Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 179-190 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi S, Ostad-Rahimi N, Rezaei A. Effect of Glycyrrhiza glabra Extract on Telomerase Enzyme and Biochemical Parameters in Elderly Rats. Pharm Biomed Res 2024; 10 (3) :179-190
URL: http://pbr.mazums.ac.ir/article-1-584-en.html
URL: http://pbr.mazums.ac.ir/article-1-584-en.html
Effect of Glycyrrhiza glabra Extract on Telomerase Enzyme and Biochemical Parameters in Elderly Rats
1- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 832 kb]
(650 Downloads)
| Abstract (HTML) (2025 Views)
Full-Text: (598 Views)
Introduction
Aging is a complex biological process that gradually reduces normal physiological functions in living organisms. Aging can lead to various diseases such as cancers, Alzheimer, Parkinson, heart diseases, and arteriosclerosis, and it is known as a risk factor in mortality [1, 2]. According to the Denham-Harman theory, the production and accumulation of free radicals in old age damages macromolecules. These damages are crucial in physiological function impairments during aging [3]. Based on the evidence, more reactive oxygen species (ROS) are produced by damaged mitochondria. The production and accumulation of ROS cause oxidative stress and accelerate aging [4]. In addition, a reduction in antioxidant status and an elevation in lipid peroxidation are obvious in the aging process; as reported in previous studies, aging suppresses the activity of catalase (CAT), superoxide dismutase, and glutathione peroxidase. However, it increases the malondialdehyde (MDA) concentration [5]. The integrity of DNA is protected by telomeres in the cell cycle. Over time, telomere length declines until the telomere becomes too short for cell division, leading to cell senescence, so telomere length is considered a landmark for biological aging [6]. Besides, oxidative stress reduces telomere length in aging, and antioxidants can affect the rate of telomere shortening [7]. Aging is identified as a significant factor in increasing the level of aminotransferase (AST), alanine aminotransferase (ALT), urea, and creatinine [8]. The antioxidants prevent the deleterious effects of ROS by eliminating free radicals. Antioxidant administration maintains a balance between oxidant and pro-oxidant and declines the aging process [2].
Nowadays, the utilization of herbal medicine has increased. Herbal medicine is administered to prevent various diseases all over the world. The origin of Glycyrrhiza glabra (GLY) is Eurasia, northern Africa, and western Asia. GLY is famous for its anticancer, anti-inflammatory, antidiabetic, antidepressant, and antioxidant properties [9]. It inhibits lipid peroxidation and is also considered an antioxidant due to its polyphenolic components [10].
Accordingly, we investigated the effects of GLY extract on biochemical parameters and oxidative stress in different tissues of aged rats.
Materials and Methods
Preparation of GLY extract
A specialist collected plant specimens and the herbarium verified it and assigned identification codes. The plants were dried in the shade and ground into powder. Then, 100 g of plant powder was mixed with 0.5 L of 50% alcohol in an Erlenmeyer flask and incubated on a shaker for 48 hours. The residue was filtered through filter paper, and the solvent was removed. The required doses were prepared in physiological serum and stored under sterile conditions [11].
Treatment of animals
Standard conditions were provided to maintain all mice, including a 12:12 h light: dark cycle, 50% relative humidity, and a 22-24 °C temperature, with free access to drinking water and food. Relevant protocols for the care and use of laboratory animals were followed according to the National Institute of Health guidelines, which the Ethics Committee of Mashhad University of Medical Sciences approved.
Thirty rats, aged 20 months, were randomly divided into three groups as follows:
1. Aging (AG) group: Elderly rats without intervention and considered as control.
2. AG- low GLY (GLY) group: Elderly rats received a daily oral dose of 150 mg/kg of body weight of GLY extract for 2 weeks using the gavage method.
3. AG- high GLY group: Elderly rats received a daily oral dose of 300 mg/kg of body weight of GLY extract for 2 weeks using the gavage method [11].
Upon completion of the treatment period, the rats were anesthetized using 10 mg/kg xylazine and 75 mg/kg ketamine, and their heart, liver, hippocampus, and right kidney tissues were extracted to evaluate oxidative stress. Additionally, the serum was separated to measure urea, creatinine, liver enzymes, telomerase enzyme, and levels of oxidants and antioxidants.
Biochemical evaluation
Tissue homogenization was carried out using a buffer solution. The concentration of MDA, thiol groups, and CAT enzyme activity in homogenized tissues and serum were measured. Serum separation was performed by centrifuging blood samples at 5000 rpm for 15 minutes. AST, ALT, urea, creatinine, and the levels of oxidative and antioxidant parameters were utilized for evaluation [12].
MDA assessment
This study employed the TBRS (thiobarbituric acid reactive substances) method to quantify MDA. The interaction between MDA and TBRS forms a red complex with a peak optical density at 532 nm. A spectrophotometer was employed to measure the absorption at a wavelength of 535 nm. The formula of MDA was used as follows (Equation 1) [13]:
1. C(M)=Absorbance/1.56×105
CAT activity assessment
The Aebi method was employed to measure CAT activity. In this method, hydrogen peroxide is decomposed at 240 nm. The procedure commenced by adding 30 mM H2O2 to an adequate homogenized tissue in a 50 mM sodium phosphate buffer. Furthermore, the absorption at 240 nm was assessed after a 3-minute interval, and the specific activity was determined in units per milligram of protein per minute at the culmination of the process [14].
Thiol concentration assessment
The reagent DTNB [5,5’-dithiobis-(2-nitrobenzoic acid)] is acknowledged as a pivotal factor for evaluating thiol groups. In this study, following adding 1 mL of Tris-EDTA buffer to 50 μL of serum. The absorption at a wavelength of 412 nm was measured against the Tris-EDTA buffer alone (A1). Subsequently, by introducing 20 μL of the DTNB reagent into the solution, the sample’s absorption was re-evaluated after a 10-minute interval (A2). The absorption of the empty DTNB reagent was considered (B). Ultimately, the following formula was used (Equation 2) [15]:
2. Total thiol concentration (mM)=(A2-A1-B)×1.07/0.05×13.6
Urea and creatinine level assessment
This study assessed kidney function using colorimetric diagnostic kits’ protocols to evaluate urea and creatinine levels (Pars Azmon Co., Iran) [16].
Liver enzymes assessment
The hepatic enzymes were assessed following the instructions in the respective kits (Pars Azmon Co., Iran) [17].
Telomerase enzyme assessment
Serum blood samples underwent evaluation following the kit’s protocol to determine telomerase enzyme activity (Elabscience) [18].
In this procedure, 100 µL of serum was introduced into the plates, and the samples were subsequently incubated, and then the antibody was added. Next, the samples were incubated for an additional hour. After three washes, HRP (Horseradish peroxidase) conjugate was introduced, and the plates were incubated at 37 °C for 30 minutes. After the final wash, 90 µL of substrate solution was added. After incubation, 50 µL of stop solution was introduced, and the absorbance was recorded at a wavelength of 450 nm.
Statistical analysis
The GraphPad Prism software, version 16 was employed for data analysis. The one-way analysis of variance (ANOVA) and Tukey test were used. The P<0.05 was regarded as noticeable in different measurements.
Results
Biochemical assessment
MDA concentration
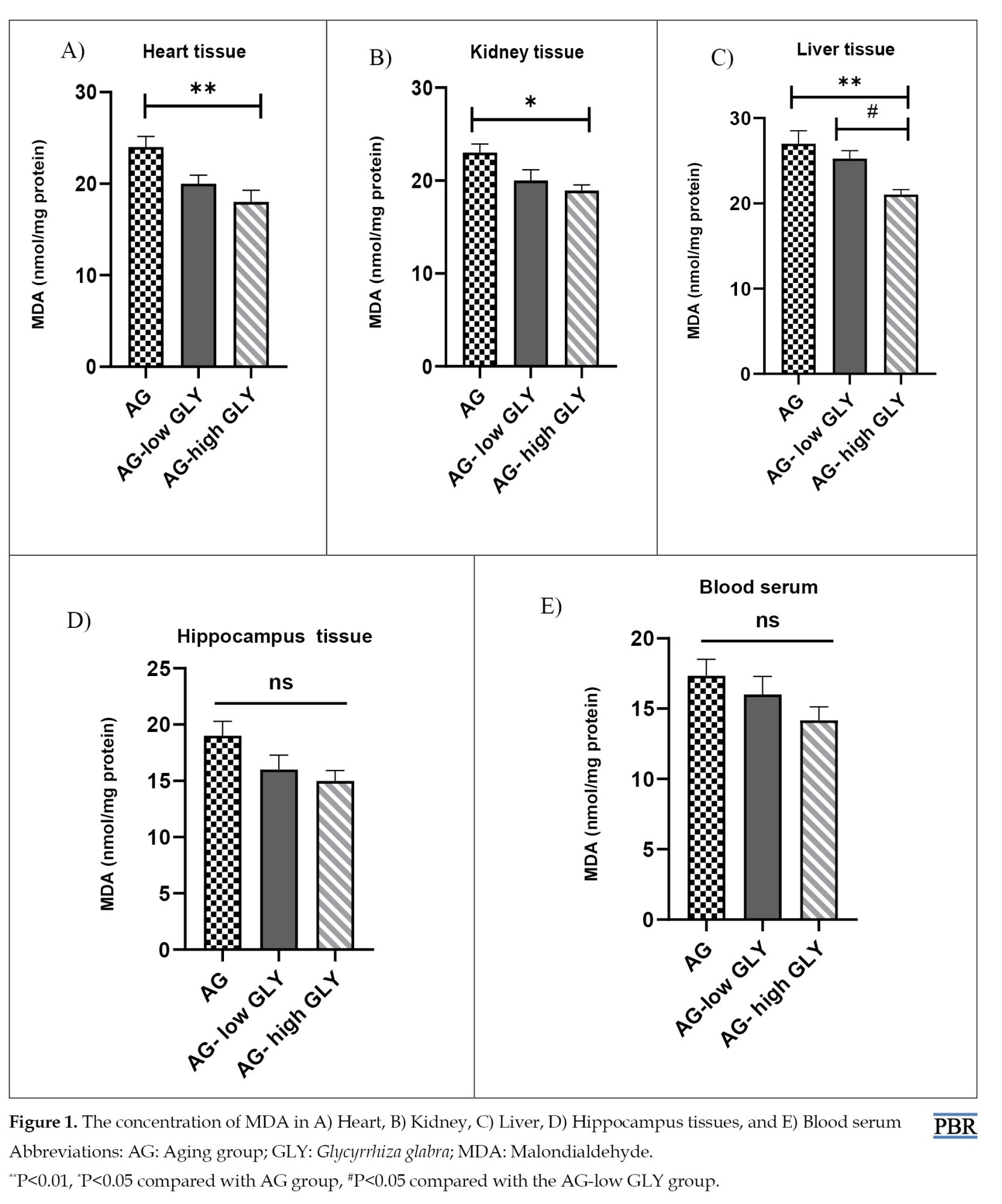
According to the results, MDA concentration in the AG-high GLY group decreased notably in comparison with the AG group in the kidney, heart (P<0.05), and liver tissues (P<0.01). In liver tissue, the level of MDA in the AG-high GLY group was remarkably lower than that in the AG-low GLY group (P<0.05). There was no significant difference in the MDA level in the hippocampus tissue and blood serum between various groups. (Figure 1A-E).
CAT activity
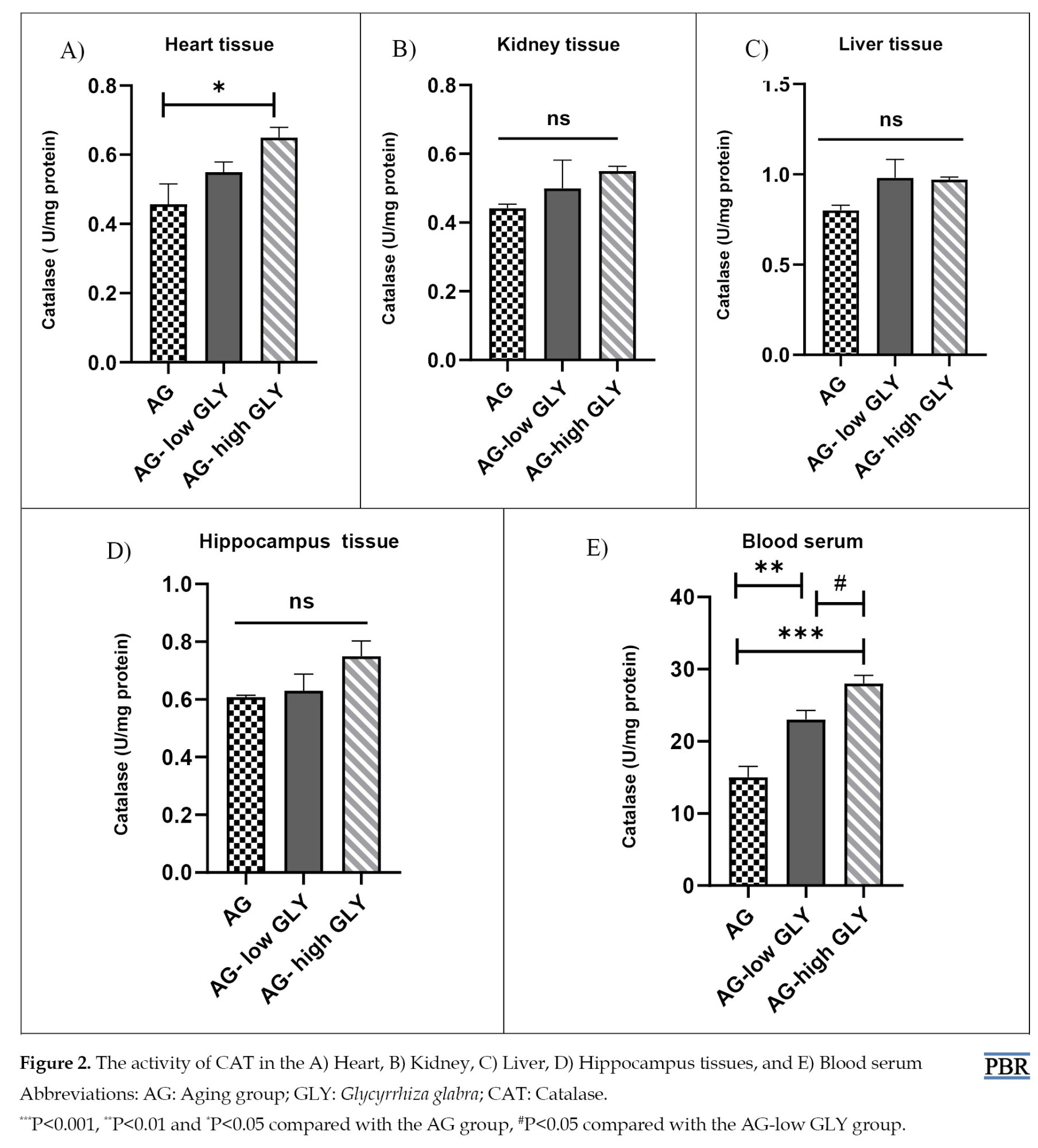
Remarkably higher CAT activity in the AG-high GLY group was seen compared to the AG group (P<0.05). In blood serum, the CAT activity in the AG-low GLY and AG-high GLY groups elevated significantly compared to the AG group (P<0.01, P<0.001, respectively). Besides, the CAT level in the AG-high GLY group was notably higher than the AG-low GLY group in blood serum (P<0.05). The change in CAT activity between different groups in the liver, kidney, and hippocampus tissues was not significant (Figure 2A-E).
Thiol concentration
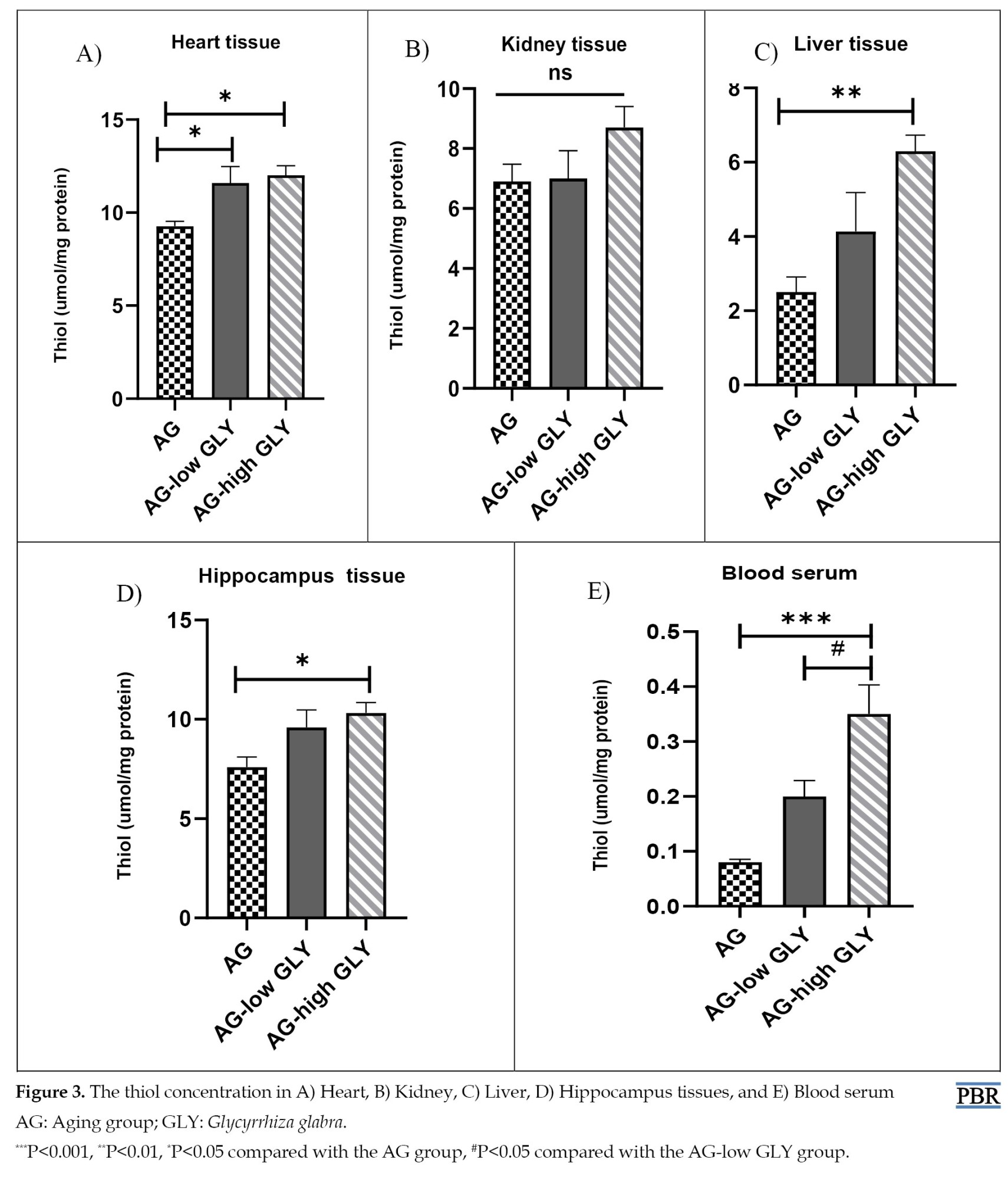
The results of thiol concentration in heart tissue showed a significant enhancement in the AG-low GLY and AG-high GLY groups compared with the AG (P<0.05). The level of thiol groups in the AG-high GLY group was higher than the AG group in the hippocampus (P<0.05) and liver tissues (P<0.01). The thiol level in the AG with high administration of GLY increased significantly compared to the AG group in blood serum (P<0.001). Moreover, in blood serum, the thiol concentration was increased in the AG-high GLY group compared to the AG-low GLY (P<0.05). No significant change in the thiol concentration between groups was observed in kidney tissue (Figure 3A-E).
Urea and creatinine level
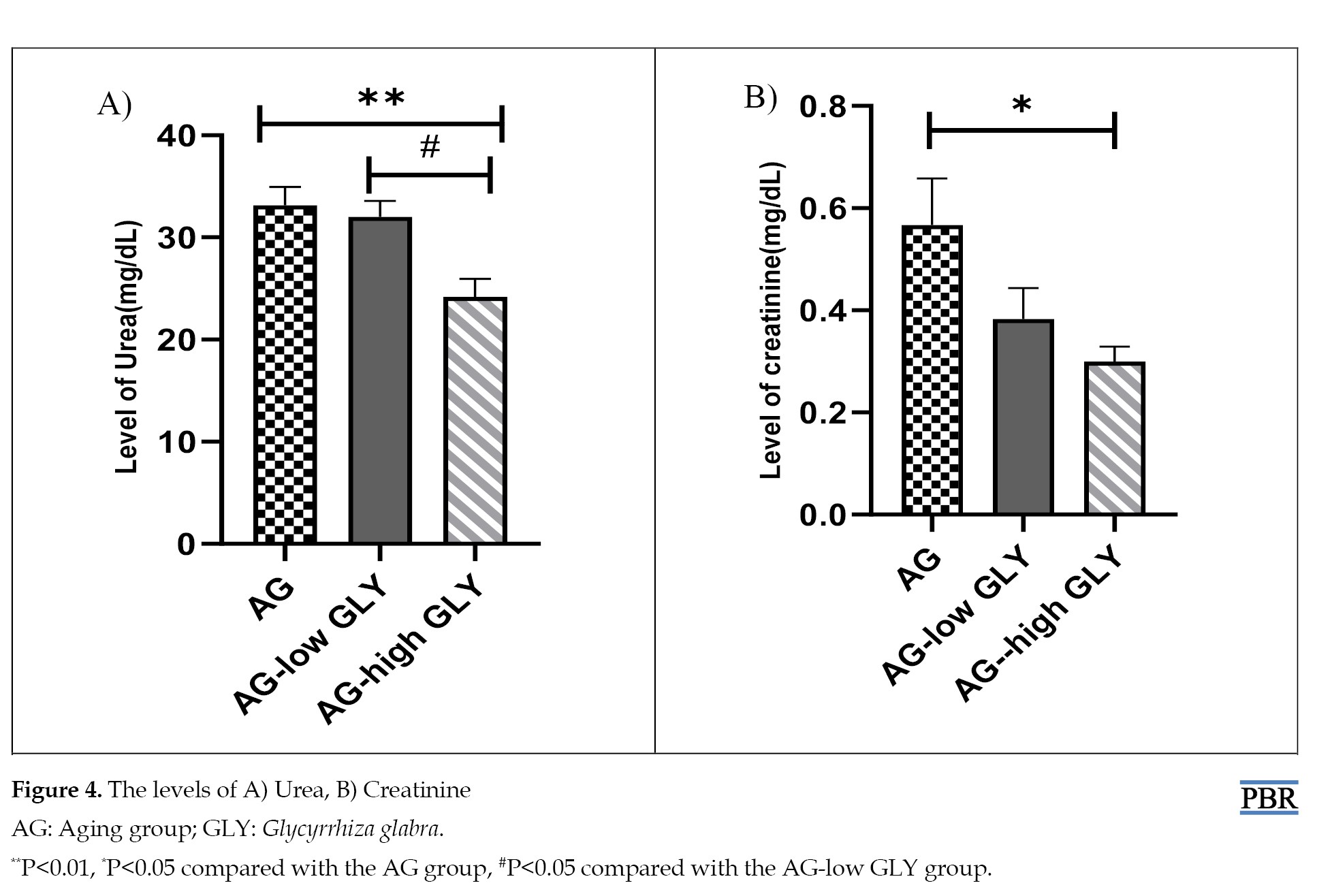
The results of kidney function evaluation demonstrated that the level of urea in the AG-high GLY group reduced remarkably compared with the AG group (P<0.01). Also, the AG-high GLY group has a significant decrement as compared to the AG-low GLY group (P<0.05) (Figure 4A). The level of creatinine in the AG-high GLY group considerably declined compared to the AG (P<0.05) (Figure 4B).
ALT and ASL level
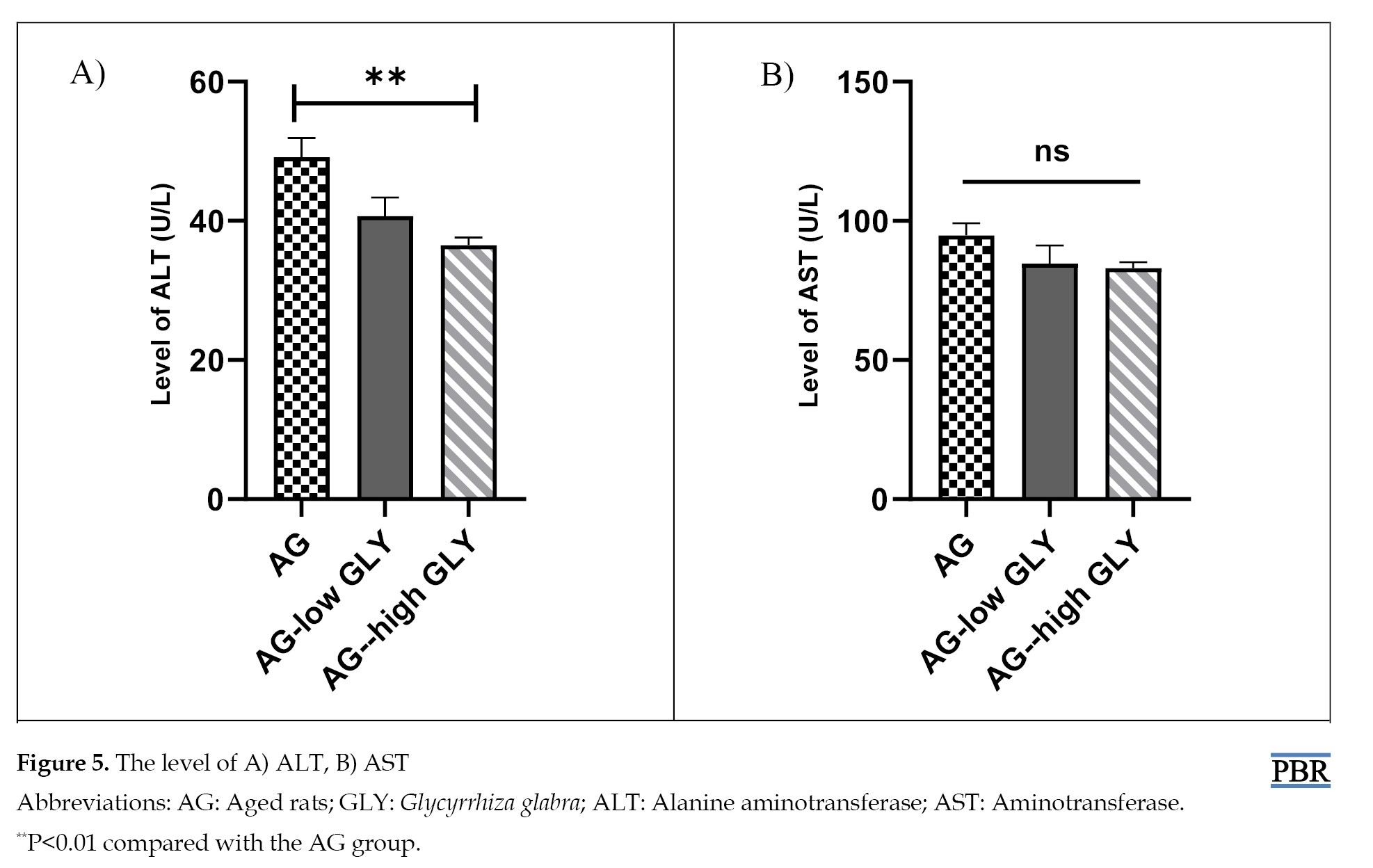
Based on the liver enzyme measurement, the level of ALT in the AG-high GLY group decreased significantly compared to the AG group (P<0.01) (Figure 5A). The change in ASL was not notable between different groups (Figure 5B).
Telomerase enzyme activity
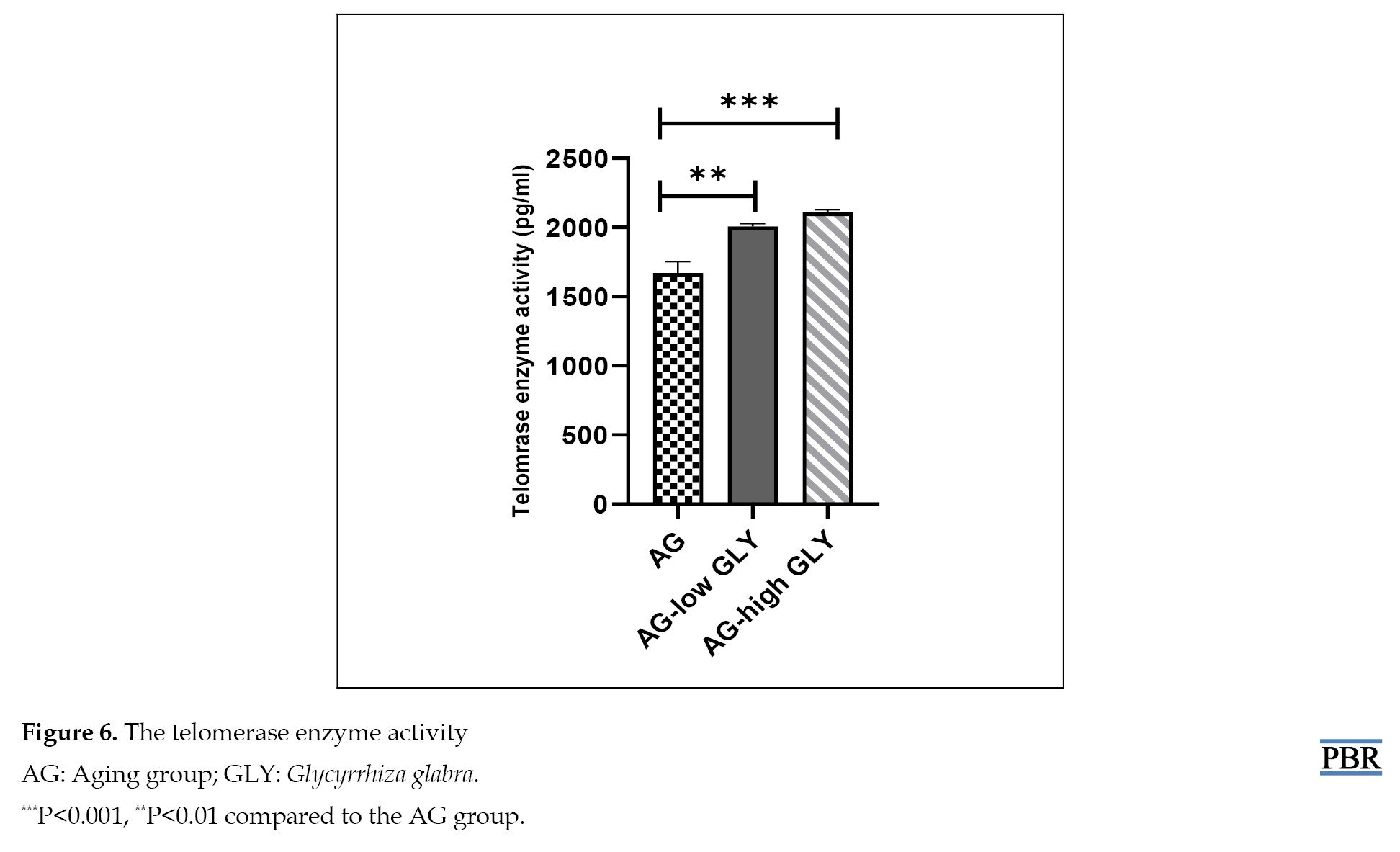
Comparison of the telomeres enzyme activity in various groups showed notably higher activity in the group in the AG-high GLY and AG-low GLY groups compared with the AG group (P<0.001, P<0.01, respectively) (Figure 6).
Discussion
The results indicate that administering a high dose of GLY extract reduces oxidative stress, notably in the tissues and blood serum. A remarkable decline in the urea, creatinine level, and ALT was observed in the aged group receiving a high dose of GLY, but the change in AST was insignificant. Receiving GLY extract in low and high doses elevated the telomerase enzyme activity remarkably.
The global aging population is steadily increasing, leading to a rise in age-related health issues. The statement underscores the critical role of anti-aging strategies in alleviating disease burdens and preserving population productivity [19]. Aging is characterized by the gradual deterioration of organ functions, often linked to ROS accumulation. Numerous antioxidants counteract ROS, thus preventing cellular and tissue damage [20]. GLY, renowned for its antioxidant properties, is significant in combating oxidative stress damage and inhibiting lipid peroxidation [21].
As people age, the efficacy of their endogenous antioxidant systems diminishes, making them more vulnerable to oxidative stress. Organs with limited replication rates and high oxygen consumption, such as the heart and brain, are primarily targeted by oxidative stress [22]. The MDA is a marker of ROS-induced tissue damage [23]. Antioxidants within the body neutralize ROS and shield against harm caused by free radicals. For example, CAT decomposes two molecules of H₂O₂ (hydrogen peroxide) into one molecule of oxygen (O₂) and two molecules of water (H₂O) in a two-step reaction. Simultaneously, thiol groups (-SH) mitigate oxidative stress by binding excess oxygen with hydrogen, effectively deactivating ROS [24-26].
A previous study reports a significant reduction in CAT activity within the elderly group, while the MDA concentration markedly increases in this demographic [27]. According to our findings, the elderly group receiving a high dose of GLY exhibits a substantial decrease in MDA concentration within the liver, kidney, and heart tissues compared to the control group. Nevertheless, no significant alterations in MDA concentration are observed in the hippocampus and serum across different groups. In heart tissue, CAT activity significantly increases in the high-dose GLY recipient group compared to the elderly group. CAT activity exhibited a significant increase in serum within both GLY recipient groups compared to the AG group. However, no notable changes in CAT activity were noted in the liver, kidney, and hippocampus tissues.
The concentrations of thiol groups (-SH) showed a significant increase in the AG-high GLY group compared to the AG group in the liver, hippocampus, and serum. Notably, thiol concentration witnessed a substantial increase in the AG-high GLY group and the AG-low GLY group compared to the AG group in heart tissue. Aslam et al. study corroborates our findings, underscoring that GLY administration, in a dose-dependent manner, reduces MDA concentration and augments CAT activity, thus functioning as an antioxidant [28].
Tissue damage associated with aging is evident in the kidneys. Glomerulosclerosis, a decrease in glomerular filtration rate, and the loss of nephron functional reserves during aging alter the structure and overall function of the kidneys. Consequently, elderly patients are more vulnerable to acute kidney injury [29]. Elevated serum urea and creatinine levels have been reported to contribute to kidney dysfunction in old age [30]. In the present study, administering high doses of GLY significantly reduces serum urea and creatinine levels in aged rats. A previous study corroborates the current research by demonstrating that dose-dependent GLY prescription effectively reduces renal damage, urea, and creatinine levels [31].
The aging process gradually alters the liver structure and affects the physiological function of cells. The serum levels of AST and ALT increase during aging [32, 33].
In this study, it is reported that in elderly rats receiving high doses of GLY, the ALT levels significantly decreased. No significant changes were observed in the ASL levels. Our findings align relatively with the study by Chauhan et al., indicating that dose-dependent GLY administration reduces AST and ALT levels and affects liver damage [34].
The terminal regions of chromosomes are recognized as telomeres. Cell senescence can harm telomeres. Genotoxic stress, reduced telomerase enzyme activity, disruptions in shelter in proteins, or changes in telomere-related RNA expression at the cellular level are detrimental to telomeres [35]. According to the study by Yao et al., telomerase enzyme activity in the testes of aged rats has significantly decreased compared to other groups [36]. The current study shows a significant increase in telomerase enzyme activity in 20-month-old rats receiving high and low doses of GLY.
Our results in this study suggest that GLY effectively improves aging-related outcomes in various tissues. Despite examining the effects in male rats, the results may vary in females and may be sex-dependent. Additionally, changes in the duration of administration can impact the results significantly. Furthermore, the molecular mechanisms of GLY action in tissues still need to be fully understood. Therefore, further studies in this area are recommended.
Conclusion
According to the current study, aging causes oxidative stress in diverse tissues and adversely influences liver and kidney function. Additionally, the telomerase activity can change during aging. Administration of GLY extract in a high dose (300 mg/kg) for 2 weeks can ameliorate the aging complications.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.MEDICAL.REC.1400.531).
Funding
This study was sponsored by the Research Vice-Chancellor of Mashhad University of Medical Sciences (Project No.: 970988).
Authors' contributions
Conceptualization and supervision: Shabnam Mohammadi; Methodology and data collection: Abolfazl Rezaei; Analysis and writing the original draft: Negar Ostad-Rahimi; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to Research Vice-Chancellor of Mashhad University of Medical Sciences.
References
Aging is a complex biological process that gradually reduces normal physiological functions in living organisms. Aging can lead to various diseases such as cancers, Alzheimer, Parkinson, heart diseases, and arteriosclerosis, and it is known as a risk factor in mortality [1, 2]. According to the Denham-Harman theory, the production and accumulation of free radicals in old age damages macromolecules. These damages are crucial in physiological function impairments during aging [3]. Based on the evidence, more reactive oxygen species (ROS) are produced by damaged mitochondria. The production and accumulation of ROS cause oxidative stress and accelerate aging [4]. In addition, a reduction in antioxidant status and an elevation in lipid peroxidation are obvious in the aging process; as reported in previous studies, aging suppresses the activity of catalase (CAT), superoxide dismutase, and glutathione peroxidase. However, it increases the malondialdehyde (MDA) concentration [5]. The integrity of DNA is protected by telomeres in the cell cycle. Over time, telomere length declines until the telomere becomes too short for cell division, leading to cell senescence, so telomere length is considered a landmark for biological aging [6]. Besides, oxidative stress reduces telomere length in aging, and antioxidants can affect the rate of telomere shortening [7]. Aging is identified as a significant factor in increasing the level of aminotransferase (AST), alanine aminotransferase (ALT), urea, and creatinine [8]. The antioxidants prevent the deleterious effects of ROS by eliminating free radicals. Antioxidant administration maintains a balance between oxidant and pro-oxidant and declines the aging process [2].
Nowadays, the utilization of herbal medicine has increased. Herbal medicine is administered to prevent various diseases all over the world. The origin of Glycyrrhiza glabra (GLY) is Eurasia, northern Africa, and western Asia. GLY is famous for its anticancer, anti-inflammatory, antidiabetic, antidepressant, and antioxidant properties [9]. It inhibits lipid peroxidation and is also considered an antioxidant due to its polyphenolic components [10].
Accordingly, we investigated the effects of GLY extract on biochemical parameters and oxidative stress in different tissues of aged rats.
Materials and Methods
Preparation of GLY extract
A specialist collected plant specimens and the herbarium verified it and assigned identification codes. The plants were dried in the shade and ground into powder. Then, 100 g of plant powder was mixed with 0.5 L of 50% alcohol in an Erlenmeyer flask and incubated on a shaker for 48 hours. The residue was filtered through filter paper, and the solvent was removed. The required doses were prepared in physiological serum and stored under sterile conditions [11].
Treatment of animals
Standard conditions were provided to maintain all mice, including a 12:12 h light: dark cycle, 50% relative humidity, and a 22-24 °C temperature, with free access to drinking water and food. Relevant protocols for the care and use of laboratory animals were followed according to the National Institute of Health guidelines, which the Ethics Committee of Mashhad University of Medical Sciences approved.
Thirty rats, aged 20 months, were randomly divided into three groups as follows:
1. Aging (AG) group: Elderly rats without intervention and considered as control.
2. AG- low GLY (GLY) group: Elderly rats received a daily oral dose of 150 mg/kg of body weight of GLY extract for 2 weeks using the gavage method.
3. AG- high GLY group: Elderly rats received a daily oral dose of 300 mg/kg of body weight of GLY extract for 2 weeks using the gavage method [11].
Upon completion of the treatment period, the rats were anesthetized using 10 mg/kg xylazine and 75 mg/kg ketamine, and their heart, liver, hippocampus, and right kidney tissues were extracted to evaluate oxidative stress. Additionally, the serum was separated to measure urea, creatinine, liver enzymes, telomerase enzyme, and levels of oxidants and antioxidants.
Biochemical evaluation
Tissue homogenization was carried out using a buffer solution. The concentration of MDA, thiol groups, and CAT enzyme activity in homogenized tissues and serum were measured. Serum separation was performed by centrifuging blood samples at 5000 rpm for 15 minutes. AST, ALT, urea, creatinine, and the levels of oxidative and antioxidant parameters were utilized for evaluation [12].
MDA assessment
This study employed the TBRS (thiobarbituric acid reactive substances) method to quantify MDA. The interaction between MDA and TBRS forms a red complex with a peak optical density at 532 nm. A spectrophotometer was employed to measure the absorption at a wavelength of 535 nm. The formula of MDA was used as follows (Equation 1) [13]:
1. C(M)=Absorbance/1.56×105
CAT activity assessment
The Aebi method was employed to measure CAT activity. In this method, hydrogen peroxide is decomposed at 240 nm. The procedure commenced by adding 30 mM H2O2 to an adequate homogenized tissue in a 50 mM sodium phosphate buffer. Furthermore, the absorption at 240 nm was assessed after a 3-minute interval, and the specific activity was determined in units per milligram of protein per minute at the culmination of the process [14].
Thiol concentration assessment
The reagent DTNB [5,5’-dithiobis-(2-nitrobenzoic acid)] is acknowledged as a pivotal factor for evaluating thiol groups. In this study, following adding 1 mL of Tris-EDTA buffer to 50 μL of serum. The absorption at a wavelength of 412 nm was measured against the Tris-EDTA buffer alone (A1). Subsequently, by introducing 20 μL of the DTNB reagent into the solution, the sample’s absorption was re-evaluated after a 10-minute interval (A2). The absorption of the empty DTNB reagent was considered (B). Ultimately, the following formula was used (Equation 2) [15]:
2. Total thiol concentration (mM)=(A2-A1-B)×1.07/0.05×13.6
Urea and creatinine level assessment
This study assessed kidney function using colorimetric diagnostic kits’ protocols to evaluate urea and creatinine levels (Pars Azmon Co., Iran) [16].
Liver enzymes assessment
The hepatic enzymes were assessed following the instructions in the respective kits (Pars Azmon Co., Iran) [17].
Telomerase enzyme assessment
Serum blood samples underwent evaluation following the kit’s protocol to determine telomerase enzyme activity (Elabscience) [18].
In this procedure, 100 µL of serum was introduced into the plates, and the samples were subsequently incubated, and then the antibody was added. Next, the samples were incubated for an additional hour. After three washes, HRP (Horseradish peroxidase) conjugate was introduced, and the plates were incubated at 37 °C for 30 minutes. After the final wash, 90 µL of substrate solution was added. After incubation, 50 µL of stop solution was introduced, and the absorbance was recorded at a wavelength of 450 nm.
Statistical analysis
The GraphPad Prism software, version 16 was employed for data analysis. The one-way analysis of variance (ANOVA) and Tukey test were used. The P<0.05 was regarded as noticeable in different measurements.
Results
Biochemical assessment
MDA concentration
According to the results, MDA concentration in the AG-high GLY group decreased notably in comparison with the AG group in the kidney, heart (P<0.05), and liver tissues (P<0.01). In liver tissue, the level of MDA in the AG-high GLY group was remarkably lower than that in the AG-low GLY group (P<0.05). There was no significant difference in the MDA level in the hippocampus tissue and blood serum between various groups. (Figure 1A-E).
CAT activity
Remarkably higher CAT activity in the AG-high GLY group was seen compared to the AG group (P<0.05). In blood serum, the CAT activity in the AG-low GLY and AG-high GLY groups elevated significantly compared to the AG group (P<0.01, P<0.001, respectively). Besides, the CAT level in the AG-high GLY group was notably higher than the AG-low GLY group in blood serum (P<0.05). The change in CAT activity between different groups in the liver, kidney, and hippocampus tissues was not significant (Figure 2A-E).
Thiol concentration
The results of thiol concentration in heart tissue showed a significant enhancement in the AG-low GLY and AG-high GLY groups compared with the AG (P<0.05). The level of thiol groups in the AG-high GLY group was higher than the AG group in the hippocampus (P<0.05) and liver tissues (P<0.01). The thiol level in the AG with high administration of GLY increased significantly compared to the AG group in blood serum (P<0.001). Moreover, in blood serum, the thiol concentration was increased in the AG-high GLY group compared to the AG-low GLY (P<0.05). No significant change in the thiol concentration between groups was observed in kidney tissue (Figure 3A-E).
Urea and creatinine level
The results of kidney function evaluation demonstrated that the level of urea in the AG-high GLY group reduced remarkably compared with the AG group (P<0.01). Also, the AG-high GLY group has a significant decrement as compared to the AG-low GLY group (P<0.05) (Figure 4A). The level of creatinine in the AG-high GLY group considerably declined compared to the AG (P<0.05) (Figure 4B).
ALT and ASL level
Based on the liver enzyme measurement, the level of ALT in the AG-high GLY group decreased significantly compared to the AG group (P<0.01) (Figure 5A). The change in ASL was not notable between different groups (Figure 5B).
Telomerase enzyme activity
Comparison of the telomeres enzyme activity in various groups showed notably higher activity in the group in the AG-high GLY and AG-low GLY groups compared with the AG group (P<0.001, P<0.01, respectively) (Figure 6).
Discussion
The results indicate that administering a high dose of GLY extract reduces oxidative stress, notably in the tissues and blood serum. A remarkable decline in the urea, creatinine level, and ALT was observed in the aged group receiving a high dose of GLY, but the change in AST was insignificant. Receiving GLY extract in low and high doses elevated the telomerase enzyme activity remarkably.
The global aging population is steadily increasing, leading to a rise in age-related health issues. The statement underscores the critical role of anti-aging strategies in alleviating disease burdens and preserving population productivity [19]. Aging is characterized by the gradual deterioration of organ functions, often linked to ROS accumulation. Numerous antioxidants counteract ROS, thus preventing cellular and tissue damage [20]. GLY, renowned for its antioxidant properties, is significant in combating oxidative stress damage and inhibiting lipid peroxidation [21].
As people age, the efficacy of their endogenous antioxidant systems diminishes, making them more vulnerable to oxidative stress. Organs with limited replication rates and high oxygen consumption, such as the heart and brain, are primarily targeted by oxidative stress [22]. The MDA is a marker of ROS-induced tissue damage [23]. Antioxidants within the body neutralize ROS and shield against harm caused by free radicals. For example, CAT decomposes two molecules of H₂O₂ (hydrogen peroxide) into one molecule of oxygen (O₂) and two molecules of water (H₂O) in a two-step reaction. Simultaneously, thiol groups (-SH) mitigate oxidative stress by binding excess oxygen with hydrogen, effectively deactivating ROS [24-26].
A previous study reports a significant reduction in CAT activity within the elderly group, while the MDA concentration markedly increases in this demographic [27]. According to our findings, the elderly group receiving a high dose of GLY exhibits a substantial decrease in MDA concentration within the liver, kidney, and heart tissues compared to the control group. Nevertheless, no significant alterations in MDA concentration are observed in the hippocampus and serum across different groups. In heart tissue, CAT activity significantly increases in the high-dose GLY recipient group compared to the elderly group. CAT activity exhibited a significant increase in serum within both GLY recipient groups compared to the AG group. However, no notable changes in CAT activity were noted in the liver, kidney, and hippocampus tissues.
The concentrations of thiol groups (-SH) showed a significant increase in the AG-high GLY group compared to the AG group in the liver, hippocampus, and serum. Notably, thiol concentration witnessed a substantial increase in the AG-high GLY group and the AG-low GLY group compared to the AG group in heart tissue. Aslam et al. study corroborates our findings, underscoring that GLY administration, in a dose-dependent manner, reduces MDA concentration and augments CAT activity, thus functioning as an antioxidant [28].
Tissue damage associated with aging is evident in the kidneys. Glomerulosclerosis, a decrease in glomerular filtration rate, and the loss of nephron functional reserves during aging alter the structure and overall function of the kidneys. Consequently, elderly patients are more vulnerable to acute kidney injury [29]. Elevated serum urea and creatinine levels have been reported to contribute to kidney dysfunction in old age [30]. In the present study, administering high doses of GLY significantly reduces serum urea and creatinine levels in aged rats. A previous study corroborates the current research by demonstrating that dose-dependent GLY prescription effectively reduces renal damage, urea, and creatinine levels [31].
The aging process gradually alters the liver structure and affects the physiological function of cells. The serum levels of AST and ALT increase during aging [32, 33].
In this study, it is reported that in elderly rats receiving high doses of GLY, the ALT levels significantly decreased. No significant changes were observed in the ASL levels. Our findings align relatively with the study by Chauhan et al., indicating that dose-dependent GLY administration reduces AST and ALT levels and affects liver damage [34].
The terminal regions of chromosomes are recognized as telomeres. Cell senescence can harm telomeres. Genotoxic stress, reduced telomerase enzyme activity, disruptions in shelter in proteins, or changes in telomere-related RNA expression at the cellular level are detrimental to telomeres [35]. According to the study by Yao et al., telomerase enzyme activity in the testes of aged rats has significantly decreased compared to other groups [36]. The current study shows a significant increase in telomerase enzyme activity in 20-month-old rats receiving high and low doses of GLY.
Our results in this study suggest that GLY effectively improves aging-related outcomes in various tissues. Despite examining the effects in male rats, the results may vary in females and may be sex-dependent. Additionally, changes in the duration of administration can impact the results significantly. Furthermore, the molecular mechanisms of GLY action in tissues still need to be fully understood. Therefore, further studies in this area are recommended.
Conclusion
According to the current study, aging causes oxidative stress in diverse tissues and adversely influences liver and kidney function. Additionally, the telomerase activity can change during aging. Administration of GLY extract in a high dose (300 mg/kg) for 2 weeks can ameliorate the aging complications.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code: IR.MUMS.MEDICAL.REC.1400.531).
Funding
This study was sponsored by the Research Vice-Chancellor of Mashhad University of Medical Sciences (Project No.: 970988).
Authors' contributions
Conceptualization and supervision: Shabnam Mohammadi; Methodology and data collection: Abolfazl Rezaei; Analysis and writing the original draft: Negar Ostad-Rahimi; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to Research Vice-Chancellor of Mashhad University of Medical Sciences.
References
- Pal S, Tyler JK. Epigenetics and aging. Sci Adv. 2016; 2(7):e1600584. [DOI:10.1126/sciadv.1600584] [PMID]
- Warraich UE, Hussain F, Kayani HUR. Aging - Oxidative stress, antioxidants and computational modeling. Heliyon. 2020; 6(5):e04107. [DOI:10.1016/j.heliyon.2020.e04107] [PMID]
- Polidori MC, Mecocci P. Modeling the dynamics of energy imbalance: The free radical theory of aging and frailty revisited. Free Radic Biol Med. 2022; 181:235-40. [DOI:10.1016/j.freeradbiomed.2022.02.009] [PMID]
- Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018; 592(5):743-58. [DOI:10.1002/1873-3468.12902] [PMID]
- Govindan S, Johnson EER, Christopher J, Shanmugam J, Thirumalairaj V, Gopalan J. Antioxidant and anti-aging activities of polysaccharides from Calocybe indica var. APK2. Exp Toxicol Pathol. 2016; 68(6):329-34. [DOI:10.1016/j.etp.2016.04.001] [PMID]
- Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget. 2017; 8(27):45008-19.[DOI:10.18632/oncotarget.16726] [PMID]
- Gavia-García G, Rosado-Pérez J, Arista-Ugalde TL, Aguiñiga-Sánchez I, Santiago-Osorio E, Mendoza-Núñez VM. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology (Basel). 2021; 10(4):253. [DOI:10.3390/biology10040253] [PMID]
- Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, et al. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012; 47(12):979-87. [DOI:10.1016/j.exger.2012.09.003] [PMID]
- Al-Snafi AE. Glycyrrhiza glabra: A phytochemical and pharmacological review. J Pharm. 2018; 8(6):1-17. [Link]
- Sharma V, Katiyar A, Agrawal RC. Glycyrrhiza glabra: Chemistry and pharmacological activity. In: Mérillon JM, Ramawat K, editors. Sweeteners. Reference series in phytochemistry. Cham: Springer; 2018. [DOI:10.1007/978-3-319-27027-2_21]
- Huo HZ, Wang B, Liang YK, Bao YY, Gu Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int J Mol Sci. 2011; 12(10):6529-43. [DOI:10.3390/ijms12106529] [PMID]
- Pallio G, Micali A, Benvenga S, Antonelli A, Marini HR, Puzzolo D, et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem Toxicol. 2019; 132:110675. [DOI:10.1016/j.fct.2019.110675] [PMID]
- Ghorbani F, Karimi S, Boustan A, Ebrahimzadeh-Bideskan A, Saburi E. Effect of melatonin on male offspring testis and sperm parameters in BALB/c mice after exposing their mother to METHamphetamine during pregnancy and lactation. Iran J Basic Med Sci. 2023; 26(7):777-84. [DOI:10.22038/IJBMS.2023.69608.15158] [PMID]
- Osatd-Rahimi N, Saburi E, Karimi S, Boustan A, Ebrahimzadeh-Bideskan A. The therapeutic effect of melatonin on female offspring ovarian reserve and quality in BALB/c mice after exposing their mother to methamphetamine during pregnancy and lactation. Iran J Basic Med Sci. 2023; 26(2):208-15. [DOI:10.22038/IJBMS.2022.66660.14636] [PMID]
- Yazdi HB, Hojati V, Shiravi A, Hosseinian S, Vaezi G, Hadjzadeh MA. Liver dysfunction and oxidative stress in streptozotocin-induced diabetic rats: Protective role of Artemisia turanica. J Pharmacopuncture. 2019; 22(2):109-14.[DOI:10.3831/KPI.2019.22.014] [PMID]
- Fahmy MA, Diab KA, Abdel-Samie NS, Omara EA, Hassan ZM. Carbon tetrachloride induced hepato/renal toxicity in experimental mice: Antioxidant potential of Egyptian Salvia officinalis L essential oil. Environ Sci Pollut Res Int. 2018; 25(28):27858-76. [DOI:10.1007/s11356-018-2820-6] [PMID]
- Dartoti HH, Firozian F, Asl SS, Ranjbar A. Protective role of Ce nanoparticles against the hepatotoxicity induced by exposure to paraquat. Avicenna J Med Biochem. 2018; 6(2):37-43. [DOI:10.15171/ajmb.2018.09]
- AlDehaini DM, Al-Bustan SA, Ali ME, Malalla ZHA, Sater M, Giha HA. Shortening of the leucocytes’ telomeres length in T2DM independent of age and telomerase activity. Acta Diabetologica. 2020;57:1287-95. [DOI:10.1007/s00592-020-01550-4]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences. 2015;112(30):E4104-E10. [DOI:10.1073/pnas.1506264112]
- Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells. 2022;11(3):552. [DOI:10.3390/cells11030552]
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBP. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy research. 2018;32(12):2323-39. [DOI:10.1002/ptr.6178]
- Conti V, Izzo V, Corbi G, Russomanno G, Manzo V, De Lise F, et al. Antioxidant supplementation in the treatment of aging-associated diseases. Frontiers in pharmacology. 2016;7:24. [DOI:10.3389/fphar.2016.00024]
- Cherian DA, Peter T, Narayanan A, Madhavan SS, Achammada S, Vynat GP. Malondialdehyde as a marker of oxidative stress in periodontitis patients. Journal of pharmacy & bioallied sciences. 2019;11(Suppl 2):S297. [DOI:10.4103/JPBS.JPBS_17_19]
- Adwas AA, Elsayed A, Azab A, Quwaydir F. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. 2019;6(1):43-7. [DOI:10.15406/jabb.2019.06.00173]
- Nandi A, Yan L-J, Jana CK, Das N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative medicine and cellular longevity. 2019; 9613090. [DOI:10.1155/2019/9613090]
- Sener S, Akbas A, Kilinc F, Baran P, Erel O, Aktas A. Thiol/disulfide homeostasis as a marker of oxidative stress in rosacea: a controlled spectrophotometric study. Cutaneous and ocular toxicology. 2019;38(1):55-8. [DOI:10.1080/15569527.2018.1517124]
- Zhao S-j, Liu X-j, Tian J-s, Gao X-x, Liu H-l, Du G-h, et al. Effects of Guilingji on aging rats and its underlying mechanisms. Rejuvenation Research. 2020;23(2):138-49. [DOI:10.1089/rej.2018.2118]
- Aslam B, Awan T, Javed I, Khaliq T, Khan J, Raza A. Gastroprotective and antioxidant potential of Glycyrrhiza glabra on experimentally induced gastric ulcers in albino mice. Int J Curr Microbiol App Sci. 2015;4(2):451-60.
- Jiao D, Qi L, Hu L, Hu D, Li X, Li G, et al. Changes in aging-induced kidney dysfunction in mice based on a metabolomics analysis. Frontiers in Endocrinology. 2022;13:959311. [DOI:10.3389/fendo.2022.959311]
- Bahri F, Khaksari M, Movahedinia S, Shafiei B, Rajizadeh MA, Nazari‐Robati M. Improving SIRT1 by trehalose supplementation reduces oxidative stress, inflammation, and histopathological scores in the kidney of aged rats. Journal of Food Biochemistry. 2021;45(10):e13931. [DOI:10.1111/jfbc.13931]
- Nassan MA, Soliman MM, Aldhahrani A, Althobaiti F, Alkhedaide AQ. Ameliorative impacts of Glycyrrhiza glabra root extract against nephrotoxicity induced by gentamicin in mice. Food Sci Nutr. 2021; 9(7):3405-13. [DOI:10.1002/fsn3.2183] [PMID]
- Wan Y, Li X, Slevin E, Harrison K, Li T, Zhang Y, et al. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 2022; 36(1):e22125. [DOI:10.1096/fj.202101426R] [PMID]
- Coelho AMM, Machado MCC, Sampietre SN, da Silva FP, Cunha JEM, D'Albuquerque LAC. Local and systemic effects of aging on acute pancreatitis. Pancreatology. 2019; 19(5):638-45. [DOI:10.1016/j.pan.2019.06.005] [PMID]
- Chauhan P, Sharma H, Kumar U, Mayachari A, Sangli G, Singh S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: An experimental approach. J Ethnopharmacol. 2020; 263:113209. [DOI:10.1016/j.jep.2020.113209] [PMID]
- Arish N, Petukhov D, Wallach-Dayan SB. The role of telomerase and telomeres in interstitial lung diseases: From molecules to clinical implications. Int J Mol Sci. 2019; 20(12):2996. [DOI:10.3390/ijms20122996] [PMID]
- Yao C, Zhao C, Zhang S, Liu S. Effect of moxibustion on testosterone secretion and apoptosis of spermatogenic cells in aging rats. Evid Based Complement Alternat Med. 2019; 2019:5186408. [DOI:10.1155/2019/5186408] [PMID]
Type of Study: Original Research |
Subject:
Traditional Medicine
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |