Volume 9, Issue 4 (2023)
Pharm Biomed Res 2023, 9(4): 297-310 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rahamouz-Haghighi S, Bagheri K, Sharafi A. Investigating a Simple and Sensitive High-performance Liquid Chromatography Method for Simultaneous Determination of Apigenin, Catalpol, and Gallic Acid Contents in Plantago Lanceolata L. and Plantago Major L.. Pharm Biomed Res 2023; 9 (4) :297-310
URL: http://pbr.mazums.ac.ir/article-1-536-en.html
URL: http://pbr.mazums.ac.ir/article-1-536-en.html
1- Department of Plant Production and Genetics, Faculty of Agriculture, University of Zanjan, Zanjan, Iran.
2- Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran.
2- Zanjan Pharmaceutical Biotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran.
Full-Text [PDF 1620 kb]
(599 Downloads)
| Abstract (HTML) (1930 Views)
Optimization of solvent systems for the extraction of apigenin, catalpol, and gallic acid
Liquid extraction was performed using various solvents to find an effective solvent for extracting apigenin, catalpol, and gallic acid. After separating the extracts, the fractions containing apigenin, catalpol, and gallic acid were analyzed using HPLC. The dichloromethane extract of the leaf part of P. lanceolata and P. major produced the highest yield of apigenin (1.08 and 0.58 µg/mg DW) compared with the root part, respectively (Table 10 and Table 11).
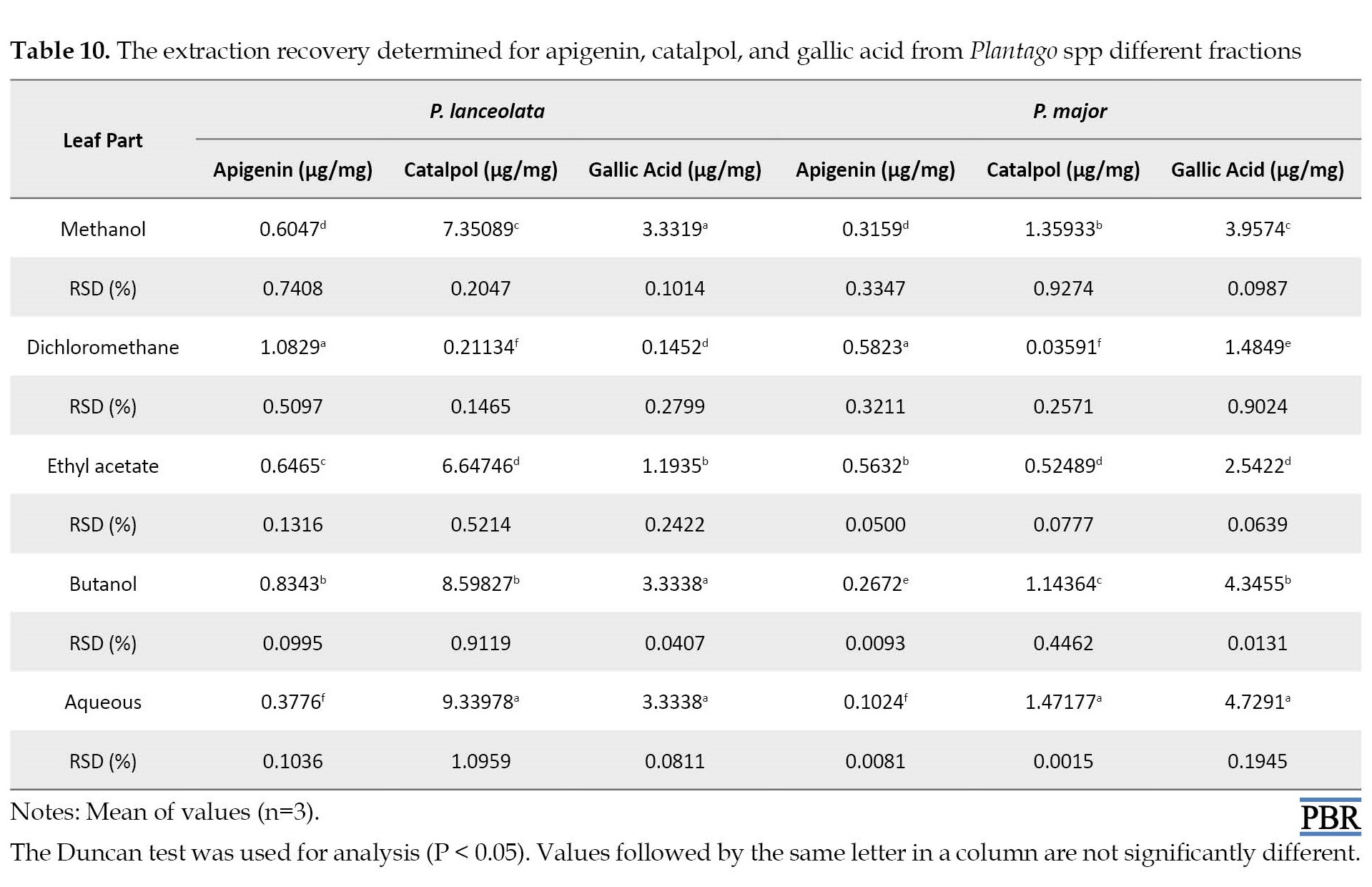

In contrast, the aqueous extract of P. lanceolata leaf and P. major root showed more catalpol content (9.339 and 2.451 µg/mg). The content of gallic acid was higher in methanolic, butanol, and aqueous extracts of P. lanceolata (3.33 µg/mg) and P. major (3.95, 4.34, and 4.72 µg/mg) (Table 10 and Table 11).
Discussion
A reliable HPLC technique was used to evaluate the levels of three pharmaceutical compounds, including the iridoid glycoside catalpol, the flavonoid apigenin, and the phenol gallic acid, in various fractions of leaf and root parts of Plantago species.
Some studies have used a methanol-water mobile phase to determine the aucubin iridoid glycoside in Plantago species using HPLC [33, 34]. Methanol can interfere with compound detection. As a result, some studies have used acetonitrile instead of methanol [35]. In this study, when methanol was used as the mobile phase, its peak interfered with the analytes’ peak. Only the internal standard peak was observed using acetonitrile as the mobile phase.
The HPLC chromatograms of P. lanceolata and P. major showed the presence of apigenin, catalpol, and gallic acid at the Rt of around 2.5, 3.7, and 5.7 min, and the RSD% of the Rt of 1.88, 0.88, and 0.76%, respectively. Other studies have been found to determine these compounds separately in two Plantago species by HPLC, such as a peak for catalpol around 4.35 min [36], 5 min [37], 13.9 min [38], and for gallic acid around 4.40 min [39] and 4.6 min in HPLC assay of P. lanceolata [40].
In our earlier research, we used the HPLC method to measure the levels of flavonoid, phenolic, and iridoid glycoside compounds in methanolic and 80% aqueous-methanol extracts of different parts of P. lanceolata and P. major. Statistical analysis of the crude extracts revealed that the aerial parts of both species contained higher levels of apigenin and gallic acid than the root parts. However, the aerial parts of P. lanceolata and the root parts of P. major had more catalpol content [5, 14, 41, 42]. Despite these findings, the methods used were not optimized, and only crude extracts were used, indicating a need for more detailed research.
As a highly polar compound, glycoside iridoid catalpol easily dissolves in water. Tong et al. (2015) investigated the typical polar biphasic solvent systems, including ethyl acetate–n-butanol–water and n-butanol–water in different amounts using thin-layer chromatography. This technique is used for the separation of a non-volatile mixture. Their study showed that a large amount of catalpol was partitioned into the aqueous phase due to its high polarity. Combining the mentioned solvents (ethyl acetate–n-butanol–water, 2:1:3, V/V/V) provided a good fraction system for separating catalpol from the purified crude extract [43]. The present study also stated that the aqueous extract of P. lanceolata leaves and P. major roots contained more catalpol content. The researchers reported that aucubin, a well-known marker of Plantago species, was present in all samples, and the highest amounts were found in P. media and P. argentea (44.27 and 39.93 mg/g DW, respectively); however, catalpol was detected only in P. altissima, P. argentea, and P. lanceolata [44, 45]. However, in the present paper, catalpol was also seen in lower amounts in P. major. Another research has shown the high content of phenolic acids in P. lanceolata aqueous-methanolic extracts and indicated that the benzoic acid derivatives hydroxybenzoate and 3,4,5-trihydroxybenzoate (gallic acid) are highly represented [46]. In the current work, the highest amount of gallic acid was obtained in aqueous, n-butanol, and methanolic fractions, indicating better solubility in polar solvents. On the other hand, the biological applications of flavonoids are limited due to low bioavailability as well as low aqueous solubility [47]. Dichloromethane fraction showed the presence of flavonoid apigenin in Orbignya peciosa Mart. leaves [48]. Similarly, dichloromethane extract also showed the highest apigenin content in Plantago species. The yield of secondary metabolites, such as flavonoid, phenol, and iridoid glycoside in the Plantago species varies under the influence of many factors, including the pattern of climatic temperature during the plant growing season, collection area, harvest time, drying method, environmental stresses, and extraction methods [41]. The data revealed the good precision and accuracy of all the proposed procedures. This protocol has advantages, including economic extraction projects, quick accuracy, ease, high sensitivity, a short chromatographic run time, and reproducibility.
Conclusions
In this study, the determination of optimum conditions for extracting these medicinal compounds, including apigenin, catalpol, and gallic acid, was developed in two species of Plantago. As far as we know, this is the first report on the simultaneous evaluation of apigenin, catalpol, and gallic acid contents in leaf and root parts of two species of this genus with various solvents. Among the important medicinal compounds in Plantago species extracts, the iridoid glycosides aucubin and catalpol can be utilized as analytical markers to evaluate the quality of different extracts due to being iridoid-containing sources. Research on available species with medicinal properties is important. In this regard, knowledge about the composition present in the plants and the most economical and simple method to measure them is essential.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The paper was extracted from the PhD dissertation of Samaneh Rahamouz-Haghighi, approved by Department of Plant Production and Genetics, University of Zanjan (Grant No: A-12-848-35).
Authors' contributions
Project administration, investigation, formal analysis, writing original draft and revising: Samaneh Rahamouz-Haghighi; Funding and supervision: Khadijeh Bagheri and Ali Sharafi.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the authority of the University of Zanjan and School of Pharmacy, Zanjan University of Medical Sciences for their support.
References
Full-Text: (563 Views)
Introduction
Recently, medicinal plants have attracted significant attention due to their therapeutic and biological properties. Numerous studies have reported the potential of medicinal plants in various traditional, alternative, and complementary systems [1]. Plantago genus belonging to the Plantaginaceae family includes about 275 species [2]. Plantago species are used commercially worldwide as food and in the traditional treatment of many diseases [2].
Ribwort plantain (Plantago lanceolata L. and great plantain (Plantago major L. are the most well-known Plantago species, which exhibited beneficial pharmaceutical properties. P. lanceolata has been used for medicinal purposes to treat various diseases, such as wound healing [3], respiratory system disorders, reproductive system, digestive organs, blood circulation, anti-inflammation [4], and anti-cancer, in addition to its anti-bacterial properties [5, 6, 7]. P. major is effective as an anti-oxidant [8], wound healing [9], anti-diabetic [10], anti-inflammatory [11], anti-bacterial, and anti-cancer [12-14]. Plantago species have many medicinally active ingredients, such as iridoids, flavonoids, phenolic acids, triterpenes, and phenylpropanoid glycosides [2].
There is currently considerable interest in studying the biological activity of plant extracts. Plants containing iridoids have attracted more attention [15, 16]. Iridoids are a large group of monoterpenoids [17] that induce a defensive role in plants [18]. Iridoids are widespread in Lamiales, such as Cornales, Gentianales, and Plantago [19]. The iridoid glycosides aucubin and catalpol are the biologically active secondary metabolites utilized as analytical and chemotaxonomic markers to determine the quality of plant-derived extracts [20]. In herbal samples, the iridoid glycosides with aucubin and catalpol are approximately 2% to 3%. The stage of plant maturity determines the content of iridoids. The iridoid content in old leaves is extremely low, while young leaves show a maximum of 9% iridoid. Aucubin is a major compound in older leaves, while catalpol is the dominant constituent in young leaves [21]. Iridoids have various biological activities, such as anti-tumor, anti-inflammatory, hepato-protective, and neuro-protective activities [22]. Catalpol has various therapeutic functions, such as protecting against liver damage and lowering high blood sugar [23]. Flavonoids, phenolic, and tannins compounds comprise most medicinal plants’ secondary metabolites [24]. Apigenin is a subclass of flavonoids and a non-mutagenic flavone with less toxicity [25]. Apigenin has notable properties, including anti-oxidant, anti-proliferative, anti-tumor, apoptosis, anti-inflammatory [26], and anti-bacterial activity [27]. Gallic acid is one of the essential bioactive agents that showed numerous medicinal properties, including anti-microbial, anti-cancer [28], anti-viral [29], anti-inflammatory, anti-oxidative, and anti-neoplastic properties [30].
Many studies have shown that apigenin, catalpol, and gallic acid present in P. lanceolata and P. major have therapeutic potential; however, the methods used to determine their amount in previous studies were not optimized and crude extracts of plants were often used, highlighting the need for further research and more detailed information. This study introduced a simple, selective, and stable method for detecting these compounds in different fractions of P. lanceolata and P. major leaves and roots using high-performance liquid chromatography (HPLC). The goal of choosing a solvent system is to find the best type of extract for detecting these compounds. To the best of the authors’ knowledge, this is the first report on the simultaneous quantification of apigenin, catalpol, and gallic acid in these species.
Materials and Methods
Instrument and high-performance liquid chromatography apparatuses
The system was equipped with a pump (Waters Corp., Milford, MA, USA) and a UV detector (WATER, model Breeze, USA) used at wavelengths 204, 210, 256, and 330 nm, with the outputs recorded using the Breeze software. The apigenin, catalpol, and gallic acid were conducted through two types of columns, the Phenomenex-C8 column (150 mm×4.6 mm, the particle size of 5 µm, USA) and Thermo Hypersil-C18 column (150 mm×4.6 mm, the particle size of 5 µm, Germany) equipped with the same packed guard column. The mobile phase includes an organic solvent, acetonitrile, and eluents orthophosphoric acid or formic acid (different ratio V/V) with flow rates of 0.4, 0.8, and 1 mL/min. An aliquot of 20 μL was injected into the HPLC system using a loop injector (Rheodyne 7725i, Cotati, CA, USA). The column temperature was kept at 25ºC.
Chemicals
Apigenin (98% purity), catalpol (96%), and gallic acid (96%) were provided by the Sigma-Aldrich (Aldrich Division; Steinheim, Germany) company. Acetonitrile, water, and methanol were HPLC, gradient grade.
Preparation of stock solutions and working solutions
Apigenin stock solutions were made in dimethyl sulfoxide (DMSO) and HPLC grade methanol (5:95%, V/V). Each standard was separately dissolved in sterile purified water to prepare catalpol and gallic acid and then was stored at 40C for gallic acid and 200C for apigenin and catalpol. Working standard solutions used to optimize and validate the method were prepared in solvents using the dilution method at concentrations of 1.5625 to 100 μg/mL for catalpol and 0.15625 to 10 μg/mL for apigenin and gallic acid.
Plant sample
P. lanceolata and P. major were collected as wild plants in September 2018 from the University of Zanjan, Iran, research field. The voucher specimens were prepared in triplicates for each analysis and authenticated at the Department of Pharmacognosy, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan. The plant samples were washed to remove dust particles. The leaf and root parts of the two species of Plantago were isolated, air-dried, and then powdered by an electric mixer-grinder.
Sample preparation by the fraction method (liquid-liquid extraction)
An amount of 250 g of dried and powdered leaf and root parts of Plantago spp were weighed, and extraction was carried out for 16 h via reflex method using petroleum ether as a solvent (to remove nonpolar compounds) followed by methanol with the same period. The methanol extract was separated using The methanol extract was separated using dichloromethane, ethyl acetate, n-butanol, and aqueous phase via the liquid-liquid extraction method in a separatory funnel [31]. Using filter paper, the aqueous fraction was filtered for plant fiber removal.
Extracts were then concentrated to dryness under a vacuum using a rotary evaporator and dried at room temperature for seven days. Dried extracts were re-dissolved in HPLC-grade methanol for HPLC analysis to obtain stock solutions, and then all of the solutions were filtered through a 0.22-μm-pore-size filter.
Standard curves
The proper volume of one of the above-mentioned working solutions for the preparation of the standard curve points equivalent to 1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL catalpol and 0.15625, 0.3125, 0.625, 1.25, 2.5, 5 and 10 μg/mL apigenin and gallic acid, respectively, was produced. The calibration curves were formed by plotting the mentioned concentrations against the corresponding peak regions.
Quality control samples preparation
Different samples were spiked with an appropriate volume of the corresponding standard solution to prepare the quality control (QC) samples daily. A final concentration equivalent to a low level (0.15625, 1.5625, 0.15625 μg/mL), middle level (1.25, 12.5, 1.25 μg/mL), and high level (10, 100, 10 μg/mL) of apigenin, catalpol, and gallic acid, were prepared, respectively. The following methods are similar to those described above.
Method validation
The well-organized methods were validated based on the USP and ICH instructions for analytical methods validation [32]. For this purpose, the procedure was confirmed for the analysis’s linearity, limit of detection (LOD) and limit of quantification (LOQ), precision, accuracy, stability, and recovery according to the Food and Drug Administration industry guideline principles.
Linearity
The linearity of the method was investigated using the regression coefficient (R2) of the calibration curves generated by seven concentrations of standards. The analysis of the blank samples was performed to ensure that no interferences existed but was not used to construct the calibration function. The ratio of signal-to-noise was used to estimate the LOD. This parameter was considered the lowest concentration level caused by a peak region of three times the baseline noise.
Precision and accuracy
Intra-run (within-run) variations and inter-run (between-run) variations were employed. The developed HPLC method was used to prepare and analyze samples in triplicate with concentrations of QCs (from low, middle, and high regions of the standard curve) in one run and on three different runs, respectively. The coefficient of variations (CV%) of the associated concentrations was then calculated in each case.
Repeatability test
Six independent samples with apigenin, catalpol, and gallic acid concentrations of 1.25, 12.5, and 1.25 μg/mL were prepared to examine the procedure repeatability and intermediate precision. The prepared samples were injected into the HPLC system, and the percentage of relative standard deviation (%RSD) between retention time (Rt) and peak areas was determined as the method’s repeatability.
Intermediate precision
Similar to a repeatability study, six samples of fresh reagents were prepared and analyzed with a different HPLC column. The %RSD was determined between six measurements, and the %RSD between 12 measurements of the repeatability and intermediate precision tests.
Reproducibility
The mean results for the analysis of the same sample between our laboratory and two various test facilities were acquired. Then, the Equation 1 was used to calculate the percentage difference between content measurements:
1. Reproducibility=(Highest value-Lowest value)/(Mean value)×100
Stability freeze and thaw stability
The three concentration levels of 1.5625, 12.5, and 100 μg/mL of QC catalpol were kept at the storage temperature (−20°C) for one day and thawed unassisted at room temperature. After being completely thawed, The samples were refrozen for one day under the same conditions. They were assayed after three freezes (−20°C)-thaw cycles (room temperature).
Short-term temperature stability
Three concentration levels of QC catalpol (1.5625, 12.5, and 100 μg/mL) were placed at room temperature for a time interval of 4 h that went beyond the catalpol routine preparation time.
Long-term stability
Three concentration levels of QC catalpol (1.5625, 12.5, and 100 μg/mL) stored at low temperatures (−20 ºC) were investigated within a time interval of two weeks.
Robustness
The method’s robustness was tested to evaluate the variation of the chromatographic conditions on catalpol determination. The mobile phase flow rate was changed to 0.4, 0.8, and 1 mL/min to determine robustness only for catalpol.
Statistical analysis
The results were reported as Mean±SD. The means were compared using the Duncan test (P<0.05) by the SPSS software, version 21.
Results
Optimization conditions
The experiment’s plan was to optimize conditions for determining the content of catalpol; however, the optimal method will also be effective in determining the content of apigenin and gallic acid. After optimization of the method, three compounds were identified in the leaf and root parts of two Plantago species by comparison with the chromatogram of the standard constituents obtained under similar conditions. Peak areas were utilized to quantify the apigenin, catalpol, and gallic acid contents in herbal samples.
Method validation
Different chromatographic variables, including kinds of columns, UV wavelengths, and different flow rates with various mobile phase compositions, were optimized for catalpol assay by HPLC-UV. Finally, optimum separation, good peak separation, and a shorter analysis time were obtained on the C8 column and 204 nm wavelength with a flow rate of 1.0 mL/min (Table 1).
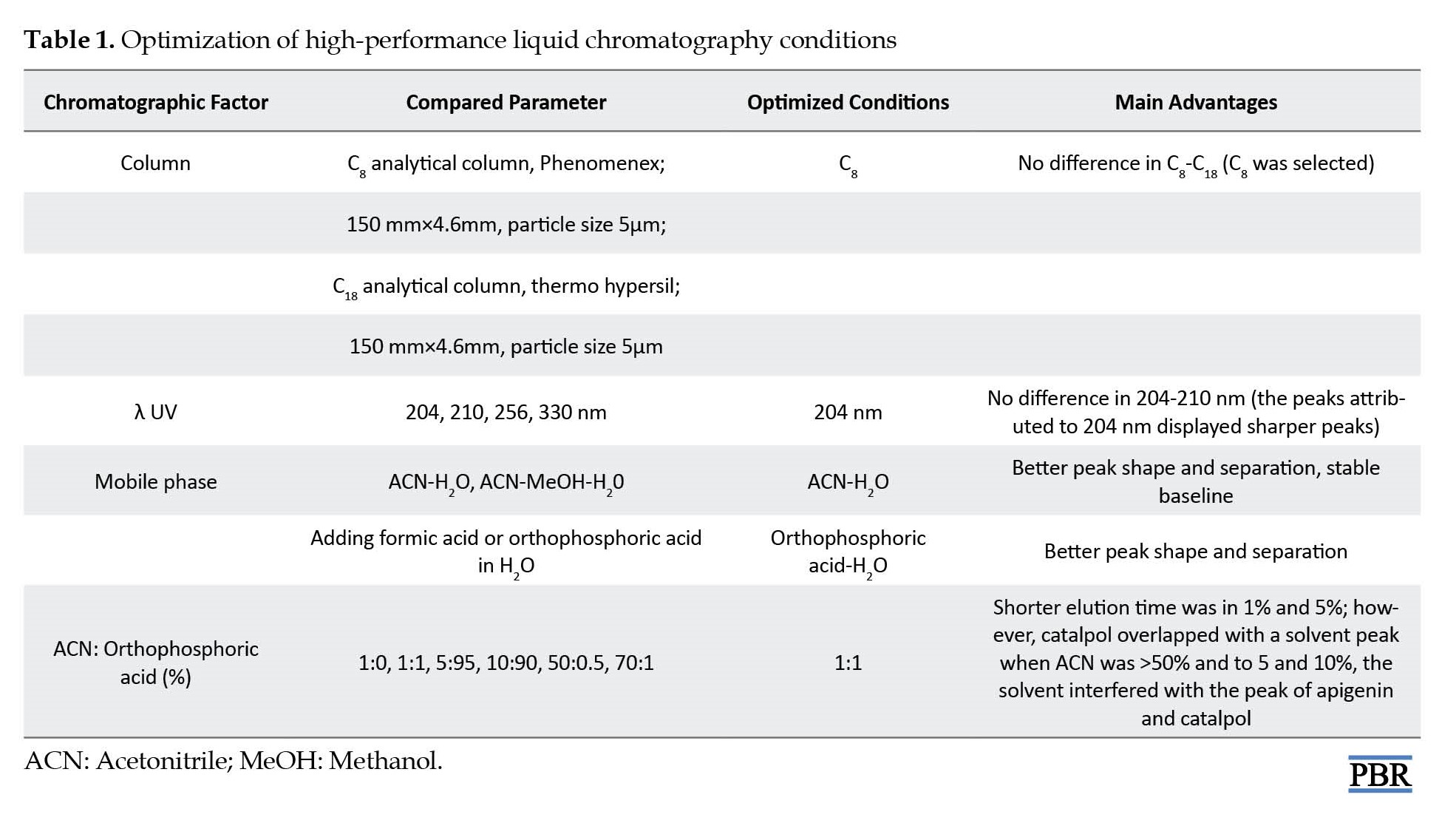
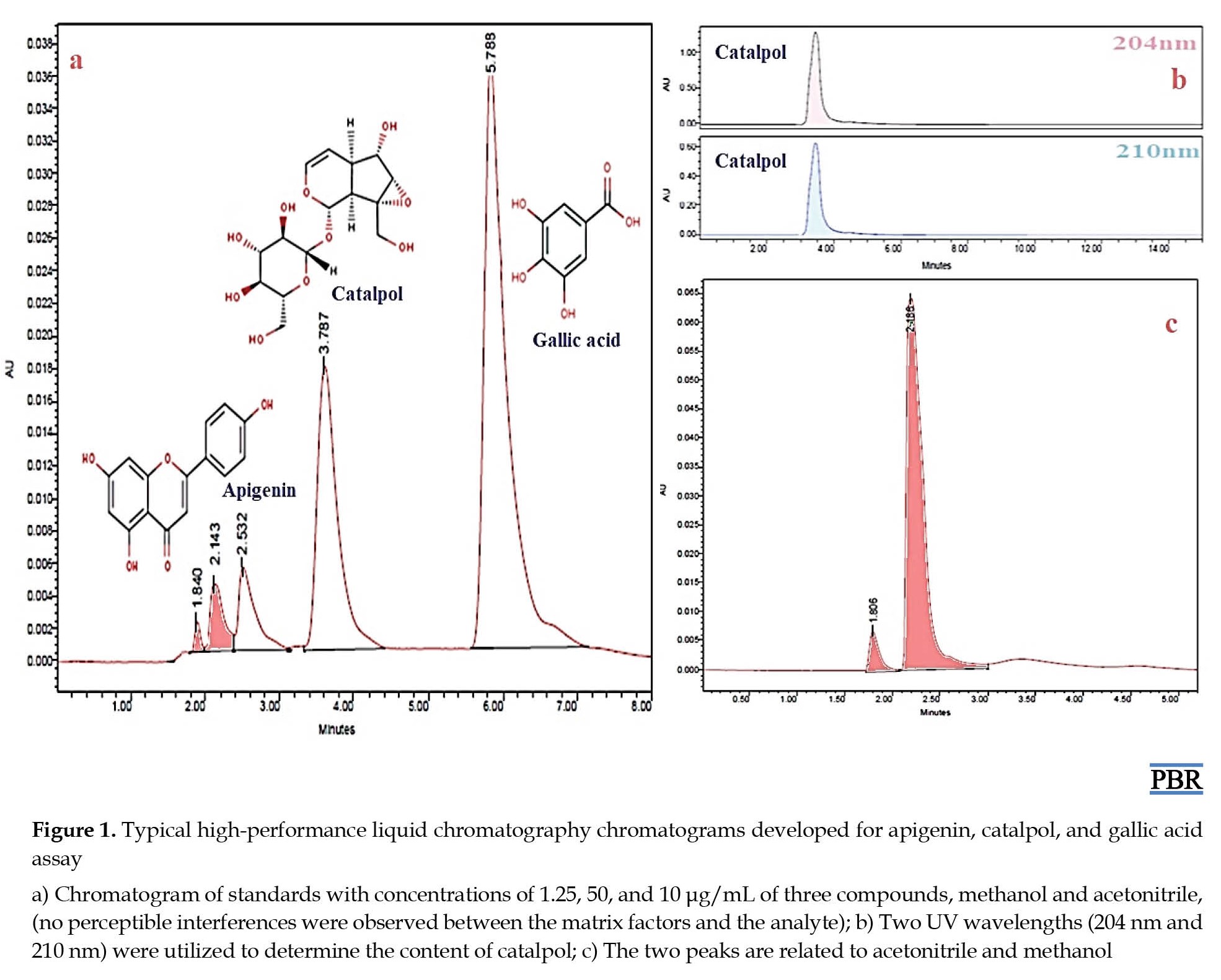
Various mobile phase compositions with different concentrations of acetonitrile, methanol, and acetonitrile–methanol were tested as organic solvents, and formic acid or orthophosphoric acid as eluents in water were compared to achieve the best separation and resolution. Acetonitrile–orthophosphoric acid (1:1%, V/V) system was the optimum choice, giving distinct resolution and sharp peaks. In this study, the methanol peak interfered with the standard peak when methanol was used as a mobile phase. In contrast, by applying acetonitrile as a mobile phase, no peak was observed except the internal standard peak (Figure 1).
Recently, medicinal plants have attracted significant attention due to their therapeutic and biological properties. Numerous studies have reported the potential of medicinal plants in various traditional, alternative, and complementary systems [1]. Plantago genus belonging to the Plantaginaceae family includes about 275 species [2]. Plantago species are used commercially worldwide as food and in the traditional treatment of many diseases [2].
Ribwort plantain (Plantago lanceolata L. and great plantain (Plantago major L. are the most well-known Plantago species, which exhibited beneficial pharmaceutical properties. P. lanceolata has been used for medicinal purposes to treat various diseases, such as wound healing [3], respiratory system disorders, reproductive system, digestive organs, blood circulation, anti-inflammation [4], and anti-cancer, in addition to its anti-bacterial properties [5, 6, 7]. P. major is effective as an anti-oxidant [8], wound healing [9], anti-diabetic [10], anti-inflammatory [11], anti-bacterial, and anti-cancer [12-14]. Plantago species have many medicinally active ingredients, such as iridoids, flavonoids, phenolic acids, triterpenes, and phenylpropanoid glycosides [2].
There is currently considerable interest in studying the biological activity of plant extracts. Plants containing iridoids have attracted more attention [15, 16]. Iridoids are a large group of monoterpenoids [17] that induce a defensive role in plants [18]. Iridoids are widespread in Lamiales, such as Cornales, Gentianales, and Plantago [19]. The iridoid glycosides aucubin and catalpol are the biologically active secondary metabolites utilized as analytical and chemotaxonomic markers to determine the quality of plant-derived extracts [20]. In herbal samples, the iridoid glycosides with aucubin and catalpol are approximately 2% to 3%. The stage of plant maturity determines the content of iridoids. The iridoid content in old leaves is extremely low, while young leaves show a maximum of 9% iridoid. Aucubin is a major compound in older leaves, while catalpol is the dominant constituent in young leaves [21]. Iridoids have various biological activities, such as anti-tumor, anti-inflammatory, hepato-protective, and neuro-protective activities [22]. Catalpol has various therapeutic functions, such as protecting against liver damage and lowering high blood sugar [23]. Flavonoids, phenolic, and tannins compounds comprise most medicinal plants’ secondary metabolites [24]. Apigenin is a subclass of flavonoids and a non-mutagenic flavone with less toxicity [25]. Apigenin has notable properties, including anti-oxidant, anti-proliferative, anti-tumor, apoptosis, anti-inflammatory [26], and anti-bacterial activity [27]. Gallic acid is one of the essential bioactive agents that showed numerous medicinal properties, including anti-microbial, anti-cancer [28], anti-viral [29], anti-inflammatory, anti-oxidative, and anti-neoplastic properties [30].
Many studies have shown that apigenin, catalpol, and gallic acid present in P. lanceolata and P. major have therapeutic potential; however, the methods used to determine their amount in previous studies were not optimized and crude extracts of plants were often used, highlighting the need for further research and more detailed information. This study introduced a simple, selective, and stable method for detecting these compounds in different fractions of P. lanceolata and P. major leaves and roots using high-performance liquid chromatography (HPLC). The goal of choosing a solvent system is to find the best type of extract for detecting these compounds. To the best of the authors’ knowledge, this is the first report on the simultaneous quantification of apigenin, catalpol, and gallic acid in these species.
Materials and Methods
Instrument and high-performance liquid chromatography apparatuses
The system was equipped with a pump (Waters Corp., Milford, MA, USA) and a UV detector (WATER, model Breeze, USA) used at wavelengths 204, 210, 256, and 330 nm, with the outputs recorded using the Breeze software. The apigenin, catalpol, and gallic acid were conducted through two types of columns, the Phenomenex-C8 column (150 mm×4.6 mm, the particle size of 5 µm, USA) and Thermo Hypersil-C18 column (150 mm×4.6 mm, the particle size of 5 µm, Germany) equipped with the same packed guard column. The mobile phase includes an organic solvent, acetonitrile, and eluents orthophosphoric acid or formic acid (different ratio V/V) with flow rates of 0.4, 0.8, and 1 mL/min. An aliquot of 20 μL was injected into the HPLC system using a loop injector (Rheodyne 7725i, Cotati, CA, USA). The column temperature was kept at 25ºC.
Chemicals
Apigenin (98% purity), catalpol (96%), and gallic acid (96%) were provided by the Sigma-Aldrich (Aldrich Division; Steinheim, Germany) company. Acetonitrile, water, and methanol were HPLC, gradient grade.
Preparation of stock solutions and working solutions
Apigenin stock solutions were made in dimethyl sulfoxide (DMSO) and HPLC grade methanol (5:95%, V/V). Each standard was separately dissolved in sterile purified water to prepare catalpol and gallic acid and then was stored at 40C for gallic acid and 200C for apigenin and catalpol. Working standard solutions used to optimize and validate the method were prepared in solvents using the dilution method at concentrations of 1.5625 to 100 μg/mL for catalpol and 0.15625 to 10 μg/mL for apigenin and gallic acid.
Plant sample
P. lanceolata and P. major were collected as wild plants in September 2018 from the University of Zanjan, Iran, research field. The voucher specimens were prepared in triplicates for each analysis and authenticated at the Department of Pharmacognosy, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan. The plant samples were washed to remove dust particles. The leaf and root parts of the two species of Plantago were isolated, air-dried, and then powdered by an electric mixer-grinder.
Sample preparation by the fraction method (liquid-liquid extraction)
An amount of 250 g of dried and powdered leaf and root parts of Plantago spp were weighed, and extraction was carried out for 16 h via reflex method using petroleum ether as a solvent (to remove nonpolar compounds) followed by methanol with the same period. The methanol extract was separated using The methanol extract was separated using dichloromethane, ethyl acetate, n-butanol, and aqueous phase via the liquid-liquid extraction method in a separatory funnel [31]. Using filter paper, the aqueous fraction was filtered for plant fiber removal.
Extracts were then concentrated to dryness under a vacuum using a rotary evaporator and dried at room temperature for seven days. Dried extracts were re-dissolved in HPLC-grade methanol for HPLC analysis to obtain stock solutions, and then all of the solutions were filtered through a 0.22-μm-pore-size filter.
Standard curves
The proper volume of one of the above-mentioned working solutions for the preparation of the standard curve points equivalent to 1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 μg/mL catalpol and 0.15625, 0.3125, 0.625, 1.25, 2.5, 5 and 10 μg/mL apigenin and gallic acid, respectively, was produced. The calibration curves were formed by plotting the mentioned concentrations against the corresponding peak regions.
Quality control samples preparation
Different samples were spiked with an appropriate volume of the corresponding standard solution to prepare the quality control (QC) samples daily. A final concentration equivalent to a low level (0.15625, 1.5625, 0.15625 μg/mL), middle level (1.25, 12.5, 1.25 μg/mL), and high level (10, 100, 10 μg/mL) of apigenin, catalpol, and gallic acid, were prepared, respectively. The following methods are similar to those described above.
Method validation
The well-organized methods were validated based on the USP and ICH instructions for analytical methods validation [32]. For this purpose, the procedure was confirmed for the analysis’s linearity, limit of detection (LOD) and limit of quantification (LOQ), precision, accuracy, stability, and recovery according to the Food and Drug Administration industry guideline principles.
Linearity
The linearity of the method was investigated using the regression coefficient (R2) of the calibration curves generated by seven concentrations of standards. The analysis of the blank samples was performed to ensure that no interferences existed but was not used to construct the calibration function. The ratio of signal-to-noise was used to estimate the LOD. This parameter was considered the lowest concentration level caused by a peak region of three times the baseline noise.
Precision and accuracy
Intra-run (within-run) variations and inter-run (between-run) variations were employed. The developed HPLC method was used to prepare and analyze samples in triplicate with concentrations of QCs (from low, middle, and high regions of the standard curve) in one run and on three different runs, respectively. The coefficient of variations (CV%) of the associated concentrations was then calculated in each case.
Repeatability test
Six independent samples with apigenin, catalpol, and gallic acid concentrations of 1.25, 12.5, and 1.25 μg/mL were prepared to examine the procedure repeatability and intermediate precision. The prepared samples were injected into the HPLC system, and the percentage of relative standard deviation (%RSD) between retention time (Rt) and peak areas was determined as the method’s repeatability.
Intermediate precision
Similar to a repeatability study, six samples of fresh reagents were prepared and analyzed with a different HPLC column. The %RSD was determined between six measurements, and the %RSD between 12 measurements of the repeatability and intermediate precision tests.
Reproducibility
The mean results for the analysis of the same sample between our laboratory and two various test facilities were acquired. Then, the Equation 1 was used to calculate the percentage difference between content measurements:
1. Reproducibility=(Highest value-Lowest value)/(Mean value)×100
Stability freeze and thaw stability
The three concentration levels of 1.5625, 12.5, and 100 μg/mL of QC catalpol were kept at the storage temperature (−20°C) for one day and thawed unassisted at room temperature. After being completely thawed, The samples were refrozen for one day under the same conditions. They were assayed after three freezes (−20°C)-thaw cycles (room temperature).
Short-term temperature stability
Three concentration levels of QC catalpol (1.5625, 12.5, and 100 μg/mL) were placed at room temperature for a time interval of 4 h that went beyond the catalpol routine preparation time.
Long-term stability
Three concentration levels of QC catalpol (1.5625, 12.5, and 100 μg/mL) stored at low temperatures (−20 ºC) were investigated within a time interval of two weeks.
Robustness
The method’s robustness was tested to evaluate the variation of the chromatographic conditions on catalpol determination. The mobile phase flow rate was changed to 0.4, 0.8, and 1 mL/min to determine robustness only for catalpol.
Statistical analysis
The results were reported as Mean±SD. The means were compared using the Duncan test (P<0.05) by the SPSS software, version 21.
Results
Optimization conditions
The experiment’s plan was to optimize conditions for determining the content of catalpol; however, the optimal method will also be effective in determining the content of apigenin and gallic acid. After optimization of the method, three compounds were identified in the leaf and root parts of two Plantago species by comparison with the chromatogram of the standard constituents obtained under similar conditions. Peak areas were utilized to quantify the apigenin, catalpol, and gallic acid contents in herbal samples.
Method validation
Different chromatographic variables, including kinds of columns, UV wavelengths, and different flow rates with various mobile phase compositions, were optimized for catalpol assay by HPLC-UV. Finally, optimum separation, good peak separation, and a shorter analysis time were obtained on the C8 column and 204 nm wavelength with a flow rate of 1.0 mL/min (Table 1).

Various mobile phase compositions with different concentrations of acetonitrile, methanol, and acetonitrile–methanol were tested as organic solvents, and formic acid or orthophosphoric acid as eluents in water were compared to achieve the best separation and resolution. Acetonitrile–orthophosphoric acid (1:1%, V/V) system was the optimum choice, giving distinct resolution and sharp peaks. In this study, the methanol peak interfered with the standard peak when methanol was used as a mobile phase. In contrast, by applying acetonitrile as a mobile phase, no peak was observed except the internal standard peak (Figure 1).
The elution times of the analytes vary with the addition of different concentrations of acetonitrile-orthophosphoric acid in the mobile phase system. As the acetonitrile concentration increased, the Rt decreased, potentially interfering with the solvent peak (Table 2).

To prevent this, we used 1% acetonitrile as the optimized ratio in the mobile phase system, which shortened the detection run time without causing interference.
Under established conditions, the longest and shortest analysis Rt is related to gallic acid and apigenin, respectively (Table 2).
Assay specificity
Typical chromatograms are generated by the developed method, and no perceptible interferences can be observed between the matrix factors and the analyte (Figure 1). Thus, the method developed to determine the concentrations of apigenin, catalpol, and gallic acid shows reliable results.
Linearity
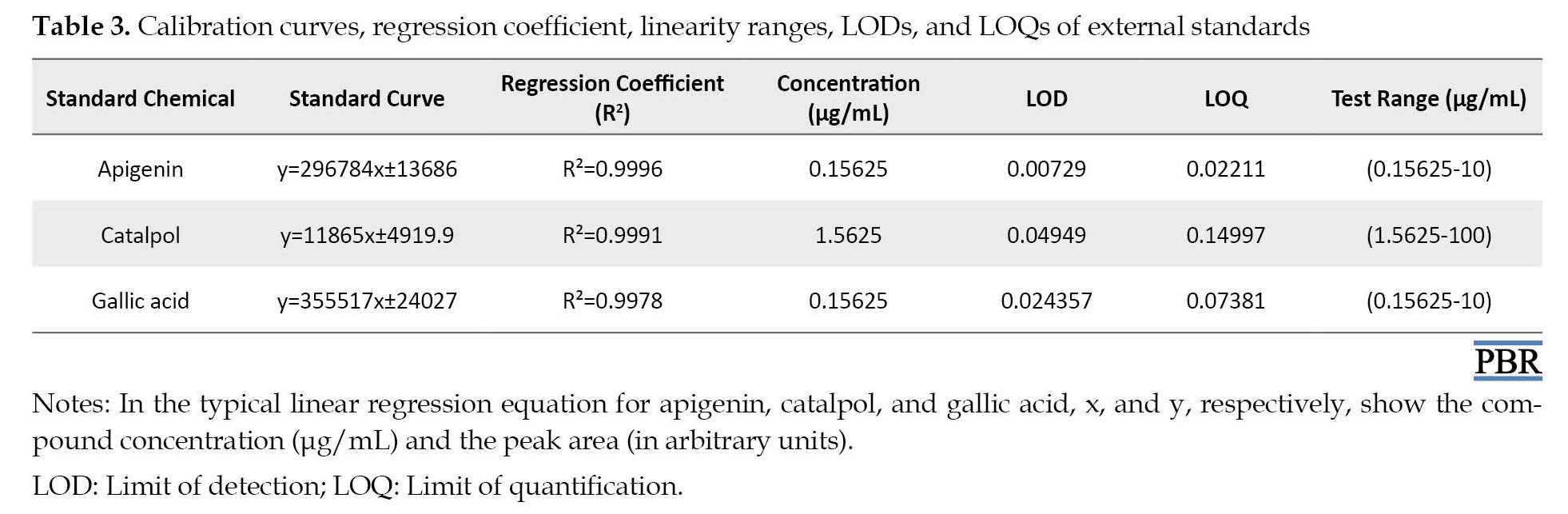
Linear responses, LOD, and LOQ, were produced for apigenin, catalpol, and gallic acid in the range concentration shown in Table 3, which is appropriate for the predesignated objectives.
Intra-day and inter-day, precision, and accuracy
The intra-day precision and accuracy of the developed HPLC method were investigated through the analysis of three sets of QC samples at low, middle, and high concentrations (Table 4).

Inter-assay precision and accuracy were analyzed over three days, while the QC samples were prepared daily (Table 5).

The RSD values of intra-assay and inter-assay precision were between 0.10 and 4.88%, and the accuracy was between 99.52% and 100.15%. These results indicate the developed method’s reproducibility and high accuracy in an analytical run.
Repeatability test
The repeatability test of the Rt and peak area is shown in Table 6.
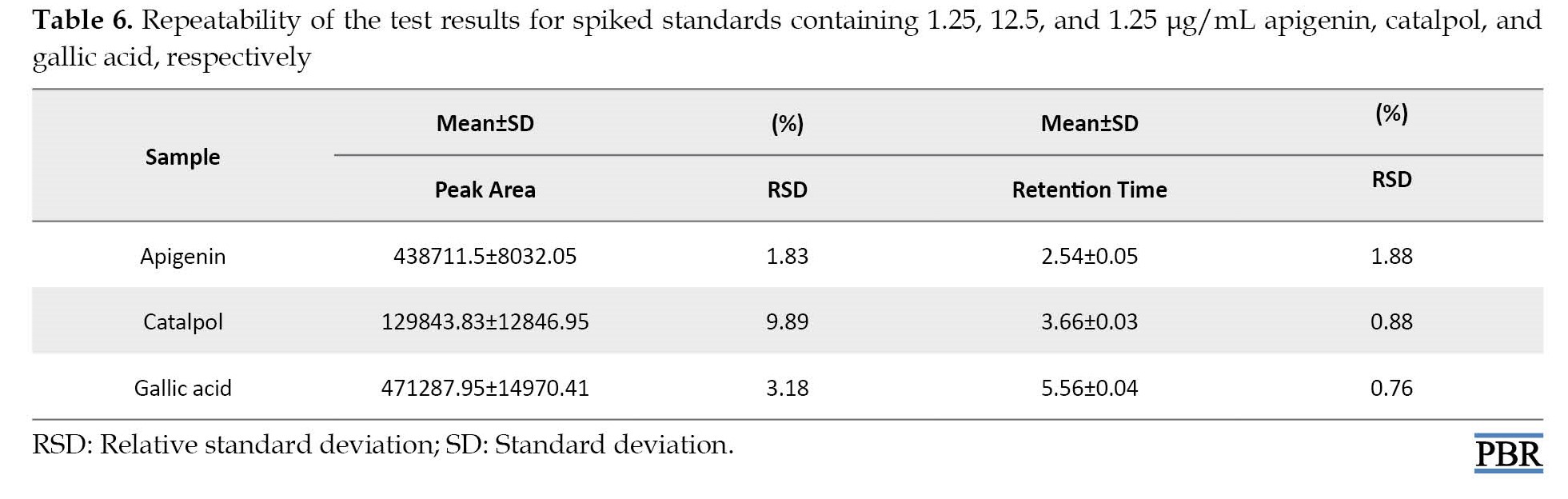
The RSD% was between 0.76 and 9.89%, respectively. This method displayed repeatability for the apigenin, catalpol, and gallic acid assay.
Intermediate precision
Table 7 shows the results of the intermediate precision test of peak area and Rt. Accordingly, an acceptable intermediate precision is shown for apigenin, catalpol, and gallic acid assay.

Reproducibility
The highest and the lowest test result of the spiked catalpol with 12.5 μg/mL catalpol was 142 646 and 132 842, respectively; the mean value was 137 372.16. The percentage difference was thus 7.13%, indicating a high reproducibility for the method.
Stability
Table 8 shows the freeze-thaw and short and long-term stability results for catalpol.

The working solution’s stability was examined at the room’s temperature for 4 h. The results from this method showed the stability of these working solutions at this time.
Robustness
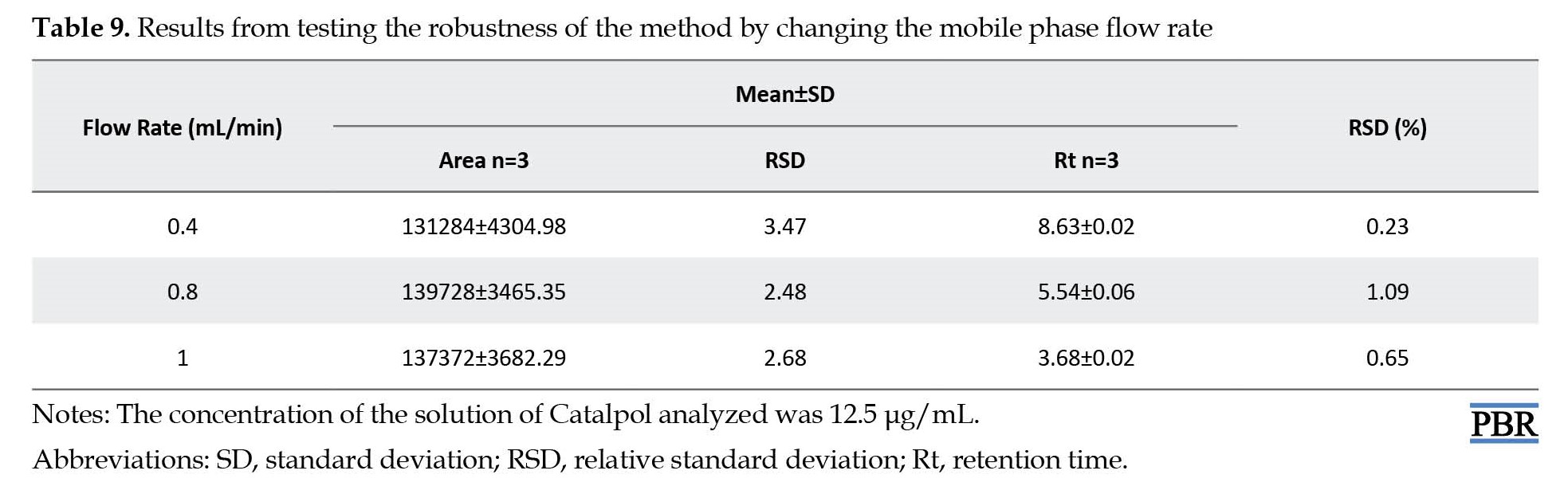
The robustness test was evaluated by changing the flow rate from 0.4, 0.8, and 1mL/min. A significant change was observed in the Rt of catalpol by changing the flow rate of the mobile phase; however, there was no significant change in the integration area of catalpol. The lowest values of the RSD, mean values of Rt, and peak area are shown in Table 9.
High-performance liquid chromatography analysis of fractions
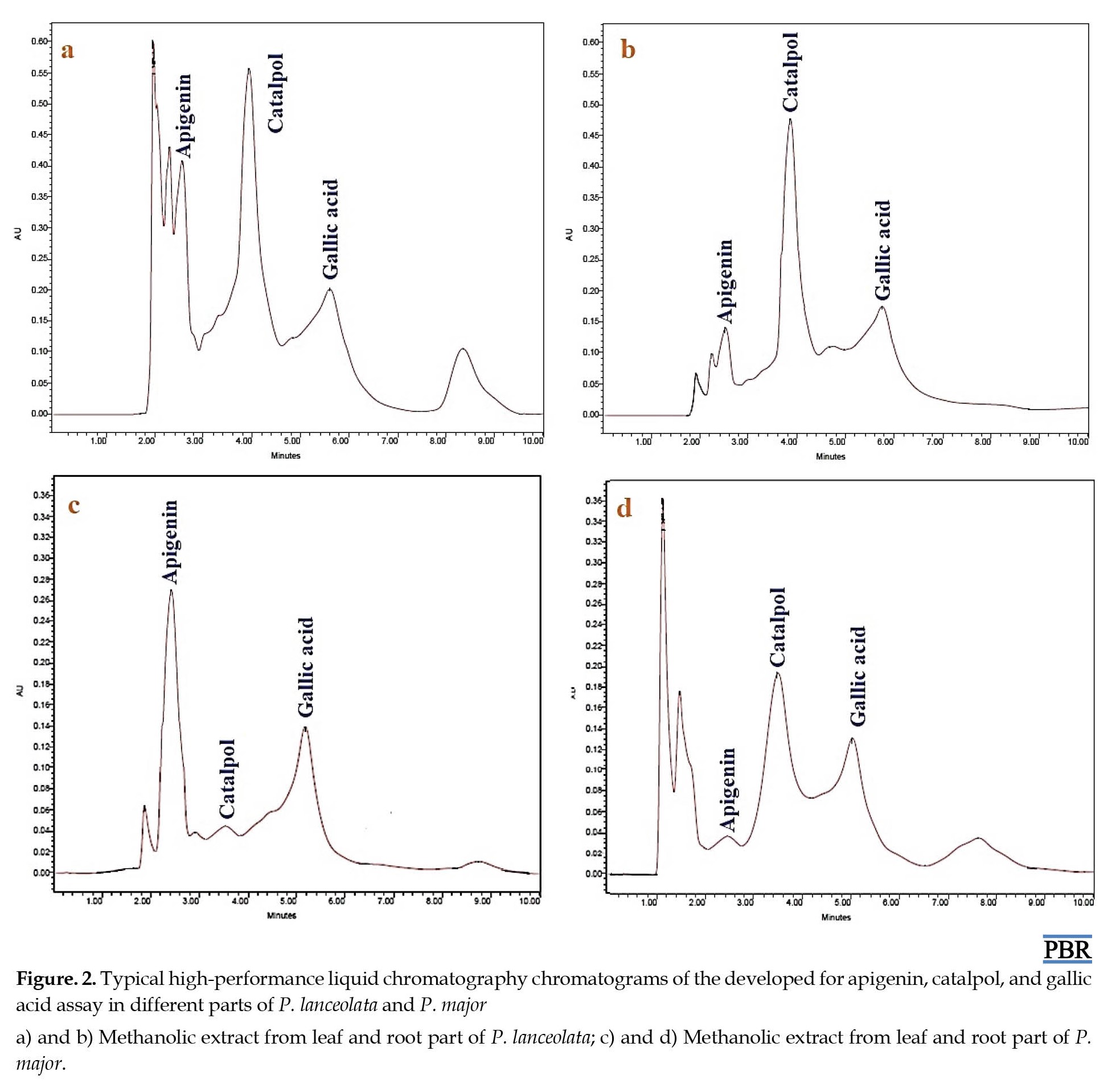
The results indicated that at 204 nm wavelength, the analyte showed no interferences per sample, and the extracts containing apigenin, catalpol, and gallic acid were represented (Figure 2).

To prevent this, we used 1% acetonitrile as the optimized ratio in the mobile phase system, which shortened the detection run time without causing interference.
Under established conditions, the longest and shortest analysis Rt is related to gallic acid and apigenin, respectively (Table 2).
Assay specificity
Typical chromatograms are generated by the developed method, and no perceptible interferences can be observed between the matrix factors and the analyte (Figure 1). Thus, the method developed to determine the concentrations of apigenin, catalpol, and gallic acid shows reliable results.
Linearity
Linear responses, LOD, and LOQ, were produced for apigenin, catalpol, and gallic acid in the range concentration shown in Table 3, which is appropriate for the predesignated objectives.

Intra-day and inter-day, precision, and accuracy
The intra-day precision and accuracy of the developed HPLC method were investigated through the analysis of three sets of QC samples at low, middle, and high concentrations (Table 4).

Inter-assay precision and accuracy were analyzed over three days, while the QC samples were prepared daily (Table 5).

The RSD values of intra-assay and inter-assay precision were between 0.10 and 4.88%, and the accuracy was between 99.52% and 100.15%. These results indicate the developed method’s reproducibility and high accuracy in an analytical run.
Repeatability test
The repeatability test of the Rt and peak area is shown in Table 6.

The RSD% was between 0.76 and 9.89%, respectively. This method displayed repeatability for the apigenin, catalpol, and gallic acid assay.
Intermediate precision
Table 7 shows the results of the intermediate precision test of peak area and Rt. Accordingly, an acceptable intermediate precision is shown for apigenin, catalpol, and gallic acid assay.

Reproducibility
The highest and the lowest test result of the spiked catalpol with 12.5 μg/mL catalpol was 142 646 and 132 842, respectively; the mean value was 137 372.16. The percentage difference was thus 7.13%, indicating a high reproducibility for the method.
Stability
Table 8 shows the freeze-thaw and short and long-term stability results for catalpol.

The working solution’s stability was examined at the room’s temperature for 4 h. The results from this method showed the stability of these working solutions at this time.
Robustness
The robustness test was evaluated by changing the flow rate from 0.4, 0.8, and 1mL/min. A significant change was observed in the Rt of catalpol by changing the flow rate of the mobile phase; however, there was no significant change in the integration area of catalpol. The lowest values of the RSD, mean values of Rt, and peak area are shown in Table 9.

High-performance liquid chromatography analysis of fractions
The results indicated that at 204 nm wavelength, the analyte showed no interferences per sample, and the extracts containing apigenin, catalpol, and gallic acid were represented (Figure 2).
Optimization of solvent systems for the extraction of apigenin, catalpol, and gallic acid
Liquid extraction was performed using various solvents to find an effective solvent for extracting apigenin, catalpol, and gallic acid. After separating the extracts, the fractions containing apigenin, catalpol, and gallic acid were analyzed using HPLC. The dichloromethane extract of the leaf part of P. lanceolata and P. major produced the highest yield of apigenin (1.08 and 0.58 µg/mg DW) compared with the root part, respectively (Table 10 and Table 11).


In contrast, the aqueous extract of P. lanceolata leaf and P. major root showed more catalpol content (9.339 and 2.451 µg/mg). The content of gallic acid was higher in methanolic, butanol, and aqueous extracts of P. lanceolata (3.33 µg/mg) and P. major (3.95, 4.34, and 4.72 µg/mg) (Table 10 and Table 11).
Discussion
A reliable HPLC technique was used to evaluate the levels of three pharmaceutical compounds, including the iridoid glycoside catalpol, the flavonoid apigenin, and the phenol gallic acid, in various fractions of leaf and root parts of Plantago species.
Some studies have used a methanol-water mobile phase to determine the aucubin iridoid glycoside in Plantago species using HPLC [33, 34]. Methanol can interfere with compound detection. As a result, some studies have used acetonitrile instead of methanol [35]. In this study, when methanol was used as the mobile phase, its peak interfered with the analytes’ peak. Only the internal standard peak was observed using acetonitrile as the mobile phase.
The HPLC chromatograms of P. lanceolata and P. major showed the presence of apigenin, catalpol, and gallic acid at the Rt of around 2.5, 3.7, and 5.7 min, and the RSD% of the Rt of 1.88, 0.88, and 0.76%, respectively. Other studies have been found to determine these compounds separately in two Plantago species by HPLC, such as a peak for catalpol around 4.35 min [36], 5 min [37], 13.9 min [38], and for gallic acid around 4.40 min [39] and 4.6 min in HPLC assay of P. lanceolata [40].
In our earlier research, we used the HPLC method to measure the levels of flavonoid, phenolic, and iridoid glycoside compounds in methanolic and 80% aqueous-methanol extracts of different parts of P. lanceolata and P. major. Statistical analysis of the crude extracts revealed that the aerial parts of both species contained higher levels of apigenin and gallic acid than the root parts. However, the aerial parts of P. lanceolata and the root parts of P. major had more catalpol content [5, 14, 41, 42]. Despite these findings, the methods used were not optimized, and only crude extracts were used, indicating a need for more detailed research.
As a highly polar compound, glycoside iridoid catalpol easily dissolves in water. Tong et al. (2015) investigated the typical polar biphasic solvent systems, including ethyl acetate–n-butanol–water and n-butanol–water in different amounts using thin-layer chromatography. This technique is used for the separation of a non-volatile mixture. Their study showed that a large amount of catalpol was partitioned into the aqueous phase due to its high polarity. Combining the mentioned solvents (ethyl acetate–n-butanol–water, 2:1:3, V/V/V) provided a good fraction system for separating catalpol from the purified crude extract [43]. The present study also stated that the aqueous extract of P. lanceolata leaves and P. major roots contained more catalpol content. The researchers reported that aucubin, a well-known marker of Plantago species, was present in all samples, and the highest amounts were found in P. media and P. argentea (44.27 and 39.93 mg/g DW, respectively); however, catalpol was detected only in P. altissima, P. argentea, and P. lanceolata [44, 45]. However, in the present paper, catalpol was also seen in lower amounts in P. major. Another research has shown the high content of phenolic acids in P. lanceolata aqueous-methanolic extracts and indicated that the benzoic acid derivatives hydroxybenzoate and 3,4,5-trihydroxybenzoate (gallic acid) are highly represented [46]. In the current work, the highest amount of gallic acid was obtained in aqueous, n-butanol, and methanolic fractions, indicating better solubility in polar solvents. On the other hand, the biological applications of flavonoids are limited due to low bioavailability as well as low aqueous solubility [47]. Dichloromethane fraction showed the presence of flavonoid apigenin in Orbignya peciosa Mart. leaves [48]. Similarly, dichloromethane extract also showed the highest apigenin content in Plantago species. The yield of secondary metabolites, such as flavonoid, phenol, and iridoid glycoside in the Plantago species varies under the influence of many factors, including the pattern of climatic temperature during the plant growing season, collection area, harvest time, drying method, environmental stresses, and extraction methods [41]. The data revealed the good precision and accuracy of all the proposed procedures. This protocol has advantages, including economic extraction projects, quick accuracy, ease, high sensitivity, a short chromatographic run time, and reproducibility.
Conclusions
In this study, the determination of optimum conditions for extracting these medicinal compounds, including apigenin, catalpol, and gallic acid, was developed in two species of Plantago. As far as we know, this is the first report on the simultaneous evaluation of apigenin, catalpol, and gallic acid contents in leaf and root parts of two species of this genus with various solvents. Among the important medicinal compounds in Plantago species extracts, the iridoid glycosides aucubin and catalpol can be utilized as analytical markers to evaluate the quality of different extracts due to being iridoid-containing sources. Research on available species with medicinal properties is important. In this regard, knowledge about the composition present in the plants and the most economical and simple method to measure them is essential.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
The paper was extracted from the PhD dissertation of Samaneh Rahamouz-Haghighi, approved by Department of Plant Production and Genetics, University of Zanjan (Grant No: A-12-848-35).
Authors' contributions
Project administration, investigation, formal analysis, writing original draft and revising: Samaneh Rahamouz-Haghighi; Funding and supervision: Khadijeh Bagheri and Ali Sharafi.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgments
The authors would like to thank the authority of the University of Zanjan and School of Pharmacy, Zanjan University of Medical Sciences for their support.
References
- Shukla S, Mehta A. Anticancer potential of medicinal plants and their phytochemicals: A review.Braz J Bot. 2015; 38(2):199-210. [DOI:10.1007/s40415-015-0135-0]
- Goncalves S, Romano A. The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind Crops Prod. 2016; 83:213-26. [DOI:10.1016/j.indcrop.2015.12.038]
- Kurt B, Bilge N, Sözmen M, Aydın U, Önyay T, Özaydın I. Effects of Plantago lanceolata L. extract on full-thickness excisional wound healing in a mouse model. Biotech Histochem. 2018; 93(4):249-57. [DOI:10.1080/10520295.2017.1421773] [PMID]
- Abate L, Bachheti RK, Tadesse MG, Bachheti A. Ethnobotanical uses, chemical constituents, and application of plantago lanceolata L. J Chem. 2022; 2022:1-17. [DOI:10.1155/2022/1532031]
- Rahamooz-Haghighi S, Bagheri K, Sharafi A, Danafar H. Establishment and elicitation of transgenic root culture of Plantago lanceolata and evaluation of its anti-bacterial and cytotoxicity activity. Prep Biochem Biotechnol. 2021; 51(3):207-24. [DOI:10.1080/10826068.2020.1805757] [PMID]
- Rahamouz-Haghighi S, Bagheri K, Mohsen-Pour N, Sharafi A. In vitro evaluation of Cytotoxicity and Antibacterial activities of ribwort plantain (Plantago lanceolata L.) root fractions and Phytochemical analysis by GC/MS. Arch Razi Inst. 2022; 77(6):2119-43. [PMID]
- Rahamouz-Haghighi S, Bagheri K, Sharafi A, Tavakolizadeh M, Mohsen-Pour N. Phytochemical screening and Cytotoxicity assessment of Plantago lanceolata L. root extracts on Colorectal cancer cell lines and Brine shrimp larvae and determination of the median lethal dose in mice. S Afr J Bot. 2022;149:740-7. [DOI:10.1016/j.sajb.2022.06.058]
- Parhizgar S, Hosseinian S, Hadjzadeh MA, Soukhtanloo M, Ebrahimzadeh A, Mohebbati R, et al. Renoprotective effect of Plantago major against nephrotoxicity and oxidative stress induced by cisplatin. Iran J Kidney Dis. 2016; 10(4):182-8. [PMID]
- Zubair M, Nybom H, Lindholm C, Brandner JM, Rumpunen K. Promotion of wound healing by Plantago major L. leaf extracts-ex-vivo experiments confirm experiences from traditional medicine. Nat Prod Res. 2016; 30(5):622-4. [DOI:10.1080/14786419.2015.1034714] [PMID]
- Abdulghani MA, Hamid I, Al-Naggar RA, Osman MT. Potential antidiabetic activity of Plantago major leaves extract in Streptozocin-induced diabetic rats. Res J Pharm Biol Chem Sci. 2014; 5(2):896-902. [Link]
- Zubair M, Widén C, Renvert S, Rumpunen K. Water and ethanol extracts of Plantago major leaves show anti-inflammatory activity on oral epithelial cells. J Tradit Complement Med. 2018; 9(3):169-71. [PMID]
- Adom MB, Taher M, Mutalabisin MF, Amri MS, Abdul Kudos MB, Wan Sulaiman MWA, et al. Chemical constituents and medical benefits of Plantago major. Biomed Pharmacother. 2017; 96:348-60. [DOI:10.1016/j.biopha.2017.09.152] [PMID]
- Rahamooz-Haghighi S, Bagheri K, Danafar H, Sharafi A. Anti-proliferative properties, biocompatibility, and chemical composition of different extracts of Plantago major medicinal plant. Iran Biomed J. 2021; 25(2):106-16. [DOI:10.29252/ibj.25.2.106] [PMID]
- Rahamouz-Haghighi S, Bagheri K, Sharafi A. In vitro elicitation and detection of apigenin, catalpol and gallic acid in hairy root culture of Plantago major L. and assessment of cytotoxicity and anti-bacterial activity of its methanolic extract. Nat Prod Res. 2023; 37(4):633-7. [DOI:10.1080/14786419.2022.2068543] [PMID]
- Viljoen A, Mncwangi N, Vermaak I. Anti-inflammatory iridoids of botanical origin. Curr Med Chem. 2012; 19(14):2104-27. [DOI:10.2174/092986712800229005] [PMID]
- West BJ, Uwaya A, Isami F, Deng S, Nakajima S, Jensen CJ. Antiglycation activity of iridoids and their food sources. Int J Food Sci. 2014; 2014:276950. [DOI:10.1155/2014/276950] [PMID]
- Nagy MM, Wang S, Farag MA. Quality analysis and authentication of nutraceuticals using near IR (NIR) spectroscopy: A comprehensive review of novel trends and applications. Trend Food Sci Technol. 2022; 123:290-309. [DOI:10.1016/j.tifs.2022.03.005]
- Divekar PA, Narayana S, Divekar BA, Kumar R, Gadratagi BG, Ray A, et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int J Mol Sci. 2022; 23(5):2690. [DOI:10.3390/ijms23052690] [PMID]
- Sertić M, Crkvenčić M, Mornar A, Pilepić KH, Nigović B, Maleš Ž. Analysis of aucubin and catalpol content in different plant parts of four Globularia species. J Appl Bot Food Qual. 2015; 88(1):209-14. [DOI:10.5073/JABFQ.2015.088.030]
- Arraché Gonçalves G, Eifler-Lima VL, von Poser GL. Revisiting nature: A review of iridoids as a potential antileishmanial class. Phytochem Rev. 2022; 21(1):101-26. [DOI:10.1007/s11101-021-09750-8] [PMID]
- Grigore A, Bubueanu C, Pirvu L, Ionita L, Toba G. Plantago lanceolata L. Crops-source of valuable raw material for various industrial applications. Scient Papers-Series A Agron. 2015; 58:207-14. [Link]
- Wang C, Gong X, Bo A, Zhang L, Zhang M, Zang E, et al. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules. 2020; 25(2):287. [DOI:10.3390/molecules25020287] [PMID]
- Park SU, Park NI, Kim YK, Suh SY, Eom SH, Lee SY. Application of plant biotechnology in the medicinal plant, Rehmannia glutinosa Liboschitz. J Med Plant Res. 2009; 3(13):1258-63. [Link]
- Dai J, Mumper RJ. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010; 15(10):7313-52. [DOI:10.3390/molecules15107313] [PMID]
- Cao X, Liu B, Cao W, Zhang W, Zhang F, Zhao H, et al. Autophagy inhibition enhances apigenin- induced apoptosis in human breast cancer cells. Chin J Cancer Res. 2013; 25(2):212-22. [PMID]
- Kashyap D, Sharma A, Tuli HS, Sak K, Garg VK, Buttar HS, et al. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J Funct Foods. 2018; 48:457-71. [DOI:10.1016/j.jff.2018.07.037]
- Kim S, Woo ER, Lee DG. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J Basic Microbiol. 2020; 60(10):862-72. [DOI:10.1002/jobm.202000432] [PMID]
- Fernandes FH, Salgado HR. Gallic acid: Review of the methods of determination and quantification. Crit Rev Anal Chem. 2016; 46(3):257-65. [DOI:10.1080/10408347.2015.1095064] [PMID]
- Govea-Salas M, Rivas-Estilla AM, Rodríguez-Herrera R, Lozano-Sepúlveda SA, Aguilar-Gonzalez CN, Zugasti-Cruz A, et al. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp Ther Med. 2016; 11(2):619-24. [DOI:10.3892/etm.2015.2923] [PMID]
- Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci. 2019; 22(3):225-37. [DOI:10.22038/ijbms.2019.32806.7897]
- Jamilah J, Sharifa AA, Sharifah NR. GC-MS analysis of various extracts from leaf of Plantago major used as traditional medicine. World Appl Sci J. 2012; 17:67-70. [Link]
- Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015; 6(3):185-7. [DOI:10.4103/0976-500X.162004] [PMID]
- Miething H, Holz W, Haensel R. HPLC content determination of aucubin in drugs and preparations. Pharm Ztg. 1986; 131:746-7. [Link]
- Guo Y, Sha M, Cao A, Yuan C. Determination of aucubin in Plantago asiatica L., P. major L., and P. depressa Willd. By HPLC. Zhongguo Zhong Yao Za Zhi. 1991; 16(12):743-4, 763. [PMID]
- Wu HK, Chuang WC, Sheu SJ. Separation of nine iridoids by capillary electrophoresis and high-performance liquid chromatography. J Chromatogr A. 1998; 803(1-2):179-87. [DOI:10.1016/S0021-9673(97)01227-2]
- Marak HB, Biere A, Van Damme JM. Direct and correlated responses to selection on iridoid glycosides in Plantago lanceolata L. J Evol Biol. 2000; 13(6):985-96. [DOI:10.1046/j.1420-9101.2000.00233.x]
- Wurst S, Wagenaar R, Biere A, Van der Putten WH. Microorganisms and nematodes increase levels of secondary metabolites in roots and root exudates of Plantago lanceolata. Plant Soil. 2010; 329(1-2):117-26. [DOI:10.1007/s11104-009-0139-2]
- Dietz M, Machill S, Hoffmann HC, Schmidtke K. Inhibitory effects of plantago lanceolata L. on soil N mineralization. Plant Soil. 2013; 368(1-2):445-58. [DOI:10.1007/s11104-012-1524-9]
- Reudler JH, Biere A, Harvey JA, Van Nouhuys S. Differential performance of a specialist and two generalist herbivores and their parasitoids on Plantago lanceolata. J chem ecol. 2011; 37(7):765-78. [DOI:10.1007/s10886-011-9983-7] [PMID]
- Varban R, Varban D. Comparative study of the active ingredients content plantago lanceolata L. ProEnvironment. 2012; 5(12):248-50. [Link]
- Rahamouz-Haghighi S, Bagheri K, Mohsen-Pour N, Sharafi A. Quantitative determination of apigenin, catalpol, and gallic acid in total extracts from different parts of plantago species by high-performance liquid chromatography. Avicenna J Pharm Res. 2021; 2(2):49-54. [DOI:doi:10.34172/ajpr.2021.10]
- Rahamouz-Haghighi S, Sharafi A, Bagheri K. In vitro organogenesis from transformed root cultures of Plantago lanceolata and phytochemical analysis by HPLC and GC-MS. J BioSci Biotechnol. 2021; 10(2):87-97. [Link]
- Tong S, Chen L, Zhang Q, Liu J, Yan J, Ito Y. Separation of catalpol from Rehmannia glutinosa Libosch. by high-speed countercurrent chromatography. J Chromatogr Sci. 2015; 53(5):725-9. [DOI:10.1093/chromsci/bmu114] [PMID]
- Majkić T, Bekvalac K, Beara I. Plantain (Plantago L.) species as modulators of prostaglandin E2 and thromboxane A2 production in inflammation. J Ethnopharmacol. 2020; 262:113140. [DOI:10.1016/j.jep.2020.113140] [PMID]
- Beara IN, Lesjak MM, Orčić DZ, Simin NĐ, Četojević-Simin DD, Božin BN, et al. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related Plantain species: Plantago altissima L. and Plantago lanceolata L. LWT - Food Sci Technol. 2012; 47(1):64-70. [DOI:10.1016/j.lwt.2012.01.001]
- Rahamouz-Haghighi S. Biological activities and analytical methods for detecting aucubin and catalpol iridoid glycosides in plantago species: A review study. Pharm Biomed Res. 2023; 9(2):85-114. [DOI:10.32598/PBR.9.2.1061.3]
- Massi A, Bortolini O, Ragno D, Bernardi T, Sacchetti G, Tacchini M, et al. Research progress in the modification of quercetin leading to anticancer agents. Molecules. 2017; 22(8):1270. [DOI:10.3390/molecules22081270] [PMID]
- Pinheiro MM, Boylan F, Fernandes PD. Antinociceptive effect of the Orbignya speciosa Mart.(Babassu) leaves: Evidence for the involvement of apigenin. Life Sci. 2012; 91(9-10):293-300. [DOI:10.1016/j.lfs.2012.06.013] [PMID]
Type of Study: Original Research |
Subject:
Phyochemistry
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |