Volume 6, Issue 2 (2020)
Pharm Biomed Res 2020, 6(2): 115-122 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadpouri J, Valipour Chahardahcharic S, Setorki M. The Effect of Adiantum Capillus-veneris L. Hydroalcoholic Extract on the Oxidative Stress Rate of Mice’s Blood and Brain in the Depression Model Caused by Acute Immobilization Stress. Pharm Biomed Res 2020; 6 (2) :115-122
URL: http://pbr.mazums.ac.ir/article-1-287-en.html
URL: http://pbr.mazums.ac.ir/article-1-287-en.html
1- Department of Biology, Izeh Branch, Islamic Azad University, Izeh, Iran.
Keywords: Acute stress disorders, Adiantum capillus-veneris linn, Antioxidant effects, Malondialdehyde, Mice, Total Antioxidant capacity
Full-Text [PDF 1097 kb]
(2133 Downloads)
| Abstract (HTML) (4513 Views)
Full-Text: (2236 Views)
Introduction
Stress is a condition caused by physical and psychological pressures. In other words, stress is the response of the individual to situations that threaten the environment [1]. Also, stress is defined as a set of general reactions to external factors which are inconsistent or unexpected, or a disruption to the system and adaptation of the body to the external environment. Based on this definition, if the balance and adaptability of organisms disappear due to external factors, they will become stressed [2]. Anxiety and depression are two psychosocial illnesses with significant comorbidity and their prevalence is increasing in the international community [3]. Monoamines play an important role in the pathophysiology of depression and anxiety, in such a way that the selection of medicine that changes the activity of the neuromuscular system of monoamines determines the treatment of depression and anxiety [4].
Homeostasis is constantly challenged by internal and external stressors [5]. The excessive production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) is stimulated under stress conditions causing a disturbance in the balance between oxidants and the antioxidant defense system and ultimately oxidative stress. In such a condition, the destructive effects of oxidants are revealed. Some destructive effects of oxidants can be the killing healthy cells, increased production of pro-inflammatory cytokines, oxidative degradation of DNA, activation of certain genes, inactivation of proteins and enzymes, oxidation of sugars and fats, and especially the production of unsaturated fatty acids and cell membrane lipoproteins [6]. Oxidative stress is involved in many degenerative diseases, including neurodegenerative diseases (Alzheimer, Parkinson, Huntington, amyotrophic lateral sclerosis, multiple sclerosis, and other aging processes), diabetes, atherosclerosis, arthritis, inflammation, and various cancers [7].
Several studies proved that exposure to stressors weakens the antioxidant defense system and increases the production of free radicals [5, 6, 8]. Plants are a rich source of terpene, phenol, flavonoids, tannins, and anthocyanin, which are the most important natural antioxidants. It has been observed that the use of a diet containing herbal compounds can reduce the oxidative damage caused by acute stress [8].
Maidenhair or Adiantum capillus-veneris (Acv) is a species of ferns belonging to the Pteridaceae family and the genus Adiantum. Persiaoshan has narrow stems and tiny leave and is located in wet areas rich in organic compounds and the margins of rivers and streams [9]. This plant is grown in southern Europe, the Alps, the Atlantic coast, and the northern and southern parts of Iran. Acv is used in Iranian traditional medicine as an anesthetic, anti-febrile dysplastic, and diuretic medicine and is widely used in the treatment of respiratory diseases and digestive disorders [10].
Laboratory studies indicated antimicrobial [9], analgesic [11], anti-inflammatory [11], and anti-oxidant [12] effects of Acv. Phytochemical analyses indicated the presence of flavonoids, alkaloids, tannins, saponins, terpenoids, glycosides, steroids, and reducing sugars in the plant extract [9]. Active compounds in this plant include rutin, quercetin, quercetin-3-O-glucoside, nicotine fluorine, niacin, astragalin, procyanidin, camphorol-3 sulfate, prodelphinidin, and saponin [13]. Some of these compounds including rutin, quercetin, and geranium showed antioxidant effects in vitro and animal models [14-16]. The in vivo effects of Acv on the central nervous system, the characteristics of oxidative stress, and antioxidant activity were studied by some researchers [17-21]. Also, the activity of antibacterial Acv was investigated by some authors in the in vitro condition [22-24].
Although another study investigated the protective effect of Acv hydroalcoholic extract on depression and anxiety due to chronic stress in adult male mice [25], since the antioxidant effect of Acv extract in the living creature has not been studied, this study was designed to evaluate the effect of Acv extract on the oxidative stress rate of blood and brain of mice in the depression model caused by acute immobilization stress.
Materials and Methods
Preparation of drugs
Acetic acid, Thiobarbituric Acid (TBA), Sodium Dodecyl Sulfate (SDS), FeCl3.6H2O, 2,4,6-Tri (2-pyridyl)-s-triazine (TPTZ) and other reagents were purchased from Sigma-Aldrich Chemical Co. (USA) and Merck Co. (Germany).
Determination of radical scavenging activity of Acv extract
Acv extracts (100, 200, 400 µg/mL) were first prepared and an equal amount of the DPPH (2,2-diphenyl-1-picrylhydrazyl) solution (1 mg/mL) was added to Acv in all concentrations. The resulting solution was kept in the dark at room temperature for 15 minutes. Finally, the optical absorbance was measured at 517 nm using a spectrophotometer and then the activity of the DPPH radical inhibition was calculated (Formula 1).
1. IC50 (%) = (Acontrol-Asample)/Acontrol×100
where IC50 is the concentration of the solution in which 50% of the DPPH radical is scavenged [26].
Ethics approval and consent to participate
This experimental study was conducted in the Experimental Animal Unit of Islamic Azad University of Izeh, Khuzestan, Iran. All animal procedures were based on the guideline for the Care and Use of Laboratory Animals. The study was reviewed by the Research Committee of Islamic Azad University of Izeh and approved by Code: 15330557962002. A standard powdered purified diet (Dampars Co, Iran) was used, which consisted of 7% protein, 20%-30% carbohydrates, 2% vegetable fat, and 10%-12% fiber. In this experimental study, 32 male Balb/C mice (25-30 g, 6-8 week) were obtained from the Animal Breeding Facility Centre of Pasteur Institute, Karaj, Iran. The mice were kept in standard conditions (21±2ºC and 12:12 h light/dark cycle) with free access to water and food of the same type. The mice were randomly divided into five groups (n=8 per group): One control group under the stress of normal saline recipient, three intervention groups under the acute stress of receiving Acv extract at doses of 100, 200, and 400 mg/kg [27] and one diazepam group. To create acute stress, a standard immobilization technique and an electric shock were used. The mice were placed in a restrictive device for water, food, and movement (Restrainer) (BorjSanat Company, Iran) for two hours. Afterward, the mice were given 0.5 mA electric shock for two minutes (each 1 second with 10 seconds of rest between each one). This process was performed for acute stress [28]. Then, the mice were treated with normal saline or extract by intraperitoneal injection for 21 days. Next, depression was assessed by forced swimming test [29]. After performing behavioral testing, the animals were subsequently put under deep anesthesia (under ether: Hakim Pharmacy, Iran). Then, their cardiac blood samples were collected, and their brains were removed. After the removal of the brain, the hippocampus, cortex, and sub-cortex were separated on ice and used for biochemical analyses. Their collected blood was centrifuged and the plasma separated was used for biochemical analyses [30].
Stress is a condition caused by physical and psychological pressures. In other words, stress is the response of the individual to situations that threaten the environment [1]. Also, stress is defined as a set of general reactions to external factors which are inconsistent or unexpected, or a disruption to the system and adaptation of the body to the external environment. Based on this definition, if the balance and adaptability of organisms disappear due to external factors, they will become stressed [2]. Anxiety and depression are two psychosocial illnesses with significant comorbidity and their prevalence is increasing in the international community [3]. Monoamines play an important role in the pathophysiology of depression and anxiety, in such a way that the selection of medicine that changes the activity of the neuromuscular system of monoamines determines the treatment of depression and anxiety [4].
Homeostasis is constantly challenged by internal and external stressors [5]. The excessive production of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) is stimulated under stress conditions causing a disturbance in the balance between oxidants and the antioxidant defense system and ultimately oxidative stress. In such a condition, the destructive effects of oxidants are revealed. Some destructive effects of oxidants can be the killing healthy cells, increased production of pro-inflammatory cytokines, oxidative degradation of DNA, activation of certain genes, inactivation of proteins and enzymes, oxidation of sugars and fats, and especially the production of unsaturated fatty acids and cell membrane lipoproteins [6]. Oxidative stress is involved in many degenerative diseases, including neurodegenerative diseases (Alzheimer, Parkinson, Huntington, amyotrophic lateral sclerosis, multiple sclerosis, and other aging processes), diabetes, atherosclerosis, arthritis, inflammation, and various cancers [7].
Several studies proved that exposure to stressors weakens the antioxidant defense system and increases the production of free radicals [5, 6, 8]. Plants are a rich source of terpene, phenol, flavonoids, tannins, and anthocyanin, which are the most important natural antioxidants. It has been observed that the use of a diet containing herbal compounds can reduce the oxidative damage caused by acute stress [8].
Maidenhair or Adiantum capillus-veneris (Acv) is a species of ferns belonging to the Pteridaceae family and the genus Adiantum. Persiaoshan has narrow stems and tiny leave and is located in wet areas rich in organic compounds and the margins of rivers and streams [9]. This plant is grown in southern Europe, the Alps, the Atlantic coast, and the northern and southern parts of Iran. Acv is used in Iranian traditional medicine as an anesthetic, anti-febrile dysplastic, and diuretic medicine and is widely used in the treatment of respiratory diseases and digestive disorders [10].
Laboratory studies indicated antimicrobial [9], analgesic [11], anti-inflammatory [11], and anti-oxidant [12] effects of Acv. Phytochemical analyses indicated the presence of flavonoids, alkaloids, tannins, saponins, terpenoids, glycosides, steroids, and reducing sugars in the plant extract [9]. Active compounds in this plant include rutin, quercetin, quercetin-3-O-glucoside, nicotine fluorine, niacin, astragalin, procyanidin, camphorol-3 sulfate, prodelphinidin, and saponin [13]. Some of these compounds including rutin, quercetin, and geranium showed antioxidant effects in vitro and animal models [14-16]. The in vivo effects of Acv on the central nervous system, the characteristics of oxidative stress, and antioxidant activity were studied by some researchers [17-21]. Also, the activity of antibacterial Acv was investigated by some authors in the in vitro condition [22-24].
Although another study investigated the protective effect of Acv hydroalcoholic extract on depression and anxiety due to chronic stress in adult male mice [25], since the antioxidant effect of Acv extract in the living creature has not been studied, this study was designed to evaluate the effect of Acv extract on the oxidative stress rate of blood and brain of mice in the depression model caused by acute immobilization stress.
Materials and Methods
Preparation of drugs
Acetic acid, Thiobarbituric Acid (TBA), Sodium Dodecyl Sulfate (SDS), FeCl3.6H2O, 2,4,6-Tri (2-pyridyl)-s-triazine (TPTZ) and other reagents were purchased from Sigma-Aldrich Chemical Co. (USA) and Merck Co. (Germany).
Determination of radical scavenging activity of Acv extract
Acv extracts (100, 200, 400 µg/mL) were first prepared and an equal amount of the DPPH (2,2-diphenyl-1-picrylhydrazyl) solution (1 mg/mL) was added to Acv in all concentrations. The resulting solution was kept in the dark at room temperature for 15 minutes. Finally, the optical absorbance was measured at 517 nm using a spectrophotometer and then the activity of the DPPH radical inhibition was calculated (Formula 1).
1. IC50 (%) = (Acontrol-Asample)/Acontrol×100
where IC50 is the concentration of the solution in which 50% of the DPPH radical is scavenged [26].
Ethics approval and consent to participate
This experimental study was conducted in the Experimental Animal Unit of Islamic Azad University of Izeh, Khuzestan, Iran. All animal procedures were based on the guideline for the Care and Use of Laboratory Animals. The study was reviewed by the Research Committee of Islamic Azad University of Izeh and approved by Code: 15330557962002. A standard powdered purified diet (Dampars Co, Iran) was used, which consisted of 7% protein, 20%-30% carbohydrates, 2% vegetable fat, and 10%-12% fiber. In this experimental study, 32 male Balb/C mice (25-30 g, 6-8 week) were obtained from the Animal Breeding Facility Centre of Pasteur Institute, Karaj, Iran. The mice were kept in standard conditions (21±2ºC and 12:12 h light/dark cycle) with free access to water and food of the same type. The mice were randomly divided into five groups (n=8 per group): One control group under the stress of normal saline recipient, three intervention groups under the acute stress of receiving Acv extract at doses of 100, 200, and 400 mg/kg [27] and one diazepam group. To create acute stress, a standard immobilization technique and an electric shock were used. The mice were placed in a restrictive device for water, food, and movement (Restrainer) (BorjSanat Company, Iran) for two hours. Afterward, the mice were given 0.5 mA electric shock for two minutes (each 1 second with 10 seconds of rest between each one). This process was performed for acute stress [28]. Then, the mice were treated with normal saline or extract by intraperitoneal injection for 21 days. Next, depression was assessed by forced swimming test [29]. After performing behavioral testing, the animals were subsequently put under deep anesthesia (under ether: Hakim Pharmacy, Iran). Then, their cardiac blood samples were collected, and their brains were removed. After the removal of the brain, the hippocampus, cortex, and sub-cortex were separated on ice and used for biochemical analyses. Their collected blood was centrifuged and the plasma separated was used for biochemical analyses [30].
Preparation of herbal extracts
Acv was bought from a valid herbal shop in Izeh, Khuzestan in summer. The plant was collected from Khuzestan Province (Figure 1).after the systematic verification by a botanist, the herbarium sample of this plant was registered in Herbarium (No. 7543) of Islamic Azad University, Izeh Branch, Khuzestan. To prepare the extract, we poured 1 kg of dried plant powder into 70% ethanol and placed on a shaker for two days at room temperature. Then, the obtained mixture was filtered and the solvent was removed by rotary evaporator (Heidolph Co. Germany), and Acv extract was obtained (yield 20%-25%). The extract was completely dried at 40°C and used to prepare the required concentrations. The extract was stored at -20°C [31]. The extract was dissolved in distilled water and given to the animals.
This test is one of the most reliable and commonly used examinations to test depression. In this method, a glass container with a length of 25 cm, a width of 12 cm, and a height of 15 cm were used. The dish was filled with water with a temperature of 25°C and the mouse was gently immersed in water. The discontinuation of the movement by the mouse’s limbs was considered as immobilization. The experiment time was 10 minutes and the first 2 minutes was considered the adaptation of the animal to existing conditions and the immobilization time was measured for the following 8 minutes [32].
The measurement of malondialdehyde of the brain and serum
A total of 100 μL of serum samples or homogeneous tissue was added with 1.5 mL of 20% acidity, 1.5 mL of 0.8% TBA, 0.8% of Sodium Dodecyl Sulfate (SDS) 1.8%, and 700 μL of distilled water to the test tubes. The tubes were placed in boiling water for 60 minutes and then 1 mL of distilled water and 5 mL of butanol or pyridine was added to the samples. Then, they were centrifuged and the light absorbance of the supernatant was measured at 532 nm [33].
The measurement of the total antioxidant capacity of the brain and serum
To measure the antioxidant capacity of serum and tissue, we used FRAP method with three solutions: buffer (1.55 mL of sodium acetate and 8 mL of concentrated acetic acid, distilled water with a volume of 500 mL), iron chloride solution (270 mg 6H2OFeCl3, distilled water with a volume of 50 mL, and a solution of triazine (47 mg of triazine dissolved in 40 mL of 40 mM HCl). The final solution was prepared by adding 10 mL of solution one, 1 mL of solution two, and 1 mL of solution three. About 25 μL of the serum or homogeneous tissue samples were added to 1.5 mL of the solution and placed on the object for 10 minutes at 37°C. Then, the optical absorption at 593 wavelengths was recorded [33].
Statistical analysis
The resulting data were analyzed in SPSS version 21. Considering that the obtained results were quantitative, the assumption of normal distribution of the frequency of data was confirmed by the Kolmogorov-Smirnov non-parametric test (P>0.05). The data were also analyzed by one-way ANOVA and the post hoc Least Significant Difference (LSD) tests. Also, the obtained results were reported together with the corresponding statistical calculations as Mean±SEM. In all cases, the difference among groups was considered significant with P<0.05.
Results
DPPH radical scavenging activities of A. capillus-veneris extract:
The results demonstrated that the anti-radical activity of Acv extract rose with increasing its concentration. Besides, the IC50 of Acv extract was obtained 54.61 ug/mL. IC50 was directly correlated with Acv extract antioxidant activity.
Results of animals
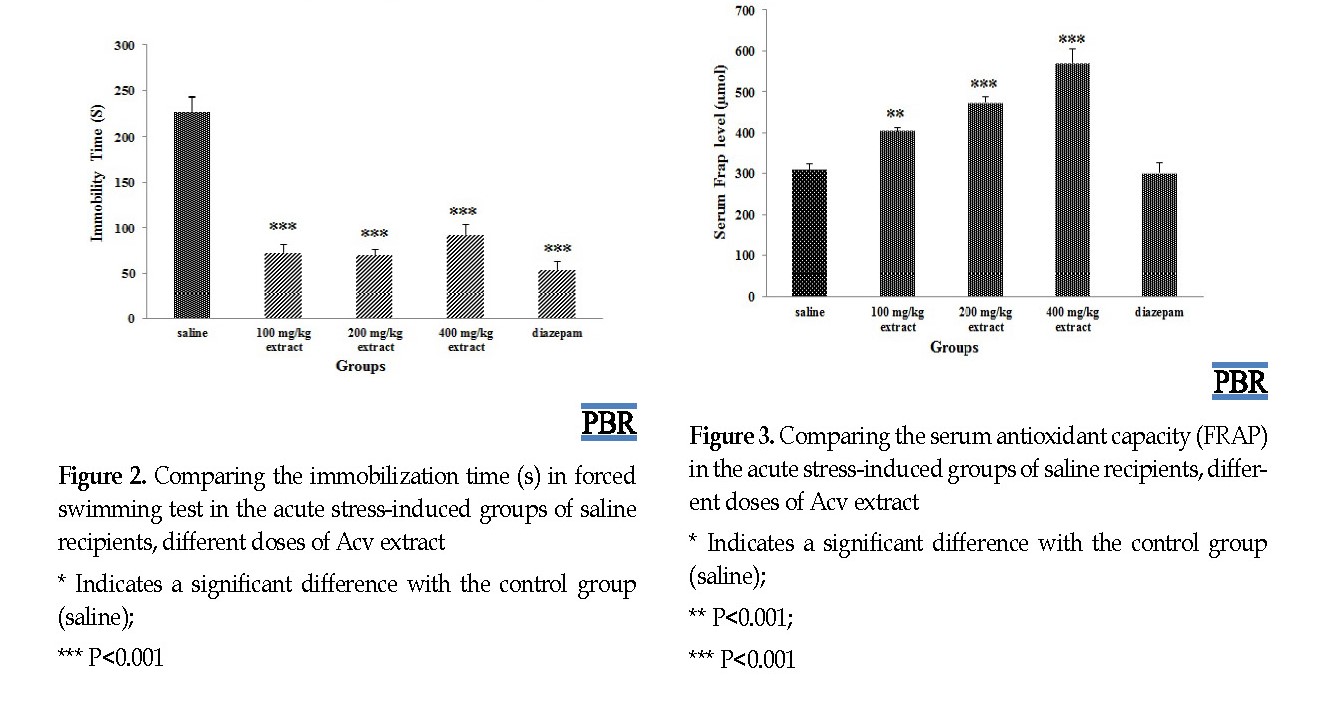
The duration of immobilization (seconds) in the forced swimming test in different groups under acute stress was investigated. The groups receiving the different doses of the extract and the diazepam showed a significant decrease in immobility period compared with the control group (saline) (P=0.000) (Figure 2).
Acv was bought from a valid herbal shop in Izeh, Khuzestan in summer. The plant was collected from Khuzestan Province (Figure 1).after the systematic verification by a botanist, the herbarium sample of this plant was registered in Herbarium (No. 7543) of Islamic Azad University, Izeh Branch, Khuzestan. To prepare the extract, we poured 1 kg of dried plant powder into 70% ethanol and placed on a shaker for two days at room temperature. Then, the obtained mixture was filtered and the solvent was removed by rotary evaporator (Heidolph Co. Germany), and Acv extract was obtained (yield 20%-25%). The extract was completely dried at 40°C and used to prepare the required concentrations. The extract was stored at -20°C [31]. The extract was dissolved in distilled water and given to the animals.
This test is one of the most reliable and commonly used examinations to test depression. In this method, a glass container with a length of 25 cm, a width of 12 cm, and a height of 15 cm were used. The dish was filled with water with a temperature of 25°C and the mouse was gently immersed in water. The discontinuation of the movement by the mouse’s limbs was considered as immobilization. The experiment time was 10 minutes and the first 2 minutes was considered the adaptation of the animal to existing conditions and the immobilization time was measured for the following 8 minutes [32].
The measurement of malondialdehyde of the brain and serum
A total of 100 μL of serum samples or homogeneous tissue was added with 1.5 mL of 20% acidity, 1.5 mL of 0.8% TBA, 0.8% of Sodium Dodecyl Sulfate (SDS) 1.8%, and 700 μL of distilled water to the test tubes. The tubes were placed in boiling water for 60 minutes and then 1 mL of distilled water and 5 mL of butanol or pyridine was added to the samples. Then, they were centrifuged and the light absorbance of the supernatant was measured at 532 nm [33].
The measurement of the total antioxidant capacity of the brain and serum
To measure the antioxidant capacity of serum and tissue, we used FRAP method with three solutions: buffer (1.55 mL of sodium acetate and 8 mL of concentrated acetic acid, distilled water with a volume of 500 mL), iron chloride solution (270 mg 6H2OFeCl3, distilled water with a volume of 50 mL, and a solution of triazine (47 mg of triazine dissolved in 40 mL of 40 mM HCl). The final solution was prepared by adding 10 mL of solution one, 1 mL of solution two, and 1 mL of solution three. About 25 μL of the serum or homogeneous tissue samples were added to 1.5 mL of the solution and placed on the object for 10 minutes at 37°C. Then, the optical absorption at 593 wavelengths was recorded [33].
Statistical analysis
The resulting data were analyzed in SPSS version 21. Considering that the obtained results were quantitative, the assumption of normal distribution of the frequency of data was confirmed by the Kolmogorov-Smirnov non-parametric test (P>0.05). The data were also analyzed by one-way ANOVA and the post hoc Least Significant Difference (LSD) tests. Also, the obtained results were reported together with the corresponding statistical calculations as Mean±SEM. In all cases, the difference among groups was considered significant with P<0.05.
Results
DPPH radical scavenging activities of A. capillus-veneris extract:
The results demonstrated that the anti-radical activity of Acv extract rose with increasing its concentration. Besides, the IC50 of Acv extract was obtained 54.61 ug/mL. IC50 was directly correlated with Acv extract antioxidant activity.
Results of animals
The duration of immobilization (seconds) in the forced swimming test in different groups under acute stress was investigated. The groups receiving the different doses of the extract and the diazepam showed a significant decrease in immobility period compared with the control group (saline) (P=0.000) (Figure 2).
The treatment of acute stress-induced mice by Acv at doses of 100, 200, 400 mg/kg resulted in a significant increase in serum antioxidant capacity compared with the control group (P=0.002, P=0.000) (Figure 3).
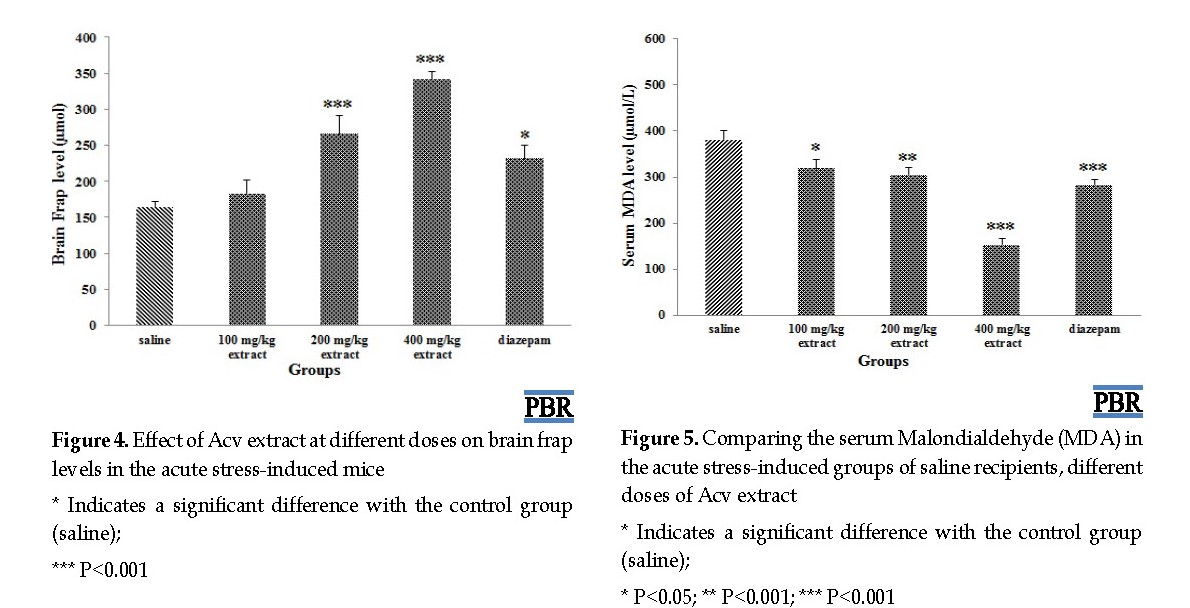
The treatment of acute stress-induced mice by Acv at doses of 200 and 400 mg/kg and diazepam significantly increased brain antioxidant capacity (P<0.05) but treatment by a dose of 100 mg/kg extract showed no significant effect on the duration of animal immobilization in the forced swimming test (P=0.444) (Figure 4).
The results of the serum and brain MDA levels in the groups studied are shown in Figures 5 and 6. The treatment of acute stress-induced mice by Acv at doses of 100, 200, 400 mg/kg and diazepam resulted in a significant decrease in serum MDA compared to the normal saline group (P=0.013, P=0.002, P=0.000) (Figure 5).
The results of the serum and brain MDA levels in the groups studied are shown in Figures 5 and 6. The treatment of acute stress-induced mice by Acv at doses of 100, 200, 400 mg/kg and diazepam resulted in a significant decrease in serum MDA compared to the normal saline group (P=0.013, P=0.002, P=0.000) (Figure 5).
The treatment of acute stress-induced mice by Acv at doses of 200 and 400 mg significantly reduced MDA in the brain (P=0.044, P=0.026) but did not have a significant effect at a dose of 100 mg/kg (P=0.642) (Figure 6).
Discussion
The present study aimed to investigate the effect of Acv extract on depression and oxidative stress indices after acute stress exposure. The results showed that the immobilization time as an indicator of depression significantly decreased in mice receiving Acv extract. The serum and brain antioxidant capacity in mice exposed to acute stress was significantly higher than those of the control group receiving saline. Besides, the levels of serum and brain malondialdehyde in the acute stress-induced groups were significantly lower than that of saline recipients. These results indicated the beneficial effects of Acv extract against oxidative stress caused by acute stress.
In general, the precise mechanism of increasing oxidative stress indices after exposure to acute stress has not been properly explained. The researchers believe that the sharp increase in the activity of the HPA axis and the levels of glucocorticoids in acute stress play some role. It has been observed that cellular contact with high levels of glucocorticoids, due to acute stress, increases the production of ROS and RNS and inhibits the dehydrogenation capacity of the enzyme endogenous antioxidants (SOD, GPX, and CAT) and non-enzymatic (glutathione) in the brain, and therefore the neural cells become susceptible to the adverse effects of ROS and RNS [6]. The increase in oxidative stress indices following acute stress has been shown in several animal studies. The exposure to acute stress increases the index of oxidative stress in the gastrointestinal tract in mice [34]. Besides, in the rats exposed to acute stress for 6 hours, an increase in oxidative stress indices was observed with increasing Nitric Oxide (NO) and induced Nitric Oxide (iNOS) [6]. In another study, the exposure of rats to acute stress has reduced the antioxidant potential of the liver, kidney, heart, and serum [5].
In general, the best and most common method for measuring free radicals and oxidative stress is to determine the products derived from the reaction of free radicals with biological molecules as biomarkers [35]. The goals of ROSs are proteins, lipids, and nucleic acids, in which their metabolites are used as the oxidative stress biomarkers in a variety of diseases [36]. Oxidative stress biomarkers are clinically prominent and the study of their rate is used in blood, urine and other body fluids to determine pathological conditions and to diagnose diseases [37]. One of the primary targets of ROSs is the fatty acids with more than one double bond or polyunsaturated fatty acids in the cell membrane, causing the oxidation of lipid or more than a dual bond through the process of lipid peroxidation. The result is lipid peroxidation of metabolites, such as MDA, which causes an alteration in cellular receptor structure and cellular damage by binding to proteins and altering their function, creating enzymatic inhibition [14].
In the present study, MDA was used as an indicator of lipid peroxidation and oxidative stress, and it was observed that treatment by Acv reduced its levels in mice under acute stress. In the present study, the protective effects of Acv extract against chronic stress-induced depression can be due to the fighting the adverse effects of oxidative stress and boosting the antioxidant defense system. Therefore, in mice under stress, Acv extract significantly reduced the serum and brain MDA levels, as a lipid peroxidation marker, and significantly increased antioxidant capacity.
It seems that the plant’s activity in reducing the symptoms of depression is due to the presence of flavonoids and phenolic compounds with biological and neuroprotective properties. We found no study that investigated the antioxidant effects of Acv extract in animal models, but the antioxidant effects of this herb were observed in several in vitro [12] and cell culture [34] studies. Acv extract is rich in flavonoids such as routine, quercetin, quercetin-3-O-glucoside, nicotine fluorine, astragalin, procyanidin, camphorol-3 sulfate, pro-delphinidin, and Paponin [13].
In rats with subarachnoid hemorrhage, quercetin treatment reduces the oxidative stress indices by reducing MDA of the brain and increasing the activity of antioxidant enzymes [38]. Furthermore, quercetin decreased oxidative stress indices in mice with ischemia-induction and strengthened the antioxidant defense of the brain. Alzheimer and Parkinson rats treated with routine have been reported to reduce the peroxidation of brain lipids and increase the activity of antioxidant enzymes and glutathione [15, 36]. Similar effects have also been reported by catechin, procyanidin [8], and camphorol [35] in animal models. It seems that Acv can cope with oxidative stress and enhance antioxidant defense due to the presence of flavonoid compounds with antioxidant effects.
Acv can improve the symptoms of depression and decrease the oxidative stress indices caused by exposure to acute stress through decreasing lipid peroxidation and enhancing antioxidant defense in mice.
Ethical Considerations
Compliance with ethical guidelines
All animal procedures were based on the guideline for the Care and Use of Laboratory Animals. The study was reviewed by the Research Committee of Islamic Azad University of Izeh and approved by Code: 15330557962002.
Funding
This article was extracted from the MSc. thesis of Jafar Ahmadpouri in Department of Biology, Izeh Branch, Islamic Azad University, Izeh.
Authors' contributions
Conceived the study: Saeid Valipour Chahardahcharic and Mahbubeh Setorki; Collected the data: Jafar Ahmadpouri; Carried out data analysis: Saeid Valipour Chahardahcharic; Wrote the paper: Mahbubeh Setorki; Drafted and finalized the paper, read and approved the final manuscript: All authors.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The researchers would like to thank the Deputy Director of Research and Technology at the Islamic Azad University of Izeh.
References
Gunnar M, Vazquez D, Cicchetti D, Cohen D. Developmental psychopathology. Psychopathol. 2006; 5(3):22-9.
Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. Develop Psychopathol. 2015; 8(5):533-77. [DOI:10.1002/9780470939390.ch13]
Park SH, Sim YB, Han PL, Lee JK, Suh HW. Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia ficus-indica var. saboten. Exp Neurobiol. 2010; 19(1):30-8. [DOI:10.5607/en.2010.19.1.30] [PMID] [PMCID]
Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacol. 2008; 55(8):1355-63. [DOI:10.1016/j.neuropharm.2008.08.026] [PMID]
Martínez-Sámano J, Torres-Durán PV, Juárez-Oropeza MA, Elías-Viñas D, Verdugo-Díaz L. Effects of acute electromagnetic field exposure and movement restraint on antioxidant system in liver, heart, kidney and plasma of Wistar rats: a preliminary report. Int J Radiat Biol. 2010; 86(12):1088-94. [DOI:10.3109/09553002.2010.501841] [PMID]
6. MadrigalJL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor κB‐mediated mechanisms. J Neurochem. 2001; 76(2):532-8. [DOI:10.1046/j.1471-4159.2001.00108.x] [PMID]
Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999; 9(1):119-31. [DOI:10.1111/j.1750-3639.1999.tb00214.x] [PMID]
Bagchi D, Garg A, Krohn R, Bagchi M, Bagchi D, Balmoori J, et al. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepaticand brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998; 30(5):771-6. [DOI:10.1016/S0306-3623(97)00332-7]
Ishaq MS, Hussain MM, Siddique Afridi M, Ali G, Khattak M, Ahmad S. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris. Sci World J. 2014; 2014. [DOI:10.1155/2014/269793] [PMID] [PMCID]
Ansari R, Ekhlasi-Kazaj K. Adiantum capillus-veneris. L: Phytochemical constituents, traditional uses and pharmacological properties: A review. J of Adv Sci Res. 2012; 3(4):15-20.
Haider S, Nazreen S, Alam MM, Gupta A, Hamid H, Alam MS. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from adiantum capillus veneris linn. J Ethnopharmacol. 2011; 138(3):741-7. [DOI:10.1016/j.jep.2011.10.012] [PMID]
Rajurkar NS, Gaikwad k. Evaluation of phytochemicals, antioxidant activity and elemental content of Adiantum capillus veneris leaves. J Chem Pharm Res. 2012; 4(1):365-74.
Akabori Y, Hasegawa M. Flavonoid pattern in the Pteridaceae II. Shokubutsugaku Zasshi. 1969; 82(973):294-7. [DOI:10.15281/jplantres1887.82.294]
Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011; 36(8):1360-71. [DOI:10.1007/s11064-011-0458-6] [PMID]
Xu Px, Wang Sw, Yu Xl, Su Yj, Wang T, Zhou Ww, et al. Rutin improves spatial memory in Alzheimer’s disease transgenic miceby reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014; 264:173-80. [DOI:10.1016/j.bbr.2014.02.002] [PMID]
Yang EJ, Kim GS, Jun M, Song KS. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014; 5(7):1395-402. [DOI:10.1039/c4fo00068d] [PMID]
Jain SK, Singh T, Pande M, Nema N. Neuropharmacological screening of fronds of Adiantum capillus veneris linn. Pharm Lett. 2014; 6(3):167-75.
Haider S, Nazreen S, Alam MM et al. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from Adiantum capillus veneris Linn. J Ethnopharmacol. 2011; 138(3):741-47. [DOI:10.1016/j.jep.2011.10.012] [PMID]
Kanchan G. Protective effect of Adiantum capillus against chemically induced oxidative stress by cisplatin. J Appl Pharm Sci. 2013; 3(2):65-68.
Jiang MZ, Yan H, Wen Y, Li XM. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. Afr J Pharm Pharmacol. 2011; 5(18):2079-85.[DOI:10.5897/AJPP11.500]
Hussain MM, Ahmad B, Rashid E, Hashim S, Marwat KB, Jan AS. In vitro antibacterial activity of methanol and water extracts of Adiantum capillus veneris and Tagetes patula against multidrug resistant bacterial strains. Pak J Bot. 2014; 46(1):363-68.
Shirazi MH, Amin G, Lavasani BA, Eshraghi SS. Study of antibacterial properties of Adiantum capillus-veneris extract on eight species of gram positive and negative bacteria. J Med Plants. 2011; 10(40):124-32.
Guha P, Mukhopadhyay R, Pal PK, Gupta K. Antimicrobial activity of crude extracts and extracted phenols from gametophyte and sporophytic plant parts of Adiantum capillus-veneris L. Allelopathy. 2004; 13(1):57-66.
Besharat M, Rahimian M, Ghaemi E, Besharat S. Effect of ethanolic extract of Adiantum capillus-veneris in comparison with Gentamicine on 3 pathogenic bacteria in vitro. Pharm Sci. 2009; 15(1):49-52.
Mokhtari S, Rabiei Z, Shahrani M, Rafieian-Kopaei M. The ameliorating effect of beta vulgaris extract on memory and learning impairment induced by lesions of the nucleus basalis of meynert in rat. Int J Clin Diagn. 2017; 11(11). [DOI:10.7860/JCDR/2017/20809.10887]
Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol. 2013; 146(1):411-6. [DOI:10.1016/j.jep.2013.01.011] [PMID]
Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: an interaction with opiate system. Behav Brain Res. 2004; 154(1):193-8. [DOI:10.1016/j.bbr.2004.02.007] [PMID]
Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. 2014; 271:116-21. [DOI:10.1016/j.bbr.2014.05.068] [PMID]
Noutarki M, Setorki M, Hooshmandi Z.The Antioxidant effect of Sidr (Zizyphusspina-Christi) leaf extract helping to improve the scopolamine induced memory impairment in male rats. Iran J Pharm Sci. 2017; 13(2):13-24
Fathi F, Oryan S, Rafieian M, Eidi A. Neuroprotective effect of Mentha longifolia L. extract on ischemia/reperfusion-induced brain injury in male wistar rats. J Med Plants. 2015; 2(54):169-82.
Aldenkamp AP, Alpherts WC, Blennow G, Elmqvist D, Heijbel J, Nilsson HL, et al. Withdrawal of antiepileptic medication in children-effects on cognitive function: The multicenter Holmfrid study. Neurol. 1993; 43(1):41-50. [DOI:10.1212/WNL.43.1_Part_1.41] [PMID]
Zahedi M, Hojjati MR, Fathpour H, Rabiei Z, Alibabaei Z, Basim A. Effect of Rheum ribes hydro-alcoholic extracton memory impairments in rat model of Alzheimer’s disease. Iran J Biopharm Res. 2015; 14(4):1197-206.
Ibraheim ZZ, Ahmed AS, Gouda YG. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J. 2011; 19(2):65-74. [DOI:10.1016/j.jsps.2011.01.007] [PMID] [PMCID]
Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, et al. Protective effects of kaempferol (3, 4′, 5, 7-tetrahydroxyflavone) against amyloid beta peptide (Aβ)-induced neurotoxicity in ICR Mice. Biosci, Biotechnol, Biochem. 2010; 74(2):397-401. [DOI:10.1271/bbb.90585] [PMID]
Khan MM, Raza SS, Javed H, Ahmad A, Khan A, Islam F, et al. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res. 2012; 22(1):1-15. [DOI:10.1007/s12640-011-9295-2] [PMID]
Ahmadpoor J , Valipour Chahardahcheric S, Setorki M. The Protective effect of hydroalcoholic extract of the southern maidenhair fern (adiantum capillus-veneris) on the depression and anxiety caused by chronic stress in adult male mice: an experimental randomized study. Iran Red Crescent Med J. 2019; 21(3):e86750
Dong Ys, Wang Jl, Feng Dy, Qin Hz, Wen H, Yin Zm, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014; 11(3):282-90. [DOI:10.7150/ijms.7634] [PMID] [PMCID]
Chtourou Y, Fetoui H, Gdoura R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res. 2014; 158(3):376-83. [DOI:10.1007/s12011-014-9948-0] [PMID]
Discussion
The present study aimed to investigate the effect of Acv extract on depression and oxidative stress indices after acute stress exposure. The results showed that the immobilization time as an indicator of depression significantly decreased in mice receiving Acv extract. The serum and brain antioxidant capacity in mice exposed to acute stress was significantly higher than those of the control group receiving saline. Besides, the levels of serum and brain malondialdehyde in the acute stress-induced groups were significantly lower than that of saline recipients. These results indicated the beneficial effects of Acv extract against oxidative stress caused by acute stress.
In general, the precise mechanism of increasing oxidative stress indices after exposure to acute stress has not been properly explained. The researchers believe that the sharp increase in the activity of the HPA axis and the levels of glucocorticoids in acute stress play some role. It has been observed that cellular contact with high levels of glucocorticoids, due to acute stress, increases the production of ROS and RNS and inhibits the dehydrogenation capacity of the enzyme endogenous antioxidants (SOD, GPX, and CAT) and non-enzymatic (glutathione) in the brain, and therefore the neural cells become susceptible to the adverse effects of ROS and RNS [6]. The increase in oxidative stress indices following acute stress has been shown in several animal studies. The exposure to acute stress increases the index of oxidative stress in the gastrointestinal tract in mice [34]. Besides, in the rats exposed to acute stress for 6 hours, an increase in oxidative stress indices was observed with increasing Nitric Oxide (NO) and induced Nitric Oxide (iNOS) [6]. In another study, the exposure of rats to acute stress has reduced the antioxidant potential of the liver, kidney, heart, and serum [5].
In general, the best and most common method for measuring free radicals and oxidative stress is to determine the products derived from the reaction of free radicals with biological molecules as biomarkers [35]. The goals of ROSs are proteins, lipids, and nucleic acids, in which their metabolites are used as the oxidative stress biomarkers in a variety of diseases [36]. Oxidative stress biomarkers are clinically prominent and the study of their rate is used in blood, urine and other body fluids to determine pathological conditions and to diagnose diseases [37]. One of the primary targets of ROSs is the fatty acids with more than one double bond or polyunsaturated fatty acids in the cell membrane, causing the oxidation of lipid or more than a dual bond through the process of lipid peroxidation. The result is lipid peroxidation of metabolites, such as MDA, which causes an alteration in cellular receptor structure and cellular damage by binding to proteins and altering their function, creating enzymatic inhibition [14].
In the present study, MDA was used as an indicator of lipid peroxidation and oxidative stress, and it was observed that treatment by Acv reduced its levels in mice under acute stress. In the present study, the protective effects of Acv extract against chronic stress-induced depression can be due to the fighting the adverse effects of oxidative stress and boosting the antioxidant defense system. Therefore, in mice under stress, Acv extract significantly reduced the serum and brain MDA levels, as a lipid peroxidation marker, and significantly increased antioxidant capacity.
It seems that the plant’s activity in reducing the symptoms of depression is due to the presence of flavonoids and phenolic compounds with biological and neuroprotective properties. We found no study that investigated the antioxidant effects of Acv extract in animal models, but the antioxidant effects of this herb were observed in several in vitro [12] and cell culture [34] studies. Acv extract is rich in flavonoids such as routine, quercetin, quercetin-3-O-glucoside, nicotine fluorine, astragalin, procyanidin, camphorol-3 sulfate, pro-delphinidin, and Paponin [13].
In rats with subarachnoid hemorrhage, quercetin treatment reduces the oxidative stress indices by reducing MDA of the brain and increasing the activity of antioxidant enzymes [38]. Furthermore, quercetin decreased oxidative stress indices in mice with ischemia-induction and strengthened the antioxidant defense of the brain. Alzheimer and Parkinson rats treated with routine have been reported to reduce the peroxidation of brain lipids and increase the activity of antioxidant enzymes and glutathione [15, 36]. Similar effects have also been reported by catechin, procyanidin [8], and camphorol [35] in animal models. It seems that Acv can cope with oxidative stress and enhance antioxidant defense due to the presence of flavonoid compounds with antioxidant effects.
Acv can improve the symptoms of depression and decrease the oxidative stress indices caused by exposure to acute stress through decreasing lipid peroxidation and enhancing antioxidant defense in mice.
Ethical Considerations
Compliance with ethical guidelines
All animal procedures were based on the guideline for the Care and Use of Laboratory Animals. The study was reviewed by the Research Committee of Islamic Azad University of Izeh and approved by Code: 15330557962002.
Funding
This article was extracted from the MSc. thesis of Jafar Ahmadpouri in Department of Biology, Izeh Branch, Islamic Azad University, Izeh.
Authors' contributions
Conceived the study: Saeid Valipour Chahardahcharic and Mahbubeh Setorki; Collected the data: Jafar Ahmadpouri; Carried out data analysis: Saeid Valipour Chahardahcharic; Wrote the paper: Mahbubeh Setorki; Drafted and finalized the paper, read and approved the final manuscript: All authors.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
The researchers would like to thank the Deputy Director of Research and Technology at the Islamic Azad University of Izeh.
References
Gunnar M, Vazquez D, Cicchetti D, Cohen D. Developmental psychopathology. Psychopathol. 2006; 5(3):22-9.
Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. Develop Psychopathol. 2015; 8(5):533-77. [DOI:10.1002/9780470939390.ch13]
Park SH, Sim YB, Han PL, Lee JK, Suh HW. Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia ficus-indica var. saboten. Exp Neurobiol. 2010; 19(1):30-8. [DOI:10.5607/en.2010.19.1.30] [PMID] [PMCID]
Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacol. 2008; 55(8):1355-63. [DOI:10.1016/j.neuropharm.2008.08.026] [PMID]
Martínez-Sámano J, Torres-Durán PV, Juárez-Oropeza MA, Elías-Viñas D, Verdugo-Díaz L. Effects of acute electromagnetic field exposure and movement restraint on antioxidant system in liver, heart, kidney and plasma of Wistar rats: a preliminary report. Int J Radiat Biol. 2010; 86(12):1088-94. [DOI:10.3109/09553002.2010.501841] [PMID]
6. MadrigalJL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor κB‐mediated mechanisms. J Neurochem. 2001; 76(2):532-8. [DOI:10.1046/j.1471-4159.2001.00108.x] [PMID]
Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999; 9(1):119-31. [DOI:10.1111/j.1750-3639.1999.tb00214.x] [PMID]
Bagchi D, Garg A, Krohn R, Bagchi M, Bagchi D, Balmoori J, et al. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepaticand brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998; 30(5):771-6. [DOI:10.1016/S0306-3623(97)00332-7]
Ishaq MS, Hussain MM, Siddique Afridi M, Ali G, Khattak M, Ahmad S. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris. Sci World J. 2014; 2014. [DOI:10.1155/2014/269793] [PMID] [PMCID]
Ansari R, Ekhlasi-Kazaj K. Adiantum capillus-veneris. L: Phytochemical constituents, traditional uses and pharmacological properties: A review. J of Adv Sci Res. 2012; 3(4):15-20.
Haider S, Nazreen S, Alam MM, Gupta A, Hamid H, Alam MS. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from adiantum capillus veneris linn. J Ethnopharmacol. 2011; 138(3):741-7. [DOI:10.1016/j.jep.2011.10.012] [PMID]
Rajurkar NS, Gaikwad k. Evaluation of phytochemicals, antioxidant activity and elemental content of Adiantum capillus veneris leaves. J Chem Pharm Res. 2012; 4(1):365-74.
Akabori Y, Hasegawa M. Flavonoid pattern in the Pteridaceae II. Shokubutsugaku Zasshi. 1969; 82(973):294-7. [DOI:10.15281/jplantres1887.82.294]
Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011; 36(8):1360-71. [DOI:10.1007/s11064-011-0458-6] [PMID]
Xu Px, Wang Sw, Yu Xl, Su Yj, Wang T, Zhou Ww, et al. Rutin improves spatial memory in Alzheimer’s disease transgenic miceby reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav Brain Res. 2014; 264:173-80. [DOI:10.1016/j.bbr.2014.02.002] [PMID]
Yang EJ, Kim GS, Jun M, Song KS. Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014; 5(7):1395-402. [DOI:10.1039/c4fo00068d] [PMID]
Jain SK, Singh T, Pande M, Nema N. Neuropharmacological screening of fronds of Adiantum capillus veneris linn. Pharm Lett. 2014; 6(3):167-75.
Haider S, Nazreen S, Alam MM et al. Anti-inflammatory and anti-nociceptive activities of ethanolic extract and its various fractions from Adiantum capillus veneris Linn. J Ethnopharmacol. 2011; 138(3):741-47. [DOI:10.1016/j.jep.2011.10.012] [PMID]
Kanchan G. Protective effect of Adiantum capillus against chemically induced oxidative stress by cisplatin. J Appl Pharm Sci. 2013; 3(2):65-68.
Jiang MZ, Yan H, Wen Y, Li XM. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. Afr J Pharm Pharmacol. 2011; 5(18):2079-85.[DOI:10.5897/AJPP11.500]
Hussain MM, Ahmad B, Rashid E, Hashim S, Marwat KB, Jan AS. In vitro antibacterial activity of methanol and water extracts of Adiantum capillus veneris and Tagetes patula against multidrug resistant bacterial strains. Pak J Bot. 2014; 46(1):363-68.
Shirazi MH, Amin G, Lavasani BA, Eshraghi SS. Study of antibacterial properties of Adiantum capillus-veneris extract on eight species of gram positive and negative bacteria. J Med Plants. 2011; 10(40):124-32.
Guha P, Mukhopadhyay R, Pal PK, Gupta K. Antimicrobial activity of crude extracts and extracted phenols from gametophyte and sporophytic plant parts of Adiantum capillus-veneris L. Allelopathy. 2004; 13(1):57-66.
Besharat M, Rahimian M, Ghaemi E, Besharat S. Effect of ethanolic extract of Adiantum capillus-veneris in comparison with Gentamicine on 3 pathogenic bacteria in vitro. Pharm Sci. 2009; 15(1):49-52.
Mokhtari S, Rabiei Z, Shahrani M, Rafieian-Kopaei M. The ameliorating effect of beta vulgaris extract on memory and learning impairment induced by lesions of the nucleus basalis of meynert in rat. Int J Clin Diagn. 2017; 11(11). [DOI:10.7860/JCDR/2017/20809.10887]
Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol. 2013; 146(1):411-6. [DOI:10.1016/j.jep.2013.01.011] [PMID]
Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: an interaction with opiate system. Behav Brain Res. 2004; 154(1):193-8. [DOI:10.1016/j.bbr.2004.02.007] [PMID]
Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res. 2014; 271:116-21. [DOI:10.1016/j.bbr.2014.05.068] [PMID]
Noutarki M, Setorki M, Hooshmandi Z.The Antioxidant effect of Sidr (Zizyphusspina-Christi) leaf extract helping to improve the scopolamine induced memory impairment in male rats. Iran J Pharm Sci. 2017; 13(2):13-24
Fathi F, Oryan S, Rafieian M, Eidi A. Neuroprotective effect of Mentha longifolia L. extract on ischemia/reperfusion-induced brain injury in male wistar rats. J Med Plants. 2015; 2(54):169-82.
Aldenkamp AP, Alpherts WC, Blennow G, Elmqvist D, Heijbel J, Nilsson HL, et al. Withdrawal of antiepileptic medication in children-effects on cognitive function: The multicenter Holmfrid study. Neurol. 1993; 43(1):41-50. [DOI:10.1212/WNL.43.1_Part_1.41] [PMID]
Zahedi M, Hojjati MR, Fathpour H, Rabiei Z, Alibabaei Z, Basim A. Effect of Rheum ribes hydro-alcoholic extracton memory impairments in rat model of Alzheimer’s disease. Iran J Biopharm Res. 2015; 14(4):1197-206.
Ibraheim ZZ, Ahmed AS, Gouda YG. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J. 2011; 19(2):65-74. [DOI:10.1016/j.jsps.2011.01.007] [PMID] [PMCID]
Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, et al. Protective effects of kaempferol (3, 4′, 5, 7-tetrahydroxyflavone) against amyloid beta peptide (Aβ)-induced neurotoxicity in ICR Mice. Biosci, Biotechnol, Biochem. 2010; 74(2):397-401. [DOI:10.1271/bbb.90585] [PMID]
Khan MM, Raza SS, Javed H, Ahmad A, Khan A, Islam F, et al. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res. 2012; 22(1):1-15. [DOI:10.1007/s12640-011-9295-2] [PMID]
Ahmadpoor J , Valipour Chahardahcheric S, Setorki M. The Protective effect of hydroalcoholic extract of the southern maidenhair fern (adiantum capillus-veneris) on the depression and anxiety caused by chronic stress in adult male mice: an experimental randomized study. Iran Red Crescent Med J. 2019; 21(3):e86750
Dong Ys, Wang Jl, Feng Dy, Qin Hz, Wen H, Yin Zm, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 2014; 11(3):282-90. [DOI:10.7150/ijms.7634] [PMID] [PMCID]
Chtourou Y, Fetoui H, Gdoura R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res. 2014; 158(3):376-83. [DOI:10.1007/s12011-014-9948-0] [PMID]
Type of Study: Original Research |
Subject:
Medicine and traditional medicine
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








 .
.