Volume 10, Issue 4 (2024)
Pharm Biomed Res 2024, 10(4): 355-366 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Darbandi N, Sadeghi A, Nazari-Serenjeh F, Ghasemzadeh Z. Interaction of Dorsal Hippocampal Nicotinic Receptors and Zinc Oxide Nanoparticles on Memory Consolidation. Pharm Biomed Res 2024; 10 (4) :355-366
URL: http://pbr.mazums.ac.ir/article-1-627-en.html
URL: http://pbr.mazums.ac.ir/article-1-627-en.html
1- Department of Biology, Faculty of Science, Arak University, Arak, Iran.
2- Department of Biology, Payame Noor University (PNU), Tehran, Iran.
3- Department of Animal Biology, School of Biology, University of Tehran, Tehran, Iran.
2- Department of Biology, Payame Noor University (PNU), Tehran, Iran.
3- Department of Animal Biology, School of Biology, University of Tehran, Tehran, Iran.
Keywords: Dorsal hippocampus, Memory, Mecamylamine, Nicotine, Rat, Zinc oxide nanoparticles (ZnO NPs)
Full-Text [PDF 2498 kb]
(633 Downloads)
| Abstract (HTML) (1436 Views)
Full-Text: (504 Views)
Introduction
Zinc oxide nanoparticles (ZnO NPs) are among the widely used metallic NPs in diverse fields of industry, medicine, and agriculture due to their exclusive physicochemical properties and also easy synthesis [1]. In biomedicine, ZnO NPs are added to oral care products as antibacterial and anti-inflammatory agents [2]. ZnO NPs have been integrated into sunscreen to protect against ultraviolet radiation [3]. In addition, ZnO NPs have been considered a therapeutic approach in cancer therapy because of their potential in drug delivery and ROS generation [4]. Moreover, ZnO NPs can increase the content and quality of crops [5]. ZnO NPs can enter the human body via skin penetration, inhalation, and digestion tract, and after distribution through circulating blood can cross the blood-brain-barrier and interact with neural cells [5]. Moreover, according to the literature, ZnO NPs can be transferred to the central nervous system via the olfactory [6] nerve translocation pathway. Thus, individuals are inevitably exposed to ZnO NPs.
One of the problems related to Zn ONPs use is their toxicity against brain tissue. According to one recent in vivo study [6], ZnO NPs induced vascular congestion in the brain of mice. In vitro studies revealed that treatment with ZnO induces oxidative stress, necrotic and apoptosis cell death, and also impaired mitochondrial function in neural cells [7, 8, 9, 10]. In addition, in vivo studies have shown that Zno NPs induce alterations in memory and cognition functions. Xiaoli et al. (2017) also reported that prenatal exposure to ZnO NPs impairs memory in rat offspring [11]. In adult mice, ZnO NPs treatment impairs long-term and passive avoidance memory [12]. Moreover, induction of neuro-inflammation and altered synaptic plasticity by ZnO NPs have been reported [13, 14]. Although some mechanisms underlying the disturbing effects of ZnO nanoparticles on memory and cognition have been introduced [12, 13], the precise mechanism of ZnO NPs–induced memory deficit has not yet been thoroughly investigated.
The limbic system is a collection of structures that work in concert for memory and emotional behavioral processing [15]. The hippocampus is a vital structure of this system that plays an important role in different stages of memory processes, such as encoding, short-term and long-term consolidation, and retrieval [16-20]. There are several lines of evidence supporting that acetylcholine is a neuromodulator molecule that involved in hippocampal-dependent memory [21-27]. Acetylcholine exerts its biological effect via two main classes of cholinergic receptors, namely nicotinic receptors and muscarinic receptors [28]. Nicotinic receptors are ligand-gated ion channels; the activation of these receptors triggers different learning-associated cell-signaling cascades and modifies hippocampal synaptic plasticity [29]. Moreover, hippocampal nicotinic receptors are involved in the effects of drug exposure on learning and memory [30-32].
Some previous studies have shown that ZnO NPs affect brain neurotransmission, including the cholinergic system [6, 33-37] consequently modulates central nervous functions. Since the involvement of hippocampal nicotinic receptors in ZnO NPs induced effect on memory consolidation has not been studied, the present study evaluates the interaction between post-training injection of nicotinic receptor agents and ZnO NPs in passive avoidance tasks. In addition, this study maintains that the distribution and retention of ZnO NPs in the brain is time-dependent [38]; thus, the memory test is performed 1 and 3 days after training.
Materials and Methods
Study animals
In this study, male Wistar rats (a total of 126 animals; body weight=220-240 g) obtained from the Pasteur Institute of Iran (Tehran, Iran) were used. The rats were housed in clean transparent plexiglas cages (3–4 per cage) on a 12-h on/off lighting schedule with unrestricted access to food and water except during experiments. The rats were kept at a temperature maintained at 22±2 °C room. The lights were on between 7:00 PM and 7:00 AM. The rats were acclimatized to the laboratory condition for 7 days before surgery. To avoid experimental deviations due to diurnal variations, all behavioral tests were carried out between 9:00 AM and 2:00 PM. All procedures were approved by the local Ethics Committee and carried out following the ethical standards and principles of laboratory animal care (NIH publication) and laws of animal protection.
Surgery procedure
One week after adaptation to laboratory condition, the rats were anesthetized with intraperitoneal administration of ketamine 10% (Alfasan, Woerden-Holand) and xylazine 2% (Alfasan, Woerden-Holand; 50 mg/kg and 5 mg/kg, respectively) cocktail. Two 22-gauge guide steel cannulas were implanted bilaterally in the dorsal hippocampus CA1 regions using the bregmatic and lambdoid sutures of the skull as a reference point (coordinates according to the Atlas of Paxions and Watson [39]: -3 to -3.5 mm posterior to the bregma, ±1.8–2 mm lateral to midline, and -2.8 to -3 mm vertical to dura). The injection needle was 0.5 mm longer than the guide cannula. The animals were handled daily for 10 min during the recovery period. The microinjection procedure was started 7 days after implantation of guide cannulas.
Drugs and microinjection procedure
ZnO NPs (20-30 nm, purity >99%) was purchased from Tecnan, Spain. Nicotine hydrogen tartrate and mecamylamine were purchased from Sigma Aldrich (St. Louis, MO purity >99%). Nicotine doses were calculated in their tartrate form and were dissolved in 0.9% saline and then the pH of the solution was adjusted to 7.2-7.4 with NaOH (0.1 normal solution). Mecamylamine was dissolved in sterile 0.9% saline. ZnO NPs were dissolved in 0.9% saline and were dispersed using the Ultrasonic Bath 2600S (Pars Nahand, Iran) for 20 min and before each injection, the compound was shaken for 1 min [40].
Immediately after training, rats were bilaterally (into the CA1) infused with 1 μL/rat of nicotine (0.3 and 0.4 µg/rat), mecamylamine (0.5 and 1 µg/rat), or vehicle. The infusion lasted 1 min (infusion rate: 0.5 μL/min, via 2 µL-Hamilton syringe) and the infusion cannula was carefully withdrawn 1 min after the end of the infusion. ZnO NPs prepared daily 30 min before the beginning of experiments and were given intraperitoneally at a dose of 0.5 mg/kg or 1 mg/kg (post-training, with 5 min intervals). The dose of drugs was selected based on our previous studies [41].
Passive avoidance test
The inhibitory avoidance apparatus consists of two compartments of the same size: One light compartment and one dark compartment which is connected by a movable guillotine. The floor of the dark compartment consists of metal grid bars (placed 1 cm away from each other, 0.5 cm diameter) and connected to a shock generator, able to generate a shock (50 Hz, 3 s) with 1 mA intensity. All experiments were done in a testing room lit by a 60 W lamp placed above the center of the apparatus.
On the training day, 30 min before a training session, the rats were allowed to become familiar with the apparatus. After 30 min, an individual rat was kept in the light compartment for 10 s, after which the door was raised, and the time taken by the rat to move from the light compartment to the dark compartment was recorded. After 2 min, the rat was placed in the light compartment again, as soon as the rat completely entered into the dark compartment, the door was closed and an electrical foot shock was applied for 3 s. The rat was then removed from the apparatus. 2 min later, the procedure was repeated. Animals that did not enter the dark compartment even after 120 s were removed from the apparatus as successful training. All animals were trained with a maximum of 3 trials. Testing day was conducted after 24 h of acquisition trial, where no shock was applied when the rat came into the dark compartment, and retrieval latency was recorded (as step-through latency) by the time taken the rat to re-enter into the dark compartment (up to 300 s).
Open field test
To assess the effects of the individual drug or drug combination on locomotor activity, the open field test was used [42]. On the first and third days after drug administration, all the individual rats were submitted to a 10-min open field test (Borj Sanat Co., Iran). The apparatus (40×40×40 cm) was equipped with 16 infrared photocells positioned every 2.5 cm. Immediately after testing on the passive avoidance apparatus, each rat was placed in the center of the box floor. The number of beam cuts was evaluated by the photocell monitoring system. After each test, the apparatus was carefully cleaned.
Experimental design
The treatments were initiated after the recovery period (7 days after surgery).
Experiment 1
In the first experiments, the rats in ZnO NPs groups after successful training were divided into 3 groups (n=7 rats per group). These rats received intra-CA1 microinjection of saline (1 µL/rat) and after 5 min, they were injected with saline (1 mg/kg, intraperitoneal) or ZnO NPs (0.5 or 1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 2
To acquire a basic understanding of the effect of nicotine microinjection into the CA1 regions of the hippocampus, another 3 groups were used as nicotine/saline groups. The rats were microinjected either vehicle (1 µL/rat, intra-CA1) or nicotine (0.3 or 0.4 µL/rat) immediately after successful training. Saline (1 mL/kg) was administered 5 min after intra-CA1 injection via intraperitoneal injection. Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 3
In experiment 3, four groups of animals were used as nicotine/ ZnO NPs groups. After successful training, the rats received vehicle (1 µL/rat, intra-CA1) or nicotine (0.3 or 0.4 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal) or ZnO NPs (1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 4
After successful training, the rats in mecamylamine treatment groups received saline (1 µL/rat, intra-CA1) or mecamylamine (0.5 or 1 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 5
Four groups of animals were used as mecamylamine / ZnO NPs groups. After successful training, the rats received saline (1 µL/rat, intra-CA1) or mecamylamine (0.5 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal) or ZnO NPs (0.5 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Histology
At the end of the experiments, the rats were all euthanized by decapitation under deep anesthesia with carbon dioxide and microinjection of 1 μL/rat of a 1% methylene-blue solution. After euthanizing, the brains were removed and fixed in the formaldehyde (10%), and stored at 4 °C until used for sectioning using a vibratome.
Data analysis
Statistical analysis was performed using SPSS Software version, 22. Data are expressed as Mean±SE of the mean. Statistical significance was evaluated by one-way analysis of variance followed by the Tukey post hoc comparisons and defined as P<0.05.
Results
Histological verifications
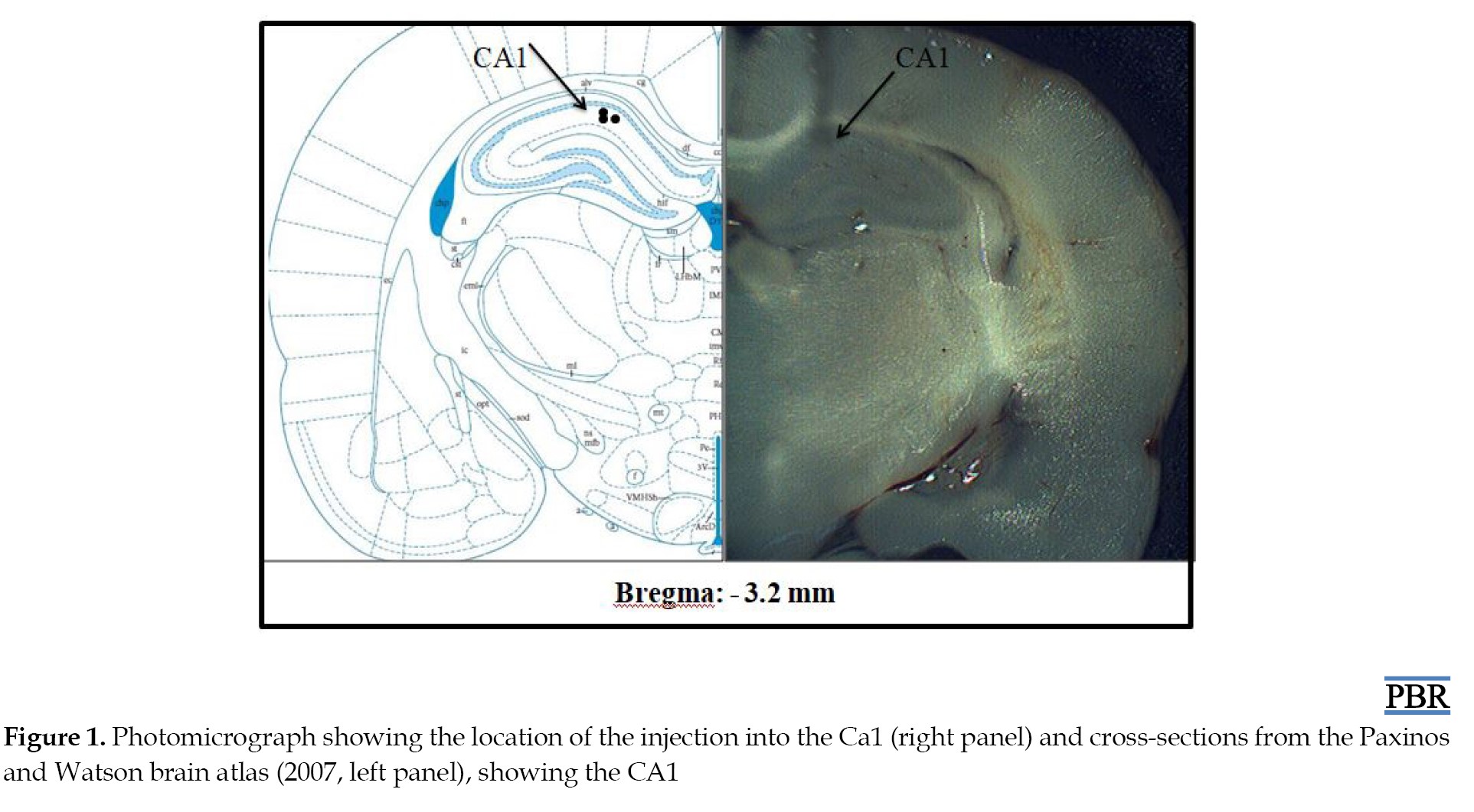
Figure 1 shows the photomicrograph of the location of the injection into the CA1 (right panel) and cross-sections of the rat brain indicating injection locations according to the Paxinos and Watson atlas [39], left panel). Black circles show injection sites inside the CA1.
Effect of post-training ZnO NPs administration on step-through latency
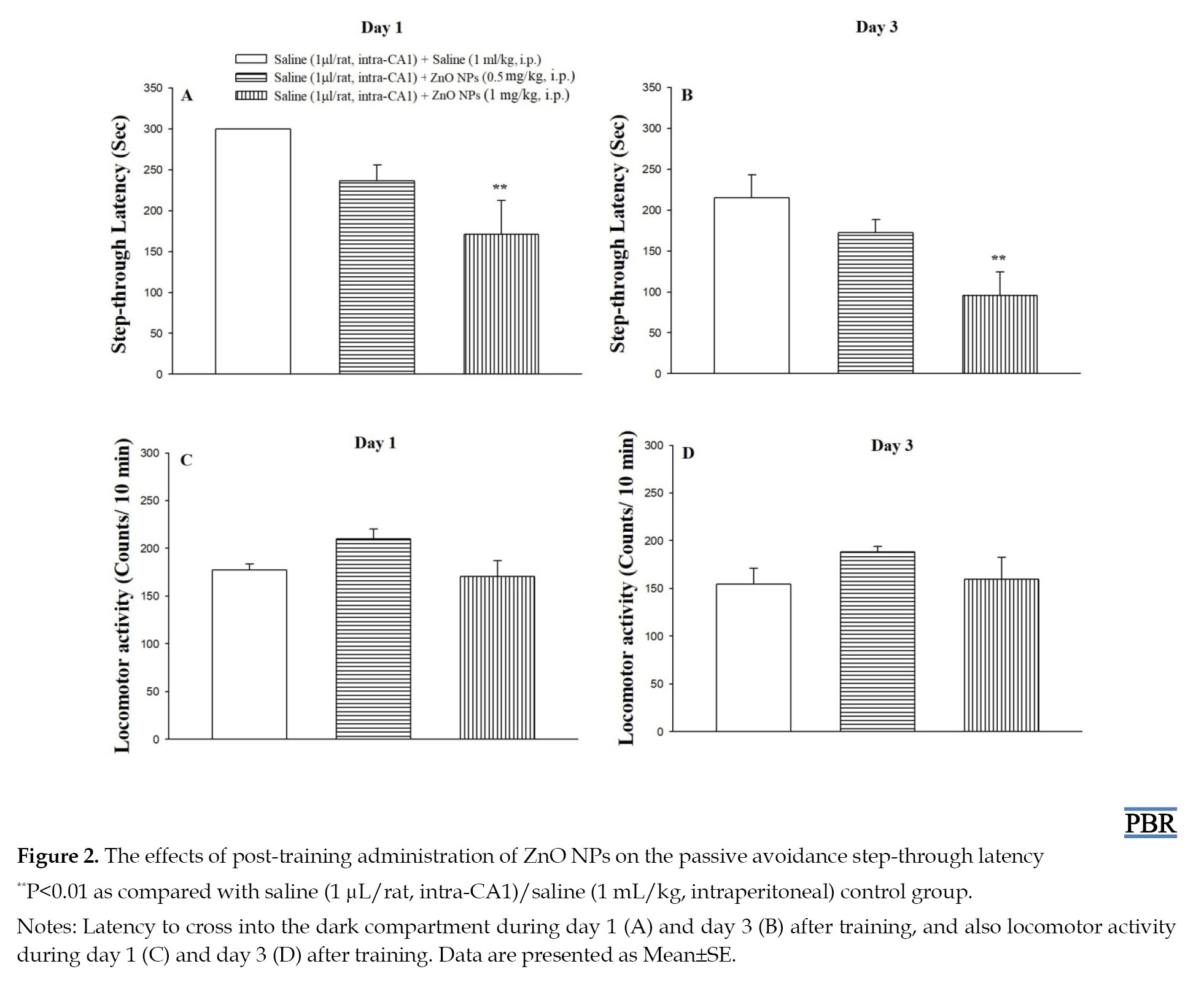
Figure 2 shows the effects of post-training administration of ZnO NPs on the latency of entrance into the dark compartment on day 1 (A) and day 3 (B) after training, and also on locomotor activity (C and D). The one-way analysis of variance in day 1 (F(2, 17)=6.06, P<0.01) and also day 3 (F(2, 17)=5.95, P<0.01) after training showed a significant difference in step-through latency among the groups. Also, post hoc analysis revealed that the groups receiving 1 mg/kg of ZnO NPs (P<0.01) spent less time to re-enter the dark compartment as compared to the control saline group, indicating ZnO NPs-induced memory loss. Moreover, the one-way analysis of variance in Figures 2C and 2D indicates that the treatments on day 1 (F(2, 17)=3.13, P>0.05) and also day 3 (F(2, 17)=1.19, P>0.05) after training did not affect locomotor activity.
Effect of post-training intra-CA1 nicotine microinjection on step-through latency
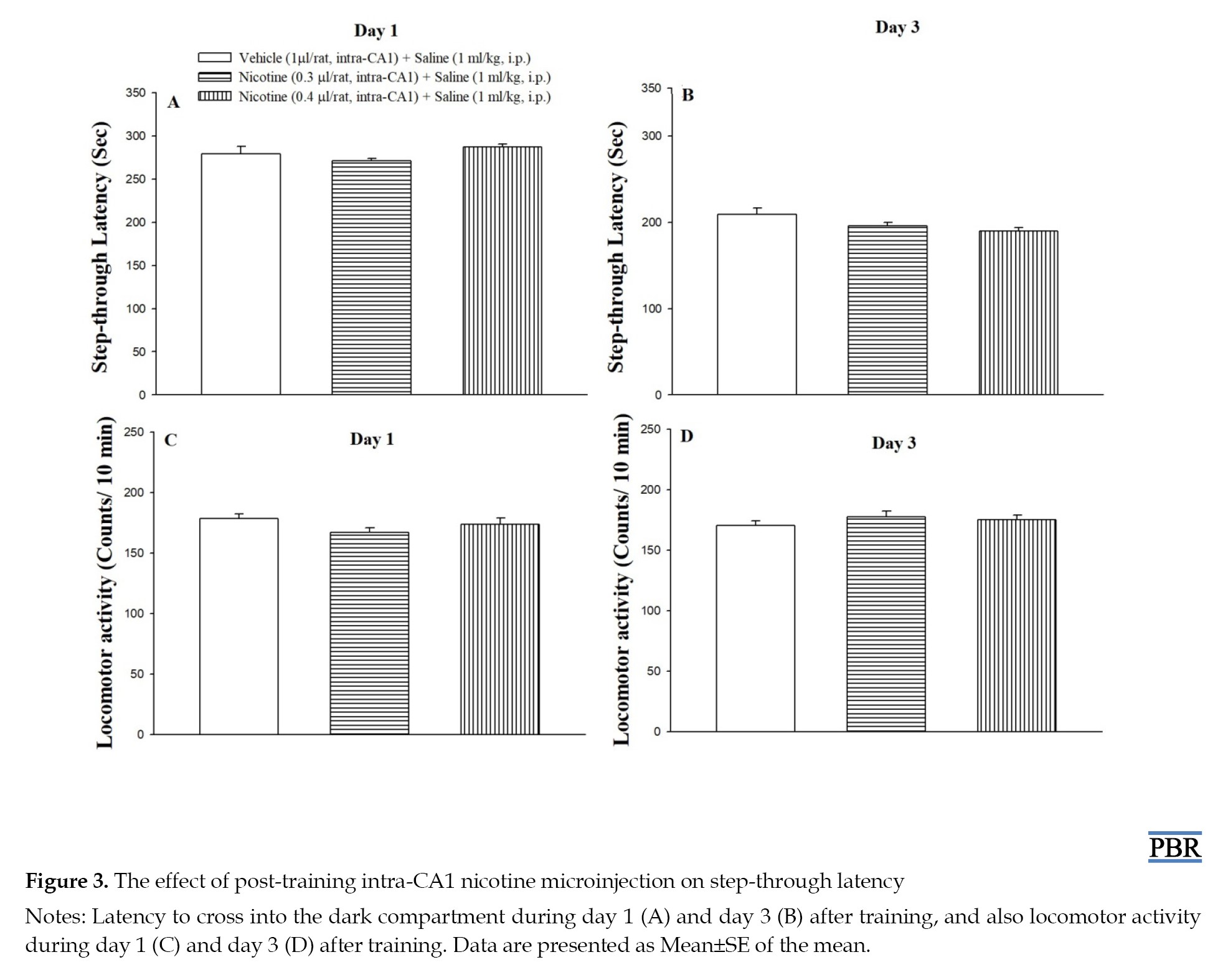
As shown in Figures 3A and 3B, post-training microinjection of nicotine (0.3 and 0.4 μg/rat, intra-CA1) had no significant effect on step-through latency at 1 (F(2, 17)=2.083, P>0.05) and 3 (F(2, 17)=3.27, P>0.05) days post-training, respectively, when compared to the control group. Figures 3C and 3D showed that the drug exposure did not alter the locomotor activity at 1 (F(2, 17)=1.71, P>0.05) and 3 (F(2, 17)=0.84, P>0.05) days post-training.
The effect of the post-training intra-CA1 nicotinic acetylcholine receptors agonist (nicotine) on the impairment effect of ZnO NPs on the passive avoidance learning.
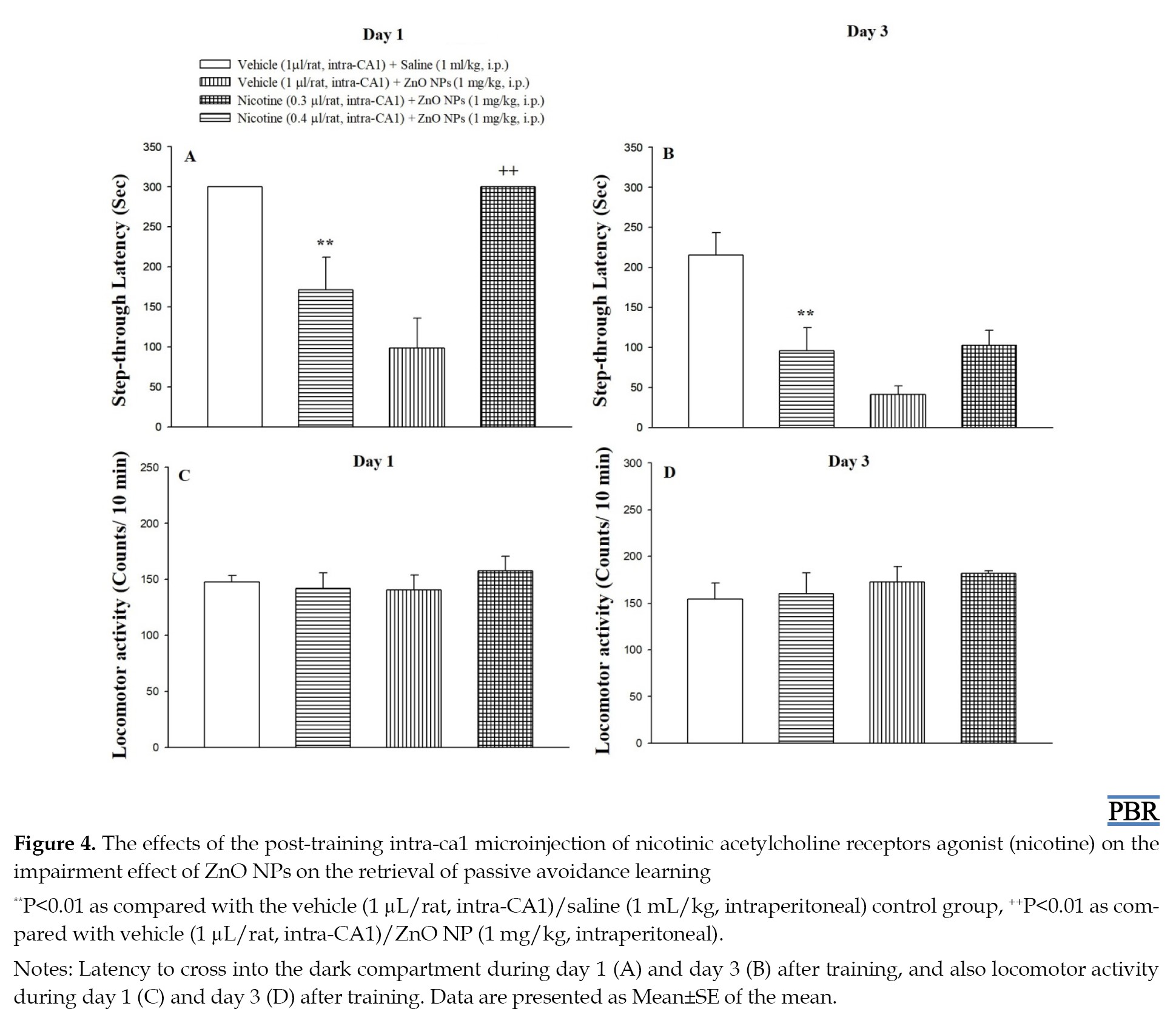
Nicotine (intra-CA1) was microinjected 5 min before ZnO NPs administration to investigate the role of the CA1 nicotinic receptors in the impairment effect of ZnO NPs on the consolidation of passive avoidance learning. The results of the one-way analysis of variance are presented in Figure 4A, indicating a statistically significant difference in step-through latency score between the groups at 1 day (F(3, 24)=12.93, P<0.001) post-training. Post-hoc analysis showed that 0.4 µg/rat of nicotine significantly increases the step-through latency (P<0.01) 1 day after training, while the group treated with 0.4 µg/rat of nicotine plus ZnO NPs showed no significant difference as compared to the control group 3 day after training (Figure 4B, F(3, 24)=10.46, P<0.001). Thus, post-training microinjection of nicotine could improve the ZnO NPs-induced reduction in the step-through latency (1 day after training). As shown in Figures 4C and 4D, the post-training microinjection of nicotine plus ZnO NPs did not affect the locomotor activity at 1 (F(3, 24)=1.71, P>0.05) and 3 (F(3, 24)=0.84, P>0.05) days post-training.
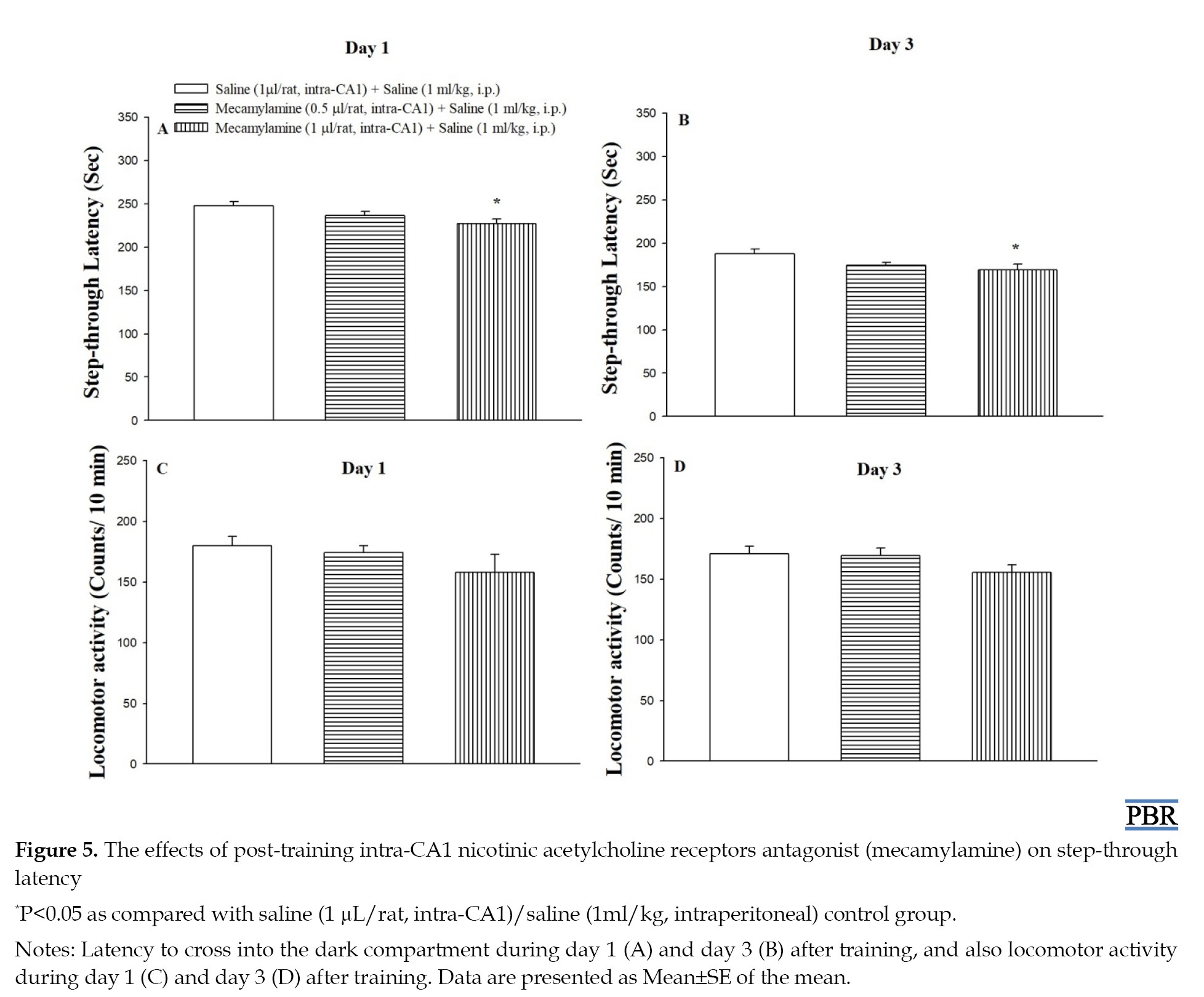
Effect of post-training intra-CA1 mecamylamine microinjection on step-through latency
As shown in Figures 5A and 5B, post-training microinjection of mecamylamine (1 μg/rat, intra-CA1) had a significant effect on step-through latency at 1 (F(2, 17)=4.14, P<0.05) and 3 (F(2, 17)=3.90, P<0.05) days post-training, respectively, when compared to the control group and decreased step-through latency. Figures 5C and 5D showed that the drug exposure did not alter the locomotor activity at 1 (F(2, 17)=1.23, P>0.05) and 3 (F(2, 17)=1.89, P>0.05) days post-training.
The effect of the post-training intra-CA1 microinjection of ineffective dose of mecamylamine with ineffective dose of ZnO NPs on the passive avoidance learning
In the last set of experiments, we determined whether the post-training intra-CA1 microinjection of an ineffective dose of mecamylamine enhances the influence of an ineffective dose of ZnO NPs on passive avoidance learning to induce memory loss. Toward this end, mecamylamine (0.5 µg/rat, intra-CA1) was microinjected 5 min before ZnO NPs (0.5 mg/kg, intraperitoneal) administration. The results of the one-way analysis of variance are presented in Figure 6A, indicating a statistical difference in step-through latency score between the groups on day 1 (F(3, 24)=5.27, P<0.001) post-training. Post-hoc analysis showed that 0.5 µg/rat of mecamylamine significantly enhances the influence of ineffective dose of ZnO NPs on step-through latency (P<0.01) 1 day and also 3 days after training (F(3, 24)=3.34, P<0.05; Figure 6B). Thus, post-training microinjection of mecamylamine could potentiate the ZnO NPs-induced reduction in the step-through latency. As shown in Figures 6C and 6D, the post-training microinjection of mecamylamine plus ZnO NPs did not affect the locomotor activity at 1 (F(3, 24)=2.31, P>0.05) and 3 (F(3, 24)=2.18, P>0.05) day post-training.
Discussion
ZnO NPs have a broad range of applications. However, certain disadvantages and toxic side effects have been reported for biomedical applications of ZnO NPs. In recent years, the majority of prior studies have emphasized that ZnO NPs modulate learning and memory ability in animals [11-13, 43, 44]. Nevertheless, Amara et al. (2014) reported acute intravenous injection of ZnO-NPs did not affect working memory in exposed rats [45]. Kesmati et al. (2020) reported that nano-ZnO increased memory in novel object recognition test [43] and Xie et al. (2012) indicated that ZnO NPs could ameliorate the cognitive impairment in mice with depressive-like behaviors [46]. As shown in Figure 2, the present study showed that post-training intraperitoneal injection of ZnO NPs dose-dependently impaired passive avoidance memory retrieval at 1 and 3 days after training. In addition, ZnO NPs injection did not affect exploratory behavior in open field tests at 1 and 3 days after training. Our result is in line with studies that conducted by Tian et al. [12] and Han et al. [13].
The research on mechanisms by which ZnO NPs influence cognitive functions is being carried out. After bypassing the blood-brain barrier or direct entrance through the nose, ZnO NPs induce zinc deposition in functional brain regions including the hippocampus. Zinc is a neuromodulator that modulates synaptic transmission [47]. However, elevated concentrations result in functional and structural changes [8, 12, 34, 48]. Moreover, exposure to ZnO-NPs alters the homeostasis of Zn and results in cell apoptosis [6]. Liu et al. [8] and Attia et al. [10] observed the hippocampal accumulation of ZnO NPs following intravenous or oral administration led to oxidative damage, inflammatory responses, and histopathological damage in the hippocampus [8, 10]. Hippocampal oxidative stress and inflammation have been closely related to impairment in synaptic plasticity and memory [49-52]. Previous studies also have demonstrated that suppression of the hippocampal cAMP/CREB signaling pathway is involved in memory impairment following ZnO NPs administration [12]. Xie et al. (2012) using isolated rat hippocampal CA3 pyramidal neurons showed that ZnO NPs increase the current amplitude and excitability of neurons that may affect physiological functions of neurons [46]. It is also suggested that ZnO NPs disturb the metal ions homeostasis in the brain [6, 46, 53]. Homeostasis of metal ions is crucial for synaptic function [54]. Han et al. [13] reported that ZnO NPs disrupt the balance between long-term potentiation and depotentiation in the dentate gyrus of the hippocampus which leads to the alteration of synaptic plasticity and memory deficits [13]. In support of this effect, it has been shown that acute administration of ZnO NPs decreases glutamate levels in the hippocampus, a main excitatory neurotransmitter involved in long-term potentiation levels in the hippocampus [33].
In the present study, we showed that post-training intra-CA1 microinjection of nicotine dose-dependently could improve the ZnO NPs-induced reduction in the step-through latency in 1 day after training, but not after 3 days (Figure 4). Combined nicotine and ZnO NPs did not affect locomotion. These results support the involvement of hippocampal nicotinic receptors in ZnO NPs-induced deficit in passive avoidance memory. Moreover, post-training microinjection of nicotine alone had no impact by itself on step-through latency or locomotor activity in all days after training (Figure 3). Using omics technologies, Guo et al. [34] nominated cholinergic neurotransmission as the main biological process affected following ZnO NPs treatment [34]. Moreover, Hsiao et al. (2008, 2001) showed that zinc modulates nicotinic receptors in a subunit composition and dose-dependent manner [37, 55]. Yadava et al. [56] reported that ZnO NPs attenuated spatial learning and memory through an increase in oxidative stress and a decrease in acetylcholinesterase enzyme activity [56]. Taking these findings together, the results confirm the involvement of nicotinic receptor mechanisms in impairing the effect of ZnO NPs on memory formation. According to Figure 4, there is a difference in the rescuing effect of nicotine on ZnO NPs at 1 day (Figure 4A) but not at 3 days, which may be related to the time-dependent neurotoxicity of ZnO NPs in the brain [10, 38]. Chen et al. (2015) reported that as time increases Zn content increases in the brain, therefore, ZnO NPs accumulate in the brain in the long term [38].
In addition, to confirm the role of nicotinic acetylcholine receptors in the effect of ZnO NPs on memory, an ineffective dose of mecamylamine (0.5 µg/rat), a nicotinic acetylcholine receptor antagonist, which alone did not affect memory (Figure 5), injected into the hippocampus before the systemic administration of an ineffective dose of ZnO-NPs (0.5 mg/kg). The results showed that a low dose of a nicotinic acetylcholine receptor antagonist can synergize with a low dose of ZO NP to produce a memory impairment that may conform to the interaction between ZnO-NPs and nicotinic receptors in memory consolidation (Figure 6). Since ZnO NPs increase Zn deposition in the hippocampus [34] and considering to modulatory effect of ZnONPs on cholinergic nicotinic receptors [37, 55], it can be concluded that interaction between ZnONPs and nicotinic receptors is involved in ZnONPs –induced effect on memory.
On the other hand, nicotine treatment prevented memory deficits in brain disorders, exerted neuroprotective effects, upregulated cAMP/CREB signaling, and reduced reactive oxygen species generation through nAChR/Erk1/2 signaling pathway [57-59]. Considering these nicotine effects, an explanation for our obtained result can be that intra-CA1 administration of nicotine ameliorates the disturbing effects of ZnO NPs on the hippocampus via different mechanisms. However, more studies are needed to precisely identify the mechanism of the modulatory effect of nicotinic receptors on ZnO NPs’ effect on the memory process.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research and Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKMU.REC.1400.206).
Funding
This work was supported by a grant from Arak University, Arak, Iran (Grant No.: 11041).
Authors' contributions
Conceptualization, methodology, formal analysis and investigation: All authors; Writing the original draft: Ahmad Sadeghi, Farzaneh Nazari-Serenjeh and Zahra Ghasemzadeh; Project administration, supervision, review and editing: Niloufar Darbandi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Arak University, Arak, Iran for its valuable cooperation.
References
Zinc oxide nanoparticles (ZnO NPs) are among the widely used metallic NPs in diverse fields of industry, medicine, and agriculture due to their exclusive physicochemical properties and also easy synthesis [1]. In biomedicine, ZnO NPs are added to oral care products as antibacterial and anti-inflammatory agents [2]. ZnO NPs have been integrated into sunscreen to protect against ultraviolet radiation [3]. In addition, ZnO NPs have been considered a therapeutic approach in cancer therapy because of their potential in drug delivery and ROS generation [4]. Moreover, ZnO NPs can increase the content and quality of crops [5]. ZnO NPs can enter the human body via skin penetration, inhalation, and digestion tract, and after distribution through circulating blood can cross the blood-brain-barrier and interact with neural cells [5]. Moreover, according to the literature, ZnO NPs can be transferred to the central nervous system via the olfactory [6] nerve translocation pathway. Thus, individuals are inevitably exposed to ZnO NPs.
One of the problems related to Zn ONPs use is their toxicity against brain tissue. According to one recent in vivo study [6], ZnO NPs induced vascular congestion in the brain of mice. In vitro studies revealed that treatment with ZnO induces oxidative stress, necrotic and apoptosis cell death, and also impaired mitochondrial function in neural cells [7, 8, 9, 10]. In addition, in vivo studies have shown that Zno NPs induce alterations in memory and cognition functions. Xiaoli et al. (2017) also reported that prenatal exposure to ZnO NPs impairs memory in rat offspring [11]. In adult mice, ZnO NPs treatment impairs long-term and passive avoidance memory [12]. Moreover, induction of neuro-inflammation and altered synaptic plasticity by ZnO NPs have been reported [13, 14]. Although some mechanisms underlying the disturbing effects of ZnO nanoparticles on memory and cognition have been introduced [12, 13], the precise mechanism of ZnO NPs–induced memory deficit has not yet been thoroughly investigated.
The limbic system is a collection of structures that work in concert for memory and emotional behavioral processing [15]. The hippocampus is a vital structure of this system that plays an important role in different stages of memory processes, such as encoding, short-term and long-term consolidation, and retrieval [16-20]. There are several lines of evidence supporting that acetylcholine is a neuromodulator molecule that involved in hippocampal-dependent memory [21-27]. Acetylcholine exerts its biological effect via two main classes of cholinergic receptors, namely nicotinic receptors and muscarinic receptors [28]. Nicotinic receptors are ligand-gated ion channels; the activation of these receptors triggers different learning-associated cell-signaling cascades and modifies hippocampal synaptic plasticity [29]. Moreover, hippocampal nicotinic receptors are involved in the effects of drug exposure on learning and memory [30-32].
Some previous studies have shown that ZnO NPs affect brain neurotransmission, including the cholinergic system [6, 33-37] consequently modulates central nervous functions. Since the involvement of hippocampal nicotinic receptors in ZnO NPs induced effect on memory consolidation has not been studied, the present study evaluates the interaction between post-training injection of nicotinic receptor agents and ZnO NPs in passive avoidance tasks. In addition, this study maintains that the distribution and retention of ZnO NPs in the brain is time-dependent [38]; thus, the memory test is performed 1 and 3 days after training.
Materials and Methods
Study animals
In this study, male Wistar rats (a total of 126 animals; body weight=220-240 g) obtained from the Pasteur Institute of Iran (Tehran, Iran) were used. The rats were housed in clean transparent plexiglas cages (3–4 per cage) on a 12-h on/off lighting schedule with unrestricted access to food and water except during experiments. The rats were kept at a temperature maintained at 22±2 °C room. The lights were on between 7:00 PM and 7:00 AM. The rats were acclimatized to the laboratory condition for 7 days before surgery. To avoid experimental deviations due to diurnal variations, all behavioral tests were carried out between 9:00 AM and 2:00 PM. All procedures were approved by the local Ethics Committee and carried out following the ethical standards and principles of laboratory animal care (NIH publication) and laws of animal protection.
Surgery procedure
One week after adaptation to laboratory condition, the rats were anesthetized with intraperitoneal administration of ketamine 10% (Alfasan, Woerden-Holand) and xylazine 2% (Alfasan, Woerden-Holand; 50 mg/kg and 5 mg/kg, respectively) cocktail. Two 22-gauge guide steel cannulas were implanted bilaterally in the dorsal hippocampus CA1 regions using the bregmatic and lambdoid sutures of the skull as a reference point (coordinates according to the Atlas of Paxions and Watson [39]: -3 to -3.5 mm posterior to the bregma, ±1.8–2 mm lateral to midline, and -2.8 to -3 mm vertical to dura). The injection needle was 0.5 mm longer than the guide cannula. The animals were handled daily for 10 min during the recovery period. The microinjection procedure was started 7 days after implantation of guide cannulas.
Drugs and microinjection procedure
ZnO NPs (20-30 nm, purity >99%) was purchased from Tecnan, Spain. Nicotine hydrogen tartrate and mecamylamine were purchased from Sigma Aldrich (St. Louis, MO purity >99%). Nicotine doses were calculated in their tartrate form and were dissolved in 0.9% saline and then the pH of the solution was adjusted to 7.2-7.4 with NaOH (0.1 normal solution). Mecamylamine was dissolved in sterile 0.9% saline. ZnO NPs were dissolved in 0.9% saline and were dispersed using the Ultrasonic Bath 2600S (Pars Nahand, Iran) for 20 min and before each injection, the compound was shaken for 1 min [40].
Immediately after training, rats were bilaterally (into the CA1) infused with 1 μL/rat of nicotine (0.3 and 0.4 µg/rat), mecamylamine (0.5 and 1 µg/rat), or vehicle. The infusion lasted 1 min (infusion rate: 0.5 μL/min, via 2 µL-Hamilton syringe) and the infusion cannula was carefully withdrawn 1 min after the end of the infusion. ZnO NPs prepared daily 30 min before the beginning of experiments and were given intraperitoneally at a dose of 0.5 mg/kg or 1 mg/kg (post-training, with 5 min intervals). The dose of drugs was selected based on our previous studies [41].
Passive avoidance test
The inhibitory avoidance apparatus consists of two compartments of the same size: One light compartment and one dark compartment which is connected by a movable guillotine. The floor of the dark compartment consists of metal grid bars (placed 1 cm away from each other, 0.5 cm diameter) and connected to a shock generator, able to generate a shock (50 Hz, 3 s) with 1 mA intensity. All experiments were done in a testing room lit by a 60 W lamp placed above the center of the apparatus.
On the training day, 30 min before a training session, the rats were allowed to become familiar with the apparatus. After 30 min, an individual rat was kept in the light compartment for 10 s, after which the door was raised, and the time taken by the rat to move from the light compartment to the dark compartment was recorded. After 2 min, the rat was placed in the light compartment again, as soon as the rat completely entered into the dark compartment, the door was closed and an electrical foot shock was applied for 3 s. The rat was then removed from the apparatus. 2 min later, the procedure was repeated. Animals that did not enter the dark compartment even after 120 s were removed from the apparatus as successful training. All animals were trained with a maximum of 3 trials. Testing day was conducted after 24 h of acquisition trial, where no shock was applied when the rat came into the dark compartment, and retrieval latency was recorded (as step-through latency) by the time taken the rat to re-enter into the dark compartment (up to 300 s).
Open field test
To assess the effects of the individual drug or drug combination on locomotor activity, the open field test was used [42]. On the first and third days after drug administration, all the individual rats were submitted to a 10-min open field test (Borj Sanat Co., Iran). The apparatus (40×40×40 cm) was equipped with 16 infrared photocells positioned every 2.5 cm. Immediately after testing on the passive avoidance apparatus, each rat was placed in the center of the box floor. The number of beam cuts was evaluated by the photocell monitoring system. After each test, the apparatus was carefully cleaned.
Experimental design
The treatments were initiated after the recovery period (7 days after surgery).
Experiment 1
In the first experiments, the rats in ZnO NPs groups after successful training were divided into 3 groups (n=7 rats per group). These rats received intra-CA1 microinjection of saline (1 µL/rat) and after 5 min, they were injected with saline (1 mg/kg, intraperitoneal) or ZnO NPs (0.5 or 1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 2
To acquire a basic understanding of the effect of nicotine microinjection into the CA1 regions of the hippocampus, another 3 groups were used as nicotine/saline groups. The rats were microinjected either vehicle (1 µL/rat, intra-CA1) or nicotine (0.3 or 0.4 µL/rat) immediately after successful training. Saline (1 mL/kg) was administered 5 min after intra-CA1 injection via intraperitoneal injection. Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 3
In experiment 3, four groups of animals were used as nicotine/ ZnO NPs groups. After successful training, the rats received vehicle (1 µL/rat, intra-CA1) or nicotine (0.3 or 0.4 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal) or ZnO NPs (1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 4
After successful training, the rats in mecamylamine treatment groups received saline (1 µL/rat, intra-CA1) or mecamylamine (0.5 or 1 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Experiment 5
Four groups of animals were used as mecamylamine / ZnO NPs groups. After successful training, the rats received saline (1 µL/rat, intra-CA1) or mecamylamine (0.5 µL/rat, intra-CA1), and after 5 min, they received saline (1 mg/kg, intraperitoneal) or ZnO NPs (0.5 mg/kg, intraperitoneal). Step-through latency and locomotor activity were measured on day 1 and day 3 after training.
Histology
At the end of the experiments, the rats were all euthanized by decapitation under deep anesthesia with carbon dioxide and microinjection of 1 μL/rat of a 1% methylene-blue solution. After euthanizing, the brains were removed and fixed in the formaldehyde (10%), and stored at 4 °C until used for sectioning using a vibratome.
Data analysis
Statistical analysis was performed using SPSS Software version, 22. Data are expressed as Mean±SE of the mean. Statistical significance was evaluated by one-way analysis of variance followed by the Tukey post hoc comparisons and defined as P<0.05.
Results
Histological verifications
Figure 1 shows the photomicrograph of the location of the injection into the CA1 (right panel) and cross-sections of the rat brain indicating injection locations according to the Paxinos and Watson atlas [39], left panel). Black circles show injection sites inside the CA1.
Effect of post-training ZnO NPs administration on step-through latency
Figure 2 shows the effects of post-training administration of ZnO NPs on the latency of entrance into the dark compartment on day 1 (A) and day 3 (B) after training, and also on locomotor activity (C and D). The one-way analysis of variance in day 1 (F(2, 17)=6.06, P<0.01) and also day 3 (F(2, 17)=5.95, P<0.01) after training showed a significant difference in step-through latency among the groups. Also, post hoc analysis revealed that the groups receiving 1 mg/kg of ZnO NPs (P<0.01) spent less time to re-enter the dark compartment as compared to the control saline group, indicating ZnO NPs-induced memory loss. Moreover, the one-way analysis of variance in Figures 2C and 2D indicates that the treatments on day 1 (F(2, 17)=3.13, P>0.05) and also day 3 (F(2, 17)=1.19, P>0.05) after training did not affect locomotor activity.
Effect of post-training intra-CA1 nicotine microinjection on step-through latency
As shown in Figures 3A and 3B, post-training microinjection of nicotine (0.3 and 0.4 μg/rat, intra-CA1) had no significant effect on step-through latency at 1 (F(2, 17)=2.083, P>0.05) and 3 (F(2, 17)=3.27, P>0.05) days post-training, respectively, when compared to the control group. Figures 3C and 3D showed that the drug exposure did not alter the locomotor activity at 1 (F(2, 17)=1.71, P>0.05) and 3 (F(2, 17)=0.84, P>0.05) days post-training.
The effect of the post-training intra-CA1 nicotinic acetylcholine receptors agonist (nicotine) on the impairment effect of ZnO NPs on the passive avoidance learning.
Nicotine (intra-CA1) was microinjected 5 min before ZnO NPs administration to investigate the role of the CA1 nicotinic receptors in the impairment effect of ZnO NPs on the consolidation of passive avoidance learning. The results of the one-way analysis of variance are presented in Figure 4A, indicating a statistically significant difference in step-through latency score between the groups at 1 day (F(3, 24)=12.93, P<0.001) post-training. Post-hoc analysis showed that 0.4 µg/rat of nicotine significantly increases the step-through latency (P<0.01) 1 day after training, while the group treated with 0.4 µg/rat of nicotine plus ZnO NPs showed no significant difference as compared to the control group 3 day after training (Figure 4B, F(3, 24)=10.46, P<0.001). Thus, post-training microinjection of nicotine could improve the ZnO NPs-induced reduction in the step-through latency (1 day after training). As shown in Figures 4C and 4D, the post-training microinjection of nicotine plus ZnO NPs did not affect the locomotor activity at 1 (F(3, 24)=1.71, P>0.05) and 3 (F(3, 24)=0.84, P>0.05) days post-training.
Effect of post-training intra-CA1 mecamylamine microinjection on step-through latency
As shown in Figures 5A and 5B, post-training microinjection of mecamylamine (1 μg/rat, intra-CA1) had a significant effect on step-through latency at 1 (F(2, 17)=4.14, P<0.05) and 3 (F(2, 17)=3.90, P<0.05) days post-training, respectively, when compared to the control group and decreased step-through latency. Figures 5C and 5D showed that the drug exposure did not alter the locomotor activity at 1 (F(2, 17)=1.23, P>0.05) and 3 (F(2, 17)=1.89, P>0.05) days post-training.
The effect of the post-training intra-CA1 microinjection of ineffective dose of mecamylamine with ineffective dose of ZnO NPs on the passive avoidance learning
In the last set of experiments, we determined whether the post-training intra-CA1 microinjection of an ineffective dose of mecamylamine enhances the influence of an ineffective dose of ZnO NPs on passive avoidance learning to induce memory loss. Toward this end, mecamylamine (0.5 µg/rat, intra-CA1) was microinjected 5 min before ZnO NPs (0.5 mg/kg, intraperitoneal) administration. The results of the one-way analysis of variance are presented in Figure 6A, indicating a statistical difference in step-through latency score between the groups on day 1 (F(3, 24)=5.27, P<0.001) post-training. Post-hoc analysis showed that 0.5 µg/rat of mecamylamine significantly enhances the influence of ineffective dose of ZnO NPs on step-through latency (P<0.01) 1 day and also 3 days after training (F(3, 24)=3.34, P<0.05; Figure 6B). Thus, post-training microinjection of mecamylamine could potentiate the ZnO NPs-induced reduction in the step-through latency. As shown in Figures 6C and 6D, the post-training microinjection of mecamylamine plus ZnO NPs did not affect the locomotor activity at 1 (F(3, 24)=2.31, P>0.05) and 3 (F(3, 24)=2.18, P>0.05) day post-training.
Discussion
ZnO NPs have a broad range of applications. However, certain disadvantages and toxic side effects have been reported for biomedical applications of ZnO NPs. In recent years, the majority of prior studies have emphasized that ZnO NPs modulate learning and memory ability in animals [11-13, 43, 44]. Nevertheless, Amara et al. (2014) reported acute intravenous injection of ZnO-NPs did not affect working memory in exposed rats [45]. Kesmati et al. (2020) reported that nano-ZnO increased memory in novel object recognition test [43] and Xie et al. (2012) indicated that ZnO NPs could ameliorate the cognitive impairment in mice with depressive-like behaviors [46]. As shown in Figure 2, the present study showed that post-training intraperitoneal injection of ZnO NPs dose-dependently impaired passive avoidance memory retrieval at 1 and 3 days after training. In addition, ZnO NPs injection did not affect exploratory behavior in open field tests at 1 and 3 days after training. Our result is in line with studies that conducted by Tian et al. [12] and Han et al. [13].
The research on mechanisms by which ZnO NPs influence cognitive functions is being carried out. After bypassing the blood-brain barrier or direct entrance through the nose, ZnO NPs induce zinc deposition in functional brain regions including the hippocampus. Zinc is a neuromodulator that modulates synaptic transmission [47]. However, elevated concentrations result in functional and structural changes [8, 12, 34, 48]. Moreover, exposure to ZnO-NPs alters the homeostasis of Zn and results in cell apoptosis [6]. Liu et al. [8] and Attia et al. [10] observed the hippocampal accumulation of ZnO NPs following intravenous or oral administration led to oxidative damage, inflammatory responses, and histopathological damage in the hippocampus [8, 10]. Hippocampal oxidative stress and inflammation have been closely related to impairment in synaptic plasticity and memory [49-52]. Previous studies also have demonstrated that suppression of the hippocampal cAMP/CREB signaling pathway is involved in memory impairment following ZnO NPs administration [12]. Xie et al. (2012) using isolated rat hippocampal CA3 pyramidal neurons showed that ZnO NPs increase the current amplitude and excitability of neurons that may affect physiological functions of neurons [46]. It is also suggested that ZnO NPs disturb the metal ions homeostasis in the brain [6, 46, 53]. Homeostasis of metal ions is crucial for synaptic function [54]. Han et al. [13] reported that ZnO NPs disrupt the balance between long-term potentiation and depotentiation in the dentate gyrus of the hippocampus which leads to the alteration of synaptic plasticity and memory deficits [13]. In support of this effect, it has been shown that acute administration of ZnO NPs decreases glutamate levels in the hippocampus, a main excitatory neurotransmitter involved in long-term potentiation levels in the hippocampus [33].
In the present study, we showed that post-training intra-CA1 microinjection of nicotine dose-dependently could improve the ZnO NPs-induced reduction in the step-through latency in 1 day after training, but not after 3 days (Figure 4). Combined nicotine and ZnO NPs did not affect locomotion. These results support the involvement of hippocampal nicotinic receptors in ZnO NPs-induced deficit in passive avoidance memory. Moreover, post-training microinjection of nicotine alone had no impact by itself on step-through latency or locomotor activity in all days after training (Figure 3). Using omics technologies, Guo et al. [34] nominated cholinergic neurotransmission as the main biological process affected following ZnO NPs treatment [34]. Moreover, Hsiao et al. (2008, 2001) showed that zinc modulates nicotinic receptors in a subunit composition and dose-dependent manner [37, 55]. Yadava et al. [56] reported that ZnO NPs attenuated spatial learning and memory through an increase in oxidative stress and a decrease in acetylcholinesterase enzyme activity [56]. Taking these findings together, the results confirm the involvement of nicotinic receptor mechanisms in impairing the effect of ZnO NPs on memory formation. According to Figure 4, there is a difference in the rescuing effect of nicotine on ZnO NPs at 1 day (Figure 4A) but not at 3 days, which may be related to the time-dependent neurotoxicity of ZnO NPs in the brain [10, 38]. Chen et al. (2015) reported that as time increases Zn content increases in the brain, therefore, ZnO NPs accumulate in the brain in the long term [38].
In addition, to confirm the role of nicotinic acetylcholine receptors in the effect of ZnO NPs on memory, an ineffective dose of mecamylamine (0.5 µg/rat), a nicotinic acetylcholine receptor antagonist, which alone did not affect memory (Figure 5), injected into the hippocampus before the systemic administration of an ineffective dose of ZnO-NPs (0.5 mg/kg). The results showed that a low dose of a nicotinic acetylcholine receptor antagonist can synergize with a low dose of ZO NP to produce a memory impairment that may conform to the interaction between ZnO-NPs and nicotinic receptors in memory consolidation (Figure 6). Since ZnO NPs increase Zn deposition in the hippocampus [34] and considering to modulatory effect of ZnONPs on cholinergic nicotinic receptors [37, 55], it can be concluded that interaction between ZnONPs and nicotinic receptors is involved in ZnONPs –induced effect on memory.
On the other hand, nicotine treatment prevented memory deficits in brain disorders, exerted neuroprotective effects, upregulated cAMP/CREB signaling, and reduced reactive oxygen species generation through nAChR/Erk1/2 signaling pathway [57-59]. Considering these nicotine effects, an explanation for our obtained result can be that intra-CA1 administration of nicotine ameliorates the disturbing effects of ZnO NPs on the hippocampus via different mechanisms. However, more studies are needed to precisely identify the mechanism of the modulatory effect of nicotinic receptors on ZnO NPs’ effect on the memory process.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research and Ethics Committee of Arak University, Arak, Iran (Code: IR.ARAKMU.REC.1400.206).
Funding
This work was supported by a grant from Arak University, Arak, Iran (Grant No.: 11041).
Authors' contributions
Conceptualization, methodology, formal analysis and investigation: All authors; Writing the original draft: Ahmad Sadeghi, Farzaneh Nazari-Serenjeh and Zahra Ghasemzadeh; Project administration, supervision, review and editing: Niloufar Darbandi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Arak University, Arak, Iran for its valuable cooperation.
References
- Gomaa EZ. Microbial mediated synthesis of zinc oxide nanoparticles, characterization and multifaceted applications. J Inorg Organomet Polym Mater. 2022; 32(11):4114-32. [DOI:10.1007/s10904-022-02406-w]
- Carrouel F, Viennot S, Ottolenghi L, Gaillard C, Bourgeois D. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials. 2020; 10(1):140. [DOI:10.3390/nano10010140] [PMID] [PMCID]
- Saber M, Hayaei-Tehrani RS, Mokhtari S, Hoorzad P, Esfandiari F. In vitro cytotoxicity of zinc oxide nanoparticles in mouse ovarian germ cells. Toxicol In Vitro. 2021; 70:105032. [DOI:10.1016/j.tiv.2020.105032] [PMID]
- Anjum S, Hashim M, Malik SA, Khan M, Lorenzo JM, Abbasi BH, et al. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers. 2021; 13(18):4570. [DOI:10.3390/cancers13184570] [PMID] [PMCID]
- Zhou XQ, Hayat Z, Zhang DD, Li MY, Hu S, Wu Q, et al. Zinc oxide nanoparticles: Synthesis, characterization, modification, and applications in food and agriculture. Processes. 2023; 11(4):1193. [DOI:10.3390/pr11041193]
- Dkhil MA, Diab MS, Aljawdah HM, Murshed M, Hafiz TA, Al-Quraishy S, et al. Neuro-biochemical changes induced by zinc oxide nanoparticles. Saudi J Biol Sci. 2020; 27(10):2863-7. [DOI:10.1016/j.sjbs.2020.07.009]
- Kao YY, Cheng TJ, Yang DM, Wang CT, Chiung YM, Liu PS. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012; 48(2):464-71. [DOI:10.1007/s12031-012-9756-y] [PMID]
- Liu H, Yang H, Fang Y, Li K, Tian L, Liu X, et al. Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci Total Environ. 2020; 705:135809. [DOI:10.1016/j.scitotenv.2019.135809] [PMID]
- Sharma AK, Singh V, Gera R, Purohit MP, Ghosh D. Zinc oxide nanoparticle induces microglial death by NADPH-oxidase-independent reactive oxygen species as well as energy depletion. Mol Neurobiol. 2017; 54(8):6273-86. [DOI:10.1007/s12035-016-0133-7] [PMID]
- Attia H, Nounou H, Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat's brain after oral exposure. Toxics. 2018; 6(2):29. [DOI:10.3390/toxics6020029] [PMID] [PMCID]
- Xiaoli F, Junrong W, Xuan L, Yanli Z, Limin W, Jia L, et al. Prenatal exposure to nanosized zinc oxide in rats: neurotoxicity and postnatal impaired learning and memory ability. Nanomedicine. 2017; 12(7):777-95. [DOI:10.2217/nnm-2016-0397] [PMID]
- Tian L, Lin B, Wu L, Li K, Liu H, Yan J, et al. Neurotoxicity induced by zinc oxide nanoparticles: Age-related differences and interaction. Sci Rep. 2015; 5:16117. [DOI:10.1038/srep16117] [PMID] [PMCID]
- Han D, Tian Y, Zhang T, Ren G, Yang Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine. 2011; 6:1453-61. [DOI:10.2147/IJN.S18507] [PMID] [PMCID]
- Liang H, Chen A, Lai X, Liu J, Wu J, Kang Y, et al. Neuroinflammation is induced by tongue-instilled ZnO nanoparticles via the Ca2+-dependent NF-κB and MAPK pathways. Part Fibre Toxicol. 2018; 15(1):39. [DOI:10.1186/s12989-018-0274-0] [PMID] [PMCID]
- Rolls ET. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015; 62:119-57. [DOI:10.1016/j.cortex.2014.04.010] [PMID]
- Voss JL, Bridge DJ, Cohen NJ, Walker JA. A closer look at the hippocampus and memory. Trends Cogn Sci. 2017; 21(8):577-88. [DOI:10.1016/j.tics.2017.05.008] [PMID] [PMCID]
- Duff MC, Covington NV, Hilverman C, Cohen NJ. Semantic memory and the hippocampus: Revisiting, reaffirming, and extending the reach of their critical relationship. Front Hum Neurosci. 2020]; 13:471. [DOI:10.3389/fnhum.2019.00471] [PMID] [PMCID]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008; 9(3):182-94. [DOI:10.1038/nrn2335] [PMID]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007; 17(1):34-48. [DOI:10.1002/hipo.20240] [PMID] [PMCID]
- Kryukov VI. The role of the hippocampus in long-term memory: Is it memory store or comparator? J Integr Neurosci. 2008; 7(1):117-84. [DOI:10.1142/S021963520800171X] [PMID]
- Koukouli F, Changeux JP. Do nicotinic receptors modulate high-order cognitive processing? Trends Neurosci. 2020; 43(8):550-64. [DOI:10.1016/j.tins.2020.06.001] [PMID]
- Solari N, Hangya B. Cholinergic modulation of spatial learning, memory and navigation. The European Journal of Neuroscience. 2018; 48(5):2199-230. [DOI:10.1111/ejn.14089] [PMID] [PMCID]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006; 16(6):710-5. [DOI:10.1016/j.conb.2006.09.002] [PMID] [PMCID]
- Maurer SV, Williams CL. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol. 2017; 8:1489. [DOI:10.3389/fimmu.2017.01489] [PMID] [PMCID]
- Haam J, Yakel JL. Cholinergic modulation of the hippocampal region and memory function. J Neurochem. 2017; 142(Suppl 2):111-21. [DOI:10.1111/jnc.14052] [PMID] [PMCID]
- Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behav Brain Res. 2011; 221(2):389-411. [DOI:10.1016/j.bbr.2010.11.036] [PMID]
- Terry AV, Callahan PM. Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob Res. 2019; 21(3):383-94. [DOI:10.1093/ntr/nty166] [PMID] [PMCID]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev. 2009; 89(1):73-120. [DOI:10.1152/physrev.00015.2008] [PMID] [PMCID]
- Kutlu MG, Gould TJ. Nicotinic receptors, memory, and hippocampus. Curr Top Behav Neurosci. 2015; 23:137-63. [DOI:10.1007/978-3-319-13665-3_6] [PMID]
- Javad-Moosavi BZ, Nasehi M, Vaseghi S, Jamaldini SH, Zarrindast MR. Activation and inactivation of nicotinic receptnors in the dorsal hippocampal region restored negative effects of total (TSD) and REM sleep deprivation (RSd) on memory acquisition, locomotor activity and pain perception. Neuroscience. 2020; 433:200-11. [DOI:10.1016/j.neuroscience.2020.03.006] [PMID]
- Raoufi M, Mirdamadi S, Mahboubi F, Ahangarani S, Mahdipoor MS, Elmkhah H. Effect of active screen plasma nitriding pretreatment on wear behavior of TiN coating deposited by PACVD technique. Appl Surf Sci. 2012; 258(20):7820-5. [DOI:10.1016/j.apsusc.2012.04.041]
- Alijanpour S, Rezayof A. Involvement of dorsal hippocampal and medial septal nicotinic receptors in cross state-dependent memory between WIN55, 212-2 and nicotine or ethanol in mice. Neuroscience. 2013; 245:61-73. [DOI:10.1016/j.neuroscience.2013.04.030] [PMID]
- Torabi M, Kesmati M, Harooni HE, Varzi HN. Different efficacy of nanoparticle and conventional ZnO in an animal model of anxiety. Neurophysiology. 2013; 45:299-305. [DOI:10.1007/s11062-013-9372-7]
- Guo Z, Zhang P, Luo Y, Xie HQ, Chakraborty S, Monikh FA, et al. Intranasal exposure to ZnO nanoparticles induces alterations in cholinergic neurotransmission in rat brain. Nano Today. 2020; 35:100977.[DOI:10.1016/j.nantod.2020.100977]
- Torabi M, Kesmati M, Galehdari H, Varzi HN, Pourreza N. MgO and ZnO nanoparticles anti-nociceptive effect modulated by glutamate level and NMDA receptor expression in the hippocampus of stressed and non-stressed rats. Physiol Behav. 2020; 214:112727. [DOI:10.1016/j.physbeh.2019.112727] [PMID]
- Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, Bayat AH, Fathi M, Vakili K, et al. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem Neurosci. 2020; 11(13):1909-13. [DOI:10.1021/acschemneuro.0c00249] [PMID]
- Hsiao B, Mihalak KB, Magleby KL, Luetje CW. Zinc potentiates neuronal nicotinic receptors by increasing burst duration. J Neurophysiol. 2008; 99(2):999-1007. [DOI:10.1152/jn.01040.2007] [PMID]
- Chen WY, Cheng YH, Hsieh NH, Wu BC, Chou WC, Ho CC, et al. Physiologically based pharmacokinetic modeling of zinc oxide nanoparticles and zinc nitrate in mice. Int J Nanomedicine. 2015; 10:6277-92. [DOI:10.2147/IJN.S86785] [PMID] [PMCID]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2007. [Link]
- Farahani ZV, Momeni H. The effects of pentoxifylline on memory in male rats treated with zinc oxide NPs. J Inflamm Dis. 2021; 25(1):1-0.[DOI:10.32598/JQUMS.25.1.1]
- Nazari-Serenjeh F, Darbandi N, Majidpour S, Moradi P. Ghrelin modulates morphine-nicotine interaction in avoidance memory: Involvement of CA1 nicotinic receptors. Brain Res. 2019; 1720:146315. [DOI:10.1016/j.brainres.2019.146315] [PMID]
- Alijanpour S, Ghasemzadeh Z, Ebrahimi-Ghiri M, Zarrindast MR. Basolateral amygdala cannabinoid CB1 receptors mediate the antinociceptive activity of harmaline in adolescent male mice. Physiol Behav. 2022; 254:113886. [DOI:10.1016/j.physbeh.2022.113886] [PMID]
- Kesmati M, Torabi M, Pourreza N, Abdollahzadeh R, Rahiminezhadseta R, Banitorof MB. Effects of nanoparticle and conventional-size suspensions of MgO and ZnO on recognition memory in mice. Neurophysiology. 2020; 52(2020):23-30. [DOI:10.1007/s11062-020-09847-4]
- Farokhcheh M, Hejazian L, Akbarnejad Z, Pourabdolhossein F, Hosseini SM, Mehraei TM, et al. Geraniol improved memory impairment and neurotoxicity induced by zinc oxide nanoparticles in male wistar rats through its antioxidant effect. Life Sci. 2021; 282:119823. [DOI:10.1016/j.lfs.2021.119823] [PMID]
- Amara S, Ben-Slama I, Mrad I, Rihane N, Jeljeli M, El-Mir L, et al. Acute exposure to zinc oxide nanoparticles does not affect the cognitive capacity and neurotransmitters levels in adult rats. Nanotoxicology. 2014; 8(Suppl 1):208-15. [DOI:10.3109/17435390.2013.879342] [PMID]
- Xie Y, Wang Y, Zhang T, Ren G, Yang Z. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J Biomed Sci. 2012; 19(1):14. [DOI:10.1186/1423-0127-19-1] [PMID] [PMCID]
- Shen Z, Haragopal H, Li YV. Zinc modulates synaptic transmission by differentially regulating synaptic glutamate homeostasis in hippocampus. Eur J Neurosci. 2020; 52(7):3710-22. [DOI:10.1111/ejn.14749] [PMID]
- Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK. Zinc effects on NMDA receptor gating kinetics. Biophys J. 2011; 100(8):1910-8. [DOI:10.1016/j.bpj.2011.02.042] [PMID] [PMCID]
- Wang S, Irving G, Jiang L, Wang H, Li M, Wang X, et al. Oxidative stress mediated hippocampal neuron apoptosis participated in carbon disulfide-induced rats cognitive dysfunction. Neurochem Res. 2017; 42(2):583-94. [DOI:10.1007/s11064-016-2113-8] [PMID]
- Ustunova S, Kilic A, Bulut H, Gurel-Gurevin E, Eris AH, Meral I. Impaired memory by hippocampal oxidative stress in rats exposed to 900 MHz electromagnetic fields is ameliorated by thymoquinone. Toxicol Environ Chem. 2022; 104(1):176-93. [DOI:10.1080/02772248.2022.2051509]
- Padurariu M, Ciobica A, Lefter R, Lacramioara Serban I, Stefanescu C, Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub. 2013; 25(4):401-9. [Link]
- Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and alzheimer's disease. J Alzheimers Dis. 2017; 57(4):1105-21. [DOI:10.3233/JAD-161088] [PMID] [PMCID]
- Sruthi S, Ashtami J, Mohanan PV. Biomedical application and hidden toxicity of Zinc oxide nanoparticles. Mater Today Chem. 2018; 10:175-86. [DOI:10.1016/j.mtchem.2018.09.008]
- Wang L, Yin YL, Liu XZ, Shen P, Zheng YG, Lan XR, et al. Current understanding of metal ions in the pathogenesis of alzheimer's disease. Transl Neurodegener. 2020; 9:10. [DOI:10.1186/s40035-019-0179-3] [PMID] [PMCID]
- Hsiao B, Dweck D, Luetje CW. Subunit-dependent modulation of neuronal nicotinic receptors by zinc. J Neurosci. 2001; 21(6):1848-56. [DOI:10.1523/JNEUROSCI.21-06-01848.2001] [PMID] [PMCID]
- Yadav E, Singh D, Yadav P, Verma A. Comparative evaluation of prosopis cineraria (L.) druce and its zno nanoparticles on scopolamine induced amnesia. Front Pharmacol. 2018; 9:549. [DOI:10.3389/fphar.2018.00549] [PMID] [PMCID]
- Dong Y, Bi W, Zheng K, Zhu E, Wang S, Xiong Y, et al. Nicotine prevents oxidative stress-induced hippocampal neuronal injury through α7-nAChR/Erk1/2 signaling pathway. Front Mol Neurosci. 2020; 13:557647. [DOI:10.3389/fnmol.2020.557647] [PMID] [PMCID]
- Alkadhi KA. Neuroprotective effects of nicotine on hippocampal long-term potentiation in brain disorders. J Pharmacol Exp Ther. 2018; 366(3):498-508. [DOI:10.1124/jpet.118.247841] [PMID]
- Ghasemzadeh Z, Sardari M, Javadi P, Rezayof A. Expression analysis of hippocampal and amygdala CREB-BDNF signaling pathway in nicotine-induced reward under stress in rats. Brain Res. 2020; 1741:146885. [DOI:10.1016/j.brainres.2020.146885] [PMID]
Type of Study: Original Research |
Subject:
Toxicology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |