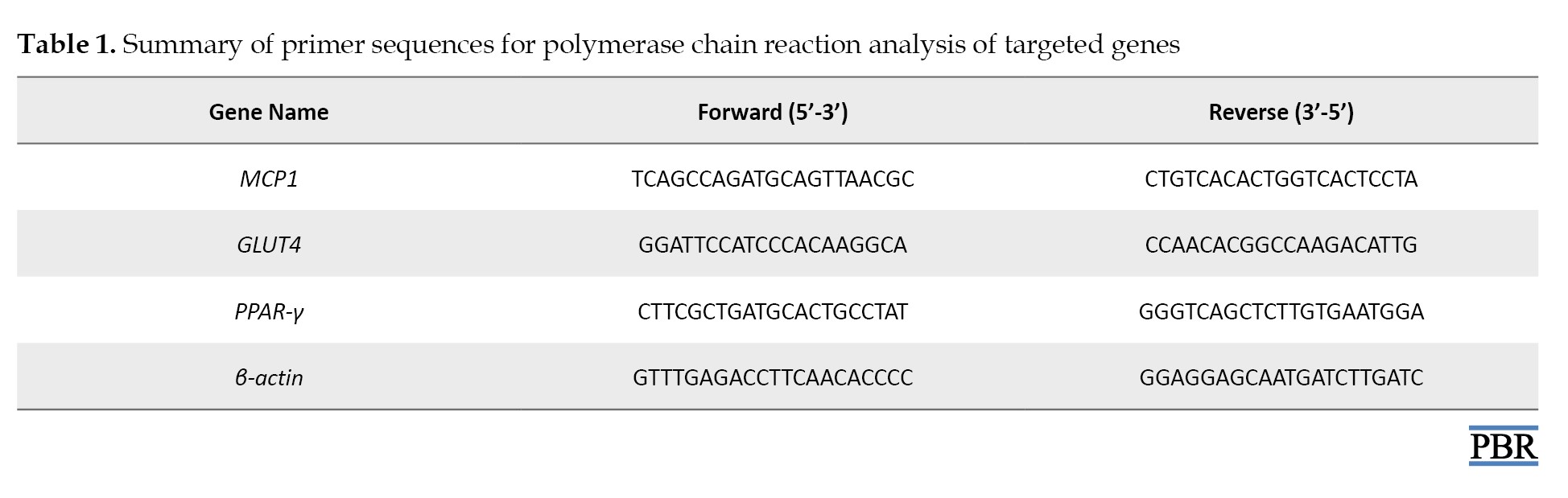
After 6 weeks of drug treatment, using the Trizol technique, messenger ribonucleic acid (mRNA) was extracted from mouse adipose tissue using Trizol T-reagent (SRL, India). Ribonucleic acid (RNA) was quantified using a Colibri Micro Volume Spectrometer (Titertek-Berthold, Germany). Protoscript-II (Biolabs Inc., New England) was then used to reverse transcribe 1 ug of RNA. Primers of a particular sequence were provided by Macrogen Inc. (Korea) (Table 1).
Quantitative polymerase chain reaction (PCR) was conducted in duplicate using the Luna Universal real-time PCR (qPCR) Master mix (Bio labs Inc. New England) in the CFX96 Touch real-time PCR detection system (Bio-Rad, US) for 40 cycles. Relative mRNA expression for each gene was calculated using the ΔΔCT method, normalized to β-actin [16-18].
Results
Development of obese mice and confirmation of obesity
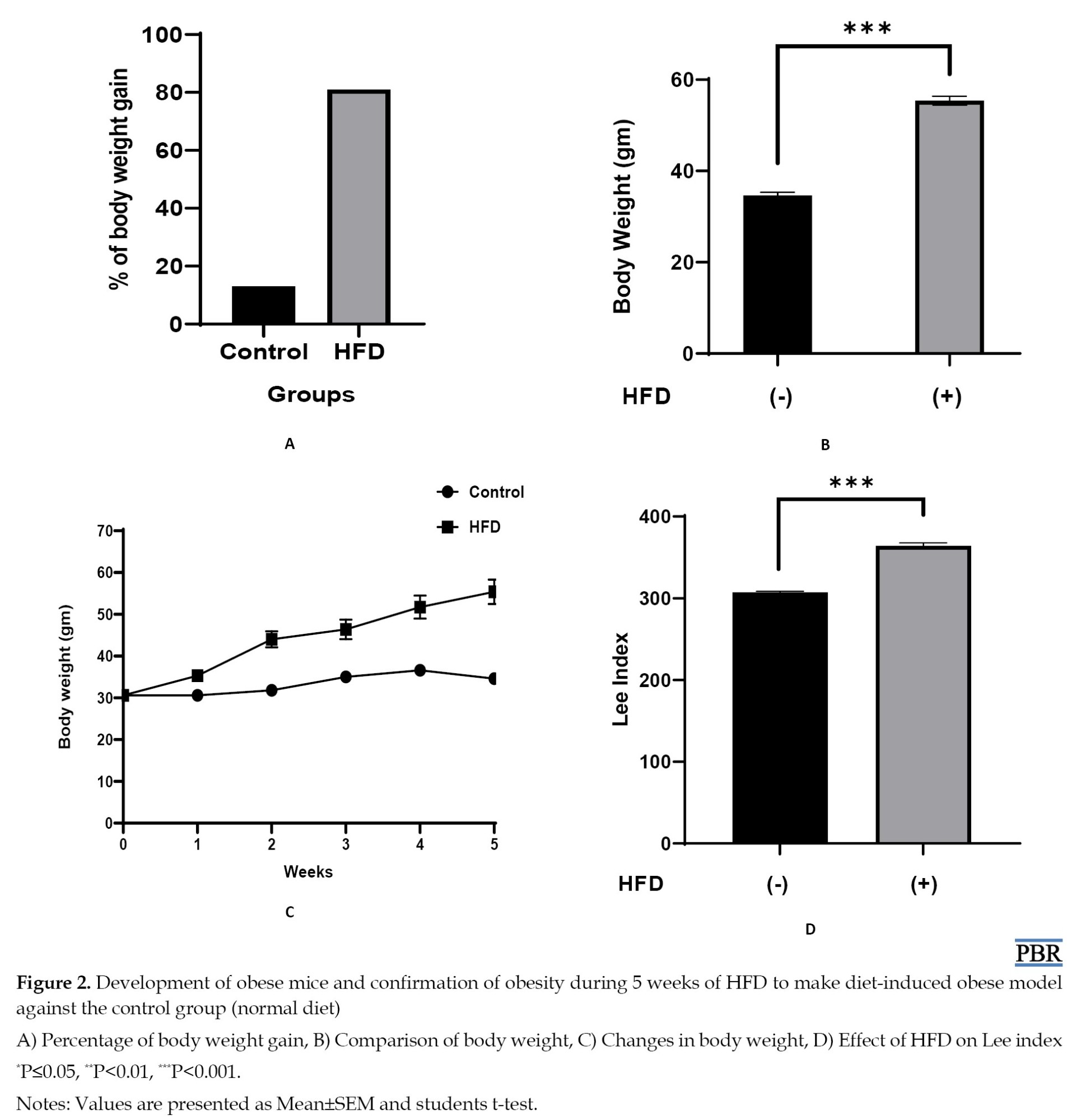
The weight of mice increased every week and at the end of the fifth week, obesity was established. After 5 weeks, the HFD group showed a significant increase in body weight than the control group (ND). For confirmation of the mice as obese, we analyzed the Lee index values and also the p-value of Lee index values of HFD groups compared to the control group. Lee considered values greater than 310 as an indicator of obesity. So, mice with Lee index ≥310 were considered as obese [19]. We found that the Lee index was higher when mice fed with HFD significantly (364) compared to the control (307) confirming the mice with HFD as obese and the control mice as non-obese. Data are presented in Figure 2.
Effect of OLA on Body weight and organ weight
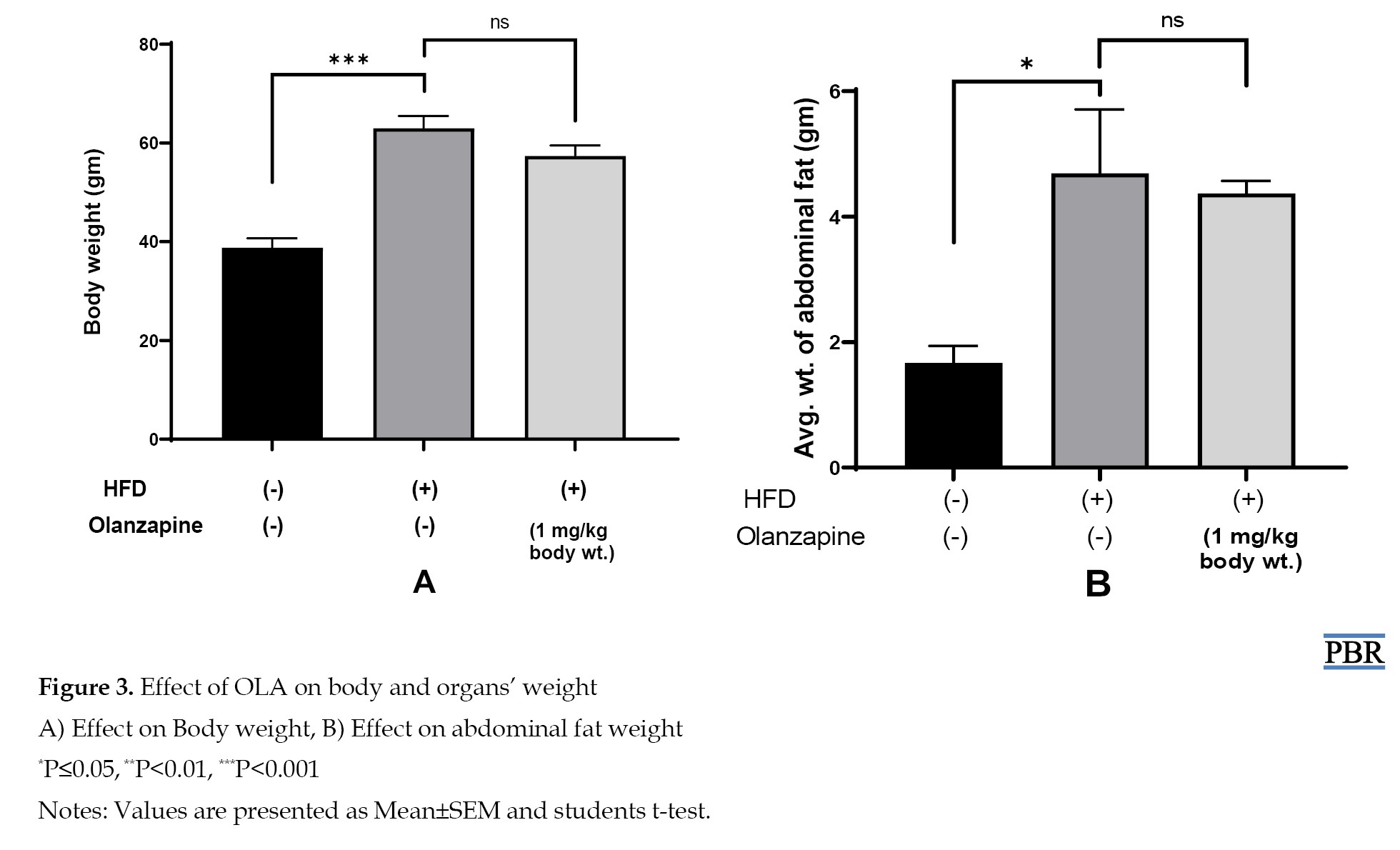
After 6 weeks of the experiment, the body weight of the mice was measured and then the mice were dissected and abdominal fat was weighed. The result of the study indicates that after drug treatment, body weight, and abdominal fat weight were increased significantly in the obese (HFD) control group than the normal diet group. But drug treatment group showed a non-significant decreasing trend in the parameters than the obese (HFD) control group. Data are presented in Figure 3.
Effect of OLA on lipid profiles
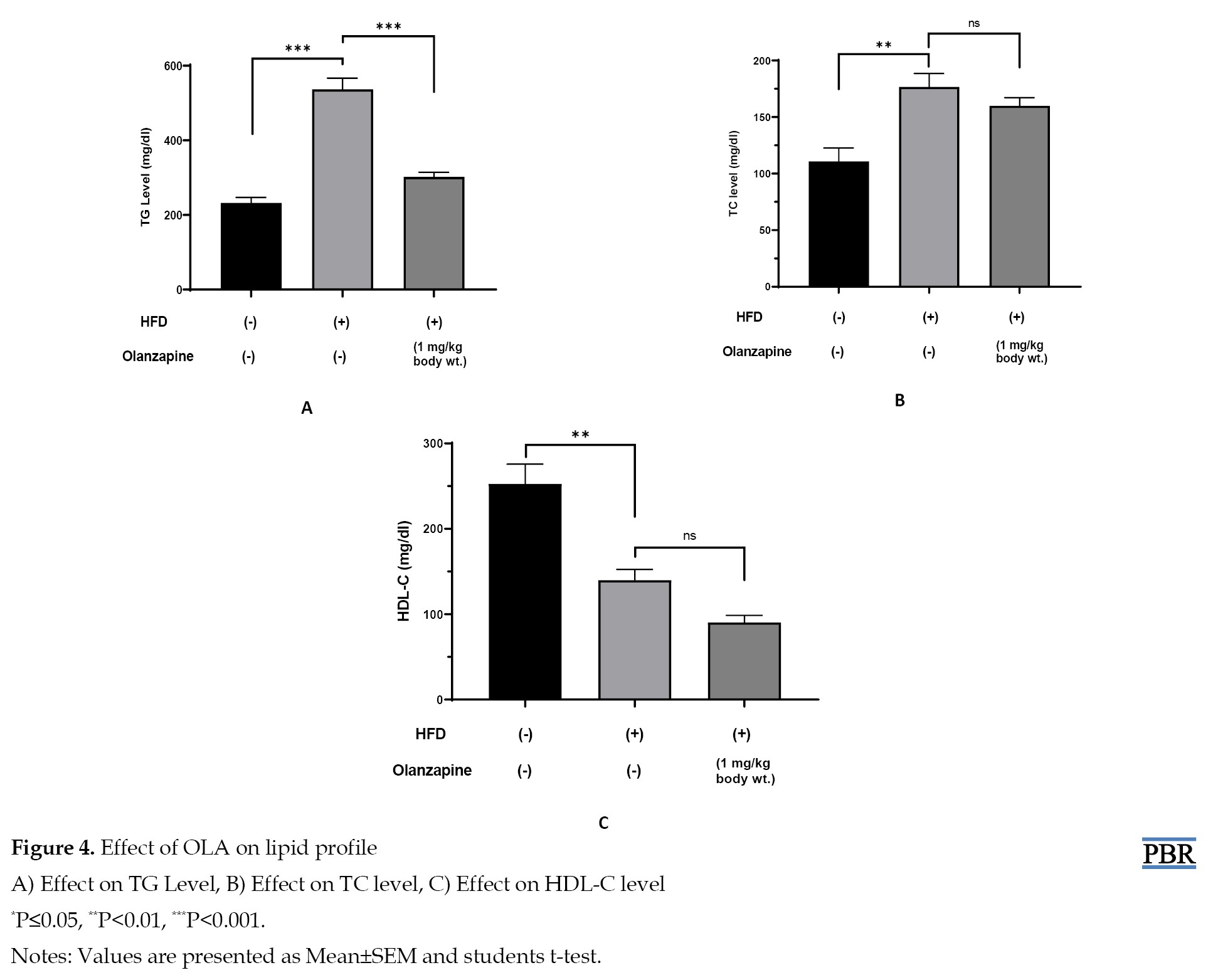
This study indicates that after 6 weeks of drug treatment, the HFD group showed a significant increase in TG and TC levels than the control group, and a significant level of declination was noticed in the HDL-C level than the control group. On the other hand, the OLA-treatment group showed a significant decrease in TG level but a non-significant decrease was noticed in TC and HDL-C levels when compared with the HFD group. Data are shown in Figure 4.
Effect of OLA on liver function
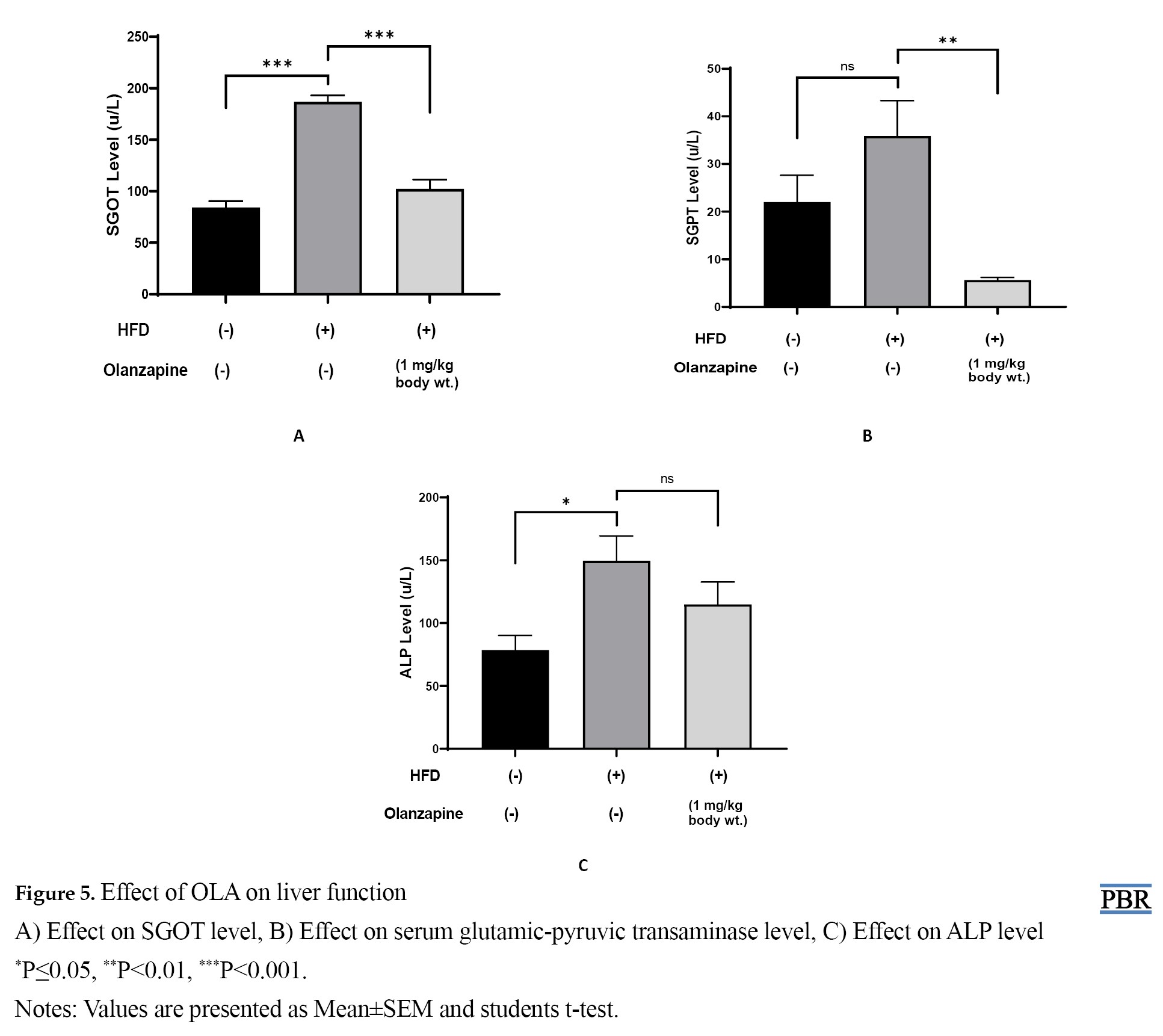
The present study indicates that after 6 weeks of drug treatment, the HFD group showed a significant increase in SGOT and ALP levels but a non-significant increase in SGPT levels compared to the control group. On the other hand, the OLA treatment group showed a significant decrease in SGOT and SGPT but a non-significant decrease in ALP levels when compared with the HFD group. Data are presented in Figure 5.
Effect of OLA on relative gene expression of MCP-1, PPAR-γ, and GLUT4
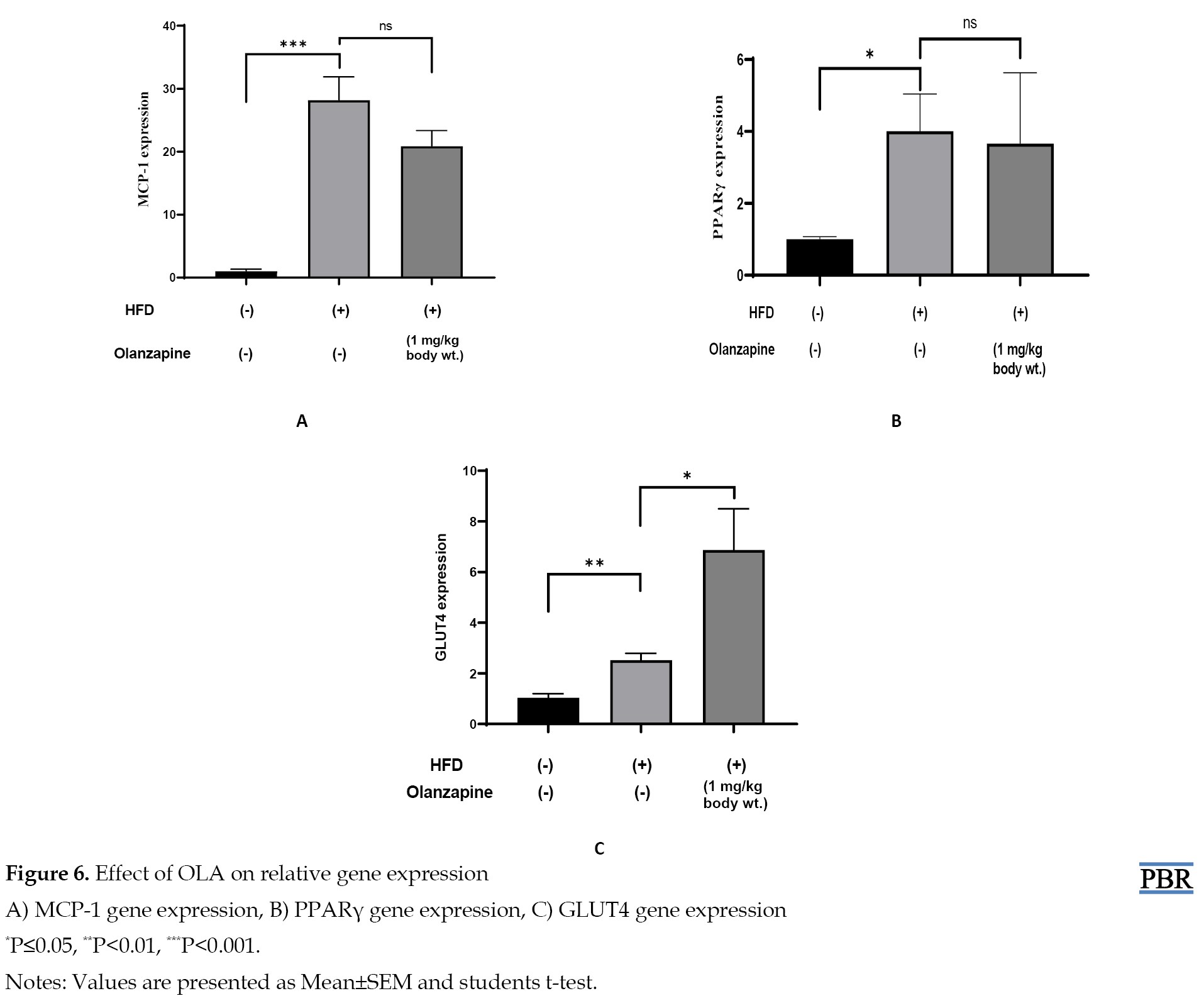
Using real-time RT-PCR and RNA isolated from adipose tissues of age- and gender-matched obese mice, we examined the expression of the MCP-1, PPAR-γ, and GLUT4 genes in vivo. In comparison to ND mice, HFD mice had a noticeably higher basal level of MCP-1 expression in their adipose tissue and a non-significant decrease in the OLA treatment group compared with the HFD group. On the other side, PPAR-γ expression in the adipose tissue was significantly elevated in HFD mice compared with ND mice and non-significantly decreased in the OLA treatment group compared with the HFD group. The result of the present study also indicates that HFD-fed mice showed a significant increase in GLUT4 expression compared to the control group and the OLA-treatment group also showed significant increasing trend in GLUT4 expression when compared with HFD group. Data are presented in Figure 6.
Discussion
Obesity as an emerging health problem is developing various chronic diseases [20, 21]. As both humans and rodents have a similar tendency to gain weight with high-calorie intake [22], we also observed that an increase in body weight significantly occurred confirming obesity by Lee index when mice fed with HFD than the control group. Besides, significant changes in body and organ weight, lipid profile, liver functions, and relative gene expressions confirmed obesity in HFD-induced obese mice. The findings in the OLA-treated group indicate a decreasing trend in body and organ weight. The OLA treatment group showed significantly less amount of serum TG than the HFD group. Serum TC is decreased after treatment but there is also a non-significant decrease in HDL-C level in the treated mice. Zhang et al. stated that OLA exerted minor effects on lipids in mice with obesity [11]. Our study also supported this but exhibited an overall lipid-lowering effect. Since SGOT, SGPT, and ALP are leakage enzymes, their elevated levels in the blood indicate severe hepatocellular injury [23]. However, in this study in case of HFD-fed mice showed a significant level of elevation in SGOT, SGPT, and ALP enzymes than the control group but a significant level of declination of them was noticed after OLA treatment. Elevated levels of serum SGOT, SGPT, and ALP in HFD-fed mice, a reliable indicator of liver damage, were dropped following OLA exposure supporting that chronic OLA is associated with declination in necrosis in liver cells.
In this study, OLA in HFD-fed mice non-significantly decreases MCP-1 mRNA expression by reducing MCP-1 gene transcription compared to only HFD-treated mice. We speculate that such effects may contribute to the salutary effect of OLA in hepatic steatosis and many other metabolic syndromes in the context of pre-existing obesity. Reduction in MCP-1 level also indicates anti-inflammatory effects of OLA exposure. The result of this study also showed a significant upregulation of PPAR-γ in HFD-fed mice than the control group and a decreasing trend non-significantly in the OLA treatment group. In this research, GLUT-4 expression showed a significant increase after OLA treatment. Notably, multiple investigations in mice/rats on HFD have found increased weight gain and metabolic disruption following acute OLA treatment [10, 24]. One possible explanation for the difference between our results and those of other research could be the significantly shorter HFD duration in those earlier trials. According to a different study, long-term exposure to OLA reduced adiposity and hepatic steatosis in obese mice [11]. This evidence supports our findings in HFD-induced obese mice, where OLA was not associated with exacerbated metabolic syndrome in female mice. However, this study suggests that when obesity is established, OLA exposure is related to reduced adiposity accumulation and liver injury. Finally, OLA exposure presents anti-obesity effects including the reduced gene expression of MCP-1 as an anti-inflammatory effect mainly. Moreover, to enhance the current study’s therapeutic significance and to have a deeper understanding of OLA’s metabolic effect, it is recommended for future research employing a larger mouse population.
Conclusion
Following prolonged exposure to OLA, the results reveal a decrease in body and organ weight, lipid levels, liver function markers, and the expression of MCP-1 and PPAR-ɣ, with the exception of GLUT4. These changes suggest that OLA influences metabolic disturbances in obese mice. Moreover, to enhance the current study’s therapeutic significance and to have a deeper understanding of OLA’s metabolic effect, it is recommended for future research employing a larger mouse population.
Ethical Considerations
Compliance with ethical guidelines
The study was reviewed and approved by the Ethics Committee of Noakhali Science and Technology University (NSTUEC), Noakhali, Bangladesh (Code: NSTU/SCI/EC/2023/158, August 03, 2023).
Funding
This work was supported partly by the Research Cell, at Noakhali Science and Technology University, Noakhali, Bangladesh.
Authors' contributions
Conceptualisation and supervision: Mohammad Salim Hossain; Study design: Tabassum Akter and Khadiza Begum; Experiments: Khadiza Begum; Data collection: Rahima Begum; Data analysis: Tabassum Akter, Md. Monirul Islam, Khadiza Begum, and Md Abdur Rahman Ripon; Writing the original draft: Md Abdur Rahman Ripon and Rahima Begum; Review and editing: Md Monirul Islam and Mohammad Salim Hossain; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to all related to this study.
References
The increased prevalence of obesity has become a major health problem worldwide for all ages. Obesity rates have risen significantly as a result of recent lifestyle changes. World Obesity Federation forecasts that if current trends continue, it will cost about US$ 1.2 trillion per year to treat the consequences of obesity by 2025 [1]. Obesity is linked to a higher risk of cardiovascular and all-cause mortality [2]. Increased adiposity increases the risk of insulin resistance, hypertension, hyperglycemia, and dyslipidemia [3]. As an endocrine organ, adipose tissue secretes a variety of hormones and messengers including adiponectin, resistin, angiotensin, leptin, tumor necrosis factor-alpha (TNF-α), and fatty acids that act at distant sites [4]. Leptin is released by adipose tissue in direct proportion to its bulk, and it binds to specific cell surface receptors in the hypothalamus, acting as a powerful inhibitor of food intake [4].
Antipsychotic medicines are widely used to treat mental diseases, such as bipolar disorder, delusional disorder, and schizophrenia. Second-generation antipsychotics (SGAs), also known as atypical antipsychotics, carry a reduced prevalence of extrapyramidal syndromes like dystonia, hypokinesia, tremors, and akathisia than do first-generation or typical antipsychotics [5]. Out of all SGAs, olanzapine (OLA) has the greatest impact on weight gain and metabolic alterations that may raise the risk of metabolic syndrome and associated diseases such as cardiovascular disease, prediabetes, and type 2 diabetes [6, 7]. It antagonizes dopamine receptor D2 and 5-HT receptor (5-HTR) 2A/C [8]. OLA is a regularly given medicine for the short-term treatment of acute psychosis, psychotic and manic-depressive disorders, and agitated states in delirium and dementia, as well as the long-term therapy of chronic psychotic disorders, such as schizophrenia [9]. However, some research reveals that OLA has severe metabolic side effects associated with obesity, including weight gain, increased adiposity, hepatosteatosis, hyperphagia, dyslipidemia, hyperglycemia, insulin resistance, increased fat oxidation and impairments in glucose homeostasis [10-12]. Thus, the prevalence of obesity is higher among people with schizophrenia than among healthy people due to the side effects of SGAs. However, if OLA and fat combine to disrupt metabolism, it may be very undesirable leading to further complications which are also supported by Townsend et al., [10]. The mechanisms behind metabolic disruption caused by SGAs are likely complex and include both the central and peripheral neural systems [13, 14].
Except for central nervous system study, little is known about the effects of OLA on peripheral organs, such as the liver and adipose tissue which are crucial for lipid metabolic homeostasis. Besides, how the status of metabolic disturbance shapes the metabolic reaction to prolonged OLA administration that changes liver function, lipid profiles, and obesity-mediated inflammation is an open question. Considering these clinically significant facts, this investigation evaluates the effect of OLA treatment on pre-existing metabolic disturbance in high-fat diet (HFD)-induced obese mice and observes the findings for future adaptation with metabolic disturbances.
Materials and Methods
Experimental animals and diets
A total number of 15 mature Swiss albino mice (female, weight=22-25 g) were used in this study. Mice were obtained from the animal house of the International Center for Diarrheal Disease Research, Bangladesh (ICDDR, B). The mice were housed in separate metal cages while maintaining constant environment and nutritional conditions and under controlled conditions of 12-h dark/light cycles. Through the particular nipple, unlimited supplies of clean, fresh drinking water were provided. Water and food were given to the mice twice a day and their cages were cleaned regularly. Seven days were dedicated to acclimatization before the start of the study. Every animal experiment was carried out at the Department of Pharmacy, Noakhali Science and Technology University, Bangladesh.
Study design
At first, a total of 15 mice (5 weeks old) were randomly categorized into two groups, namely the HFD group which consisted of 10 mice with HFD for five weeks in a row, and the normal diet (ND) group, which consisted of 5 mice given normal diets for five weeks in a row. The study design has been summarized and represented in Figure 1. Throughout the duration, the body weight of all mice was determined by a weight balance machine daily and the average weight of each group of mice was determined. After 5 weeks, the HFD group was further divided into two groups with 5 mice. One remained with the previous name HFD group. Another was renamed with HFD+OLA which was given 1 mg/kg dose of OLA via oral gavage for 6 weeks. All of these two groups were continued with regular HFD and the previous ND group was continued with normal diets. Accordingly, the overall mice groups after 5 weeks in this study design are as follows: Group 1 (n=5), including a control group, fed with only ND and water: Group 2 (n=5), including the HFD group, was provided with HFD (normal diet along with 20% cow fat) and water; and group 3 (n=5), including HFD with OLA (HFD + OLA) group, was given HFD along with OLA (1 mg/kg of body weight) and water.
Method of serum collection for biochemical tests and liver function tests
The mice’s heart was used to extract blood, which was then left to stand at room temperature for 30 min to allow it to clot. After that, blood samples were centrifuged in a centrifuge machine for 10 min at 2600 rounds per min (rpm) to extract serum for tests of liver function (aspartate transferase [AST]/serum glutamic-oxaloacetic transaminase [SGOT], alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase [SGPT], and alkaline phosphatase [ALP] level) and biochemical parameters (triglycerides [TG], total cholesterol [TC], and high-density lipoprotein cholesterol [HDL-C] level) according to the procedure applied in our previous study [15]. Before analysis, the serum was finally transferred into an Eppendorf tube and kept in a freezer at -80 ºC. Using Human Diagnostics kits (Germany) in a semi-automated chemistry analyzer (Mindray BA-88A), the test was conducted following the guidelines provided in the relevant manufacturing protocol.
Determination of organ weight
After 6 weeks of drug treatment, the mice were anesthetized by using chloroform and dissected by using a dissection box, and their abdominal fat was collected and weighed separately.
Extraction of messenger ribonucleic acid from adipose tissue, quantification and analysis
After 6 weeks of drug treatment, using the Trizol technique, messenger ribonucleic acid (mRNA) was extracted from mouse adipose tissue using Trizol T-reagent (SRL, India). Ribonucleic acid (RNA) was quantified using a Colibri Micro Volume Spectrometer (Titertek-Berthold, Germany). Protoscript-II (Biolabs Inc., New England) was then used to reverse transcribe 1 ug of RNA. Primers of a particular sequence were provided by Macrogen Inc. (Korea) (Table 1).

Quantitative polymerase chain reaction (PCR) was conducted in duplicate using the Luna Universal real-time PCR (qPCR) Master mix (Bio labs Inc. New England) in the CFX96 Touch real-time PCR detection system (Bio-Rad, US) for 40 cycles. Relative mRNA expression for each gene was calculated using the ΔΔCT method, normalized to β-actin [16-18].
Results
Development of obese mice and confirmation of obesity
The weight of mice increased every week and at the end of the fifth week, obesity was established. After 5 weeks, the HFD group showed a significant increase in body weight than the control group (ND). For confirmation of the mice as obese, we analyzed the Lee index values and also the p-value of Lee index values of HFD groups compared to the control group. Lee considered values greater than 310 as an indicator of obesity. So, mice with Lee index ≥310 were considered as obese [19]. We found that the Lee index was higher when mice fed with HFD significantly (364) compared to the control (307) confirming the mice with HFD as obese and the control mice as non-obese. Data are presented in Figure 2.
Effect of OLA on Body weight and organ weight
After 6 weeks of the experiment, the body weight of the mice was measured and then the mice were dissected and abdominal fat was weighed. The result of the study indicates that after drug treatment, body weight, and abdominal fat weight were increased significantly in the obese (HFD) control group than the normal diet group. But drug treatment group showed a non-significant decreasing trend in the parameters than the obese (HFD) control group. Data are presented in Figure 3.
Effect of OLA on lipid profiles
This study indicates that after 6 weeks of drug treatment, the HFD group showed a significant increase in TG and TC levels than the control group, and a significant level of declination was noticed in the HDL-C level than the control group. On the other hand, the OLA-treatment group showed a significant decrease in TG level but a non-significant decrease was noticed in TC and HDL-C levels when compared with the HFD group. Data are shown in Figure 4.
Effect of OLA on liver function
The present study indicates that after 6 weeks of drug treatment, the HFD group showed a significant increase in SGOT and ALP levels but a non-significant increase in SGPT levels compared to the control group. On the other hand, the OLA treatment group showed a significant decrease in SGOT and SGPT but a non-significant decrease in ALP levels when compared with the HFD group. Data are presented in Figure 5.
Effect of OLA on relative gene expression of MCP-1, PPAR-γ, and GLUT4
Using real-time RT-PCR and RNA isolated from adipose tissues of age- and gender-matched obese mice, we examined the expression of the MCP-1, PPAR-γ, and GLUT4 genes in vivo. In comparison to ND mice, HFD mice had a noticeably higher basal level of MCP-1 expression in their adipose tissue and a non-significant decrease in the OLA treatment group compared with the HFD group. On the other side, PPAR-γ expression in the adipose tissue was significantly elevated in HFD mice compared with ND mice and non-significantly decreased in the OLA treatment group compared with the HFD group. The result of the present study also indicates that HFD-fed mice showed a significant increase in GLUT4 expression compared to the control group and the OLA-treatment group also showed significant increasing trend in GLUT4 expression when compared with HFD group. Data are presented in Figure 6.
Discussion
Obesity as an emerging health problem is developing various chronic diseases [20, 21]. As both humans and rodents have a similar tendency to gain weight with high-calorie intake [22], we also observed that an increase in body weight significantly occurred confirming obesity by Lee index when mice fed with HFD than the control group. Besides, significant changes in body and organ weight, lipid profile, liver functions, and relative gene expressions confirmed obesity in HFD-induced obese mice. The findings in the OLA-treated group indicate a decreasing trend in body and organ weight. The OLA treatment group showed significantly less amount of serum TG than the HFD group. Serum TC is decreased after treatment but there is also a non-significant decrease in HDL-C level in the treated mice. Zhang et al. stated that OLA exerted minor effects on lipids in mice with obesity [11]. Our study also supported this but exhibited an overall lipid-lowering effect. Since SGOT, SGPT, and ALP are leakage enzymes, their elevated levels in the blood indicate severe hepatocellular injury [23]. However, in this study in case of HFD-fed mice showed a significant level of elevation in SGOT, SGPT, and ALP enzymes than the control group but a significant level of declination of them was noticed after OLA treatment. Elevated levels of serum SGOT, SGPT, and ALP in HFD-fed mice, a reliable indicator of liver damage, were dropped following OLA exposure supporting that chronic OLA is associated with declination in necrosis in liver cells.
In this study, OLA in HFD-fed mice non-significantly decreases MCP-1 mRNA expression by reducing MCP-1 gene transcription compared to only HFD-treated mice. We speculate that such effects may contribute to the salutary effect of OLA in hepatic steatosis and many other metabolic syndromes in the context of pre-existing obesity. Reduction in MCP-1 level also indicates anti-inflammatory effects of OLA exposure. The result of this study also showed a significant upregulation of PPAR-γ in HFD-fed mice than the control group and a decreasing trend non-significantly in the OLA treatment group. In this research, GLUT-4 expression showed a significant increase after OLA treatment. Notably, multiple investigations in mice/rats on HFD have found increased weight gain and metabolic disruption following acute OLA treatment [10, 24]. One possible explanation for the difference between our results and those of other research could be the significantly shorter HFD duration in those earlier trials. According to a different study, long-term exposure to OLA reduced adiposity and hepatic steatosis in obese mice [11]. This evidence supports our findings in HFD-induced obese mice, where OLA was not associated with exacerbated metabolic syndrome in female mice. However, this study suggests that when obesity is established, OLA exposure is related to reduced adiposity accumulation and liver injury. Finally, OLA exposure presents anti-obesity effects including the reduced gene expression of MCP-1 as an anti-inflammatory effect mainly. Moreover, to enhance the current study’s therapeutic significance and to have a deeper understanding of OLA’s metabolic effect, it is recommended for future research employing a larger mouse population.
Conclusion
Following prolonged exposure to OLA, the results reveal a decrease in body and organ weight, lipid levels, liver function markers, and the expression of MCP-1 and PPAR-ɣ, with the exception of GLUT4. These changes suggest that OLA influences metabolic disturbances in obese mice. Moreover, to enhance the current study’s therapeutic significance and to have a deeper understanding of OLA’s metabolic effect, it is recommended for future research employing a larger mouse population.
Ethical Considerations
Compliance with ethical guidelines
The study was reviewed and approved by the Ethics Committee of Noakhali Science and Technology University (NSTUEC), Noakhali, Bangladesh (Code: NSTU/SCI/EC/2023/158, August 03, 2023).
Funding
This work was supported partly by the Research Cell, at Noakhali Science and Technology University, Noakhali, Bangladesh.
Authors' contributions
Conceptualisation and supervision: Mohammad Salim Hossain; Study design: Tabassum Akter and Khadiza Begum; Experiments: Khadiza Begum; Data collection: Rahima Begum; Data analysis: Tabassum Akter, Md. Monirul Islam, Khadiza Begum, and Md Abdur Rahman Ripon; Writing the original draft: Md Abdur Rahman Ripon and Rahima Begum; Review and editing: Md Monirul Islam and Mohammad Salim Hossain; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are thankful to all related to this study.
References
- Global obesity epidemic to cost US$1.2 trillion annually by 2025-food tank [Internet]. 2024 [Updated 2024 December 11]. Available from: [Link]
- Smith KB, Smith MS. Obesity statistics. Prim Care. 2016; 43(1):121-35. [DOI:10.1016/j.pop.2015.10.001] [PMID]
- Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity. 2007; 15(4):798-808. [DOI:10.1038/oby.2007.608] [PMID]
- Celi FS, Shuldiner AR. The role of peroxisome proliferator-activated receptor gamma in diabetes and obesity. Curr Diab Rep. 2002; 2(2):179-85. [DOI:10.1007/s11892-002-0078-2] [PMID]
- Salviato Balbão M, Cecílio Hallak JE, Arcoverde Nunes E, Homem de Mello M, Triffoni-Melo Ade T, Ferreira FI, et al. Olanzapine, weight change and metabolic effects: A naturalistic 12-month follow up. Ther Adv Psychopharmacol. 2014; 4(1):30-6. [DOI:10.1177/2045125313507738] [PMID] [PMCID]
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry. 1999; 156(11):1686-96. [DOI:10.1176/ajp.156.11.1686] [PMID]
- Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006; 51(8):480-91. [DOI:10.1177/070674370605100803] [PMID]
- Łukasiewicz S, Błasiak E, Szafran-Pilch K, Dziedzicka-Wasylewska M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics - in vitro studies. J Neurochem. 2016; 137(4):549-60. [DOI:10.1111/jnc.13582] [PMID]
- Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: A critical overview. CMAJ. 2005; 172(13):1703-11. [DOI:10.1503/cmaj.1041064] [PMID] [PMCID]
- Townsend LK, Peppler WT, Bush ND, Wright DC. Obesity exacerbates the acute metabolic side effects of olanzapine. Psychoneuroendocrinology. 2018; 88:121-8. [DOI:10.1016/j.psyneuen.2017.12.004] [PMID]
- Zhang X, Zhao Y, Liu Y, Yuan Y, Shao H, Zheng X. Regulation of obesity-associated metabolic disturbance by the antipsychotic drug olanzapine: Role of the autophagy-lysosome pathway. Biochem Pharmacol. 2018; 158:114-25. [DOI:10.1016/j.bcp.2018.10.001] [PMID]
- Li H, Peng S, Li S, Liu S, Lv Y, Yang N, et al. Chronic olanzapine administration causes metabolic syndrome through inflammatory cytokines in rodent models of insulin resistance. Sci Rep. 2019; 9(1):1582. [DOI:10.1038/s41598-018-36930-y] [PMID] [PMCID]
- Tighe S, Dinan T. An overview of the central control of weight regulation and the effect of antipsychotic medication. J Psychopharmacol. 2005; 19(6 Suppl):36-46. [DOI:10.1177/0269881105058679] [PMID]
- Kowalchuk C, Castellani LN, Chintoh A, Remington G, Giacca A, Hahn MK. Antipsychotics and glucose metabolism: How brain and body collide. Am J Physiol Endocrinol Metab. 2019; 316(1):E1-15 [DOI:10.1152/ajpendo.00164.2018] [PMID]
- Akter T, Islam MM, Begum K, Begum R, Roy S, Ripon MAR, et al. Risperidone upregulates the expression of adhesion molecules VCAM-I, and P-selectin in high-fat diet-induced obese mice. J Affect Disord Rep. 2023; 13:100583. [DOI:10.1016/j.jadr.2023.100583]
- Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010; 2010(6):pdb.prot5439. [DOI:10.1101/pdb.prot5439] [PMID]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009; 55(4):611-22. [DOI:10.1373/clinchem.2008.112797] [PMID]
- Roy S, Ripon MAR, Begum R, Bhowmik DR, Amin MT, Islam MA, et al. Arachidonic acid supplementation attenuates adipocyte inflammation but not adiposity in high fat diet induced obese mice. Biochem Biophys Res Commun. 2022; 608:90-95. [DOI:10.1016/j.bbrc.2022.03.089] [PMID]
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010; 23(2):270-99. [DOI:10.1017/S0954422410000168] [PMID]
- Baceviciene M, Reklaitiene R, Tamosiūnas A. Effect of excess body weight on quality of life and satisfaction with body image among middle-aged lithuanian inhabitants of Kaunas city. Medicina. 2009; 45(7):565-73. [DOI:10.3390/medicina45070075] [PMID]
- Blüher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019; 15(5):288-98. [DOI:10.1038/s41574-019-0176-8] [PMID]
- Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007; 41(1):111-9. [DOI:10.1258/002367707779399518] [PMID]
- Huang TW, Chang CL, Kao ES, Lin JH. Effect of hibiscus sabdariffa extract on high fat diet-induced obesity and liver damage in hamsters. Food Nutr Res. 2015; 59:29018. [DOI:10.3402/fnr.v59.29018] [PMID] [PMCID]
- Minet-Ringuet J, Even PC, Lacroix M, Tomé D, de Beaurepaire R. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology. 2006; 187(4):447-54. [DOI:10.1007/s00213-006-0433-0] [PMID]
Type of Study: Original Research |
Subject:
Clinical Pharmacy
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |