Volume 10, Issue 3 (2024)
Pharm Biomed Res 2024, 10(3): 167-178 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kumar G N, D R B, K C, B M J, Ahmed S S. Revolutionizing Care: A Comprehensive Review of Modern Diabetes Research Advancements. Pharm Biomed Res 2024; 10 (3) :167-178
URL: http://pbr.mazums.ac.ir/article-1-583-en.html
URL: http://pbr.mazums.ac.ir/article-1-583-en.html
1- Department of Pharmacology, Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University, Nagamangala, India
Keywords: Diabetes mellitus (DM), Biomarkers, Precision medicine, Innovative treatment approaches, Telemedicine
Full-Text [PDF 1612 kb]
(818 Downloads)
| Abstract (HTML) (2755 Views)
Full-Text: (773 Views)
Introduction
Diabetes mellitus (DM) is an increasingly prevalent chronic disease characterized by either a total or partial deficiency of insulin, leading to elevated blood glucose levels, dyslipidemia, and neurovascular system damage [1]. Pancreatic beta cells generate the hormone insulin with various functions in the body. Insulin aids the body’s cells in utilizing glucose as fuel. DM is caused by a deficiency in insulin synthesis or sensitivity [2]. Type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (GDM) are the three primary types of the DM. The first kind of diabetes, commonly known as “juvenile/childhood-onset diabetes” or “insulin-dependent diabetes,” is categorized by the body’s inability to produce insulin. Regular injection of insulin analogs is required for the treatment of this DM [3]. Scientific and technological advancements are essential to identify novel therapeutic agents since DM is a significant socioeconomic burden in many nations. As a result, new therapeutic classes such as amylin analogs, incretin mimics, gastric inhibitory peptide analogs, and inhibitors of the dipeptidyl peptidase-4 and the peroxisome proliferator-activated receptor have been introduced, which are potential targets for drugs used to treat diabetes [2].
The frequency of T2DM has been steadily rising over the past few decades, significantly contributing to the dramatic rise in the incidence of DM. Statistics from the World Health Organization (WHO) show that more than 422 million people worldwide had DM in 2014. It is anticipated that DM prevalence will continue to climb. By 2040, 642 million people will have diabetes worldwide, as predicted by the International Diabetes Federation (IDF). Recent epidemiological data that offers global estimates for the incidence of DM is considered in this research [3].
Prevalence
According to current research, the prevalence of DM has risen dramatically over the last ten years and might be regarded as an epidemic. The diagnosis of DM has been made in 8.8% of the adult population. If this trend continues, it is predicted that by 2040, 6.93 million individuals worldwide, or 9.9% of the population, will be living with diabetes.
According to IDF projections, the incidence of diabetes is predicted to ascend by 1.1% by 2045. Additionally, 1105500 T1DM patients are in the 0 to 19 age range, compared to 132600 newly diagnosed cases in children and young adults. According to current statistics, there are 352.1 million IGT (impaired glucose tolerance) sufferers globally, and 587 million people aged 20 to 79 are expected to get IGT by the year 2050. Last but not least, 21.3 million live births were associated with hyperglycemia in pregnancy in 2017. The majority of these instances (86.4%) were caused by GDM, which primarily affects people in the age range of 40 to 49; the remaining cases were associated with other kinds of DM [3].
Rates of diabetic metabolic syndrome by age and sex
The most current statistics show that more than 326 million individuals in the work population have diabetes, compared to 122.8 million people over the age of 65. In the following decades, these figures are predicted to upsurge to 438.2 and 253.4 million, respectively. The number of men is on the verge of declining regarding gender distribution. In 2017, 8.4% of women aged 20 to 79 were diagnosed with DM, compared to 9.1% of males in the same age range; this discrepancy will likely narrow dramatically in the next few years as the aforementioned numbers are predicted to increase to 9.7% and 10%, respectively [2].
Emerging biomarkers for the early detection and risk assessment of diabetes
Gene expression profiling can precisely correlate with certain disease states because of the development of technologies that recuperate, amplify, and identify the nucleic acids in the plasma. Numerous investigations have found that a particular miRNA expression in circulation is possibly associated with diabetic problems.
New biomarkers
In clinical settings, non-invasive biomarkers detect early signs of islet stress and disorders before beta-cell damage occurs. Established biomarkers of autoimmunity in T1DM include autoantibodies against glutamate decarboxylase, islet cell autoantibodies, islet β-cell secretory granule membrane protein-zinc transporter 8, islet antigens insulin, and protein tyrosine phosphate phosphohydrolase protein [4].
Autoantibodies
To forecast disease and aid in diagnosing T1DM, beta-cell peptides, and proteins are now frequently targeted by autoantibodies. Zinc transporter-8, glutamic acid decarboxylase, islet antigen-2 and insulin are the primary targets of autoantibodies utilized as T1DM indicators in clinical settings [5].
Non-coding ribonucleic acid (RNA)
The non-coding RNAs are now recognized as influential biomarkers in disease diagnosis and clinical management, offering unique functionalities that impact biological processes ahead of glucose level changes. This increased recognition is supported by technological advancements, enhancing the detectability and accuracy of non-coding RNAs [6].
MiRNAs as biomarkers
Specialized short ncRNAs called miRNAs inhibit target mRNA from being translated. According to recent studies, circulating miRNAs are valuable as illness biomarkers. MiRNAs are released by cells under various circumstances, either as free molecules or as tiny vesicles that additional cell types might ingest. The extracellular miRNAs operate as crucial cell-to-cell messengers and regulate different biological processes, such as angiogenesis, tumor cell invasion, and immune response. MiRNAs are trustworthy biomarkers due to their resistance to oxidation in circulation and abundance in particular organs, which can be used to pinpoint the basis of circulating miRNAs. Due to developments in DNA amplification, DNA sequencing, and analysis, we have recognized the miRNAs generated and released by islets under stress. RNA sequencing, quantitative polymerase chain reaction and microarray, and data from extensive human studies have been compiled for this review to present the possibility of using miRNA signatures to identify pre-DM and or staging evolution and mode of diabetic complications in the future [4].
Biomarkers associated with diabetic nephropathy (DN) pathogenesis
By developing advanced glycation products and stimulating the polyol pathway, protein kinase C, hexosamine pathwaym and angiotensin II, hyperglycemia promotes the electron transport chain to enhance too many reactive oxygen species. The oxidative stress is then initiated or enhanced by ROS, which leads to an inflammatory reaction and the development of fibrosis. Additional factors that contribute to the occurrence of DN involve abnormal metabolism of lipids, the renin-angiotensin-aldosterone system (RAAS) stimulation, persistent and glomerular hypertension, deterioration of insulin signaling, increased levels of developmental factors and pro-inflammatory cytokines, and stimulation of intracellular signaling pathways [32].
Potential of precision medicine in tailoring diabetes therapies
Monogenic DM can account for as much as 3% of cases in children, with HNF1A transcription factor gene mutations being the most common cause. Rare penetrant HNF1A mutant carriers frequently manifest diabetes without insulin dependence before the age of 25. They have a significant family history of the disease. Clinical evaluation typically finds such people to be C-peptide positive but autoantibody negative. An early case study demonstrated that patients with DM brought on by HNF1A changes had great sympathy for sulfonylureas, which was neatly backed by a randomized precise study, serving as the initial instance of individualized treatment in diabetes. Even though the exact molecular mechanism underlying the sensitivity of individuals with HNF1A mutations to this particular class of oral diabetes medications is still unknown, it has been proposed that the closure of the KATP channel by sulfonylureas bypasses the main locations of cell dysfunction that are ambitious of the KATP channel, activating the secretion of insulin. Because potassium inwardly-rectifying channel subfamily J member 11 and ATP-binding cassette transporter subfamily C member 8, activating mutations prevent the KATP channels from closing in retort to ATP generated by glycolysis, sulfonylureas are also the initial treatment for people with neonatal diabetes [7].
Personalized treatment recommendations using evidence from research trials and pharmacogenomics
Recognizing the value of replication is crucial. We should be just as skeptical of the latest findings from pharmacogenetic analyses as we are of research focusing on the possibility of T2DM. Most scientific research concentrates on small-scale investigations of candidate genes or single nucleotide polymorphisms rather than objective analyses of the influence of genetic diversity on therapeutic response across the genome [7].
Metformin
Metformin is the most frequently prescribed and thoroughly researched medication for the treatment of T2DM. Additionally, the study of metformin comprises pharmacogenomics, genetic investigations of metformin transport, and molecular targets for metformin activity. SLC22A1, SLC22A2 and SLC47A have been discovered as genetic drivers of metformin pharmacokinetic variability, tissue uptake, and clearance; nevertheless, their therapeutic value in predicting metformin interaction in T2DM patients [7].
Sulfonylureas
Several T2DM guidelines suggest insulin and sulfonylureas as second-line blood sugar-lowering medications. This widespread history and current use result from years of clinical study. Information from the GoDARTS study revealed that carriers of the TCF7L2 T2DM risk variant are less probable than non-carriers to enhance glycaemic goal line in reaction to sulfonylureas. This treatment effect was not obtained for metformin [7]. Change at the TCF7L2 gene location is probably the most well-studied example of genetics determining pharmacological responses. Additionally, genetic variation among the genes crucial for sulfonylurea metabolism, the sulfonylurea receptor, beta-cell function, and insulin action, such as CYP2C9, KCNJ1, CDKAL1, CDKN2B and NOS1AP, has also been studied [7].
Thiazolidinediones
Thiazolidinediones slowed the progression of nonalcoholic fatty liver disease in subsets of patients in studies lasting up to 24 months despite questions about the relative advantages and risks of these drugs for T2DM patients’ glucose control. There is still a limited understanding of the genetic factors influencing the medicinal effects of thiazolidinediones, especially pioglitazone and rosiglitazone. Variations in solute carrier organic anion-transporter, which codes for the organic anion transporting polypeptide 1B1, had an impact on the glucose tolerance to the medication in 833 patients who were evaluated from 1 to 18 months into treatment, in contrast to CYP2C8, which encodes the cytochrome P450 2C8 metabolizing enzyme. Surprisingly, these genetic variations did not similarly impact how well patients responded to pioglitazone medication [8].
Innovative treatment approaches
Treatments for T2DM patients include reducing blood sugar levels, shedding pounds, and decreasing blood pressure through GLP-1 receptor analogs and SGLT2 inhibitors. According to results from cardiovascular outcome examinations, these drugs reduce the hazard of heart failure hospitalization, lower cardiovascular disease, and offer protection against severe cardiovascular disease to those with chronic heart disease [9]. Studies that use hard renal endpoint kidney disease instead of substitute indicators are currently being conducted to define the renoprotective benefits of both drugs. SGLT2 inhibitors should be avoided in conditions where there is a potential of developing diabetic ketoacidosis because they operate best when used to lower blood sugar when the predictable rate of glomerular filtration is greater than 60 mL/min/173 m2 at the time of treatment.
SGLT2 inhibitors
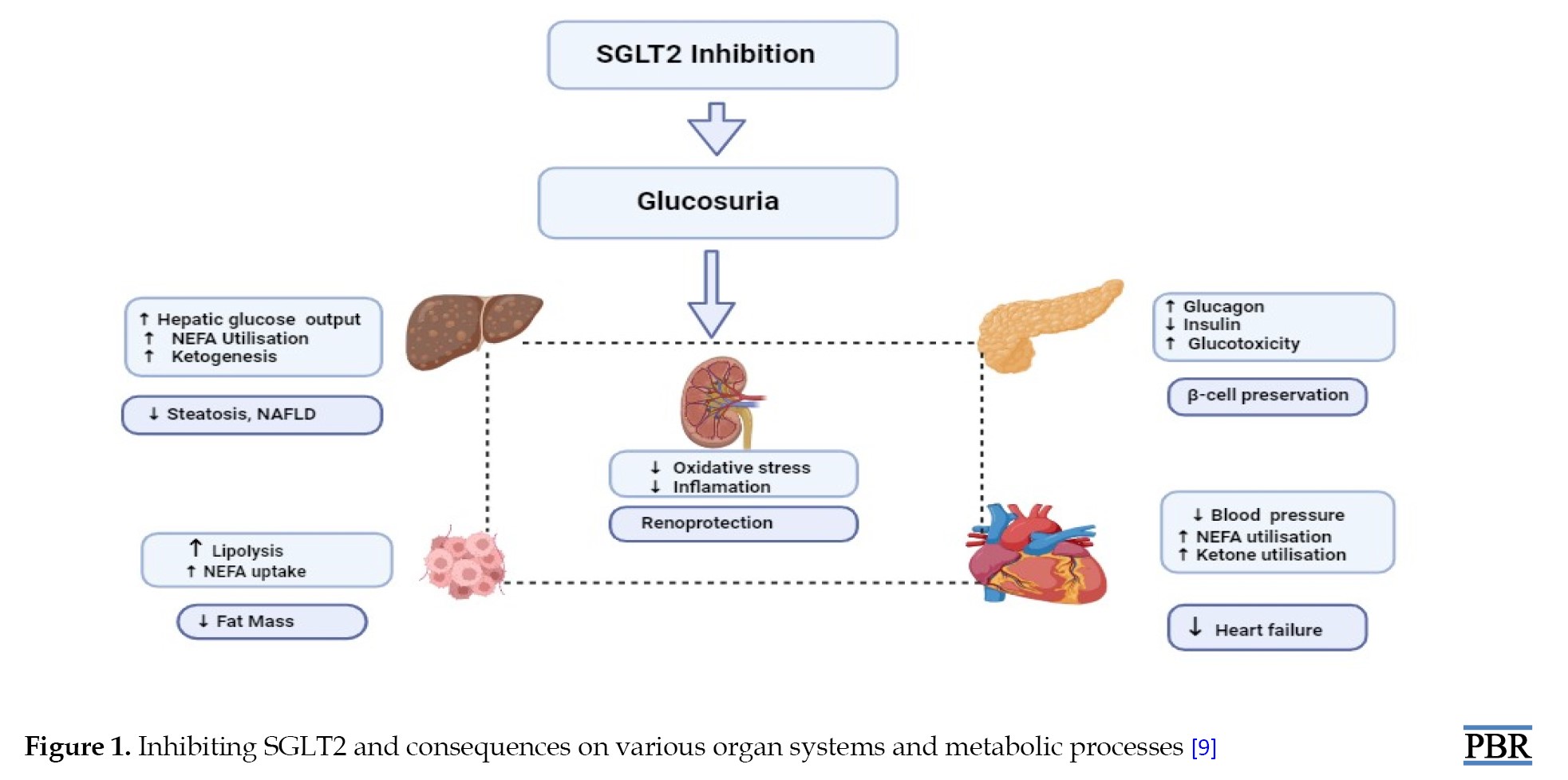
A person with diabetes has urine glucose reabsorption that is substantially higher than the normal value of 180 g/d. SGLT2 inhibition causes around 70-90 g of urine glucose excretion, with an accompanying energy forfeiture of about 300 kcal/d, because it inhibits only about 50 percent of the filtered glucose load. This finding contradicts the frequently cited statistic that SGLT2 is in charge of about 90% of renal glucose absorption. This outcome can be caused by partial SGLT2 blockage or additional upregulation of SGLT1 expression following SGLT2 therapy (Figure 1) [9]. All presently accessible SGLT2i lower blood sugar and HbA1c by 0.6% to 1%, relying on the baseline HbA1c and experimental design. Generally speaking, these medications work independently of other glucose-dropping drugs and can be coupled with further treatments, including insulin. In the presence of severe renal impairment, the effects of decreasing HbA1c are diminished, and none of them are presently advised for use once the eGFR is <45 mL/min. A weight loss of two to three kg and a minor decrease in blood pressure are also predicted. The foremost side effects are an elevated hazard of bacterial and fungal urinary tract infections, particularly in women, as well as adverse blood pressure-lowering side effects like giddiness and postural hypotension. More common side effects comprise diabetic ketoacidosis, more seen in people with T1DM. Concerns have also been upstretched lower limb amputees, bladder cancer, and crepitus upon palpation. Still, new statistics from the DECLARE trial propose the hazard for these side effects may not be augmented for dapagliflozin [10]. The eradication of urinary glucose excretion (UGE) when the filtered glucose level comes in below the transport capacity of SGLT1, together with the compensatory metabolic changes observed, which includes increased production of liver glucose and the circumstance that these medications do not stimulate insulin secretion [9], all contribute to the fact that the menace of lower the blood sugar level with SGLT2 inhibition.
GLP-1 receptor agonists
In reaction to foods, mainly glucose and fat, intestinal epithelial L-cells produce the peptide GLP-1 derived from the gut. Multiple target organs are affected physiologically by GLP-1. It is an incretin that increases the amount of insulin secreted by pancreatic cells in response to glucose, increases neogenesis, prevents apoptosis, and decreases the cells’ ability to secrete glucagon. GLP-1 does not cause hypoglycemia because insulin production is only elevated overhead about 3 to 5 mmol/L of blood glucose. GLP-1 also affects the hypothalamus, which results in an increase in satiety and a reduction in food intake. The adipose tissue, heart, and stomach are additional areas where GLP-1 has an impact.
Lixisenatide, exenatide, dulaglutide, liraglutide and semaglutide are a few GLP-1 receptor agonists that have been accepted for use in T2DM. Although oral semaglutide is accessible, all are directed as subcutaneous injections. Exendin-4, human GLP-1 or the salivary peptide from the Gila monster (Heloderma suspectum) served as the basis for the development of these molecules, which have biological characteristics that are indicative of important structural alterations to the amino acid order that confer resistance to dipeptidyl peptidase-4 deprivation. Different clinical outcomes result from these molecular variations. Short-acting GLP-1 receptor agonists are the first class of receptor agonists and have a structural similarity to exendin-4 of 90%–97%. These substances alter postprandial plasma glucose concentrations more drastically than long-acting GLP-1 receptor agonists due to their dramatic slowing of stomach emptying and lessening impact on insulin production [8]. Long-acting GLP-1 receptor agonists can have a more pronounced effect on fasting blood sugar levels and 24-hour glucose profiles. This outcome is because they provide a continuous and sustained level of GLP-1 receptor activation throughout the day, helping to regulate blood sugar levels in both fasting and postprandial states. It is important to note that the choice between short-acting and long-acting GLP-1 receptor agonists depends on individual patient characteristics, preferences, and treatment goals. Healthcare professionals consider factors such as the patient’s lifestyle, adherence to medication, and the overall management plan for diabetes when prescribing these medications [11].
Mesenchymal stem cell-based therapy
Multipotent adult stem cells (MSCs) can be produced from tissues in both adult and neonatal individuals. MSCs can be found in practically all tissues with a perivascular region, although adult bone marrow is the most common source. MSCs are produced from the mesodermal germ layer and can develop in vitro into adipocytes, osteoblasts, and chondroblasts, all connective tissue types [12].
Artificial intelligence (AI) and predictive analytics
AI research is advancing quickly, and its applicability to the global diabetes pandemic could fundamentally alter how this chronic illness is identified and treated. Prognostic mock-ups for the chance of receiving diabetes or its difficulties have been established utilizing machine learning principles. AI enables continuous, effortless remote monitoring of the patient’s signs and biomarkers. Social media platforms and online forums also increase patient involvement in diabetes care. Technological improvements have enhanced the distribution of resources in the field of diabetes. These ingenious technical advancements have improved glycaemic control by lowering fasting and postprandial blood glucose levels, glycosylated hemoglobin, and glucose excursions. AI will enable a paradigm shift in diabetes management from current methods to building targeted, based on data meticulousness care [15].
Case-based reasoning
Diabetes management uses case-based reasoning, an AI technique, to answer novel issues based on learning from analogous prior experiences [13, 14]. A case-based reasoning approach employed in the treatment of DM is the diabetes management system. This system intends to mechanically recognize blood glucose control problems, make recommendations for fixing them, and keep the path of positive and negative remedies designed for each patient individually [13]. For various meal settings in diabetes, case-based reasoning has been utilized to optimize and personalize insulin administration.
Artificial neural networks
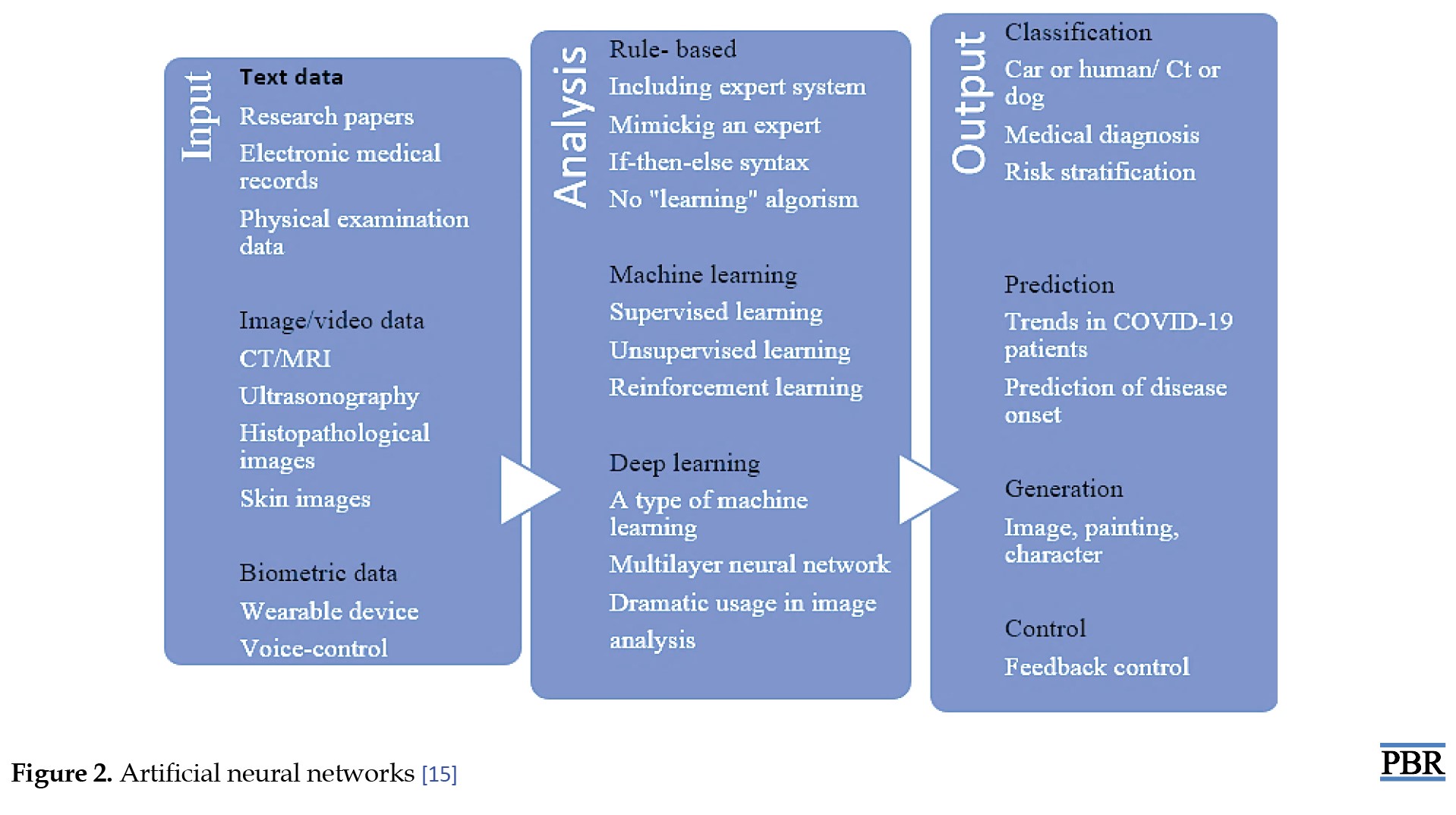
Diabetes diagnosis has made extensive and unique use of neural network techniques. Intelligent algorithms have been developed to investigate how different factors affect glycemic indices (Figure 2) [15].
Advances in continuous glucose monitoring (CGM)
Patients with chronic renal disease have an option that provides a more thorough and reliable glycaemic assessment called CGM. A tiny filament is frequently inserted into subcutaneous tissue to monitor glucose in interstitial fluid in most commercially accessible CGM devices, which are minimally invasive. Because diffusion is concentration gradient-dependent, there is a dynamic equilibrium among the blood’s interstitial fluid glucose and sugar levels. The filament of the CGM takes up the interstitial glucose through capillaries. An electrochemical response in the sensor calculates the amount of interstitial glucose present. A reader or smartphone app sends and shows minute-by-minute interstitial glucose measurements on a mobile device [16] diabetes is the leading cause of chronic kidney disease (CKD).
Closed-loop insulin delivery system
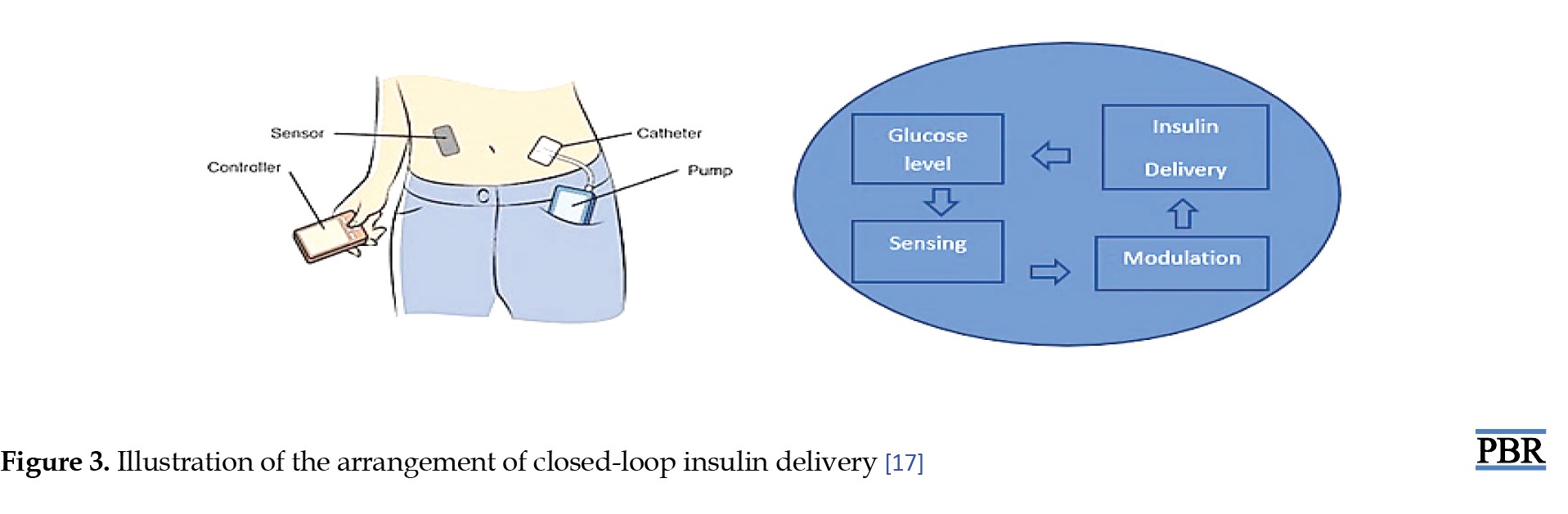
Automation of closed-loop insulin delivery systems has been made possible by developments in diabetes technology. Numerous composites of closed-loop systems are being commercialized, demonstrating the quick progression of this rapidly developing expertise from investigative into experimental practice, progressively revolutionizing the treatment of T1DM in children and adults. The clinical treatment of T1DM continuously changes because of hybrid closed-loop insulin delivery systems. They include a subcutaneously worn continuous glucose monitor instrument that interacts with a program that alters the subcutaneous insulin infusion ingested by an insulin pump in retort to variations in sensing blood sugar levels in actual time (Figure 3) [17].
Efficacy and safety of hybrid closed-loop systems
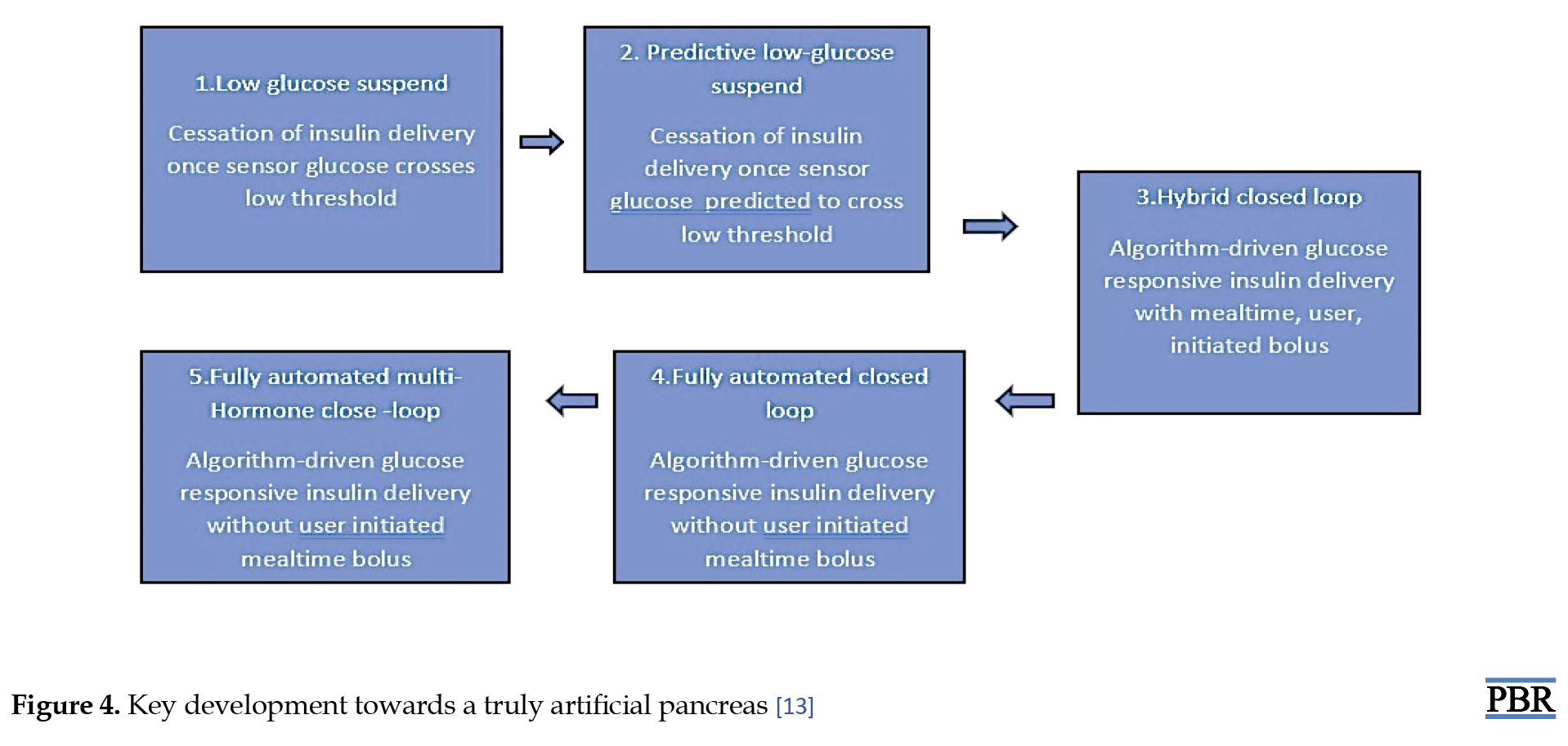
Larger randomized precise trials of unobstructed home usage, conducted over six months or longer, have replaced smaller, carefully supervised investigations conducted overnight or over 24 hours in research centers in experimental studies to examine the safety and effectiveness of hybrid closed loop systems. Initial closed-loop systems have resulted in a 9.6% recovery in time spent in the intended blood sugar range (3.9-10.0 mmol/L) associated with comparator therapies (>2 additional h/d) as well as a 1.5% decrease in time consumed in hypoglycemia (3.9 mmol/L) associated to control treatment. In studies lasting longer than 8 weeks for each intervention, hybrid closed-loop systems positively impact HbA1c, with an overall decrease of 0.3%-0.4% associated with control treatment. The fact that there was a decrease in hypoglycemia in several studies and study participants’ HbA1c levels were low at recruitment, indicates adequate baseline glycaemic control and makes this effect seem small (Figure 4). Similar advantages were noted in a meta-analysis of 25 approaches in the pediatric group [13].
Diabetic complication and intervention
Over time, high blood sugar levels can lead to various complications, affecting different body parts. Here are some common diabetic complications and interventions to manage and prevent them.
Diabetic nephropathy (DN)
DN denotes a clinical condition defined by persistent albuminuria and a continuous decline in renal function that suggests an identifiable course of glomerular disease. DN is the prominent reason for end-stage kidney disease in various nations, with rates of 44% in the United States and 38% in Australia. It has been identified in 20% to 50% of people with diabetes. The results for patients with T1DM or T2DM who acquire DN are much inferior than those who do not [14]. DN is often linked with arterial hypertension and amplified cardiovascular morbidity and mortality.
Clinical method for diabetic kidney disease diagnosis
Diabetic kidney disease is frequently a clinical diagnosis. The most common test for prognosis and diagnosis is a kidney biopsy; however, in most clinics, this procedure is typically only carried out when a different renal disease has been identified [18].
Diabetic ketoacidosis
Diabetic ketoacidosis is the most common acute hyperglycaemic complication in people with DM. The trifecta of high blood sugar levels, metabolic acidosis, and ketosis usually result from a complete or relative absence of insulin and a parallel rise of counter-regulatory hormones. Variable levels of circulatory volume depletion are frequently present as well. Under stressful conditions, such as severe medical or surgical diseases, Diabetic ketoacidosis can happen in people with poorly managed T2DM also in teenagers with newly diagnosed T2DM. Diabetic ketoacidosis is more prevalent in individuals with T1DM, which is uncontrolled and is brought on by an autoimmune breakdown of the islets of Langerhans cells [19].
Diagnosis
The trio of high blood sugar levels, ketosis, and metabolic acidosis is utilized for the detection of diabetic ketoacidosis. Even though the American Diabetic Association (ADA), the Joint British Diabetes Societies (JBDS) for Inpatient Care, and the International Society of Paediatric and Adolescent Diabetes accept that the foremost indicative feature of diabetic ketoacidosis is the elevation of overall blood ketone levels, other analytical measures, such as plasma glucose and bicarbonate values, vary depending on the severity of the elevated total blood ketone content. Studies demonstrate that approximately 3% and 8.7% of those diagnosed with diabetic ketoacidosis present with normal or only modestly increased glucose levels, a state described as euglycemic diabetic ketoacidosis [19].
Lifestyle intervention and behavioral changes
The relevance of education on self-management, patients’ ability to develop self-care and the effects of lifestyle modification therapies are all factors in lifestyle modification [20].
DM patients’ empowerment
Empowerment increases a person's ability to make decisions and engage in self-care practices for their health [20]. Education on the long-term complications of diabetes can motivate individuals to adhere to their treatment plans and make lifestyle changes that can prevent or delay complications such as cardiovascular disease and kidney disease [21]. The empowerment tactic aims to progress an individual's ability for critical thought and self-care decision-making, relies heavily on mutual involvement, awareness-raising, giving essential information, taking social understanding and linguistic differences into account, and open announcement. Allowing patients to discover their goals, comforts, and needs might progress their treatment adherence by involving them in healthcare decisions. According to studies, greater nutritional education is associated with improved glycemic control because those who are aware of nutrition are more likely to make choices appropriate for their health [22].
Lifestyle modification
Adopting and maintaining a healthy lifestyle is often a key component of chronic disease management. This action may include changes in diet, regular exercise, and stress management. Individuals may need to learn specific self-management techniques tailored to their condition, such as monitoring blood sugar levels or managing dietary restrictions [23].
Nutrition therapy and weight management
Bodyweight control is a crucial component of lifestyle adjustment, especially for DM patients who are overweight or obese. The length of the trial is a significant consideration in nutritional intervention studies. There is frequently a greater upgrading in short-term results because it is known that participants' commitment to dietary adjustments decreases over a period [22].
Combined lifestyle interventions
Several studies have investigated the effects of healthy nutrition, management of weight, and regular exercise on risk variables for cardiovascular disease or the development of cardiovascular morbidity in individuals with DM, in addition to research looking at these effects in combination [22].
Telemedicine and digital health solutions
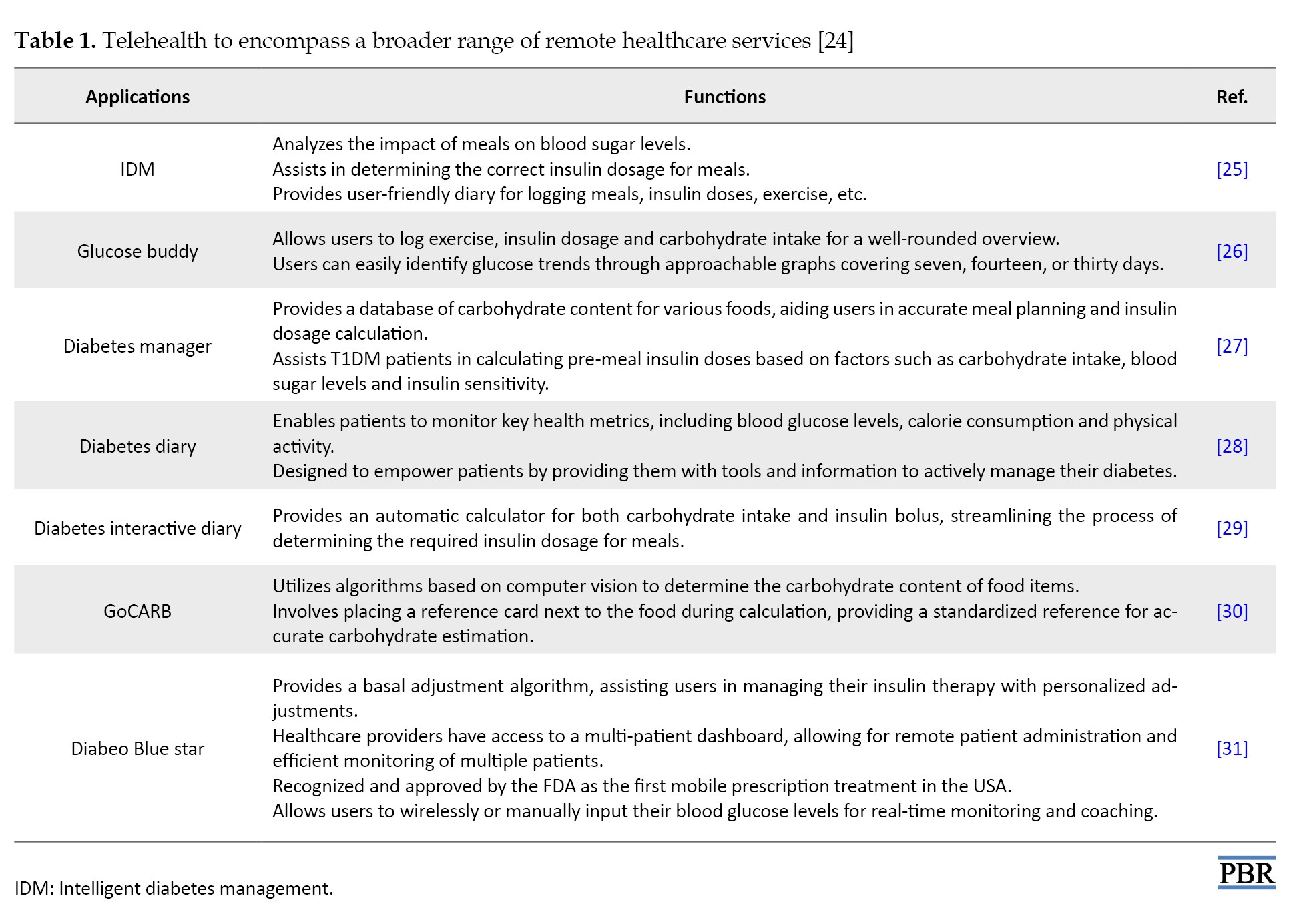
The University of Alberta created and released the smartphone software intelligent diabetes management (IDM) in 2014 for individuals suffering from T1DM. Many apps for both iOS and Android claim to make the essential calculations. Still, most are not accepted by the United States Food and Drug Administration (FDA) and other pertinent regulatory bodies. The most recent smartphone insulin dosage calculator programs examined in randomized clinical trials are described in the following section [24] (Table 1).
Conclusion
Contemporary advancements in diabetes research have shown a new era of hope and improved management for individuals with diabetes. These breakthroughs encompass personalized medicine, innovative technologies like artificial pancreas and continuous glucose monitoring, novel drug therapies, digital health tools, and precision nutrition. While these advancements hold great promises, ongoing research and collaboration among scientists, healthcare providers, and patients are essential to progress toward better diabetes prevention, treatment, and, ultimately, a potential cure.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the support of the Department of Pharmacology, Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University, Bellur, India.
References
Diabetes mellitus (DM) is an increasingly prevalent chronic disease characterized by either a total or partial deficiency of insulin, leading to elevated blood glucose levels, dyslipidemia, and neurovascular system damage [1]. Pancreatic beta cells generate the hormone insulin with various functions in the body. Insulin aids the body’s cells in utilizing glucose as fuel. DM is caused by a deficiency in insulin synthesis or sensitivity [2]. Type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (GDM) are the three primary types of the DM. The first kind of diabetes, commonly known as “juvenile/childhood-onset diabetes” or “insulin-dependent diabetes,” is categorized by the body’s inability to produce insulin. Regular injection of insulin analogs is required for the treatment of this DM [3]. Scientific and technological advancements are essential to identify novel therapeutic agents since DM is a significant socioeconomic burden in many nations. As a result, new therapeutic classes such as amylin analogs, incretin mimics, gastric inhibitory peptide analogs, and inhibitors of the dipeptidyl peptidase-4 and the peroxisome proliferator-activated receptor have been introduced, which are potential targets for drugs used to treat diabetes [2].
The frequency of T2DM has been steadily rising over the past few decades, significantly contributing to the dramatic rise in the incidence of DM. Statistics from the World Health Organization (WHO) show that more than 422 million people worldwide had DM in 2014. It is anticipated that DM prevalence will continue to climb. By 2040, 642 million people will have diabetes worldwide, as predicted by the International Diabetes Federation (IDF). Recent epidemiological data that offers global estimates for the incidence of DM is considered in this research [3].
Prevalence
According to current research, the prevalence of DM has risen dramatically over the last ten years and might be regarded as an epidemic. The diagnosis of DM has been made in 8.8% of the adult population. If this trend continues, it is predicted that by 2040, 6.93 million individuals worldwide, or 9.9% of the population, will be living with diabetes.
According to IDF projections, the incidence of diabetes is predicted to ascend by 1.1% by 2045. Additionally, 1105500 T1DM patients are in the 0 to 19 age range, compared to 132600 newly diagnosed cases in children and young adults. According to current statistics, there are 352.1 million IGT (impaired glucose tolerance) sufferers globally, and 587 million people aged 20 to 79 are expected to get IGT by the year 2050. Last but not least, 21.3 million live births were associated with hyperglycemia in pregnancy in 2017. The majority of these instances (86.4%) were caused by GDM, which primarily affects people in the age range of 40 to 49; the remaining cases were associated with other kinds of DM [3].
Rates of diabetic metabolic syndrome by age and sex
The most current statistics show that more than 326 million individuals in the work population have diabetes, compared to 122.8 million people over the age of 65. In the following decades, these figures are predicted to upsurge to 438.2 and 253.4 million, respectively. The number of men is on the verge of declining regarding gender distribution. In 2017, 8.4% of women aged 20 to 79 were diagnosed with DM, compared to 9.1% of males in the same age range; this discrepancy will likely narrow dramatically in the next few years as the aforementioned numbers are predicted to increase to 9.7% and 10%, respectively [2].
Emerging biomarkers for the early detection and risk assessment of diabetes
Gene expression profiling can precisely correlate with certain disease states because of the development of technologies that recuperate, amplify, and identify the nucleic acids in the plasma. Numerous investigations have found that a particular miRNA expression in circulation is possibly associated with diabetic problems.
New biomarkers
In clinical settings, non-invasive biomarkers detect early signs of islet stress and disorders before beta-cell damage occurs. Established biomarkers of autoimmunity in T1DM include autoantibodies against glutamate decarboxylase, islet cell autoantibodies, islet β-cell secretory granule membrane protein-zinc transporter 8, islet antigens insulin, and protein tyrosine phosphate phosphohydrolase protein [4].
Autoantibodies
To forecast disease and aid in diagnosing T1DM, beta-cell peptides, and proteins are now frequently targeted by autoantibodies. Zinc transporter-8, glutamic acid decarboxylase, islet antigen-2 and insulin are the primary targets of autoantibodies utilized as T1DM indicators in clinical settings [5].
Non-coding ribonucleic acid (RNA)
The non-coding RNAs are now recognized as influential biomarkers in disease diagnosis and clinical management, offering unique functionalities that impact biological processes ahead of glucose level changes. This increased recognition is supported by technological advancements, enhancing the detectability and accuracy of non-coding RNAs [6].
MiRNAs as biomarkers
Specialized short ncRNAs called miRNAs inhibit target mRNA from being translated. According to recent studies, circulating miRNAs are valuable as illness biomarkers. MiRNAs are released by cells under various circumstances, either as free molecules or as tiny vesicles that additional cell types might ingest. The extracellular miRNAs operate as crucial cell-to-cell messengers and regulate different biological processes, such as angiogenesis, tumor cell invasion, and immune response. MiRNAs are trustworthy biomarkers due to their resistance to oxidation in circulation and abundance in particular organs, which can be used to pinpoint the basis of circulating miRNAs. Due to developments in DNA amplification, DNA sequencing, and analysis, we have recognized the miRNAs generated and released by islets under stress. RNA sequencing, quantitative polymerase chain reaction and microarray, and data from extensive human studies have been compiled for this review to present the possibility of using miRNA signatures to identify pre-DM and or staging evolution and mode of diabetic complications in the future [4].
Biomarkers associated with diabetic nephropathy (DN) pathogenesis
By developing advanced glycation products and stimulating the polyol pathway, protein kinase C, hexosamine pathwaym and angiotensin II, hyperglycemia promotes the electron transport chain to enhance too many reactive oxygen species. The oxidative stress is then initiated or enhanced by ROS, which leads to an inflammatory reaction and the development of fibrosis. Additional factors that contribute to the occurrence of DN involve abnormal metabolism of lipids, the renin-angiotensin-aldosterone system (RAAS) stimulation, persistent and glomerular hypertension, deterioration of insulin signaling, increased levels of developmental factors and pro-inflammatory cytokines, and stimulation of intracellular signaling pathways [32].
Potential of precision medicine in tailoring diabetes therapies
Monogenic DM can account for as much as 3% of cases in children, with HNF1A transcription factor gene mutations being the most common cause. Rare penetrant HNF1A mutant carriers frequently manifest diabetes without insulin dependence before the age of 25. They have a significant family history of the disease. Clinical evaluation typically finds such people to be C-peptide positive but autoantibody negative. An early case study demonstrated that patients with DM brought on by HNF1A changes had great sympathy for sulfonylureas, which was neatly backed by a randomized precise study, serving as the initial instance of individualized treatment in diabetes. Even though the exact molecular mechanism underlying the sensitivity of individuals with HNF1A mutations to this particular class of oral diabetes medications is still unknown, it has been proposed that the closure of the KATP channel by sulfonylureas bypasses the main locations of cell dysfunction that are ambitious of the KATP channel, activating the secretion of insulin. Because potassium inwardly-rectifying channel subfamily J member 11 and ATP-binding cassette transporter subfamily C member 8, activating mutations prevent the KATP channels from closing in retort to ATP generated by glycolysis, sulfonylureas are also the initial treatment for people with neonatal diabetes [7].
Personalized treatment recommendations using evidence from research trials and pharmacogenomics
Recognizing the value of replication is crucial. We should be just as skeptical of the latest findings from pharmacogenetic analyses as we are of research focusing on the possibility of T2DM. Most scientific research concentrates on small-scale investigations of candidate genes or single nucleotide polymorphisms rather than objective analyses of the influence of genetic diversity on therapeutic response across the genome [7].
Metformin
Metformin is the most frequently prescribed and thoroughly researched medication for the treatment of T2DM. Additionally, the study of metformin comprises pharmacogenomics, genetic investigations of metformin transport, and molecular targets for metformin activity. SLC22A1, SLC22A2 and SLC47A have been discovered as genetic drivers of metformin pharmacokinetic variability, tissue uptake, and clearance; nevertheless, their therapeutic value in predicting metformin interaction in T2DM patients [7].
Sulfonylureas
Several T2DM guidelines suggest insulin and sulfonylureas as second-line blood sugar-lowering medications. This widespread history and current use result from years of clinical study. Information from the GoDARTS study revealed that carriers of the TCF7L2 T2DM risk variant are less probable than non-carriers to enhance glycaemic goal line in reaction to sulfonylureas. This treatment effect was not obtained for metformin [7]. Change at the TCF7L2 gene location is probably the most well-studied example of genetics determining pharmacological responses. Additionally, genetic variation among the genes crucial for sulfonylurea metabolism, the sulfonylurea receptor, beta-cell function, and insulin action, such as CYP2C9, KCNJ1, CDKAL1, CDKN2B and NOS1AP, has also been studied [7].
Thiazolidinediones
Thiazolidinediones slowed the progression of nonalcoholic fatty liver disease in subsets of patients in studies lasting up to 24 months despite questions about the relative advantages and risks of these drugs for T2DM patients’ glucose control. There is still a limited understanding of the genetic factors influencing the medicinal effects of thiazolidinediones, especially pioglitazone and rosiglitazone. Variations in solute carrier organic anion-transporter, which codes for the organic anion transporting polypeptide 1B1, had an impact on the glucose tolerance to the medication in 833 patients who were evaluated from 1 to 18 months into treatment, in contrast to CYP2C8, which encodes the cytochrome P450 2C8 metabolizing enzyme. Surprisingly, these genetic variations did not similarly impact how well patients responded to pioglitazone medication [8].
Innovative treatment approaches
Treatments for T2DM patients include reducing blood sugar levels, shedding pounds, and decreasing blood pressure through GLP-1 receptor analogs and SGLT2 inhibitors. According to results from cardiovascular outcome examinations, these drugs reduce the hazard of heart failure hospitalization, lower cardiovascular disease, and offer protection against severe cardiovascular disease to those with chronic heart disease [9]. Studies that use hard renal endpoint kidney disease instead of substitute indicators are currently being conducted to define the renoprotective benefits of both drugs. SGLT2 inhibitors should be avoided in conditions where there is a potential of developing diabetic ketoacidosis because they operate best when used to lower blood sugar when the predictable rate of glomerular filtration is greater than 60 mL/min/173 m2 at the time of treatment.
SGLT2 inhibitors
A person with diabetes has urine glucose reabsorption that is substantially higher than the normal value of 180 g/d. SGLT2 inhibition causes around 70-90 g of urine glucose excretion, with an accompanying energy forfeiture of about 300 kcal/d, because it inhibits only about 50 percent of the filtered glucose load. This finding contradicts the frequently cited statistic that SGLT2 is in charge of about 90% of renal glucose absorption. This outcome can be caused by partial SGLT2 blockage or additional upregulation of SGLT1 expression following SGLT2 therapy (Figure 1) [9]. All presently accessible SGLT2i lower blood sugar and HbA1c by 0.6% to 1%, relying on the baseline HbA1c and experimental design. Generally speaking, these medications work independently of other glucose-dropping drugs and can be coupled with further treatments, including insulin. In the presence of severe renal impairment, the effects of decreasing HbA1c are diminished, and none of them are presently advised for use once the eGFR is <45 mL/min. A weight loss of two to three kg and a minor decrease in blood pressure are also predicted. The foremost side effects are an elevated hazard of bacterial and fungal urinary tract infections, particularly in women, as well as adverse blood pressure-lowering side effects like giddiness and postural hypotension. More common side effects comprise diabetic ketoacidosis, more seen in people with T1DM. Concerns have also been upstretched lower limb amputees, bladder cancer, and crepitus upon palpation. Still, new statistics from the DECLARE trial propose the hazard for these side effects may not be augmented for dapagliflozin [10]. The eradication of urinary glucose excretion (UGE) when the filtered glucose level comes in below the transport capacity of SGLT1, together with the compensatory metabolic changes observed, which includes increased production of liver glucose and the circumstance that these medications do not stimulate insulin secretion [9], all contribute to the fact that the menace of lower the blood sugar level with SGLT2 inhibition.
GLP-1 receptor agonists
In reaction to foods, mainly glucose and fat, intestinal epithelial L-cells produce the peptide GLP-1 derived from the gut. Multiple target organs are affected physiologically by GLP-1. It is an incretin that increases the amount of insulin secreted by pancreatic cells in response to glucose, increases neogenesis, prevents apoptosis, and decreases the cells’ ability to secrete glucagon. GLP-1 does not cause hypoglycemia because insulin production is only elevated overhead about 3 to 5 mmol/L of blood glucose. GLP-1 also affects the hypothalamus, which results in an increase in satiety and a reduction in food intake. The adipose tissue, heart, and stomach are additional areas where GLP-1 has an impact.
Lixisenatide, exenatide, dulaglutide, liraglutide and semaglutide are a few GLP-1 receptor agonists that have been accepted for use in T2DM. Although oral semaglutide is accessible, all are directed as subcutaneous injections. Exendin-4, human GLP-1 or the salivary peptide from the Gila monster (Heloderma suspectum) served as the basis for the development of these molecules, which have biological characteristics that are indicative of important structural alterations to the amino acid order that confer resistance to dipeptidyl peptidase-4 deprivation. Different clinical outcomes result from these molecular variations. Short-acting GLP-1 receptor agonists are the first class of receptor agonists and have a structural similarity to exendin-4 of 90%–97%. These substances alter postprandial plasma glucose concentrations more drastically than long-acting GLP-1 receptor agonists due to their dramatic slowing of stomach emptying and lessening impact on insulin production [8]. Long-acting GLP-1 receptor agonists can have a more pronounced effect on fasting blood sugar levels and 24-hour glucose profiles. This outcome is because they provide a continuous and sustained level of GLP-1 receptor activation throughout the day, helping to regulate blood sugar levels in both fasting and postprandial states. It is important to note that the choice between short-acting and long-acting GLP-1 receptor agonists depends on individual patient characteristics, preferences, and treatment goals. Healthcare professionals consider factors such as the patient’s lifestyle, adherence to medication, and the overall management plan for diabetes when prescribing these medications [11].
Mesenchymal stem cell-based therapy
Multipotent adult stem cells (MSCs) can be produced from tissues in both adult and neonatal individuals. MSCs can be found in practically all tissues with a perivascular region, although adult bone marrow is the most common source. MSCs are produced from the mesodermal germ layer and can develop in vitro into adipocytes, osteoblasts, and chondroblasts, all connective tissue types [12].
Artificial intelligence (AI) and predictive analytics
AI research is advancing quickly, and its applicability to the global diabetes pandemic could fundamentally alter how this chronic illness is identified and treated. Prognostic mock-ups for the chance of receiving diabetes or its difficulties have been established utilizing machine learning principles. AI enables continuous, effortless remote monitoring of the patient’s signs and biomarkers. Social media platforms and online forums also increase patient involvement in diabetes care. Technological improvements have enhanced the distribution of resources in the field of diabetes. These ingenious technical advancements have improved glycaemic control by lowering fasting and postprandial blood glucose levels, glycosylated hemoglobin, and glucose excursions. AI will enable a paradigm shift in diabetes management from current methods to building targeted, based on data meticulousness care [15].
Case-based reasoning
Diabetes management uses case-based reasoning, an AI technique, to answer novel issues based on learning from analogous prior experiences [13, 14]. A case-based reasoning approach employed in the treatment of DM is the diabetes management system. This system intends to mechanically recognize blood glucose control problems, make recommendations for fixing them, and keep the path of positive and negative remedies designed for each patient individually [13]. For various meal settings in diabetes, case-based reasoning has been utilized to optimize and personalize insulin administration.
Artificial neural networks
Diabetes diagnosis has made extensive and unique use of neural network techniques. Intelligent algorithms have been developed to investigate how different factors affect glycemic indices (Figure 2) [15].
Advances in continuous glucose monitoring (CGM)
Patients with chronic renal disease have an option that provides a more thorough and reliable glycaemic assessment called CGM. A tiny filament is frequently inserted into subcutaneous tissue to monitor glucose in interstitial fluid in most commercially accessible CGM devices, which are minimally invasive. Because diffusion is concentration gradient-dependent, there is a dynamic equilibrium among the blood’s interstitial fluid glucose and sugar levels. The filament of the CGM takes up the interstitial glucose through capillaries. An electrochemical response in the sensor calculates the amount of interstitial glucose present. A reader or smartphone app sends and shows minute-by-minute interstitial glucose measurements on a mobile device [16] diabetes is the leading cause of chronic kidney disease (CKD).
Closed-loop insulin delivery system
Automation of closed-loop insulin delivery systems has been made possible by developments in diabetes technology. Numerous composites of closed-loop systems are being commercialized, demonstrating the quick progression of this rapidly developing expertise from investigative into experimental practice, progressively revolutionizing the treatment of T1DM in children and adults. The clinical treatment of T1DM continuously changes because of hybrid closed-loop insulin delivery systems. They include a subcutaneously worn continuous glucose monitor instrument that interacts with a program that alters the subcutaneous insulin infusion ingested by an insulin pump in retort to variations in sensing blood sugar levels in actual time (Figure 3) [17].
Efficacy and safety of hybrid closed-loop systems
Larger randomized precise trials of unobstructed home usage, conducted over six months or longer, have replaced smaller, carefully supervised investigations conducted overnight or over 24 hours in research centers in experimental studies to examine the safety and effectiveness of hybrid closed loop systems. Initial closed-loop systems have resulted in a 9.6% recovery in time spent in the intended blood sugar range (3.9-10.0 mmol/L) associated with comparator therapies (>2 additional h/d) as well as a 1.5% decrease in time consumed in hypoglycemia (3.9 mmol/L) associated to control treatment. In studies lasting longer than 8 weeks for each intervention, hybrid closed-loop systems positively impact HbA1c, with an overall decrease of 0.3%-0.4% associated with control treatment. The fact that there was a decrease in hypoglycemia in several studies and study participants’ HbA1c levels were low at recruitment, indicates adequate baseline glycaemic control and makes this effect seem small (Figure 4). Similar advantages were noted in a meta-analysis of 25 approaches in the pediatric group [13].
Diabetic complication and intervention
Over time, high blood sugar levels can lead to various complications, affecting different body parts. Here are some common diabetic complications and interventions to manage and prevent them.
Diabetic nephropathy (DN)
DN denotes a clinical condition defined by persistent albuminuria and a continuous decline in renal function that suggests an identifiable course of glomerular disease. DN is the prominent reason for end-stage kidney disease in various nations, with rates of 44% in the United States and 38% in Australia. It has been identified in 20% to 50% of people with diabetes. The results for patients with T1DM or T2DM who acquire DN are much inferior than those who do not [14]. DN is often linked with arterial hypertension and amplified cardiovascular morbidity and mortality.
Clinical method for diabetic kidney disease diagnosis
Diabetic kidney disease is frequently a clinical diagnosis. The most common test for prognosis and diagnosis is a kidney biopsy; however, in most clinics, this procedure is typically only carried out when a different renal disease has been identified [18].
Diabetic ketoacidosis
Diabetic ketoacidosis is the most common acute hyperglycaemic complication in people with DM. The trifecta of high blood sugar levels, metabolic acidosis, and ketosis usually result from a complete or relative absence of insulin and a parallel rise of counter-regulatory hormones. Variable levels of circulatory volume depletion are frequently present as well. Under stressful conditions, such as severe medical or surgical diseases, Diabetic ketoacidosis can happen in people with poorly managed T2DM also in teenagers with newly diagnosed T2DM. Diabetic ketoacidosis is more prevalent in individuals with T1DM, which is uncontrolled and is brought on by an autoimmune breakdown of the islets of Langerhans cells [19].
Diagnosis
The trio of high blood sugar levels, ketosis, and metabolic acidosis is utilized for the detection of diabetic ketoacidosis. Even though the American Diabetic Association (ADA), the Joint British Diabetes Societies (JBDS) for Inpatient Care, and the International Society of Paediatric and Adolescent Diabetes accept that the foremost indicative feature of diabetic ketoacidosis is the elevation of overall blood ketone levels, other analytical measures, such as plasma glucose and bicarbonate values, vary depending on the severity of the elevated total blood ketone content. Studies demonstrate that approximately 3% and 8.7% of those diagnosed with diabetic ketoacidosis present with normal or only modestly increased glucose levels, a state described as euglycemic diabetic ketoacidosis [19].
Lifestyle intervention and behavioral changes
The relevance of education on self-management, patients’ ability to develop self-care and the effects of lifestyle modification therapies are all factors in lifestyle modification [20].
DM patients’ empowerment
Empowerment increases a person's ability to make decisions and engage in self-care practices for their health [20]. Education on the long-term complications of diabetes can motivate individuals to adhere to their treatment plans and make lifestyle changes that can prevent or delay complications such as cardiovascular disease and kidney disease [21]. The empowerment tactic aims to progress an individual's ability for critical thought and self-care decision-making, relies heavily on mutual involvement, awareness-raising, giving essential information, taking social understanding and linguistic differences into account, and open announcement. Allowing patients to discover their goals, comforts, and needs might progress their treatment adherence by involving them in healthcare decisions. According to studies, greater nutritional education is associated with improved glycemic control because those who are aware of nutrition are more likely to make choices appropriate for their health [22].
Lifestyle modification
Adopting and maintaining a healthy lifestyle is often a key component of chronic disease management. This action may include changes in diet, regular exercise, and stress management. Individuals may need to learn specific self-management techniques tailored to their condition, such as monitoring blood sugar levels or managing dietary restrictions [23].
Nutrition therapy and weight management
Bodyweight control is a crucial component of lifestyle adjustment, especially for DM patients who are overweight or obese. The length of the trial is a significant consideration in nutritional intervention studies. There is frequently a greater upgrading in short-term results because it is known that participants' commitment to dietary adjustments decreases over a period [22].
Combined lifestyle interventions
Several studies have investigated the effects of healthy nutrition, management of weight, and regular exercise on risk variables for cardiovascular disease or the development of cardiovascular morbidity in individuals with DM, in addition to research looking at these effects in combination [22].
Telemedicine and digital health solutions
The University of Alberta created and released the smartphone software intelligent diabetes management (IDM) in 2014 for individuals suffering from T1DM. Many apps for both iOS and Android claim to make the essential calculations. Still, most are not accepted by the United States Food and Drug Administration (FDA) and other pertinent regulatory bodies. The most recent smartphone insulin dosage calculator programs examined in randomized clinical trials are described in the following section [24] (Table 1).

Conclusion
Contemporary advancements in diabetes research have shown a new era of hope and improved management for individuals with diabetes. These breakthroughs encompass personalized medicine, innovative technologies like artificial pancreas and continuous glucose monitoring, novel drug therapies, digital health tools, and precision nutrition. While these advancements hold great promises, ongoing research and collaboration among scientists, healthcare providers, and patients are essential to progress toward better diabetes prevention, treatment, and, ultimately, a potential cure.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contribute to preparing all parts of the research.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the support of the Department of Pharmacology, Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University, Bellur, India.
References
- Copur S, Onal EM, Afsar B, Ortiz A, van Raalte DH, Cherney DZ, et al. Diabetes mellitus in chronic kidney disease: Biomarkers beyond HbA1c to estimate glycemic control and diabetes-dependent morbidity and mortality. J Diabetes Complications. 2020; 34(11):107707. [DOI:10.1016/j.jdiacomp.2020.107707] [PMID]
- Vasu S, Kumano K, Darden CM, Rahman I, Lawrence MC, Naziruddin B. MicroRNA signatures as future biomarkers for diagnosis of diabetes states. Cells. 2019; 8(12):1533. [DOI:10.3390/cells8121533] [PMID] [PMCID]
- Zhang J, Liu J, Qin X. Advances in early biomarkers of diabetic nephropathy. Rev Assoc Med Bras. 2018; 64(1):85-92. [DOI:10.1590/1806-9282.64.01.85] [PMID]
- Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med. 2020; 30(3):030502. [DOI:10.11613/BM.2020.030502] [PMID] [PMCID]
- Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: A meta-analysis. JAMA Intern Med. 2017; 177(5):633-40. [DOI:10.1001/jamainternmed.2016.9607] [PMID] [PMCID]
- Gauthier BR, Cobo-Vuilleumier N, López-Noriega L. Roles of extracellular vesicles associated non-coding RNAs in diabetes mellitus. Front Endocrinol. 2022; 13:1057407. [DOI:10.3389/fendo.2022.1057407] [PMID] [PMCID]
- Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018; 6(11):891-900. [DOI:10.1016/S2213-8587(18)30052-4] [PMID]
- Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet. 2021; 398(10296):262-76 [DOI:10.1016/S0140-6736(21)00536-5] [PMID]
- Brown E, Rajeev SP, Cuthbertson DJ, Wilding JPH. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019; 21(Suppl 2):9-18. [DOI:10.1111/dom.13650] [PMID]
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380(4):347-57. [DOI:10.1056/NEJMoa1812389] [PMID]
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019; 139(17):2022-31. [DOI:10.1161/CIRCULATIONAHA.118.038868] [PMID]
- de Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol. 2021; 12:631463. [DOI:10.3389/fendo.2021.631463] [PMID] [PMCID]
- Karageorgiou V, Papaioannou TG, Bellos I, Alexandraki K, Tentolouris N, Stefanadis C, et al. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism. 2019; 90:20-30. [DOI:10.1016/j.metabol.2018.10.002] [PMID]
- Evans K, Pyart R, Steenkamp R, Whitlock T, Stannard C, Gair R, et al. UK renal registry 20th annual report: Introduction. nephron. 2018; 139(Suppl 1):1-12. [DOI:10.1159/000490958] [PMID]
- Nomura A, Noguchi M, Kometani M, Furukawa K, Yoneda T. Artificial intelligence in current diabetes management and prediction. Curr Diab Rep. 2021; 21(12):61. [DOI:10.1007/s11892-021-01423-2] [PMID] [PMCID]
- Ling J, Ng JKC, Chan JCN, Chow E. Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol. 2022; 13:869899. [DOI:10.3389/fendo.2022.869899] [PMID] [PMCID]
- Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia. 2021; 64(5):1007-15. [DOI:10.1007/s00125-021-05391-w] [PMID] [PMCID]
- American Diabetes Association. 11. Microvascular complications and foot care: Standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S124-38. [DOI:10.2337/dc19-S011] [PMID]
- Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020; 6(1):40. [DOI:10.1038/s41572-020-0165-1] [PMID]
- Cheng LJ, Wang W, Lim ST, Wu VX. Factors associated with glycaemic control in patients with diabetes mellitus: A systematic literature review. J Clin Nurs. 2019; 28(9-10):1433-50. [DOI:10.1111/jocn.14795] [PMID]
- Li Z, Chen Q, Yan J, Liang W, Wong WCW. Effectiveness of motivational interviewing on improving care for patients with type 2 diabetes in China: A randomized controlled trial. BMC Health Serv Res. 2020; 20(1):57. [DOI:10.1186/s12913-019-4776-8] [PMID] [PMCID]
- Lambrinou E, Hansen TB, Beulens JW. Lifestyle factors, self-management and patient empowerment in diabetes care. Eur J Prev Cardiol. 2019; 26(2_suppl):55-63. [DOI:10.1177/2047487319885455] [PMID]
- Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M. Management of type 2 diabetes: Current strategies, unfocussed aspects, challenges, and alternatives. Med Princ Pract. 2021; 30(2):109-21. [DOI:10.1159/000511002] [PMID] [PMCID]
- Doupis J, Festas G, Tsilivigos C, Efthymiou V, Kokkinos A. Smartphone-based technology in diabetes management. Diabetes Ther. 2020; 11(3):607-19. [DOI:10.1007/s13300-020-00768-3] [PMID] [PMCID]
- Ryan EA, Holland J, Stroulia E, Bazelli B, Babwik SA, Li H, et al. Improved A1c levels in type 1 diabetes with smartphone app use. Can J Diabetes. 2017; 41(1):33-40. [DOI:10.1016/j.jcjd.2016.06.001] [PMID]
- Loretelli C, Assi E, Seelam AJ, Ben Nasr M, Fiorina P. Cell therapy for type 1 diabetes. Expert Opin Biol Ther. 2020; 20(8):887-97. [DOI:10.1080/14712598.2020.1748596] [PMID]
- Shariful Islam SM, Mishra V, Siddiqui MU, Moses JC, Adibi S, Nguyen L, et al. Smartphone apps for diabetes medication adherence: Systematic review. JMIR Diabetes. 2022;7(2):1-14. [DOI:10.2196/33264] [PMID] [PMCID]
- Ersotelos NT, Margioris AN, Zhang X, Dong F. Review of mobile applications for optimizing the follow-up care of patients with diabetes. Hormones. 2018; 17(4):541-50. [DOI:10.1007/s42000-018-0062-0] [PMID] [PMCID]
- Kumar S, Moseson H, Uppal J, Juusola JL. A diabetes mobile app with in-app coaching from a certified diabetes educator reduces A1C for Individuals With Type 2 diabetes. Diabetes Educ. 2018; 44(3):226-36.[DOI:10.1177/0145721718765650] [PMID]
- Vasiloglou MF, Mougiakakou S, Aubry E, Bokelmann A, Fricker R, Gomes F, et al. A Comparative study on carbohydrate estimation: GoCARB vs. dietitians. Nutrients. 2018; 10(6):741. [DOI:10.3390/nu10060741] [PMID] [PMCID]
- Franc S, Hanaire H, Benhamou PY, Schaepelynck P, Catargi B, Farret A, et al. DIABEO system combining a mobile app software with and without telemonitoring versus standard care: A randomized controlled trial in diabetes patients poorly controlled with a basal-bolus insulin regimen. Diabetes Technol Ther. 2020; 22(12):904-11. [DOI:10.1089/dia.2020.0021] [PMID] [PMCID]
- Mohamed MM, Eissa S, Mostafa M, Hegazy MGA. Diabetic nephropathy assessment: Microtubule-associated protein 1 light-chain 3B a new promising biomarker. Indian J Clin Biochem. 2019; 34(4):472-8. [DOI:10.1007/s12291-018-0773-7] [PMID] [PMCID]
Type of Study: Review article |
Subject:
Pharmacology
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |