Volume 6, Issue 1 (2020)
Pharm Biomed Res 2020, 6(1): 45-52 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezaie M, Azarbayjani M A, Peeri M, Hosseini S A. The Effect of Exercise, Ozone, and Mesenchymal Stem Cells Therapy on CB-1 and GABA Gene Expression in the Cartilage Tissue of Rats With Knee Osteoarthritis. Pharm Biomed Res 2020; 6 (1) :45-52
URL: http://pbr.mazums.ac.ir/article-1-275-en.html
URL: http://pbr.mazums.ac.ir/article-1-275-en.html
1- Department of Sport Physiology, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
2- Department of Sport Physiology, Marvdasht Branch, Islamic Azad University, Marvdasht, Iran.
2- Department of Sport Physiology, Marvdasht Branch, Islamic Azad University, Marvdasht, Iran.
Full-Text [PDF 720 kb]
(1584 Downloads)
| Abstract (HTML) (4776 Views)
Full-Text: (2013 Views)
Introduction
Osteoarthritis (OA) is one of the most painful arthritic diseases that result in disability. Recent studies have shown that overweight, aging, genetics, and diabetes are risk factors for this disease [1, 2]. Increased inflammation following OA can disrupt the sympathetic nervous system [3]. Pain is one of the main symptoms of this disease. Increased inflammation and free radicals pressurize the nerves of this area, induce pain, and thereby reduce the motor ability and quality of life in patients with OA [4, 5].
On the other hand, since the identification of endocannabinoid receptors, many researchers have paid attention to this anti-nociceptive pathway. The endocannabinoid system includes cannabinoid receptors 1 and 2 (CB-1 and CB-2), binding to G proteins that are activated by cannabinoid binding and inhibit adenylyl cyclase and cyclic AMP production. As a result, it inhibits gamma-aminobutyric acid (GABA) and glutamate transport that, in turn, suppress the excitability of the nervous system and pain sensation [4, 6].
Despite much research, no effective treatment has been found for OA yet. However, recent studies have investigated the effect of mesenchymal stem/stromal cells (MSCs) on experimental models of cartilage injury. It has been reported that intra-articular injection of stem cells can reduce pain and protect cartilage. MSCs appear to express a variety of anti-inflammatory chemokines and cytokines in the cartilage tissue and are effective in the treatment of osteoarthritis by modulating cartilage metabolism and coping with inflammation and apoptosis [7]. Some studies have investigated the effect of MSCs in the treatment of OA. For example, the researchers in a study concluded that treatment with MSCs for 12 months reduced pain, inflammatory and anti-inflammatory factors, and improved metabolic factors in the cartilage tissue in patients with knee OA. These researchers also reported that the favorable effects were dependent on the dose and duration of treatment such that short-term treatment of fewer than 3 months had no significant impact on these variables [8, 9].
Given that many patients are old age, the duration of treatment and the economic costs will cause problems for them. So using complementary therapies to achieve the desired results is a worthwhile solution. In this regard, out of various therapeutic approaches, ozone therapy is shown to improve knee cartilage metabolism because of its anti-inflammatory properties and increased tissue oxygenation. Studies have reported that ozone therapy help improves inflammatory factors and reduce pain in patients with knee OA [10]. However, the results of a study showed that ozone therapy had no significant effect on pain in patients with OA for the first three months, and it shows its effects after six months [11].
Patients with knee OA usually have a reduced ability to perform their daily activities, and the most important factor in improving their quality of life and improving their condition is helping them to perform daily activities and reducing pain. Therefore, health activities have been considered as a non-invasive and low-cost treatment by health researchers. Regular and aerobic exercise increases joint metabolism and synovial fluid and has antioxidant and anti-inflammatory effects [12].
According to studies, complementary therapies reduce pain and improve the quality of life of these patients, but the mechanisms of their effects are not fully evident. Also, little information is known about the concurrent use of ozone therapy, MSCs, and ET on anti-nociceptive pathways. So, it seems necessary to conduct a study in this regard. We to investigate the effect of concurrent use of ET, ozone therapy, and MSCs on CB-1 and GABA gene expression in the cartilage tissue of rats with knee osteoarthritis.
Materials and Methods
Animals
In this experimental study, 45 rats, aged 40-45 weeks, weighing 250-300 g, were purchased and transferred to the animal house. During the research period, standard food plates and water were provided ad libitum. All rats were kept in the standard situation (temperature of 23±2°C, light/dark cycle of 12:12 h, and the humidity of 45%-55%). After one week of adaptation to the laboratory environment, OA was induced in all rats on the eighth day. The researchers received introduction letters from Islamic Azad University, Marvdasht Branch (IR.MIAU.RECE.1396.132).
Study experiment
In the present study, OA was surgically induced, according to Zhao et al. study [13]. In this method, rats were first anesthetized by ketamine and xylazine, then their right knee hairs were completely shaved, and a 1-cm area was cut in the knee joint. After removing the skin, the internal lateral ligament of the knee was removed to observe the internal meniscus. An incomplete cutting then resulted in rupture and injury to the meniscus and the area was. The rats were then fed with standard food for three weeks and kept in a laboratory environment for recovery. Then, they were divided into eight groups of 5 rats each: 1. osteoarthritis; 2. MSCs, 3. ozone, 4. ET, 5. ozone+MSCs; 6. ET+ozone, 7. ET+MSCs, and 8. ET+MSCs+ozone. Also, 5 rats were assigned to the healthy control group. Table 1 presents all procedures done in the experimental groups of ET, MSCs, ozone, and mix groups.
.jpg)
Study design
After grouping, MSC, ozone+MSCs, ET+MSCs, and ET+MSCs+ozone groups received intra- articular injection of 1×106 cells/kg MSCs into their right knee joint. Ozone, ozone+MSC, ET+ozone, and ET+MSC+ozone groups received 20 μg/mL ozone once weekly for 3 weeks. Besides, ET, ET+ozone, ET+MSCs, and ET+MSCs+ozone groups performed ET for 8 weeks, 3 sessions per week and 30-50 minutes per session.
Endurance training protocol
Before the training program, the rats in the ET group were adapted to a rodent treadmill for one week (three times per week, 60%-70% of VO2max at a speed of 16 m/min at 0% inclination for 10 min/d). Briefly, the training program was initiated with a 30-min run on a treadmill without slope at a speed of 16 m/min in the first week, which gradually reached 50 minutes in the eighth week. Warm-up and cool-down were performed for 5 m/min at the beginning and end of the training [14].
Mesenchymal stem cells therapy
On the other hand, in the MSCs groups, bone marrow-derived MSCs were extracted from healthy male Wistar rats after being anesthetized with ketamine (30-50 mg/kg) and xylazine (3-5 mg/kg). Briefly, their femur and tibia bones were cut off from the back limbs, remove the skin and muscles. The femurs and tibias were transferred to a 10-cm dish containing Dulbecco’s Modified Eagle Medium (DMEM). Each bone was then held with tweezers, and the two ends were cut open with a scissor. A 22-G needle was attached to a 3-mL syringe and filled with DMEM. Then the marrow was flushed into a 50-mL tube by inserting the needle to one open end of the bone. The procedure is repeated 2~3 times for each bone. When all marrows were obtained, the cells were resuspended, and the suspension was passed through a 70-μm cell strainer to remove the bone debris and blood aggregates. The cells were spun down at 200g, 4°C for 5 min and the supernatant was removed by aspiration. The cells were resuspended in 25-mL MSC medium (DMEM containing 10% Fetal Bovine Serum [FBS] and 1% Pen-Strep). An amount of 10 mL cell suspension was seeded into each 10-cm culture dish for a total of two dishes. The culture dishes were kept at 37°C and 5% CO2 incubator for 1~2 weeks. The medium was changed every 2~3 days.
The isolated MSCs were cultured in DMEM medium containing 20% FBS and incubated at 37°C overnight to select the adherent cells. The culture medium in the flasks was changed every 3 days, and MSCs were passaged 3-4 times. After MSCs reached >90% confluency, they were selected for injection purposes. Rats in the MSCs groups received an intraarticular injection of 1×106 cells/kg into their right knee joint [15].
Ozone therapy
A low-intensity electric discharge also produced ozone, and its concentration was measured at 254 nm using ultraviolet radiation. Ozone was injected into the knee tibia-femoral articular line at a concentration of 20 μg/mL once a week for 3 weeks starting at 21 days after the OA induction [16].
Polymerase chain reaction
Forty-eight hours after the last treatment, the rats were anesthetized with ketamine (30-50 mg/kg) and xylazine (3-5 mg/kg). Their cartilage tissues were isolated and homogenized in phosphate buffer saline (0.01 M; pH 7.0) at 4°C with a homogenizer (Hielscher, UP100H). RNA was extracted from cartilage tissues using the RNX-Plus (SinaClon; RN7713C) kit. The quantity and quality of extracted RNAs were examined with the NanoDrop ND-1000 spectrophotometer (Thermo Sci., Newington, NH) method. cDNA was synthesized from RNA samples using RevertAid Reverse Transcriptase (Thermo science, Germany) at 42°C for 1 h and random hexamer primers (Thermo science, Germany). A Rotor-Gene 6000 (Corbett Research, Australia) thermocycler and Real qPCR 29 Master Mix Kit (Amplicon, Denmark) in 40 cycles were applied for amplification. Each reaction included 5 μL master mix and 100 nm primers. Table 2 presents the sequence of the primers used to measure the expression level of the genes in the study.
.jpg)
Data analysis
The Shapiro-Wilk test was used to investigate the normality of the findings. Also, to examine the effect of OA induction on research variables, we used the independent samples t test to compare the difference between the healthy control and OA group. Besides, 3-way ANOVA was employed to investigate the effect of training, MSCs, ozone therapy, the combination of ET and MSCs, the combination of ET and ozone therapy, the combination of MSCs and ozone therapy, as well as the combination of ET, ozone therapy, and MSCs (P≤0.05).
Results
The levels of CB-1 and GABA gene expression are reported in Figures 1 and 2, respectively. The results showed that CB-1 (P=0.001) and GABA (P=0.001) levels were significantly lower in the control group compared with the healthy group.
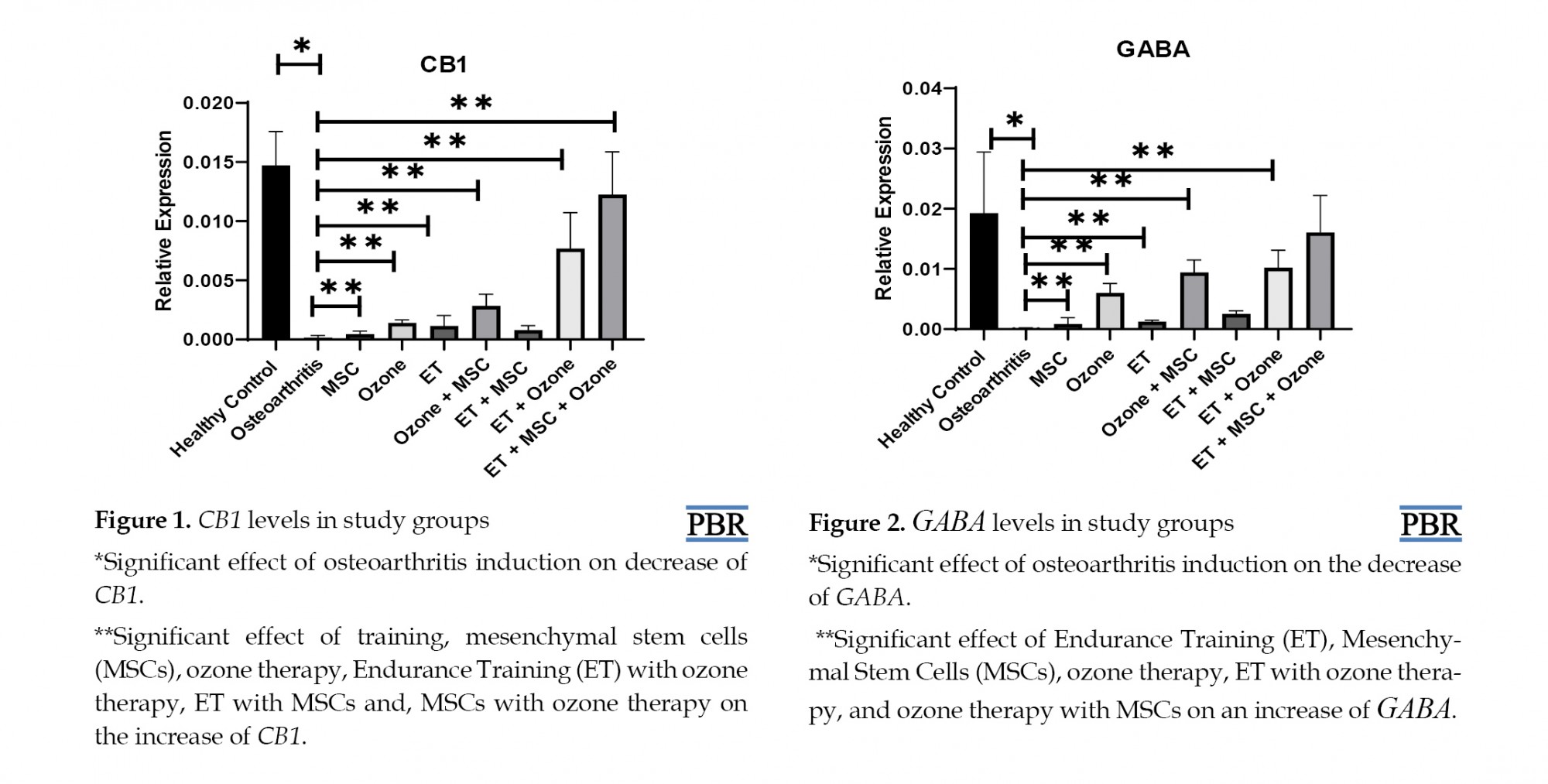
ET (P=0.001), MSCs (P=0.003,) and ozone therapy (P=0.001) had significant effect on the increase of CB-1 gene expression compared with OA group. ET with ozone therapy (P=0.001), as well as MSCs with ozone therapy (P=0.004), had a significant effect on the increase of CB-1 gene expression compared with the OA group. Nevertheless, ET with MSCs did not have a significant effect on CB-1 gene expression (P=0.189). Also, ET, MSCs with ozone therapy (P=0.048) had a significant effect on the increase of CB-1 gene expression compared with the OA group (Table 3).
.jpg)
ET (P=0.001), MSCs (P=0.001), and ozone therapy (P=0.001) had a significant effect on the increase of GABA gene expression compared with the OA group. ET with ozone therapy (P=0.003), as well as MSCs with ozone therapy (P=0.006), had a significant effect on the increase of GABA gene expression compared with the OA group. However, ET with MSCs (P=0.206) and ET+MSCs+ozone therapy (P=0.507) did not have a significant effect on the increase of GABA gene expression compared with the OA group (Table 3).
Discussion
The results of this study showed that CB-1 and GABA levels were significantly lower in the control group than in the healthy group. In support of the findings of the present study, Hammell et al. (2016), Burston et al. (2013), and Yuan et al. (2018) stated that OA decreases cannabinoid receptors and decreases GABA and serotonin by increasing inflammation pathway [17, 18, 19]. On the other hand, the results showed that aerobic training, ozone therapy, and MSCs alone increased CB-1 and GABA gene expression in the cartilage tissue of rats with knee OA. The anti-nociceptive signal pathway of exercise training is dependent on the expression of neurotransmitters in the nervous system, which include increased expression of serotonin, opioids, increased activity of micro-opioids, cannabinoid receptors, increased N-methyl-D-aspartate and carriers of serotonin [20].
Consistent with the present study, regular and aerobic exercise training reduced and alleviated pain by increasing serotonergic and opioid activity following induction of alloxan-induced neuropathy [21]. Also, voluntary training with rotational wheel alleviated pain in rats with the same mechanism [22]. On the other hand, the researchers believe that the anti-nociceptive effects of exercise training are dependent on the intensity of exercise and the induction of fatigue caused by exercise. Therefore, vigorous exercise increases pain levels in athletes [23], and in individuals with OA that acts through a reduced cannabinoid receptors pathway [24].
Vigorous exercise seems to be a factor in increasing free radicals and inflammation that decrease cannabinoid receptors and GABA. Besides, ozone therapy with the mechanism of reducing arthritis inflammation enhances oxygen delivery to the injured tissue and induces the anti-nociceptive effects [10]. The precise mechanism of the anti-nociceptive effects of ozone therapy has not been fully understood. However, based on Cuadros study, two months of ozone therapy had no significant effect on pain in patients with knee OA [10].
The main reasons for inconsistency in these studies appear to be the differences in the statistical population, consumption rate, and duration of treatment. In another study, longer treatment with ozone reduced joint damage and inflammation [25]. Because of the simultaneous anti-inflammatory effects of ET and ozone therapy in this study, they were able to have synergistic effects on CB-1 and GABA receptor elevation. No studies have been found that investigated the concurrent effect of ozone therapy and ET on the variables of the present study; however, Asadi et al. reported that ET and ozone therapy alone increased interleukin-10 levels as an anti-inflammatory cytokine and reduced tumor necrosis factor-α in the cartilage tissue of rats with knee OA [2].
The researchers also suggested that MSCs could modulate bone growth and metabolic factors such as insulin-like growth factor-1, morphological proteins of bone growth by reducing the pro-inflammatory and inflammatory cytokines, as well as increasing anti-inflammatory cytokines, that result in modulation of serotonin and cannabinoid receptors in patients with osteoarthritis [26]. Also, the synergistic effects of MSCs and ozone therapy with affecting the pathway of arthritis and regulating metabolic factors and bone repair [2, 10] could increase CB-1 and GABA receptors.
Regarding the contradictory results of exercise training [20, 22, 23] on the similar molecular, cellular pathway as well as the therapeutic effects of MSCs that depend on the amount and duration of treatment [8, 9], a closer look failed to find an investigation on the interactive effect of training and stem cells simultaneously on cannabinoid receptors and GABA. Thus, the interactive effect of these two interventions is still unclear. However, Asadi et al. reported that the combination of ET and MSCs on the reduction of inflammatory factors in rats with OA was not significant [2]. Nonetheless, the most important factor in the lack of significant synergistic effects between ET and MSCs, as well as between ET, MSCs, and ozone therapy, can be attributed to the drug interference that has received particular attention in medical science.
Interferences that cause a drug to lose its positive effect and even exacerbate the drug’s adverse effects. In this regard, it is suggested that these three interventions be investigated in different time periods in future studies. Also, since CB-1 and GABA receptors are related to the anti-nociceptive pathway, one of the limitations of the present study is the lack of evaluation of the pain tolerance threshold in rats. Thus it is recommended that the test be measured along with related physiological variables. Another limitation of this study is the lack of measurement of physiological variables related to pain induction pathways such as inflammation and anti-nociceptive pathways such as serotonergic and GABAergic pathways. Therefore, it is suggested that in future studies, the mentioned signaling pathways be considered and evaluated.
Conclusion
ET, ozone therapy, and MSCs alone can have favorable effects on the CB-1 and GABA gene expressions. Also, the combination of ET and ozone therapy, as well as the combination of ozone therapy and MSCs, have favorable effects on the anti-nociceptive pathway in the animal model of OA. However, further studies should be conducted to investigate the combinatory effects of ET and MSCs and those of ET, ozone therapy, and MSCs.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Islamic Azad University, Central Tehran Branch, Iran.
Funding
Present study is a part of the doctoral dissertation approved by Central Tehran Branch of Islamic Azad University.
Authors' contributions
Conceptualization: Maryam Rezaie, Maghsoud Peeri; Methodology, funding acquisition, and resources: Maryam Rezaie; Investigation: Mohammad Ali Azarbayjani, Maghsoud Peeri; Writing-original draft: Seyed Ali Hosseini; Writing-review & editing: Seyed Ali Hosseini, Mohammad Ali Azarbayjani; Supervision: Mohammad Ali Azarbayjani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The present study is a part of the PhD. dissertation approved by Central Tehran Branch of Islamic Azad University. The authors express their gratitude and appreciation for the support of the Research and Technology Department.
References
Osteoarthritis (OA) is one of the most painful arthritic diseases that result in disability. Recent studies have shown that overweight, aging, genetics, and diabetes are risk factors for this disease [1, 2]. Increased inflammation following OA can disrupt the sympathetic nervous system [3]. Pain is one of the main symptoms of this disease. Increased inflammation and free radicals pressurize the nerves of this area, induce pain, and thereby reduce the motor ability and quality of life in patients with OA [4, 5].
On the other hand, since the identification of endocannabinoid receptors, many researchers have paid attention to this anti-nociceptive pathway. The endocannabinoid system includes cannabinoid receptors 1 and 2 (CB-1 and CB-2), binding to G proteins that are activated by cannabinoid binding and inhibit adenylyl cyclase and cyclic AMP production. As a result, it inhibits gamma-aminobutyric acid (GABA) and glutamate transport that, in turn, suppress the excitability of the nervous system and pain sensation [4, 6].
Despite much research, no effective treatment has been found for OA yet. However, recent studies have investigated the effect of mesenchymal stem/stromal cells (MSCs) on experimental models of cartilage injury. It has been reported that intra-articular injection of stem cells can reduce pain and protect cartilage. MSCs appear to express a variety of anti-inflammatory chemokines and cytokines in the cartilage tissue and are effective in the treatment of osteoarthritis by modulating cartilage metabolism and coping with inflammation and apoptosis [7]. Some studies have investigated the effect of MSCs in the treatment of OA. For example, the researchers in a study concluded that treatment with MSCs for 12 months reduced pain, inflammatory and anti-inflammatory factors, and improved metabolic factors in the cartilage tissue in patients with knee OA. These researchers also reported that the favorable effects were dependent on the dose and duration of treatment such that short-term treatment of fewer than 3 months had no significant impact on these variables [8, 9].
Given that many patients are old age, the duration of treatment and the economic costs will cause problems for them. So using complementary therapies to achieve the desired results is a worthwhile solution. In this regard, out of various therapeutic approaches, ozone therapy is shown to improve knee cartilage metabolism because of its anti-inflammatory properties and increased tissue oxygenation. Studies have reported that ozone therapy help improves inflammatory factors and reduce pain in patients with knee OA [10]. However, the results of a study showed that ozone therapy had no significant effect on pain in patients with OA for the first three months, and it shows its effects after six months [11].
Patients with knee OA usually have a reduced ability to perform their daily activities, and the most important factor in improving their quality of life and improving their condition is helping them to perform daily activities and reducing pain. Therefore, health activities have been considered as a non-invasive and low-cost treatment by health researchers. Regular and aerobic exercise increases joint metabolism and synovial fluid and has antioxidant and anti-inflammatory effects [12].
According to studies, complementary therapies reduce pain and improve the quality of life of these patients, but the mechanisms of their effects are not fully evident. Also, little information is known about the concurrent use of ozone therapy, MSCs, and ET on anti-nociceptive pathways. So, it seems necessary to conduct a study in this regard. We to investigate the effect of concurrent use of ET, ozone therapy, and MSCs on CB-1 and GABA gene expression in the cartilage tissue of rats with knee osteoarthritis.
Materials and Methods
Animals
In this experimental study, 45 rats, aged 40-45 weeks, weighing 250-300 g, were purchased and transferred to the animal house. During the research period, standard food plates and water were provided ad libitum. All rats were kept in the standard situation (temperature of 23±2°C, light/dark cycle of 12:12 h, and the humidity of 45%-55%). After one week of adaptation to the laboratory environment, OA was induced in all rats on the eighth day. The researchers received introduction letters from Islamic Azad University, Marvdasht Branch (IR.MIAU.RECE.1396.132).
Study experiment
In the present study, OA was surgically induced, according to Zhao et al. study [13]. In this method, rats were first anesthetized by ketamine and xylazine, then their right knee hairs were completely shaved, and a 1-cm area was cut in the knee joint. After removing the skin, the internal lateral ligament of the knee was removed to observe the internal meniscus. An incomplete cutting then resulted in rupture and injury to the meniscus and the area was. The rats were then fed with standard food for three weeks and kept in a laboratory environment for recovery. Then, they were divided into eight groups of 5 rats each: 1. osteoarthritis; 2. MSCs, 3. ozone, 4. ET, 5. ozone+MSCs; 6. ET+ozone, 7. ET+MSCs, and 8. ET+MSCs+ozone. Also, 5 rats were assigned to the healthy control group. Table 1 presents all procedures done in the experimental groups of ET, MSCs, ozone, and mix groups.
.jpg)
Study design
After grouping, MSC, ozone+MSCs, ET+MSCs, and ET+MSCs+ozone groups received intra- articular injection of 1×106 cells/kg MSCs into their right knee joint. Ozone, ozone+MSC, ET+ozone, and ET+MSC+ozone groups received 20 μg/mL ozone once weekly for 3 weeks. Besides, ET, ET+ozone, ET+MSCs, and ET+MSCs+ozone groups performed ET for 8 weeks, 3 sessions per week and 30-50 minutes per session.
Endurance training protocol
Before the training program, the rats in the ET group were adapted to a rodent treadmill for one week (three times per week, 60%-70% of VO2max at a speed of 16 m/min at 0% inclination for 10 min/d). Briefly, the training program was initiated with a 30-min run on a treadmill without slope at a speed of 16 m/min in the first week, which gradually reached 50 minutes in the eighth week. Warm-up and cool-down were performed for 5 m/min at the beginning and end of the training [14].
Mesenchymal stem cells therapy
On the other hand, in the MSCs groups, bone marrow-derived MSCs were extracted from healthy male Wistar rats after being anesthetized with ketamine (30-50 mg/kg) and xylazine (3-5 mg/kg). Briefly, their femur and tibia bones were cut off from the back limbs, remove the skin and muscles. The femurs and tibias were transferred to a 10-cm dish containing Dulbecco’s Modified Eagle Medium (DMEM). Each bone was then held with tweezers, and the two ends were cut open with a scissor. A 22-G needle was attached to a 3-mL syringe and filled with DMEM. Then the marrow was flushed into a 50-mL tube by inserting the needle to one open end of the bone. The procedure is repeated 2~3 times for each bone. When all marrows were obtained, the cells were resuspended, and the suspension was passed through a 70-μm cell strainer to remove the bone debris and blood aggregates. The cells were spun down at 200g, 4°C for 5 min and the supernatant was removed by aspiration. The cells were resuspended in 25-mL MSC medium (DMEM containing 10% Fetal Bovine Serum [FBS] and 1% Pen-Strep). An amount of 10 mL cell suspension was seeded into each 10-cm culture dish for a total of two dishes. The culture dishes were kept at 37°C and 5% CO2 incubator for 1~2 weeks. The medium was changed every 2~3 days.
The isolated MSCs were cultured in DMEM medium containing 20% FBS and incubated at 37°C overnight to select the adherent cells. The culture medium in the flasks was changed every 3 days, and MSCs were passaged 3-4 times. After MSCs reached >90% confluency, they were selected for injection purposes. Rats in the MSCs groups received an intraarticular injection of 1×106 cells/kg into their right knee joint [15].
Ozone therapy
A low-intensity electric discharge also produced ozone, and its concentration was measured at 254 nm using ultraviolet radiation. Ozone was injected into the knee tibia-femoral articular line at a concentration of 20 μg/mL once a week for 3 weeks starting at 21 days after the OA induction [16].
Polymerase chain reaction
Forty-eight hours after the last treatment, the rats were anesthetized with ketamine (30-50 mg/kg) and xylazine (3-5 mg/kg). Their cartilage tissues were isolated and homogenized in phosphate buffer saline (0.01 M; pH 7.0) at 4°C with a homogenizer (Hielscher, UP100H). RNA was extracted from cartilage tissues using the RNX-Plus (SinaClon; RN7713C) kit. The quantity and quality of extracted RNAs were examined with the NanoDrop ND-1000 spectrophotometer (Thermo Sci., Newington, NH) method. cDNA was synthesized from RNA samples using RevertAid Reverse Transcriptase (Thermo science, Germany) at 42°C for 1 h and random hexamer primers (Thermo science, Germany). A Rotor-Gene 6000 (Corbett Research, Australia) thermocycler and Real qPCR 29 Master Mix Kit (Amplicon, Denmark) in 40 cycles were applied for amplification. Each reaction included 5 μL master mix and 100 nm primers. Table 2 presents the sequence of the primers used to measure the expression level of the genes in the study.
.jpg)
Data analysis
The Shapiro-Wilk test was used to investigate the normality of the findings. Also, to examine the effect of OA induction on research variables, we used the independent samples t test to compare the difference between the healthy control and OA group. Besides, 3-way ANOVA was employed to investigate the effect of training, MSCs, ozone therapy, the combination of ET and MSCs, the combination of ET and ozone therapy, the combination of MSCs and ozone therapy, as well as the combination of ET, ozone therapy, and MSCs (P≤0.05).
Results
The levels of CB-1 and GABA gene expression are reported in Figures 1 and 2, respectively. The results showed that CB-1 (P=0.001) and GABA (P=0.001) levels were significantly lower in the control group compared with the healthy group.

ET (P=0.001), MSCs (P=0.003,) and ozone therapy (P=0.001) had significant effect on the increase of CB-1 gene expression compared with OA group. ET with ozone therapy (P=0.001), as well as MSCs with ozone therapy (P=0.004), had a significant effect on the increase of CB-1 gene expression compared with the OA group. Nevertheless, ET with MSCs did not have a significant effect on CB-1 gene expression (P=0.189). Also, ET, MSCs with ozone therapy (P=0.048) had a significant effect on the increase of CB-1 gene expression compared with the OA group (Table 3).
.jpg)
ET (P=0.001), MSCs (P=0.001), and ozone therapy (P=0.001) had a significant effect on the increase of GABA gene expression compared with the OA group. ET with ozone therapy (P=0.003), as well as MSCs with ozone therapy (P=0.006), had a significant effect on the increase of GABA gene expression compared with the OA group. However, ET with MSCs (P=0.206) and ET+MSCs+ozone therapy (P=0.507) did not have a significant effect on the increase of GABA gene expression compared with the OA group (Table 3).
Discussion
The results of this study showed that CB-1 and GABA levels were significantly lower in the control group than in the healthy group. In support of the findings of the present study, Hammell et al. (2016), Burston et al. (2013), and Yuan et al. (2018) stated that OA decreases cannabinoid receptors and decreases GABA and serotonin by increasing inflammation pathway [17, 18, 19]. On the other hand, the results showed that aerobic training, ozone therapy, and MSCs alone increased CB-1 and GABA gene expression in the cartilage tissue of rats with knee OA. The anti-nociceptive signal pathway of exercise training is dependent on the expression of neurotransmitters in the nervous system, which include increased expression of serotonin, opioids, increased activity of micro-opioids, cannabinoid receptors, increased N-methyl-D-aspartate and carriers of serotonin [20].
Consistent with the present study, regular and aerobic exercise training reduced and alleviated pain by increasing serotonergic and opioid activity following induction of alloxan-induced neuropathy [21]. Also, voluntary training with rotational wheel alleviated pain in rats with the same mechanism [22]. On the other hand, the researchers believe that the anti-nociceptive effects of exercise training are dependent on the intensity of exercise and the induction of fatigue caused by exercise. Therefore, vigorous exercise increases pain levels in athletes [23], and in individuals with OA that acts through a reduced cannabinoid receptors pathway [24].
Vigorous exercise seems to be a factor in increasing free radicals and inflammation that decrease cannabinoid receptors and GABA. Besides, ozone therapy with the mechanism of reducing arthritis inflammation enhances oxygen delivery to the injured tissue and induces the anti-nociceptive effects [10]. The precise mechanism of the anti-nociceptive effects of ozone therapy has not been fully understood. However, based on Cuadros study, two months of ozone therapy had no significant effect on pain in patients with knee OA [10].
The main reasons for inconsistency in these studies appear to be the differences in the statistical population, consumption rate, and duration of treatment. In another study, longer treatment with ozone reduced joint damage and inflammation [25]. Because of the simultaneous anti-inflammatory effects of ET and ozone therapy in this study, they were able to have synergistic effects on CB-1 and GABA receptor elevation. No studies have been found that investigated the concurrent effect of ozone therapy and ET on the variables of the present study; however, Asadi et al. reported that ET and ozone therapy alone increased interleukin-10 levels as an anti-inflammatory cytokine and reduced tumor necrosis factor-α in the cartilage tissue of rats with knee OA [2].
The researchers also suggested that MSCs could modulate bone growth and metabolic factors such as insulin-like growth factor-1, morphological proteins of bone growth by reducing the pro-inflammatory and inflammatory cytokines, as well as increasing anti-inflammatory cytokines, that result in modulation of serotonin and cannabinoid receptors in patients with osteoarthritis [26]. Also, the synergistic effects of MSCs and ozone therapy with affecting the pathway of arthritis and regulating metabolic factors and bone repair [2, 10] could increase CB-1 and GABA receptors.
Regarding the contradictory results of exercise training [20, 22, 23] on the similar molecular, cellular pathway as well as the therapeutic effects of MSCs that depend on the amount and duration of treatment [8, 9], a closer look failed to find an investigation on the interactive effect of training and stem cells simultaneously on cannabinoid receptors and GABA. Thus, the interactive effect of these two interventions is still unclear. However, Asadi et al. reported that the combination of ET and MSCs on the reduction of inflammatory factors in rats with OA was not significant [2]. Nonetheless, the most important factor in the lack of significant synergistic effects between ET and MSCs, as well as between ET, MSCs, and ozone therapy, can be attributed to the drug interference that has received particular attention in medical science.
Interferences that cause a drug to lose its positive effect and even exacerbate the drug’s adverse effects. In this regard, it is suggested that these three interventions be investigated in different time periods in future studies. Also, since CB-1 and GABA receptors are related to the anti-nociceptive pathway, one of the limitations of the present study is the lack of evaluation of the pain tolerance threshold in rats. Thus it is recommended that the test be measured along with related physiological variables. Another limitation of this study is the lack of measurement of physiological variables related to pain induction pathways such as inflammation and anti-nociceptive pathways such as serotonergic and GABAergic pathways. Therefore, it is suggested that in future studies, the mentioned signaling pathways be considered and evaluated.
Conclusion
ET, ozone therapy, and MSCs alone can have favorable effects on the CB-1 and GABA gene expressions. Also, the combination of ET and ozone therapy, as well as the combination of ozone therapy and MSCs, have favorable effects on the anti-nociceptive pathway in the animal model of OA. However, further studies should be conducted to investigate the combinatory effects of ET and MSCs and those of ET, ozone therapy, and MSCs.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Islamic Azad University, Central Tehran Branch, Iran.
Funding
Present study is a part of the doctoral dissertation approved by Central Tehran Branch of Islamic Azad University.
Authors' contributions
Conceptualization: Maryam Rezaie, Maghsoud Peeri; Methodology, funding acquisition, and resources: Maryam Rezaie; Investigation: Mohammad Ali Azarbayjani, Maghsoud Peeri; Writing-original draft: Seyed Ali Hosseini; Writing-review & editing: Seyed Ali Hosseini, Mohammad Ali Azarbayjani; Supervision: Mohammad Ali Azarbayjani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The present study is a part of the PhD. dissertation approved by Central Tehran Branch of Islamic Azad University. The authors express their gratitude and appreciation for the support of the Research and Technology Department.
References
- Villafañe JH. Exercise and osteoarthritis: An update. J Exerc Rehabil. 2018; 14(4):538-9. [DOI:10.12965/jer.1836352.176] [PMID] [PMCID]
- Asadi S, Farzanegi P, Azarbayjani MA. Effect of exercise, ozone and mesenchymal stem cells therapies on expression of IL-10 and TNF-α in the cartilage tissue of overweight rats with knee osteoarthritis. Soc Determ Heal. 2018; 4(3):162-70.
- Lowin T, Straub RH. Cannabinoid-based drugs targeting CB 1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res Ther. 2015; 17(1):1-13. [DOI:10.1186/s13075-015-0743-x] [PMID] [PMCID]
- Barrie N, Manolios N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur J Rheumatol. 2017; 4(3):210-8. [DOI:10.5152/eurjrheum.2017.17025] [PMID] [PMCID]
- Vedekoi J, Dongmo Selestin S, Juliette K, Pierre K. Effects of ethanol extract of the resin exudate of boswellia dalzielii hutch on pain in mice. Pharm Biomed Res. 2019; 5(2):32-7. [DOI:10.18502/pbr.v5i2.1583]
- Farhadi Moghaddam B, Fereidouni M, Abdollahi A. The effect of intraperitoneal administration of hydroalcoholic extracts of heated and unheated and hexanic extract of heated Cannabis sativa flowers on pain in rats (Cannabis sativa). Daneshvar. 2016; 23(121):53-60.
- Mancuso P, Raman S, Glynn A, Barry F, Murphy JM. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol. 2019; 7:9. [DOI:10.3389/fbioe.2019.00009] [PMID] [PMCID]
- Ham O, Lee C, Kim R, Lee J, Oh S, Lee M, et al. Therapeutic potential of differentiated mesenchymal stem cells for treatment of osteoarthritis. Int J Mol Sci. 2015; 16(7):14961-78. [DOI:10.3390/ijms160714961] [PMID] [PMCID]
- Chahal J, Gómez Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. 2019; 8(8):746-57. [DOI:10.1002/sctm.18-0183] [PMID] [PMCID]
- Cuadros MEF, Moro OSP, Florin MJA, Canelo JAM. Ozone improves pain, function and quality of life in patients with knee osteoarthritis: A prospective quasi-experimental before-after study. Middle East J Rehabil Heal. 2017; 4(1):e41821 [DOI:10.17795/mejrh-41821]
- Dernek B, Kesiktas FN. Efficacy of combined ozone and platelet-rich-plasma treatment versus platelet-rich-plasma treatment alone in early stage knee osteoarthritis. J Back Musculoskelet Rehabil. 2019; 32(2):307-11. [DOI:10.3233/BMR-181354] [PMID]
- Jahanjoo F, Eftekharsadat B, Bihamta A, Babaei-Ghazani A. Efficacy of balance training in combination with physical therapy in rehabilitation of knee osteoarthritis: A randomized clinical trial. Crescent J Med Biol Sci. 2019; 6(3):225-334.
- Zhao Y, Liu B, Liu CJ. Establishment of a surgically-induced model in mice to investigate the protective role of progranulin in osteoarthritis. J Vis Exp. 2014; (84):e50924. [DOI:10.3791/50924] [PMID] [PMCID]
- Liu S, Zhou P, Zhang Y. Abnormal expression of key genes and proteins in the canonical Wnt/β-catenin pathway of articular cartilage in a rat model of exercise-induced osteoarthritis. Mol Med Rep. 2016; 13(3):1999-2006. [DOI:10.3892/mmr.2016.4798] [PMID] [PMCID]
- Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016; 7(1):1-13. [DOI:10.1186/s13287-016-0420-2] [PMID] [PMCID]
- de Jesus CCL, dos Santos FC, de Jesus LMOB, Monteiro I, Sant’Ana MSSC, Trevisani VFM. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS One. 2017; 12(7):e0179185. [DOI:10.1371/journal.pone.0179185] [PMID] [PMCID]
- Yuan X-C, Zhu B, Jing X-H, Xiong L-Z, Wu C-H, Gao F, et al. Electroacupuncture potentiates cannabinoid receptor-mediated descending inhibitory control in a mouse model of knee osteoarthritis. Front Mol Neurosci. 2018; 11:112. [DOI:10.3389/fnmol.2018.00112] [PMID] [PMCID]
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, et al. Transdermal cannabidiol reduces inflammation and pain related behaviours in a rat model of arthritis. Eur J Pain. 2016; 20(6):936-48. [DOI:10.1002/ejp.818] [PMID] [PMCID]
- Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, et al. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One. 2013; 8(11):e80440. [DOI:10.1371/journal.pone.0080440] [PMID] [PMCID]
- Lima L V, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol. 2017; 595(13):4141-50. [DOI:10.1113/JP273355] [PMID] [PMCID]
- Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain modelrole of endogenous opioids. Anesthesiol J Am Soc Anesthesiol. 2011; 114(4):940-8. [DOI:10.1097/ALN.0b013e318210f880] [PMID] [PMCID]
- Bement MKH, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005; 86(9):1736-40. [DOI:10.1016/j.apmr.2005.03.029] [PMID]
- Geva N, Defrin R. Enhanced pain modulation among triathletes: A possible explanation for their exceptional capabilities. Pain. 2013; 154(11):2317-23. [DOI:10.1016/j.pain.2013.06.031] [PMID]
- Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain. 2017; 33(5):395-404. [DOI:10.1097/AJP.0000000000000418] [PMID]
- García AG, Gandía L. El condroitín sulfato y la glucosamina frenan la progresión de la artrosis y disminuyen la necesidad de prótesis. Actual en Farmacol y Ter. 2014; 12(3):145-53.
- Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, et al. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy-a review. BMC Musculoskelet Disord. 2016; 17(1):1-13. [DOI:10.1186/s12891-016-1085-9] [PMID] [PMCID]
Type of Study: Original Research |
Subject:
General
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








