Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 191-202 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ohiemi Amedu N, Olim Obu M. Assessment of Potential Acetylcholinesterase Inhibitors: An In Silico Study. Pharm Biomed Res 2024; 10 (3) :191-202
URL: http://pbr.mazums.ac.ir/article-1-619-en.html
URL: http://pbr.mazums.ac.ir/article-1-619-en.html
1- Department of Anatomy, Faculty of Basic Medical Sciences, Adeleke University, Ede, Nigeria.
2- Department of Anatomy, College of Basic Medical Sciences, Chrisland University, Abeokuta, Nigeria.
2- Department of Anatomy, College of Basic Medical Sciences, Chrisland University, Abeokuta, Nigeria.
Full-Text [PDF 3360 kb]
(761 Downloads)
| Abstract (HTML) (1713 Views)
Full-Text: (968 Views)
Introduction
In 2016, the global incidence of dementia was estimated at 43.8 million, marking a substantial increase from the 20.2 million cases reported in 1990. Projections indicate that this figure will surpass 100 million by 2050 [1, 2]. Dementia ranked as the 5th leading cause of death worldwide in 2016. It is a major socioeconomic challenge associated with aging populations [3]. The most common cause of dementia is Alzheimer disease (AD), which accounts for 50%–80% of all dementia cases [3].
AD is a neurodegenerative condition marked by progressive impairment in cognition, emotion, language, and memory [4]. Biomarkers detect the pathophysiological abnormalities of AD in vivo [5]. The risk factors for AD are diverse, including genetic and environmental elements. Treatable medical conditions such as type 2 diabetes, traumatic brain injury, epilepsy, and depression have been associated with AD [4, 6]. Various cardiovascular diseases, like hypertension, atrial fibrillation, and atherosclerosis, are associated with elevated levels of amyloid beta, which contributes to neurodegeneration [6]. The use of cholinesterase inhibitors represents one therapeutic approach for managing AD [7].
Acetylcholinesterase (AChE) is an enzyme that hydrolyzes acetylcholine (ACh) into acetate and choline, thereby terminating signal transmission [8]. Data from an earlier study show a strong connection between low acetylcholine levels and dysfunction of the cholinergic system, as well as a decline in cognitive capacity, learning, and memory [9]. AChE inhibitors (AChEIs) prevent the degradation of ACh, resulting in the accumulation of Ach, which in turn leads to a response that helps ameliorate the symptoms of AD [4, 7]. Currently, the main cholinesterase inhibitors (ChEIs) approved for AD treatment include donepezil, galantamine, and rivastigmine [3, 4, 7].
Donepezil, otherwise known as Aricept, is a piperidine-derived AChEI used in managing the dementia of AD, and other forms of dementia [10, 11]. It can selectively and reversibly inhibit AChE enzyme, which normally breaks down acetylcholine (ACh). The activity of donepezil directly influences the ACh level [3]. Apart from being an AChEI, donepezil opposes glutamate-induced excitatory transmission via downregulation of NMDA (N-methyl-D-aspartate) receptors and regulates amyloid proteins [12]. Donepezil exerts neuroprotective effects by inhibiting various inflammatory signaling pathways [13, 14]. Even though many approved ChEIs exist, donepezil has some advantages, such as its novel structure, strong anti-AChE activity, consistent beneficial effects on cognitive function, once-daily usage, and long-lasting efficacy [15-17].
Using in silico simulations in drug development results in a faster and more efficient drug development process [18]. 6U34 is a crystalloid structure of AChE obtained from the Protein Data Bank (PDB) or designed via homology modeling. According to experimental data from various PDB, 6U34 is not mutated and has a resolution of 2.40 Å. Furthermore, 6U34 has an average protein factor of 41.0, 242 water molecules, and a bond angle 0.521˚ [19]. Data from earlier studies has shown that both 1-indanone and piperidine on the chemical structure of donepezil are responsible for inhibiting the AChE [20].
In light of donepezil’s structure, pharmacological profile, and molecular interactions, this study set out to identify compounds that share similar structures with donepezil. The hypothesis was that compounds with a similar structure, pharmacological profile, and molecular interaction as donepezil would likely inhibit AChE or have the same effect. Thus, the study aimed to evaluate the cholinesterase inhibitory potentials of selected test compounds against AChE via in silico methods.
Materials and Methods
Ligands and proteins collection
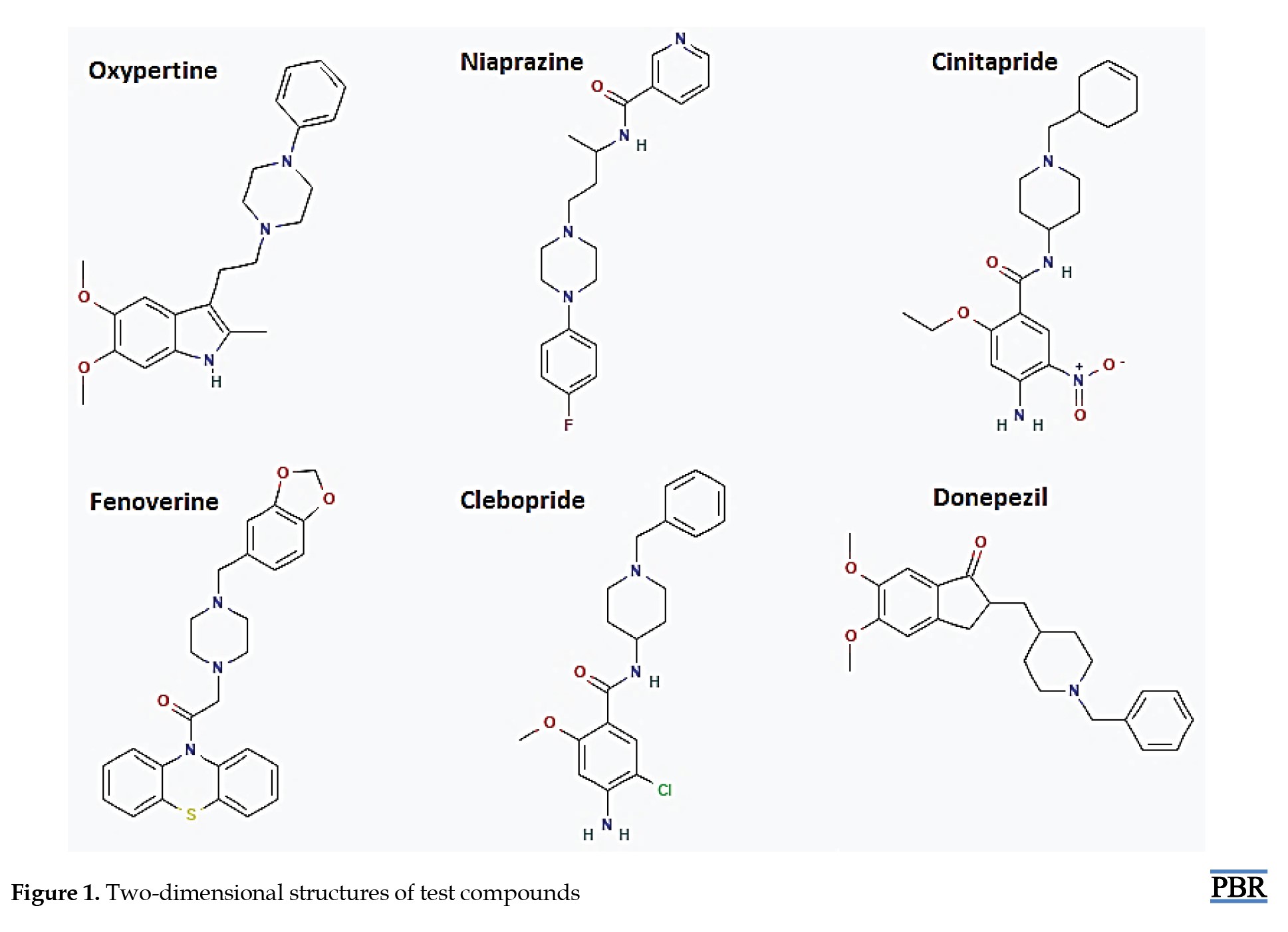
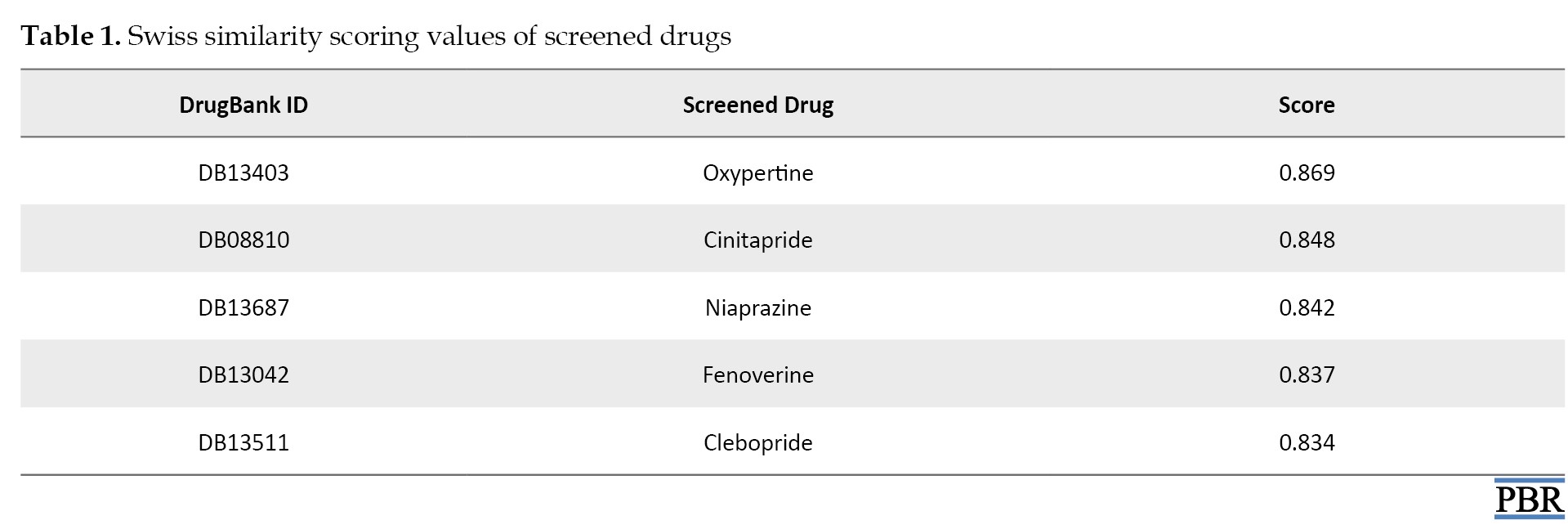
Donepezil, a standard drug that inhibits the activity of AChE, was used to screen a ligand library for compounds similar in shape (electroshape) and chemistry. Evidently, compounds with electrostatic potentials similar to donepezil are more likely to interact with AChE comparably. This similarity can lead to effective inhibition of the enzyme, thereby increasing acetylcholine levels. Furthermore, molecules with shapes similar to donepezil can fit into the AChE active site more snugly, enhancing their inhibitory potential. This shape complementarity ensures that the molecules can occupy the same spatial region as donepezil, facilitating similar interactions. Five test compounds, comprising oxypertine, cinitapride, niaprazine, fenoverine, and clebopride, were identified using the SwissSimilarity web server [21]. The top five similar compounds were chosen based on their scores (0.869-0.834) (Table 1).
The compounds’ structure data file (SDF) format was retrieved from the PubChem database. The crystal structure of AD protein biomarker AChE (hAChE PDB ID: 6U34; binding sites TRP 86, TYR 337, and TYR 124) was obtained from RCSB PDB. The target protein was prepared for docking using Discovery Studio 2020 [22]. Protein was prepared by removing water and heteroatoms from the protein and adding polar hydrogen atoms. Energy minimization and PDBQT format of the SDF files were generated using Open Babel in PyRx software [23].
Virtual screening
Docking-based virtual screening of ligands against the target protein was done using AutoDock Vina in PyRx [23]. This procedure was performed to gain more insight into the binding mode of the compounds. The AutoDock Vina grid box was set to incorporate the entire active site of the protein structure of AchE (with coordinates of x=69.65, y=145.56, z=-11.65). The protein-ligand docked complexes were visualized and analyzed with Discovery Studio 2020 software [22].
Pharmacology parameters
An in silico integrative model (SwissADME server) was utilized to determine the ADMET (absorption, distribution, metabolism, elimination, and toxicity) properties of the test compounds [24], while the ProTox-II server [25] was used to predict toxicities.
Results
The 2D structures of the test compounds (oxypertine, cinitapride, niaprazine, fenoverine, clebopride) (Figure 1) and an established drug (donepezil) were modeled and used as a target for docking studies against the target proteins (AChE). The physicochemical properties of test compounds (Table 2) show that their molecular weights (MW) ranged from 379.49 g/mol (donepezil) to 459.56 g/mol (fenoverine).
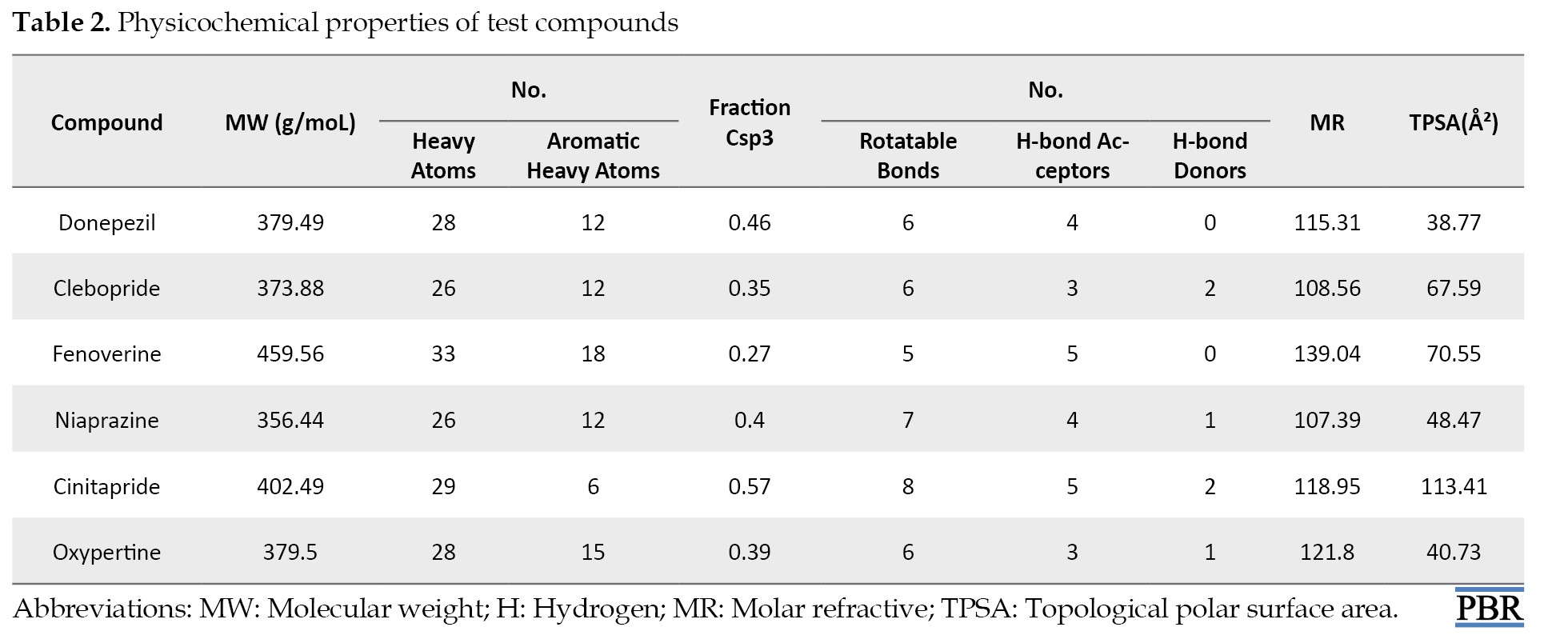
The molar refractive (MR) of donepezil was 115.31, while those of fenoverine, clebopride, cinitapride, niaprazine, and oxypertine were 139.04, 108.56, 118.95, 107.39 and 121.8 m3/mol, respectively. The topological polar surface area (TPSA) of test compounds showed that donepezil had 38.77 Ų while fenoverine, clebopride, cinitapride, niaprazine, and oxypertine had 70.55, 67.59, 113.41, 48.47, and 40.73 Ų, respectively (Table 2). The number of rotatable bonds, H-bond donors, and H-bond acceptors of test compounds were ≥8, ≥5, and ≥2, respectively (Table 2).
The gastrointestinal absorption of all the test compounds is high (Table 3).
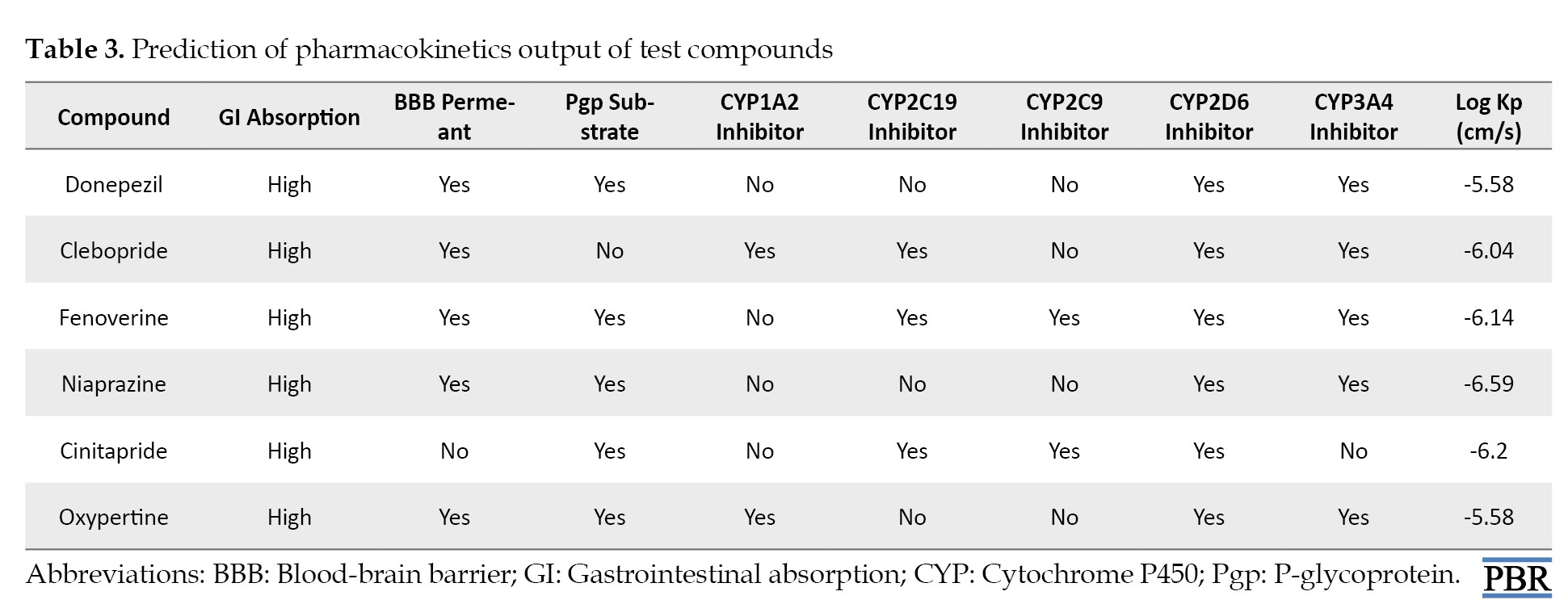
Similarly, all test compounds except cinitapride are permeable to the blood-brain barrier (Table 3). Except for clebopride, all test compounds use P-glycoprotein (Pgp) as a transport substrate. All test compounds except clebopride and oxypertine inhibit the activity of CYP1A2. Furthermore, all test compounds are inhibitors of CYP2D6. CYP3A4 is inhibited by all test compounds except cinitapride. According to Table 3, the skin permeation rate of donepezil was -5.58 cm/s, while fenoverine, clebopride, cinitapride, niaprazine, and oxypertine were -6.14, -6.04, -6.2, -6.59, and -5.58 cm/s, respectively.
The prediction of lipophilicity of test compounds (Table 4) showed that donepezil has a consensus LogP of 4, followed by fenoverine (3.74).
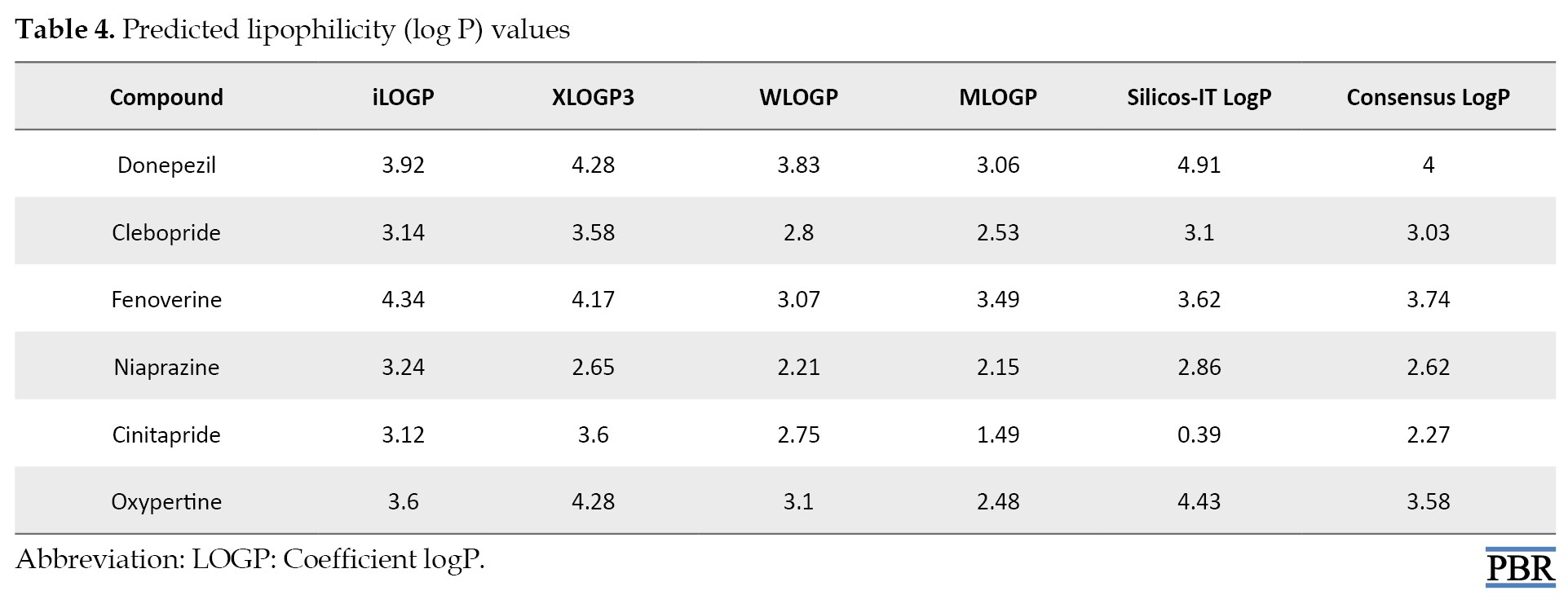
Others are 3.03, 2.27, 2.62, and 3.58 (clebopride, cinitapride, niaprazine, and oxypertine, respectively).
A prediction of drug-likeness of the test compounds based on Lipinski’s rule of five indicated no violation of the rule by any test compound (Table 5).
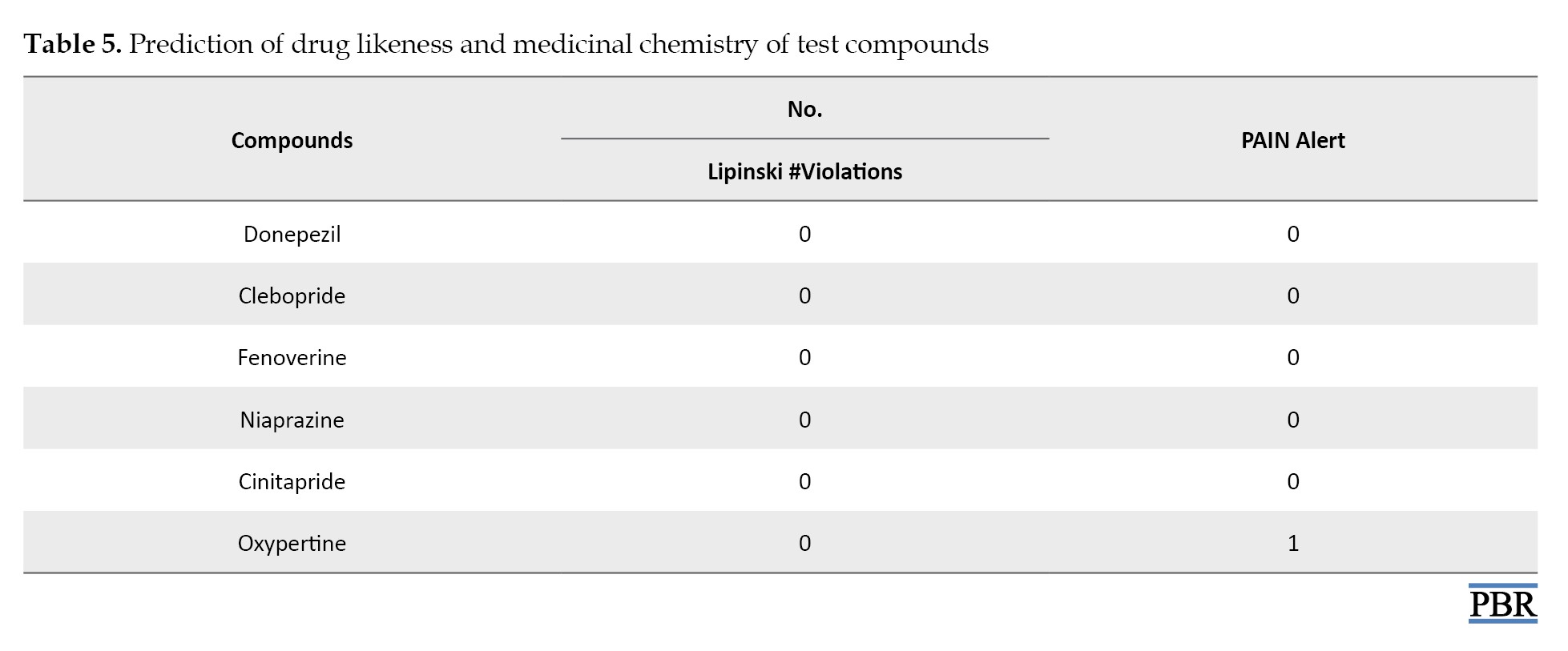
The Lipinski rule of five specifies that compounds that are considered acceptable drugs have the following characteristics: Molecular weight of ≤500, number of hydrogen bond donors of ≤5, number of hydrogen bond acceptors of ≤10, and lipophilicity (LogP) of ≤5. Except for oxypertine, other test compounds are unlikely to cause pain (Table 5).
Water solubility (ESOL) prediction of test compounds (Table 6) showed that donepezil was moderately soluble in water (1.55×10-5 mol/L).
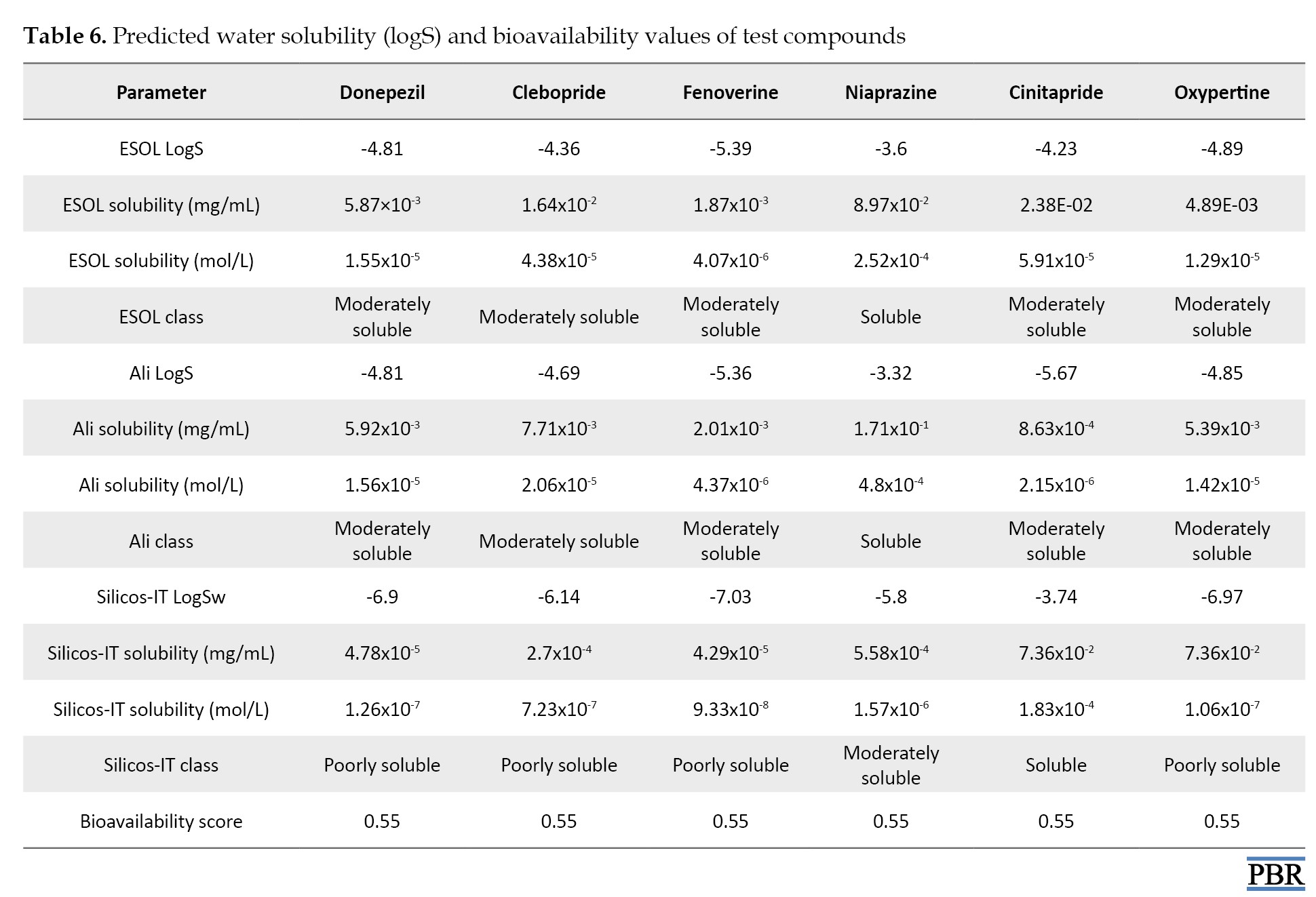
Similarly, Fenoverine (4.07 ×10-6 mol/L), Clebopride (4.38×10-5 mol/L), cinitapride (5.91×10-5 mol/L), and oxypertine (1.29×10-5 mol/L) were moderately solubilized in water. Niaprazine was soluble in water (2.52×10-4 mol/L). All the tested compounds showed a bioavailability score 0.55 (Table 6).
The binding affinity results of the ligands against AChE are presented in Table 7.
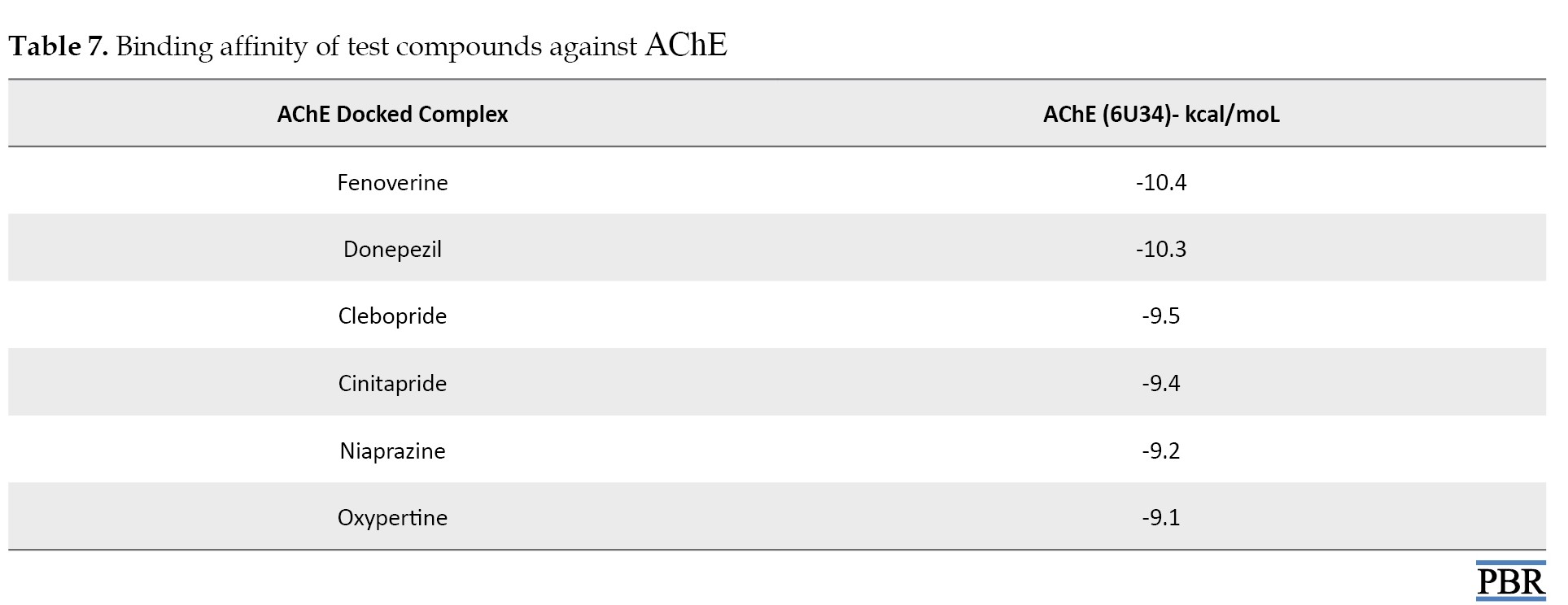
Fenoverine attained the highest binding affinity score of −10.40 kcal/mol, followed by donepezil, with docking scores of −10.30 kcal/mol. Furthermore, the binding affinities of clebopride, cinitapride, niaprazine, and oxypertine are -9.50, -9.40, -9.20, and -9.10 kcal/mol, respectively.
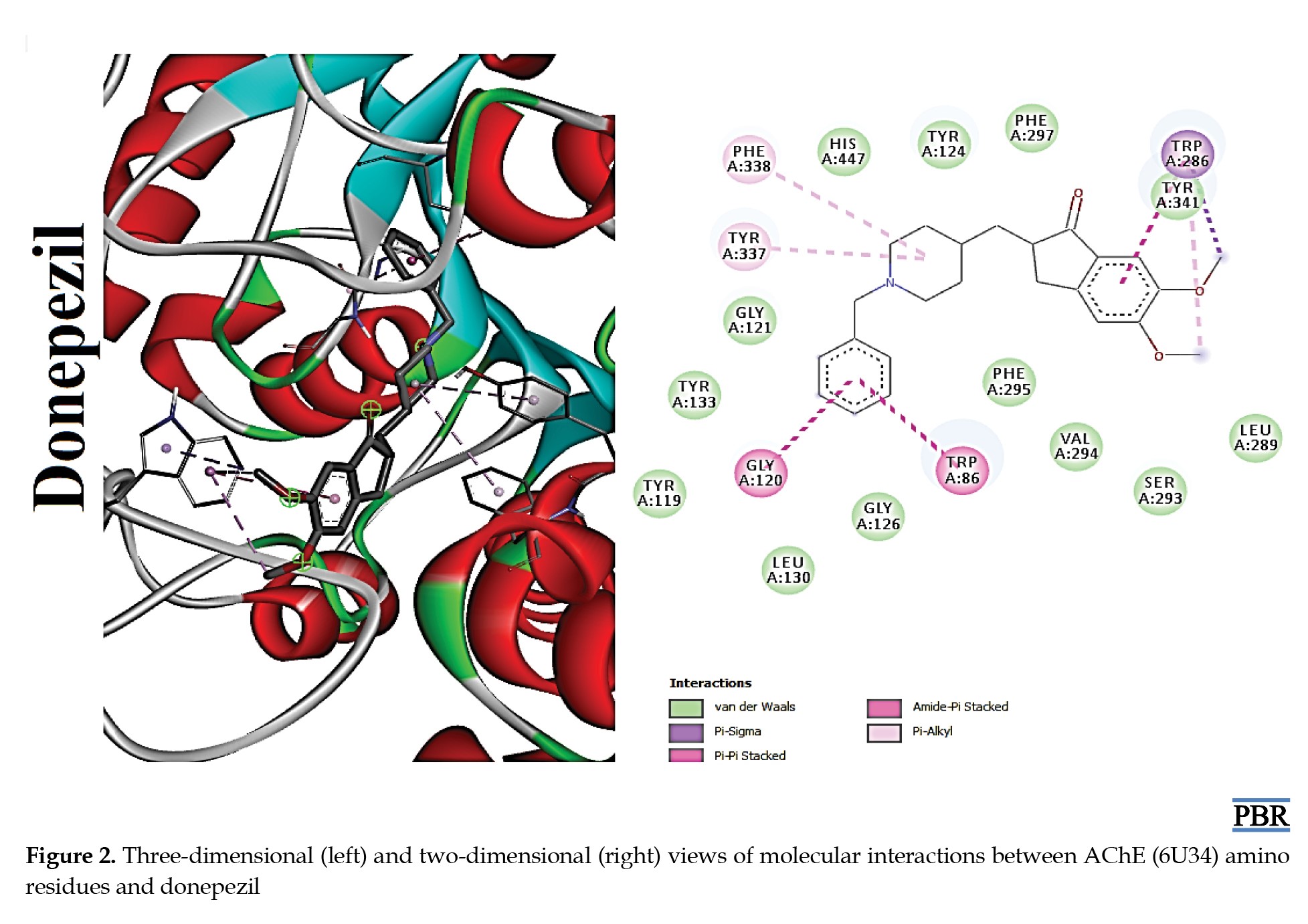
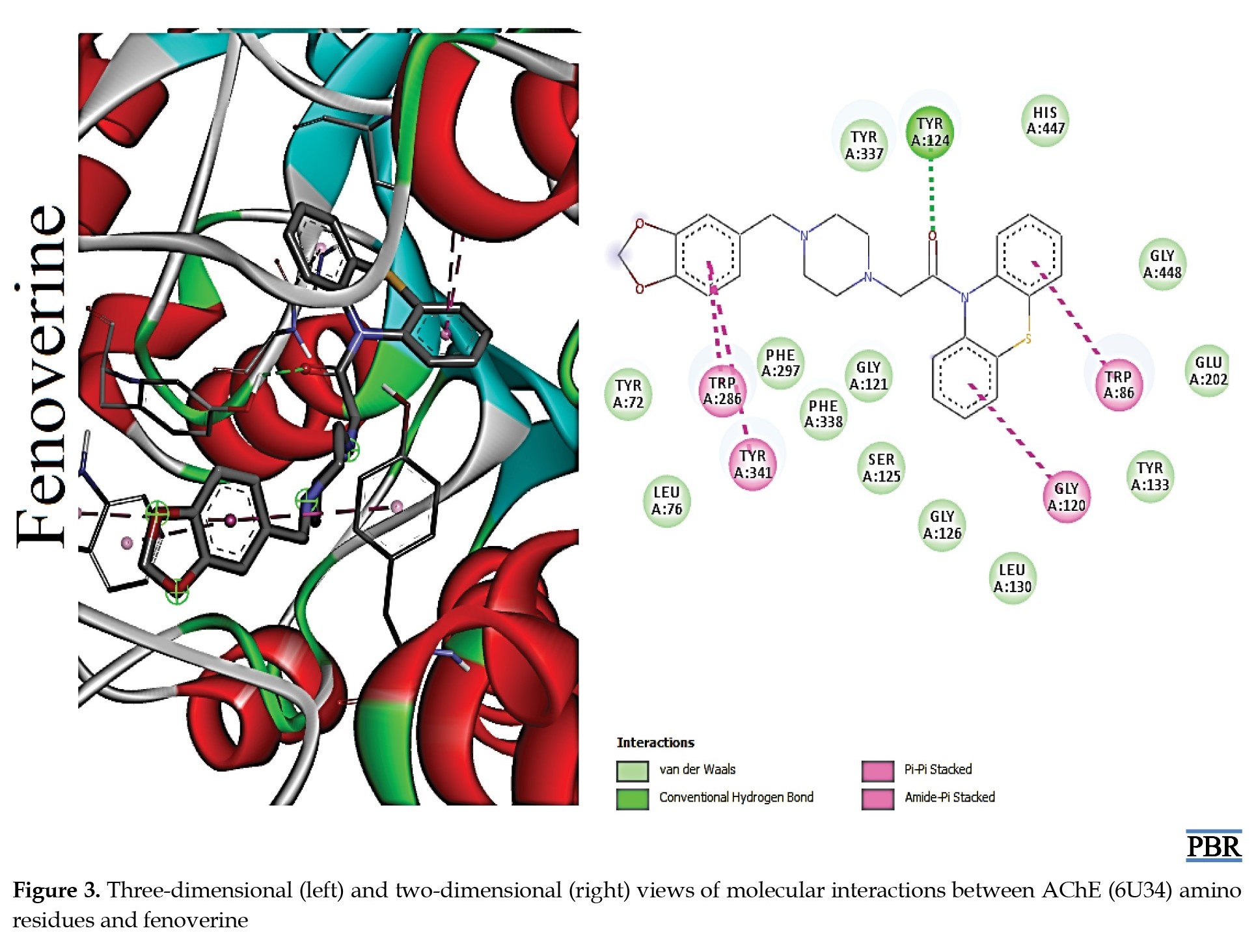
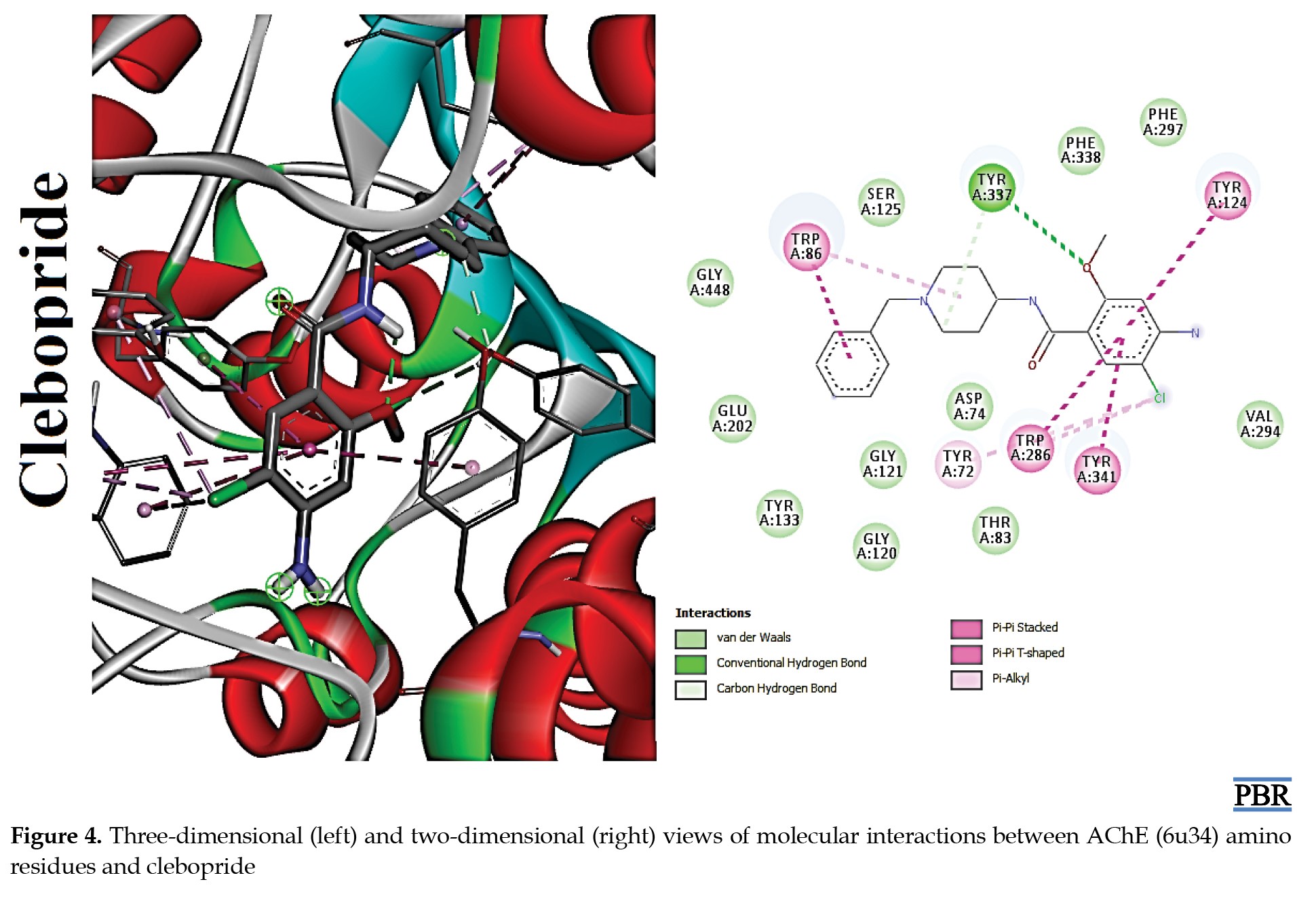
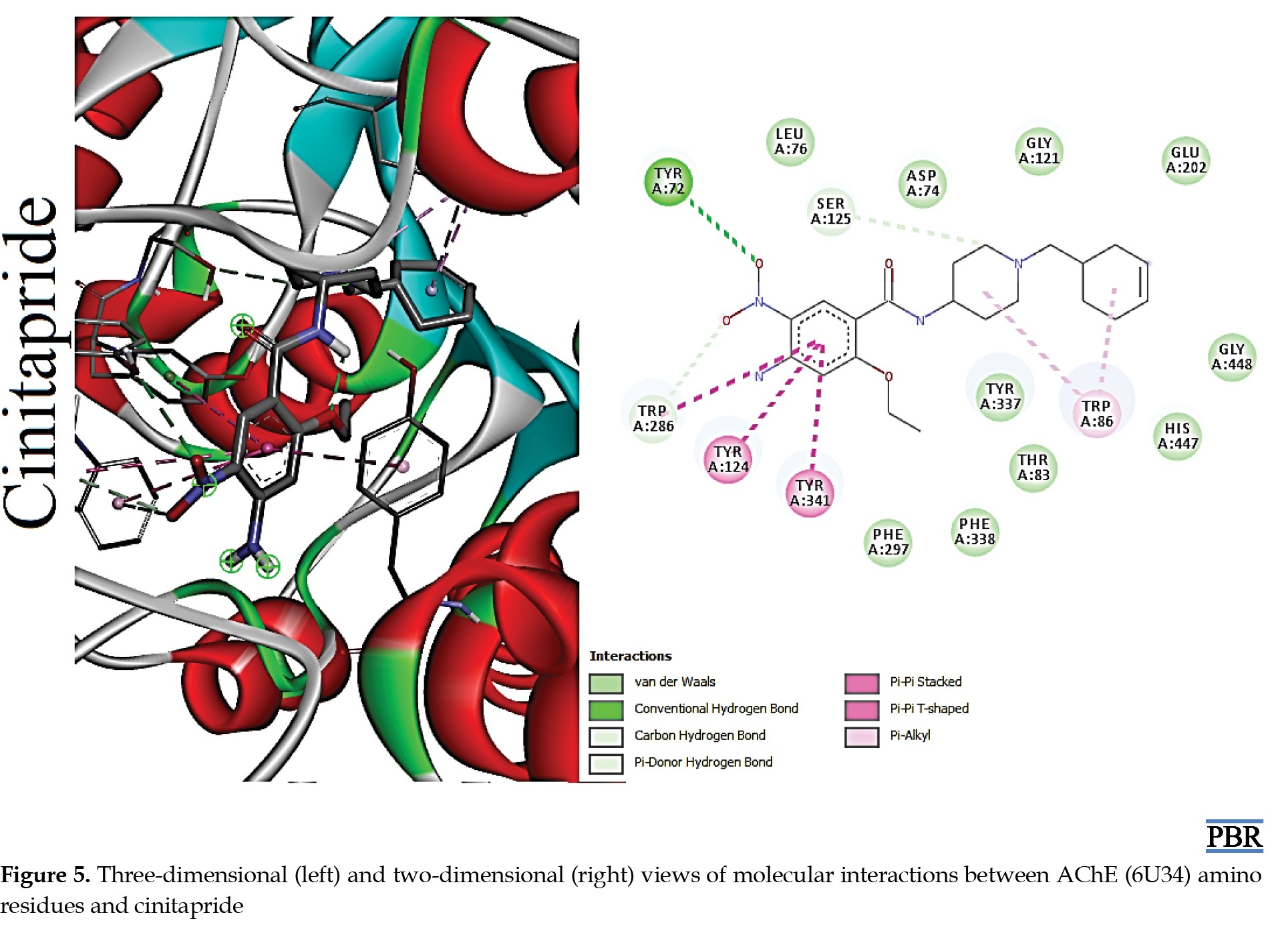
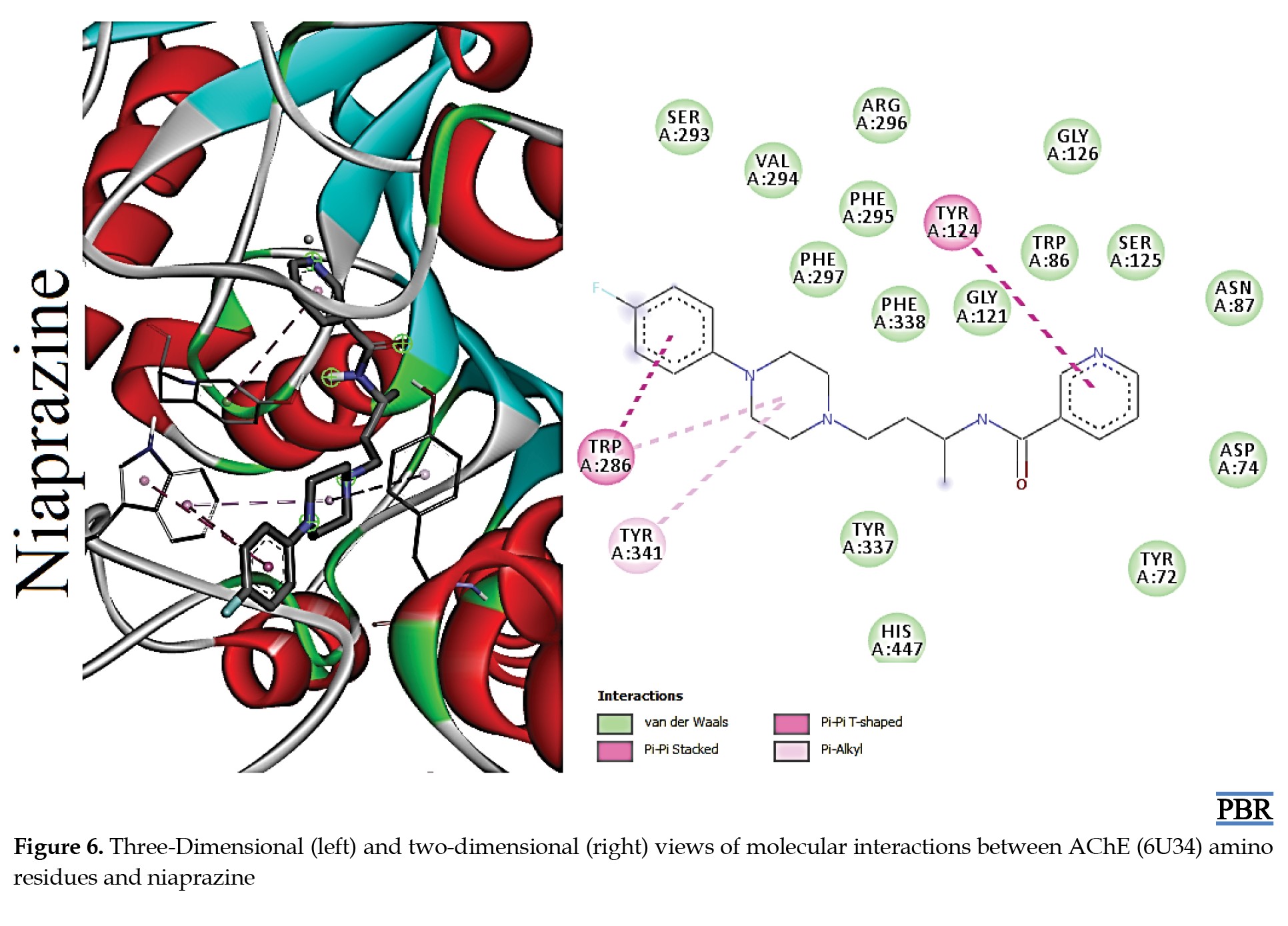
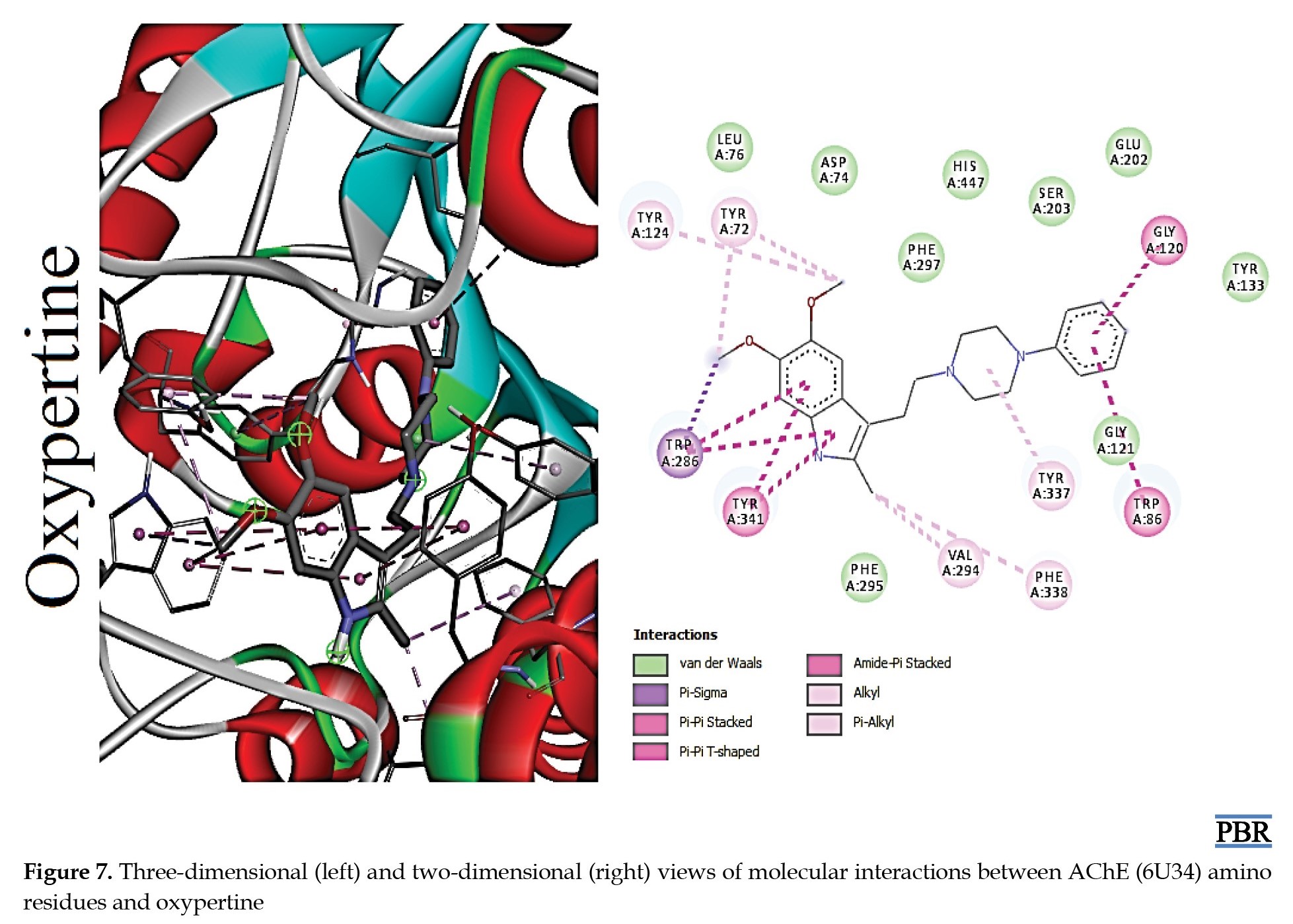
The molecular interactions (3D and 2D) of the amino acid residues of AChE with oxypertine, cinitapride, niaprazine, fenoverine, clebopride, and donepezil (standard) are presented in Figures 2, 3, 4, 5, 6 and 7. The compounds interacted with several amino residues via numerous forces such as conventional hydrogen bonds, carbon-hydrogen bonds, and π-interactions (i.e. π-alkyl bonds, π-sigma, amide-π stacking, alkyl, π-π stacking). Donepezil interacts with amino residues of AChE at HIS A:447, TRY A: 124, PHE A:297, TRY A 341, LEU A: 289, SER A: 293, VAL A: 294 PHE A:295, GLY A:126, LEU A:130, TYR A: 119, TRY A: 133, and GLY A: 121 (Van der Waals); others are PHE A: 338, TYR A: 337, GLY A: 120, TRP A: 86, and TRP A: 286 (π-interactions). The interaction of AChE with fenoverine is at TYR A: 124 (conventional H-bond), GLY A: 121 (Van der Waal), TRP A: 286, TYR A:341, GLY A:120, and TRP A:86 (π-interactions). Furthermore, clebopride binds with AChE at TYR A: 337 (conventional H-bond and carbon H-bond), TRP A: 86, TRP A: 286, TYR A: 124, TYR A: 341, and TYR A: 72 (π-interactions). The amino residues of AChE formed interactions with cinitapride at TYR A: 72 (conventional H-bond), SER A:125 (carbon H-bond), TRP A:286 (carbon H-bond and π-interaction), TYR A: 124, TYR A: 341, and TRP A: 86 (π-interactions). Niaprazine interacted with AChE at TRP A: 286, TYR A: 124, and TYR A: 341 (π-interactions). Oxypertine had many π-interactions (TYR 124, TYR 72, TYR 341, TYR 337, TRP 286, TRP 86, VAL 294, PHE 338, and GLY 120) with AChE (Figure 2).
Discussion
In this study, we screened a ligand library to identify known drugs and bioactive compounds that share similarities with donepezil. Donepezil is an AChE inhibitor that is effective in the management of AD [4, 7]. Some of the selected bioactive compounds are potential novel inhibitors currently being tested clinically for various health conditions. For example, fenoverine is an anti-spasmodic agent used to treat irritable bowel syndrome and a potent selective BChE inhibitor [26]. Cinitapride is typically prescribed to treat gastrointestinal motility disorders such as gastroesophageal reflux disease and non-ulcer dyspepsia and as a potential therapeutic agent for AD [27, 28]. Niaprazine is a selective brain catecholamine depletor [29], while clebopride is a dopamine antagonist used to treat symptoms associated with functional gastrointestinal disorders [30]. Oxypertine is an indole derivative used to treat various central nervous system disorders [31].
Molecular docking is invaluable for exploring molecular interactions between proteins and ligands. Docking analysis was performed in the current study to recognize the various forms of interaction between amino acid residues in the active sites of AChE and selected compounds [10, 26]. All the compounds docked well within the binding pocket of AChE. Fenoverine interacted better with AChE (-10.40 kcal/mol) than other compounds, including donepezil (-10.30 kcal/mol). This result suggests that fenoverine is more likely to produce more effective actions. The reason lies in its specific binding affinity for AChE. It could be recommended as a suitable alternative to donepezil.
Drug pharmacokinetics encompasses the kinetics of drug absorption, distribution, biotransformation/metabolism, and excretion [32]. A druglike character for a molecule entails a molecular weight of 400 sufficient water solubility to be dispersed in aqueous media with concomitant lipophilic properties [33]. The physicochemical properties of a compound influence the absorption, distribution, and metabolism of such compound. For example, higher TPSA and molecular weight of a drug result in lower penetration through biological barriers [10]. In this study, the TPSA of cinitapride was (301.13 Ų) highest when compared with other test molecules. This condition indicates that the penetration of cinitapride will be slower than other test molecules. The level of blood-brain barrier penetration of cinitapride in this study supports this observation.
The blood-brain barrier is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the brain’s extracellular fluid. All the test compounds in this study, except cinitapride, are permeant to BBB. This condition implies that all test compounds except cinitapride could reach the brain by crossing into the brain’s extracellular fluid from the blood.
Metabolism prediction of drug compounds is a key process in drug discovery, including optimizing drug candidates’ pharmacokinetics, pharmacodynamics, and safety profiles [34]. The cytochrome P450 (CYP) enzymes are membrane-bound hemoproteins that detoxify xenobiotics, cellular metabolism, and homeostasis. CYP induction or inhibition is a major mechanism that underlies drug-drug interactions [35]. Enzyme inhibition impairs biotransformation or clearance and leads to toxicity [35, 36]. This study shows that fenoverine, niaprazine, cinitapride, and donepezil do not interfere with CYP1A2. Conversely, clebopride and oxypertine inhibit CYP1CYP1A2. Furthermore, the test compounds inhibit CYP2D6 and CYP3A4. This result suggests that clebopride and oxypertine would likely impair the biotransformation activity of CYP1A2. Also, it indicates that all test compounds would likely impair the biotransformation activities of CYP2D6 and CYP3A4.
Drug solubility is one of the pre-formulation properties that control the desired drug concentration in the systemic circulation [37]. Poor solubility leads to poor bioavailability. The ability of a drug compound to dissolve is impacted by its LogS value, and the lower value is better than the higher value [10]. Except for niaprazine, all the test compounds are moderately soluble and have the same level of bioavailability. This property suggests that all the compounds may have the same concentration level in the systemic circulation.
Lipophilicity or LogP is the partition coefficient logarithm of a drug compound in an organic or liquid phase [10]. According to Lipinski’s rule of five, the partition coefficient should be positive but less than ≤5 [38]. Increased lipophilicity implies an increased likelihood of binding to unwanted cellular targets and a decreased degradation rate of drug compounds in the body [39, 40]. The Log P of all the test compounds was positive and ≤5 in this study, with cinitapride showing the lowest Log P. Based on this result, the likelihood of cinitapride binding to unwanted cellular targets is low compared to other test compounds.
Lipinski’s rule of five efficiently assesses drug-likeness in drug discovery [41]. This rule indicates the merits of a viable drug candidate. To avoid violating this rule, the drug candidates must have the following properties: Molecular weight: ≤500; the number of hydrogen bond donors: ≤5; the number of hydrogen bond acceptors: ≤10; lipophilicity (expressed as LogP): ≤5; and molar refractivity of 40 to 130 [42]. This study found that all test compounds obeyed Lipinski’s rule of five. This condition, therefore implies that all the test compounds are suitable as drug candidates. However, the docking scores from the current study indicate fenoverine as a better choice based on the binding affinity to the target protein.
Conclusion
Based on molecular docking scores and pharmacological parameters (such as ADMET), fenoverine may be a therapeutic alternative to donepezil. This outcome is based on simulations; hence, in vivo studies and other relevant techniques are required to validate the present results.
Ethical Considerations
Compliance with ethical guidelines
The research project approval was obtained from the Research Ethics Committee of Adeleke University, Ede, Nigeria (Code: 00760).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology and supervision: Nathaniel Ohiemi Amedu; Investigation and writing the original draft: Michael Obu; Data collection, analysis, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Javis Computational Biology Center for their technical assistance.
References
In 2016, the global incidence of dementia was estimated at 43.8 million, marking a substantial increase from the 20.2 million cases reported in 1990. Projections indicate that this figure will surpass 100 million by 2050 [1, 2]. Dementia ranked as the 5th leading cause of death worldwide in 2016. It is a major socioeconomic challenge associated with aging populations [3]. The most common cause of dementia is Alzheimer disease (AD), which accounts for 50%–80% of all dementia cases [3].
AD is a neurodegenerative condition marked by progressive impairment in cognition, emotion, language, and memory [4]. Biomarkers detect the pathophysiological abnormalities of AD in vivo [5]. The risk factors for AD are diverse, including genetic and environmental elements. Treatable medical conditions such as type 2 diabetes, traumatic brain injury, epilepsy, and depression have been associated with AD [4, 6]. Various cardiovascular diseases, like hypertension, atrial fibrillation, and atherosclerosis, are associated with elevated levels of amyloid beta, which contributes to neurodegeneration [6]. The use of cholinesterase inhibitors represents one therapeutic approach for managing AD [7].
Acetylcholinesterase (AChE) is an enzyme that hydrolyzes acetylcholine (ACh) into acetate and choline, thereby terminating signal transmission [8]. Data from an earlier study show a strong connection between low acetylcholine levels and dysfunction of the cholinergic system, as well as a decline in cognitive capacity, learning, and memory [9]. AChE inhibitors (AChEIs) prevent the degradation of ACh, resulting in the accumulation of Ach, which in turn leads to a response that helps ameliorate the symptoms of AD [4, 7]. Currently, the main cholinesterase inhibitors (ChEIs) approved for AD treatment include donepezil, galantamine, and rivastigmine [3, 4, 7].
Donepezil, otherwise known as Aricept, is a piperidine-derived AChEI used in managing the dementia of AD, and other forms of dementia [10, 11]. It can selectively and reversibly inhibit AChE enzyme, which normally breaks down acetylcholine (ACh). The activity of donepezil directly influences the ACh level [3]. Apart from being an AChEI, donepezil opposes glutamate-induced excitatory transmission via downregulation of NMDA (N-methyl-D-aspartate) receptors and regulates amyloid proteins [12]. Donepezil exerts neuroprotective effects by inhibiting various inflammatory signaling pathways [13, 14]. Even though many approved ChEIs exist, donepezil has some advantages, such as its novel structure, strong anti-AChE activity, consistent beneficial effects on cognitive function, once-daily usage, and long-lasting efficacy [15-17].
Using in silico simulations in drug development results in a faster and more efficient drug development process [18]. 6U34 is a crystalloid structure of AChE obtained from the Protein Data Bank (PDB) or designed via homology modeling. According to experimental data from various PDB, 6U34 is not mutated and has a resolution of 2.40 Å. Furthermore, 6U34 has an average protein factor of 41.0, 242 water molecules, and a bond angle 0.521˚ [19]. Data from earlier studies has shown that both 1-indanone and piperidine on the chemical structure of donepezil are responsible for inhibiting the AChE [20].
In light of donepezil’s structure, pharmacological profile, and molecular interactions, this study set out to identify compounds that share similar structures with donepezil. The hypothesis was that compounds with a similar structure, pharmacological profile, and molecular interaction as donepezil would likely inhibit AChE or have the same effect. Thus, the study aimed to evaluate the cholinesterase inhibitory potentials of selected test compounds against AChE via in silico methods.
Materials and Methods
Ligands and proteins collection
Donepezil, a standard drug that inhibits the activity of AChE, was used to screen a ligand library for compounds similar in shape (electroshape) and chemistry. Evidently, compounds with electrostatic potentials similar to donepezil are more likely to interact with AChE comparably. This similarity can lead to effective inhibition of the enzyme, thereby increasing acetylcholine levels. Furthermore, molecules with shapes similar to donepezil can fit into the AChE active site more snugly, enhancing their inhibitory potential. This shape complementarity ensures that the molecules can occupy the same spatial region as donepezil, facilitating similar interactions. Five test compounds, comprising oxypertine, cinitapride, niaprazine, fenoverine, and clebopride, were identified using the SwissSimilarity web server [21]. The top five similar compounds were chosen based on their scores (0.869-0.834) (Table 1).

The compounds’ structure data file (SDF) format was retrieved from the PubChem database. The crystal structure of AD protein biomarker AChE (hAChE PDB ID: 6U34; binding sites TRP 86, TYR 337, and TYR 124) was obtained from RCSB PDB. The target protein was prepared for docking using Discovery Studio 2020 [22]. Protein was prepared by removing water and heteroatoms from the protein and adding polar hydrogen atoms. Energy minimization and PDBQT format of the SDF files were generated using Open Babel in PyRx software [23].
Virtual screening
Docking-based virtual screening of ligands against the target protein was done using AutoDock Vina in PyRx [23]. This procedure was performed to gain more insight into the binding mode of the compounds. The AutoDock Vina grid box was set to incorporate the entire active site of the protein structure of AchE (with coordinates of x=69.65, y=145.56, z=-11.65). The protein-ligand docked complexes were visualized and analyzed with Discovery Studio 2020 software [22].
Pharmacology parameters
An in silico integrative model (SwissADME server) was utilized to determine the ADMET (absorption, distribution, metabolism, elimination, and toxicity) properties of the test compounds [24], while the ProTox-II server [25] was used to predict toxicities.
Results
The 2D structures of the test compounds (oxypertine, cinitapride, niaprazine, fenoverine, clebopride) (Figure 1) and an established drug (donepezil) were modeled and used as a target for docking studies against the target proteins (AChE). The physicochemical properties of test compounds (Table 2) show that their molecular weights (MW) ranged from 379.49 g/mol (donepezil) to 459.56 g/mol (fenoverine).

The molar refractive (MR) of donepezil was 115.31, while those of fenoverine, clebopride, cinitapride, niaprazine, and oxypertine were 139.04, 108.56, 118.95, 107.39 and 121.8 m3/mol, respectively. The topological polar surface area (TPSA) of test compounds showed that donepezil had 38.77 Ų while fenoverine, clebopride, cinitapride, niaprazine, and oxypertine had 70.55, 67.59, 113.41, 48.47, and 40.73 Ų, respectively (Table 2). The number of rotatable bonds, H-bond donors, and H-bond acceptors of test compounds were ≥8, ≥5, and ≥2, respectively (Table 2).
The gastrointestinal absorption of all the test compounds is high (Table 3).

Similarly, all test compounds except cinitapride are permeable to the blood-brain barrier (Table 3). Except for clebopride, all test compounds use P-glycoprotein (Pgp) as a transport substrate. All test compounds except clebopride and oxypertine inhibit the activity of CYP1A2. Furthermore, all test compounds are inhibitors of CYP2D6. CYP3A4 is inhibited by all test compounds except cinitapride. According to Table 3, the skin permeation rate of donepezil was -5.58 cm/s, while fenoverine, clebopride, cinitapride, niaprazine, and oxypertine were -6.14, -6.04, -6.2, -6.59, and -5.58 cm/s, respectively.
The prediction of lipophilicity of test compounds (Table 4) showed that donepezil has a consensus LogP of 4, followed by fenoverine (3.74).

Others are 3.03, 2.27, 2.62, and 3.58 (clebopride, cinitapride, niaprazine, and oxypertine, respectively).
A prediction of drug-likeness of the test compounds based on Lipinski’s rule of five indicated no violation of the rule by any test compound (Table 5).

The Lipinski rule of five specifies that compounds that are considered acceptable drugs have the following characteristics: Molecular weight of ≤500, number of hydrogen bond donors of ≤5, number of hydrogen bond acceptors of ≤10, and lipophilicity (LogP) of ≤5. Except for oxypertine, other test compounds are unlikely to cause pain (Table 5).
Water solubility (ESOL) prediction of test compounds (Table 6) showed that donepezil was moderately soluble in water (1.55×10-5 mol/L).

Similarly, Fenoverine (4.07 ×10-6 mol/L), Clebopride (4.38×10-5 mol/L), cinitapride (5.91×10-5 mol/L), and oxypertine (1.29×10-5 mol/L) were moderately solubilized in water. Niaprazine was soluble in water (2.52×10-4 mol/L). All the tested compounds showed a bioavailability score 0.55 (Table 6).
The binding affinity results of the ligands against AChE are presented in Table 7.

Fenoverine attained the highest binding affinity score of −10.40 kcal/mol, followed by donepezil, with docking scores of −10.30 kcal/mol. Furthermore, the binding affinities of clebopride, cinitapride, niaprazine, and oxypertine are -9.50, -9.40, -9.20, and -9.10 kcal/mol, respectively.
The molecular interactions (3D and 2D) of the amino acid residues of AChE with oxypertine, cinitapride, niaprazine, fenoverine, clebopride, and donepezil (standard) are presented in Figures 2, 3, 4, 5, 6 and 7. The compounds interacted with several amino residues via numerous forces such as conventional hydrogen bonds, carbon-hydrogen bonds, and π-interactions (i.e. π-alkyl bonds, π-sigma, amide-π stacking, alkyl, π-π stacking). Donepezil interacts with amino residues of AChE at HIS A:447, TRY A: 124, PHE A:297, TRY A 341, LEU A: 289, SER A: 293, VAL A: 294 PHE A:295, GLY A:126, LEU A:130, TYR A: 119, TRY A: 133, and GLY A: 121 (Van der Waals); others are PHE A: 338, TYR A: 337, GLY A: 120, TRP A: 86, and TRP A: 286 (π-interactions). The interaction of AChE with fenoverine is at TYR A: 124 (conventional H-bond), GLY A: 121 (Van der Waal), TRP A: 286, TYR A:341, GLY A:120, and TRP A:86 (π-interactions). Furthermore, clebopride binds with AChE at TYR A: 337 (conventional H-bond and carbon H-bond), TRP A: 86, TRP A: 286, TYR A: 124, TYR A: 341, and TYR A: 72 (π-interactions). The amino residues of AChE formed interactions with cinitapride at TYR A: 72 (conventional H-bond), SER A:125 (carbon H-bond), TRP A:286 (carbon H-bond and π-interaction), TYR A: 124, TYR A: 341, and TRP A: 86 (π-interactions). Niaprazine interacted with AChE at TRP A: 286, TYR A: 124, and TYR A: 341 (π-interactions). Oxypertine had many π-interactions (TYR 124, TYR 72, TYR 341, TYR 337, TRP 286, TRP 86, VAL 294, PHE 338, and GLY 120) with AChE (Figure 2).
Discussion
In this study, we screened a ligand library to identify known drugs and bioactive compounds that share similarities with donepezil. Donepezil is an AChE inhibitor that is effective in the management of AD [4, 7]. Some of the selected bioactive compounds are potential novel inhibitors currently being tested clinically for various health conditions. For example, fenoverine is an anti-spasmodic agent used to treat irritable bowel syndrome and a potent selective BChE inhibitor [26]. Cinitapride is typically prescribed to treat gastrointestinal motility disorders such as gastroesophageal reflux disease and non-ulcer dyspepsia and as a potential therapeutic agent for AD [27, 28]. Niaprazine is a selective brain catecholamine depletor [29], while clebopride is a dopamine antagonist used to treat symptoms associated with functional gastrointestinal disorders [30]. Oxypertine is an indole derivative used to treat various central nervous system disorders [31].
Molecular docking is invaluable for exploring molecular interactions between proteins and ligands. Docking analysis was performed in the current study to recognize the various forms of interaction between amino acid residues in the active sites of AChE and selected compounds [10, 26]. All the compounds docked well within the binding pocket of AChE. Fenoverine interacted better with AChE (-10.40 kcal/mol) than other compounds, including donepezil (-10.30 kcal/mol). This result suggests that fenoverine is more likely to produce more effective actions. The reason lies in its specific binding affinity for AChE. It could be recommended as a suitable alternative to donepezil.
Drug pharmacokinetics encompasses the kinetics of drug absorption, distribution, biotransformation/metabolism, and excretion [32]. A druglike character for a molecule entails a molecular weight of 400 sufficient water solubility to be dispersed in aqueous media with concomitant lipophilic properties [33]. The physicochemical properties of a compound influence the absorption, distribution, and metabolism of such compound. For example, higher TPSA and molecular weight of a drug result in lower penetration through biological barriers [10]. In this study, the TPSA of cinitapride was (301.13 Ų) highest when compared with other test molecules. This condition indicates that the penetration of cinitapride will be slower than other test molecules. The level of blood-brain barrier penetration of cinitapride in this study supports this observation.
The blood-brain barrier is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the brain’s extracellular fluid. All the test compounds in this study, except cinitapride, are permeant to BBB. This condition implies that all test compounds except cinitapride could reach the brain by crossing into the brain’s extracellular fluid from the blood.
Metabolism prediction of drug compounds is a key process in drug discovery, including optimizing drug candidates’ pharmacokinetics, pharmacodynamics, and safety profiles [34]. The cytochrome P450 (CYP) enzymes are membrane-bound hemoproteins that detoxify xenobiotics, cellular metabolism, and homeostasis. CYP induction or inhibition is a major mechanism that underlies drug-drug interactions [35]. Enzyme inhibition impairs biotransformation or clearance and leads to toxicity [35, 36]. This study shows that fenoverine, niaprazine, cinitapride, and donepezil do not interfere with CYP1A2. Conversely, clebopride and oxypertine inhibit CYP1CYP1A2. Furthermore, the test compounds inhibit CYP2D6 and CYP3A4. This result suggests that clebopride and oxypertine would likely impair the biotransformation activity of CYP1A2. Also, it indicates that all test compounds would likely impair the biotransformation activities of CYP2D6 and CYP3A4.
Drug solubility is one of the pre-formulation properties that control the desired drug concentration in the systemic circulation [37]. Poor solubility leads to poor bioavailability. The ability of a drug compound to dissolve is impacted by its LogS value, and the lower value is better than the higher value [10]. Except for niaprazine, all the test compounds are moderately soluble and have the same level of bioavailability. This property suggests that all the compounds may have the same concentration level in the systemic circulation.
Lipophilicity or LogP is the partition coefficient logarithm of a drug compound in an organic or liquid phase [10]. According to Lipinski’s rule of five, the partition coefficient should be positive but less than ≤5 [38]. Increased lipophilicity implies an increased likelihood of binding to unwanted cellular targets and a decreased degradation rate of drug compounds in the body [39, 40]. The Log P of all the test compounds was positive and ≤5 in this study, with cinitapride showing the lowest Log P. Based on this result, the likelihood of cinitapride binding to unwanted cellular targets is low compared to other test compounds.
Lipinski’s rule of five efficiently assesses drug-likeness in drug discovery [41]. This rule indicates the merits of a viable drug candidate. To avoid violating this rule, the drug candidates must have the following properties: Molecular weight: ≤500; the number of hydrogen bond donors: ≤5; the number of hydrogen bond acceptors: ≤10; lipophilicity (expressed as LogP): ≤5; and molar refractivity of 40 to 130 [42]. This study found that all test compounds obeyed Lipinski’s rule of five. This condition, therefore implies that all the test compounds are suitable as drug candidates. However, the docking scores from the current study indicate fenoverine as a better choice based on the binding affinity to the target protein.
Conclusion
Based on molecular docking scores and pharmacological parameters (such as ADMET), fenoverine may be a therapeutic alternative to donepezil. This outcome is based on simulations; hence, in vivo studies and other relevant techniques are required to validate the present results.
Ethical Considerations
Compliance with ethical guidelines
The research project approval was obtained from the Research Ethics Committee of Adeleke University, Ede, Nigeria (Code: 00760).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology and supervision: Nathaniel Ohiemi Amedu; Investigation and writing the original draft: Michael Obu; Data collection, analysis, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate Javis Computational Biology Center for their technical assistance.
References
- Korsnes MS, Winkler AS. Global, regional, and national burden of dementia, 1990-2016: Predictions need local calibration. Neurology. 2020; 94(16), 718-9. [DOI:10.1212/WNL.0000000000009301] [PMID]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019; 18(1):88-106. [DOI:10.1016/S1474-4422(18)30403-4] [PMID]
- Mitić M, Lazarević-Pašti,T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin Drug Metab Toxicol. 2021; 17(7):841-56. [DOI:10.1080/17425255.2021.1931681] [PMID]
- Se Thoe E, Fauzi A, Tang YQ, Chamyuang S, Chia AYY. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021; 276:119129. [DOI:10.1016/J.LFS.2021.119129] [PMID]
- Jack CR, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013; 80(6):1347-58. [DOI:10.1016/j.neuron.2013.12.003] [PMID]
- Edwards Iii GA, Gamez N, Escobedo G Jr, Calderon O, Moreno-Gonzalez I. Modifiable risk factors for Alzheimer’s disease. Front Aging Neurosci. 2019; 11:146. [DOI:10.3389/fnagi.2019.00146] [PMID]
- Ahmed S, Khan ST, Zargaham MK, Khan AU, Khan S, Hussain A, et al. Potential therapeutic natural products against Alzheimer’s disease with Reference of Acetylcholinesterase. Biomed Pharmacother. 2021; 139:111609. [DOI:10.1016/J.BIOPHA.2021.111609] [PMID]
- Thapa S, Lv M, Xu H. Acetylcholinesterase: A primary target for drugs and insecticides. Mini Rev Med Chem. 2017; 17(17):1665-76. [DOI:10.2174/1389557517666170120153930] [PMID]
- Maurer SV, Williams CL. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol. 2017; 8:1489. [DOI:10.3389/FIMMU.2017.01489] [PMID]
- Ojo OA, Ojo AB, Okolie C, Nwakama MA, Iyobhebhe M, Evbuomwan IO, et al. Deciphering the interactions of bioactive compounds in selected traditional medicinal plants against Alzheimer’s Diseases via Pharmacophore Modeling, Auto-QSAR, and Molecular Docking Approaches. Molecules (Basel, Switzerland). 2021; 26(7):1996. [DOI:10.3390/MOLECULES26071996] [PMID]
- Doody RS, Cummings JL, Farlow MR. Reviewing the role of donepezil in the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2012; 9(7):773-81. [DOI:10.2174/156720512802455412] [PMID]
- Murphy MP, LeVine H 3rd. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010; 19(1):311-23. [DOI:10.3233/JAD-2010-1221] [PMID]
- Hwang J, Hwang H, Lee HW, Suk K. Microglia signaling as a target of donepezil. Neuropharmacology. 2010; 58(7), 1122-9. [DOI:10.1016/j.neuropharm.2010.02.003] [PMID]
- Kim J, Lee HJ, Park SK, Park JH, Jeong HR, Lee S, et al. Donepezil Regulates LPS and Aβ-stimulated neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int J Mol Sci. 2021; 22(19):10637. [DOI:10.3390/IJMS221910637] [PMID]
- Sugimoto H, Ogura H, Arai Y, Limura Y, Yamanishi Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn J Pharmacol. 2002; 89(1):7-20. [DOI:10.1254/jjp.89.7] [PMID]
- Tsuno N. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Rev Neurother. 2009; 9(5):591-8. [DOI:10.1586/ern.09.23] [PMID]
- Cheewakriengkrai L, Gauthier S. A 10-year perspective on donepezil. Expert Opin Pharmacother. 2013; 14(3):331-8. [DOI:10.1517/14656566.2013.760543] [PMID]
- Detalle L, Vanheusden K, Sargentini-Maier ML, Stöhr T. Translational aspects in drug discovery. In: Chackalamannil S, Rotella D, Ward SE, editors. Comprehensive Medicinal chemistry III. Amsterdam: Elsevier; 2017. [DOI:10.1016/B978-0-12-409547-2.12335-2]
- Gorecki L, Gerlits O, Kong X, Cheng X, Blumenthal DK, Taylor P, et al. Rational design, synthesis, and evaluation of uncharged, “smart” bis-oxime antidotes of organophosphate-inhibited human acetylcholinesterase. J Biol Chem. 2020; 295(13):4079-92. [DOI:10.1074/JBC.RA119.012400] [PMID]
- Sağlık BN, Ilgın S, Özkay Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur J Med Chem. 2016; 124:1026-40. [DOI:10.1016/J.EJMECH.2016.10.042] [PMID]
- Bragina ME, Daina A, Perez MAS, Michielin O, Zoete V. The SwissSimilarity 2021 Web Tool: Novel chemical libraries and additional methods for an enhanced ligand-based virtual screening experience. Int J Mol Sci. 2022; 23(2):811. [DOI:10.3390/ijms23020811]
- Pawar SS, Sachin HR. Review on discovery studio: An important Tool for Molecular Docking. Asian J Res Chem. 2021; 14(1):86-8. [DOI:10.5958/0974-4150.2021.00014.6]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015; 1263:243-50.[DOI:10.1007/978-1-4939-2269-7_19] [PMID]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7:42717. [DOI:10.1038/srep42717] [PMID]
- Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018; 46(W1):W257-63. [DOI:10.1093/nar/gky318]
- Li S, Li AJ, Travers J, Xu T, Sakamuru S, Klumpp-Thomas C, et al. Identification of compounds for butyrylcholinesterase inhibition. SLAS Discov. 2021; 26(10):1355-64.[DOI:10.1177/24725552211030897] [PMID]
- Hassan M, Raza H, Abbasi MA, Moustafa AA, Seo SY. The exploration of novel Alzheimer’s therapeutic agents from the pool of FDA approved medicines using drug repositioning, enzyme inhibition and kinetic mechanism approaches. Biomed Pharmacother. 2019; 109:2513-26. [DOI:10.1016/J.BIOPHA.2018.11.115] [PMID]
- Liang L, Yu J, Xiao L, Wang G. Comparative efficacy of various pharmacological interventions in the treatment of functional dyspepsia: A network meta-analysis. Dig Dis Sci. 2022; 67(1):187-207. [DOI:10.1007/S10620-021-06846-1] [PMID]
- Keane PE, Benedetti MS. Niaprazine, a selective brain catecholamine depletor. Neuropharmacology. 1979; 18(7):595-600. [DOI:10.1016/0028-3908(79)90110-2] [PMID]
- Choi JK, Hong JY. Acute cervical dystonia induced by clebopride. Case Rep Neurol Med. 2017; 2017:2834349.[DOI:10.1155/2017/2834349] [PMID]
- Saini T, Kumar S, Narasimhan B. Central nervous system activities of indole derivatives: An overview. Cent Nerv Syst Agents Med Chem. 2015; 16(1):19-28. [DOI:10.2174/1871524915666150608103224] [PMID]
- Saghir SA, Ansari RA. Pharmacokinetics. Reference module in biomedical sciences. Amsterdam: Elsevier; 2018. [DOI:10.1016/B978-0-12-801238-3.62154-2]
- Kenakin TP. Pharmacokinetics. In: Kenakin TP, editor. A pharmacology primer. Massachusetts: Academic Press; 2019. [DOI:10.1016/B978-0-12-813957-8.00009-6]
- Zhang Z, Tang W. Drug metabolism in drug discovery and development. Acta Pharm Sin B. 2018; 8(5):721-32.[DOI:10.1016/J.APSB.2018.04.003] [PMID]
- Manikandan P, Nagini S. Cytochrome P450 structure, function and clinical significance: A review. Curr Drug Targets. 2018; 19(1):38-54. [DOI:10.2174/1389450118666170125144557] [PMID]
- Kumar S, Sharma R, Roychowdhury A. Modulation of cytochrome-P450 inhibition (CYP) in drug discovery: A medicinal chemistry perspective. Curr Med Chem. 2012; 19(21):3605-21. [DOI:10.2174/092986712801323180]
- Chaudhary N, Tripathi D, Rai AK. A technical approach of solubility enhancement of poorly soluble drugs: Liquisolid technique. Curr Drug Deliv. 2020; 17(8):638-50. [DOI:10.2174/1567201817666200516155733] [PMID]
- Liu Z, Wang S, Hu M. Oral Absorption Basics: Pathways, physico-chemical and biological factors affecting absorption. In: Qiu Y, Chen Y, Porter WR, editor. Developing solid oral dosage forms. Massachusetts: Academic Press; 2009. [DOI:10.1016/B978-0-444-53242-8.00011-4]
- Savaser A, Esim O, Kurbanoglu S, Ozkan SA, Ozkan Y. Current perspectives on drug release studies from polymeric nanoparticles. In: Mihai Grumezescu A, editor. Organic materials as smart nanocarriers for drug delivery. William Andrew: New York; 2018. [DOI:10.1016/B978-0-12-813663-8.00003-8]
- Stephens C, Lucena MI, Andrade RJ. Idiosyncratic drug-induced liver injury: Mechanisms and susceptibility factors. In: McQueen CA, editor. Comprehensive toxicology. Amsterdam: Elsevier; 2018. [DOI:10.1016/B978-0-12-801238-3.64089-8]
- Kiss DJ, Pándy-Szekeres G, Keserű GM. Computational medicinal chemistry to target GPCRs. Compr Pharm. 2022; 84-114. [DOI:10.1016/B978-0-12-820472-6.00208-5]
- Román GC, Rogers SJ. Donepezil: A clinical review of current and emerging indications. Expert Opin Pharmacother. 2004; 5(1):161-80. [DOI:10.1517/14656566.5.1.161]
Type of Study: Original Research |
Subject:
Mollecular Modeling
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |